Abstract
Aliphatic biodegradable polyesters have been the most widely used synthetic polymers for developing biodegradable devices as alternatives for the currently used permanent medical devices. The performances during biodegradation process play crucial roles for final realization of their functions. Because physiological and biochemical environment in vivo significantly affects biodegradation process, large numbers of studies on effects of mechanical loads on the degradation of aliphatic biodegradable polyesters have been launched during last decades. In this review article, we discussed the mechanism of biodegradation and several different mechanical loads that have been reported to affect the biodegradation process. Other physiological and biochemical factors related to mechanical loads were also discussed. The mechanical load could change the conformational strain energy and morphology to weaken the stability of the polymer. Besides, the load and pattern could accelerate the loss of intrinsic mechanical properties of polymers. This indicated that investigations into effects of mechanical loads on the degradation should be indispensable. More combination condition of mechanical loads and multiple factors should be considered in order to keep the degradation rate controllable and evaluate the degradation process in vivo accurately. Only then can the degradable devise achieve the desired effects and further expand the special applications of aliphatic biodegradable polyesters.
Keywords: aliphatic biodegradable polyesters, mechanical load, degradation
Introduction
With the development of degradable biomaterials science during the last decades, biodegradable devices have been developed and investigated as alternatives for the currently used scaffolds, drug delivery system and permanent implanted devices for optimization purpose. Because of their good biodegradability and biocompatibility, aliphatic biodegradable polyesters, mainly including polyglycolic acid (PGA), polylactic acid (PLA) and their random block copolymers poly(lactide-co-glycolide) acid (PLGA), have been the most widely used synthetic degradable biomaterials for biodegradable devices approved by the US Food and Drug Administration [1–4] (Fig. 1).
Figure 1.
Structure of (a) PLA, (b) PGA and (c) PLGA
With respect to the chemical and mechanical properties [5–11] as shown in Table 1 and their good processabilities, PGA, PLA and PLGA have been developed for different prospective commercial applications. In the latter half of 1960s [12], aliphatic biodegradable polyesters were first utilized for synthetic biodegradable sutures. Since then, these polymers have been applied to fabricate temporary prostheses [13–17], 3D porous films and scaffolds [18–45] for tissue engineering, regenerative medicine, gene therapy and bionanotechnology, controlled/sustained release drug delivery system vehicles [46–64], surgical sutures and staples [65–67] for wound closure and implantable orthopedic fixation devices [68–70]. Particularly, as cardiovascular incidents are dramatically increasing, the applications in the field of heart patches [71] and percutaneous angioplasty and stenting treatment have been drawn more and more attention. As illustrated in Table 2, these polymers can be designed for coating drug-eluting stents (DESs) and manufacturing biodegradable stents (BDSs) [58, 72–85].
Table 1.
Chemical and mechanical properties of PGA, PLA and PLGA [5–11]
| PGA | PLLAa | PDLLAa | PLGA | |
|---|---|---|---|---|
| Crystallinity(%) | 45-55 | ∼37 | / | / |
| TM (°C) | >200 | ∼175 | / | / |
| Tg (°C) | 35–40 | 60–65 | 55–60 | / |
| Modulus(GPa) | 12.5 | ∼4.8 | 1.9 | / |
| Lose strength | 1–2 months | 2-5.6 years in vivo | 1–2 months | 50/50: 1–2 months |
| Mass loss | 6–12 months | 6–12 months | 75/25: 4–5 months | |
| 85:15: 5–6 months |
TM, melting point; Tg, glass transition temperature.
aAlthough PLA exists in four stereoisomeric forms: poly(L-lactic acid) (PLLA), poly(D-lactic acid)(PDLA), poly(D,L-lactic acid) (PDLLA) and meso-poly(lactic acid), only PLLA and PDLLA have been extensively used for biomedical applications so far.
Table 2.
| Stent name | Manufacturer | Stent platform | Polymer |
|---|---|---|---|
| Axxess | Devax Inc. | Nickel- titaniumNitinol | Bioabsorbable, abluminal PLA |
| Custom NX | Xtent | Cobalt-chromium | Bioabsorbable, PLA |
| Supralimus (Infinium stent) | Sahajan and Medical | Stainless steel | Bioabsorbable, containing poly-L- lactide,polyvinyl pyrrolidone, polylactide-co-caprolactone, and PLGA |
| Excel stent | JW Medical System | Stainless steel | Bioabsorbable, PLa |
| NEVO | Johnson & Johnson | Cobalt-chromium | Bioabsorbable, polylactide-co-glycolide |
| BioMime | Meril Life Science | Cobalt-chromium | PLLA + PLGA |
| BioMatrix | Biosensors | Stainless steel | Abluminal PLa |
| NOBORI | Terumo | Stainless steel | Abluminal PLA |
| Orsiro | Biotronik | Cobalt-chromium | PLLA + silicon carbide layer |
| DESyne BD | Elixir Medical) | Cobalt-chromium | PLA |
| AXXESS | Devax Inc. | Nitinol | Abluminal PLA |
| Elixir Myolimus | Elixir Medical | Cobalt-chromium | Abluminal PLA |
| JACTAX HD | Boston Scientific | Stainless steel | Biodegradable abluminal PLA polymer |
| CORACTO | ALVIMEDICA | Stainless steel | Polylactic-co-glycolic acid copolymer |
| DREAMS I& II | Biotronik | Mg | PLGA |
| Igaki-TamaiStent | Kyoto Medical Planning Co, Ltd | No | PLLA |
| AbsorbBVS 1.0& 1.1 | Abbott Vascular | No | PLLA |
| DESolve 1stgeneration DESolve2ndgeneration | Elixir Medical Corp. | No | PLLA |
| Amaranth | Amaranth Medical Inc. | No | PLLA |
| ART18ZBRS | Arterial Remodeling Tech., | No | PLLA,PDLA |
| XinsorbBRS | Shandong Hua An Biotech., Co. Ltd., | No | Poly-lactic acid, poly--caprolactone,poly-glycolicacid |
| AcuteBRS | Orbus Neich | No | PLLA,L-latic-co--caprolactone,PDLA |
A better understanding of the mechanism of biodegradation and factors affecting the degradation process is critical for the design and preparation of aliphatic biodegradable polyesters and optimization of biodegradable devices. As a biodegradable device, aliphatic biodegradable polyester is supposed to maintain suitable degradation rate, appropriate integrity and mechanical properties during the degradation process to match the rates of bone healing, drug release and tissue regeneration. However, during the maintenance, the degradation rates of aliphatic biodegradable polyesters are closely related to the complex physiological and biomechanical environment from internal and external. Extensive investigations have been launched during last twenty years in view of how physiological and biochemical environment in vitro and in vivo significantly affects biodegradation process [86–95]. The mechanical load is one of the most important factors that may cause the polymer degrade not as predetermined and lead to the devise fracture. It has drawn considerable attention recently when scientists are designing, preparing and optimizing implantable orthopedic fixation devises and cardiovascular BDSs. The uncontrollable degradation rate affected by unpredicted mechanical loads may cause the orthopedic fixation plates/screws and cardiovascular BDSs degrade too fast to keep the integrity and mechanical properties to match with the bone self-healing and vessel remodeling process, making the plates/screws or stents fracture before an expected life, which may result in bone refracture, blood vessel elastic recoil or distal vascular blockage by stent fragments. Hence, a lot of studies on effects of different mechanical loads on the degradation of aliphatic biodegradable polyesters have been carried out yet, but no systematic summary has been done.
The objective of this article is to outline the mechanism of biodegradation and several different mechanical loads that have been reported to affect the biodegradation process. Other physiological and biochemical factors related to mechanical loads will be also discussed.
Mechanism of biodegradation
It has been evidenced that there are hydrolytically labile chemical bonds in the backbone of PGA, PLA and PLGA, so these polymer primarily undergo bulk degradation in vivo via the chemical random scission of the hydrolytically unstable ester backbone into lactic acid or glycolic acid (GA) monomers, which can be broken down into carbon dioxide and water in the urine and eliminated from the body safely by the tricarboxylic acid cycle [96]. As shown in Fig. 2, the biodegradation process is elucidated to complete in four consecutive steps [97–100]: (i) Hydration. The aqueous medium penetrates the polymer matrix and disrupts the secondary forces, which lead to the relaxation and the decrease of the glass transition temperature [101]; (ii) Initial degradation. After hydrolysis, in the hydrated region of the polymer, the cleavage of the covalent bonds in the polymer backbone begins, resulting in the molecular weight decrease of the polymer. As hydrolysis goes on, the hydrolysis reaction inside the polymer matrix were auto-catalyzed by more and more carboxylic end-groups [102], leading to the continuously decrease of the molecular weight of the polymer. The polymer loses its mechanical strength along with the decrease of the molecular weight, but the integrity of the polymer maintains. (iii) Further degradation. The molecular weight of the polymer keeps falling to a threshold that the integrity of the polymer no longer can be held [97]. So, significant mass loss begins. (iv) Solubilization or erosion [103]. The polymer loses its weight and the fragments are further cleaved to molecular which are soluble in the medium [97].
Figure 2.

Schematic representation of hydrolytic degradation of polymer
Effects of mechanical loads
After implantation, the degradation rates of biodegradable medical devices such as orthopedic fixation devices, cardiovascular stents, grafts and heart valves which are composed of aliphatic biodegradable polyesters have been reported to be affected by various local and gross mechanical loads from different surrounding tissues, with conflicting results. On the contrary, the micro and macro structural, mechanical and morphological properties of aliphatic biodegradable polyesters have also been influenced during the degradation process.
Tensile, compressive and cyclic loads
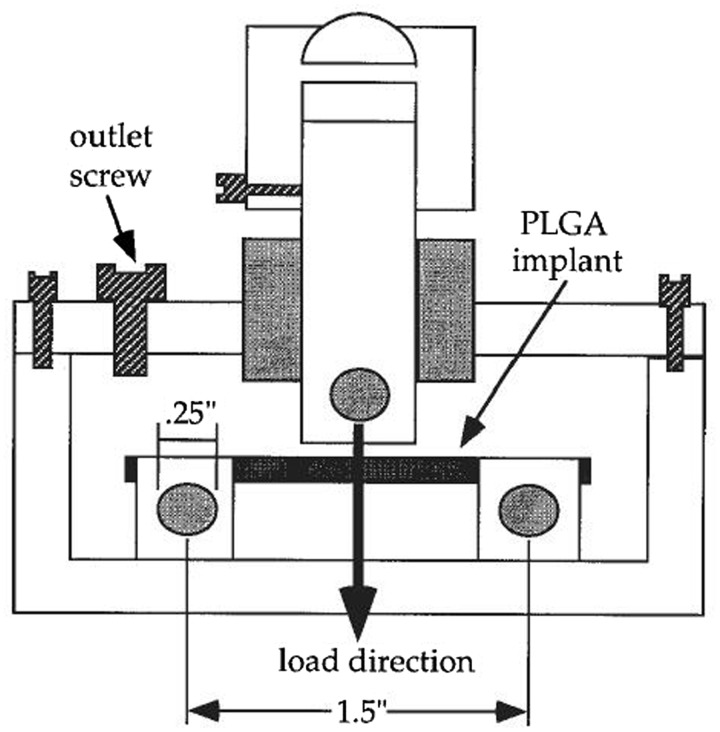
The effects of tensile, compressive and cyclic loads, as the most common types of mechanical loading in vivo, on the degradation process have been extensively investigated. Bikales [104] first proposed that mechanical stresses may accelerate the chain scission, crosslinking and other changes in biodegradable polymers’ chemical and physical properties. Otherwise, these changes may influence the mechanical properties of polymers substantially. Miller and William [105] demonstrated that the degradation rate of PGA sutures was dependent on the magnitude of a pre-imposed strain. As reported, the degradation of PGA sutures characterized by the changes in the tensile load at break was considerably enhanced both in vivo and in vitro by pre-straining the specimen to one half of the normal extension at break. Daniels [106] reported that the cyclical mechanical stress could accelerate the degradation rates of several polymers. Then a test methodology was developed for poly(ortho ester) to characterize the effect of a simulated mechanical and chemical body environment with aerated tris-buffered saline (pH 7.4 and 37°C) on the degradation rate, mainly focusing on the changes of the stress-strain behavior. The results showed that cyclic loading in air alone had no effect on the rate of the change of the mechanical property. However, the flexural yield strength decreased by 29% in static load group and 75% in cyclic loading group respectively, while the modulus of elasticity reduced to 80% and 25% of the original value in static load group and cyclic loading group separately after 40 days when specimens exposed to tris-buffered saline simultaneously. This is the first attempt to investigate multiple factors including pH, oxygen concentration, temperature and mechanical loads [107]. However, in contrary, the cyclic tensile loading presented no effect on the degradation of a PLA–PGA copolymer in Agrawal and Kennedy’s work [108]. Zhong et al. [109] found that 4% applied strain increased the degradation rate of a PLA/PGA copolymer compared with unloaded samples both in the water and hydrogen peroxide solution. Thompson et al. [110] examined the in vitro mechanical properties of a PLA/PGA (50/50) two phase implant under a cyclic compressive load over 6 weeks compared with no loading conditions. The dynamic compressive load collapsed the pores in the polymer matrix, resulting in a reduction in volume, so the more compact structure presented a smaller surface area for hydrolysis. Though the manifestation that the polymer underwent a surface deformation to be more stiffness occurred, there was a threshold that the polymer could no longer maintain the mechanical properties and started to collapse as hydrolysis broke down the polymer chains. A cyclic three-point bending loading of 720 cycles/day at 0.4 Hz for 2 weeks was conducted by Arm and Tencer [111] utilized a self-design chamber shown in Fig. 3 to biodegradable PLGA cylindrical implants. But there was no significant change in their mass loss nor swelling and molecular weight during the period. Remarkably, the superficial pores in the highest stress region were elongated into cracks. This demonstrated that the pores probably acted as stress risers to initiate cracks. Besides, the pore and crack density was greater for loaded implants, but no relation with the magnitude of deformation was found. Fan et al. [86] investigated the mechanism of how the different continuous loads affected the hydrolytic degradation of poly(D,L-lactic acid) (PDLLA) foam gasket in phosphate-buffered saline (PBS) solution (pH 7.4 at 37°C) by the self-made load-providing devices shown in Fig. 4. Two different magnitudes of tensile loads (15 N and 25 N) combined with 0 and 100 N compressive loads were used to mark the changes of the surface morphology, molecular weight, elastic modulus, tensile strength and mass loss when compared with those with no load. Within 3-month observation, it has been concluded that the mechanical load played an important role in accelerating the degradation rate. The load-induced degradation rate of polymers was faster than the rate of unloaded ones and the combinative load affected the rate more distinctly. The changes in Morphologies of PDLLA were shown in Fig. 5. Afterward, similar work about the degradation behavior of porous PLLA/β-TCP and PLGA/β-TCP composite scaffolds under the dynamic loading and static condition in PBS solution (pH 7.4 at 37°C) for 12 weeks was examined by Kang [87] and Yang [24]. The dynamic loading condition accelerated the degradation process with respect to more rapid reductions in mass, height, diameter and number-average relative molecular mass compared with that under the static conditions with no stress. Similarly, with the same methods, the cyclic loading was also found to accelerate the in vitro degradation of porous PLGA scaffolds incubated in PBS solution (pH 7.4 at 37°C) for 12 weeks, accounting for the faster mass loss, dimensions and shape change, morphological variations and reduction in mechanical properties [88]. After that, Li et al. [89] demonstrated that the tensile elastic modulus and ultimate strength of electrospun PLGA scaffolds in tensile loaded group decreased faster than that with no load, after a dramatical increase in both groups, during the 7-week degradation in PBS solution (pH 7.4 at 37°C). Moreover, changes in their molecular weight, thermal properties, lactic acid release and morphology property indicted the tensile loading accelerate the degradation rate. In addition, Zhao et al. [90] reported the accelerated degradation of electrospun PLLA membranes when subjected to the cyclic stretch loading in Tris-HCl buffer solution containing proteinase K. Furthermore, a quantitative investigation of the tensile stress and in vitro degradation rate of PLGA membranes has been conducted by Guo et al. [91]. Tensile stress in levels of 0.1–0.5 MPa and deionized water was applied. As the magnitude of tensile stress increased, more loss in the mass and mechanical properties, elastic modulus and tensile strength, were observed.
Figure 3.
Schematic diagram of a chamber used to load a PLGA implant in three-point bending. The implant rests on two roller end supports and is loaded at its center, vertically downward by a plunger. The magnitude of the plunger displacement can be varied. (Reproduced from ref. [111], with permission from Wiley)
Figure 4.
Self-made load-providing devices: (a) tensile load-providing device; (b) compressive load-providing device and (c) tensile-compressive combined load providing device. (Reproduced from ref. [86], with permission from Elsevier)
Figure 5.
Morphologies of PDLLA before and after degradation (magnification of 200×) Part (A): tensile loaded (15 N) and compressive loaded (100 N): (a) before degradation; unloaded degradation after (b) 1 month, (c) 2 months and (d) 3 months; tensile loaded degradation after (e) 1 month, (f) 2 months and (g) 3 months; tensile-compressive combined loaded degradation after (h) 1 month, (i) 2 months and (j) 3 months. Part (B): tensile loaded (25 N) and compressive loaded (100 N):(a) before degradation; unloaded degradation after (b) 1 month, (c) 2 months and (d) 3 months; tensile loaded degradation after (e) 1 month, (f) 2 months and (g) 3 months; tensile-compressive combined loaded degradation after (h) 1 month, (i) 2 months and (j) 3 months. (Reproduced from ref. [86], with permission from Elsevier)
Fluid shear stress
Fluid shear stress is one type of the main mechanical loadings generated by fluid flow and also has been proved to be effective to the degradation rate. Agrawal et al. [92] examined the effects of fluid flow of 0.25 ml/min on the in vitro degradation characteristics and kinetics of PLA-PGA scaffolds with different porosity and permeability in PBS solution (pH 7.4 at 37°C) for up to 6 weeks. The changes in mass, molecular weight and elastic modulus indicated that the increasement of porosity/permeability and fluid flow could decrease the degradation rate significantly. This can be attributed to the mass transportation of fluid flow and the autocatalysis of the degradation reaction generated by the acidic degradation products, although the fluid shear stress is too small and negligible. Besides, a much clearer comparative study was done by Huang et al. [93] on the degradation of PLGA 50/50 cylinder subjected to Hank’s simulated body fluid (Hank’s SBF) under static and body fluid flow condition. Significant decrease of weight-average molecular weight began rapidly in static SBF but this happened until 10 days in the dynamic system. Moreover, significant mass loss occurred from 20 days in the static condition while little changed in the dynamic one during the 30 days. With respect to the morphology change, a slower degradation rate in the dynamic system was indicated. Furthermore, Chu et al. [94] did a series of quantitative work on the effect of different steady fluid shear stresses on the degradation of PLGA in deionized water (pH 7.4 at 37°C) for 20 days. The viscosity of the degradation solution in the loaded condition subjected to fluid shear stress was more severely affected. Raising the fluid shear stress could speed up the loss of ultimate strength and slowed down the decrease of tensile elastic modulus as well. Similarly, the fluid shear stress did have effect on the morphology change as shown in Fig. 5. Subsequently, the effect of different patterns of fluid shear stress on the degradation was investigated [95]. Steady, sinusoid and squarewave fluid shear stress with the same average magnitude and the different maximum fluid shear stress and ‘window’ of effectiveness were applied. The results showed that the maximum fluid shear stress accelerated the loss of molecular fragments in the solution while the ‘window’ of effectiveness affected as well in the early stage. In addition, the maximum fluid shear stress and ‘window’ of effectiveness accelerated the reduction of tensile modulus and ultimate strength while the maximum fluid shear stress acted the leading role in the decrease of tensile modulus at the early degradation stage. However, there was no clear evidence showing that different patterns of fluid shear stress influenced the morphology property (Fig. 6).
Figure 6.
PLGA morphology before and after degradation with different fluid shear stress (magnification of 300×). (a) before degradation. (b–e) unloaded degradation after (b) 5 days, (c) 10 days, (d) 15 days and (e) 20 days. (f–i) at a fluid shear stress of 12 dyn/cm2 after (f) 5 days, (g) 10 days, (h) 15 days and (i) 20 days. (j–m) at a fluid shear stress of 30 dyn/cm2 after (j) 5 days, (k) 10 days, (l) 15 days and (m) 20 days. (Reproduced from ref. [94], with permission from Wiley)
Factors related to mechanical loads
It’s worth noting that only the factor of mechanical loads in all researches aforementioned was considered due to single factor analysis method. But it is well known that the degradation rates are difficult to be ideal because of the inherent properties and complex environmental factors in vivo. The degradation process suffers a combined impact of mechanical loads and these factors. So understand the effect of each variable on the degradation rate is the foundation to evaluate the degradation process in vivo under the condition of multiple factors.
Inherent physical factors
Accordingly, several inherent properties are important factors that affecting the degradation rate, including the copolymer composition, molecular weight, shape, and indirect factors of glass transition temperature and crystallinity which are dependent on the copolymer composition.
Copolymer ratio
Miller et al. [112–113] first examined the rate modification with the changes in copolymer ratios and confirmed that PLGA 50/50 was very hydrolytically unstable. After that, Park [114] prepared a wide range of PLGA microspheres with different copolymer compositions with no active ingredients. The degradation behaviors of PDLLGA 90/10, PDLLGA 80/20, PDLLGA70/30, PDLLGA50/50 and PDLA were compared in an Eppendorf centrifuge tube incubated at 37°C with PBS up to 53 days. As reported, the hydrolytic scission preferentially occurs between the ester bonds linked with the GA unit (glycolic–glycolic acid or glycolic–lactic acid).Similarly, Wang and Wu [115] studied the degradation process of three different PLGA samples with the ratio of 46/54, 65/35 and 72/28. The results showed a positive correlation between the mass loss and increase of GA residue in the oligomers. Afterwards, they [116] reported a systematic study of the effect of copolymer composition. With similar molecular weights, PLGA 50/50, 65/35, 75/25, 85/15 and PLLA were compared. The absolute value of the biodegradation rate constants were evidenced to rise with increasing the GA content. This is in clear agreement with the results reported by Li [117]. In summary, due to the great hydrophilicity, the ester bonds linked with GA unit affect the degradation rate and there is a positive correlation between the content and the rate.
Molecular weight
Park [114] also examined the degradation behaviors of two PDLA microspheres with molecular weight of 17 and 41 kDa respectively. The results exhibited that the degradation behaviors were greatly depended on the molecular weight of raw PDLA during the 53-day incubation. Microspheres with the lower molecular weight showed a significant degradation with reduced Tg. However, because of their glassy state, microspheres with the higher molecular weight show no detectable change during in the 53 days’ degradation. Wang et al. [118] investigated the effect of molecular weights of 1317 and 3025 Da on the biodegradation of two different LGA oligomers 72/28 in tubes incubated at 37°C with PBS (pH 7.4) shaking at 30 rpm. A slower weight loss of LGA oligomer with the higher molecular weight was found than that having the lower molecular weight counterpart. On the contrary, Cam et al. [119] used four PLLAs with different molecular weights of 300, 450, 650 and 3000 kDa to study the effect of molecular weight on degradation in 0.01 NNaOH alkaline solution (pH 11.8) at 37°C. The crystallinity of samples decreased from 30 to 3% with an increase in molecular weight. The films had higher molecular weight prior to hydrolysis and degraded at a higher rate. Another study done by Wu and Wang [116] investigated a group of PLGAs with the same composition of 75/25 but different molecular weights of 12 876, 31 403, 66 946, 124 450 and 166 630 Da, respectively. The first order biodegradation reaction rate constant observed were 0.0472, 0.0681, 0.0834, 0.0961 and 0.0969 day−1separately. After the initial stage, PLGA with higher molecular weights degraded faster than those with lower ones. All above, the molecular weight has a considerable effect on the biodegradation rate in three ways. First, lower molecular weight polymers have more carboxylic end groups per unit weight and are more hydrophilic than higher molecular weight counterpart. Second, the Tg is frequently influenced by molecular weight. Higher molecular weight polymers usually have higher Tg than 37°C [120]. Third, the higher molecular weight polymers have longer polymer chains. The chances being attacked by water molecules is increased because of the longer chains [121].
Shape
Li et al. [122–126] investigated the degradation of PLA and PDLLA parallelepiped devises and found, for the first time, that the degradation process was significantly faster in the inner part than at the surface both in vivo and in vitro [127]. Grizzi et al. [128] reported that instruments with dimensions smaller than the thickness of the more stable outer layer could degrade slower than larger ones and they testified this hypothesis on compression moulded plates, millimetric beads and submillimetric microspheres and cast films. A critical thickness of 200–300 μm was proposed. Similarly, Witt and Kissel [129] compared the degradation rates of microspheres, films, rods and tablets with different dimensions but the same material of PLGA 50/50, and the apparent constant rate of degradation were shown to be 0.041, 0.093, 0.115 and 0.1035 day−1, respectively. Lu et al. [130] also reported that thick films degraded faster than thin ones and indicated that the degradation rate of porous foams could be designed by differing the pore wall thickness and pore surface/volume ratio [131] for the use of tissue engineering scaffolds. He and Xiong [27] investigated the in vitro degradation process of three-dimensional porous and films made from PLGA 85/15 and demonstrated that the films degraded much faster. It can be reasonably concluded that, due to acid catalysis of carboxylic end groups, the degradation rate of aliphatic biodegradable polyesters can be affected by shape.
Environmental factors
Some biochemical environmental factors such as pH value and temperature were evidenced to affect the rate as well. Belbella et al. [132] proved that degradation of PDLLA was related to the pH value (pH value of 2.2, 4.2, 6.0, 7.4, 8.4 and 10.1 were used) and the hydrolysis was much more catalysed at acidic and alkaline pH than at neutral one. Wang et al. [118] found that the degradation of the LGA oligomer 72/28 is faster in phosphate buffer (pH 7.4, 0.2 M) than in Na2B2O7 10 H2O buffered solution (pH 9.4, 0.1 M). Holy et al. [133] demonstrated that the rate of macroporous PLGA 75/25 was much faster in pH 5.0 than in pH 6.4 and 7.4 after 16 weeks of in vitro degradation. Wu and Wang [116] also examined the degradation of PLGA 50/50 with a weight-average molecular weight of 13134 D in three different buffers including pH 5.0 phosphate buffer (0.2 M), pH 7.4 phosphate buffer (0.2 M) and pH 9.24 sodium borate buffer (0.1 M). The results showed that the biodegradation rate decreased when the pH was 9.24 while increased in an acidic one (pH 5.0) from the third week. This is in agreement with the result reported by Yoo [134].This can be concluded that aliphatic biodegradable polyesters degrade faster in acidic medium than in alkaline or neutral one. 37 and 100°C were applied by Jamishidi [135] to study the effect of temperature on the degradation behavior of PLLA fibers in PBS. The tensile strength was observed reducing to half at 100°C after 10 h while no changes was observed at 37°C. In agreement, Aso et al. [136] reported that the molecular weight of PDLLA discs and microspheres decreased rapidly at 50°C. In Belbella’s work [132], the degradation of PDLLA nanospheres at pH 7.4 was much faster at 37°C than at 4 and −18°C. In addition, Hakkarainen et al. [137] also reported a dramatic acceleration of degradation of PLLA and PLGAs at 60°C. As such, the degradation rate of aliphatic biodegradable polyesters is highly dependent on the temperature, especially when it is higher than the glass transition temperature of polymers. Deng [138, 139] also found that an elevated temperature would accelerate the degradation process of 90/10 poly(glycolide-co-L-lactide) multifilament braids in PBS solution.
Besides, other environment factors including the addition of drug [140–143], sterilization [144–147] and enzymes [148–157] and so on are reviewed by Alexis [121] and a lot of these facts presented controversial results in so far.
Conclusion and prospects
In general, though the mechanical load may not be able to initiate the degradation process independently, it is reasonable to conclude that the mechanical load can influence the degradation of aliphatic biodegradable polyesters. The mechanical load can get the polymer extended for more cavities. Therefore the water molecular can be much easier to diffuse into the inner part to scissor the chain segments, leading to a faster hydrolysis. Then, under the action of stretch or compression, the conformational strain energy change might change the length or angle of the bonds, resulting in weakening of the stability. Furthermore, the load could affect the intrinsic mechanical properties of the polymer. Besides, the fluid shear stress of different patterns with the maximum fluid shear stress and the ‘window’ of effectiveness could accelerate the loss of ultimate strength and delay the decrease of tensile elastic modulus. The conclusions all above indicated that investigations into the effects of mechanical loads on the degradation should be very indispensable for appropriately designing and preparing not only aliphatic biodegradable polyesters but also other biodegradable polymers for targeted applications.
Till date various studies about one of the various physiological and biochemical factors have been carried out. However, the degradation rates of aliphatic biodegradable polyesters suffer a combined impact of mechanical loads and other complex inherent and environmental factors in vivo. It can be anticipated that more in vivo experiments on the degradation behavior under a single kind of mechanical loads and more combination condition of mechanical loads and multiple factors should be considered during the elucidating process of the degradation behavior in future in vitro work.
It is much urgent to propose the mechanism of degradation of aliphatic biodegradable polyesters affected by combined factors both in vitro and in vivo, which is the foundation to keep the degradation rate controllable and evaluate the degradation process in vivo accurately. Only then can the degradable devise achieve the desired effects and further expand the special applications of aliphatic biodegradable polyesters.
Funding
This work was supported by the National Key Technology R&D Program (Nos. 2014BAI11B02, 2014BAI11B03, 2012BAI18B01), National Natural Science Foundation of China (Nos. 11120101001, 11421202, 31370959, 11572029, 31470915), National key research and development program in China (No. 2016YFC1100704, 2016YFC1102202, 2016YFC1101100), Beijing Nova Programme Interdisciplinary Cooperation Project (No. xxjc201616), Key Laboratory of Advanced Materials of Ministry of Education of China (Tsinghua University), Fok Ying Tung Education Foundation (No. 141039) and International Joint Research Center of Aerospace Biotechnology and Medical Engineering, Ministry of Science and Technology of China, and the 111 Project (No. B13003).
Conflict of interest statement. None declared.
References
- 1. Kohn J, Langer R.. Bioresorbable and bioerodible materials In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds). Biomaterials Science: An Introduction to Materials in Medicine. San Diego, CA: Academic Press, 1996,64–73. [Google Scholar]
- 2. Piskin E. Biodegradable polymers as biomaterials. J Biomat Sci Polym Ed 1995;6:775–95. [DOI] [PubMed] [Google Scholar]
- 3. Domb AJ, Wiseman DM, eds. Handbook of Biodegradable Polymers. Boca Raton: CRC Press, 1998. [Google Scholar]
- 4. Shalaby SW, Burg KJL, eds. Absorbable and Biodegradable Polymers (Advances in Polymeric Materials). Boca Raton: CRC press, 2003. [Google Scholar]
- 5. Maurus PB, Kaeding CC.. Bioabsorbable implant material review. Oper Tech Sport Med 2004;12:158–60. [Google Scholar]
- 6. Nair LS, Laurencin CT.. Biodegradable polymers as biomaterials. Prog Polym Sci (Oxford) 2007;32:762–98. [Google Scholar]
- 7. Middleton JC, Tipton AJ.. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000;21:2335–46. [DOI] [PubMed] [Google Scholar]
- 8. Bergsma JE, Rozema FR, Bos RR. et al. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polyactide particles. Biomaterials 1995;16:267–74. [DOI] [PubMed] [Google Scholar]
- 9. Middleton JC, Tipton AJ.. Synthetic biodegradable polymers as medical devices. Med Plast Biomater 1998;31–8. [DOI] [PubMed] [Google Scholar]
- 10. Ulery BD, Nair LS, Laurencin CT.. Biomedical applications of biodegradable polymers. J Polym Sci B Polym Phys 2011;49:832–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silva ATCR, Cardoso BCO, Freitas RFS. et al. Synthesis, Characterization, and Study of PLGA Copolymer, in vitro, Degradation. J Biomater Nanobiotechnol 2015;6:8–19. [Google Scholar]
- 12. Barbucci R. Integrated Biomaterial Science. New York: Kluwer Academic/Plenum Publishers, 2002. [Google Scholar]
- 13. Angelova N, Hunkeler D.. Rationalizing the design of polymeric biomaterials. Trends Biotechnol 1999;17:409–21. [DOI] [PubMed] [Google Scholar]
- 14. Dee KC, Puleo DA, Bizios R, eds. An Introduction to Tissue Biomaterial Interactions. New York: John Wiley and Sons, 2002. [Google Scholar]
- 15. Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, eds. Biomaterials Science: An Introduction to Materials in Medicine. Amsterdam: Elsevier Academic Press, 2004. [Google Scholar]
- 16. Neut D, Dijkstra RJ, Thompson JI. et al. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur Cells Mater 2015;29:42–56. [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Liu T, Liu S. et al. Feasibility of biodegradable PLGA common bile duct stents: an in vitro and in vivo study. J. Mater. Sci. 2009;20:1167–73. [DOI] [PubMed] [Google Scholar]
- 18. Freed LE, Gordana VN, Langer R.. Biodegradable polymer scaffolds for tissue engineering. Biotechnology 1994;12:689–93. [DOI] [PubMed] [Google Scholar]
- 19. Chu CC. Biodegradation properties In: Chu CC, von Fraunhofer JA, Greisler HP (eds). Wound Closure Biomaterials and Devices. Boca Raton, FL: CRC Press, 1997, 182–83. [Google Scholar]
- 20. Peter SJ, Miller MJ, Yasko AW. et al. Polymer concepts in tissue engineering. J Biomed Mater Res 1998;43:422–7. [DOI] [PubMed] [Google Scholar]
- 21. Holy CE, Shoichet MS, Davies JE.. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. J Biomed Mater Res 2000;51:376–82. [DOI] [PubMed] [Google Scholar]
- 22. Riddle KW, Mooney DJ.. Role of poly(lactide-co-glycolide) particle size on gas-foamed scaffolds. J Biomater Sci Polym Ed 2004;15:1561–70. [DOI] [PubMed] [Google Scholar]
- 23. Yue H, Zhang L, Wang Y. et al. Proliferation and differentiation into endothelial cells of human bone marrow mesenchymal stem cells (MSCs) on poly-dl-lactic-co-glycolic acid (PLGA) films. Chinese Sci Bull 2006;51:1328–33. [Google Scholar]
- 24. Yang Y, Zhao Y, Tang G. et al. In vitro degradation of porous poly(Llactide-co-glycolide)/b-tricalcium phosphate (PLGA/b-TCP) scaffolds under dynamic and static conditions. Polym Degrad Stab 2008;93:1838–45. [Google Scholar]
- 25. Yu D, Li Q, Mu X. et al. Bone regeneration of critical calvarial defect in goat model by PLGA/TCP/rhBMP-2 scaffolds prepared by low-temperature rapid-prototyping technology. Int J Oral Max Surg 2008;37:929–34. [DOI] [PubMed] [Google Scholar]
- 26. Hu X, Shen H, Yang F. et al. Preparation and cell affinity of microtubular orientation-structured PLGA (70/30) blood vessel scaffold. Biomaterials 2008;29:3128–36. [DOI] [PubMed] [Google Scholar]
- 27. He ZQ, Xiong LZ.. A comparative study on in vitro degradation behaviors of poly(l-lactide-co-glycolide) scaffolds and films. J Macromol Sci Part B 2010;49:66–74. [Google Scholar]
- 28. Li XM, Yang Y, Fan YB. et al. Biocomposites reinforced by fibers or tubes, as scaffolds for tissue engineering or regenerativemedicine. J Biomed Mater Res Part A 2014;102:1580–94. [DOI] [PubMed] [Google Scholar]
- 29. Li XM, Wang Z, Zhao TX. et al. A novel method to in vitro evaluate biocompatibility of nanoscaled scaffolds for tissue engineering with neutrophils. J Biomed Mater Res Part A 2016;104:2117–25. [DOI] [PubMed] [Google Scholar]
- 30. Ma PX, Choi JW.. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng 2001;7:23–33. [DOI] [PubMed] [Google Scholar]
- 31. Karp JM, Shoichet MS, Davies JE.. Bone formation on two-dimensional poly(DL-lactide-co-glycolide) (PLGA) films and three-dimensional PLGA tissue engineering scaffolds in vitro. J Biomed Mater Res Part A 2003;64:388–96. [DOI] [PubMed] [Google Scholar]
- 32. Wu L, Ding J.. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004;25:5821–30. [DOI] [PubMed] [Google Scholar]
- 33. Wang SG, Wan YQ, Cai Q. et al. Molecular design of synthetic biodegradable polymers as cell scaffold materials. Chem Res Chin Univ 2004;20:191–4. [Google Scholar]
- 34. Sung HJ, Meredith C, Johnson C. et al. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004; 25:5735–42. [DOI] [PubMed] [Google Scholar]
- 35. Wu L, Ding J.. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. J Biomed Mater Res Part A 2005;75:767–77. [DOI] [PubMed] [Google Scholar]
- 36. Oh SH, Kang SG, Jin HL.. Degradation behavior of hydrophilized PLGA scaffolds prepared by melt-molding particulate-leaching method: Comparison with control hydrophobic one. J Mater Sci 2006;17:131–7. [DOI] [PubMed] [Google Scholar]
- 37. Yang F, Cui WJ, Xiong Z. et al. Poly (l, l-lactide-co-glycolide)/tricalcium phosphate composite scaffold and its various changes during degradation in vitro. Polym Degrad Stab 2006;91:3065–73. [Google Scholar]
- 38. Kang YQ, Xu XJ, Yin GF. et al. A comparative study of the in vitro, degradation of poly (l -lactic acid)/β-tricalcium phosphate scaffold in static and dynamic simulated body fluid. Eur Polym J 2007;43:1768–78. [Google Scholar]
- 39. Yoshioka T, Kawazoe N, Tateishi T. et al. In vitro evaluation of biodegradation of poly(lactic- co -glycolic acid) sponges. Biomaterials 2008;29:3438–43. [DOI] [PubMed] [Google Scholar]
- 40. Zhao W, Li J, Jin K. et al. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater Sci Eng C 2015;59:1181–94. [DOI] [PubMed] [Google Scholar]
- 41. Li XM, Feng QL.. Dynamic rheological behaviors of the bone scaffold reinforced by chitin fibres. Mater Sci Forum 2005;475-479:2387–90. [Google Scholar]
- 42. Li XM, Liu HF, Niu XF. et al. The use of carbon nanotubes to induce osteogenic differentiation of human adipose-derived MSCs in vitro and ectopic bone formation in vivo. Biomaterials 2012;33:4818–27. [DOI] [PubMed] [Google Scholar]
- 43. Li XM, Wang L, Fan YB. et al. Nanostructured scaffolds for bone tissue engineering. J Biomed Mater Res Part A 2013;101A:2424–35. [DOI] [PubMed] [Google Scholar]
- 44. Li XM, Huang Y, Zheng LS. et al. Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro. J Biomed Mater Res Part A 2014;102A:1092–101. [DOI] [PubMed] [Google Scholar]
- 45. Li XM, Liu W, Sun LW. et al. Effects of physicochemical properties of nanomaterials on their toxicity. J Biomed Mater Res Part A 2015;103A:2499–507. [DOI] [PubMed] [Google Scholar]
- 46. Cohen S, Yoshioka T, Lucarelli M. et al. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res 1991;8:713–20. [DOI] [PubMed] [Google Scholar]
- 47. Park TG, Lu WQ, Crotts G.. Importance of in vitro experimental conditions on protein release kinetics, stability and polymer degradation in protein encapsulated poly(d,l-lactic-co-glycolic acid) microspheres. J Control Release 1995;33:211–22. [Google Scholar]
- 48. Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000;21:2475–90. [DOI] [PubMed] [Google Scholar]
- 49. Friess W, Schlapp M.. Release mechanisms from gentamicin loaded poly(lactic-co-glycolic acid) (PLGA) microparticles. J Pharm Sci 2002;91:845–55. [DOI] [PubMed] [Google Scholar]
- 50. Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier Syst 2002;19:73–98. [DOI] [PubMed] [Google Scholar]
- 51. Taluja A, Yu SY, You HB.. Novel approaches in microparticulate PLGA delivery systems encapsulating proteins. J Mater Chem 2007;17:4002–14. [Google Scholar]
- 52. Semete B, Booysen L, Lemmer Y.. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed Nanotechnol Biol Med 2010;6:662–71. [DOI] [PubMed] [Google Scholar]
- 53. Li XM, Zhao TX, Sun LW. et al. The applications of conductive nanomaterials in the biomedical field. J Biomed Mater Res Part A 2016;104:320–37. [DOI] [PubMed] [Google Scholar]
- 54. Li XM, Wei JR, Aifantis KE. et al. Current investigations into magnetic nanoparticles for biomedical applications. J Biomed Mater Res Part A 2016;104:1285–96. [DOI] [PubMed] [Google Scholar]
- 55. Santoveña A, Alvarez-Lorenzo C, Concheiro A. et al. Rheological properties of PLGA film-based implants: correlation with polymer degradation and SPf66 antimalaric synthetic peptide release. Biomaterials 2004;25:925–31. [DOI] [PubMed] [Google Scholar]
- 56. Galeska I, Kim TK, Patil SD. et al. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J 2005;7:E231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Estey T, Kang J, Schwendeman SP. et al. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J Pharm Sci 2006;95:1626–39. [DOI] [PubMed] [Google Scholar]
- 58. Westedt U, Wittmar M, Hellwig M. et al. Paclitaxel releasing films consisting of poly(vinyl alcohol)-graft-poly(lactide-co-glycolide) and their potential as biodegradable stent coatings. J Control Release 2006;111:235–46. [DOI] [PubMed] [Google Scholar]
- 59. Houchin ML, Neuenswander SA, Topp EM.. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide. J Control Release 2007;117:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patterson J, Stayton PS, Li X.. In situ characterization of the degradation of PLGA microspheres in hyaluronic acid hydrogels by optical coherence tomography. IEEE Trans Med Imaging 2009;28:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu L, Xie S, Dong Z. et al. Effects of poly(lactic-co-glycolic acid) on preparation and characteristics of plasmid DNA-loaded solid lipid nanoparticles. Iet Nanobiotechnol 2011;5:79–85. [DOI] [PubMed] [Google Scholar]
- 62. Klose D, Delplace C, Siepmann J.. Unintended potential impact of perfect sink conditions on PLGA degradation in microparticles. Int J Pharm 2011; 404:75–82. [DOI] [PubMed] [Google Scholar]
- 63. Keles H, Naylor A, Clegg F. et al. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym Degrad Stab 2015;119:228–41. [Google Scholar]
- 64. Tesfamariam B. Bioresorbable vascular scaffolds: Biodegradation, drug delivery and vascular remodeling. Pharmacol Res 2016;107:163–71. [DOI] [PubMed] [Google Scholar]
- 65. Benicewicz BC, Hopper PK.. Polymers for absorbable surgical sutures. J Bioact Compat Polym 1991;6:64–94. [Google Scholar]
- 66. Zacchi V, Soranzo C, Cortivo R. et al. In vitro engineering of human skin-like tissue. J Biomed Mater Res 1997;36:17–28. [DOI] [PubMed] [Google Scholar]
- 67. Khorsand-Ghayeni M, Sadeghi A, Nokhasteh S, Molavi AM. Collagen modified PLGA nanofibers as wound-dressing. In: The International Conference on Nanostructures; 2016.
- 68. Gibbons DF. Tissue response to resorbable synthetic polymers In: Plank H, Dauner M, Renardy M (eds). Degradation Phenomena on Polymeric Biomaterials. New York: Springer, 1992, 97–104. [Google Scholar]
- 69. Kleinschmidt JC, Marden LJ, Kent D. et al. A multiphase system bone implant for regenerating the calvaria. Plast Reconstr Surg 1993;91:581–8. [DOI] [PubMed] [Google Scholar]
- 70. Ishaug-Riley SL, Crane GM, Gurlek A. et al. Ectopic bone formation by marrow stromal osteoblast transplantation using poly(dl-lactic-coglycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res 1997;36:1–8. [DOI] [PubMed] [Google Scholar]
- 71. Tallawi M, Zebrowski DC, Rai R. et al. Poly(glycerol sebacate)/poly(butylene succinate-dilinoleate) (PGS/PBS-DLA) fibrous scaffolds for cardiac tissue engineering. Tissue Eng C 2015;21:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang XT, Venkatraman SS, Boey FYC. et al. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials 2006;27:5588–95. [DOI] [PubMed] [Google Scholar]
- 73. Zhu XX, Braatz RD.. Modeling and analysis of drug-eluting stents with biodegradable PLGA coating: Consequences on intravascular drug delivery. J Biomech Eng 2014;136:111004-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu XX, Braatz RD.. A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion. J Biomed Mater Res Part A 2014;103:2269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jain AK, Lotan C, Meredith IT. et al. Twelve-month outcomes in patients with diabetes implanted with a zotarolimus-eluting stent: results from the E-Five Registry. Heart 2010;96:848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Guagliumi G, Ikejima H, Sirbu V. et al. Impact of drug release kinetics on vascular response to different zotarolimus-eluting stents implanted in patients with long coronary stenoses: the LongOCT Study (Optical Coherence Tomography in Long Lesions). JACC Cardiovasc Interv 2011;4:778–85., [DOI] [PubMed] [Google Scholar]
- 77. Basalus MW, Tandjung K, van Houwelingen KG. et al. TWENTE Study: The Real-World Endeavor Resolute Versus Xience V Drug-Eluting Stent Study in Twente: study design, rationale and objectives. Netherlands Heart Journal 2010;18:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chevalier B, Di Mario C, Neumann FJ. et al. A randomized, controlled, multicenter trial to evaluate the safety and efficacy of zotarolimus-versus paclitaxel-eluting stents in de novo occlusive lesions in coronary arteries: the ZoMaxx I trial. JACC Cardiovasc Interv 2008;1:524–32. [DOI] [PubMed] [Google Scholar]
- 79. Waseda K, Hasegawa T, Ako J. et al. Comparison of vascular response to zotarolimuseluting stent vs paclitaxel-eluting stent implantation: pooled IVUS results from the ZoMaxx I and II trials. Circ J 2010;74:2334–9. [DOI] [PubMed] [Google Scholar]
- 80. Waseda K, Ako J, Yamasaki M. et al. Impact of polymer formulations on neointimal proliferation after zotarolimus-eluting stent with different polymers: insights from the RESOLUTE, trial. Circ Cardiovasc Interv 2011;4:248–55. [DOI] [PubMed] [Google Scholar]
- 81. Kereiakes DJ, Cannon LA, Ormiston JA. et al. Propensity-matched patientlevel comparison of the TAXUS Liberte and TAXUS Element (ION) paclitaxeleluting stents. Am J Cardiol 2011;108:828–37. [DOI] [PubMed] [Google Scholar]
- 82. Martin DM, Boyle FJ.. Drug-eluting stents for coronary artery disease: a review. Med Eng Phys 2011;33: 148–63. [DOI] [PubMed] [Google Scholar]
- 83. Khan W, Farah S, Domb AJ.. Drug eluting stents: developments and current status. J Control Release 2012;161:703–12. [DOI] [PubMed] [Google Scholar]
- 84. Wiebe J, Nef HM, Hamm CW.. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol 2014;64:2541–51. [DOI] [PubMed] [Google Scholar]
- 85. Huang Y, Ng HC, Ng XW. et al. Drug-eluting biostable and erodible stents. J Control Release 2014;193:188–201. [DOI] [PubMed] [Google Scholar]
- 86. Fan YB, Li P, Zeng L. et al. Effects of mechanical load on the degradation of poly(D,L-lactic acid) foam. Polym Degrad Stab 2008;9:677–83. [Google Scholar]
- 87. Kang Y, Yao Y, Yin G. et al. A study on the in vitro degradation properties of poly(L-lactic acid)/beta-tricalcuim phosphate (PLLA/beta-TCP) scaffold under dynamic loading. Med Eng Phys 2009;31:589–94. [DOI] [PubMed] [Google Scholar]
- 88. Yang YF, Tang GW, Zhao YH. et al. Effect of cyclic loading on in vitro degradation of poly(L-lactide-co-glycolide) scaffolds. J Biomater Sci Polym Ed 2010;21:53–66. [DOI] [PubMed] [Google Scholar]
- 89. Li P, Feng XL, Jia XL. et al. Influences of tensile load on in vitro degradation of an electrospun poly(lactide-co-glycolide) scaffold. Acta Biomater 2010;6:2991–6. [DOI] [PubMed] [Google Scholar]
- 90. Zhao YH, Qiu DP, Yang YF. et al. Degradation of electrospun poly(L-lactide) membranes under cyclic loading. J Appl Polym Sci 2012;124:E258–66. [Google Scholar]
- 91. Guo M, Chu ZW, Yao J. et al. The effects of tensile stress on degradation of biodegradable PLGA membranes: a quantitative study. Polym Degrad Stab 2016;124:95–100. [Google Scholar]
- 92. Agrawal CM, Mckinney JS, Lanctot D. et al. Effects of fluid flow on the in vitro degradation kinetics of biodegradable scaffolds for tissue engineering. Biomaterials 2000;21:2443–52. [DOI] [PubMed] [Google Scholar]
- 93. Huang YY, Qi M, Zhang M. et al. Degradation mechanisms of poly (lactic-co-glycolic acid) films in vitro under static and dynamic environment. Trans Nonferrous Met Soc China 2006;16:293–7. [Google Scholar]
- 94. Chu ZW, Zheng Q, Guo M. et al. The effect of fluid shear stress on the in vitro degradationof poly(lactide-co-glycolide) acid membranes. J Biomed Mater Res Part A 2016;104A:2315–24. [DOI] [PubMed] [Google Scholar]
- 95. Chu ZW, Li XM, Li Y. et al. Effects of different fluid shear stress patterns on the in vitro degradation of poly(lactide-co-glycolide) acid membranes. J Biomed Mater Res Part A 2016;105:23–20. [DOI] [PubMed] [Google Scholar]
- 96. Athanasiou KA, Niederauer GG, Agrawal CM.. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996;17:93–102. [DOI] [PubMed] [Google Scholar]
- 97. Wu XS. In: Wise DL, Trantolo DJ, Altobelli DE, Yaszemski MJ, Gresser JD, Schwartz ER (eds). Encyclopedic Handbook of Biomaterials and Bioengineering, Part A: Materials. Marcel Dekker, New York, 1995, 1015. [Google Scholar]
- 98. Kumar GS. Biodegradable Polymers: Prospects and Progress. New York: Marcel Dekker; 1987, 44. [Google Scholar]
- 99. Schnabel W. Polymer Degradation -Principles and Practical Applications. Germany: Hanser International, 1981, 179. [Google Scholar]
- 100. Kronenthal RL. In: Kronenthal RL, Oser Z, Martin E (eds). Polymer Science and Technology, Vol. 8, Polymers in Medicine and Surgery. New York: PlenumPress, 1975, 119. [Google Scholar]
- 101. Ginde RM, Gupta RK.. In vitro chemical degradation of poly(glycolic acid) pellets and fibers. J Appl Polym Sci 1987;33:2411–29. [Google Scholar]
- 102. Çatıker E, Gümüşderelioğlu M, Güner A.. Degradation of PLA, PLGA homo- and copolymers in the presence of serum albumin: a spectroscopic investigation. Polym Int 2000;49:728–34. [Google Scholar]
- 103. Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials 1996;17:103–14. [DOI] [PubMed] [Google Scholar]
- 104. Bikales NM. Mechanical Properties of Polymers. New York: John Wiley & Sons, 1971. [Google Scholar]
- 105. Miller ND, Williams DF.. The in vivo and in vitro degradation of poly(glycolic acid) suture material as a function of applied strain. Biomaterials 1984;5:365–8. [DOI] [PubMed] [Google Scholar]
- 106. Daniels AU, Smutz WP, Andriano KP, Chang MKO, Heller J.. “Dynamic environmental exposure testing of biodegradable polymers” In: Specter M. (ed). Transactions of the 15th Meeting of the Society for Biomaterials, Vol. 12 Birmingham, AL: Society for Biomaterials, 1989, 74. [Google Scholar]
- 107. Smutz WP, Daniels AU, Andriano KP. et al. Mechanical test methodology for environment exposure testing of biodegradable polymers. J Appl Biomater 1991;2:13. [Google Scholar]
- 108. Agrawal CM, Kennedy ME. The effects of fatigue loading on the biodegradation of a copolymer used for implants. Proceedings of the 12th Southern Biomedical Engineering Conference, New Orleans, LA, 1993, p. 266.
- 109. Zhong SP, Doherty PJ, Williams DF.. The effects of applied strain on the degradation of absorbable suture in vitro. Clin Mater 1993;14:183–9. [Google Scholar]
- 110. Thompson DE, Agrawal CM, Athanasiou K.. The effects of dynamic compressive loading on biodegradable implants of 50-50% polylactic Acid-polyglycolic Acid. Tissue Eng 1996;2:61–74. [DOI] [PubMed] [Google Scholar]
- 111. Arm DM, Tencer AF.. Effects of cyclical mechanical stress on the controlled release of proteins from a biodegradable polymer implant. J Biomed Mater Res Part A 1997;35:433–41. [DOI] [PubMed] [Google Scholar]
- 112. Miller RA, Brady JM, Cutright DE.. Degradation rates of oral resorbable implants (polylactates and polyglycolates: rate modification with changes in PLA/PGA copolymer ratios. J Biomed Mater Res 1977;11:711–9. [DOI] [PubMed] [Google Scholar]
- 113. Gunatillake P, Mayadunne R, Adhikari R.. Recent developments in biodegradable synthetic polymers. Biotechnol Ann Rev 2006;12:301–47. [DOI] [PubMed] [Google Scholar]
- 114. Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials 1995;16:1123–30. [DOI] [PubMed] [Google Scholar]
- 115. Wang N, Wu XS. Tailored polymeric materials for controlled delivery systems. In: Mc Culloch I, Shalaby SW (eds). ACS Symposium Series 709, Washington DC, 1998, 255–65.
- 116. Wu XS, Wang N.. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: Biodegradation. J Biomater Sci Polym Ed 2001;12:21–34. [DOI] [PubMed] [Google Scholar]
- 117. Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J Biomed Mater Res Part A 1999;48:342–53. [DOI] [PubMed] [Google Scholar]
- 118. Wang N, Qiu JS, Wu XS. Tailored polymeric materials for controlled delivery systems. In: Mc Culloch I, Shalaby SW (eds). ACS Symposium Series 709, ACS, Washington DC, 1998, 242–54.
- 119. Cam D, Hyon SH, Ikada Y.. Degradation of high molecular weight poly(L-lactide) in alkaline medium. Biomaterials 1995;16:833–43. [DOI] [PubMed] [Google Scholar]
- 120. Vert M, Li S, Garreau H.. More about the degradation of LA/GA-derived matrices in aqueous media. J Control Release 1991;16:15–26. [Google Scholar]
- 121. Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)]. Polym Int 2005;54:36–46. [Google Scholar]
- 122. Li SM, Garreau H, Vert M.. Structure-property relationships in the case of the degradation of massive aliphatic poly(a-hydroxyacids) in aqueous media. Part 1: poly(DL-lactic acid). J Mater Sci Mater Med 1990;1:123–30. [Google Scholar]
- 123. Li SM, Garreau H, Vert M.. Structure-property relationships in the case of the degradation of massive aliphatic poly(a-hydroxyacids) in aqueous media. Part 2: degradation of lactide/glycolide copolymers: PLA37.5GA25. and PLA75GA25. J Mater Sci Mater Med 1990;1:131–9. [Google Scholar]
- 124. Li SM, Garreau H, Vert M.. Structure-property relationships in the case of the degradation of massive aliphatic poly(a-hydroxyacids) in aqueous media. Part 3: influence of the morphology of poly(L-lactic acid). J Mater Sci Mater Med 1990;1:198–206. [Google Scholar]
- 125. Vert M, Li SM, Garreau H.. More about the degradation of LA/GA-derived matrices in aqueous media. J Control Rel 1991;16:15–26. [Google Scholar]
- 126. Li S, Girod-Holland S, Vert M.. Hydrolytic degradation of poly (dl -lactic acid) in the presence of caffeine base. J Control Release 1996;40:41–53. [Google Scholar]
- 127. Therin M, Christel P, Li SM. et al. In vivo degradation of massive poly(a-hydroxyacids): validation of in vitro findings. Biomaterials 1992;13:594–600. [DOI] [PubMed] [Google Scholar]
- 128. Grizzi I, Garreau H, Li S. et al. Hydrolytic degradation of devices based on poly (dl -lactic acid) size-dependence. Biomaterials 1995;16:305–11. [DOI] [PubMed] [Google Scholar]
- 129. Witt C, Kissel T.. Morphological characterization of microspheres, films and implants prepared from poly(lactide-co-glycolide) and ABA triblock copolymers: is the erosion controlled by degradation, swelling or diffusion?. Eur J Pharm Biopharm 2001;51:171–81. [DOI] [PubMed] [Google Scholar]
- 130. Lu L, Garcia CA, Mikos AG.. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J Biomed Mater Res Part A 1999;46:236–44. [DOI] [PubMed] [Google Scholar]
- 131. Lu L, Peter SJ, Lyman MD. et al. In vitro degradation of porous poly(L-lactic acid) foams. Biomaterials 2000;21:1595–605. [DOI] [PubMed] [Google Scholar]
- 132. Belbella A, Vauthier C, Fessi H. et al. In vitro degradation of nanospheres from poly(D,L-lactides) of different molecular weights and polydispersities. Int J Pharm 1996;129:2545–8. [Google Scholar]
- 133. Holy CE, Dang SM, Davies JE. et al. In vitro degradation of a novel poly(lactide-co-glycolide) 75/25 foam. Biomaterials 1999;20:1177–85. [DOI] [PubMed] [Google Scholar]
- 134. Yoo JY, Kim JM, Seo KS. et al. Characterization of degradation behavior for PLGA in various pH condition by simple liquid chromatography method. Bio-Med Mater Eng 2005;15:279–88. [PubMed] [Google Scholar]
- 135. Jamishidi K, Hyon S-H, Nakamura T, Ikada Y, Shimizu Y, Teramatsu T. In: Christel P, Meunier A, Lee AJC (eds). Biological Performance of Biomaterials. Amsterdam: Elsevier Science, 1986, 227. [Google Scholar]
- 136. Aso Y, Yoshioka S, Po ALW. et al. Effect of temperature on mechanisms of drug release and matrix degradation of poly (d,l -lactide) microspheres. J Control Release 1994;31:33–9. [Google Scholar]
- 137. Hakkarainen M, Albertsson AC, Karlsson S.. Weight losses and molecular weight changes correlated with the evolution of hydroxyacids in simulated in vivo, degradation of homo- and copolymers of PLA and PGA. Polym Degrad Stab 1996;52:283–91. [Google Scholar]
- 138. Deng M, Zhou J, Chen G. et al. Effect of load and temperature on in vitro degradation of poly(glycolide-co-l-lactide) multifilament braids. Biomaterials 2005;26:4327–36. [DOI] [PubMed] [Google Scholar]
- 139. Deng M, Chen G, Burkley D. et al. A study on in vitro degradation behavior of a poly(glycolide-co-l-lactide) monofilament. Acta Biomater 2008;4:1382–91. [DOI] [PubMed] [Google Scholar]
- 140. Giunchedi P, Conti B, Scalia S. et al. In vitro degradation study of polyester microspheres by a new HPLC method for monomer release determination. J Control Release 1998;56:53–62. [DOI] [PubMed] [Google Scholar]
- 141. Zhang Y, Zale S, Sawyer L. et al. Effects of metal salts on poly(DL-lactide-co-glycolide) polymer hydrolysis. J Biomed Mater Res Part A 1997;34:531–8. [DOI] [PubMed] [Google Scholar]
- 142. Bodmeier R, Oh KH, Chen H.. The effect of the addition of low molecular weight poly(dl-lactide) on drug release from biodegradable poly(dl-lactide) drug delivery systems. Int J Pharm 1989;51:1–8. [Google Scholar]
- 143. Sung KC, Han RY, Hu OYP. et al. Controlled release of nalbuphine prodrugs from biodegradable polymeric matrices: influence of prodrug hydrophilicity and polymer composition. Int J Pharm 1998;172:17–25. [Google Scholar]
- 144. Rothen-Weinhold A, Besseghir K, Gurny R.. Analysis of the influence of polymer characteristics and core loading on the in vivo release of a somatostatin analogue. Eur J Pharm Sci 1997;5:303–13. [Google Scholar]
- 145. Hausberger AG, Kenley RA, Deluca PP.. Gamma Irradiation Effects on Molecular Weight and in vitro, Degradation of Poly(D,L-Lactide- CO -Glycolide) Microparticles. Pharm Res 1995;12:851–6. [DOI] [PubMed] [Google Scholar]
- 146. Hooper KA, Cox JD, Kohn J.. Comparison of the effect of ethylene oxide and γ-irradiation on selected tyrosine-derived polycarbonates and poly(L-lactic acid). J Appl Polym Sci 1997;63:1499–510. [Google Scholar]
- 147. Faisant N, Siepmann J, Oury P. et al. The effect of gamma-irradiation on drug release from bioerodible microparticles: a quantitative treatment. Int J Pharm 2002;242:281–4. [DOI] [PubMed] [Google Scholar]
- 148. Vert M, Li SM, Spenlehauer G. et al. Bioresorbability and Biocompatibility of Aliphatic Polyesters. J Mater Sci Mater Med 1992;3:432–46. [Google Scholar]
- 149. Li S, Girard A, Garreau H. et al. Enzymatic degradation of polylactide stereocopolymers with predominant d -lactyl contents. Polym Degrad Stab 2000;71:61–7. [Google Scholar]
- 150. Hooper KA, Macon ND, Kohn J.. Comparative histological evaluation of new tyrosine-derived polymers and poly (L-lactic acid) as a function of polymer degradation. J Biomed Mater Res Part A 1998;41:443–54. [DOI] [PubMed] [Google Scholar]
- 151. Leenslag JW, Pennings AJ, Bos RRM. et al. Resorbable materials of poly (l -lactide): VII. In vivo, and in vitro, degradation. Biomaterials 1987;8:311–4. [DOI] [PubMed] [Google Scholar]
- 152. Miyajima M, Koshika A, Okada J. et al. The effects of drug physico-chemical properties on release from copoly (lactic/glycolic acid) matrix. Int J Pharm 1998;169:255–63. [Google Scholar]
- 153. Miyajima M, Koshika A, Okada J, Ikeda M.. Mechanism of drug release from poly(L-lactic acid) matrix containing acidic or neutral drugs. J Control Release 1999;60:199–209. [DOI] [PubMed] [Google Scholar]
- 154. Gümüşderelioglu M, Deniz G.. Sustained release of mitomycin-C from poly(DL-lactide)/poly(DL-lactide-co-glycolide) films. J Biomater Sci Polym Ed 2000;11:1039–50. [DOI] [PubMed] [Google Scholar]
- 155. Cai Q, Shi G, Bei J. et al. Enzymatic degradation behavior and mechanism of poly(lactide-co-glycolide) foams by trypsin. Biomaterials 2003;24:629–38. [DOI] [PubMed] [Google Scholar]
- 156. Kemme M, Prokesch I, Heinzel-Wieland R.. Comparative study on the enzymatic degradation of poly(lactic-co-glycolic acid) by hydrolytic enzymes based on the colorimetric quantification of glycolic acid. Polym Test 2011;30:743–8. [Google Scholar]
- 157. Żenkiewicz M, Richert A, Malinowski R, Moraczewski K.. A comparative analysis of mass losses of some aliphatic polyesters upon enzymatic degradation. Polym Test 2013;32:209–14. [Google Scholar]