Abstract
Pedersen et al. (Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, et al. 2016. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 535: 376–381.) report that human serum levels of branched-chain amino acids (BCAA) and N-acetylglucosamine (GlcNAc) increase in proportion to insulin resistance. They focus on the microbiome and the contributing subset of microbe species, thereby demonstrating disease causality in mice. As either oral GlcNAc or BCAA in mice are known to increase insulin resistance and weight gain, we note that recently published molecular data argues for a cooperative interaction.
Keywords: branched-chain amino acids, insulin resistance, microbiome, mTOR, N-acetylglucosamine
In large scale molecular screens, there is a wealth of data, often beyond our capacities for integration into a single coherent story for publication. We are generally forced to select a few top hits for experimental validation of the screen and biological hypothesis, while leaving others for another time. With our interests in N-glycosylation and metabolic regulation of mTor signaling, we were excited to read Pedersen et al. (2016) and find elevated serum N-acetyl d-glucosamine (GlcNAc) (unexplored therein), as well as branched chain amino acids (BCAA) (the focus of validation) in the Supplementary Table of metabolites associated with insulin resistance (IR). Oral supplements with either GlcNAc (Ryczko et al. 2016) or BCAA (Newgard et al. 2009) are reported to increase IR in rodents. Pedersen et al. (2016) reveal the human gut microbiota as an important source of these metabolites in serum, which we posit herein are likely to interact cooperatively.
IR increases with obesity, hypertension and a sedentary life-style, and it portends a higher risk of type 2 diabetes and cardiovascular disease; all-too-common medical histories in the industrialized world. Recent advances in gut microbiota research suggest certain species correlate with these chronic conditions, and are perhaps causal through their metabolic capacities. To address this question, Pedersen et al. (2016) conducted a broad survey of water soluble metabolites and lipids in serum by untargeted mass spectrometry. The 277 non-diabetic individuals in the study were ranked by homeostatic model assessment-IR (HOMA-IR), a widely applied measure of IR. Mass spectrometry data for serum metabolites were clustered to reveal correlative changes, and association with IR. GlcNAc clustered with leucine, isoleucine, valine and proline; and as a cluster (P < 10−13) or individually, increasing levels of these metabolites correlated with HOMA-IR severity (Supplementary Table (tab ST4 and ST6), see cluster M10). Genomic sequences of gut microbiomes from these subjects were assessed for biosynthetic capacities that might contribute to changes in serum metabolites associated with IR. Overall, 41 of 567 KEGG microbiome pathway modules were significantly associated with HOMA-IR, and further data analysis suggested that HOMA-IR and the modules for BCAAs biosynthesis were largely driven by Prevotella copri followed by Bacteroides vulgatus, while other species such as Butyrivibrio crossotus and Eubacterium Siraeum showed a reduced capacity for bacterial BCAA uptake. Thus, as a community, more BCAAs are made available to the host and less is consumed by the microbiota.
As a test of causality, mice on a high-fat diet were challenged with P. copri, which supported the hypothesis by showing increased serum BCAAs, glucose intolerance and IR. However, the authors note that P. copri gavage had a relatively modest effect on serum BCAA levels (Pedersen et al. 2016), leaving the reader to ponder what else may play a role. As a likely coconspirator, GlcNAc is an under-appreciated amino-sugar found widely on glycoproteins synthesized in the secretory pathway, and on cytoplasmic and nuclear proteins subject to O-GlcNAcylation (Figure 1). HOMA-IR increases by ~four times when GlcNAc (0.5 mg/mL) is added to the drinking water of B57BL/6 mice on a 9% fat diet; a dosage of GlcNAc that represents an insignificant source of calories, suggesting its effects are mediated through protein glycosylation (Ryczko et al. 2016). In addition to the de novo hexosamine biosynthesis pathway to UDP-GlcNAc, the salvage of GlcNAc from dietary and glyconjugate turnover makes a significant contribution to cellular UDP-GlcNAc levels (Hascall et al. 2014). The KEGG pathways for microbial production of GlcNAc did not reach significance in the Pedersen study. Nonetheless, P. copri and B. vulgatus are Bacteroidetes, a phylum known to encode the most numerous collections of glycoside hydrolases and polysaccharide lyases (El Kaoutari et al. 2013). Thus, IR-related increases in serum GlcNAc may come from a similar community of gut microbiota as BCAA, via greater release of dietary and host glycans.
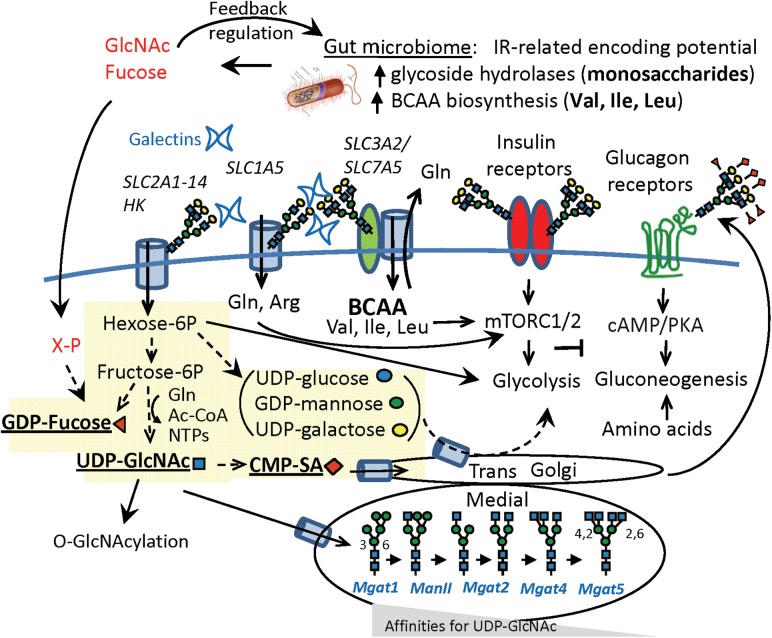
Fig. 1.
Possible synergy of GlcNAc and BCAA leading to IR: de novo nucleotide-sugar biosynthesis in yellow. Nucleotide-hexoses (brackets) contribute to glycoconjugate biosynthesis, and upon turnover the hexoses are available to host energy harvest, whereas fucose and GlcNAc are phosphorylated and returned to the nucleotide-sugar pools. The Golgi N-acetylglucosyltransferases (MGAT1, 2, 4 and 5) form a linear pathway displaying an increasing dependence on UDP-GlcNAc concentrations (Km increasing), whereas galectins bind to with affinities proportional to N-glycan branching, and stabilizing receptors and transporters against loss to endocytosis (Lau et al. 2007). These kinetics combine for ultrasensitive responses to changes in UDP-GlcNAc. SLC1A5 and SLC2A1-4 import glutamine and glucose, respectively. SLC7A5/SLC3A2 is an antiporter for BCAA/glutamine (Nicklin et al. 2009).
Indeed, recent reports indicate that sugars and glycoconjugates mediate communication and symbiosis between the microbiota and host immunity with broad clinical significance (Kashyap et al. 2013; Pickard et al. 2014). The microbiota stimulates expression of α1-2fucosyltransferase 2 (FUT2) by intestine epithelial cells beginning at birth. This provides a source of fucose from host mucosa, which is liberated by microbial glycoside hydrolases, and acts to reduce the expression of bacterial virulence genes and protect the commensal bacteria (Goto et al. 2014; Pickard et al. 2014). Both GlcNAc and fucose are accessible to microbial catabolism, and have regulatory effects on microbial gene expression with the potential to impact virulence (Naseem et al. 2012). However, GlcNAc (Wellen et al. 2010) and fucose (Becker and Lowe 2003) are not catabolized in host cells, but rather salvaged into the nucleotide-sugar pools and used in posttranslational modifications (Figure 1).
Oral gavage of mice with 13C-GlcNAc revealed maximum levels in serum at ~30 min followed rapidly by labeling of hepatic UDP-GlcNAc (Ryczko et al. 2016), but specific intestinal transporters have yet to be identified. GlcNAc in the drinking water increased hepatic UDP-GlcNAc levels by ~25%, as well as GlcNAc-branching of N-glycan on multiple cell surface glycoproteins, including the glucagon receptor (Ryczko et al. 2016). The more branched N-glycans bind galectins with higher affinity at the cell surface, which slows receptor trafficking and increases responsiveness to cognate ligands (Partridge et al. 2004). Mgat5−/− mice are deficient in one of the four possible branches, and display hyposensitivity to glucagon, and resistance to weight-gain on a 9% fat diet (Johswich et al. 2014). GlcNAc salvage and Mgat5 over-expression are synergistic in promoting hepatic sensitivity to glucagon signaling and weight-gain in mice. Weight gain on oral GlcNAc occurs without an increase in caloric intake, suggesting that more efficient extraction of nutrients, possibly at the level of nutrient transporter activity may be the cause. Indeed, GlcNAc salvage and Mgat5 over-expression in cultured cells stimulate uptake of glutamine, essential amino acids (Abdel Rahman et al. 2015) and promote responsiveness to growth factors in cell culture (Lau et al. 2007; Wellen et al. 2010). Nutrient transporters commonly have 8–14 transmembrane segments, and an extended luminal loop modified with N-glycans where the degree of branching regulates trafficking thereby cell surface residency and activity (such as with SLC2A2 and SLC2A4) (Ohtsubo et al. 2005; Lau et al. 2007). Recent reports suggest that N-acetylglucosaminyltransferases in the same and different pathways compete for the available UDP-GlcNAc, which impacts the global glycan profile and downstream effector functions of glycoproteins (Mkhikian et al. 2016; Araujo et al. 2017). O-GlcNAc, N-glycosylation and proteoglycans have been implicated in IR (Bernelot Moens et al. 2014; Hardiville and Hart 2014), perhaps suggesting we need to account for multiple glycosylation pathways and their coregulation by nucleotide-sugar availability.
Insulin signaling activates mammalian Target of Rapamycin Complexes (mTORC1 and mTORC2), Ser/Thr-kinase activities that regulate anabolic and catabolic pathways (Zoncu et al. 2011). Transporter dependent uptake of extracellular leucine (Nicklin et al. 2009), alongside arginine (Rebsamen et al. 2015; Wang et al. 2015), glutamine (Jewell et al. 2015) and energy charge are enabling; they serve as a coincidence detector for threshold concentrations of these key resources, tuning mTORC1 sensitivity to insulin receptor signaling. Similarly, microbiota-dependent increases in BCAA and GlcNAc may cooperate, whereby the conversion of GlcNAc into N-glycan branching on amino acid transporters and growth factor receptors (Lau et al. 2007; Wellen et al. 2010) increases BCAA uptake and mTORC1-driven negative feedback to the insulin receptor. In the liver, elevated BCAA and GlcNAc driven mTORC1 activity may weaken insulin-mTORC2-AKT signaling, which opposes gluconeogenesis while enhancing glucagon signaling, a common imbalance observed with increasing IR (Hagiwara et al. 2012).
Polymorphisms in the pathway to UDP-GlcNAc and quantitative trait locus for a Golgi UDP-GlcNAc transporter are associated with IR and obesity (Yazbek et al. 2011). However, a recent large DNA-sequencing study of type 2 diabetes concluded that the contribution of common genetic variants are limited to ~10% with little evidence of additional rare variants (Fuchsberger et al. 2016); and while epigenetic mechanism are also likely to contribute (Barres and Zierath 2016), a significant portion of risk may be found in evolved symbiotic interactions between host–microbiota–diet and environmental stresses. Dietary fiber is a broad category, and labeling of fiber in processed foods obscures the use of additives such as chitin, a GlcNAc polymer (Shahidi and Abuzaytoun 2005). Benefits to stability and texture are touted by the food industry, but chitinases encoded by the microbiome and a mammalian gene (CHIT1) are both associated with IR and inflammatory bowel disease (Kanneganti et al. 2012). IR-associated B. vulgatus identified in (Pedersen et al. 2016) encodes multiple GH18 and GH20 family enzymes, annotated by carbohydrate-active enzymes (CAZymes) as chitinases (El Kaoutari et al. 2013). The microbiota readily adapts to diet. For example, the chitin-rich krill consumed by Baleen whales, promotes a microbiota enriched in chitinases, thereby allowing GlcNAc harvest to account for ~10% of total energy equivalence, as well as GlcNAc's possible regulatory activities on both the host and microbiota (Sanders et al. 2015). Co-evolution of whale, diet and microbiota may have contributed to their distinction as the largest mammals on earth by promoting GlcNAc-dependent effects on insulin growth signaling and nutrient transport. A wider examination of host–diet–microbiota symbiosis in different species should reveal more molecular interactions, and possible clinical opportunities.
Acknowledgments
We thank Dr. Jim Woodgett for helpful comments.
Supplementary data
Supplementary data is available at Glycobiology online.
Funding
Canadian Institutes of Health Research (CIHR) (MOP-62,975), and J.W.D. is Canada Research Chair in Glycobiology. G.G.H. is supported by a Basic Research Fellowship from Parkinson Canada.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Abbreviations
BCAA, branched-chain amino acids; GlcNAc, N-acetylglucosamine; HOMA-IR, homeostatic model assessment-IR; IR, insulin resistance.
REFERENCES
- Abdel Rahman AM, Ryczko M, Nakano M, Pawling J, Rodrigues T, Johswich A, Taniguchi N, Dennis JW. 2015. Golgi N-glycan branching N-acetylglucosaminyltransferases I, V and VI promote nutrient uptake and metabolism. Glycobiology. 25:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo L, Khim P, Mkhikian H, Mortales CL, Demetriou M. 2017. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. Elife. 6:e21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres R, Zierath JR. 2016. The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nat Rev Endocrinol. 12:441–451. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Lowe JB. 2003. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 13:41R–53R. [DOI] [PubMed] [Google Scholar]
- Bernelot Moens SJ, Mooij HL, Hassing HC, Kruit JK, Witjes JJ, van de Sande MA, Nederveen AJ, Xu D, Dallinga-Thie GM, Esko JD et al. 2014. Carriers of loss-of-function mutations in EXT display impaired pancreatic beta-cell reserve due to smaller pancreas volume. PLoS One. 9:e115662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. 2013. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 11:497–504. [DOI] [PubMed] [Google Scholar]
- Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ et al. 2016. The genetic architecture of type 2 diabetes. Nature. 536:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Obata T, Kunisawa J, Sato S, Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki T et al. 2014. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 345:1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Ruegg MA, Hall MN. 2012. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 15:725–738. [DOI] [PubMed] [Google Scholar]
- Hardiville S, Hart GW. 2014. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 20:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, Vigetti D, Hanson RW, Hart GW. 2014. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol. 35:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. 2015. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 347:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johswich A, Longuet C, Pawling J, Rahman AA, Ryczko M, Drucker DJ, Dennis JW. 2014. N-glycan remodeling on glucagon receptor is an effector of nutrient sensing by the hexosamine biosynthesis pathway. J Biol Chem. 289:15927–15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti M, Kamba A, Mizoguchi E. 2012. Role of chitotriosidase (chitinase 1) under normal and disease conditions. J Epithel Biol Pharmacol. 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, Costello EK, Higginbottom SK, Domino SE, Holmes SP, Relman DA et al. 2013. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA. 110:17059–17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. 2007. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 129:123–134. [DOI] [PubMed] [Google Scholar]
- Mkhikian H, Mortales CL, Zhou RW, Khachikyan K, Wu G, Haslam SM, Kavarian P, Dell A, Demetriou M. 2016. Golgi self-correction generates bioequivalent glycans to preserve cellular homeostasis. Elife. 5:e14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem S, Parrino SM, Buenten DM, Konopka JB. 2012. Novel roles for GlcNAc in cell signaling. Commun Integr Biol. 5:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA et al. 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9:311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C et al. 2009. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 136:521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. 2005. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 123:1307–1321. [DOI] [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 306:120–124. [DOI] [PubMed] [Google Scholar]
- Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G et al. 2016. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 535:376–381. [DOI] [PubMed] [Google Scholar]
- Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ et al. 2014. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 514:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M et al. 2015. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 519:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryczko MC, Pawling J, Chen R, Abdel Rahman AM, Yau K, Copeland JK, Zhang C, Surendra A, Guttman DS, Figeys D et al. 2016. Metabolic reprogramming by hexosamine biosynthetic and Golgi N-glycan branching pathways. Sci Rep. 6:23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JG, Beichman AC, Roman J, Scott JJ, Emerson D, McCarthy JJ, Girguis PR. 2015. Baleen whales host a unique gut microbiome with similarities to both carnivores and herbivores. Nat Commun. 6:8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Abuzaytoun R. 2005. Chitin, chitosan, and co-products: Chemistry, production, applications, and health effects. Adv Food Nutr Res. 49:93–135. [DOI] [PubMed] [Google Scholar]
- Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W et al. 2015. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 347:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. 2010. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24:2784–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazbek SN, Buchner DA, Geisinger JM, Burrage LC, Spiezio SH, Zentner GE, Hsieh CW, Scacheri PC, Croniger CM, Nadeau JH. 2011. Deep congenic analysis identifies many strong, context-dependent QTLs, one of which, Slc35b4, regulates obesity and glucose homeostasis. Genome Res. 21:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. 2011. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]