Abstract
α-Lytic protease is a bacterial serine protease widely studied as a model system of enzyme catalysis. Here we report that lyophilization induces a structural change in the enzyme that is not reversed by redissolution in water. The structural change reduces the mobility of the active-site histidine residue and the catalytic activity of the enzyme. The application of mild pressure to solutions of the altered enzyme reverses the lyophilization-induced structural change and restores the mobility of the histidine residue and the enzyme's catalytic activity. This effect of lyophilization permits a unique opportunity for investigating the relationship between histidine ring dynamics and catalytic activity. The results demonstrate that His57 in resting enzymes is more mobile than previously thought, especially when protonated. The histidine motion and its correlation to enzyme activity lend support to the reaction-driven ring flip hypothesis.
Keywords: catalytic triad, lyophilization, serine protease mechanism
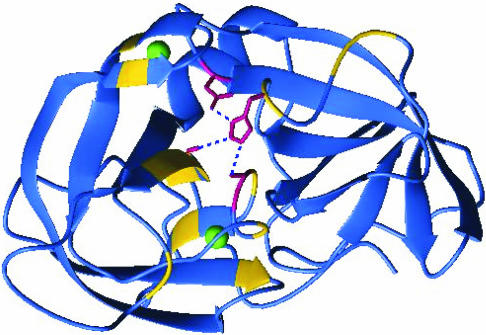
The abundance of enzymes containing the Asp-His-Ser catalytic triad at their active sites (>300 enzymes from at least four unrelated clans) suggests that this structure is an especially effective catalytic apparatus (Fig. 1). How does it work? The conserved Asp-His and His-Ser H-bonds have long been thought to be the key to the mechanism, as illustrated by the charge relay (1), modified charge relay (2), and low barrier H-bond hypotheses (3), as well as the general acid-base mechanism currently found in most textbooks (4). All of the above mechanisms assume that the side chains remain stationary during catalysis.
Fig. 1.
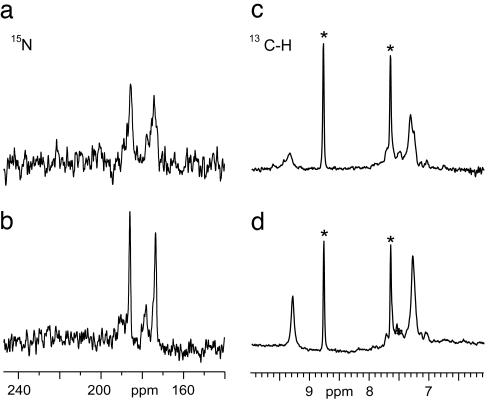
Selected NMR resonances of the catalytic triad of resting α-lytic protease at “low” and “high” pH values. pH values are 4.0 (Left) and 9.0 (Right).
Wang (5), Polgar (6), and Jencks (7) have pointed out that any mechanism devised under this assumption has an inherent deficiency, namely that it cannot satisfactorily explain how catalytic assistance can be provided to both the formation and the productive breakdown of the tetrahedral intermediate. More recently, a third conserved H-bond involving the central histidine has been discovered (8, 9); this one links the imidazole Cε1 proton and the oxygen of a backbone carbonyl group (Fig. 1). The fact that this H-bond has emerged with the triad through at least three and perhaps as many as five independent evolutionary origins indicates that it must be as important to catalysis as the Asp-His and His-Ser H-bonds. We proposed that it may be the key to resolving the Wang–Polgar–Jencks dilemma by making a reaction-driven histidine ring flip (RDRF) mechanism possible (8). It makes this mechanism possible by providing for the selective stabilization of the histidine ring in a flipped configuration when the ring becomes protonated. Thus, transfer of the proton from serine to histidine in the first step of the catalytic pathway, according to this hypothesis, would trigger the ring flip, placing Nδ1-H in position to transfer its proton to the substrate amide nitrogen. In addition to providing for the redirection of the proton, the flipped ring would also help to block the reverse reaction through the formation of a H-bond between Cε1-H of His57 and Oγ of Ser195. After transfer of the proton to the substrate and expulsion of the amidate ion, the flipped orientation then aids deacylation by providing an optimally located nucleophile for the activation of water. If correct, this mechanism would transform our view of the serine proteinases from rigid templates that are designed to bind single transition states to mechanical devices that are able to guide the reaction through multiple transition states.
The most recent, high-resolution structures of an acylated intermediate of elastase show His57 in the standard conformation (10), which could be construed as evidence against the RDRF mechanism. However, only a few percent or less of the histidine would need to be in the flipped configuration at any time in the tetrahedral intermediate or acyl enzyme complexes to account for catalysis. Structural analysis alone, therefore, cannot rule out the RDRF mechanism.
What is also needed is information on the dynamics of His57, because the RDRF mechanism predicts that this histidine should be more mobile than predicted in previous catalytic models, even in resting enzymes and especially with histidine protonated. However, experimental data on the dynamic properties of these histidines is surprisingly scarce, considering how much attention the catalytic triad has attracted over the years (11). Although crystallographic B factors are normally thought to be a measure of mobility, Depristo et al. (12) have explained in detail why B factors are unreliable for evaluating structural heterogeneity of the type represented by a histidine ring flip. A recent computational analysis (13, 14) has concluded that His57 is likely to move during catalysis. This analysis favors more limited movement but does not rule out the RDRF mechanism because the simulation of Topf et al. (13, 14) was of insufficient duration to encompass a ring flip.
Here we report NMR data from α-lytic protease, a bacterial serine proteinase, demonstrating that its histidine is highly mobile in the resting enzyme, especially at low pH, and that the mobility is consistent with ring flipping. We also report the discovery that lyophilization induces a structural change in this enzyme that is not reversed by redissolution in water and selectively slows both His57 mobility and the rate of catalysis. The original structure, histidine mobility, and catalytic activity can all be restored by the application of mild pressure to enzyme solutions. This reversible structural effect is unprecedented and potentially important per se, especially in light of protein-misfolding diseases. However, it also provides a unique window for examining the relationship between histidine ring mobility and catalytic activity.
Methods
Preparation of α-Lytic Protease. α-Lytic protease containing histidine uniformly 15N- and/or 13C-labeled (Cambridge Isotope Laboratories, Cambridge, MA) was purified from the supernatant of a histidine auxotroph of Lysobacter enzymogenes by using defined media containing amino acids and several salts (15). α-Lytic protease uniformly 15N-labeled was grown in Celtone-N media (Isotec) supplemented with 30 g/l sucrose and several salts. Before separation on an SP Sepharose FF column (Amersham Biosciences), the supernatants were treated with polyethyleneimine (Sigma) to a final concentration of 0.1% and spun down in a Beckman Coulter JA-21 rotor at 12,500 rpm. Desalting of the sample was completed on a 60 × 1.6-cm S200 column (Amersham Biosciences) as described in refs. 15 and 16.
Enzymatic Activity Measurements. Enzyme activities in aqueous solution were measured by using Ac-Ala-Pro-Ala-p-nitroanilide (Bachem) and monitoring the change in absorbance at 410 nm. Quantification of the activity was performed in the manner of Hunkapiller et al. (17).
Calculation of Specific Activity. Specific activity is defined as the units of activity per unit of protein. We normalized for the amount of protein by using the Pierce Protein Plus assay. The specific activity of freshly prepared enzyme is defined as activity per units of protein and is considered fully active (control). All other specific activity measurements are expressed as a percentage of this control value. Measurements of absorbance and activity of each sample were taken in duplicate, and each experiment was repeated at least three times.
NMR Sample Preparation, Lyophilization, and Pressure Treatment. Enzyme NMR samples were 2 mM in 90% H2O/10% D2O with 0.05 M KCl. Samples for lyophilization were diluted 4-fold with deionized water (Millipore) before shelling of the liquid sample in a dry ice/ethanol bath. Samples were kept under high vacuum for at least 18 h, until the sample was white and fluffy and the bottle was no longer cold to the touch. For pressure treatment, samples at pH 4.0 and 4°C were subjected to 60 psi [≈4 atm (1 atm = 101.3 kPa)] of nitrogen gas for 8 h in a chamber improvised from a 25-ml Amicon concentration cell (Millipore) by replacing the usual porous ultrafiltration membrane with an impermeable Teflon membrane. Samples were allowed to sit at 4°C for up to 8 h before running the NMR spectra.
NMR Experiments. 15N NMR experiments were carried out at 50.68 MHz on a Bruker (Billerica, MA) AMX-500 spectrometer equipped with a 10-mm probe by using the Bruker pulse sequence “aring” for cancellation of acoustic ringing (18). 1H-detected NMR experiments were acquired with a 5-mm triple resonance inverse probe by using a Bruker AMX-500 (nongradient) and a Bruker DRX-600 spectrometer with triple axis gradients. Low-field spectra were acquired either on the AMX-500 by using the “hard 1–1” pulse sequence (19) with oversampling (spectral width of 26.3 ppm, 32,768 data points) (20) or on the DRX-600 by using the “watergate” water-suppression sequence (21) with a spectral width of 40 ppm and 16,384 data points. 1H 1D 13C-selective heteronuclear multiple-quantum correlation spectroscopy spectra used a principal delay of 0.5/(1JCH) = 2.5 ms. 15N/1H-heteronuclear sequential quantum correlation (HSQC) spectra were acquired on the AMX-500 by using the Bruker pulse sequence “invitp” with an added spin-lock pulse for water suppression on the y axis (22). Each spectrum was taken with 256 rows, 32 scans, 2,048 data points, and a 1-s recycle delay. All 1H chemical shifts were referenced to water at 4.766 ppm at 25°C and at 5.004 ppm at 5°C.
Results
The first clue that lyophilization induces a conformational change in α-lytic protease that is not reversed when the lyophilized powder is redissolved in water came when we discovered its effects on the low-field proton resonance. This resonance, a hallmark of serine protease NMR, arises from the proton in the Asp-His H-bond. It is typically found at ≈17–18 ppm when the catalytic histidine is protonated and at ≈13.8–15 ppm when it is neutral, depending on the protease. In α-lytic protease, it titrates from 17.1–13.8 ppm between pH 4.0 and 8.5 (23). To our surprise, the 17.1-ppm signal expected in pH-4.0 samples is not detectable with preparations of α-lytic protease that have not undergone lyophilization but can be made to appear in these same samples by lyophilizing and redissolving them in water (Fig. 2Ab). Lyophilization also affects the 13.8-ppm signal at high pH, although less dramatically, broadening its linewidth ≈2-fold, from 35 to 60–80 Hz (Fig. 2B). It also affects the specific catalytic activity, decreasing it to about one-third that of non-lyophilized enzyme (NONLYO) (Fig. 2C).
Fig. 2.
The reversible effect of lyophilization. (A) 1H NMR spectra of 2 mM α-lytic protease in 0.05 M KCl at 600 MHz, 5°C, and pH 4.0 showing the reversible effects of lyophilization and pressure upon the low-field 1H. Each spectrum represents 1,024 scans using the watergate water-suppression sequence. Spectra shown are for: freshly prepared sample (a), after lyophilization (b), after 60-psi pressure treatment (c), and after a second lyophilization (d). (B) 1H NMR spectra of 2 mM α-lytic protease in 0.05 M KCl at 500 MHz, 5°C, and pH 8.5 showing the broadening of the high-pH species after lyophilization. Each spectrum represents 512 scans using the hard 1-1 water suppression sequence. Spectra shown are for: freshly prepared sample (a) and after lyophilization (b). (C) Effect on specific activity. Each bar represents the percentage of specific activity of the treatment group compared with untreated enzyme (control) on the substrate Ac-Ala-Pro-Ala-p-nitroanilide (14). Data are shown for: NONLYO with no treatment assayed at the same time points as the other samples (a), freshly lyophilized enzyme resuspended in water (b), lyophilized enzyme subsequently treated with 60 psi of N2 gas pressure overnight (c), resuspended lyophilized enzyme subsequently relyophilized (d), and twice-lyophilized enzyme that was treated with 60 psi of pressure overnight (e). Each bar represents an average of six experiments. Pressure had no effect on NONLYO (data not shown).
The two forms of the enzyme are quite stable in a low-salt (50 mM) aqueous solution, maintaining the above differences when stored for days or weeks in solution at 4°C and pH 4.0. However, lyophilized enzyme (LYO) can be converted back to NONLYO by the application of mild pressure to the enzyme solution (Fig. 2 Ac), i.e., the 17.1-ppm signal disappears from low-pH samples, the 13.8-ppm signal sharpens in high-pH samples, and the specific catalytic activity is restored. Relyophilization of pressure-treated enzyme produces results equivalent to a first lyophilization. Thus, the two forms, although quite stable, can be interconverted through the reverse effects of lyophilization and pressurization, limited only by the irreversible loss of enzyme, because α-lytic protease, like all proteases, autolyzes slowly but continually.
A second lyophilization and reconstitution in water without an intervening pressurization step does not reduce the specific activity or change the NMR spectrum further, indicating that lyophilization is not damaging the enzyme but is converting it to an alternate form. Thus, once conversion is complete, further lyophilization has no effect. The above findings indicate the existence of two independent, conformationally distinct forms of α-lytic protease. 1H/15N HSQC spectra confirm this. Each form of the enzyme (LYO and NONLYO) exhibits a single set of HSQC crosspeaks, one for each of the 189 amino acids comprising the protein. Sixteen of the crosspeaks, however, occur in significantly different positions in the two spectra (i.e., >0.1 ppm difference in either the 1H or 15N dimension). These 16 are clustered primarily in a single β-sheet located near the active site, highlighted in yellow in Fig. 3 (HSQC in Fig. 7, which is published as supporting information on the PNAS web site). Another difference between the two forms, revealed by a special type of NOESY experiment (24, 25), is that LYO contains at least one more protein-bound water molecule than NONLYO (Fig. 4). Like the low-field proton spectra and the catalytic activity, the two HSQC spectra and the NOESY spectra showing the additional water are reversibly interconverted through lyophilization and pressurization.
Fig. 3.
Mapping of the shifted backbone residues onto the 2ALP structure (33). Royal blue ribbon represents the backbone residues that were unchanged upon lyophilization; yellow indicates backbone amides that shifted >0.1 ppm in the 1H or 15N dimension of the HSQC spectra. Crystal structures of α-lytic protease may all be of LYO conformation because lyophilization has been routinely used in the purification of α-lytic protease for many years (34). If so, the most likely candidates for the additional sequestered water (Fig. 4) are molecule nos. 4 and 13, displayed in green, the only interior water molecules found within 10 Å of His57. The catalytic triad is rendered in deep pink, with the blue dotted lines representing the conserved H-bond network of the catalytic triad. The figure was prepared with the program molmol (35).
Fig. 4.
Two-dimensional 1H modified NOESY spectrum (24) of 2 mM α-lytic protease in 0.05 M KCl at 600 MHz, 25°C, and pH 3.9 in NONLYO (a) and LYO (b). The LYO spectrum contains an additional water protein crosspeak, at 7.7 ppm, indicated by the arrow. The gradient strength in each spectrum was 2%.
Why is the proton resonance from the functionally crucial Asp-His H-bond at pH 4.0 missing from the more active form of the enzyme (NONLYO) but present in the less active form (LYO)? The observability of this resonance has long been attributed to the Asp-His H-bond stabilizing the ordinarily labile Nδ1-H proton against solvent exchange in addition to displacing its chemical shift into the low-field region, where it can be observed unobstructed by other resonances.
One possibility is that the low-field H-bond is simply not present in NONLYO at low pH. To address this question we examined the 15N NMR signals of the imidazole ring of His57. The chemical shifts of these resonances are very informative about H-bonding interactions in the catalytic triad. In particular, the H-bond to Asp102 has been shown to displace the 15Nδ1 resonance downfield ≈10 ppm at pH 4.0 (26). This study, like most previous NMR studies of α-lytic protease, was performed on LYO. Fig. 5 a and b shows that the histidine 15N shifts of NONLYO are essentially identical to those of LYO, including the 10-ppm downfield displacement of 15Nδ1, which demonstrates that the Asp-His H-bond is indeed present in NONLYO and is about equally strong to the one in LYO. Although the chemical shifts are the same, the 15N spectra are not identical at low pH, and the 15N lines are substantially sharper in NONLYO than in LYO (Table 1). Nδ1 is sharp enough in NONLYO to show the absence of 1J splitting from the attached proton, a result that further confirms that this proton is in fast exchange with solvent in this form of the enzyme. In contrast, the Nδ1 linewidth of LYO is broad enough to obscure the ≈80–100 Hz 1J splitting that has been observed previously in this form of the enzyme at lower magnetic fields (26). The above results indicate that the insusceptibility of the Nδ1-H proton to NMR detection at low pH in NONLYO is due to its short lifetime, owing to rapid exchange with solvent rather than to the Asp-His H-bond being weakened or broken in this form of the enzyme.
Fig. 5.
The effect of lyophilization on the H-bonding network at low pH values. (Left) 15N NMR spectra of 1.5 mM α-lytic protease in 0.05 M KCl at 50.68 MHz and 5°C. Each spectrum represents 60,000 scans. Spectra shown are for: LYO at pH 4.6 (a) and NONLYO at pH 4.6 (b). (Right) Carbon-filtered 1D 1H spectra of 1.5 mM α-lytic protease in 0.05M KCl at 600 MHz and 25°C. Each spectrum represents 1,024 scans. Spectra shown are for: LYO at pH 4.0 (c) and NONLYO at pH 4.0 (d). Asterisks denote degradation peaks and background from other aromatic side chains (as discussed in ref. 8). The enzyme samples are isotopically enriched at His57 only.
Table 1. Linewidth measurements of selected His57 resonances in hertz.
| Low pH (4.0)
|
High pH (8.9)
|
|||||
|---|---|---|---|---|---|---|
| Resonance | LYO | NONLYO | LYO | NONLYO | Solvent exchangeable? | Flippable? |
| 15Nδ1* | 160-180 | 40-45 | 150-165 | 150-165 | No | Yes |
| 15Nε2* | 90-100 | 60-70 | 85-95 | 85-95 | No | Yes |
| 13Cε1-H (25°C) | 84 | 33 | 40 | 26 | No | Yes |
| 13Cε1-H | 170 | 46 | nd | 29 | No | Yes |
| (CMK)15Nδ1-H | 35 | 35 | † | † | Yes | No |
| 15Nδ1-H | 110 | >500 | 60-80 | 35-40 | Yes | Yes |
All measurements were done on the Bruker DRX-600 MHz spectrum at 5°C, unless otherwise noted. Ranges are provided for peaks that were not consistently defined by the xwinnmr deconvolution software. Peaks without ranges were consistently defined by xwinnmr by using a Lorentzian algorithm, error ≈2%. nd, not determined.
Linewidth measurements were taken at 50.68 MHz with the 1H decoupler off; low pH was defined as 4.6.
Low- and high-pH species are identical because of the elevated pKa value of His57 (>9.5).
The non-solvent-exchangeable carbon-bound protons of His-57, like the 15N signals, are also significantly broadened in LYO at low pH. The Cε1 proton (Fig. 5 c and d) is more severely broadened, which helps to explain why this “true” signal was missed for many years, until its discovery in the NONLYO form (8), where it is sharper and therefore easier to detect. In LYO, its chemical shift is even larger than in NONLYO, confirming the presence of the Cε1-H ... O C H bond first identified by NMR in NONLYO. At high pH where the imidazole ring is neutral, the effects of lyophilization are not as dramatic (Table 1 and Fig. 8, which is published as supporting information on the PNAS web site). The Cε1 proton is broadened <2-fold, and the Nδ1-H proton is broadened ≈2-fold; the 15N resonances, however, are not detectably broadened.
C H bond first identified by NMR in NONLYO. At high pH where the imidazole ring is neutral, the effects of lyophilization are not as dramatic (Table 1 and Fig. 8, which is published as supporting information on the PNAS web site). The Cε1 proton is broadened <2-fold, and the Nδ1-H proton is broadened ≈2-fold; the 15N resonances, however, are not detectably broadened.
The linewidths of the backbone N-H groups do not differ between LYO and NONLYO (Fig. 9, which is published as supporting information on the PNAS web site), indicating that lyophilization selectively affects the linewidths of His57 resonances. The lack of broadening of the backbone N-H groups also rules out dimerization or aggregation as the cause of the effects on His57. NMR pH titrations show that the pKa value of His57 is essentially the same in the two enzyme forms, ruling out pKa perturbations as the basis of the lyophilization-induced effects on His57 (Fig. 10, which is published as supporting information on the PNAS web site).
A change in the internal mobility of His57 is left as the most likely explanation for the observed lyophilization effects on His57. To test this hypothesis, we examined Ac-Ala-Pro-Val-chloromethyl ketone (CMK)-inhibited complexes of the enzyme. CMK inhibitors form covalent bonds to both the serine and histidine (Fig. 6A). Such tethering of His57 to Ser195 will prevent His57 from undergoing 180° ring flips. The tethering does not alter the normal position of the imidazole ring, because the Asp-His and histidine Cε1-H ... O C H bonds remain intact, demonstrated by their 1H chemical shifts of 16.76 and 9.59 ppm, respectively (Fig. 6A). The results show that the Nδ1 proton signal is visible at low pH in CMK complexes of both LYO and NONLYO (Fig. 6 B and C) and that the linewidths are the same and quite small (≈35 Hz). Thus, hindering His57 motion by chemically linking the His57 Nε2 nitrogen to Ser195 by means of CMK modification in NONLYO at low pH mimics lyophilization in rendering the Nδ1-H proton observable. It also precludes further lyophilization-induced effects. These results, therefore, support the idea that His57 motion plays an important role in solvent exchange of the Nδ1-H proton in NONLYO at low pH and that lyophilization slows this solvent exchange by reducing His57 mobility.
C H bonds remain intact, demonstrated by their 1H chemical shifts of 16.76 and 9.59 ppm, respectively (Fig. 6A). The results show that the Nδ1 proton signal is visible at low pH in CMK complexes of both LYO and NONLYO (Fig. 6 B and C) and that the linewidths are the same and quite small (≈35 Hz). Thus, hindering His57 motion by chemically linking the His57 Nε2 nitrogen to Ser195 by means of CMK modification in NONLYO at low pH mimics lyophilization in rendering the Nδ1-H proton observable. It also precludes further lyophilization-induced effects. These results, therefore, support the idea that His57 motion plays an important role in solvent exchange of the Nδ1-H proton in NONLYO at low pH and that lyophilization slows this solvent exchange by reducing His57 mobility.
Fig. 6.
The effect of CMK inhibition on the low-field proton. (A) Selected NMR resonances of CMK-inhibited α-lytic protease. (B–C) Low-field 1H NMR spectra of 2 mM α-lytic protease with 10 mM Ac-AAPV-CMK (Bachem) in 0.05 M KCl at 600 MHz, 25°C, and pH 4.0. Each spectrum represents 1,024 scans using the watergate water-suppression sequence (21). Spectra shown are for LYO (B) and NONLYO (C).
The temperature dependence of the Nδ1-H proton linewidths is also different in LYO at high and low pH values (Fig. 11, which is published as supporting information on the PNAS web site). At high pH, the signal exhibits classical temperature-dependent behavior for a solvent-exchangeable resonance, broadening and disappearing as the temperature is raised. At low pH, the behavior is more complicated, with the linewidth staying essentially constant with increasing temperature while the area decreases.
Discussion
For the His57 Nδ1 proton to be undetectable in NONLYO at low pH, the linewidth would need to be ≥500 Hz. Lyophilization, therefore, slows exchange with solvent sufficiently to narrow the linewidth to ≈100 Hz, the linewidth observed in LYO at low pH, corresponding to a 7-fold increase in proton lifetime from <0.6 to ≈4.0 ms. What is the mechanism through which lyophilization causes this change?
The two most likely mechanisms, a lyophilization-induced dimerization (or aggregation) or increase in His57 pKa, have been ruled out by the NMR results. The next most likely mechanism is a lyophilization-induced structural change that reduces the mobility of His57. For a H-bonded proton to exchange with solvent, the H-bond must first break, at least transiently (27, 28). To break the His57-Asp102 H-bond, one or both of these side chains must move. A His57 ring flip would be an especially effective mechanism for solvent exchange because it would transiently place the Nδ1-H proton in direct contact with solvent. However, other less dramatic His57 motions, such as a “wobble,” could also suffice. The HSQC spectra demonstrate that lyophilization does induce a structural change and that it is localized near the active site. One possibility is that lyophilization causes a tightening of the protein structure. Whatever the structural change, it must selectively hinder His57 motion, thereby slowing the exchange rate of the Nδ1-H proton sufficiently to render it visible in NMR spectra.
The slowness with which the LYO form of the enzyme converts back to NONLYO when the lyophilized enzyme is redissolved in water is consistent with the model of a tightened structure viewed as having constricted passageways that would hinder water reentry. The additional enzyme-bound water found in LYO also supports the hypothesis, because it demonstrates that at least one passageway becomes constricted enough in LYO to trap a water molecule that in NONLYO has free access to solvent. The need to “reinflate” the tightened LYO structure will also contribute to an activation energy barrier for the LYO-to-NONLYO reaction and thus helps to explain the slowness of this reaction. The fact that pressure accelerates the conversion supports this model, because pressurization would supply the energy needed to “push” the water back into the protein during reinflation.
Lyophilization-induced structural changes in subtilisin and chymotrypsin have been reported previously. These conformational changes are maintained when the enzyme is resuspended in organic solvents (29, 30) but apparently are readily reversed when redissolved in water (31). A lyophilization-induced conformational change in a protein that is not readily reversed when the protein is redissolved in water, as reported here for α-lytic protease, is, however, not unprecedented. The efficacy of a clinical antibody was reported to be reduced by 80–90% on lyophilization and traced to a reduction in conformational flexibility (32), suggesting that small but functionally significant lyophilization-induced conformational changes may be quite common but have been largely overlooked until now, perhaps owing to difficulties in detection. In any case, to our knowledge, ours is the first report on the reversibility of this phenomenon by application of mild pressure.
A key test of this hypothesis of reduced His57 mobility in LYO arises from the effect of lyophilization on the non-solvent-exchangeable His57 NMR resonances (i.e., from 15N and from 1H atoms bound to carbon). That is because lyophilization is predicted to have the opposite effect on these resonances than it has on the solvent-exchangeable Nδ1-H proton resonance at low pH, i.e., that it should broaden, not narrow, their linewidths. Whether their linewidths be determined by dipolar relaxation, chemical shift anisotropy, or incomplete chemical shift averaging of multiple conformations, all these mechanisms would cause increased linewidth with decreased mobility. The observed result is, indeed, broadening of all these non-solvent-exchangeable resonances, especially at low pH and 5°C, where the 15N signals are broadened ≈4-fold (from 40 Hz in NONLYO to 160 Hz in LYO) and the Cε1-H proton is broadened nearly 4-fold (from ≈46 to 170 Hz).
The less dramatic line broadening exhibited by the non-solvent-exchangeable resonances at high pH (only 2-fold for the Cε2-H and undetectable for the 15N signals) suggests that lyophilization may slow His57 motion less when the imidazole ring is neutral. This reduced slowing might be the result of neutral His57 being less mobile than protonated His57 to begin with and, therefore, less susceptible to being slowed. The fact that the 15N linewidths are nearly as broad in NONLYO at high pH as they are in lyo at low pH (Table 1) supports this idea. A His57 that is less mobile when neutral is consistent with the RDRF mechanism because this mechanism predicts that protonation of His57 enables ring flipping.
The difference in NMR observability of the Nδ1-H proton in NONLYO between low (not visible) and high (visible) pH could also be due to a difference in mobility between neutral and protonated His57. It must also reflect the difference in pKa between imidazolium and imidazole, because, all else being equal, the intrinsic release rate will be much slower from imidazole with a pKa of ≈14 than from imidazolium with a pKa of ≈6–7. Interestingly, this proton resonance, which is sharpened by lyophilization at low pH, is broadened at high pH. The broadening is modest, i.e., ≈2-fold, about the same as that of the Cε2-H proton. Nevertheless, the fact that it becomes broader rather than sharper on lyophilization shows that at high pH, the linewidth is not dominated by solvent exchange, as it is at low pH. The temperature dependence of this resonance further indicates that the mechanism of solvent exchange is likely to be fundamentally different at high and low pH. At high pH, the Nδ1-H resonance broadens with increasing temperature before disappearing, as would be predicted for most relatively simple exchange mechanisms. However, at low pH (in LYO), the line-width remains essentially constant with increasing temperatures as the intensity decreases. These results suggest that the broadening expected from increasing the solvent exchange rate is being offset by some other factor, such as ring flipping, for example, which would be expected to increase with temperature and work to narrow the linewidth. Defining the basis of these interesting linewidth effects, however, will require more extensive investigations.
The histidine ring movement facilitating solvent exchange of the Nδ1 proton at pH 4.0 could be as small as a wobble or as large as a flip (further discussion on exchange mechanisms, with examples, is provided in Supporting Text and Schemes 1–3, which are published as supporting information on the PNAS web site). When inhibited with a CMK, the motion of His57 is restricted, and the imidazole ring is formally protonated owing to the alkylation of Nε2. It is therefore fair to compare the Nδ1 proton exchange rates in these complexes to those of the uninhibited enzymes at low pH. The comparison shows that CMK immobilization of the histidine slows solvent exchange even more than does lyophilization, indicating that the scale of motion underlying the fast exchange in NONLYO is larger than the scale of movement allowed when His57 is tethered to Ser195 by the CMK inhibitor. Various aspartate-catalyzed exchange mechanisms involving a stationary or slightly moving histidine can be ruled out as responsible for at least the fastest solvent exchange mechanism, because they are freely available to the inhibited enzyme. Because the linewidth in the CMK complex is narrower than in resting LYO, the results also show that the histidine mobility in LYO, although reduced, has not been eliminated. We believe the reduced motion accounts for why the activity of LYO is reduced but not eliminated.
In summary, the present work shows that α-lytic protease can exist in two distinct conformations, termed LYO and NONLYO, that can be interconverted through the opposite effects of lyophilization and pressurization. LYO is characterized by a less mobile histidine, slower Nδ1 proton exchange with solvent, at least one additional enzyme-bound water molecule, and lower specific catalytic activity. This particular lyophilization-induced structural effect may be unique among serine proteases to α-lytic protease, owing to some peculiarity in its interaction with water. Nevertheless, the information that it reveals about the relationship between His57 dynamics and mechanisms should apply to all serine proteases. This information includes that: (i) His57 is more mobile in resting enzymes at low pH than at high pH and that (ii) the mobility is consistent with ring flipping and (iii) is correlated to enzyme activity. The results, therefore, provide experimental support for the RDRF mechanism.
Supplementary Material
Author contributions: K.C.H. designed research; K.C.H. and D.A.B. performed research; K.C.H. and J.L.S. analyzed data; K.C.H., J.L.S., and W.W.B. wrote the paper; and J.L.S. contributed new reagents/analytic tools.
Abbreviations: RDRF, reaction-driven histidine ring flip; CMK, chloromethyl ketone; HSQC, heteronuclear sequential quantum correlation; LYO, conformation of α-lytic protease after it has been lyophilized and redissolved in water; NONLYO, conformation of α-lytic protease, either freshly purified and nonlyophilized or lyophilized after a mild pressure treatment.
References
- 1.Blow, D. M., Birktoft, J. J. & Hartley, B. S. (1969) Nature 221, 337–340. [DOI] [PubMed] [Google Scholar]
- 2.Hunkapiller, M. W., Smallcombe, S. H., Whitaker, D. R. & Richards, J. H. (1973) J. Biol. Chem. 248, 8306–8308. [PubMed] [Google Scholar]
- 3.Frey, P. A., Whitt, S. A. & Tobin, J. B. (1994) Science 264, 1927–1930. [DOI] [PubMed] [Google Scholar]
- 4.Stryer, L. (1981) Biochemistry (Freeman, San Francisco).
- 5.Wang, J. H. (1970) Proc. Natl. Acad. Sci. USA 66, 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polgar, L. (1972) Acta Biochim. Biophys. Acad. Sci. Hung. 7, 29–34. [PubMed] [Google Scholar]
- 7.Jencks, W. P. (1970) Biochem. J. 117, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ash, E. L., Sudmeier, J. L., Day, R. M., Vincent, M., Torchilin, E. V., Haddad, K. C., Bradshaw, E. M., Sanford, D. G. & Bachovchin, W. W. (2000) Proc. Natl. Acad. Sci. USA 97, 10371–10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derewenda, Z. S., Derewenda, U. & Kobos, P. M. (1994) J. Mol. Biol. 241, 83–93. [DOI] [PubMed] [Google Scholar]
- 10.Katona, G., Wilmouth, R. C., Wright, P. A., Berglund, G. I., Hajdu, J., Neutze, R. & Schofield, C. J. (2002) J. Biol. Chem. 277, 21962–21970. [DOI] [PubMed] [Google Scholar]
- 11.Bachovchin, W. W. (2001) Magn. Reson. Chem. 39, Suppl., S199–S213. [Google Scholar]
- 12.DePristo, M. A., de Bakker, P. I. W. & Blundell, T. L. (2004) Structure 12, 831–838. [DOI] [PubMed] [Google Scholar]
- 13.Topf, M., Varnai, P. & Richards, G. (2001) Theor. Chim. Acta 106, 146–151. [Google Scholar]
- 14.Topf, M., Varnai, P., Schofield, C. J. & Richards, W. G. (2002) Proteins Struct. Funct. Genet. 47, 357–369. [DOI] [PubMed] [Google Scholar]
- 15.Bachovchin, W. W. & Roberts, J. D. (1978) J. Am. Chem. Soc. 100, 8041–8047. [Google Scholar]
- 16.Tsilikounas, E., Kettner, C. A. & Bachovchin, W. W. (1993) Biochemistry 32, 12651–12655. [DOI] [PubMed] [Google Scholar]
- 17.Hunkapiller, M. W., Forgac, M. D. & Richards, J. H. (1976) Biochemistry 15, 5581–5588. [DOI] [PubMed] [Google Scholar]
- 18.Patt, S. L. (1982) J. Magn. Reson. 49, 161–163. [Google Scholar]
- 19.Clore, G. M., Kimber, B. J. & Gronenborn, A. M. (1983) J. Magn. Reson. 54, 170–173. [Google Scholar]
- 20.Delsuc, M. A. & Lallemand, J. Y. (1986) J. Magn. Reson. 69, 504–507. [Google Scholar]
- 21.Piotto, M., Saudek, V. & Sklenar, V. (1992) J. Biomol. NMR 2, 661–665. [DOI] [PubMed] [Google Scholar]
- 22.Messerle, B. A., Wider, G., Otting, G., Weber, C. & Wuethrich, K. (1989) J. Magn. Reson. 85, 608–613. [Google Scholar]
- 23.Robillard, G. & Shulman, R. G. (1974) J. Mol. Biol. 86, 519–540. [DOI] [PubMed] [Google Scholar]
- 24.Doetsch, V. & Wider, G. (1995) J. Am. Chem. Soc. 117, 6064–6070. [Google Scholar]
- 25.Otting, G. & Wuethrich, K. (1989) J. Am. Chem. Soc. 111, 1871–1875. [Google Scholar]
- 26.Bachovchin, W. W. (1986) Biochemistry 25, 7751–7759. [DOI] [PubMed] [Google Scholar]
- 27.Perrin, C. L., Dwyer, T. J., Rebek, J. J. & Duff, R. J. (1990) J. Am. Chem. Soc. 112, 3122–3125. [Google Scholar]
- 28.Redfield, A. G. & Waelder, S. (1979) J. Am. Chem. Soc. 101, 6151–6162. [Google Scholar]
- 29.Carrasquillo, K. G., Sanchez, C. & Griebenow, K. (2000) Biotechnol. Appl. Biochem. 31, 41–53. [DOI] [PubMed] [Google Scholar]
- 30.Kwon, O. H., Imanishi, Y. & Ito, Y. (2000) Bull. Chem. Soc. Jpn. 73, 1277–1282. [Google Scholar]
- 31.Desai, U. R., Osterhout, J. J. & Klibanov, A. M. (1994) J. Am. Chem. Soc. 116, 9420–9422. [Google Scholar]
- 32.Taschner, N., Muller, S. A., Alumella, V. R., Goldie, K. N., Drake, A. F., Aebi, U. & Arvinte, T. (2001) J. Mol. Biol. 310, 169–179. [DOI] [PubMed] [Google Scholar]
- 33.Fujinaga, M., Delbaere, L. T. J., Brayer, G. D. & James, M. N. G. (1985) J. Mol. Biol. 184, 479–502. [DOI] [PubMed] [Google Scholar]
- 34.Whitaker, D. R. (1965) Can. J. Biochem. 43, 1935–1954. [DOI] [PubMed] [Google Scholar]
- 35.Koradi, R., Billeter, M. & Wuethrich, K. (1996) J. Mol. Graphics 14, 51–55; plates, 29–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.