Abstract
It has been well recognized that the modification of biomaterials with appropriate bioactive peptides could further enhance their functions. Especially, it has been shown that peptide-modified bone repair materials could promote new bone formation more efficiently compared with conventional ones. The purpose of this article is to give a general review of recent studies on bioactive peptide-modified materials for bone tissue repair. Firstly, the main peptides for inducing bone regeneration and commonly used methods to prepare peptide-modified bone repair materials are introduced. Then, current in vitro and in vivo research progress of peptide-modified composites used as potential bone repair materials are reviewed and discussed. Generally speaking, the recent related studies have fully suggested that the modification of bone repair materials with osteogenic-related peptides provide promising strategies for the development of bioactive materials and substrates for enhanced bone regeneration and the therapy of bone tissue diseases. Furthermore, we have proposed some research trends in the conclusion and perspectives part.
Keywords: bone repair material, peptide, osteogenic activity
Introduction
Recently, the biomaterials community has increasingly embraced the concept that implanted substrates should not only provide structural support for damaged tissues, but also integrate with the surrounding tissues and promote the desired tissue regeneration [1–3]. In order to achieve this goal, researchers have been not only trying their best to choose appropriate raw materials and utilize advanced preparation methods, but also making great efforts to further functionalise the materials with effective optimizing techniques. Up to now, a large panel of natural and synthetic materials has been investigated for bone tissue engineering applications. However, no single material fulfils all the criteria of biocompatibility and bioactivity [4]. Although it was previously thought that materials should present a relatively biotolerant surface in order to minimize immune and fibrotic responses, more and more evidence now suggests that interactive, biomimetic surfaces often exhibit enhanced performances [5]. For instance, the efficacy of osseointegration is mostly dependent on the interactions between the implants and osteogenic macromolecules in blood [6]. Some biomaterials do not readily adsorb blood proteins to their surface, and therefore do not support well bone-related cell activities, potentially leading to limited bone formation. Consequently, these materials need to be further optimized to enhance and accelerate bone ingrowth [7]. The successful development of high-performance biomaterials must take into consideration how their surfaces will interact with in vivo substances, which has prompted a burgeoning effort to engineer materials with biomolecules or modify their surfaces with biologic elements [8, 9]. One common approach is to functionalise biomaterial surfaces with osteoinductive molecules, including growth factors or peptides [4]. For the design of biomaterials, it has been well recognized that desired cell responses are crucial [10]. The immobilization of certain molecules on scaffolds has been shown very effective to improve not only recruiting stem cells but also triggering their osteogenic differentiation because these molecules can provide osteogenesis-stimulating signals for the cells [4, 11]. The practical advantage of using peptides, rather than native proteins, for biomaterial surface functionalization, is due to the fact they can be produced synthetically with precise control of their chemical composition, thereby avoiding pathogenic contamination from animal sources. In general, peptides are more resistant than high-molecular weight proteins, with respect to the denaturation caused by pH or temperature variations. Moreover, they are easier to manipulate during the grafting procedure. Especially, the research for bioactive peptides in orthopaedics is growing rapidly in recent years.
In this article, we have reviewed recent studies on bioactive peptide-modified materials for bone tissue repair. Firstly, the main peptides for inducing bone regeneration and commonly-used methods to prepare peptide-modified bone repair materials are introduced. Then, current in vitro and in vivo research progress of peptide-modified composites used as potential bone repair materials are reviewed and discussed. Furthermore, the related research trends are proposed in the conclusion and perspectives part.
The main peptides used for bone tissue repair
In the past two decades, a variety of bioactive peptides have been studied and applied for the promotion of bone regeneration to repair local bone defects or treat other bone diseases. In this section, we divided these peptides into three categories, including extracellular matrix (ECM)-derived peptides, bone morphogenetic proteins (BMPs)-derived peptides and others, and respectively introduced and discussed the current progress of investigations into them as follows.
ECM-derived peptides
ECM is an aggregation of extracellular molecules from different cells to provide biochemical and structural supports [12]. The ECM-derived peptides with signalling domains, which are capable of connecting with receptors on the surface of cell membrane, is a hotspot of research. Variety of ECM-related peptides shown in Table 1 have been studied for bone tissue repair and regeneration [13].
Table 1.
In Vitro and in vivo studies presenting the osteogenic effects of ECM-derived peptides
| Active peptides | Abbreviation | Composition | Binding sites | Upregulation / Downregulation of proteins or genes | Final functions | References |
|---|---|---|---|---|---|---|
| PepGen P-15 | P-15 | 15 amino acids | type I collagens-binding sites | upregulating the expression of ALP, BMP-2, BMP-7; RUNX2, COL1, OSTRX and BSP; a2 integrin | upregulating proliferation and osteogenic differentiation; cell attachment, migration and survival; extracellular matrix production | 14–16, 20 |
| arginyl-glycyl- aspartic acid | RGD | 3 amino acids | integrin-binding sites | upregulating the expression of ALP, RUNX2, osteocalcin, osteopontin and BSP; Sox9, Aggrecan, fibronectin and clloagen II | upregulation proliferation; osteogenic differentiation and mineralization; cell attachment and survival | 21–25 |
| Ser-Val-Val-Tyr- Gly-Leu-Arg | SVVYGLR | 7 amino acids | RGD-binding sites | upregulating αvβ3 integrin; suppressing NFAT activity and expression of osteoclastogenesis-related mRNAs | upregulating proliferation and neovascularization; angiogenesis and osteogenesis; adhesion, migration and tube formation of endothelial cells | 26–28 |
| glycine-phenylalanine- hydroxyproline-arginine | GFOGER | 4 amino acids | α2β1 integrin-binding sites | upregulating α2β1 integrin binding | upregulating differentiation, bone regeneration and osseointegration | 29–32 |
| Asp-Gly-Glu-Ala | DGEA | 4 amino acids | α2β1 integrin-binding sites | upregulating ALP | cell adhesion, spreading and osteogenic differentiation | 33–37 |
| collagen-binding motif | CBM | 28 amino acids | collagen-binding sites | inducing sustained activation of ERK; the transactivation of SRE, CRE, and AP-1; expression of type X collagen | upregulating bone-related cell adhesion and growth; osteogenic differentiation | 38–39 |
| lysine-arginine- serine-arginine | KRSR | 4 amino acids | heparin-binding sites | upregulating osteogenic gene expression | upregulating bone-related cell spreading, adhesion and mineralization | 40–46 |
| Phe-His-Arg-Arg- Ile-Lys-Ala | FHRRIKA | 10 amino acids | putative heparin-binding sites | – | upregulating bone-related cell spreading, adhesion and mineralization | 47, 48 |
| Fibronectin-derived peptides | FN-derived peptides | 7 amino acids | – | – | upregulating bone-related cell spreading, adhesion and mineralization | 49–51 |
The PepGen P-15 (P-15) is a kind of peptide containing 15 amino acid sequences (766-GTPGPQGIAGQRGVV-780) and identical to the cell-binding region of type I collagens [14]. Its capacity of supporting bone growth is approved by several studies, such as stimulating osteoblast proliferation and differentiation by enhancing cell attachment to bone repair materials and upregulating ECM production [15, 16]. Different studies have shown that the P-15 could increase not only osteogenic gene expression but also the expression of osteogenic alkaline phosphatase (ALP) proteins and matrix mineralization due to upregulating runt-related transcription factor-2 (RUNX2), collagen 1(COL1), osterix (OSTRX) and bone sialoprotein (BSP) [17–19]. Furthermore, in the study of Nguyen et al. [20], they found that P-15 could significantly enhance the gene expression of BMP-2 and BMP-7 in human osteosarcoma cell (HOS).
The arginyl-glycyl-aspartic acid (RGD) peptide, which is comprised of 3 amino acid residues, is the cardinal integrin-binding domain and presents in many extracellular matrix proteins, such as fibronectin and vitronectin [21, 22]. As a part of cell surface signalling, RGD peptide can enhance the expression of ALP, Runx2, osteocalcin (OCN), osteopontin (OPN) and BSP to ensure osteoblast proliferation, differentiation and mineralization. For example, Huang et al. found that RGD increased cell attachment, and enhanced cellular proliferation [23]. Furthermore, Rammelt and his group further used RGD peptide to enhance the adhesion and growth of cells [24, 25].
The Ser-Val-Val-Tyr-Gly-Leu-Arg (SVVYGLR) peptide that are adjacent to RGD sequence in osteopontin can not only significantly improve the proliferation of MSCs, but also upregulate neovascularization [26–28]. The relative results can be found in the studies of Egusa et al. [27] and Hamada et al. [28] who investigated bioactive function of a SVVYGLR peptide in osteoclasts and osteoprogenitor cells.
The GFOGER (glycine-phenylalanine-hydroxyproline-arginine) as a collagen-mimetic peptide can improve osteogenic differentiation, bone regeneration and osseointegration [29–31]. In the study of Shekaran et al., they showed that GFOGER could accelerate and increase bone formation in the femoral defect model [29–31]. Moreover, Mhanna et al. showed that GFOGER played a crucial role in the proliferation and osteogenic differentiation of mesenchyal stem cells (MSCs) [32].
The Asp-Gly-Glu-Ala (DGEA) that is a kind of collagen peptide can bind to α2β1 integrin, which is an extracellular matrix receptor for collagens and/or laminins, and promote cell adhesion, spreading and osteogenic differentiation [33]. The study of Hennessy et al. showed that DGEA stimulated osteogenic differentiation of MSCs [34]. In the study of Ceylan et al., they synthesized peptide nanofibers with DGEA to promote the osteogenic differentiation of progenitor stem cells and bone-like mineralization [35]. Moreover, it was shown that DGEA peptide enhanced adhesion and osteogenic differentiation of bone marrow stem cells [35–37].
As a cleavage product of osteopontin, the collagen-binding motif (CBM) can promote osteogenic differentiation and migration through some specific signalling pathways [38, 39]. For example, the evidence provided by the study of Shin et al. indicated that CBM could promote osteogenic differentiation through Ca2+/CaMKII/ERK/AP-1 signalling pathway in hMSCs. Furthermore, they also found that CBM stimulated the migration of hMSCs by suppressing cell proliferation [38].
In bone sialoprotein, vitronectin, fibronectin, osteopontin and thrombospondin, researchers found that lysine-arginine-serine-arginine (KRSR) as a heparin-binding site could increase osteogenic gene expression and osteoblast adhesion [40–44]. Moreover, in the study of Dettin et al. [45] and Hasenbein et al. [46] it was shown that KRSR motif was selective for osteoblast attachment, not for endothelial cells or fibroblasts. In addition, they also found that KRSR could enhance osteoblast spreading.
The Phe-His-Arg-Arg-Ile-Lys-Ala (FHRRIKA) is a putative heparin-binding domain of bone sialoprotein. It can enhance the ability of osteoblast adhesion, spreading and mineralization [47]. In the work of Gentile et al, they showed that FHRRIKA could induce cell adhesion and bone mineralization [48].
Fibronectin (FN)-derived peptides could improve osteoblast adhesion, spreading and mineralization [49]. Lee et al. used model of rabbit calvarial defect to find new bone formation enhanced by a fibrin-binding synthetic oligopeptide derived from FN [50]. In addition, Martino et al. reported the regenerative effects of FN III9-10/12-14 on enhancing platelet-derived growth factor-BB (PDGF-BB) and BMP-2 in a critical-size bone defect model [51].
BMPs-derived peptides
BMPs are a group of growth factors to induce the formation of bone or cartilage [52]. Bone-repairing responses of BMPs-derived peptides have been widely studied by scholars [53]. These peptides have been mainly derived from BMP-2, BMP-7 and BMP-9 to promote bone-repairing responses. BMP-2-derived peptide as residues of BMP-2, including P17, P20 and P24, can regulate bone-repairing responses [4, 54–59]. The effect of BMP-2-derived peptide on bone tissue repair and regeneration as a key point is studied. For example, Zhang et al. found the synthetic peptides derived from BMP-2 residues 32-48 (P17-BMP-2) could significantly upregulate bone-repairing response [60]. In the study of Zhou et al. residues 73-92 of BMP-2 was used to induce osteogenic differentiation and bone regeneration [61]. The study of Kim et al. [62] also revealed the positive effects of BMP-2 peptides on osteogenic differentiation of hMSCs. Moreover, Lin et al. [63] synthesized BMP-2-derived peptide, P24, and found that it could promote osteogenic differentiation of BMSCs.
In order to discover the functions of other BMPs-derived peptides to stimulate bone formation, Kim et al. [64] found a new BMP-7-derived peptide, BFP-1. By adding BFP-1 into culture medium of BMSCs, they found that BFP-1 could enhance Ca2+ content in the cells and induced their ALP activity. In the study of Bo et al. [65], they used BMP-7 mimetic peptide to accelerate bone regeneration.
Moreover, Lord et al. [66] used human white preadipocytes (HWP) to determine the effects of BMP-9-derived peptide, pBMP-9, and found that the pBMP-9 did not affect the number of apoptotic cells along with reducing the proliferation of HWP. They also found that the pBMP-9 inhibited the proliferation and induced osteogenic differentiation of preosteoblasts when its content was about 400 ng/ml.
Other peptides
In addition to the peptides derived from ECM and BMPs, as shown above, other ones shown in Table 2 have also been studied and developed to induce bone regeneration.
Table 2.
In Vitro and in vivo studies presenting the osteogenic effects of other peptides except those derived from ECM and BMPs
| Active peptides | Abbreviation | Composition | Potential pathways | Upregulation / Downregulation of proteins or genes | Final functions | References |
|---|---|---|---|---|---|---|
| calcitonin gene-related peptides | CGRP | 37 amino acids | the cAMP, Wnt and AMPK-eNOS pathways | upregulating expression of IGF-1, IGF-1 receptor and BMP-2 receptor; ALP, OC and COLLA1 | upregulating cell proliferation, osteogenic differentiation and angiogenesis; downregulating apoptosis and inflammation | 67-85 |
| Parathyroid hormone (1-34) | PTH1-34 | 34 amino acids | G(q)-signalling, β-arrestin recruitment, ERK1/2 phosphorylation and phospholipase C pathway | upregulating expression of Runx2 and COL2A1; downregulating expression of ALP and BMP-2 | upregulating cell proliferation and chondrogenesis | 86-88 |
| osteogenic growth peptides | OGP | 14 amino acids | the G1 protein-MAPK and RhoA/ROCK pathway | upregulating osteocalcin, collagen, BMP-2, ALP and mineralization; upregulating TGF β1, β2, β3, FGF-2, IGF-I | upregulating cell proligeration and osteogenic differentiation; cartilage-to-bone transition; downregulating adipogenic differentiation | 89-96 |
| thrombin peptide 508 | TP508 | 23 amino acids | cell cycle-G1/S checkpoint, JAK/STAT, NF-kappaB, PDGF, PI3K/AKT, PTEN, and ERK/MAPK | upregulating the expression of Runx2 and OPN | upregulating cell proliferation and osteogenic differentiation; chemotaxis, angiogenesis and revascularization; downregulating apoptosis, the effect of hypoxia | 22, 97-99 |
| NEMO-binding domain peptide | NBD | 6 amino acids | NF-κB pathway | downregulating TRAP activity, actin rings; RANKL-induced c-Src kinase activity | upregulating osteogenic differentiation of cells; downregulating bone resorption | 100-103 |
| Cell penetrating peptide | CPP | 30 amino acids | – | – | transcriptional factor to deliver bone-regenerating related proteins or factors into cells | 104-106 |
| AcN-RADARADARADARADA-CONH2 | RADA16-I | 16 amino acids | – | upregulating expression of Runx2 genes, ALP and osteocalcin | transcriptional factor to deliver bone-regenerating related proteins or factors into cells | 107-109 |
The calcitonin gene-related peptide (CGRP) has been widely studied due to their positive effects on bone-repairing response. CGRP is discovered in bone marrow, metaphysis and periosteum, etc. Two different forms, CGRP-α and CGRP-β, are derived from separate genes [67]. Compared with CGRP-β, CGRP-α can enhance osteoblast proliferation and bone regeneration [68–71]. The previous studies showed that CGRP could not only enhance osteoblast proliferation and differentiation because it could bond with functional receptors and transporters on the bone-related cells, but also stimulate the production of growth factors, including BMP-2 and IGF-1. In addition, the CGRP could reduce the apoptosis and inflammation [72–83]. Its positive effects on the bone-related cells and new bone formation can be found in many studies. For instance, Lv et al. found that CGRP was an important factor and could enhance bone density to improve the quality of regenerated alveolar bone [84]. Zhou et al. elucidated the important role of CGRP by spinal fusion model, and found that expression of CGRP was located around the interface between allograft and fibrous tissue to induce the osteogenic differentiation of cells and new bone formation [85].
With 34 amino acids, the peptide derived from parathyroid hormone (PTH1-34) is one of the earliest artificially synthesized amino acid fragments [86]. It was shown that the PTH1-34 peptide could upregulate proliferation and osteogenic differentiation of cells by enhancing the expressions of Runx2 and OPN. Alkhiary et al. used animal models to demonstrate that PTH1-34 could improve bone regeneration by enhancing bone density, mineral content and strength [87]. In addition, it could also increase angiogenesis and revascularization [88]. Therefore, PTH1-34 is one of effective peptides for the improvement of fracture-healing.
As H4 histone-related peptide, osteogenic growth peptide (OGP) is highly conserved with 14 amino acids, which was found in serum [89, 90]. It could activate an intracellular Gi-protein-MAP kinase signalling pathway [91, 92]. OGP have been found to increase the proliferation, osteogenic differentiation and matrix mineralization of bone-related cells [93–96]. Brager et al. showed that OGP regulated TGF-β1, β2, β3, IGF-I, FGF-2 and aggrecan in vivo [93]. Bab et al. found OGP increased overall bone mass and bone formation quality by exerting an anabolic effect on bone cells [89].
With 23 amino acids, thrombin peptide 508 (TP508) is a synthetic peptide that represents the non-proteolytic receptor binding domain of thrombin. TP508 enhances not only proliferation and osteogenic differentiation of human osteoblasts, but also increased angiogenesis [97, 98]. For instance, Hanratty et al. [99] improved healing effect of murine fracture by injecting the TP508 peptide into the fracture gap. The similar result can be found in the study of Cakarer et al., which showed that the TP508 peptide could enhance bone consolidation in tibial distraction osteogenesis [22].
Kappa-B kinase (IKK) is an inhibitor of nuclear factor with two catalytic subunits, IKK-1 and IKK-2. Strnad et al. reported it was a non-catalytic regulatory subunit NF-kB essential modulator (NEMO or IKK-γ), and the NEMO-binding domain (NBD) is an interacting region with 6-amino acids where IKK subunits interacts with NEMO [100, 101]. Furthermore, the studies of Li et al. [102] and Jimi et al. [103] showed that this peptide could induce osteogenic differentiation of cells and inhibit bone resorption.
Cell penetrating peptides (CPPs) can transport various molecules into the cytoplasm through cell membranes [104, 105]. With the above properties, CPPs can behave as a transcriptional factor to deliver bone-repairing relevant proteins or factors into cells. For example, in a calvarial defect model, Park et al. used the CPPs to transfer recombinant adenovirus expressing BMP-2 into MSCs to promote new bone formation [106].
RADA16-I (AcN-RADARADARADARADA-CONH2) is generated by spontaneous assembly of several fragments into ordered nanostructures [107]. The studies of Li et al. [108] and Hou et al. [109] showed that MSCs could express higher level of Runx2 genes, ALP and osteocalcin proteins in demineralized bone matrix (DBM) modified with RADA16-I compared to the bare DBM.
The commonly-used methods to prepare peptide-modified bone repair materials
The preparation method of peptide-modified bone repair materials determines the manner, in which the peptide is bonded to the substrate material, and has a significant effect on the final osteogenic activity of the composite. Currently, the commonly-used preparation methods include electrodeposition, covalent immobilization, physical adsorption and others.
Electrodeposition
Traditionally, electrodeposition is an electrochemically process, during which a material is deposited from its compound aqueous solution, non-aqueous solution or molten salt. It is necessary that both positive and negative poles should be inert because the cations in the solution need to be introduced to the cathode. It has been shown that this technique has been used to compound peptides with artificial biomaterials. In order to prepare peptide-modified materials by electrodeposition, we need to take the following steps. At first, the terminally modified polymers, containing both –NH2 and –COOH at terminals, should be fabricated. Then, the polymers are dissolved in the electrolyte solution (eg. NaCl, PBS, etc.), in which – and –COO– are formed on the polymers by hydrolysis of –NH2 and –COOH. And then, – of the polymers are combined with –OH- of cathode by an ionic bond, N–HO under the action of an electric field. Finally, the materials are immersed in an aqueous solution containing peptides where – of the peptides are connected with –COO– of the polymers through an ionic bond, resulting in the immobilization of the peptides on the materials.
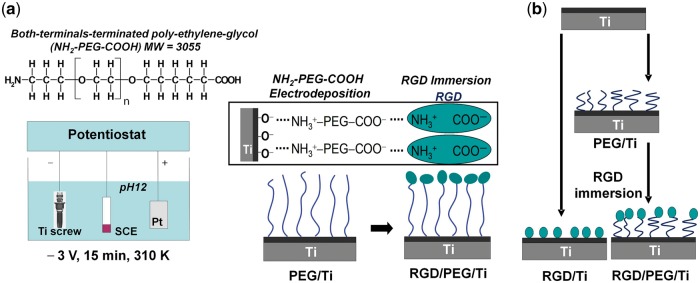
Oya et al. [18] fabricated RGD peptide-modified titanium (Ti) materials by electrodeposition. At first, poly(ethylene glycol) (PEG) was terminated with two kinds of functional groups, –NH2 and –COOH, and the NH2–PEG–COOH was dissolved in a 0.5 mol/l NaCl solution with a concentration of 0.2 mass%. Meanwhile, glycine (Gly) with both –NH2 and –COOH was selected as a control, which was dissolved in the same solution with a concentration of 0.0076 mass%. They found that the polymer could be most effectively electrodeposited when pH of the solution was adjusted to 12. Under the action of the electric field 3.0 V for 900 s at 37°C, the NH2–PEG–COOH and glycine respectively migrated to the cathode Ti and were immobilized on it by an ionic bond. Then, each specimen was immersed in a 0.001 mass% RGD aqueous solution with pH 12 at 37°C for 24 h. After immobilization, the specimens were rinsed and dried. The chemical structure formulae of NH2–PEG–COOH and schematic illustration of RGD immobilization on the Ti implant was shown in Fig. 1. The thickness of the immobilized layer on Ti of each specimen was determined by ellipsometry, which showed that more RGD molecules were immobilized on PEG/Ti than on Gly/Ti, indicating that PEG is a kind of satisfactory polymer for preparing peptide-modified materials by electrodeposition.
Figure 1.
(a) Process illustration of covalent immobilization of the RGD peptide on Ti bone repair material by electrodeposition, (b) simple description of the difference between RGD/PEG/Ti and RGD/Ti materials (adapted with permission from ref. [17]. Copyright 2011 Elsevier Ltd)
Moreover, it has been shown that the surface morphology of the materials was obviously changed during the whole electrodeposition, which was observed by Park et al. with scanning probe microscopy [17]. The nano-scale clumps could be observed when only PEG or RGD was introduced on Ti. However, when RGD was immobilized on PEG/Ti, the surface became smooth.
Above all, it is obvious that the deposition of the polymers on the cathode is very important for the successful preparation of the peptide-modified materials by electrodeposition. In this process, it has been shown that the terminals of polymer and the pH of the electrolyte have significant effects on the deposition efficiency of the polymers, which is bound to determine the final bonding results of peptides. Tanaka et al. electrodeposited two kinds of PEG polymers terminated with different chemical groups on titanium oxide, and characterized the deposition efficiency by x-ray photoelectron spectroscopy (XPS) with an angle-resolved technique and glow discharge optical emission spectroscopy (GD-OES), which showed that the polymers with two kinds of terminal groups, –NH2 and –COOH, could be deposited on the substrate more stable than those with only one terminal group [110, 111]. They also studied the effects of the pH of the electrolyte on the deposition efficiency of the PEG polymer with two kinds of terminal groups, –NH2 and –COOH. They selected five different pH values, 2, 4, 7, 10 and 12. Their final results showed that when the pH of the electrolyte was 12, electrostatic reactivity between the polymer and substrate was the highest and the thickness of the polymer layer was the largest. The terminals of PEG were oriented perpendicular to the substrate and formed stable U–shape immobilization [112]. Conformational changes in the adsorbed RGD peptides are dependent on the surface energy and nanotopography of the Ti surface. The RGD was more firmly immobilized on the materials through PEG terminated with two kinds of functional groups.
Covalent immobilization
The preparation of peptide-modified materials by covalent immobilization generally requires the chemical reactions between functional groups on substrate materials and aminos or carboxies of peptides. This process normally includes two steps. Firstly, some specific functional groups need to be introduced to the substrate materials. Then, the substrate materials are immersed into the peptide solution with or without ultrasonic treatment to form the covalent bonds between the substrate materials and peptides. During the process of this preparation method, a variety of different techniques have been used to achieve the above effects, which include epoxy linkages, silane couplings, polydopamine-assisted immobilization, and thiol-ene click reactions, etc. [62, 113–117].
For the preparation by epoxy linkages, there are normally two different procedures to follow. The first one is that the chemical reactions between some specific epoxy compounds and substrate materials with –COOH are launched to produce O-acylurea on the substrate materials, -C = O- of which is then made to react with –NH2 of the peptides to form amido bond, thereby achieving covalent immobilization of the peptides on the substrate materials. Seo et al. [113] fabricated RGD peptide covalently modified titanium surface by this procedure. The titanium surface, which has been modified by polyacrylic acid (PAA) to graft the carboxyl group (Ti/COOH), was immersed in 100 mg of EDC (1-Ethyl-3-[3dimethylaminopropyl] carbodiimide hydrochloride) with NHS (N-hydroxysuccinimide) mixture and 10 ml of phosphate-buffered saline (PBS) for 24 h with gentle shaking at 0–4°C to generate O-acylurea. Then, the modified materials were immersed in 0.1 mg/ml RGD solution for 36 h with mild stirring at 0–4°C to achieve a covalent bond between RGD peptide and Ti substrate. Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) showed N-H peak and the weak C = O peak of the Ti/COOH/RGD at 3400 cm−1 and 1700 cm−1 respectively, which was related to the amide carboxyl group. The strong C = O peak near 1700 cm−1 of the Ti/COOH substrate was related to carboxyl groups. The result showed that the –NH2 groups of the RGD peptide had effectively reacted with the –COOH group on the Ti/COOH surface.
The other procedure to prepare covalently modified material by epoxy linkages is that epoxy polymer are deposited on the substrate, and then their epoxy groups are made to react with –NH2 of the peptides to achieve covalent bonds. Kim et al. [62] fabricated BMP-2 peptide covalently modified nanopatterned polyurethane acrylate (PUA) substrates by this procedure. Firstly, they coated an epoxy compound, poly(glycidyl methacrylate) (pGMA) on PUA through initiated chemical vapor deposition (iCVD) technique, thereby achieving the polymerization of the epoxy compound and substrate. Then, epoxy groups of the pGMA-PUA substrates were covalently bonded with –NH2 of BMP-2 peptides by immersing the substrates in BMP-2 peptide solution (100 mM) for 2 h at room temperature, which is shown in Fig. 2. XPS analysis showed a strong nitrogen (N1s) atomic elemental peak at 401 eV, which confirmed that the BMP-2 peptide was successfully covalently conjugated to the substrate.
Figure 2.
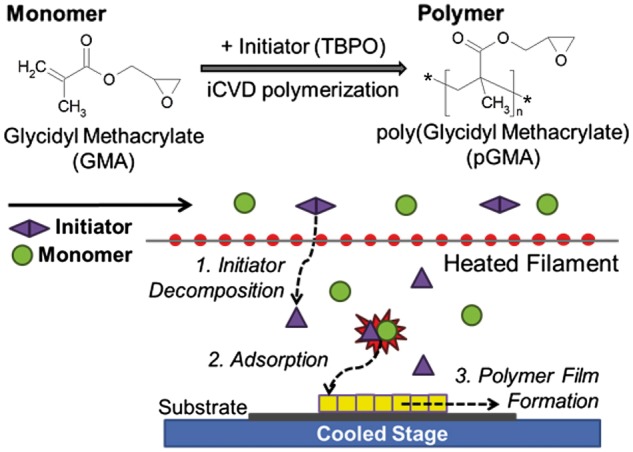
Preparation of poly(glycidyl methacrylate)-polyurethane acrylate (pGMA-PUA) nanopatterned substrate materials. pGMA was deposited onto the PUA substrates via the initiated chemical vapor deposition (iCVD) polymerization process, which was synthesized with GMA monomer and initiator (TBPO) (adapted with permission from ref. [62]. Copyright 2013 Elsevier Ltd)
For the preparation by silane couplings, it is normally performed by means of a three-step reaction. At first, materials are silanized with silane coupling agent. Then, –NH2 of silylated modified materials are made to react with –COOH of a crosslinker. Finally, the outer maleimide groups of the crosslinker are made to react with the thiolgroups of the peptide. Acharya et al. [115] prepared matrix extracellular phosphoglycoprotein (MEPE) peptide covalently modified hydroxyapatite/β-tricalcium phosphate (HA/β-TCP) composites by this method. At first, the HA/β-TCP composite particles were soaked in a silane coupling agent, 3-aminopropyl-triethoxysilane (APTES) solution to be silanized. Then, –NH2 of the silanized particles were made to react with –COOH of a crosslinker, polyethylene glycol disuccinimidyl succinate (SS-PEG-SS), by soaking them in the 10 mM SS-PEG-SS solution. Finally, the modified HA/β-TCP was soaked in MEPE peptide solution to achieve the covalent reaction between the outer maleimide groups of the SS-PEG-SS and thiol groups of MEPE peptides. FTIR analysis showed that the absorbance peak of NH2, C = C, COO-, and C = O bonds increased in the MEPE peptide-immobilized HA/β-TCP, which confirmed that the MEPE peptides were successfully immobilized to the HA/β-TCP via covalent bonding.
For the fabrication by polydopamine-assisted immobilization, there are usually two steps to follow. The first step is the dopamine crosslinking on the surface of substrate materials. The cross-linking principle is that the phenolic structure of dopamine is oxidized into the quinone structure, and then crosslinked by Michael addition reactions between the quinone structure with the primary amine group of dopamine. The second step is the quinone structure of the crosslinked dopamine are reacted with the –NH2 of the peptide through Michael addition reactions. Pan et al. [116] prepared BMP-2-derived peptide (P24) covalently modified poly(lactic-co-glycolic acid (PLGA)-[Asp-PEG]n scaffolds by this method. At first, the PLGA-[Asp-PEG]n scaffolds were soaked in a DA solution (2 mg/mL 10 mM Tris–HCl, pH 8.5) overnight, in order to be crosslinked. Then, the scaffolds were immersed in the P24 peptide solution (1 mg/mL in10 mM Tris–HCl, pH 8.5) for 4 h, with shaking at room temperature, thereby achieving the covalent bond between the dopamine and peptide. HPLC analysis showed that this method, compared with physical adsorption, could load more peptides, and that the peptides could be released more slowly and continuously.
For the preparation by thiol-ene click reactions, at first some special polymers containing C = C need to be attached to the substrate materials by radical polymerization, and then the propionamide group on the polymers is reacted with the thiol group at the terminal of the peptide. Yang et al. [117] fabricated Arg-Glu-Asp-Val (REDV) peptide covalently modified polycarbonate urethane (PCU) materials by this method. Firstly, the PCU materials were immersed in a mixed solution of N-(2-hydroxypropyl)methacrylamide (HPMA) and eugenyl methacrylate (EgMA) at 30°C to achieve copolymers by radical polymerization. Then, the REDV peptide was directly covalently immobilized onto the substrate surface by a thiol–ene click reaction, which was carried out at 30°C in a nitrogen atmosphere for 30 min under the exposure of a 365 nm UVlamp (300 W) from a distance of 30 cm. The results showed the copolymer with higher EgMA content could immobilize a larger amount of REDV peptide. The result indicates that copolymer with high EgMA content contributes to the immobilization of the peptides because EgMA contains catechol.
It is obvious that peptides can be bonded to substrates firmly by this method. The modified materials have good stability. Peptides can be released slowly, the rate of which can be controlled. However, this method requires the participation of reagents with special functional groups, and the reaction mechanism is complicated.
Physical adsorption
Physical Adsorption means that molecules or ions are attracted and attached to the surface of substrates in liquid or gas mediums by electrostatic force or Van der Waals force [118]. Using this method to prepare peptide-modified materials, we need to prepare substrates with high surface energy, and then immerse them in a supersaturated solution of the peptides. For example, Feng et al. [65] prepared chitosan/nano-hydroxyapatite/collagen (nHAC) composites modified by BMP-7 derived peptides by physical adsorption. Firstly, they prewetted the chitosan/nHAC composites in pure ethanol. Then, the ethanol was replaced with excess water. Subsequently, the samples were shaken continuously for 24 hours in the water. Finally the prewetted composites were impregnated with 1 mg of the BMP-7 derived peptide in 100 μL water to adsorb the peptide, followed by vacuum dried. Their subsequent in vivo study showed that significantly improved bone regeneration and better bone repair effectiveness were achieved with the composites loaded with BMP-7 derived peptide compared to the original composites.
In addition, Reyes et al. [29] coated tissue culture-treated polystyrene dishes with 300 Å of pure titanium using an electron beam evaporator, and then put 20 mg/ml GFOGER (Gly-Phe-Hyp-Gly-Glu-Arg) peptide solution (the peptide in PBS) into the dishes, which were incubated for 1 hour to make the titanium surface adsorb the peptide. Their subsequent in vitro study showed that the peptide treated material surface significantly enhanced osteogenic differentiation and mineralization of bone marrow stromal cells, compared to the unmodified titanium surface.
Others
In addition to the above-mentioned methods, there are other ways to prepare peptide-modified materials for bone tissue repair, such as solvent extraction technique, molecular plasma deposition, etc.
Extraction means the separation of a substance from a mixture or solvent. In order to prepare peptide-modified materials by this technique, we need to take the following steps. The peptide is dissolved in water and the material, which is to be modified, is dissolved in an organic solvent. Then, the two solutions are mixed and emulsified to form a water/oil emulsion, which is then transferred to another aqueous phase containing an emulsifier to prepare water/oil/water emulsion. And then, the emulsion is constantly stirred to vaporize the organic solvent, which causes that the material is solidified into microspheres with the peptide inside. Finally the peptide-modified microspheres are washed and dried. For example, Hedberg et al. [119] fabricated thrombin peptide 508 (TP508)-modified PLGA/PEG microsphere using the solvent extraction technique. Firstly, PLGA and PEG were dissolved in 1 ml of CH2Cl2 solution. Then, 125 μl of TP508 solution was added into the PLGA/PEG solutions, and the two solutions were mixed to form a water/oil emulsion, which was then added to 1.5 ml 0.3% poly(vinyl alcohol) (PVA, an emulsifier) aqueous solution to produce a water/oil/water emulsion. And then, the water/oil/water emulsion was added to 100 ml of 0.2% aqueous isopropanol and 98.5 ml of 0.3% aqueous PVA, and stirred rapidly for one hour, which led to the formation of PLGA/PEG microspheres with the TP508 peptide inside. At last, the microspheres were separated from the solution by centrifuged at 180 × g for one min, followed by washed and dried. Their subsequent study on release kinetics indicated that the TP508 peptide-modified microspheres could release the peptide slowly and steadily, the rate of which could be well controlled by changing the preparation parameters of the microspheres.
The molecular plasma deposition (MPD) enables the deposition of uniform coatings onto substrates using corona discharge under high voltage. Peptide-modified bone repair materials can be prepared with this method by the steps as follows. First, the substrate is placed inside of a vacuum chamber, and the peptide solution is put into a reservoir, which is then added to a metallic needle. Then, the peptide solution is dispensed under a high voltage between the substrate and needle that induces a corona discharge, which then ionizes the peptide solution. And then, the ionized solution is introduced onto the substrate (positive pole) inside the vacuum chamber under the high voltage. Finally, the peptide-modified material is removed from the chamber, followed by washed and dried. For example, Balasundaram et al. [120] deposited arginine-glycine-aspartic acid-serine (RGDS), lysine-arginine-serine-arginine (KRSR), and isoleucinelysine-valine-alanine-valine (IKVAV) peptides on the surface of anodized titanium substrate respectively by this method. A high voltage of 20 kV was applied between the substrate and the needle. Electrospray ionization data demonstrated that the ionization process did not alter the original characters of the peptides. Their subsequent in vitro study showed that the peptide-modified anodized titanium substrates improved osteoblast adhesion and proliferation compared to the substrate without the introduction of the peptides.
The current research progress of peptide-modified materials for bone tissue repair
Usually, after the preparation of bone repair materials, some in vitro and in vivo experiments need to be carried out to research its impact on cellular functions and new bone formation, thereby evaluating their osteogenic activity. In this section, we will review and discuss the current in vitro and in vivo research progress of peptide-modified composites used as potential bone repair materials.
In vitro evaluations
In this subsection, we will mainly present and discuss the recent researches on the in vitro evaluations of peptide-modified composites used as potential bone repair materials, focusing on the effects of the materials on the functions of cultured cells in vitro, such as adhesion, spreading, proliferation, differentiation and mineralization, etc.
ECM-derived peptides modified materials
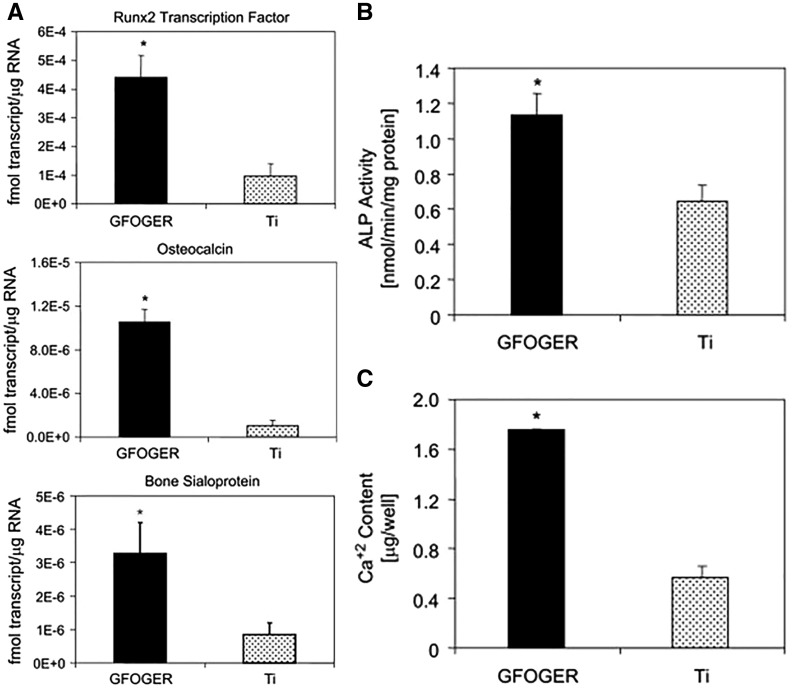
Numerous studies have indicated that the ECM-derived peptides modified materials can significantly improve the desired functions of bone-related cells in vitro. To date, calcium phosphate modified by ECM-derived peptides have attracted more and more attention. Hennessy et al. [34] studied that the efficacy of hydroxyapatite (HA) disks coated with a kind of ECM-derived peptide, DGEA, in promoting the osteogenic-related functions of MSCs, the results of which showed that cells grown on HA disks coated with DGEA (test group) exhibited greater ALP activity and OCN secretion than those on bare HA disks (control group). However, there was no obvious difference on cell adhesion between test group and control group. Hydrogel composites modified by ECM-derived peptides are another kind of materials, which have been researched widely by cell culture in vitro to evaluate the possibility of their application as bone repair materials. For example, Stile et al. [121] modified poly(N-isopropyl acrylamide-co-acrylic acid) [P(NIPAAm-co-AAc)] hydrogels with FHRRIKA peptide and studied the effects of FHRRIKA peptide addition on the cell viability of rat calvarial osteoblasts (RCO) in vitro. Their results showed that more cells attached and spread on the peptide-modified hydrogels than on the untreated ones, indicating that the FHRRIKA peptide addition improved the cell viability of the [P(NIPAAm-co-AAc)] hydrogels. Moreover, Cavalcanti-Adam et al. [122] investigated into the effects of the RGD peptide-modified silicone membranes on the osteogenic-related functions of osteoblast-like MC3T3-E1 cells in vitro. Their results showed that cells exhibited higher level of ALP activity after 8 days cultured on the RGD peptide-modified silicone membrane surface, indicating that the cells differentiated further into osteogenic ones better. Meanwhile, based on alizarin red staining and FTIR analysis, the cells cultured on the RGD-modified silicone membrane have better ability to generate biological apatite mineral deposition. Therefore, their results indicated that the RGD peptide addition significantly enhanced the osteogenic functions of the cells. In addition to the above-mentioned studies, researchers have investigated into metal biomaterials modified by ECM-derived peptides by cell culture in vitro. For example, Liu et al. [123] investigated into the osteogenic functions of preosteocyte MLO-A5 cells and mesenchymal cell (MSC) C3H10T1/2 on titanium surface modified by the P15 peptide with microscopies, real-time reverse transcription- polymerase chain reaction (qRT-PCR) analysis, western blotting and immunohistochemical analysis, etc., the results of which showed that the modification of titanium with P15 significantly increased not only the adhesion, spreading, and proliferation but also the maturation and osteogenic differentiation of the cells. Similarly, Reyes et al. [29] investigated into the osteogenic functions of bone marrow stromal cells on the titanium surfaces modified by GFOGER peptide in vitro. Their results showed that the cells cultured on titanium surfaces coated with the peptide achieved significantly higher expression of multiple osteoblast-specific genes (Fig. 3a), greater ALP activity (Fig. 3b), and biomineralized better (Fig. 3c) than those on untreated titanium surfaces.
Figure 3.
Compared to untreated titanium (Ti), GFOGER peptide coated Ti much more significantly promoted specific osteogenic gene expression (a), enhanced ALP activity (B) and biomineralization (C) of the cultured bone marrow stromal cells (adapted with permission from ref. [29]. Copyright 2007 Elsevier Ltd)
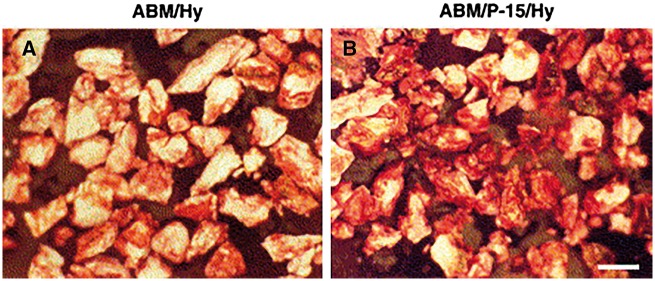
Besides the pure peptide-modified materials as described above, composites of them and other materials have been also widely researched by cell culture in vitro to evaluate the possibility of their application as bone repair materials. For instance, Nguyen et al. [20] modified anorganic bovine-derived mineral (ABM) with a typical ECM-derived peptide, P15, and then suspended them into hyaluronate (Hy) hydrogels, thereby preparing ABM/P15/Hy composites. Subsequently, they studied the effects of the P15 addition on the behaviours of osteoblast-like HOS cells in vitro. Their results showed that more cells adhered to ABM/P-15/Hy composites compared to ABM/Hy ones, and that the cells on ABM/P-15/Hy formed better surface coverage and had more stress fibers, suggesting that the P-15 addition promoted and strengthened cell adhesion. Most importantly, the cells cultured on ABM/P-15/Hy achieved significantly higher osteogenic gene expression of alkaline phosphatase and bone morphogenetic proteins, and biomineralized better (Fig. 4), indicating that the P-15 addition successfully enhanced the osteogenic differentiation and biomineralization of the cells.
Figure 4.
Alizarin red staining images of ABM/hy(a) and ABM/P-15/hy (B) cultured with HOS cells for 2 weeks. Bar =500 μm (adapted with permission from ref. [20]. Copyright 2003 Elsevier Ltd)
BMPs-derived peptides modified materials
Up to now, many scholars have confirmed that BMPs-derived peptides modified materials could promote desired osteogenic functions of cultured cells in vitro. In their studies, single polymer modified by BMP-derived peptides is one kind of main material. For example, Luo et al. [124] investigated into behaviours of osteoblast-like MG-63 cells cultured on porous alginate scaffolds (PAS) modified by BMP-7-derived peptide, BFP-1, with scanning electron microscope (SEM), confocal laser scanning microscopy (CLSM), and ALP activity assay, etc., the results of which showed that the peptide introduction significantly increased not only the adhesion, spreading, proliferation, and aggregation but also osteogenic differentiation (Fig. 5) of the cells.
Figure 5.
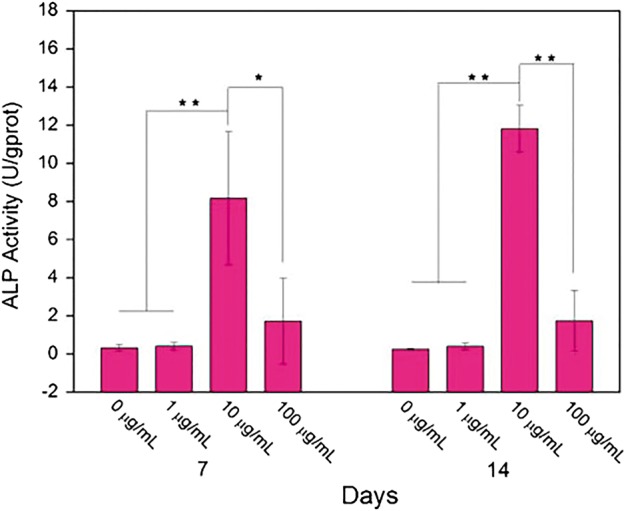
ALP Activity of MG-63 cells cultured on pure PAS and peptide-modified PAS with different incorporated concentrations for 7 and 14 days (adapted with permission from ref. [124]. Copyright 2016 Elsevier Ltd)
Besides single polymers, copolymers have also been used as substrates for the preparation of BMP-derived peptide-modified bone repair materials. For example, using PEG, amino acid units (ASP) and PLGA, Lin et al. [63] synthesized the PLGA-(PEG-ASP)n copolymer, which was then modified by P24. Their results showed that P24/PLGA-(PEG-ASP)n could improve better attachment of the bone MSCs and increase more significantly the expression of their osteogenic genes than PLGA-(PEG-ASP)n and PLGA groups.
Moreover, composites modified by BMP-derived peptides have recently attracted more and more attention. For instance, Zhang et al.[60] investigated the effects of nHAC scaffolds modified by BMP-2-derived peptides, P17-BMP-2, on rabbit marrow stromal cells in vitro, the results of which showed that P17-BMP-2 compounded into the nHAC not only retained its activity, but also significantly upregulated the expression level ofosteogenic-related genes of the cells, such as OPN and OCN. Meanwhile, the cells could attach better on P17-BMP-2/nHAC than on nHAC. Moreover, Li et al. [125] in vitro studied true bone ceramics (TBC)/collagen I composites modified by another BMP-2-derived peptides, P24, by culturing bone marrow stromal cells. Based on SEM, energy dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD) analysis, etc., they found that the P24 addition significantly enhanced the level of hydroxylapatite crystal mineralization with a Ca/P molar ratio of 1.63.
In addition to the above-mentioned studies, researchers have investigated into particles modified by BMP-derived peptides as a delivery system to find out their potentials as bone repair materials. For example, Bergeron et al. [126] compounded pBMP-9 peptide into collagen/45S5 Bioglass® microspheres for their controlled release, the results of which showed that the collagen/45S5 Bioglass® group could release proteins more slowly than the pure collagen group (control). Moreover, the collagen/45S5 Bioglass® microspheres containing the pBMP-9 peptide could induce the osteogenic differentiation of MC3T3-E1 preosteoblasts better than those compounded with rhBMP-2. Similarly, Zhou et al. [61] synthesized the residues 73-92 of BMP-2 covalently functionalized mesoporous silica (MSNs-pep) via an aminosilane linker. The cell viability of MSNs-pep was tested by bone MSCs exposure to different particle concentrations in vitro. The results revealed that the modified MSNs had better cytocompatibility, and that the cellular uptake efficiency of MSNs-pep was significantly larger than that of bare MSNs. Moreover, it was shown that the peptide addition significantly enhanced the osteogenic differentiation of the bone MSCs, based on the data of ALP activity, calcium deposition, and expression of bone-related proteins, etc.
Materials modified by other peptides
Compared to the materials modified by ECM or BMP-derived peptides, those modified by other active peptides were researched less in vitro. Recently, calcium phosphate minerals modified by other peptides have been used as coatings of bone repair materials. For example, Chen et al. [127] prepared OGP modified mixture of CaO and HA coatings on titanium substrates, and then investigated into the effects of the coatings on the behaviours of MSCs with XPS, SEM, and CLSM, etc., the results of which showed that the OGP addition significantly increased not only the adhesion and proliferation but also the maturation and osteogenic differentiation of the cells. Moreover, collagen matrix composites modified by self-assembly peptides have recently attracted more and more attention. For instance, Li et al. [108] developed RADA16-I peptide-modified demineralized bone matrix (DBM) material for bone repair, and studied them in vitro by culturing MSCs, which showed that the levels of expression of ALP, OCN, and Runx2 gene in DBM modified by RADA16-I were significantly higher than those in unmodified DBM at 14 days. Besides the above studies, biomacromoleculars modified by other active peptides have also researched in vitro to evaluate the potential of their application as bone repair materials. For instance, Suh et al. [128] prepared CPP peptide-modified a typical protein, PDZ-binding motif (TAZ), and then studied the effects of the CPP addition on the osteogenic differentiation of hMSCs. Their results showed that the cells cultured on the TAZ protein modified by CPP peptide achieved significantly higher expression of multiple osteoblast-specific genes (ALP, OCN, and Runx2), and biomineralized better than those on the untreated TAZ.
In vivo evaluations
Currently, besides the in vitro studies, many researchers have investigated into peptide-modified materials by animal experiments in vivo to find out their potentials as bone repair materials, providing more direct data for their possible further clinical applications.
ECM-derived peptides modified materials
In recent years, many researchers have tried their best to heal bone defects of animals using calcium phosphate modified by ECM-derived peptides. For instance, Lindley et al. [129] created 4 mm diameter defects by drill bits in the tibiae of rabbits. Then, the animals were divided into four groups, which were respectively implanted with ABM/P-15/hyaluronate hydrogel, ABM/hyaluronate hydrogel, hyaluronate hydrogel alone, and nothing. Histomorphometric analyses showed that defects treated with ABM/P-15 had significantly larger areas of new bone formation than the other three groups at 2, 6, and 8 weeks after surgery. However, some other researchers got opposite outcomes of repairing bone defects with ABM/P-15. For example, Sarahrudi et al. [130] created 5 mm defects by high-speed oscillating saw in the femur of rabbits. Then, the animals were divided into two groups, which were respectively implanted with ABM/P-15 and nothing (control). Histomorphometry analyses showed that the ABM/P-15 groups have a smaller amount of new bone formation (1.56 ± 0.27 mm2) than the control group (2.5 ± 0.2 mm2) at 12 weeks after operation.
Beside the above studies, researchers have also investigated the osteogenic activity in vivo of collagen modified by ECM-derived peptides. For example, Egusa et al. [27] implanted atelocollagen sponge containing either 10 mg SVVYGLR peptide or PBS (as control) into the calvaria defects (5 mm in diameter and 0.5 mm in depth) of rats created by dental round burr. Their results showed that the number of osteoblasts in the SVVYGLR modified implants at 3 weeks after surgery was significantly higher than that in the control group. Meanwhile, newly formed blood vessels in the peptide-modified graft groups were more evident than those in the control group. Moreover, at five weeks after surgery, although both groups showed new bone formation in the cavity surrounding the sponge graft, more compact woven-like bone formed in animals treated with the atelocollagen sponge modified by SVVYGLR peptide.
In addition to pure peptide-modified materials as described above, composites containing them have been also researched in vivo. For example, Bitschnau et al. [131] prepared RGD-modified HA coatings on stainless steel K-wires, and then investigated into the effects of their implantation into the intramedullary canal of the rabbit tibia on the new bone formation and implant bone contact with quantitative and qualitative histology analysis, the results of which showed that RGD-HA and pure HA coated K-wires displayed higher new bone formation and implant bone contact than the uncoated ones after 12 weeks. There were no significant differences between the RGD-HA and the pure HA coated K-wires in new bone formation and implant bone contact after 4 and 12 weeks, the reason of which might be that the strong osteoconductive effect of the HA coating ‘‘over-whelms’’ the potential RGD effect.
BMPs-derived peptides modified materials
To date, many scholars have also studied the potential of in vivo repairing bone defects in animals of BMP-derived peptide-modified materials. For instance, Bergeron et al. [132] evaluated the bone repair ability of the pBMP-9 peptide bonded to type I collagen and chitosan by injecting them into mouse quadriceps. Histological analyses clearly demonstrated that more lamellar bone formed in animals treated with the chitosan modified by pBMP-9.
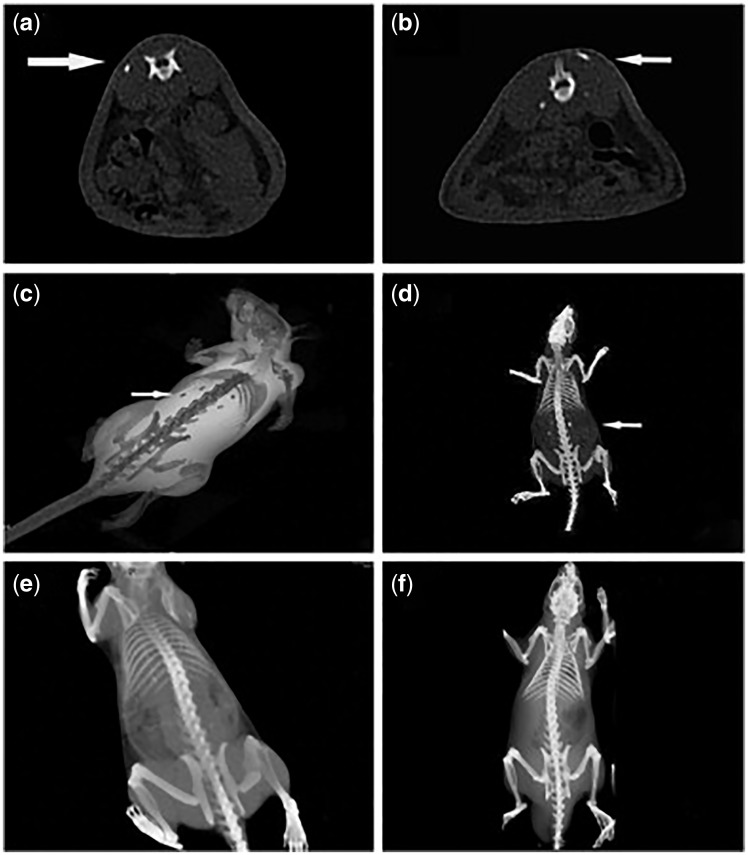
Besides single polymers, copolymers modified by BMPs-derived peptides have also been researched in vivo. For example, Lin et al. [63] respectively implanted P24/PLGA-(PEG-ASP)n, PLGA-(PEG-ASP)n and gelatin sponge into the dorsal muscle of rats. Radiographic examination showed that the P24/PLGA-(PEG-ASP)n group had block-like shadows on the CT image at 12 weeks after the surgery (Fig. 6a–d) while no high-density shadows were observed in groups of PLGA-(PEG-ASP)n (Fig. 6e) and gelatin sponge (Fig. 6f). Subsequently, histological examinations confirmed that new bone formed at the subcutaneous layers, where high-density shadows were shown by the CT scans.
Figure 6.
CT Images of P24/PLGA-(PEG-ASP)n (a-d), PLGA-(PEG-ASP)n (e), and gelatin sponge (f) after implanted into the dorsal muscle of rats for 12 weeks after operation. Arrows indicated the new bone formation (adapted with permission from ref. [63]. Copyright 2010 Elsevier Ltd)
Moreover, BMP-derived peptide-modified composites have also been tried to repair bone defects in vivo. For example, Li et al. [125] created 10 mm unilateral segmental bone defect with burr drill in rabbit radius. Then, the animals were divided into three groups, which were respectively implanted with P24/(true bone ceramics) TBC/collagen I composite (Group A), TBC/collagen I composite (Group B), and TBC (Group C). Based on histological examination at 8 and 12 weeks after surgery, more newly formed bone was observed in Group A (Fig. 7a and b) than those in Group B (Fig. 7c and d) and Group C (Fig. 7e and f). In addition, Li et al. [133] implanted nHAC/PLLA containing either P24, rhBMP-2, or nothing into the cranial bone defects (5 mm diameter) of rats created by trephine drill. The results of the radiographic and three-dimensional CT evaluations and the histological examinations showed that the P24 addition much more significantly enhanced the bone defect repair effectiveness.
Figure 7.
The histological images of the implanted materials at two time points: (a) P24/TBC/collagen I, (c) TBC/collagen I, (e) TBC at 8 weeks; (b) P24/TBC/collagen I, (d) TBC/collagen I, and (f) TBC at 12 weeks (magnification: 200×) (adapted with permission from ref. [125]. Copyright 2010 Elsevier Ltd)
Materials modified by other peptides
At present, in vivo bone repair ability of materials modified by other active peptides, besides ECM or BMPs-derived ones, have been also studied. For example, Sheller et al. [134] prepared PLGA microspheres modified by TP508 peptide, and implanted them into the defects of rabbit forelimbs (0.5 cm in length) to evaluate their bone repair ability. The radiographs showed a significantly higher degree of bone repair in the animals treated with PLGA microspheres modified by the peptide. Three-dimensional synchrotron tomography showed that the new bone in animal treated with the peptide-modified PLGA microspheres had a less porous surface appearance and more open marrow spaces than that in the animal treated with unmodified PLGA microspheres, indicating that the implantation of the peptide-modified material could get a better result of bone remodeling. In addition, Ma et al. [135] created 1.5 cm defects by saw in the rabbit radius. Then, the animals were divided into two groups, which were respectively implanted with OGP/PLGA scaffold and bare PLGA scaffold. Radiographic images and histomorphological analysis showed that more new bone formed in animals treated with the PLGA scaffolds modified by the OGP peptide.
Moreover, hydrogels modified by other peptides have been researched in vivo. For instance, Jung et al. [136] synthesized the polyethylene glycol (PEG) hydrogel, which was then modified by PTH1-34 and RGD peptides. In their study, circumferential bone defects were created in foxhound mandibular premolar, and then the animals were randomly divided into four groups, which were respectively implanted with PEG containing PTH1-34 and RGD peptides, PEG, autogenous bone, and nothing (control). Histomorphometric analysis showed that newly formed bone in the peptide-modified graft groups were much more evident than that in the groups of PEG or control at 4 and 12 weeks after surgery. Furthermore, it was indicated that the peptide-modified materials could get almost the same bone repair effectiveness compared with autogenous bone.
Conclusion and perspective
In this article, recent studies on bioactive peptide-modified materials for bone tissue repair have been generally reviewed. Currently, many kinds of peptides, including ECM-derived ones, BMPs-derived ones, etc., have been developed and investigated as valid candidates for bone healing. These peptides can activate some specific signalling pathways that control osteogenic-related cellular functions. Meanwhile, a lot of studies have been launched to modify bone repair materials with these peptides with many different methods, such as electrodeposition, covalent immobilization, physical adsorption, etc. In combination with the peptides, the materials have been generally shown to possess enhanced osteogenic ability, presenting to induce osteogenic-related cellular responses and further promote new bone formation and osseointegration. Generally speaking, the recent related studies have fully suggested that the modification of bone repair materials with osteogenic-related peptides provide promising strategies for the development of bioactive materials and substrates for enhanced bone regeneration and the therapy of bone tissue diseases.
Although great achievements have been got, there is still a lot of work to do. Firstly, more detailed systematic studies to figure out more specific characteristics and potential functions of each related peptide are necessary. Secondly, more satisfactory techniques need to be developed to prepare the bioactive peptide-modified materials for different applications, in which the amount of loaded peptides can be flexibly controlled. Furthermore, the loaded peptides should be able to release at a controllable rate. Thirdly, since it has been shown that the preparation method of the peptide-modified materials has significant effects on the bioactivity of the peptides, the systematic investigations into the possible influential mechanisms should be launched. Especially, although there have been already many methods to covalently bond peptides to materials, hardly any publication on how the different covalent bonding methods affect the bioactivity of the same peptide can be found. Fifthly, there are many factors that influence the function realization of the peptide-modified materials, among which degradation of the substrate materials is a crucial one. Future studies on this aspect are very necessary. Sixthly, for the bone repair materials, it is well recognized that their positive effects on the biomineralization is one of most important evaluation standards of their quality. However, the current investigations into the effects of peptide-modified materials for bone repair on the biomineralization in vitro and in vivo are very inadequate. Finally, the efforts to ensure the loaded peptides on the bone repair materials to entirely reach the targeted sites to utmostly promote new bone formation still need to be made in future studies.
Funding
National Natural Science Foundation of China (Nos. 31370959, 11421202 and 61227902), Fok Ying Tung Education Foundation (No. 141039), Beijing Nova Programme Interdisciplinary Cooperation Project (No. xxjc201616), Key Laboratory of Advanced Materials of Ministry of Education of China (Tsinghua University), International Joint Research Center of Aerospace Biotechnology and Medical Engineering, Ministry of Science and Technology of China, and the 111 Project (No. B13003).
Conflict of interest statement. None declared.
References
- 1. Qiu ZY, Chen C, Wang XM. et al. Advances in the surface modification techniques of bone-related implants for last 10 years. Regen Biomater 2014; 1:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li XM, Yang Y, Fan YB. et al. Biocomposites reinforced by fibers or tubes, as scaffolds for tissue engineering or regenerative medicine. J Biomed MaterResPart A 2014;102A:1580–94. [DOI] [PubMed] [Google Scholar]
- 3. Shi S, Jiang WB, Aifantis KE. et al. The application of nanomaterials in controlled drug delivery for bone regeneration. J Biomed Mat Res Part A 2015;103:3978–92. [DOI] [PubMed] [Google Scholar]
- 4. Bilem I, Chevallier P, Plawinski L. et al. RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater 2016; 36:132–42. [DOI] [PubMed] [Google Scholar]
- 5. Yi J, Xiong F, Li BB. et al. Degradation characteristics, cell viability and host tissue responses of PDLLA-based scaffold with PRGD and β-TCP nanoparticles incorporation. Regen Biomater 2016; 3:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Punet X, Mauchauffé R, Rodríguez-Cabello JC. et al. Biomolecular functionalization for enhanced cell–material interactions of poly (methyl methacrylate) surfaces. Regen Biomater 2015; 2:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sathy BN, Olvera D, Gonzalez-Fernandez T. et al. RALA complexed a-TCP nanoparticle delivery to mesenchymal stem cells induces bone formation in tissue engineered constructs in vitro and in vivo. J. Mater. Chem. B 2017; 5:1753–64. [DOI] [PubMed] [Google Scholar]
- 8. Li XM, Liu HF, Niu XF. et al. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012; 33:4818–27. [DOI] [PubMed] [Google Scholar]
- 9. Zhao ZY, Shao L, Zhao HM. et al. Osteogenic growth peptide accelerates bone healing during distraction osteogenesis in rabbit tibia. J Int Med Res 2011; 39:456– 63. [DOI] [PubMed] [Google Scholar]
- 10. Li XM, Wang L, Fan YB. et al. Nanostructured scaffolds for bone tissue engineering. J Biomed Mater Res Part A 2013; 101:2424–35. [DOI] [PubMed] [Google Scholar]
- 11. Ko E, Yang K, Shin J. et al. Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules 2013; 14: 3202–13. [DOI] [PubMed] [Google Scholar]
- 12. Michel G, Thierry T, Delphine SJ, Mark C. et al. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytologist 2010; 188:82–97. [DOI] [PubMed] [Google Scholar]
- 13. Falcigno L, D'Auria G, Calvanese L. et al. Osteogenic properties of a short BMP-2 chimera peptide. J Pept Sci 2015; 21:700–9. [DOI] [PubMed] [Google Scholar]
- 14. Emam HA, Behiri G, El-Alaily M. et al. The efficacy of a tissue-engineered xenograft in conjunction with sodium hyaluronate carrier in maxillary sinus augmentation: a clinical study. Int J Oral Maxillofac Surg 2015; 44:1287–94. [DOI] [PubMed] [Google Scholar]
- 15. Yang XB, Bhatnagar RS, Li S. et al. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng 2004; 10:1148–59. [DOI] [PubMed] [Google Scholar]
- 16. Bhatnagar RS, Qian JJ, Wedrychowska A. et al. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng 1999; 5:53–65. [DOI] [PubMed] [Google Scholar]
- 17. Park JW, Kurashima K, Tustusmi Y. et al. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly(ethylene glycol) in rabbit cancellous bone. Acta Biomater 2011; 7:3222–9. [DOI] [PubMed] [Google Scholar]
- 18. Oya K, Tanaka Y, Saito H. et al. Calcification by MC3T3-E1 cells on RGD peptide immobilized on titanium through electrodeposited PEG. Biomaterials 2009; 30:1281– 6. [DOI] [PubMed] [Google Scholar]
- 19. Chollet C, Chanseau C, Remy M. et al. The effect of RGD density on osteoblast and endothelial cell behavior on RGD-grafted polyethylene terephthalate surfaces. Biomaterials 2009; 30:711– 20. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen H, Qian JJ, Bhatnagar RS. et al. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun 2003; 311:179–86. [DOI] [PubMed] [Google Scholar]
- 21. Chen Z, Wang X, Shao Y. et al. Synthetic osteogenic growth peptide promotes differentiation of human bone marrow mesenchymal stem cells to osteoblasts via RhoA/ROCK pathway. Mol Cell Biochem 2011; 358:221– 7. [DOI] [PubMed] [Google Scholar]
- 22. Cakarer S, Olgac V, Aksakalli N. et al. Acceleration of consolidation period by thrombin peptide 508 in tibial distraction osteogenesis in rats. Br J Oral Maxillofac Surg 2010; 48:633–6. [DOI] [PubMed] [Google Scholar]
- 23. Huang H, Zhao Y, Liu Z. et al. Enhanced osteoblast functions on RGD immobilized surface. J Oral Implantol 2003; 29:73–9. [DOI] [PubMed] [Google Scholar]
- 24. Rammelt S, Illert T, Bierbaum S. et al. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006; 27:5561–71. [DOI] [PubMed] [Google Scholar]
- 25. Priddy LB, Chaudhuri O, Stevens HY. et al. Oxidized alginate hydrogels for bone morphogenetic protein-2 delivery in long bone defects. Acta Biomater 2014; 10:4390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamada Y, Yuki K, Okazaki M. et al. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent Mater J 2004; 23:650–5. [DOI] [PubMed] [Google Scholar]
- 27. Egusa H, Kaneda Y, Akashi Y. et al. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009; 30:4676–86. [DOI] [PubMed] [Google Scholar]
- 28. Park KM, Lee Y, Son JY. et al. In situ SVVYGLR peptide conjugation into injectable gelatin-poly(ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjug Chem 2012; 23:2042–50. [DOI] [PubMed] [Google Scholar]
- 29. Reyes CD, Petrie TA, Burns KL. et al. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007; 28:3228– 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reyes CD, Garcia AJ.. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A 2004; 69:591– 600. [DOI] [PubMed] [Google Scholar]
- 31. Shekaran A, Garcia JR, Clark AY. et al. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014; 35:5453– 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mhanna R, Öztürk E, Vallmajomartin Q. et al. GFOGER-modified MMP-sensitive polyethylene glycol hydrogels induce chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A 2014; 20:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madamanchi A, Santoro SA, Zutter MM.. α2β1 Integrin. Adv Exp Med Biol 2014; 819:41–60. [DOI] [PubMed] [Google Scholar]
- 34. Hennessy KM, Pollot BE, Clem WC. et al. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials 2009; 30:1898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ceylan H, Kocabey S, Unal Gulsuner H. et al. Bone-like mineral nucleating peptide nanofibers induce differentiation of human mesenchymal stem cells into mature osteoblasts. Biomacromolecules 2014; 15:2407–18. [DOI] [PubMed] [Google Scholar]
- 36. Yoo SY, Kobayashi M, Lee PP. et al. Early osteogenic differentiation of mouse preosteoblasts induced by collagen-derived DGEA-peptide on nanofibrous phage tissue matrices. Biomacromolecules 2011; 12:987–96. [DOI] [PubMed] [Google Scholar]
- 37. Culpepper BK, Phipps MC, Bonvallet PP. et al. Enhancement of peptidecoupling to hydroxyapatite and implant osseointegration through collagenmimetic peptide-modified with a polyglutamate domain. Biomaterials 2010; 31:9586– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shin MK, Kim MK, Bae YS. et al. A novel collagenbinding peptide promotes osteogenic differentiation via Ca2+/calmodulindependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell Signal 2008; 20:613–24. [DOI] [PubMed] [Google Scholar]
- 39. Lee JY, Choo JE, Park HJ. et al. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochem Biophys Res Commun 2007; 357:68–74. [DOI] [PubMed] [Google Scholar]
- 40. Palchesko RN, Romeo JD, McGowan KA. et al. Increased osteoblast adhesion on physically optimized KRSR modified calcium aluminate. J Biomed Mater Res A 2012; 100:1229–38. [DOI] [PubMed] [Google Scholar]
- 41. Sun S, Yu W, Zhang Y. et al. Increased preosteoblast adhesion and osteogenic gene expression on TiO2 nanotubes modified with KRSR. J Mater Sci Mater Med 2013;24:1079–91. [DOI] [PubMed] [Google Scholar]
- 42. Gentile P, Ferreira AM, Callaghan JT. et al. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Adv Healthc Mater2017; DOI:10.1002/adhm.201601182. [DOI] [PubMed]
- 43. Nelson M, Balasundaram G, Webster TJ.. Increased osteoblast adhesion on nanoparticulate crystalline hydroxyapatite functionalized with KRSR. Int J Nanomedicine 2006; 1:339–49. [PMC free article] [PubMed] [Google Scholar]
- 44. Balasundaram G, Webster TJ.. Increased osteoblast adhesion on nanograined Ti modified with KRSR. J Biomed Mater Res A 2007; 80:602–11. [DOI] [PubMed] [Google Scholar]
- 45. Dettin M, Conconi MT, Gambaretto R. et al. Novel osteoblast-adhesive peptides for dental/orthopedic biomaterials. J Biomed Mater Res 2002; 60:466–71. [DOI] [PubMed] [Google Scholar]
- 46. Hasenbein ME, Andersen TT, Bizios R.. Micropatterned surfaces modified with select peptides promote exclusive interactions with osteoblasts. Biomaterials 2002; 23:3937–42. [DOI] [PubMed] [Google Scholar]
- 47. Rezania A, Healy KE.. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol Prog 1999; 15:19–32. [DOI] [PubMed] [Google Scholar]
- 48. Gentile P, Ghione C, Tondaturo C. et al. Peptide functionalisation of nanocomposite polymer for bone tissue engineering using plasma surface polymerisation. RSC Advances 2015; 5:80039–47. [Google Scholar]
- 49. Kim YJ, Park YJ, Lee YM. et al. The biological effects of fibrinbinding synthetic oligopeptides derived from fibronectin on osteoblast-like cells. J Periodontal Implant Sci 2012; 42:113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee JA, Ku Y, Rhyu IC. et al. Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects. J Periodontal Implant Sci 2010;40:211– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martino MM, Tortelli F, Mochizuki M. et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 2011; 3:100–89. [DOI] [PubMed] [Google Scholar]
- 52. Reddi AH, Reddi A.. Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev 2009; 20:341–2. [DOI] [PubMed] [Google Scholar]
- 53. Devescovi V, Leonardi E, Ciapetti G. et al. Growth factors in bone repair. Chir Organi Mov 2008; 92:161– 8. [DOI] [PubMed] [Google Scholar]
- 54. Ma Y, Policastro GM, Li Q. et al. Concentration-Dependent hMSC Differentiation on Orthogonal Concentration Gradients of GRGDS and BMP-2 Peptides. Biomacromolecules 2016; 17:1486–95. [DOI] [PubMed] [Google Scholar]
- 55. Beauvais S, Drevelle O, Lauzon MA. et al. Modulation of MAPK signalling by immobilized adhesive peptides: effect on stem cell response to BMP-9-derived peptides. Acta Biomater 2015; 31:241–51. [DOI] [PubMed] [Google Scholar]
- 56. Liu Z, Tang Y, Kang T. et al. Synergistic effect of HA and BMP-2 mimicking peptide on the bioactivity of HA/PMMA bone cement. Colloids Surf B Biointerfaces 2015; 131:39-39.. [DOI] [PubMed] [Google Scholar]
- 57. Moeinzadeh S, Barati D, Sarvestani SK. et al. Experimental and computational investigation of the effect of hydrophobicity on aggregation and osteoinductive potential of BMP-2-derived peptide in a hydrogel matrix. Tissue Eng Part A 2014; 21:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pan H, Zheng Q, Yang S. et al. Effects of functionalization of PLGA-[Asp-PEG]n copolymer surfaces with Arg-Gly-Asp peptides, hydroxyapatite nanoparticles, and BMP-2-derived peptides on cell behavior in vitro. J Biomed Mat Res Part A 2014; 102:4526–35. [DOI] [PubMed] [Google Scholar]
- 59. Madl CM, Mehta M, Duda GN. et al. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules 2014; 15:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang X, Guo WG, Cui H. et al. In vitro and in vivo enhancement of osteogenic capacity in a synthetic BMP-2-derived peptide-coated mineralized collagen composite. J Tissue Eng Regen Med 2016; 10:99–107. [DOI] [PubMed] [Google Scholar]
- 61. Zhou X, Feng W, Qiu K. et al. BMP-2-derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Appl Mater Interfaces 2015; 7:15777–89. [DOI] [PubMed] [Google Scholar]
- 62. Kim MJ, Lee B, Yang K. et al. BMP-2 peptide-functionalized nanopatterned substrates for enhanced osteogenic differentiation of human mesenchymal stem cells. Biomaterials 2013; 34:7236–46. [DOI] [PubMed] [Google Scholar]
- 63. Lin ZY, Duan ZX, Guo XD. et al. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J Control Release 2010; 144:190–5. [DOI] [PubMed] [Google Scholar]
- 64. Kim HK, Kim JH, Park DS. et al. Osteogenesis induced by a bone forming peptide from the prodomain region of BMP-7. Biomaterials 2012; 33:7057–63. [DOI] [PubMed] [Google Scholar]
- 65. Feng B, Hu D, Zhang Y.. Accelerated bone regeneration by chitosan/nanometer hydroxyapatite/collagen composite incorporating BMP-7 mimetic peptide. J Hard Tissue Biol 2012; 21:481–7. [Google Scholar]
- 66. Lord E, Bergeron E, Senta H. et al. Effect of BMP-9 and its derived peptide on the differentiation of human white preadipocytes. Growth Factors 2010; 28:149–56. [DOI] [PubMed] [Google Scholar]
- 67. Wang YH, Zhang L, Jia L. et al. Calcitonin gene-related peptide in aerobic exercise induces collateral circulation development in rat ischemia myocardium. Biomed Pharmacother 2016; 82:561–7. [DOI] [PubMed] [Google Scholar]
- 68. Villa I, Dal Fiume C, Maestroni A. et al. Human osteoblast-like cell proliferation induced by calcitonin-related peptides involves PKC activity. Am J Physiol Endocrinol Metab 2003; 284:E627–33. [DOI] [PubMed] [Google Scholar]
- 69. Cornish J, Callon KE, Lin CQ. et al. Comparison of the effects of calcitonin gene-related peptide and amylin on osteoblasts. J Bone Miner Res 1999; 14:1302–9. [DOI] [PubMed] [Google Scholar]
- 70. Valentijn K, Gutow AP, Troiano N. et al. Effects of calcitonin gene-related peptide on bone turnover in ovariectomized rats. Bone 1997; 21:269–74. [DOI] [PubMed] [Google Scholar]
- 71. Ballica R, Valentijn K, Khachatryan A. et al. Targeted expression of calcitonin gene related peptide to osteoblasts increases bone density in mice. J Bone Miner Res 1999; 14:1067–74. [DOI] [PubMed] [Google Scholar]
- 72. Mrak E, Guidobono F, Moro G. et al. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. J Physiol 2010; 225:701–8. [DOI] [PubMed] [Google Scholar]
- 73. Lerner UH. Neuropeptidergic regulation of bone resorption and bone formation. J Musculoskel Neuron Interact 2002; 2:440–7. [PubMed] [Google Scholar]
- 74. Hay DL. What makes a CGRP2 receptor?. Clin Exp Pharmacol Physiol 2007; 34:963–71. [DOI] [PubMed] [Google Scholar]
- 75. Sample SJ, Hao Z, Wilson AP. et al. Role of calcitonin gene-related peptide in bone repair after cyclic fatigue loading. Plos One 2011; 6:e20386.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Villa I, Melzi R, Pagani F. et al. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. Eur J Pharmacol 2000; 409:273–8. [DOI] [PubMed] [Google Scholar]
- 77. Kruger L, Silverman JD, Mantyh PW. et al. Peripheral patterns of calcitonin-gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol 1989; 280:291–302. [DOI] [PubMed] [Google Scholar]
- 78. Wang L, Shi X, Zhao R. et al. Calcitoningene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone 2010; 46:1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Calland JW, Harris SE, Carnes Jr DL.. Human pulp cells respond to calcitonin gene-related peptide in vitro. J Endod 1997; 23:485–9. [DOI] [PubMed] [Google Scholar]
- 80. Vignery A, McCarthy TL.. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone 1996; 18:331–5. [DOI] [PubMed] [Google Scholar]
- 81. Millet I, Vignery A.. The neuropeptide calcitonin gene-related peptide inhibits TNF-alpha but poorly induces IL-6 production by fetal rat osteoblasts. Cytokine 1997; 9:999–1007. [DOI] [PubMed] [Google Scholar]
- 82. Xu G, Jiang D.. The role and mechanism of exogenous calcitonin generelated peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell Biochem Biophys 2014; 69:369–78. [DOI] [PubMed] [Google Scholar]
- 83. Tian G, Zhang G, Tan YH.. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin 2013; 34:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lv L, Wang Y, Zhang J. et al. Healing of periodontal defects and calcitonin gene related peptide expression following inferior alveolar nerve transection in rats. J Mol Histol 2014; 45:311–20. [DOI] [PubMed] [Google Scholar]
- 85. Zhou XY, Xu XM, Wu SY. et al. Spatiotemporal Changes of Calcitonin Gene-Related Peptide Innervation in Spinal Fusion. BioMed Res Int 2016;2016:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fujita T, Fukase M, Baba H. et al. New actions of parathyroid hormone through its degradation. J Endocrinol Invest 1992; 15:121–7. [PubMed] [Google Scholar]
- 87. Alkhiary YM, Gerstenfeld LC, Krall E. et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am 2005; 87:731–41. [DOI] [PubMed] [Google Scholar]
- 88. Whitfield JF, Morley P, Willick GE.. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat Endocrinol 2002; 1:175–90. [DOI] [PubMed] [Google Scholar]
- 89. Bab I, Gazit D, Chorev M. et al. Histone H4-related osteogenic growth peptide (OGP): a novel circulating stimulator of osteoblastic activity. Embo J 1992; 11:1867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Greenberg Z, Gavish H, Muhlrad A. et al. Isolation of osteogenic growth peptide from osteoblastic MC3T3 E1 cell cultures and demonstration of osteogenic growth peptide binding proteins. J Cell Biochem 1997; 65:359– 63. [PubMed] [Google Scholar]
- 91. Bab I, Gavish H, Namdar-Attar M. et al. Isolation of mitogenically active C-terminal truncated pentapeptide of osteogenic growth peptide from human plasma and culture medium of murine osteoblastic cells. J Pept Res 1999; 54:408–14. [DOI] [PubMed] [Google Scholar]
- 92. Gabarin N, Gavish H, Muhlrad A. et al. Mitogenic G(i) protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: activation by C-terminal pentapeptide of osteogenic growth peptide [OGP(10-14)] and attenuation of activation by cAMP. J Cell Biochem 2001; 81:594–603. [DOI] [PubMed] [Google Scholar]
- 93. Brager MA, Patterson MJ, Connolly JF. et al. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. J Orthop Res 2000; 18:133–9. [DOI] [PubMed] [Google Scholar]
- 94. Fei Q, Guo C, Xu X. et al. Osteogenic growth peptide enhances the proliferation of bone marrow mesenchymal stem cells from osteoprotegerin-deficient mice by CDK2/cyclin A. Acta Biochim Biophys Sin Shanghai 2010; 42:801–6. [DOI] [PubMed] [Google Scholar]
- 95. Spreafico A, Frediani B, Capperucci C. et al. Osteogenic growth peptide effects on primary human osteoblast cultures: potential relevance for the treatment of glucocorticoid-induced osteoporosis. J Cell Biochem 2006; 98:1007–20. [DOI] [PubMed] [Google Scholar]
- 96. An G, Xue Z, Zhang B. et al. Expressing osteogenic growth peptide in the rabbit bone mesenchymal stem cells increased alkaline phosphatase activity and enhanced the collagen accumulation. Eur Rev Med Pharmacol Sci 2014; 18:1618–24. [PubMed] [Google Scholar]
- 97. Li G, Cui Y, McIlmurray L. et al. rhBMP-2, rhVEGF(165), rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res 2005; 23:680–5. [DOI] [PubMed] [Google Scholar]
- 98. Vordemvenne T, Paletta JR, Hartensuer R. et al. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix-derived synthetic peptides in human osteoblasts. BMC Musculoskelet Disord 2011; 12:263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Trasatti C, Spears R, Gutmann JL. et al. Increased Tgf-beta1 production by rat osteoblasts in the presence of PepGen P-15 in vitro. J Endod 2004; 30:213–7. [DOI] [PubMed] [Google Scholar]
- 100. Strnad J, McDonnell PA, Riexinger DJ. et al. NEMO binding domain of IKK-2 encompasses amino acids 735-745. J Mol Recognit 2006; 19:227–33. [DOI] [PubMed] [Google Scholar]
- 101. Soysa NS, Alles N, Shimokawa H. et al. Inhibition of the classical NF-kappaB pathway prevents osteoclast bone-resorbing activity. J Bone Miner Metabol 2009; 27:131–9. [DOI] [PubMed] [Google Scholar]
- 102. Li W, Yu B, Li M. et al. NEMO-binding domain peptide promotes osteoblast differentiation impaired by tumor necrosis factor alpha. Biochem Biophys Res Commun 2010; 391:1228–33. [DOI] [PubMed] [Google Scholar]
- 103. Jimi E, Aoki K, Saito H. et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med 2004; 10:617–24. [DOI] [PubMed] [Google Scholar]
- 104. Karagiannis ED, Urbanska AM, Sahay G. et al. Rational design of a biomimetic cell penetrating peptide library. ACS Nano 2013; 7:8616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jo J, Hong S, Choi WY. et al. Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymal stromal cells. Sci Rep 2014; 4:4378.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Park SH, Doh J, Park SI. et al. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene Ther 2010; 17:1052–61. [DOI] [PubMed] [Google Scholar]
- 107. Zhang S. Emerging biological materials through molecular self-assembly. Biotechnol Adv 2002; 20:321–39. [DOI] [PubMed] [Google Scholar]
- 108. Li ZQ, Hou TY, Luo F. et al. Bone marrow enriched graft, modified by self-assembly peptide, repairs critically-sized femur defects in goats. Int Orthop 2014; 38:2391–8. [DOI] [PubMed] [Google Scholar]
- 109. Hou TY, Li ZQ, Luo F. et al. A composite demineralized bone matrix-self assembling peptide scaffold for enhancing cell and growth factor activity in bone marrow. Biomaterials 2014; 35:5689–99. [DOI] [PubMed] [Google Scholar]
- 110. Tanaka Y, Doi H, Kobayashi E. et al. T. Determination of the immobilization manner of amine-terminated poly (ethylene glycol) electrodeposited on a titanium surface with XPS and GD-OES. Mater Trans 2007; 48:287–92. [Google Scholar]
- 111. Tanaka Y, Matsuo Y, Komiya T. et al. Characterization of the spatial immobilization manner of poly (ethylene glycol) to a titanium surface with immersion and electrodeposition and its effects on platelet adhesion. J Biomed Mater Res Part A 2010; 92:350–8. [DOI] [PubMed] [Google Scholar]
- 112. Tanaka Y, Saito H, Tsutsumi Y. et al. Effect of pH on the interaction between zwitterions and titanium oxide. J Colloid Interface Sci 2009; 330:138–43. [DOI] [PubMed] [Google Scholar]
- 113. Seo HS, Ko YM, Shim JW. et al. Characterization of bioactive RGD peptide immobilized onto poly (acrylic acid) thin films by plasma polymerization. Appl Surf Sci 2010; 257:596–602. [Google Scholar]
- 114. Liu H, Webster TJ.. Ceramic/polymer nanocomposites with tunable drug delivery capability at specific disease sites. J Biomed Mater Res Part A 2010; 93:1180–92. [DOI] [PubMed] [Google Scholar]
- 115. Acharya B, Chun SY, Kim SY. et al. Surface immobilization of MEPE peptide onto HA/β-TCP ceramic particles enhances bone regeneration and remodeling. J Biomed Mater Res B Appl Biomater 2012; 100:841–9. [DOI] [PubMed] [Google Scholar]
- 116. Pan H, Zheng Q, Guo X. et al. Polydopamine-assisted BMP-2-derived peptides immobilization on bioderived copolymer scaffold for enhanced bone induction in vitro and in vivo. Colloids Surf B 2016; 142:1–9. [DOI] [PubMed] [Google Scholar]
- 117. Yang J, Khan M, Zhang L. et al. Antimicrobial surfaces grafted random copolymers with REDV peptide beneficial for endothelialization. J Mater Chem B 2015; 3:7682–97. [DOI] [PubMed] [Google Scholar]
- 118. Wojtowicz AM, Shekaran A, Oest ME. et al. Coating of biomaterial scaffolds with the collagen-derived peptide GFOGER for bone defect repair. Biomaterials 2010; 31:2574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hedberg EL, Tang A, Crowther RS. et al. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J Control Release 2002; 84:137–50. [DOI] [PubMed] [Google Scholar]
- 120. Balasundaram G, Shimpi TM, Sanow WR. et al. Molecular plasma deposited peptides on anodized nanotubular titanium: an osteoblast density study. J Biomed Mater Res Part A 2011; 98:192–200. [DOI] [PubMed] [Google Scholar]
- 121. Stile RA, Healy KE.. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules 2001; 2:185– 94. [DOI] [PubMed] [Google Scholar]
- 122. Cavalcanti-Adam EA, Shapiro IM, Composto RJ. et al. RGD peptides immobilized on a mechanically deformable surface promote osteoblast differentiation. J Bone Miner Res 2002; 17:2130– 40. [DOI] [PubMed] [Google Scholar]
- 123. Liu Q, Limthongkul W, Sidhu G. et al. Covalent Attachment of P15 Peptide to Titanium Surfaces Enhances Cell Attachment, Spreading, and Osteogenic Gene Expression. J Orthopaedic Res 2012; 30:1626–33. [DOI] [PubMed] [Google Scholar]
- 124. Luo ZY, Yang Y, Deng Y. et al. Peptide-incorporated 3D porous alginate scaffolds with enhanced osteogenesis for bone tissue engineering. Colloids Surf B 2016; 143:243– 51. [DOI] [PubMed] [Google Scholar]
- 125. Li JF, Lin ZY, Zheng QX. et al. Repair of rabbit radial bone defects using true bone ceramics combined with BMP-2-related peptide and type I collagen. Mater Sci Eng C 2010; 30:1272– 9. [Google Scholar]
- 126. Bergeron E, Marquis ME, Chretien I. et al. Differentiation of preosteoblasts using a delivery system with BMPs and bioactive glass microspheres. J Mater Sci Mater Med 2007; 18:255– 63. [DOI] [PubMed] [Google Scholar]
- 127. Chen C, Kong XD, Zhang SM. et al. Characterization and in vitro biological evaluation of mineral/osteogenic growth peptide nanocomposites synthesized biomimetically on titanium. Appl Surf Sci 2015; 334:62– 8. [Google Scholar]
- 128. Suh JS, Lee JY, Choi YJ. et al. Intracellular delivery of cell-penetrating peptide-transcriptional factor fusion protein and its role in selective osteogenesis. Int J Nanomed 2014; 9:1153– 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lindley EM, Guerra FA, Krauser JT. et al. Small peptide (P-15) bone substitute efficacy in a rabbit cancellous bone model. J Biomed Mater Res B Appl Biomater 2010; 94:463– 8. [DOI] [PubMed] [Google Scholar]
- 130. Sarahrudi K, Mousavi M, Grossschmidt K. et al. Combination of anorganic bovine-derived hydroxyapatite with binding peptide does not enhance bone healing in a critical-size defect in a rabbit model. J Orthop Res 2008; 26:759– 63. [DOI] [PubMed] [Google Scholar]
- 131. Bitschnau A, Alt V, Bohner F. et al. Comparison of new bone formation, implant integration, and biocompatibility between RGD-hydroxyapatite and pure hydroxyapatite coating for cementless joint prostheses-an experimental study in rabbits. J Biomed Mater Res B Appl Biomater 2009; 88:66– 74. [DOI] [PubMed] [Google Scholar]
- 132. Bergeron E, Leblanc E, Drevelle O. et al. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng A 2012; 18:342–52. [DOI] [PubMed] [Google Scholar]
- 133. Li J, Hong J, Zheng Q. et al. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. J Orthop Res 2011; 29:1745– 52. [DOI] [PubMed] [Google Scholar]
- 134. Sheller MR, Crowther RS, Kinney JH. et al. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res 2004; 22:1094– 9. [DOI] [PubMed] [Google Scholar]
- 135. Ma SQ, Wang KZ, Dang XQ. et al. Osteogenic growth peptide incorporated into PLGA scaffolds accelerates healing of segmental long bone defects in rabbits. J Plast Reconstr Aesthet Surg 2008; 61:1558– 60. [DOI] [PubMed] [Google Scholar]
- 136. Jung RE, Cochran DL, Domken O. et al. The effect of matrix bound parathyroid hormone on bone regeneration. Clin. Oral Impl. Res 2007; 18:319– 25. [DOI] [PubMed] [Google Scholar]