Abstract
Melanopsin is the photopigment that confers light sensitivity on intrinsically photosensitive retinal ganglion cells. Mammalian intrinsically photosensitive retinal ganglion cells are involved in the photic synchronization of circadian rhythms to the day–night cycle. Here, we report molecular components of melanopsin signaling using the cultured Xenopus dermal melanophore system. Photo-activated melanopsin is shown to initiate a phosphoinositide signaling pathway similar to that found in invertebrate photo-transduction. In melanophores, light increases the intracellular level of inositol trisphosphate and causes the dispersion of melanosomes. Inhibition of phospholipase C and protein kinase C and chelation of intracellular calcium block the effect of light on melanophores. At least four proteins, 43, 74, 90, and 134 kDa, are phosphorylated by protein kinase C upon light stimulation. This provides evidence of an invertebrate-like light-activated signaling cascade within vertebrate cells.
Keywords: melanophore, phosphoinositide, phospholipase C, photoreception
The canonical visual photoreceptors (rods and cones) are not the only photosensitive cells in the mammalian retina. A subpopulation of retinal ganglion cells are intrinsically photosensitive, depolarizing in response to light (1). These cells project to the master circadian clock, the hypothalamic suprachiasmatic nucleus, and other brain sites known to participate in nonvisual responses to light (2–4). It has been demonstrated that these intrinsically photosensitive retinal ganglion cells (ipRGC) are important for photic regulation of the circadian oscillator, acute suppression of pineal melatonin, and acute suppression of activity (masking) in rodents (5, 6). Melanopsin (gene symbol Opn4), a recently identified opsin-based photopigment (7, 8), is expressed in ipRGC and is required for their photosensitivity (9). Melanopsin was initially cloned from cultured photosensitive dermal melanophores derived from Xenopus laevis embryos (7). Its peptide sequence is consistent with melanopsin being a member of the superfamily of heptahelical G protein-coupled receptors; specifically, the family of photopigment proteins known as opsins. Despite its vertebrate source, melanopsin's predicted peptide sequence bears greater homology to invertebrate than to vertebrate opsins (7). Although phototransduction is well understood in vertebrate visual photoreceptors and the photoreceptors of some invertebrates, virtually nothing is known currently about intracellular melanopsin-initiated signaling pathways (1).
Photosensitive amphibian melanophores are an ideal model in which to investigate melanopsin signal transduction. These cells grow at room temperature by using atmospheric air and show a robust melanosome dispersion in response to illumination. Scoring of this photoresponse can be automated through absorbance monitoring in a microtiter plate reader (10–12). Although ipRGC and Xenopus melanophores naturally express melanopsin, the ultimate cellular responses to melanopsin activation are quite different; melanophores redistribute melanosomes while ipRGC produce a membrane depolarization, suggesting that the terminal effectors of the signaling pathways are different. However, the high degree of amino acid homology between amphibian and mammalian melanopsins, particularly within the domains that interact with G proteins, predicts conservation of upstream signaling.
Although rhodopsin has been reported in some photoaggregating tailfin melanophores (13), among the known Xenopus opsins, only melanopsin message was detected by real-time PCR in the cultured melanophores used in this study. Melanophores derived from transgenic Xenopus that constitutively overexpress melanopsin are 100-fold more sensitive to light than nontransgenic control melanophores (12), demonstrating that melanopsin mediates the melanosome photodispersion response. In this study, we demonstrate that light-stimulated melanopsin activates a phosphoinositide cascade, resulting in melanosome granule dispersion within Xenopus melanophores.
Materials and Methods
Characterization of the Model. Melanophores were derived from X. laevis embryos in 1992 and were maintained in culture as described in ref. 12. Briefly, cells were kept in 60% L-15 medium, 480 mg/liter galactose, 5 mg/liter insulin/transferrin/selenium, 4 mg/liter uridine, 87.6 mg/liter l-glutamine, 25 mg/liter l-asparagine, 152 mg/liter CaCl2, 49.6 mg/liter MgCl2, 51.7 mg/liter MgSO4, 0.4× MEM nonessential amino acids solution, 0.2× MEM amino acids solution, 0.3× MEM vitamin solution, 2× HT supplement, 0.05% penicillin/streptomycin, and 5% noninactivated FCS (all from GIBCO/BRL) (pH 7.5) at 25°C.
To determine the EI50 irradiance (irradiance necessary to evoke 50% of the maximal pigment dispersing response), Xenopus melanophores were seeded in 96-well plates in 60% L-15 medium supplemented with 2% serum and 10–7 M all-trans-retinaldehyde (Sigma) and kept in the dark for a minimum of 3 days. The addition of retinaldehyde has been shown to maintain the photosensitivity of the cells (14). Before the assay, the cells were incubated with 10–9 M melatonin for 90 min to fully aggregate the melanosomes. Cells were then exposed to increasing intensities of white light, and the photoresponse, melanosome dispersion, was quantified as absolute absorbance in a plate reader (Sunrise, Tecan, Durham, NC) and expressed as a percentage of the maximal absorbance value obtained with 10–8 M α-melanocyte-stimulating hormone (α-MSH), a potent melanosome-dispersing hormone. The EI50 irradiance (obtained by nonlinear regression, prism v.2.01, GraphPad, San Diego) was used in subsequent pharmacologic assays.
A spectral sensitivity curve was obtained by using 1-inch interference filters ranging in peak transmission wavelengths from 420 to 520 nm (half-peak bandwidths of 10 nm; Thermo Oriel, Stratford, CT).
Total RNA was extracted with TRIzol (Invitrogen) and reverse transcribed (SuperScript II Reverse-Transcriptase Preamplification System, Invitrogen). PCR was performed for Xenopus known opsins by using specific primers (melanopsin, forward 5′-AGC AAG TTC AGC CTG GTT AAG-3′, reverse 5′-TTA TGA GTA TTT CTT CCA GGG-3′; rhodopsin, forward 5′-CAA TGC TCA TGC GGA GTA GA-3′, reverse 5′-AAG TTA GAG CCC TGG TGG GT-3′; red, forward 5′-TCT CTG ACG GTC ATT GCT TG-3′, reverse 5′-CTC TTG GCA AAG TAG GCA GG-3′; violet, forward 5′-ATG GGC CAC AAT ACC ACA TT-3′, reverse 5′-CAA CAG CCA AAG CAT GAG AA-3′; and green, forward 5′-AGT AAA GGC CGT CCA GAC CT-3′, reverse 5′-GAT TGC AAG AGG GAA TCC AA-3′).
Second Messenger Determination. For cyclic nucleotide assays, Xenopus melanophores were seeded in 96-well plates as described above. Cells were then preincubated in 10–4 M phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX, Sigma) for 15 min, followed by exposure to light for 1 min in the presence of IBMX. Additional triplicates were kept in the dark and received 10–8 M α-MSH (Sigma), as a positive control for cAMP, or the nitric oxide-independent guanylyl cyclase activator YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (Biomol)], as a positive control for cGMP. Cells subsequently were lysed with kit reagents, and the manufacturer's protocol was followed as described for total cAMP (Biotrak Enzymeimmunoassay System, code RPN 225, Amersham Pharmacia Biosciences) or total cGMP (Biotrak Enzymeimmunoassay System, code RPN 225, Amersham Pharmacia Biosciences) measurements. The data were derived from standard curves to obtain cAMP or cGMP concentrations, expressed as femtomoles per 104 cells.
To assay inositol 1,4,5-trisphosphate (IP3), Xenopus melanophores were seeded in six-well plates as described above. After exposure to 15 min of light, the cells were lysed with 20% perchloric acid (0.2-vol equivalents of the cell suspension volume) and centrifuged (2,000 × g for 15 min), and the supernatant was neutralized to pH 7.5 (Method A, kit TRK1000, Amersham Pharmacia Biosciences). Upon completion of the kit procedure, the amount of [3H]IP3 in the experimental samples and standards was measured in a β-scintillation counter (Beckman, model LS5801). Experimental values were derived from the standard curve and expressed as percentage of the dark control.
Melatonin-aggregated Xenopus melanophores were also treated with the cell-permeable cGMP analogue, 8-bromo-cGMP (Biomol), at a 10–4 M concentration for 30 and 60 min in the dark. The absorbances were compared with those of melatonin-treated cells exposed to light for the same durations of time.
Inhibitors of Signaling Pathway Components. To test the inhibitory effects of various pharmacological agents on the light response, Xenopus melanophores were seeded in 96-well plates as described above. Before the assays, the cells were treated with 10–9 M melatonin for 90 min, and a signaling pathway inhibitor was added to the medium in the last 30 min of this period. Plates were then exposed to light, and control triplicates were kept in the dark. Absorbance was measured after 30 and 60 min, and the responses were expressed as the percentage of the maximal response obtained after 60 min of exposure to EI50 (considered as 100%) in the absence of inhibitors. The following signaling pathway inhibitors were used: KT 5823 and DT-2 (protein kinase G), SQ 22536 [9-(tetrahydro-2′-furyl)adenine, adenylyl cyclase], H-89 {N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide·2HCl, protein kinase A}, U-73122 [1-(6-(17-3-methoxyestra-1,3,5(10)-trien-17-yl)amino) hexyl-1H-pyrrole-2,5-dione, phospholipase C (PLC)], Ro 31-8220 {2-[1-3(-(amidinothio)propyl)-1H-indol-3-yl]-3-(1-methylindol-3-yl)maleimide methanesulfonate, PKC}, and BAPTA-AM [1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid tetraacetoxymethyl ester, calcium chelator] (all from Biomol).
PKC Activity and Western Blot Analysis. For PKC activity assays, Xenopus melanophores were seeded in six-well plates and kept in the dark or exposed to 15 min of light or 10–5 M phorbol 12,13-dibutyrate in the absence or presence of 10–5 M Ro 31-8220. Cells were lysed with 200 μl of PhosphoSafe extraction buffer (Novagen) containing protease inhibitors (Roche) and incubated for 15 min at 37°C in the kit buffers (Protein Kinase C Enzyme Assay system, RPN77, Amersham Pharmacia Biosciences) supplemented with 40 μCi/ml [γ-32P]ATP (1 Ci = 37 GBq). The reaction was stopped with the appropriate kit reagent, and 35 μl of the reaction was transferred to paper discs provided in the kit. After rinsing with 75 mM orthophosphoric acid, the 32P incorporated into the synthetic peptide PKC substrate (provided in the kit) was measured in a β-scintillation counter (Beckman, model LS5801) and expressed as nanomoles of phosphate transferred per minute.
For Western blot analysis, Xenopus melanophores were seeded and treated as described above. After lysis with 300 μlof PhosphoSafe extraction buffer containing protease inhibitors (Roche), the protein concentration was determined with the BCA protein assay kit (Pierce), and the mixture of loading gel, antioxidant agent (Invitrogen), and 25 μg of protein was heat-denatured (70°C for 10 min) and loaded into a 10% NuPAGE Tris-Bis gel (Invitrogen). Electrophoresis was performed at 200 V and 100 mA for 50 min. After transfer (30 V and 170 mA) to PDVF membranes, the blot was developed by using the WesternBreeze chromogenic immunodetection kit (Invitrogen). Briefly, nonspecific sites were blocked (1 h), and the membranes were incubated overnight at 4°C in a mixture of phospho-(Ser) PKC substrate antibody (1:1,000 dilution; Cell Signaling Technology) and a loading control antibody against β-actin (1:1,000 dilution; Abcam). Membranes were then rinsed (four times, 5 min) with the kit antibody washing solution, incubated in the alkaline phosphatase-conjugated anti-rabbit secondary antibody for 1 h at room temperature, rinsed again (four times, 5 min), and finally placed in a solution of 5-bromo-4-chloro-3-indolyl-1-phosphate and nitro blue tetrazolium (BCIP/NBT) substrate for alkaline phosphatase until bands appeared. After rinsing in water, the membranes were air-dried, scanned, and digitalized, and the optical densities were quantified by using nih image software (Version 1.62, by Wayne Rasband, National Institutes of Health, Bethesda). Band densities were normalized to that of the anti-β-actin immunoreactive band within the same lane.
Statistical Analyses. The data were compared by one-way ANOVA, followed by a Student–Newman–Keuls test (significance at P < 0.05).
Results
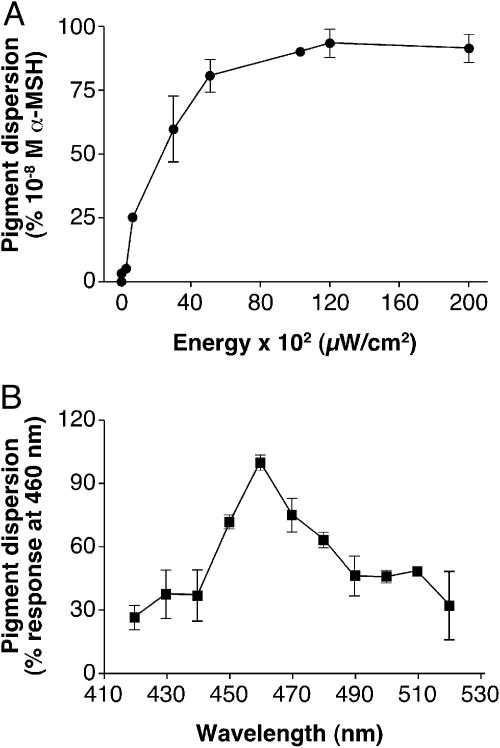
To characterize the effects of light on the melanophores, we used a subsaturating stimulus to allow discrimination between different pharmacologic manipulations. An irradiance–response curve was obtained with white light, and the EI50 irradiance was determined to be 20.82 × 102 μW/cm2 (Fig. 1A). This irradiance was used for subsequent pharmacologic experiments. We also characterized the spectral sensitivity of the response by administering equal-photon stimuli at a range of wavelengths. We found peak spectral sensitivity between 450 and 470 nm (Fig. 1B). (The 10-nm half-peak bandwidth of our interference filters did not permit a finer resolution of spectral sensitivity.)
Fig. 1.
Xenopus melanophores were seeded in 96-well plates and kept in the dark for a minimum of 3 days. Before the assay, the cells were incubated with 10–9 M melatonin for 90 min to fully aggregate the melanosome. (A) Cells were exposed to increasing intensities of white light, and the photoresponse, melanosome dispersion, was quantified as absolute absorbance in a plate reader and expressed as the percentage of the absorbance of cells treated with 10–8 M α-MSH (considered as 100%), a potent melanosome-dispersing hormone. The EI50 was determined to be 20.82 × 102 μW/cm2. This nonsaturating irradiance was used in all pharmacologic assays. Each point is the mean ± SE (n = 6) at the irradiance values noted. (B) Cells were exposed to a range of wavelengths, and melanosome dispersion was quantified as the absolute absorbance and expressed as the percentage of the absorbance of cells irradiated with 460 nm (considered as 100%). The number of photons was kept constant (1 × 1018 s–1·cm–2). Each point is the mean ± SE (n = 6) at the wavelength noted.
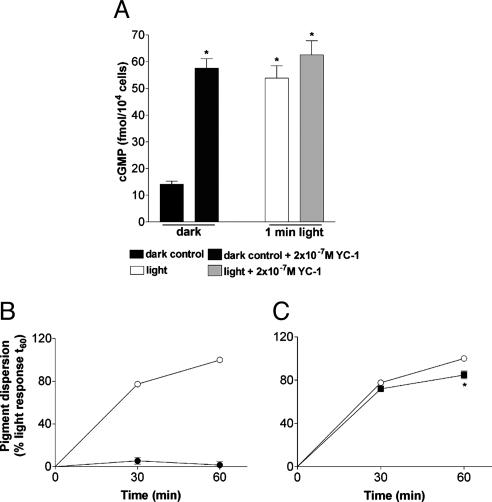
Because cGMP is involved in phototransduction of most ciliary (15) and some rhabdomeric photoreceptors (16), we first investigated the role of cGMP in the melanosome photoresponse. We observed a 4-fold increase in cGMP after a 1-min exposure to light, similar to that which we found after the application of the guanylyl cyclase activator YC-1 in the dark (Fig. 2A); YC-1 did not augment the light-induced cGMP increase. Application of the cell-permeable cGMP analog 8-bromo-cGMP in the dark did not evoke dispersion (Fig. 2B). Inhibition of protein kinase G (PKG) with the PKG inhibitor KT8525 (data not shown) also had no effect after 30 and 60 min of light exposure. DT-2, a membrane-permeable PKG-specific peptide blocker had no effect after 30 min of light exposure and caused a <20% decrease of the response after 60 min (Fig. 2C). It is unlikely, therefore, that the light-induced increase in cGMP triggers melanosome dispersion.
Fig. 2.
Xenopus melanophores were seeded in 96-well plates and kept in the dark for a minimum of 3 days. (A) cGMP determination in Xenopus melanophores in the dark and after a 1-min light exposure, in the absence or presence of the guanylyl cyclase activator, YC-1, at 2 × 10–7 M. Each bar is the mean ± SE (n = 6) of femtomoles per 104 cells. Asterisks indicate a significant difference from the dark control. (B) Melanosome dispersion in response to 10–4 M 8-bromo-cGMP in the dark (•), as compared with the light-evoked response (○). (C) Light-evoked melanosome dispersion in Xenopus melanophore in the absence (○) or presence (▪) of the PKG inhibitor, DT-2, at 10–5 M. In B and C the cells were pretreated with 10–9 M melatonin for 90 min, and the absorbance readings were expressed as a percentage of the absorbance of cells exposed to the EI50 for 60 min (considered as 100%). Each point is the mean ± SE (n = 6) at the times noted. Asterisks indicate a significant difference from the light response.
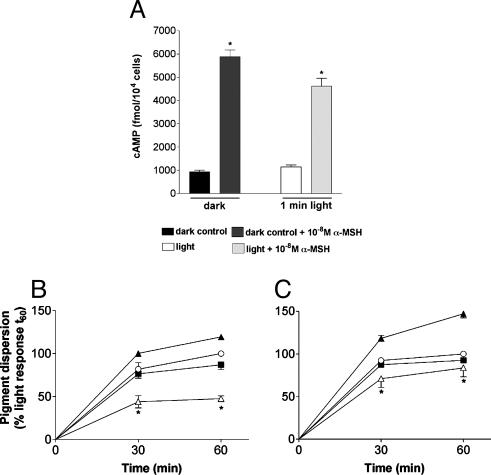
Previously, it had been shown that Xenopus melanophores disperse melanosomes in response to α-MSH through a cAMP signaling pathway (ref. 17; for a review, see ref. 18). We did not observe an increase in cAMP after exposure of our melanophore cultures to 1 min of light, although a 6-fold increase in cAMP was induced by α-MSH (Fig. 3A). The adenylyl cyclase inhibitor SQ 22536 (Fig. 3B) and the protein kinase A inhibitor H-89 (Fig. 3C) had no effect on the photodispersion response to light. However, these compounds markedly suppressed the dispersion response to α-MSH (Fig. 3 B and C). Thus, light-induced melanosome dispersion in our melanophores is not triggered by cAMP signaling.
Fig. 3.
Xenopus melanophores were seeded in 96-well plates and kept in the dark for a minimum of 3 days. (A) cAMP determination in Xenopus melanophores in the dark and after a 1-min light exposure, in the absence or presence of 10–8 M α-MSH. Each bar is the mean ± SE (n = 6). Asterisks indicate a significant difference from the dark control. (B) Light-evoked (○) or 10–8 M α-MSH-evoked (▴) melanosome dispersion in the presence of the adenylyl cyclase inhibitor, SQ-22536, at 10–4 M(▪, light plus SQ-22536; ▵, α-MSH plus SQ-22536). (C) Light-evoked (○) or 10–8 M α-MSH-evoked (▴) melanosome dispersion in the presence of the PKA inhibitor, H-89, at 10–5 M(▪, light plus H-89; ▵, α-MSH plus H-89). In B and C the cells were pretreated with 10–9 M melatonin for 90 min, and the absorbance readings were expressed as a percentage of the absorbance of cells exposed to the EI50 for 60 min (considered as 100%). Each point is the mean ± SE (n = 6) at the times noted. Asterisks indicate a significant difference from the α-MSH-evoked response.
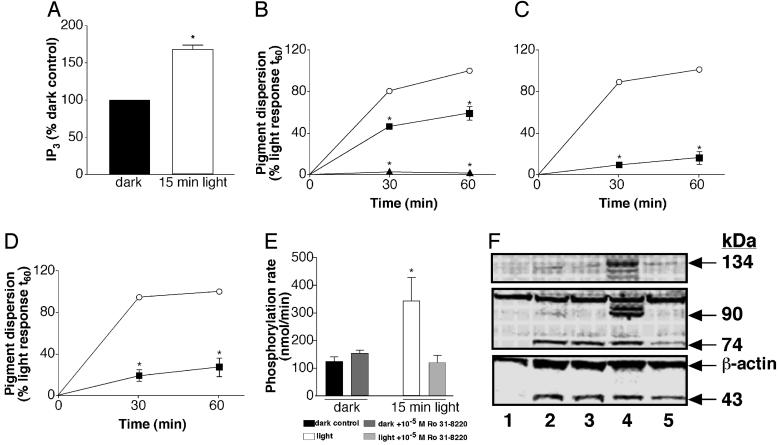
We then investigated the phosphoinositide-signaling pathway, a second known pathway leading to melanosome dispersion in amphibians (18). A 15-min light stimulus increased IP3 levels to 170% of dark controls (Fig. 4A). The PLC inhibitor U-73122, at 5 × 10–5 M, completely inhibited light-induced melanosome dispersion (Fig. 4B), and the intracellular calcium chelator BAPTA-AM blocked 90% and 84% of the photoresponse after 30- and 60-min light exposure, respectively (Fig. 4C). In addition, the PKC-specific inhibitor Ro 31-8220 reduced the photodispersion response to 26% of the control (Fig. 4D), and furthermore, light increased PKC activity, and this increase in enzymatic activity can be blocked by Ro 31-8220 (Fig. 4E). We found that 43-, 74-, 90-, and 134-kDa proteins are phosphorylated on PKC-specific Ser residues by light or phorbol esters (Fig. 4F). These results demonstrate that light-activated melanopsin triggers PLC activation, IP3 production, intracellular Ca2+ increase, and PKC activation. The subsequent phosphorylation events promoted by this Ca2+-dependent PKC result in melanosome dispersion.
Fig. 4.
Xenopus melanophores were seeded in six-well plates and kept in the dark for a minimum of 3 days. (A) IP3 determination in Xenopus melanophores in the dark and after a 15-min light exposure. Each bar is the mean ± SE (n = 3) percentage of the dark control, considered as 100%. Asterisks indicate a significant difference from the dark control. (B–D) Light-evoked melanosome dispersion in cells pretreated with 10–9 M melatonin for 90 min, in the absence (○) or presence of the PLC inhibitor, U-73122, at 5 × 10–6 M(▪) and 5 × 10–5 M(▴)(B); the Ca2+ chelator, BAPTA-AM, at 10–5 M(▪)(C); or the PKC inhibitor, Ro 31-8220, at 10–5M ▪)(D). The absorbance readings were expressed as the percentage of the absorbance of cells exposed to the EI50 for 60 min (considered as 100%). Each point is the mean ± SE (n = 6) at the times noted. Asterisks indicate a significant difference from the light response. (E) PKC activity in the dark and after a 15-min light exposure, in the absence or presence of the PKC inhibitor, Ro 31-8220, at 10–5 M. Each point is the mean ± SE (n = 6) phosphorylation rate in nmol/min. Asterisks indicate a significant difference from the dark control. (F) Representative Western blot of PKC-phosphorylated substrates in Xenopus melanophores maintained in the dark or after a 15-min light exposure or in the presence of 10–5 M phorbol 12,13-dibutyrate, in the absence or presence of the PKC inhibitor, Ro 31-8220, at 10–5 M. Lane 1, dark control; lane 2, light; lane 3, light plus 10–5 M Ro 31-8220; lane 4, 10–5 M phorbol ester; lane 5, 10–5 M phorbol ester plus 10–5 M Ro 31-8220.
The previously described overexpression studies (12), our observed spectral sensitivity, and the absence of other opsins in our cell line all indicate that melanopsin mediates the photodispersion response. Future melanopsin “knock-down” studies should confirm this finding.
Discussion
The cultured X. laevis melanophores used in this study respond to light with melanosome dispersion, the characteristic photoresponse in melanophores derived from Xenopus embryos (12). Our observed EI50 (20.82 × 102 μW/cm2) is similar to the value found by Rollag and collaborators (12) in this cell line, using the melanophore index as a response parameter. The photoresponse has an action spectrum suggesting involvement of an opsin (19), which is consistent with the report that the photic response depends on retinaldehyde (14). In our hands, the spectral maximal response is between 450 and 470 nm.
The cell-permeable cGMP analogue, 8-bromo cGMP, did not evoke melanosome dispersion in Xenopus melanophores, and the light response was mildly affected by PKG blockers. However, a 4-fold increase in cGMP level was observed after illumination and was not augmented by pharmacologic activation of guanylyl cyclase, suggesting that the maximal production of the cyclic nucleotide had been achieved with light stimulation. It is interesting to note that in rods and cones there is a light-induced decrease of cGMP promoted by the activation of photoreceptor-specific phosphodiesterases (resulting in cation channel closing and hyperpolarization), whereas in scallop photoreceptors and in the vertebrate parietal eye, cGMP increases with light, eliciting either a K+ inward current in scallop (20) or Na+ channel opening in the parietal eye of reptiles (21). Thus, with respect to cGMP, melanopsin signal transduction more closely matches that of invertebrate photoreceptors.
Two biochemical routes can lead to hormone-induced melanosome dispersion in amphibian melanophores: increase of cAMP and subsequent PKA activation (22, 23), or increase of diacylglycerol (DAG) and subsequent PKC stimulation (17). Although it had been reported that light triggers an increase of cAMP in Xenopus melanophores (19), we were not able to reproduce those results with our cell line. No increase in cAMP was seen after light stimulation either in the absence or presence of melatonin, whereas positive controls with α-MSH in the dark or light exhibited 4- to 6-fold increases in the cyclic nucleotide concentration, even in the presence of the antagonist hormone, melatonin. Additional confirmation that the cAMP/PKA pathway plays no role in the melanosome photodispersion is evident from the lack of inhibition in the presence of adenylyl cyclase and PKA inhibitory agents. Again, positive controls were performed with α-MSH, demonstrating the integrity of the system and the efficacy of the pharmacologic agents.
We then investigated the IP3/DAG pathway. U-73122, a well established PLC inhibitor, completely blocked the melanosome-dispersing response to light. The catalytic activity of PLC cleaves membrane phospholipids, mainly phosphatidylinositol 4,5 bisphosphate (PIP2), to IP3 and DAG. We observed an increase in PLC activity as demonstrated by the 70% increase in IP3 generated after light exposure, similar to what has been reported in the literature for other Gq-coupled receptors (24). IP3 opens Ca2+ channels in the endoplasmic reticulum, thus leading to an increase of cytoplasmic Ca2+ concentration. Furthermore, IP3 may be converted to a variety of inositol phosphates, which in turn, may also trigger other intracellular signals (25). Chelating free intracellular Ca2+ with BAPTA-AM totally blocked the response to light in Xenopus melanophores, demonstrating the crucial role of an increase in intracellular Ca2+ in the signaling pathway.
On the other arm of the cascade, DAG may activate PKC. PKCs are usually subdivided in three categories: (i) classic PKC (cPKC), responsive to DAG, Ca2+, phosphatidylcholines, and phorbol esters; (ii) new PKC (nPKC), activated by phosphatidylcholines, DAG, and phorbol esters but insensitive to Ca2+; and (iii) atypical PKC (aPKC), activated only by phosphatidylserines (26). In Xenopus melanophores, Ro 31-8220, a potent PKC inhibitor, almost totally blocked light-induced pigment granule dispersion, proving the importance of PKC activation in the photoresponse. In fact, the kinase activity was increased 3-fold in melanophores exposed to light. In addition, the Western blot assays, using a specific antibody against PKC substrates, demonstrated light-induced phosphorylation of at least four proteins of varying sizes by PKC, whose phosphorylation in the presence of Ro 31-8220 or in the dark controls was partial or absent. We have previously demonstrated that three PKC isoforms are present in X. laevis melanophores, the cPKCs, beta 1 and beta 2, and the atypical PKC zeta (27). Phorbol esters mimic light-induced phosphorylation in addition to phosphorylating other proteins. Assuming that Xenopus melanophore PKC is phorbol- and Ca2+-sensitive, and its activation is being promoted by DAG, the participation of the zeta isoform may be ruled out, making the beta isoforms strong candidates for phosphorylating light-activated pathway components.
The primary finding of these studies is that the phosphoinositide signaling cascade mediates photodispersion of melanosomes in amphibian dermal melanophores that selectively express melanopsin. Photic activation of a phosphoinositide second messenger system is common among invertebrate photoreceptors but not previously described in a vertebrate system. According to a comparative molecular analysis of photoreceptor gene families, RGC, horizontal cells, and amacrine cells have evolved from a rhabdomeric photoreceptor ancestor, unlike rods and cones, which are derived from a common ciliary photoreceptor precursor (28). Thus, the fact that light signaling in ipRGC uses the phosphoinositide pathway may reflect this evolutionary history. In fact, the coexistence of ciliary and rhabdomeric photoreceptor cells has been demonstrated in the rag-worm Platynereis, indicating that both types arose early in evolution (29).
Coupling of melanopsin with the inositol trisphosphate signaling pathway is also consistent with melanopsin's higher degree of amino acid homology to rhabdomeric opsins than to ciliary opsins. Our data, together with the recent literature (28, 29), are making clear that the distinction has to be made between rhabdomeric and ciliary photoreceptors rather than between vertebrate and invertebrate photoreceptors. The terminal effector of rhabdomeric photoreception is typically a transient receptor potential (trp) channel (16). These channels mediate light-induced calcium influx and could be activated by melanopsin coupling to a phosphoinositide second messenger system in mammalian ganglion cells, cells that have an action spectrum similar to the spectral sensitivity we observed in the amphibian melanophore cell line studied here.
Acknowledgments
This work was supported by Uniformed Services University Grant H070PY (to I.P.), National Institutes of Health Grant R01 NS052112 (formerly R01 MH62405) (to I.P.), Fundaçao de Amparo à Peçquisa do Estado de Sao Paulo Grant 01/02460-1 (to A.M.d.L.C.), and Conselho Nacional de Pesquisas Grant 550676/2002-3 (to A.M.d.L.C.).
Author contributions: M.C.I., M.D.R., A.M.d.L.C., and I.P. designed research; M.C.I. and A.M.d.L.C. performed research; M.C.I., M.D.R., A.M.d.L.C., and I.P. analyzed data; M.C.I., M.D.R., A.M.d.L.C., and I.P. wrote the paper; M.D.R. contributed new reagents/analytic tools.
Abbreviations: α-MSH, α-melanocyte-stimulating hormone; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; ipRGC, intrinsically photosensitive retinal ganglion cells; PLC, phospholipase C.
References
- 1.Berson, D.M., Dunn, F.A. & Takao, M. (2002) Science 295, 1070–1073. [DOI] [PubMed] [Google Scholar]
- 2.Hattar, S., Liao, H.N., Takao, M., Berson, M.D. & Yau, K.-W. (2002) Science 295, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooley, J. J., Lu, J., Fischer, D. & Saper, C. B. (2003) J. Neurosci. 23, 7093–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morin, L. P., Blanchard, J. H. & Provencio, I. (2003) J. Comp. Neurol. 465, 401–416. [DOI] [PubMed] [Google Scholar]
- 5.Panda, S., Sato, T. K., Castrucci, A. M. L., Rollag, M. D., DeGrip, W. J., Hogenesch, J. B., Provencio, I. & Kay, S. A. (2002) Science 298, 2213–2216. [DOI] [PubMed] [Google Scholar]
- 6.Panda, S., Provencio, I., Tu, D. C., Pires, S. S., Rollag, M. D., Castrucci, A. M. L., Pletcher, M. T., Sato, T. K., Wiltshire, T., Andahary, M., et al. (2003) Science 301, 525–527. [DOI] [PubMed] [Google Scholar]
- 7.Provencio, I., Jiang, G., DeGrip, W., Hayes, W. P. & Rollag, M. D. (1998) Proc. Natl. Acad. Sci. USA 95, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provencio, I., Rollag, M. D. & Castrucci, A. M. (2002) Nature 415, 493. [DOI] [PubMed] [Google Scholar]
- 9.Lucas, R. J., Hattar, S., Takao, M., Berson, D. M., Foster, R. M. & Yau, K. M. (2003) Science 299, 245–247. [DOI] [PubMed] [Google Scholar]
- 10.Lerner, M. R. (1994) Trends Neurosci. 17, 142–146. [DOI] [PubMed] [Google Scholar]
- 11.McClintock, T. S. & Lerner, M. R. (1997) Brain Res. Brain Res. Prot. 2, 59–68. [DOI] [PubMed] [Google Scholar]
- 12.Rollag, M. D., Provencio, I., Sugden, D. & Green, C. B. (2000) Methods Enzymol. 316, 291–309. [DOI] [PubMed] [Google Scholar]
- 13.Moriya, T., Miyashita, Y., Arai, J., Kusunoki, S., Abe, M. & Asami, K. (1996) Exp. Zool. 276, 11–18. [DOI] [PubMed] [Google Scholar]
- 14.Rollag, M. D. & Lynch, G. R. (1993) J. Exp. Zool. 265, 488–495. [DOI] [PubMed] [Google Scholar]
- 15.Finn, J. T., Solessio, E. C. & Yau, K.-W. (1997) Nature 385, 815–819. [DOI] [PubMed] [Google Scholar]
- 16.Garger, A. V., Richard, E. A. & Lisman, J. E. (2004) BMC Neurosci. 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugden, D. & Rowe, S. J. (1992) J. Cell Biol. 119, 1515–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nery, L. E. & Castrucci, A. M. L. (1997) Comp. Biochem. Physiol. A Physiol. 118, 1135–1144. [DOI] [PubMed] [Google Scholar]
- 19.Daniolos, A., Lerner, A. B. & Lerner, M. R. (1990) Pigm. Cell Res. 3, 38–43. [DOI] [PubMed] [Google Scholar]
- 20.Gomez, M. P. & Nasi, E. (2000) J. Neurosci. 20, 5254–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong, W.-H., Solessio, E. C. & Yau, K.-W. (1998) Nat. Neurosci. 1, 359–365. [DOI] [PubMed] [Google Scholar]
- 22.McGuire, J., Moellmann, G. & McKeon, F. (1972) J. Cell Biol. 52, 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, B. H., Sekura, R. D. & Rollag, M. D. (1987) J. Comp. Physiol. 157, 153–159. [DOI] [PubMed] [Google Scholar]
- 24.Jin, N., Packer, C. S., English, D. & Rhoades, R. A. (1993) Am. J. Physiol. 264, L160–L164. [DOI] [PubMed] [Google Scholar]
- 25.Rhee, S. G. (2001) Annu. Rev. Biochem. 70, 281–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizuka, Y. (1988) Nature 334, 661–665. [DOI] [PubMed] [Google Scholar]
- 27.Isoldi, M.C., Castrucci, A. M. L., Lima, L. H. R. G., Visconti, M. A. & Rebouças, N. A. (2003) Pigm. Cell Res. 16, 639–643. [DOI] [PubMed] [Google Scholar]
- 28.Arendt, D. (2003) Int. J. Dev. Biol. 47, 563–571. [PubMed] [Google Scholar]
- 29.Arendt, D., Tessmar-Raible, K., Snyman, H., Dorresteijn, A. W. & Wittbrodt, J. (2004) Science 306, 869–871. [DOI] [PubMed] [Google Scholar]