Abstract
Siglecs are transmembrane sialoglycan binding proteins, most of which are expressed on leukocyte subsets and have inhibitory motifs that translate cell surface ligation into immune suppression. In humans, Siglec-8 on eosinophils, mast cells and basophils and Siglec-9 on neutrophils, monocytes and some T-cells, mediate immune cell death, inhibition of immune mediator release and/or enhancement of anti-inflammatory mediator release. Endogenous sialoglycan ligands in tissues, mostly uncharacterized, engage siglecs on leukocytes to inhibit inflammation. Glycan array analyses demonstrated that Siglec-8, Siglec-9 and their mouse counterparts Siglec-F and Siglec-E (respectively) have distinct glycan binding specificities, with Siglec-8 more structurally restricted. Since siglecs are involved in lung inflammation, we studied Siglec-8 and Siglec-9 ligands in human lungs and airways. Siglec-8 ligands are in tracheal submucosal glands and cartilage but not airway epithelium or connective tissues, whereas Siglec-9 ligands are broadly distributed. Mouse airways do not have Siglec-8 ligands, whereas Siglec-9 ligands are on airways of both species. Extraction of human airways and lung followed by electrophoretic resolution and siglec blotting revealed Siglec-8 ligands in extracts of human trachea and cultured tracheal gland cells, but not parenchyma or cultured airway epithelial cells whereas Siglec-9 ligands were extracted from all airway and lung tissues and cells tested. Siglec-8 and Siglec-9 ligands in airways appear to be high molecular weight O-linked sialoglycoproteins. These data reveal differential glycan specificities of Siglec-8, Siglec-9 and their mouse counterparts Siglec-F and Siglec-E, and the tissue distributions and molecular characteristics of Siglec-8 and Siglec-9 sialoglycan ligands on human airways and lungs.
Keywords: airways, sialic acid, siglec, submucosal glands, trachea
Introduction
Siglecs (sialic acid binding immunoglobulin-like lectins) are among the immune regulatory molecules that control ongoing inflammation (Macauley et al. 2014; Schnaar 2016). All siglecs bind to sialoglycans and most are immune inhibitory, with intracellular immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Data support the hypothesis that Siglec-8, which is expressed on eosinophils, mast cells and basophils and Siglec-9, which is expressed on neutrophils, monocytes, dendritic cells and natural killer cells are important to the resolution of ongoing inflammation, such as that which occurs in inflammatory lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) (Schleimer et al. 2016; Schnaar 2016). Ligation of Siglec-8 on eosinophils or Siglec-9 on neutrophils induces apoptosis or regulated necrosis, and in both cases prior activation sensitizes the leukocytes to siglec-mediated death (von Gunten et al. 2005; Nutku-Bilir et al. 2008).
Several human siglecs, including Siglec-8 and Siglec-9, have evolved so rapidly that matching their structures and functions to the smaller family of mouse siglecs is not straightforward, leading to the use of letter designations for mouse siglecs rather than number designations as for human siglecs (Angata 2006; Macauley et al. 2014). Mouse Siglec-F, a functional paralogue of human Siglec-8, is expressed by mouse eosinophils (as well as certain resident macrophages) where it appears to have similar functions (Tateno et al. 2005). Mouse Siglec-E and its ortholog human Siglec-9 have similar cell type expression and may have similar roles in the immune system (McMillan et al. 2013). In animal models of allergic lung inflammation, mice lacking Siglec-F displayed exacerbated eosinophil recruitment (Zhang et al. 2007; Cho et al. 2010) whereas in models of acute lung inflammation Siglec-E-deficient mice exhibited exaggerated neutrophil recruitment (McMillan et al. 2013; Schwarz et al. 2015). The conclusion from these studies is that sialoglycans in the lung engage siglecs on incoming activated leukocytes to resolve the inflammatory response. This conclusion is further supported by the finding that knockout of the sialyltransferase responsible for synthesis of Siglec-F ligands in the mouse lung, ST3Gal-III, results in exacerbated eosinophilic inflammation similar to that in Siglec-F deficient mice (Kiwamoto et al. 2014a). Addition of a synthetic multivalent Siglec-8 binding sialoglycan to primary human eosinophils in vitro resulted in their apoptosis (Hudson et al. 2009). Together, these findings imply that multivalent sialoglycans in the lung engage and crosslink siglecs on leukocytes as a normal mechanism to downregulate inflammation.
The endogenous sialoglycan ligands for Siglec-8 and Siglec-9 on normal human lung are unknown. Several prior studies used glycan arrays to provide insights into their glycan binding specificities and that of their mouse counterparts (Bochner et al. 2005; Tateno et al. 2005; Redelinghuys et al. 2011; Kiwamoto et al. 2014b; Macauley et al. 2014; Siddiqui et al. 2016). The current study used chimeric Fc-tagged soluble expressed forms of Siglec-8 and Siglec-9 and their mouse counterparts Siglec-F and Siglec-E (respectively) first to compare their distinct glycan array specificities and then to probe the tissue distributions and molecular characteristics of human lung ligands for Siglec-8 and Siglec-9.
Results
Comparative siglec binding to glycan arrays
Plasmids were constructed to express the complete extracellular domains of Siglec-8, -9, -E, and -F, each as a chimera in frame with the Fc region of human IgG1. Purified soluble fusion proteins were transferred to the Consortium for Functional Glycomics (CFG) and tested for binding to Version 5.1 (http://glycomics.scripps.edu/CoreH/CoreHarray070112V5.1.pdf) of their printed glycan array (Blixt et al. 2004), which carries 610 synthetic glycans. All four siglecs were screened on the same day using the same reagents and protocols.
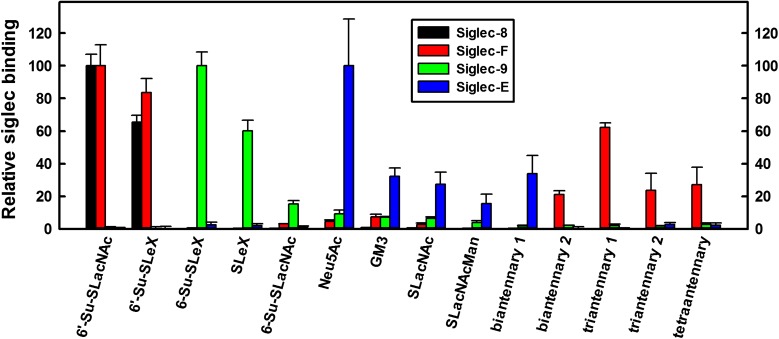
Consistent with prior screens, Siglec-8-Fc bound to only two glycans (Figure 1, Supplementary Table 1), both of which are terminated with a 6-sulfated, 3-sialylated galactose residue: 6′-Su-SLacNAc and 6′-Su-SLex. The mouse paralog of Siglec-8, Siglec-F, bound to these two glycans, but also bound to a select set of α2,3-sialylated biantennary, triantennary and tetraantennary N-linked glycans. Siglec-9-Fc and Siglec-E-Fc binding were distinct from the other siglecs and from each other. Siglec-9-Fc bound to SLex and 6-Su-SLex (which carries the sulfate on the GlcNAc residue) and more modestly to 6-Su-SLacNAc whereas Siglec-E-Fc bound selectively to sialic acid itself (Neu5Ac) and less so to a defined set of glycans with terminal Neu5Acα2,3Galβ1,4Glc(NAc) sequences.
Fig. 1.
Binding of human Fc chimeras of Siglec-8, -F, -9 and -E to the Consortium for Functional Glycomics (CFG) glycan array. Siglec-human Fc chimeras were overlaid on the CFG printed microarray version 5.1 (610 glycans, http://glycomics.scripps.edu/CoreH/CoreHarray070112V5.1.pdf) and binding detected using fluorescently labeled anti-human IgG secondary antibody. Binding of each siglec is normalized to its maximum binding glycan. The 14 sialoglycans that supported ≥15% of maximum binding to any of the four siglecs are included in the graph. Values are reported as mean ± SEM of four replicate spots. The average maximum and background (average binding to the lowest 305 glycans, in parentheses) relative fluorescence values for each siglecs was: Siglec-8, 19200 (8); Siglec-F, 7105 (8); Siglec-9, 3453 (13) and Siglec-E, 1562 (14). Glycan abbreviations are shown in Supplemental Table 1. This figure is available in black and white in print and in color at Glycobiology online.
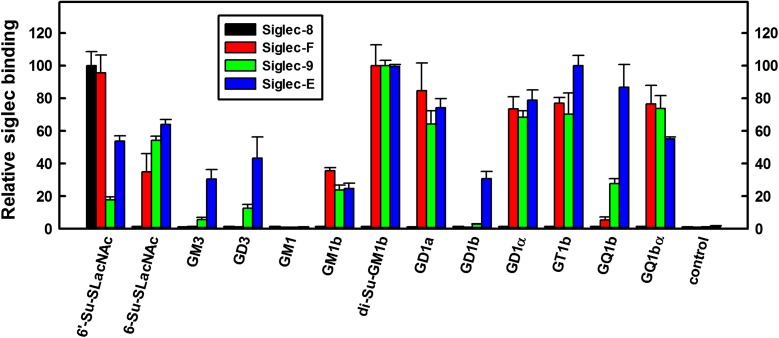
In a partially overlapping cross-platform comparison, the same four siglec chimeras were tested for binding to a limited microplate glycolipid array (Lopez and Schnaar 2006). Again, Siglec-8-Fc bound only to a synthetic neoglycolipid structure with a 6-sulfated, 3-sialylated galactose terminus (Figure 2) whereas Siglec-F-Fc bound to the same glycan, less so to 6-Su-LacNAc, and robustly to a series of natural glycolipids terminating in Neu5Acα2,3Galβ1,3GalNAc (e.g., GD1a, GT1b, Figure 2 and Supplementary Table 1). Siglec-9-Fc had a similar glycolipid binding pattern to Siglec-F-Fc, whereas Siglec-E-Fc also bound to Neu5Acα2,8Neu5Ac terminated structures (GD3, GQ1b and GD1b). We conclude that each of these four siglecs has its own distinct binding pattern on glycan arrays, with Siglec-8 being the most selective. Siglec overlay histochemistry supports the conclusion that each of these siglecs has distinct endogenous ligands.
Fig. 2.
Binding of human Fc chimeras of Siglec-8, -F, -9 and -E to a custom glycolipid microplate array. Glycolipids were co-adsorbed with carrier lipids (phosphatidylcholine and cholesterol) as a monolayer on polystyrene 96-well microwells (Lopez and Schnaar 2006). Glycans included phosphatidylethanolamine-based synthetic neoglycolipids (6’-Su-SLacNAc, 6-Su-SLacNAc), synthetic ceramide-based glycosphingolipids (GD1α, GQ1bα, GM1b and di-Su-GM1b) and naturally sourced ceramide-based gangliosides (GM3, GD3, GM1, GD1a, GD1b, GT1b and GQ1b). Control wells were adsorbed with carrier lipids only. Binding of each siglec is normalized to its maximum binding glycan. Values are reported as mean ± SEM for triplicate wells. Average maximum and background binding (relative colorimetric values, background in parentheses) for each of the siglecs was: Siglec-8, 59 (0.7); Siglec-F, 254 (2); Siglec-9, 256 (3) and Siglec-E, 305 (4). This figure is available in black and white in print and in color at Glycobiology online.
Comparative siglec ligand expression in human and mouse airways
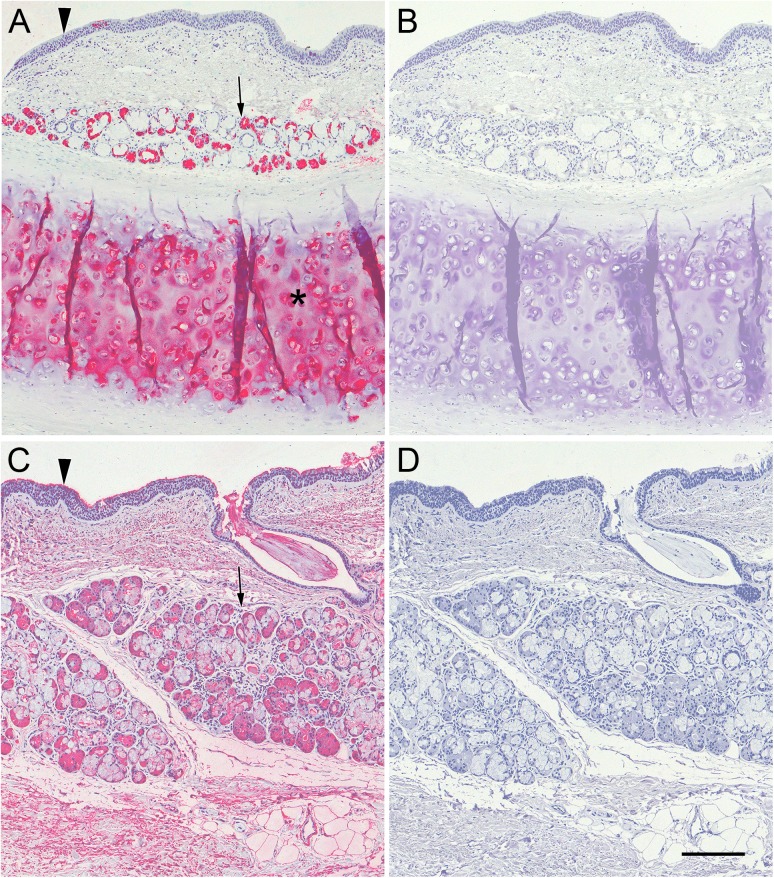
To determine whether human airway and lung express detectable ligands for the human siglecs Siglec-8 and Siglec-9 fixed tissue sections were overlaid with Siglec-8-Fc or Siglec-9-Fc. Specific binding in these experiments was defined as binding that was sensitive to pretreatment of tissue sections with sialidase. Using human tracheal cross sections, Siglec-8-Fc bound robustly to cells in the submucosal glands and to cartilage (Figure 3A), but not to airway epithelium or connective tissue. In contrast, Siglec-9-Fc bound to the surface of the epithelium, to cells in the submucosal glands, and to connective tissue (Figure 3C). All tissue binding by both Siglec-8-Fc and Siglec-9-Fc was completely reversed by sialidase treatment (Figure 3B, D).
Fig. 3.
Siglec-8-Fc and Siglec-9-Fc overlay of human trachea cross sections. Cross sections of human trachea were stained with Siglec-8-Fc (A,B) or Siglec-9-Fc (C,D) precomplexed with AP-conjugated anti-human-Fc. Lectin binding was detected using Vector Red stain and sections counterstained using Hematoxylin QS. Prior to overlay, matched tissues sections (B,D) were incubated in 100 mU/mL sialidase in PBS for 2.5 h at 37°C. Arrowheads: airway epithelium; arrows: submucosal glands; asterisk: cartilage. Scale bar, 200 μm. This figure is available in black and white in print and in color at Glycobiology online.
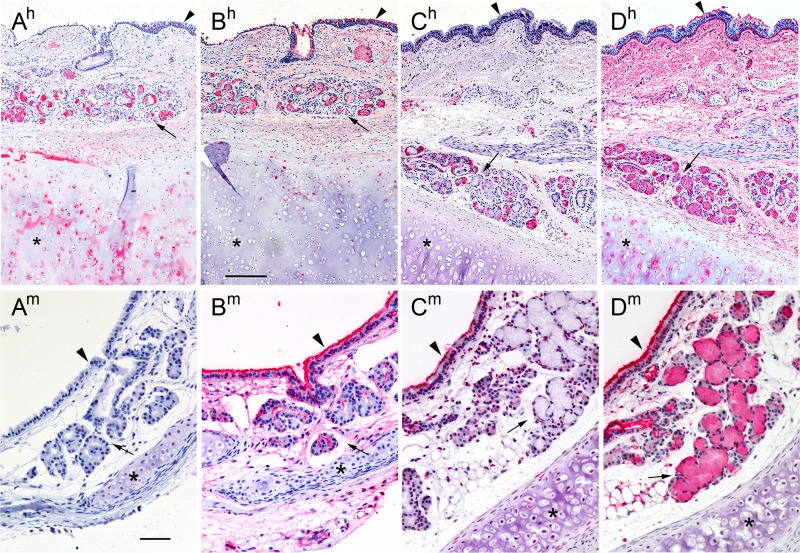
Siglec overlay histochemistry was extended to human and mouse tracheal cross-sections using the Fc chimeras of Siglec-8, -F, -9 and -E to compare the distribution of siglec ligands across species (Figure 4). Whereas Siglec-8-Fc bound to submucosal glands and cartilage in human tracheal cross sections, no Siglec-8 ligands were detected in mouse trachea (Figure 4A). In contrast, human Siglec-9-Fc bound broadly to ligands on the airway epithelium, submucosal glands, and connective tissues of both human and mouse trachea (Figure 4B). Mouse Siglec-E bound robustly to the airway epithelium and some submucosal cells in mouse airway, but only to submucosal cells in the human airway (Figure 4C). Finally, mouse Siglec-F bound robustly to airway epithelium and submucosal cells of mouse airway, and even more broadly to human airway where connective tissue and cartilage were also intensely stained (Figure 4D). The distinct binding patterns of these four siglecs across species emphasize the diversity of their glycan ligands and demonstrate the need to use human tissues to investigate human siglec ligands, most obviously for Siglec-8.
Fig. 4.
Siglec overlay of human and mouse trachea cross sections. Cross sections of human (superscript h) and mouse (superscript m) trachea were stained with Siglec-8-Fc (A), Siglec-9-Fc (B), Siglec-E-Fc (C) or Siglec-F-Fc (D) precomplexed with AP-conjugated anti-human-Fc. Lectin binding was detected using Vector Red stain and sections counterstained using Hematoxylin QS. Images captured using different siglec-Fc chimeras were linearly adjusted to maximize the dynamic staining range within the section. Arrowheads: airway epithelium; arrows: submucosal glands; asterisks: cartilage. Scale bars, 250 μm top row (human), 50 μm bottom row (mouse). This figure is available in black and white in print and in color at Glycobiology online.
Differential binding of Siglec-8 and Siglec-9 to histological sections of human trachea, bronchus and lung parenchyma
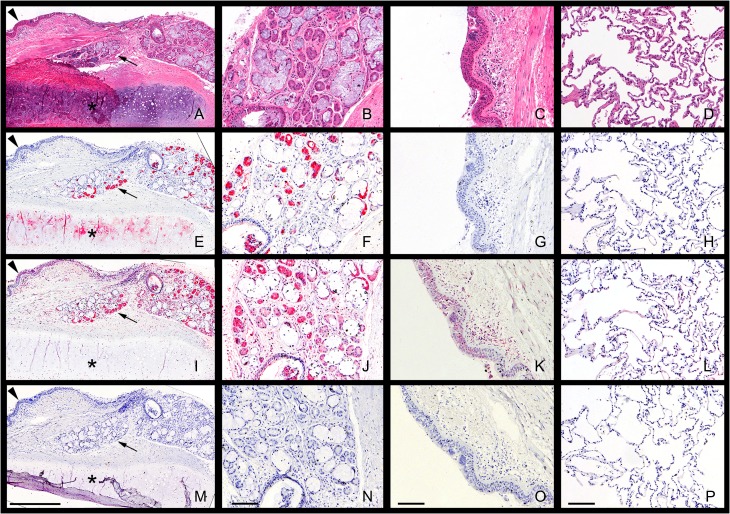
Binding of Siglec-8-Fc and Siglec-9-Fc to human trachea, bronchus and lung parenchyma (LP) revealed further distinctive patterns of siglec ligand distributions (Figure 5). Low power microscopic images of serial tracheal cross sections captured the airway epithelium, underlying submucosal glands, and airway cartilage as seen in routine hematoxylin–eosin (H&E) histological staining (Figure 5A). Siglec-8-Fc binding (Figure 5E) was highly selective, with intense staining of a subpopulation of cells in submucosal glands but no binding to the airway epithelium or connective tissue of the lamina propria. Siglec-8-Fc binding was also detected in airway cartilage. Higher power images of Siglec-8-Fc binding (Figure 5F–H) demonstrated selective staining of cells with the characteristics of serous cells in submucosal glands, but little or no staining in airway epithelium or LP. Intense Siglec-9-Fc binding was also seen in submucosal glands, but Siglec-9-Fc also bound to the airway epithelium and connective tissue of the lamina propria (Figure 5I), as well as in cartilage. Higher power images revealed the broader distribution on Siglec-9, with staining of many cells in the airway (Figure 5J, K) as well as alveolar cells in the LP (Figure 5L). Staining of tissue sections was completely absent using secondary antibody alone (Figure 5M–P) or after treatment of the sections with 100 mU/mL sialidase for 2.5 h at 37°C (Figure 3).
Fig. 5.
Siglec overlay of human airway and lung parenchyma (LP) histological sections. Serial sections of paraffin-embedded human airway (trachea) and LP were stained with H&E to reveal histology or with Siglec-8-Fc or Siglec-9-Fc precomplexed to AP-conjugated anti-human Fc to reveal siglec ligand distributions. Low-power microscopic images of cross sections of human trachea (panels A,E,I,M) and higher power images of airway submucosal glands (panels B,F,J,N), airway epithelium (C,G,K,O) and LP (D,H,L,P) stained with H&E (A–D) or overlaid with precomplexed Siglec-8-Fc (E–H), Siglec-9-Fc (I–L) or secondary antibody (control, AP-conjugated anti-human Fc, M–P) are presented. Lectin binding was detected using Vector Red AP stain and sections counterstained using Hematoxylin QS. Images captured using different siglec-Fc chimeras were linearly adjusted to maximize the dynamic staining range within the section. Scale bars are 0.5 mm (A,E,I,M) and 100 μm for all other panels. Arrowheads: airway epithelium; arrows: submucosal glands; asterisks: cartilage. This figure is available in black and white in print and in color at Glycobiology online.
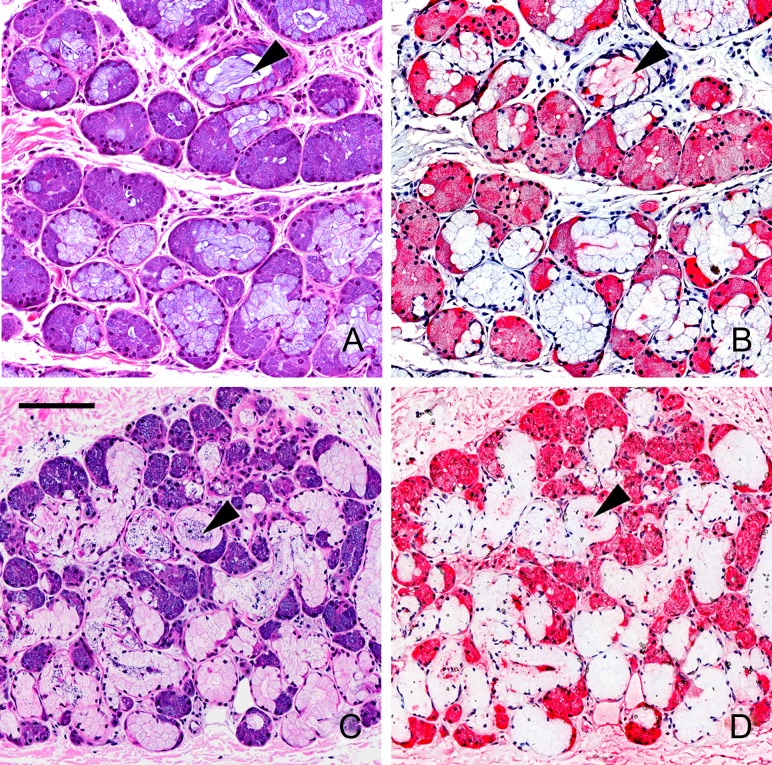
More detailed images of binding of Siglec-8-Fc and Siglec-9-Fc to human submucosal glands are shown in Figure 6. H&E staining revealed gland acini with serous cells stained dark purple and mucous cells light purple (Figure 6A, C). The acinar cells surround mucous-filled ducts which are visible in some of the acini in the stained sections. Whereas Siglec-8-Fc staining of serous cells in the acini was intense, staining of mucous cells and connective tissue was essentially absent (Figure 6B). Siglec-8-Fc staining of material within the ducts was also frequently seen. Siglec-9-Fc intensely stained serous cells in the submucosal gland as well as cells in the connective tissue surrounding the acini (Figure 6D). Siglec-9-Fc staining is also seen faintly in a subpopulation of mucous cells and in ducts.
Fig. 6.
Siglec overlay of human airway submucosal glands. Cross sections of human trachea were stained with H&E (panels A,C) or with Siglec-8-Fc (B), or Siglec-9-Fc (D) precomplexed with AP-conjugated anti-human Fc. Lectin binding was detected using Vector Red stain and sections counterstained using Hematoxylin QS. Images captured using different siglec-Fc chimeras were linearly adjusted to maximize the dynamic staining range within the section. Arrowheads: selected examples of cross sections of submucosal gland ducts. Scale bar, 100 μm. This figure is available in black and white in print and in color at Glycobiology online.
Siglec-Fc staining of bronchi was comparable to that in trachea (Supplementary Figure S1); Siglec-8-Fc stained submucosal glands and cartilage, whereas Siglec-9-Fc stained epithelium, connective tissue and submucosal glands.
Differential binding of Siglec-8-Fc and Siglec-9-Fc to proteins extracted from human trachea, lung parenchyma, cultured human airway cells and to cultured airway secretions
Proteins were extracted from human trachea (Tr) and LP. LP was extracted with detergent-containing buffer, whereas Tr was subjected to a dual extraction procedure to account for tissue elasticity. The longitudinally opened trachea was immersed in detergent-containing buffer and luminal tissue scraped and homogenized. The remaining elastic tissue was snap-frozen, pulverized and extracted with 6M guanidinium hydrochloride (GuHCl). Aliquots of protein extracts were resolved by SDS-PAGE, blotted and ligands detected by overlay with Siglec-8-Fc or Siglec-9-Fc precomplexed with HRP-conjugated secondary antibody for subsequent chemiluminescent detection. Since siglec tissue overlay histochemistry revealed sialidase-dependent binding, replicate aliquots were treated with sialidase and only those bands that were sialidase sensitive were considered specific. Although sialidase insensitive bands may or may not be physiological, they were not studied further.
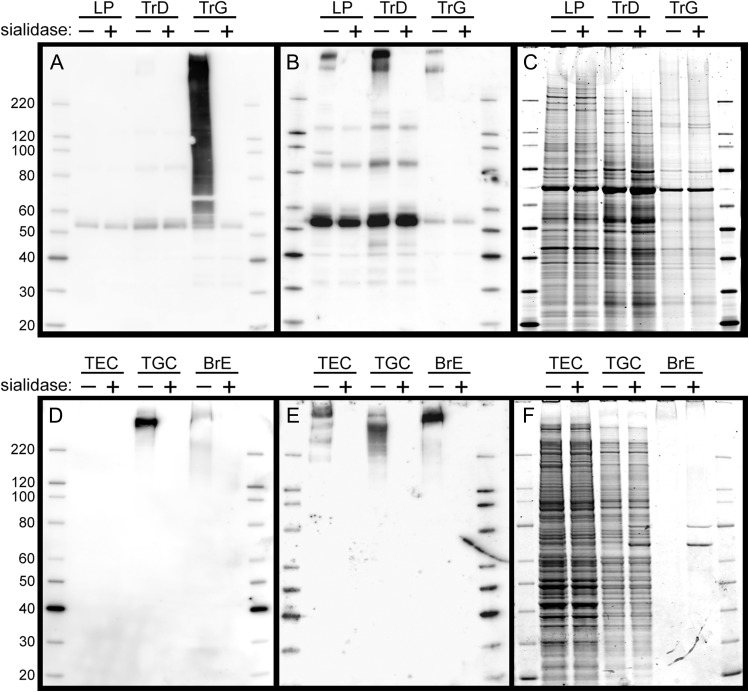
Blotting of SDS-PAGE-resolved proteins with Siglec-8-Fc revealed intense sialic acid-dependent binding to components in the Tr GuHCl extract with much lower binding to the Tr detergent extract and no detectable sialic acid-dependent binding to LP extract (Figure 7A). Short-exposure images (not shown) reveal that the most intense Siglec-8-Fc binding in the Tr GuHCl extract migrates well above the highest molecular weight marker (220 kDa). Sialic acid-dependent Siglec-9-Fc binding was strongest to components in the Tr detergent extract, with binding also readily apparent in the LP and Tr GuHCl extracts (Figure 7B). Sialic acid-dependent binding was predominantly but not exclusively to components that migrated well above the highest molecular weight marker.
Fig. 7.
Siglec-8-Fc and Siglec-9-Fc lectin blotting of extracts from human airway, LP, and cultured airway cells. Panels A–C: Tissue extracts. Detergent extract of human LP, detergent extract of scraped human trachea luminal tissue (TrD), and subsequent GuHCl extract of the pulverized remaining trachea (TrG) were resolved by SDS-PAGE on replicate 4–12% polyacrylamide gels, blotted to PVDF and overlaid with Sigec-8-Fc precomplexed to HRP-conjugated anti-human Fc (Panel A) or similarly precomplexed Siglec-9-Fc (Panel B) prior to enhanced chemiluminescent detection. A replicate gel stained with SYPRO ruby protein gel stain is shown in Panel C. As indicated at the top of each blot, protein extracts in alternate lanes (+) were pretreated with sialidase prior to SDS-PAGE. Panels D–F: Cultured cells and tissue. Detergent extracts of cultured tracheal epithelial cells (TEC), tracheal gland cells (TGC) and secretions from cultured intact bronchus (BrE) were resolved by SDS-PAGE, blotted and overlaid with precomplexed Siglec-8-Fc (Panel D) or Siglec-9-Fc (Panel E) prior to enhanced chemiluminescent detection. A replicate gel stained with SYPRO ruby is shown in Panel F. As indicated at the top of each blot, protein extracts in alternate lanes (+) were pretreated with sialidase prior to SDS-PAGE.
Probing detergent extracts of cultured human airway cells provided complementary data (Figure 7D, E). Siglec-8-Fc bound robustly to a high molecular weight component extracted from tracheal gland cells (TGC) but failed to bind to any component in extract from tracheal epithelial cells (TEC). In contrast, Siglec-9-Fc bound to high molecular weight components in extracts of both TEC and TGC. Both Siglec-8-Fc and Siglec-9-Fc bound to high molecular weight components exuded into the medium of cultured intact human bronchus (Figure 7D, E). Using fixed primary TGC cultures (Siglec-8 and Siglec-9) and primary TEC (Siglec-9 only) mosaic staining of subsets of cultured cells was observed (data not shown).
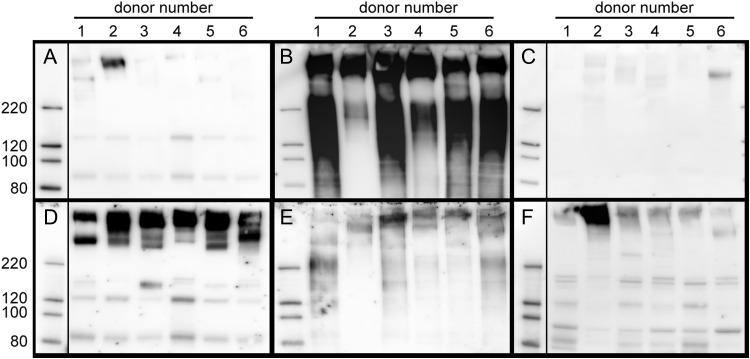
Subject-to-subject reproducibility of siglec ligand extraction was tested on like tissue samples prepared in the same manner from six organ donors (donor information listed in Table I). Equal amounts of extracted protein (1 µg) were loaded in each SDS-PAGE gel lane (Figure 8). The most consistent findings were that Siglec-8-Fc bound most robustly to components in the Tr GuHCl extract (Figure 8B), whereas Siglec-9-Fc bound most robustly to components in the Tr detergent extract (Figure 8D). Binding of Siglec-8-Fc to detergent-extracted Tr was variable, and consistently low compared to the GuHCl extract of the same tissue, whereas Siglec-9-Fc binding to GuHCl extracts was consistent but less robust than to components that had first been extracted with detergent from the same tissue. Binding of Siglec-8-Fc to components in detergent-extracted LP was low to absent in most samples. Siglec-9-Fc bound to components in every LP extract, but the degree of binding was variable.
Table I.
Tissue donors
| Donor | Sex | Age (yr) |
|---|---|---|
| 1 | F | 45 |
| 2 | F | 39 |
| 3 | M | 53 |
| 4 | F | 28 |
| 5 | F | 57 |
| 6 | M | 45 |
Fig. 8.
Siglec-8-Fc and Siglec-9-Fc lectin blotting of extracts from human airways and LP different donors. Tissues from six donors (Table I) are compared. Human LP was detergent extracted and tracheas were subjected to a two-stage detergent/GuHCl extraction as described in Materials and methods. Protein (1 µg based on Pierce BCA protein assay) from tracheal detergent-extracts (Panels A and D), tracheal GuHCl extracts (Panels B and E) and LP detergent-extracts (Panels C and F) were resolved by SDS-PAGE on replicate 4–12% polyacrylamide gels, blotted to PVDF and overlaid with Sigec-8-Fc precomplexed to HRP-conjugated anti-human Fc (Panels A–C) or similarly precomplexed Siglec-9-Fc (Panels D–F) prior to enhanced chemiluminescent detection.
Siglec-8 and Siglec-9 ligands are primarily O-linked glycans on high molecular weight glycoproteins
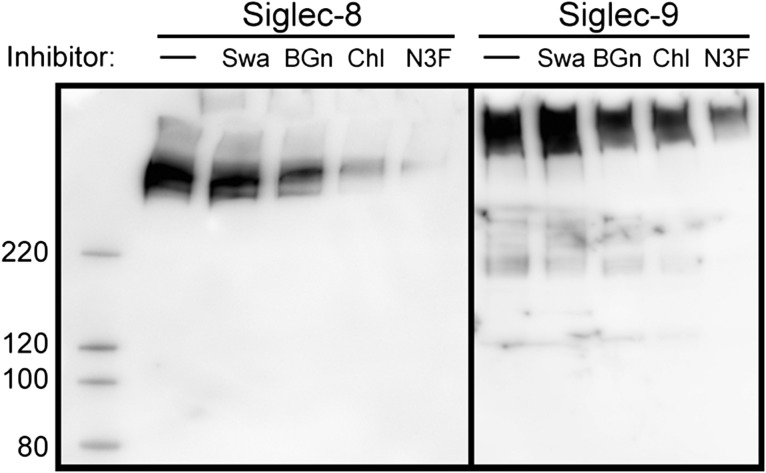
Based on the above findings, airway cell cultures (TEC and TGC) were pretreated with the N-glycan processing inhibitor swainsonine, which altered N-glycan expression as detected using lectin blotting (data not shown). This treatment did not significantly diminish Siglec-8-Fc binding to TGC extract or Siglec-9-Fc binding to TEC extract (Figure 9). In contrast, treatment of airway cells in culture with benzyl-α-GalNAc to circumvent O-linked glycan extension diminished both Siglec-8-Fc binding (to TGC extracts) and Siglec-9-Fc binding (to TEC extracts), as did treatment of cells with sodium chlorate to diminish sulfation, or 3Fax-Neu5Ac to inhibit sialyltransferases (Figure 9).
Fig. 9.
Metabolic inhibitors indicate Siglec-8 and Siglec-9 ligands include O-linked sulfated and sialylated glycans. Metabolic inhibitor treatment of TGC cultures (Siglec-8, left panel) or TEC cultures (Siglec-9, right panel). Cell cultures were pretreated for 24 h with swainsonine (Swa) to inhibit N-glycan processing, benzyl-α-GalNAc (BGn) to inhibit O-linked glycan extension, sodium chlorate (Chl) to inhibit sulfation, or 3Fax-Neu5Ac (N3F) to inhibit sialyltransferases (as described in the text) prior to extraction, resolution by SDS-PAGE, and detection using precomplexed Siglec-8-Fc or Siglec-9-Fc overlay as indicated. Swainsonine efficacy was confirmed by lectin overlay, which showed increased ConA binding and decreased RCA and WGA binding of extracted proteins (data not shown).
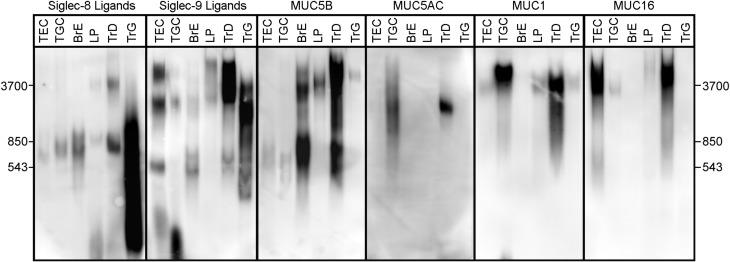
Comparative electrophoretic migration of siglec ligands and endogenous mucins in human airway, lung parenchyma and cultured human airway cells and tissues
Since Siglec-8 and Siglec-9 ligands may be sialylated, sulfated large molecular weight glycoproteins, properties shared by some mucins, a direct comparison of Siglec-8-Fc and Siglec-9-Fc binding to tissue and cell extracts resolved by SDS-PAGE with the migration of major airway mucins was conducted (Figure 10). Composite agarose-acrylamide gels were used, since they allow enhanced resolution and blotting of very high molecular weight proteins up to ≥4 MDa (Issa et al. 2011; Jia et al. 2015). Siglec ligands from airway and lung ranged in size from ~250 kDa to >4 MDa by composite gel electrophoresis. Of the two major secreted airway mucins (MUC5B and MUC5AC) and two major membrane-bound mucins (MUC1 and MUC16), no single mucin tracked quantitatively tissue-to-tissue with either Siglec-8 or Siglec-9 ligands, although the family of mucins was well represented in all sources of Siglec-8 and Siglec-9 ligands except GuHCl extracted proteins from trachea. These data suggest that no one major mucin isoform carries Siglec-8 and Siglec-9 sialoglycan ligands in airways and lung tissues, although the roles of multiple mucins or minor mucin isoforms, or mucins that are not well represented in immuno-overlay, remain valid possibilities.
Fig. 10.
Comparison of siglec ligands and mucins in human airway and lung tissue extracts and human airway cells. Equivalent aliquots of extracts of cultured human airway cells (TEC and TGC), secretions from cultured intact bronchus (BrE), detergent extracts of LP and sequential detergent and GuHCl extracts of trachea (TrD, TrG) were resolved on replicate composite agarose-acrylamide gels, blotted to PVDF membranes, and replicate blots probed with precomplexed Siglec-8-Fc or Siglec-9-Fc or with antibodies to the indicated human mucins followed by appropriate HRP-conjugated secondary antibodies prior to enhanced chemiluminescent detection. Migration positions of very high molecular weight markers purified from rat soleus muscle are shown on each side of the panels.
Discussion
Many members of the siglec family, most of which are involved in immune regulation, have undergone rapid evolution that may reflect the evolutionary pressure of pathogens that circumvent immune regulatory mechanisms resulting in evolutionary responses in host populations (Varki 2006; Varki and Angata 2006). This results in differences in siglecs and their ligands not only between rodents and humans, but even among primates (Padler-Karavani et al. 2014). Based on overlapping expression on leukocyte subsets and overlapping functions, mouse Siglec-F is considered a functional paralog of human Siglec-8 (Tateno et al. 2005) and mouse Siglec-E a functional ortholog of human Siglec-9 (McMillan et al. 2013). Siglec-8, -F, -9 and -E control inflammation by regulating the leukocyte subsets on which they are expressed (Nutku et al. 2005; von Gunten et al. 2005; Zhang et al. 2007; Nutku-Bilir et al. 2008; Cho et al. 2010; McMillan et al. 2013). This concept is supported not only by in vitro and mouse genetic studies, but by the observation that SIGLEC8 polymorphism is associated with asthma (Gao et al. 2010). Together, these findings support the concept that siglec glycan binding specificities and endogenous ligands are relevant to the regulation of inflammation and to understanding inflammatory disorders.
Glycan array data (Figures 1 and 2) confirm and expand prior findings of glycan recognition by these siglecs (Bochner et al. 2005; Tateno et al. 2005; Redelinghuys et al. 2011; Kiwamoto et al. 2014b; Macauley et al. 2014; Propster et al. 2016; Siddiqui et al. 2016). Concurrent testing of a matched set of human Fc chimeras revealed overlapping but distinct patterns of recognition, with Siglec-8 notable for its extraordinarily narrow binding specificity, requiring an α2,3 sialylated 6-O-sulfated galactose. Whereas Siglec-F binds to the same determinant (Tateno et al. 2005; Kiwamoto et al. 2014b), its glycan binding specificity is much broader with robust binding to certain N-linked branched sialoglycans and gangliosides with a terminal α2,3 sialic acid. This may explain why mice engineered to lack the enzymes required for galactose 6-sulfation retain Siglec-F ligands (Patnode et al. 2013), and sialylated 6-sulfated galactose was absent from mouse TEC mucins that bind Siglec-F (Kiwamoto et al. 2014b).
Binding to the printed glycan array (Figure 1) indicates that each siglec tested has a different set of preferred sialoglycan ligands, whereas glycolipid microarray binding using the same chimeras (Figure 2) reveals considerable overlap between Siglec-9, Siglec-F and Siglec-E binding. This may be due in part to the different structures tested, but also may reflect the ability of glycolipids adsorbed as a lipid monolayer to diffuse laterally and achieve quaternary associations that support siglec binding. Nevertheless, Siglec-8 remains the only siglec tested that required a sialylated 6-sulfated galactose determinant for binding. A basis for this structural specificity was confirmed using solution NMR (Propster et al. 2016), implicating sialylated 6-sulfated galactose as the key Siglec-8 binding determinant.
Cross-species airway binding of siglec chimeras supports the conclusion that each has different sialoglycan binding specificities. Mouse airway fails to express Siglec-8 binding determinants, indicating that sialylated 6-sulfated galactose residues are absent from or inappropriately expressed on the mouse airway or are inaccessible to exogenously added soluble siglec chimeras. This emphasizes the evolutionary divergence of siglec ligands, and compels the use of human tissues to explore human siglec ligands. Notably, the airway tissue distribution of Siglec-F ligands is akin to a combination of Siglec-8 and Siglec-9 binding, with all major subcompartments stained in both human and mouse.
The histological pattern of Siglec-8 ligands in the human trachea was striking, with intense staining of serous cells in airway submucosal glands and in cartilage, similar to our prior findings in nasal airway (Jia et al. 2015). The borders of Siglec-8 staining in gland acini were sharp, often with little or no staining in adjoining connective tissue or other areas of the airway or lung. Since the proposed physiological role of Siglec-8 ligands is to downregulate ongoing allergic inflammation by engaging and crosslinking Siglec-8 on the surface of eosinophils and mast cells (Schleimer et al. 2016), Siglec-8 ligands must come into physical contact with those cells. The presence of Siglec-8 stained material in submucosal ducts implies that production of Siglec-8 ligands in submucosal gland serous cells leads to their secretion via submucosal ducts to the surface of the airway where leukocytes are encountered. The absence of Siglec-8 ligands on the epithelial surface may indicate that Siglec-8 ligands in the airway mucus layer are washed away during tissue acquisition and processing. Further testing of this hypothesis will require the study of siglec ligands in airway mucus acquired from live subjects. The relative absence of Siglec-8 ligands in the LP is consistent with the appearance of activated eosinophils in the trachea, bronchi and bronchioles rather than in alveoli in asthma and allergic diseases (Jeffery 2000). The function of Siglec-8 ligands in cartilage is not clear, although cartilage damage downstream of eosinophilic inflammation is well-established (Naik and Wala 2013).
The histological pattern of Siglec-9 ligands detected in this study was broader than for Siglec-8 ligands, with staining throughout the airway and on alveolar cells. The broader lung distribution of Siglec-9 ligands is consistent with neutrophilic inflammation, which occurs throughout the lung from alveoli to airways in different lung inflammatory diseases such as COPD and asthma (Jeffery 2000; Meijer et al. 2013; Ciepiela et al. 2015). Siglec-9 binds to sialylated glycans with a 6-sulfated GlcNAc, several nonsulfated sialoglycans (Paulson et al. 2012), and even to the nonsialylated nonsulfated anionic polysaccharide hyaluronic acid (Secundino et al. 2016). In a prior study of detergent extracted nasal tissues, MUC5B (among several sialoglycoproteins) was found to carry Siglec-9 (but not Siglec-8) sialoglycan ligands (Jia et al. 2015).
Analyses of extracted airway and lung proteins by siglec overlay is consistent with the histological findings. Siglec-8-Fc bound consistently to sialoglycoproteins extracted from trachea but not parenchyma and to proteins extracted from cultured TGCs but not epithelial cells, whereas Siglec-9-Fc bound to sialoglycoproteins extracted from all of these sources. Binding of Siglec-8 and Siglec-9 to proteins extracted from trachea was consistent among donors, whereas binding to extracted LP was variable. This may relate to the small sample volume of LP from any one donor, or to physiological or pathological factors not captured in this study. It is notable that both Siglec-8-Fc and Siglec-9-Fc staining of airway was increased in chronically inflamed nasal tissues compared to tissues from noninflamed subjects (Jia et al. 2015). The local expression of siglec ligands in lung and airways may be under inflammatory regulation, as was found for Siglec-9 using a human airway cell line (Jia et al. 2015). Although not a focus of the current study, no differences in binding patterns were detected in tissues from human smokers and nonsmokers.
Most of the sialoglycoprotein ligands extracted from airways and LP were large (>250 kDa to >4 MDa). On composite agarose-acrylamide gels that resolve very large glycoproteins and enhance their blotting, prominent siglec ligands nearly all exceeded 250 kDa. In this size range, mucins predominate. Although immunoblotting of any one human mucin failed to consistently track with either Siglec-8-Fc or Siglec-9-Fc binding, it remains a valid hypothesis that minor glycoforms of major mucins may be siglec ligands. Although the protein carriers and specific glycan structures on Siglec-8 and Siglec-9 lung and airway ligands await further study, the findings here implicate sialylated and sulfated O-linked glycans.
In exploring functional lectin ligands it is worth recapitulating the criteria articulated by Varki (1997), who proposed four conditions to identify “real” lectin ligands: The ligands should be present in the right place at the right time; removal or blockade of the ligand should abrogate biologically relevant interactions; the absence of ligand should abrogate the function of the lectin; and the ligand should be recognized with selectivity, relatively high affinity and well-defined stoichiometry. Most of these criteria have yet to be met for Siglec-8 and Siglec-9 ligands in the human lung, although several have been met for Siglec-F and/or Siglec-E in the mouse (Zhang et al. 2007; Cho et al. 2010; Kiwamoto et al. 2014a). While mice appear to be an excellent model for siglec functions on leukocytes, they do not provide a compelling model for the study of endogenous Siglec-8 (or perhaps Siglec-9) ligands. Although MUC5B is a carrier of Siglec-F ligands in the mouse (Kiwamoto et al. 2014b), that does not implicate a role for MUC5B as a carrier of human Siglec-8 ligands.
Siglec-8 and Siglec-9 binding in airways and lungs, both histological and biochemical, may relate to siglec functions. As yet, a direct connection of the binding reported here to physiology or pathology is lacking. Nevertheless, the implications of such knowledge may be relevant to asthma and COPD, both of which are chronic inflammatory diseases of the lung (Barnes 2008; Postma et al. 2014). Asthma is dominated by eosinophilic inflammation and mast cell sensitization, COPD by neutrophilic inflammation accompanied by macrophage accumulation (Barnes 2008; Vlahos and Bozinovski 2014), and asthma–COPD overlap is well established (Slats and Taube 2016). As further studies reveal the structures of endogenous human sialoglycans responsible for inhibition of ongoing inflammation via both Siglec-8 and Siglec-9, insights to enhance the design of anti-inflammatory sialoglycans that target siglecs may emerge (Angata et al. 2015; Bochner 2016; Bull et al. 2016).
Materials and methods
Reagents
Siglec-Fc chimeras were produced by cloning the entire extracellular domain of each siglec in frame with the human Fc domain of IgG1 behind an EF1α promoter. Fusion proteins were transiently transfected and expressed in human embryonic kidney 293T cells, and expressed soluble constructs purified from the medium using Protein G chromatography. Alternatively (as indicated) Siglec-8-(human)Fc was produced as described (Kikly et al. 2000), and Siglec-9-(human)Fc, Siglec-F-(human)Fc and Siglec-E-(mouse)Fc were purchased from R&D Systems (Minneapolis, MN).
Pronase and dispase/collagenase were purchased from Roche Life Science (Indianapolis, IN). Protease inhibitor cocktail was from Sigma-Aldrich (St. Louis, MO). Human Fc receptor blocker was from Innovex Biosciences (Richmond, CA). Recombinant V. cholerae sialidase was produced in E. coli from a plasmid as described (Mountney et al. 2010). Glycan biosynthetic inhibitors swainsonine, sodium chlorate, and benzyl-N-acetyl-α-d-galactosaminide (benzyl-α-GalNAc) were from Sigma-Aldrich. The sialyltransferase inhibitor peracetylated 3Fax-Neu5Ac (Rillahan et al. 2012) was from EMD Millipore (Billerica, MA). PNGase F was purchased from New England Biolabs (Ipswich, MA). Anti-human MUC5B (SC 20119), anti-human MUC5AC (SC-20118), anti-human MUC16 (SC-52095), and anti-human MUC1 (SC-7313) were purchased from Santa Cruz Biotechnology (Dallas, TX).
Glycan array binding
Expressed and purified siglec-(human Fc) chimeras were transferred to the Protein-Glycan Interaction Core of the CFG to test binding to 610 immobilized glycans on Version 5.1 of their printed glycan array (http://glycomics.scripps.edu/CoreH/CoreHarray070112V5.1.pdf). Binding was detected using Alexa Fluor 488-labeled antibody to human IgG. Fluorescence intensity on six replicates of each glycan was quantified, the highest and lowest values discarded, and the mean and SEM of binding to the remaining four values calculated. Data were normalized to the highest-binding glycan for each siglec. Average intensities for glycans that failed to bind ≥15% of maximum for any of the four siglecs tested are not presented, but are available online at the CFG data repository (http://www.functionalglycomics.org/glycomics/publicdata/primaryscreen.jsp). Glycans that bound any of the siglecs tested at ≥15% of maximum for that siglec are listed in Supplementary Table 1 with binding data shown in Figure 1.
Glycolipid microwell arrays were prepared by co-adsorbing glycolipids (25 pmol/well) as a monolayer along with phosphatidylcholine (25 pmol/well) and cholesterol (100 pmol/well) onto polystyrene microwells as described (Lopez and Schnaar 2006). Control wells were adsorbed with the carrier lipids without glycolipid. Glycan structures of the glycolipids are listed in Supplementary Table 1, and include naturally sourced purified glycosphingolipids (GM3, GD3, GM1, GD1a, GD1b, GT1b and GQ1b) from Sigma-Aldrich or Matreya LLC (Pleasant Gap, PA), and synthetic glycosphingolipids (GD1α, GQ1α, GM1b, di-sulfo-GM1b) prepared as described (Ito et al. 2003). Aminoethyl glycosides of Neu5Ac(α2-3)Gal(β1-4)(6-O-sulfo)GlcNAc (6-Su-SLacNAc) and Neu5Ac(α2-3)(6-O-sulfo)Gal(β1-4)GlcNAc (6′-Su-SLacNAc) were prepared as previously described (Cheng et al. 2015). Neoglycolipids were prepared by coupling the aminoethyl glycosides to N-(succinimidyloxy-glutaryl)-l-α-phosphatidylethanolamine (COATSOME FE-8080SU5, NOF America Corp., White Plains, NY) in CH2Cl2-DMSO (1:1) containing 2 molar equivalents of diisopropylethylamine. Neoglycolipids were purified using Sep-Pak C18 cartridges (Waters Corp., Milford, MA) washed with H2O, methanol, then eluted with CH2Cl2-methanol (1:1).
Siglec-(human Fc) chimeras (20 µg/mL) were premixed with alkaline phosphatase (AP) labeled anti-human Fc antibody (10 µg/mL) for 30 min at 37°C, diluted 10-fold, and 50 µL added to each lipid-adsorbed well. After incubation for 1 h at ambient temperature, binding was detected colorimetrically using p-nitrophenylphosphate as substrate. Binding of each siglec was normalized to the highest binding glycan and is reported as the mean and SEM for triplicate determinations.
Tissues
Lungs were obtained from six human organ donors (Table I) within 24 h of removal. None of the donors were reported to have had chronic respiratory disease, but all were reported to have smoked tobacco. Organs were flushed and stored in HTK solution (donor #4) or UW solution (all other donors) and kept ice cold for up to 24 h prior to dissection (Latchana et al. 2014). Parenchyma, trachea and bronchus were dissected, transferred to ice cold RPMI-1640 containing antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin), and further processed for histology, extraction, or intact cell isolation. To test tobacco use as a causative factor resulting in siglec-Fc binding, a single 47-year old female donor who was reported to be tobacco-free was used and no differences to other donors were apparent (e.g., see Supplementary Figure S1). For comparative siglec overlay histochemistry, adult wild type C57BL/6 mice were deeply anesthetized with isoflurane then tracheas dissected and further processed for histology.
Siglec overlay histochemistry
Tissues were fixed in neutral 4% paraformaldehyde in Dulbecco's phosphate-buffered saline (PBS) at 4°C for 16 h, embedded in paraffin, sectioned to 5 µm and captured on glass slides. Following deparaffinization, the slides were heated briefly in 10 mM sodium citrate (pH 6.0) for antigen retrieval. Subsequent steps were performed at ambient temperature. Slides were incubated in endogenous enzyme Blocking Reagent (Dako North America, Carpinteria, CA) for 10 min, and then in Fc Receptor Blocker (Innovex Biosciences, Richmond, CA) for 30 min. Siglec-8-Fc (160 μg/mL) or Siglec-9-Fc (120 μg/mL) was pre-incubated in PBS with AP-conjugated goat anti-human antibody (16 μg/mL, product 109-055-008, Jackson Immunoresearch, West Grove, PA) for 30 min at ambient temperature. The solution was diluted 8-fold in PBS, then overlaid on blocked slides and incubated 60 min. Slides were washed with PBS, bound lectin conjugate detected with Vector Red AP substrate (Vector Laboratories, Burlingame, CA), slides counterstained with Hematoxylin QS (Vector Laboratories), dehydrated, mounted in Krystalon (EMD Millipore) and imaged using a Nikon Eclipse 90i microscope. Prior to blocking, some slides were overlaid with a solution of 100 mU/mL V. cholerae sialidase in PBS for 2.5 h at 37°C to destroy siglec ligands. Images captured using different siglec-Fc chimeras were linearly adjusted to maximize the dynamic staining of the entire image.
For comparative siglec overlay of mouse and human airway sections, the above procedure was modified as follows. Prior to treatment with blocking reagents, slides were incubated in PBS supplemented with 0.1% Tween-20 and 10 mg/mL BSA (Sigma-Aldrich) for 30 min. Siglec-8-Fc (15 μg/mL), Siglec-9-Fc (15 μg/mL), or Siglec-F-Fc (5 μg/mL) in the same buffer were pre-incubated with AP-conjugated goat anti-human IgG (2 μg/mL, product 109-055-044, Jackson Immunoresearch, West Grove, PA) for 30 min at 4°C. Alternatively, Siglec-E-(mouse Fc) (5 µg/mL) was similarly pre-incubated with AP-conjugated goat anti-mouse IgG (2 μg/mL, product 115-055-003, Jackson Immunoresearch). Preconjugated siglec-Fc chimeras were pipetted onto the washed slides and incubated for 16 h at 4°C. Slides were washed with PBS/0.1% Tween-20, then with 100 mM Tris-HCl (pH 8.3)/0.1% Tween 20 for 10 min prior to conjugate detection, counterstaining and imaging as above.
Protein extracts
Tracheae were immersed in Detergent Buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM CaCl2, 2 mM MgCl2, 0.3% CHAPS, 1% NP-40 and protease inhibitor cocktail) and the luminal surface scraped to dislodge soft tissues. Elastic tissue was removed and the remaining suspension homogenized by passing through a 20-gauge syringe needle. Homogenates were centrifuged at 20,000 × g for 5 min, and then supernatants collected and stored at −70°C. Elastic tissue was pulverized under liquid nitrogen and incubated 16 h at 4°C in 10 mM sodium phosphate (pH 6.5), 6 M GuHCl (OminPur, EMD Millipore), 5 mM EDTA, and 0.1 mM phenylmethylsulfonyl fluoride. The extract was centrifuged at 22,000 × g for 30 min and the supernatant stored at −20°C. LP was homogenized in Detergent Buffer using a Potter-Elvehjem glass-Teflon homogenizer on ice. In some experiments intact bronchus and bronchioles were dissected clean of surrounding parenchyma and incubated in RPMI-1640 containing 100 U/mL penicillin and 100 μg/mL streptomycin for 48 h at 4°C. Tissue was removed and soluble exudate analyzed. Protein concentrations in extracts and exudates was determined by Pierce BCA protein assay (ThermoFisher Scientific, Waltham, MA).
Primary airway epithelial and submucosal gland cells
Isolation of airway epithelial and submucosal gland cells was performed as described (Sommerhoff and Finkbeiner 1990; Casalino-Matsuda et al. 2004). The trachea was rinsed in sterile Ham's F-12 medium, opened longitudinally, and then treated with 0.1% pronase in Ham's F12 medium containing antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL fungizone) at 4°C overnight to release epithelial cells, which were collected as a suspension. Gland-rich submucosal tissue was dissected from the remaining airway and incubated for 24 h in 0.01% dispase/collagenase in Dulbecco's modified Eagle medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin and 50 μg/mL gentamicin (DMEM-AB). Each strip was scraped after digestion to release the remaining gland cells. Cells were collected by centrifugation, washed in DMEM-AB, and then resuspended in trypsin-EDTA (0.25%, ThermoFisher Scientific) and triturated to dissociate remaining clumps of cells. Digestion was stopped by addition of fetal bovine serum, cells collected by centrifugation and resuspended in DMEM-AB. Gland cells were plated on collagen-coated plates (BD Biosciences, Billerica, MA) in 1:1 DMEM-AB-Ham's F-12 supplemented with hydrocortisone (0.5 μg/mL), insulin (5 μg/mL), transferrin (10 μg/mL), epinephrine (0.5 μg/mL), triiodothyronine (6.5 ng/mL) and human EGF (25 ng/mL). Cultures were maintained as submerged cultures at 37°C in an atmosphere of 5% CO2 in air with medium changed every third day.
Airway epithelial cells were plated on collagen-coated T25 flasks and cultured in DMEM/F12 (ThermoFisher Scientific) containing 20% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL fungizone until confluent. Cells were then dissociated with trypsin-EDTA and plated on 6-well Falcon 0.4 μm porous culture inserts (Corning Inc., Corning, NY). The cultures were maintained at an air–liquid interface with medium below the culture insert (DMEM-BEGM (1:1) plus SingleQuots (Lonza Inc, Mapleton, IL)). Culture medium was replaced every third day.
Cultures were maintained for 10 days prior to mechanical release, collection by centrifugation and detergent extraction. As indicated, cultures were treated 1 day prior to collection with swainsonine (20 μM), sodium chlorate (50 mM), benzyl-α-GalNAc (2 mM), or peracetylated 3Fax-Neu5Ac (200 μM).
Lectin blotting and immunoblotting
Samples extracted with GuHCl were first dialyzed against 1 M urea, 20 mM sodium phosphate pH 7.4 before loading on a denaturing gel. Proteins in cell and tissue extracts and exudates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 4–12% NuPAGE Bis-Tris precast gels (ThermoFisher Scientific) or 2% agarose/1.5% acrylamide composite gels (Jia et al. 2015) and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% nonfat milk in PBS/0.1% Tween-20 for 30 min and overlaid with a solution of siglec-Fc precomplexed with horseradish peroxidase (HRP)-conjugated anti-human Fc (Sigma-Aldrich) prepared as follows. A solution containing Siglec-8-Fc or Siglec-9-Fc (20 μg/mL) and HRP-conjugated anti-human Fc (14 μg/mL) in PBS/0.1% Tween-20 was incubated for 30 min on ice, diluted 40-fold with the same buffer, and blots were incubated with the solution for 16 h at 4°C. Membranes were washed and developed using enhanced chemiluminescence (Amersham ECL Prime Western Blotting Detection Reagent, GE Healthcare, Pittsburgh, PA). Alternatively, blots were incubated with anti-human mucin antibodies as indicated and binding detected using HRP-conjugated anti-rabbit (for anti-MUC5B and anti-MUC5AC) or anti-mouse (for anti-MUC1 and anti-MUC16) secondary antibodies with detection by enhanced chemiluminescence. Molecular weight marker for Bis-Tris precast gels (up to 220 kDa) was MagicMark XP Western protein standard (ThermoFisher Scientific). Molecular weight markers for composite gels (up to 3700 kDa) were prepared from rat soleus muscle extracts as described (Greaser and Warren 2012).
Supplementary Material
Acknowledgments
The authors thank Mary G. Motari for valuable technical assistance and support. Neoglycolipids were prepared by the Shared Resources Core of the Lung Inflammatory Disease Program of Excellence in Glycoscience (http://lidpeg.org). Printed glycan microarray analyses were performed by the Protein-glycan Interaction Resource of the CFG (GM098791) at Emory University School of Medicine, Atlanta, GA, USA.
Supplementary data
Funding
This work was performed under the auspices of the Lung Inflammatory Disease Program of Excellence in Glycoscience (LIDPEG) supported by the National Heart, Lung, and Blood Institute (Grant HL107151). A.G.G. and R.N.P. were supported in part by The Chemistry-Biology Interface Program at Johns Hopkins University (T32 GM080189).
Conflict of interest statement
The authors declare no financial conflicts of interest.
Abbreviations
AP, alkaline phosphatase; benzyl-α-GalNAc, benzyl-N-acetyl-α-d-galactosaminide; CFG, Consortium for Functional Glycomics; DMEM, Dulbecco's modified Eagle medium; DMEM-AB, DMEM supplemented with antibiotics; GuHCl, guanidinium hydrochloride; H&E, hematoxylin–eosin stain; HRP, horseradish peroxidase; LP, lung parenchyma; PBS, Dulbecco's phosphate-buffered saline; PVDF, polyvinylidene difluoride; TEC, tracheal epithelial cells; TGC, tracheal gland cells; Tr, trachea.
References
- Angata T. 2006. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol Divers. 10:555–566. [DOI] [PubMed] [Google Scholar]
- Angata T, Nycholat CM, Macauley MS. 2015. Therapeutic targeting of siglecs using antibody- and glycan-based approaches. Trends Pharmacol Sci. 36:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. 2008. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 8:183–192. [DOI] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J et al. 2004. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 101:17033–17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS. 2016. “Siglec”ting the allergic response for therapeutic targeting. Glycobiology. 26:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. 2005. Glycan array screening reveals a candidate ligand for siglec-8. J Biol Chem. 280:4307–4312. [DOI] [PubMed] [Google Scholar]
- Bull C, Heise T, Adema GJ, Boltje TJ. 2016. Sialic acid mimetics to target the sialic acid-siglec axis. Trends Biochem Sci. 41:519–531. [DOI] [PubMed] [Google Scholar]
- Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. 2004. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem. 279:21606–21616. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Chou CC, Hsieh HW, Tu Z, Lin CH, Nycholat C, Fukuda M, Khoo KH. 2015. Efficient mapping of sulfated glycotopes by negative ion mode nanoLC-MS/MS-based sulfoglycomic analysis of permethylated glycans. Anal Chem. 87:6380–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. 2010. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 11:154–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciepiela O, Ostafin M, Demkow U. 2015. Neutrophils in asthma – a review. Respir Physiol Neurobiol. 209:13–16. [DOI] [PubMed] [Google Scholar]
- Gao PS, Shimizu K, Grant AV, Rafaels N, Zhou LF, Hudson SA, Konno S, Zimmermann N, Araujo MI, Ponte EV et al. 2010. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 18:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser ML, Warren CM. 2012. Protein electrophoresis in agarose gels for separating high molecular weight proteins. Methods Mol Biol. 869:111–118. [DOI] [PubMed] [Google Scholar]
- Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. 2009. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6’-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 330:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa SM, Schulz BL, Packer NH, Karlsson NG. 2011. Analysis of mucosal mucins separated by SDS-urea agarose polyacrylamide composite gel electrophoresis. Electrophoresis. 32:3554–3563. [DOI] [PubMed] [Google Scholar]
- Ito H, Ishida H, Collins BE, Fromholt SE, Schnaar RL, Kiso M. 2003. Systematic synthesis and MAG-binding activity of novel sulfated GM1b analogues as mimics of Chol-1 (alpha-series) gangliosides: highly active ligands for neural siglecs. Carbohydr Res. 338:1621–1639. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. 2000. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest. 117:251S–260S. [DOI] [PubMed] [Google Scholar]
- Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS et al. 2015. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 135:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D'alessio KJ, Holmes SD, Abrahamson JA, Erickson-Miller CL, Murdock PR et al. 2000. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 105:1093–1100. [DOI] [PubMed] [Google Scholar]
- Kiwamoto T, Brummet ME, Wu F, Motari MG, Smith DF, Schnaar RL, Zhu Z, Bochner BS. 2014. a. Mice deficient in the St3gal3 gene product alpha2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 133:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, Zhu Z, Tiemeyer M, Bochner BS. 2014. b. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 135:1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchana N, Peck JR, Whitson B, Black SM. 2014. Preservation solutions for cardiac and pulmonary donor grafts: a review of the current literature. J Thorac Dis. 6:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PH, Schnaar RL. 2006. Determination of glycolipid-protein interaction specificity. Methods Enzymol. 417:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Crocker PR, Paulson JC. 2014. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 14:653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan SJ, Sharma RS, McKenzie EJ, Richards HE, Zhang J, Prescott A, Crocker PR. 2013. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling. Blood. 121:2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M, Rijkers GT, van Overveld FJ. 2013. Neutrophils and emerging targets for treatment in chronic obstructive pulmonary disease. Exp Rev Clin Immunol. 9:1055–1068. [DOI] [PubMed] [Google Scholar]
- Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. 2010. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci USA. 107:11561–11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik SR, Wala SM. 2013. Inflammation, allergy and asthma, complex immune origin diseases: mechanisms and therapeutic agents. Recent Pat Inflamm Allergy Drug Discov. 7:62–95. [PubMed] [Google Scholar]
- Nutku E, Hudson SA, Bochner BS. 2005. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 336:918–924. [DOI] [PubMed] [Google Scholar]
- Nutku-Bilir E, Hudson SA, Bochner BS. 2008. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 38:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padler-Karavani V, Hurtado-Ziola N, Chang YC, Sonnenburg JL, Ronaghy A, Yu H, Verhagen A, Nizet V, Chen X, Varki N et al. 2014. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 28:1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode ML, Cheng CW, Chou CC, Singer MS, Elin MS, Uchimura K, Crocker PR, Khoo KH, Rosen SD. 2013. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem. 288:26533–26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JC, Macauley MS, Kawasaki N. 2012. Siglecs as sensors of self in innate and adaptive immune responses. Ann NY Acad Sci. 1253:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS, Reddel HK, ten Hacken NH, van den BM. 2014. Asthma and chronic obstructive pulmonary disease: similarities and differences. Clin Chest Med. 35:143–156. [DOI] [PubMed] [Google Scholar]
- Propster JM, Yang F, Rabbani S, Ernst B, Allain FH, Schubert M. 2016. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci USA. 113:E4170–E4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelinghuys P, Antonopoulos A, Liu Y, Campanero-Rhodes MA, McKenzie E, Haslam SM, Dell A, Feizi T, Crocker PR. 2011. Early murine T-lymphocyte activation is accompanied by a switch from N-glycolyl- to N-acetyl-neuraminic acid and generation of ligands for siglec-E. J Biol Chem. 286:34522–34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, Dell A, Haslam SM, Paulson JC. 2012. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 8:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleimer RP, Schnaar RL, Bochner BS. 2016. Regulation of airway inflammation by Siglec-8 and Siglec-9 sialoglycan ligand expression. Curr Opin Allergy Clin Immunol. 16:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL. 2016. Glycobiology simplified: diverse roles of glycan recognition in inflammation. J Leukoc Biol. 99:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Pearce OM, Wang X, Samraj AN, Laubli H, Garcia JO, Lin H, Fu X, Garcia-Bingman A, Secrest P et al. 2015. Siglec receptors impact mammalian lifespan by modulating oxidative stress. Elife. 4:e06184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secundino I, Lizcano A, Roupe KM, Wang X, Cole JN, Olson J, Ali SR, Dahesh S, Amayreh LK, Henningham A et al. 2016. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med (Berl). 94:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S, Schwarz F, Springer S, Khedri Z, Yu H, Deng L, Verhagen A, Naito-Matsui Y, Jiang W, Kim D et al. 2016. Studies on the detection, expression, glycosylation, dimerization and ligand binding properties of mouse Siglec-E. J Biol Chem. 292:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slats A, Taube C. 2016. Asthma and chronic obstructive pulmonary disease overlap: asthmatic chronic obstructive pulmonary disease or chronic obstructive asthma. Ther Adv Respir Dis. 10:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerhoff CP, Finkbeiner WE. 1990. Human tracheobronchial submucosal gland cells in culture. Am J Respir Cell Mol Biol. 2:41–50. [DOI] [PubMed] [Google Scholar]
- Tateno H, Crocker PR, Paulson JC. 2005. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6’-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 15:1125–1135. [DOI] [PubMed] [Google Scholar]
- Varki A. 1997. Selectin ligands: will the real ones please stand up. J Clin Invest. 99:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. 2006. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 126:841–845. [DOI] [PubMed] [Google Scholar]
- Varki A, Angata T. 2006. Siglecs--the major subfamily of I-type lectins. Glycobiology. 16:1R–27R. [DOI] [PubMed] [Google Scholar]
- Vlahos R, Bozinovski S. 2014. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 5:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seger R, Takala J, Villiger PM, Simon HU. 2005. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 106:1423–1431. [DOI] [PubMed] [Google Scholar]
- Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. 2007. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 109:4280–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.