Abstract
Fucose is a 6-deoxy hexose in the l-configuration found in a large variety of different organisms. In mammals, fucose is incorporated into N-glycans, O-glycans and glycolipids by 13 fucosyltransferases, all of which utilize the nucleotide-charged form, GDP-fucose, to modify targets. Three of the fucosyltransferases, FUT8, FUT12/POFUT1 and FUT13/POFUT2, are essential for proper development in mice. Fucose modifications have also been implicated in many other biological functions including immunity and cancer. Congenital mutations of a Golgi apparatus localized GDP-fucose transporter causes leukocyte adhesion deficiency type II, which results in severe developmental and immune deficiencies, highlighting the important role fucose plays in these processes. Additionally, changes in levels of fucosylated proteins have proven as useful tools for determining cancer diagnosis and prognosis. Chemically modified fucose analogs can be used to alter many of these fucose dependent processes or as tools to better understand them. In this review, we summarize the known roles of fucose in mammalian physiology and pathophysiology. Additionally, we discuss recent therapeutic advances for cancer and other diseases that are a direct result of our improved understanding of the role that fucose plays in these systems.
Keywords: cancer, development, fucose, fucosyltransferase, immunology
Introduction
Fucose is an unusual sugar that is present in a variety of glycolipids and glycoproteins produced by mammalian cells. It is unique in having an l-configuration, whereas all other naturally occurring sugars in mammals exist in the d-conformation (Figure 1). It is also structurally distinct in lacking a hydroxyl group on the C-6 carbon (note contrast with d-galactose in Figure 1). A study of 3299 mammalian oligosaccharides revealed that fucose is found in 7.2% of oligosaccharides studied, second only to sialic acid, making fucose a relatively common component of glycan modifications on proteins and lipids (Werz et al. 2007).
Fig. 1.
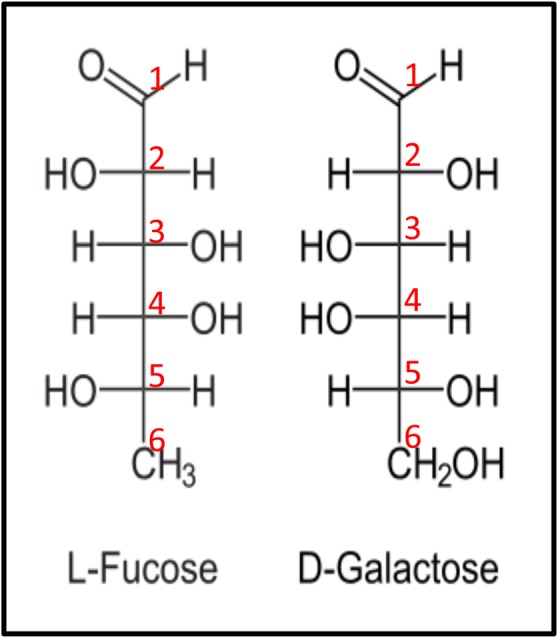
Fischer projection formula of l-fucose. The six carbons of fucose are numbered. Note that most naturally occurring sugars, such as galactose, are present in the d-configuration, as can be determined by the arrangement of the hydroxyl group bound to the C-5 carbon. Note further that the C-6 carbon of l-fucose lacks a hydroxyl group present at the C-6 position of d-galactose. l-Fucose can also be described as 6-deoxy-l-galactose. This figure is available in black and white in print and in color at Glycobiology online.
Fucose can be incorporated into the terminal portions of N-, O- or lipid-linked oligosaccharide chains, modify the core of complex N-glycans, or can be linked directly to serine or threonine residues in some proteins. N-glycans are extremely structurally diverse, but all contain a 5-saccharide core with an N-acetylglucosamine (GlcNAc) attached to the amide nitrogen of asparagine within the appropriate consensus sequence (Asn-X-Ser/Thr) of target proteins (Stanley et al. 2009). Two types of O-glycans can be modified with fucose: mucin O-GalNAc glycans are initiated by the attachment of N-acetylgalactosamine (GalNAc) to the hydroxyl group of a serine or threonine; alternatively fucose can be directly attached to serine or threonine residues within the appropriate consensus sequence of a subset of proteins. There are 13 known fucosyltransferases responsible for the synthesis of this group of fucosylated glycans (Figure 2). The addition of fucose by these enzymes plays an important role in a variety of biological systems, many of which are discussed here. Knockout of three of these fucosyltransferases, FUT8, POFUT1 and POFUT2, is lethal to mice, demonstrating their biologic importance (Shi and Stanley 2003; Wang et al. 2005; Du et al. 2010).
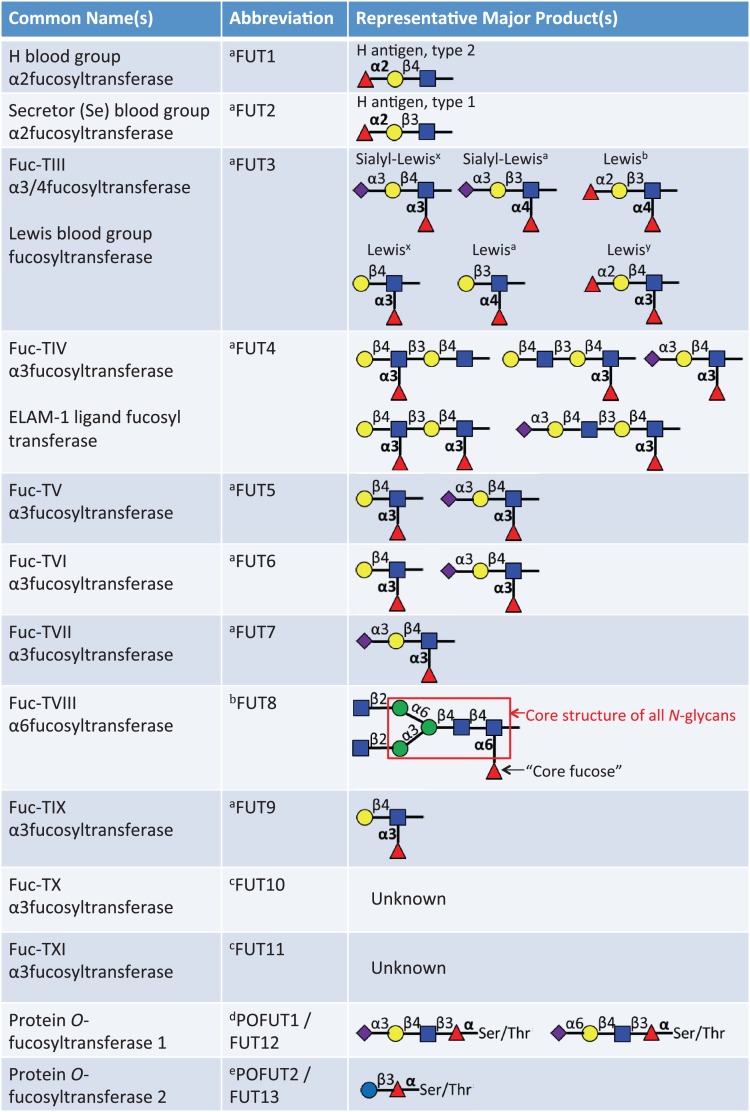
Fig. 2.
List of 13 known fucosyltransferases in humans. Major representative products of each fucosyltransferase are listed. The linkage of the fucose added by each enzyme appears in bold.aThese enzymes can add fucose to oligosaccharide chains on glycolipids, N-glycans or mucin O-glycans. bThis enzyme only adds the core fucose to N-glycans. cThese are putative α3-fucosyltransferases. Acceptor substrates have not been clearly defined. dThis modification is only observed in O-fucose consensus sequences on EGF repeats (C2XXXX(S/T)C3), see Figure 4A. eThis modification is only observed in O-fucose consensus sequences on TSRs (C1−2XX(S/T)C2−3), see Figure 4B. This figure is available in black and white in print and in color at Glycobiology online.
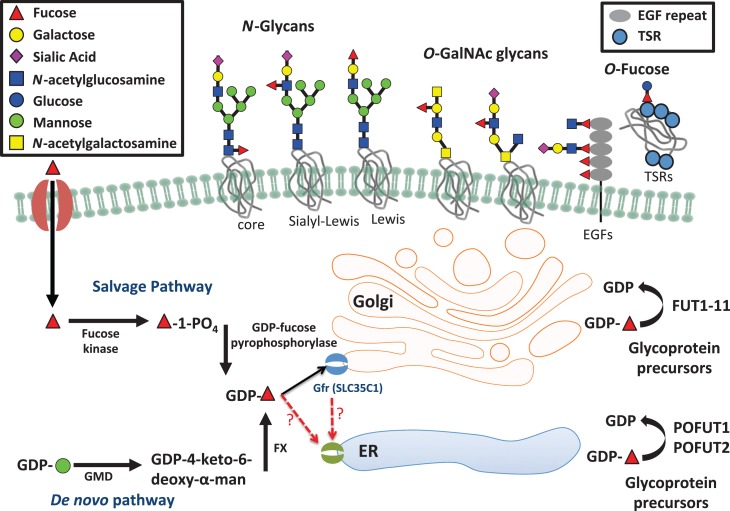
All fucosyltransferases utilize a nucleotide-charged form of fucose, GDP-fucose, to modify target proteins or lipids. In mammals, GDP-fucose is synthesized through two pathways—the de novo synthesis pathway and the fucose salvage pathway (Figure 3). The de novo pathway synthesizes GDP-fucose from GDP-mannose through a three-step reaction catalyzed by two enzymes, GDP-mannose 4,6-dehydratase and GDP-keto-6-deoxymannose 3,5 epimerase (the FX protein) (Ginsburg 1960; Tonetti et al. 1996). It is estimated that ~90% of GDP-fucose in mammals is generated by the de novo pathway under ordinary circumstances (Yurchenco and Atkinson 1975). The fucose salvage pathway utilizes free fucose derived from dietary sources or added to culture medium (Coffey et al. 1964; Kaufman and Ginsburg 1968). Fucose is transported across the plasma membrane through a poorly understood mechanism, perhaps l-fucose-specific facilitated diffusion (Wiese et al. 1994). A two-step mechanism catalyzed by two alternative enzymes then converts fucose to GDP-fucose (Ishihara et al. 1968). Once synthesized, GDP-fucose is transported into the lumen of the Golgi or endoplasmic reticulum (ER) to be used by fucosyltransferases. The Golgi transporter has been identified as SLC35C1, mutations in which result in the human disorder leukocyte adhesion deficiency type II (LAD2; see below) (Lühn et al. 2001). An ER-localized GDP-fucose transporter has been identified in Drosophila (Ishikawa et al. 2010), but the human ortholog of this gene has been shown to be a UDP-xylose/GlcNAc transporter (Ashikov et al. 2005). Identification of a candidate for a mammalian ER GDP-fucose transporter remains an open question. Fucose metabolism and function has been previously reviewed in detail (Becker and Lowe 2003). The remainder of this review will summarize the physiological and pathophysiological significance of fucose. Several very recent observations and their potential implications not covered in the earlier review will be emphasized.
Fig. 3.
Fucose metabolism pathways and variation in types of fucosylated glycans. This figure illustrates the de novo fucose synthesis pathway, which converts GDP-mannose to GDP-fucose and the fucose salvage pathway, which converts free fucose taken up from outside the cell to GDP-fucose. GDP-fucose can then be taken up into the Golgi apparatus by the GDP-fucose transporter (SLC35C1) and possibly into the ER by an as yet unknown transporter. Proteins are then modified with GDP-fucose and other carbohydrates within the Golgi and ER and can then be secreted or expressed on the cell surface. This figure is available in black and white in print and in color at Glycobiology online.
Terminal fucosylation
Terminal fucosylation is a common modification found on many N-glycans, mucin O-GalNAc glycans and glycolipids. The processing and maturation of these glycans is quite complex and is carried out by the concerted action of a staggering number of enzymes. Ten fucosyltransferases (FUT1–7 and FUT9–11) are responsible for the addition of terminal fucose to these oligosaccharide chains. These fucosyltransferases are all localized to the Golgi apparatus and add fucose to oligosaccharides by α(1,2)-linkage to a terminal galactose or α(1,3/4)-linkage to a subterminal GlcNAc to generate blood group and Lewis antigens (Figure 2). Many of these enzymes serve redundant functions and thus, despite the biological importance of these modifications, loss of function for any one of these enzymes is not lethal in mice.
ABO blood groups
The ABO blood group antigens are perhaps the most well-known fucosylated glycans. Two α(1,2)fucosyltransferases, the H-transferase (FUT1) and the Secretor (Se) transferase (FUT2), synthesize the glycan known as the H-antigen by adding fucose to a terminal galactose residue (Lowe 1993). The H-transferase is expressed in erythroid precursors and is responsible for the generation of H-antigen on red blood cells (RBCs). The Se transferase is expressed in epithelial tissues and salivary glands and is responsible for the formation of H-antigen in saliva and other bodily secretions. Individuals without at least one copy of a functional FUT2 gene are considered nonsecretors and do not produce soluble H-antigen.
ABO locus-encoded glycosyltransferases can modify the H-antigen to generate A and B antigens in A, B or AB blood type individuals. In O blood type individuals, only unmodified H-antigen is expressed. These antigens are highly immunogenic and are found in high quantities on glycoproteins and glycolipids in RBCs. As a result, they notoriously prevent successful blood transfusion between incompatible individuals.
Patients lacking functional copies of both α(1,2)-FucT enzymes (FUT1 and FUT2), display the rare “Bombay phenotype” (present in only ~0.01% of the population) (Dipta and Hossain 2011), and are entirely deficient in type A, type B and H blood group antigens (Kelly et al. 1994). These individuals contain robust anti-A, anti-B and anti-H antibody titers and can only receive blood transfusions from other Bombay individuals (Davey et al. 1978). Similarly “para-Bombay” individuals lack functional copies of FUT1, but still have functional Se transferase (FUT2), resulting in the absence of blood group antigens only in RBCs (Wang et al. 1997). These individuals may have low titers of antibodies against the H-antigen, but can typically receive normal blood transfusions without complication (Lin-Chu and Broadberry 1990). Aside from potential issues with blood transfusions, these individuals appear unaffected, prompting questions about the physiological importance of these antigens.
Although the functional significance of ABO antigen expression remains unclear, ABO blood type has been linked with other processes, suggesting medical importance beyond blood typing. ABO blood type and ability to secrete soluble H-antigen have been linked with plasma von Willebrand Factor levels, a protein vital to the process of blood coagulation (Levy and Ginsburg 2001). Consequently, these characteristics are also related to von Willebrand disease and other related coagulopathies. ABO blood type has also been linked to increased risk for several types of cancer (Slater et al. 1993; Edgren et al. 2010; Wolpin et al. 2010), possibly suggesting a role in the immunogenicity of tumors and the associated opportunity for host recognition. The blood groups also appear to affect susceptibility to a number of pathogens (Ilver et al. 1998; Hutson et al. 2002; Huang et al. 2005; Wands et al. 2015) (discussed further below), suggesting that variation in blood types among individuals in a population might help to prevent the spread of disease.
Host–microbe interactions
Blood group antigens fucosylated by the Se transferase (FUT2) and Lewis fucosyltransferase (FUT3) also play an important role in mediating host–microbe interactions. Helicobacter pylori, a pathogen that can lead to peptic ulcer disease and gastric cancer, utilizes host expression of the Lewisb antigen, generated by the joint action of the Se and Lewis fucosyltransferases, to recognize and attach to gastric epithelial tissue (Ilver et al. 1998). Other pathogens including Norovirus (Xu et al. 2003; Huang et al. 2005) and Vibrio cholera (Wands et al. 2015) also take advantage of specific blood group antigens to attach to host cells. Additionally, Bacteroides thetaiotaomicron, a prominent resident of the human intestinal tract, can sense low fucose availability in the gut and induce expression of host fucosyltransferases. It is able to harvest fucose from secreted oligosaccharides using α-fucosidases (Xu et al. 2003). Other bacteria exploit the release of free fucose by B. thetaiotaomicron using their own fucose sensors (Pacheco et al. 2012).
Fucosyltransferases also play an important role in maintaining the gut microbiome. The activity of Se fucosyltransferase (FUT2) promotes normal microbial diversity and composition in the gut (Kashyap et al. 2013). Its up-regulation during illness serves as a protective mechanism to increase tolerance to infection and maintain host-microbiome symbiosis (Pham et al. 2014; Pickard et al. 2014). Inactivating FUT2 mutations, seen in about 20% of the human population (Hoskins 1967; Ikehara et al. 2001), result in a nonsecretor phenotype that is associated with a distinct community of bacteria in the gut. Among the notable distinctions in nonsecretors is an increased association with the genus Prevotella, which can promote breakdown of the gut's mucus barrier (Rho et al. 2005; Rausch et al. 2011). Conversely, bacteria thought to promote good intestinal health including members of the Lactobacillus and Bifidobacterium genera are decreased in nonsecretors (Rausch et al. 2011; Wacklin et al. 2011). Abnormal gut microbiome composition with a disproportionately increased segment of bacteria associated with the nonsecretor phenotype can result in dysregulation of the local immune response (Xavier and Podolsky 2007) and is associated with increased risk of Crohn's disease, a chronic inflammatory bowel disease (Serpa et al. 2004; van Heel et al. 2004; McGovern et al. 2010).
Learning, memory and cognitive processes
Synaptic plasticity, neurite outgrowth and neuron morphology are regulated by fucosylation and are responsible for many cognitive processes including learning and memory. It was initially recognized that fucosylation of structures in the hippocampus was a component of learning and long-term potentiation (LTP) (Pohle et al. 1987). Further, injections of l-fucose enhance LTP in the rat brain (Krug et al. 1994). Additional work demonstrated that fucose α(1,2)-linkages formed by FUT1 and FUT2 were directly involved in synapse formation and neurite outgrowth (Kalovidouris et al. 2005). These fucose modifications can also direct neurite migration and mediate pathfinding for sensory neurons, including those in the olfactory bulb (Lipscomb et al. 2003; St John et al. 2006).
One glycoprotein involved in these processes that has been well characterized is Synapsin I, a protein involved in neurotransmitter release and the formation of new synapses. Fucosylation regulates turnover and stability of this protein (Murrey et al. 2006). Fucosylation of neural cell adhesion molecule has also been suggested to regulate its function (Pestean et al. 1995; Liedtke et al. 2001). More recent work suggests that a wide array of olfactory bulb proteins involved in cell adhesion, ion and solute transport, ATP binding, synaptic vesicle formation, and cell signaling are all modified with α(1,2)-fucose (Murrey et al. 2009). Fucosylation of these proteins contributes to olfactory bulb development (Murrey et al. 2009).
Leukocyte rolling and extravasation
Leukocyte trafficking is a process mediated by selectins and their counter-receptors (reviewed previously in Lowe (1997)). E-, P- and L-selectins are expressed in platelets (P-selectin), leukocytes (L-selectin) and endothelial cells (E- and P-selectins) allowing for their adhesion to oligosaccharide-containing ligands expressed by specialized endothelial cells lining postcapillary venules. Mucin O-GalNAc glycans can make up ~70% of these ligands by mass and are heavily decorated with fucose (Imai et al. 1991; Lowe 1997). Two α(1,3) fucosyltransferases, encoded by the FUT4 and FUT7 genes, are responsible for the addition of these fucose residues (Homeister et al. 2001). Inactivation of FUT7, in particular, causes a severe deficit in selectin-dependent endothelial cell adhesion and lymphocyte homing (Malý et al. 1996). Fucose modifications on glycolipid E-selectin receptors are required for neutrophil extravasation during inflammation (Malý et al. 1996; Nimrichter et al. 2008).
LAD2, a rare congenital disorder of glycosylation caused by mutation of the gene encoding a GDP-fucose transporter in the Golgi apparatus (SLC35C1), exemplifies the importance of fucose in leukocyte trafficking. LAD2 is characterized by immunodeficiency, leukocytosis without pus formation, mental retardation and growth retardation, all directly attributed to the absence of neutrophil sialyl LewisX, of which fucose is an essential component (Yakubenia et al. 2008). Dietary supplementation with fucose can reduce symptoms of LAD2 in some patients (Marquardt et al. 1999; Etzioni et al. 2002), including some with mutations causing complete inactivation of SLC35C1 (Hidalgo et al. 2003), suggesting that at high concentrations GDP-fucose might be transported to the Golgi by the more recently described SLC35C2 (Lu et al. 2010) or other as yet unknown transporters (Figure 3).
Cancer metastasis
As a byproduct of their role in promoting selectin-mediated rolling and adhesion, Sialyl Lewis antigens play an important role in promoting cancer migration and metastasis (Kannagi 1997). These antigens are upregulated in a variety of cancer types including lung (Zenita et al. 1988), breast (Jeschke et al. 2005) and colorectal (Kudo et al. 1998; Zipin et al. 2004) cancers and serve as prognostic factors for increased risk of metastasis (Itai et al. 1991). Studies have shown that elimination of terminal fucose from these antigens with an α-l-fucosidase can impair their ability to roll within endothelial tissue and decrease cancer cell invasion (Yuan et al. 2008). Additionally, one study demonstrated that preventing terminal fucosylation by knocking down FUT1 and FUT4 inhibits tumor growth (Zhang et al. 2008). Similarly, endogenous fucosidases have been shown to play a role in preventing cancer cell proliferation. Decreased expression of α-l-fucosidase 1 (FUCA1) has been identified in a number of different cancer types including colorectal (Otero-Estévez et al. 2013; Ezawa et al. 2016), gastric (Liu et al. 2009) and breast (Cheng et al. 2015) cancers.
Altered fucosylation has also been implicated in affecting TNF-related apoptosis inducing ligand activity in colon cancer, a ligand important for promoting destruction of transformed cells. Although the precise role for fucose in the regulation of this signaling pathway remains unclear (Haltiwanger 2009), defects in the de novo synthesis of GDP-fucose cause increased tumor growth and metastasis of colon cancer in mice (Moriwaki et al. 2009).
Fertilization and development
Fucosylated N-glycans in the zona pellucida facilitate sperm binding in a variety of mammalian species (Lefebvre et al. 1997; Yonezawa et al. 1997; Johnston et al. 1998), including humans (Pang et al. 2011). Fucosylated LewisX antigens also promote cell–cell adhesion in early stage embryos (Bird and Kimber 1984). Fuc-TIX encoded by the FUT9 gene and responsible for the generation of LewisX in the brain plays an important role in neural development and promotes normal migration of motor neuron progenitors (Ohata et al. 2009). Fut9 knockout in mice results in development of anxiety-like behavior (Kudo et al. 2007). Additionally, knockout of Fut2 in mice resulted in altered hepatic vasculature and hepatic fibrosis resulting in microcirculatory disturbances and sensitivity toward bile salt toxicity (Maroni et al. 2017).
Core fucosylation
Fucosylation on the GlcNAc linked to asparagine in the core of N-glycans (core fucosylation) is the most common type of fucose modification. It occurs exclusively on N-glycans. Like terminal fucosylation, core fucosylation occurs in the Golgi and is characterized by α(1,6)-linkage to the innermost GlcNAc of the N-glycan core (Figure 2). However, while enzymes responsible for terminal fucosylation may catalyze the formation of redundant linkages, FUT8 is the sole enzyme responsible for catalyzing this reaction. Fut8 knockout mice lack core fucose, and while born with no apparent anomalies, about 70% die within three days of birth due to major developmental growth and respiratory defects (Wang et al. 2005, 2006b). Survivors display severe growth retardation and emphysema-like changes in the lungs. Core fucosylation of α3β1 integrin also plays a critical role in kidney and lung organogenesis (Kreidberg et al. 1996).
Inflammation and the immune system
Core fucosylation of N-glycans plays several important roles in regulating the immune system. Perhaps of greatest interest is the observation that antibody dependent cellular cytotoxicity (ADCC) is inhibited by the presence of fucose on the Fc region of IgG1 antibodies. Core fucose on IgG1 N-glycans causes a 50- to 100-fold reduction in binding to FcγRIIIa (CD16), an Fc receptor found on the surface of natural killer cells and macrophages that is partially responsible for crosslinking these immune effector cells with antibody-bound cells targeted for destruction (Shields et al. 2002). A co-crystal structure demonstrated that the addition of this core fucose causes a steric clash that weakens carbohydrate–carbohydrate interactions required for high affinity receptor recognition (Ferrara et al. 2011). This observation is of particular importance because therapeutic antibodies, used in the treatment of cancer and other diseases, can be generated without this core fucose to significantly enhance their potency (Shields et al. 2002; Shinkawa et al. 2003).
Several pharmaceutical companies have begun to take advantage of this knowledge and glycoengineered monoclonal antibodies (mAb) are being developed for therapeutic purposes (Yamane-Ohnuki and Satoh 2009). Two afucosylated mAbs have already been approved by the FDA for use in cancer patients: mogamulizumab and obinutuzumab. Mogamulizumab targets chemokine receptor 4, an important signal transducer that is upregulated in T-cell leukemia and lymphoma (Ishii et al. 2010; Beck and Reichert 2012). Obinutuzumab is an afucosylated mAb against CD20, an antigen found on developing B-cells, and has been effective for the treatment of chronic lymphocytic leukemia (CLL). Rituximab, a mAb also targeting CD20, has been approved for use in autoimmune diseases and CLL since 1997. However, obinutuzumab has been shown to be more effective in CLL treatment due to more efficient promotion of ADCC (Illidge et al. 2015). Inspired by these successes, drug companies have continued development of similarly glycoengineered mAbs and have more than 20 currently in clinical trials (Hamadani et al. 2013; Wei et al. 2013; Sathish 2014; Gardai et al. 2015).
While enhanced activation of ADCC by afucosylated antibodies has proven useful in the development of cancer therapeutics, in the setting of dengue virus infection the same phenomenon contributes to antibody dependent enhancement of disease. Only about 15% of individuals infected by dengue virus progress to more severe hemolytic disease (dengue hemorrhagic fever or dengue shock syndrome) (Vaughn et al. 2000). A recent report has demonstrated that patients with a high percentage of afucosylated antibodies targeting a dengue envelope protein are more likely to develop acute hemolytic disease (Wang et al. 2017).
Additionally, inflammatory cytokines TGFβ1 and α3β1 require core fucose to function (Kreidberg et al. 1996; Wang et al. 2005). Down-regulation of these signaling pathways causes enhanced matrix metalloproteinase expression and inflammation. Lack of core fucosylation also disrupts epidermal growth factor receptor (Wang et al. 2006a) and vascular endothelial growth factors mediated signaling (Wang et al. 2009). Core fucosylation is vital for appropriate growth factor receptor signaling (Wang et al. 2005, 2006b).
Cancer and cancer biomarkers
Many fucosylated glycans on glycoproteins serve as important cancer biomarkers (Miyoshi et al. 2008; Adamczyk et al. 2012). Elevated α-fetoprotein (AFP) levels are a well-established marker for hepatocellular carcinoma. Unfortunately, elevated AFP is not entirely specific for cancer, and may also be associated with other forms of benign liver disease (i.e., cirrhosis or hepatitis). Only in hepatocellular carcinoma, however, is the fraction of core fucosylated AFP elevated, making this a more reliable biomarker for cancer (Aoyagi et al. 1998; Flores and Marrero 2014). In prostate cancer, prostate-specific antigen (PSA) is another well-established “tumor-specific” biomarker that lacks true specificity as it may also be elevated in benign prostatic hyperplasia (BPH), a very common diagnostic confounder. In patients with prostate cancer, the fraction of core fucosylated PSA is significantly increased relative to patients with BPH (Saldova et al. 2011), again increasing the value of this biomarker. Increases in core fucosylation of serum proteins have also been associated with increased risk of metastasis in prostate cancer (Kyselova et al. 2007). In pancreatic cancer, core fucosylated haptoglobin is another potential biomarker for cancer detection (Okuyama et al. 2006; Miyoshi and Nakano 2008). Pancreatic cancer has a very poor prognosis largely due to a lack of reliable early detection methods, so the discovery and development of more reliable detection biomarkers would be of tremendous clinical utility (Goggins 2005).
Additionally, increased core fucosylation of N-glycans on E-cadherin and integrins has been shown to decrease cell adhesion and promote cell migration and metastasis in cancer (Zhao et al. 2006, 2008). Increased expression of FUT8 promotes this mechanism causing increased tumor growth and metastasis in nonsmall cell lung cancer and ovarian cancer (Yan et al. 2010; Chen et al. 2013). FUT8 inhibitors might rationally be developed as antineoplastic agents in this context.
O-Fucosylation
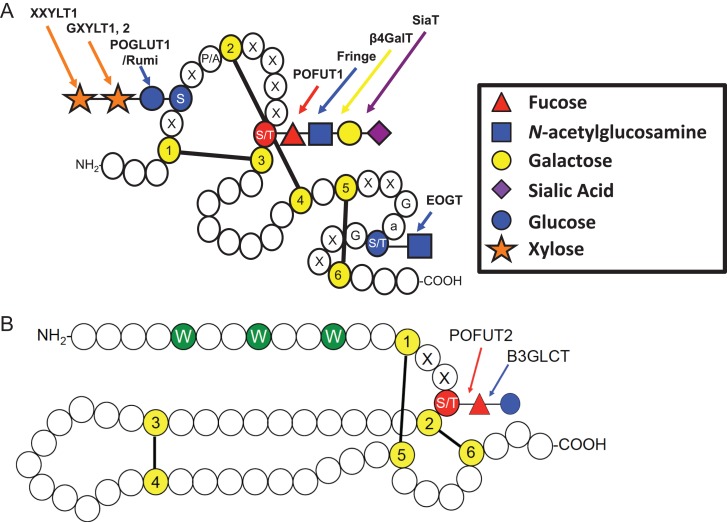
Fucose is also added directly to serine or threonine residues on proteins by two protein O-fucosyltransferases: POFUT1 (FUT12) or POFUT2 (FUT13). Both POFUT1 and POFUT2 are essential for development in mice and are widely expressed in embryonic and adult tissues (Shi and Stanley 2003; Du et al. 2010). POFUT1 is responsible for the addition of fucose to epidermal growth factor-like (EGF) repeats containing the consensus sequence C2XXXX(S/T)C3, where C2 and C3 are the second and third conserved cysteines of the EGF repeat and X represents any amino acid (Shao et al. 2003; Müller et al. 2014) (Figure 4A). EGF repeats can also be modified with O-glucose and O-GlcNAc at distinct consensus sequences. POFUT2 is responsible for transferring fucose to serine or threonine on thrombospondin type 1 repeats (TSRs) with the consensus sequence C1XX(S/T)C2 in group 1 TSRs and C2XX(S/T)C3 in group 2 TSRs (Luo et al. 2006a; 2006b; Valero-González et al. 2016) (Figure 4B). TSRs can also be modified with C-mannosylation of tryptophans (de Peredo et al. 2002). Both EGF repeats and TSRs contain six conserved cysteines, which form three disulfide bonds that are crucial for the structure of these motifs. POFUT1 and POFUT2 only modify properly folded EGF repeats and TSRs, respectively (Wang and Spellman 1998; Luo et al. 2006). Over 100 proteins contain EGF repeats with consensus sequences for O-fucose modification by POFUT1 (Rampal et al. 2007) (Table I) and about 50 proteins contain TSRs with O-fucose consensus sequences for modification by POFUT2 (Leonhard-Melief and Haltiwanger 2010) (Table II). Modification of many of these proteins remains unconfirmed and much remains to be determined about roles of O-fucose on these proteins. Unlike the other fucosyltransferases in Figure 2, the protein O-fucosyltransferases are localized in the ER (Luo and Haltiwanger 2005; Okajima et al. 2005; Luo et al. 2006). The fact that POFUT1 and POFUT2 only modify properly folded modules and are ER-localized has led to the hypothesis that both enzymes participate in quality control (Vasudevan and Haltiwanger 2014).
Fig. 4.
Key features of EGF repeats and TSRs. (A) Cartoon showing a single EGF repeat. Each circle represents one amino acid. Conserved cysteines (yellow) are numbered and disulfide bonds are indicated. O-Glucose and O-GlcNAc sites are shaded blue and the O-fucose site is shaded red. Enzymes responsible for the addition of each sugar are indicated. Modified from Rana and Haltiwanger (2011). Used with permission. Elsevier. (B) Cartoon showing a typical TSR. Conserved cysteines (yellow) and disulfide bonds are indicated. C-Mannose sites are shown in green and the O-fucose site is shaded red. (S) Serine; (T) Threonine; (G) Glycine; (W) Tryptophan; (X) any amino acid, (a) any aromatic amino acid. Modified with permission from Haltiwanger (2004). ©Elsevier. This figure is available in black and white in print and in color at Glycobiology online.
Table I.
List of putative human gene targets of POFUT1
| Name and UNIPROT ID | Consensus/total | Known human pathology (if any) |
|---|---|---|
| AGRIN (O00468) | 2/4 | Myasthenia, limb-girdle, familial (Huze et al. 2009; Maselli et al. 2012) |
| ATRAID (Q6UW56) | 1/1 | --- |
| CD93 (Q9NYP3) | 1/5 | --- |
| CD97 (P48960) | 1/5 | --- |
| CELSR1 (Q9NYQ6) | 2/8 | Neural tube defects (Robinson et al. 2012) |
| CELSR2 (Q9HCU4) | 2/7 | --- |
| CELSR3 (Q9NYQ7) | 2/8 | --- |
| CFC1 (P0CG37) | 1/1 | Heterotaxy, visceral, 2, autosoma; transposition of the great arteries dextro-looped 2; Conotruncal heart malformations (Bamford et al. 2000; Goldmuntz et al. 2002) |
| CFC1B (P0CG36) | 1/1 | --- |
| CNTNAP5 (Q8WYK1) | 1/2 | --- |
| CRB1 (P82279) | 8/19 | Retinitis pigmentosa 12; Leber congenital amaurosis 8; Pigmented paravenouschorioretinal atrophy (den Hollander et al. 1999, 2001; McKay et al. 2005) |
| CRB2 (Q5IJ48) | 8/15 | --- |
| CSPG2 (P13611) | 2/2 | Wagner vitreoretinopathy (Miyamoto et al. 2005; Kloeckener-Gruissem et al. 2013) |
| CUBN (O60494) | 4/7 | Recessive hereditary megaloblastic anemia 1 (Aminoff et al. 1999; Kristiansen et al. 2000) |
| DLK1 (P80370) | 3/6 | --- |
| DLK2 (Q6UY11) | 1/6 | --- |
| DLL1 (O00548) | 4/8 | --- |
| DLL3 (Q9NYJ7) | 2/6 | Spondylocostaldysostosis 1, autosomal recessive (Bulman et al. 2000) |
| DLL4 (Q9NR61) | 5/8 | --- |
| DNER (Q8NFT8) | 6/10 | --- |
| EDIL3 (O43854) | 1/3 | --- |
| EGF (P01133) | 1/9 | Hypomagnesemia 4 (Groenestege et al. 2007) |
| EGFL7 (Q9UHF1) | 1/2 | --- |
| EGFLAM (Q63HQ2) | 2/3 | --- |
| EMR1 (Q14246) | 4/6 | --- |
| EMR2 (Q9UHX3) | 1/5 | --- |
| EYS (Q5T1H1) | 11/27 | Retinitis pigmentosa 25 (Abd El-Aziz et al. 2008; Collin et al. 2008; Audo et al. 2010; Huang et al. 2010) |
| F7 (P08709) | 1/2 | Factor VII deficiency (O'Brien et al. 1991; Bernardi et al. 1994; Leonard et al. 1998; Girelli et al. 2000; Landau et al. 2009; Jiang et al. 2011) |
| F9 (P00740) | 1/2 | Hemophilia B; Thrombophilia, X-linked, due to factor IX defect (Green et al. 1989; Suehiro et al. 1989; de la Salle et al. 1993; Simioni et al. 2009) |
| F12 (P00748) | 1/2 | Factor XII deficiency; Hereditary angioedema 3 (Bernardi et al. 1987; Schloesser et al. 1995; Cichon et al. 2006; Dewald and Bork 2006) |
| FAT1 (Q14517) | 2/5 | --- |
| FAT2 (Q9NYQ8) | 1/2 | --- |
| FAT3 (Q8TDW7) | 3/4 | --- |
| FAT4 (Q6V0I7) | 5/6 | Van Maldergem syndrome 2 (Cappello et al. 2013) |
| FBLN1 (P23142) | 1/9 | Complex type of synpolydactyly; associated with human breast cancer (Debeer et al. 2002; Greene et al. 2003) |
| FBLN7 (Q53RD9) | 1/3 | --- |
| FBN2 (P35556) | 1/47 | Arthrogryposis, distal 9 (Putnam et al. 1995; Babcock et al. 1998; Park et al. 1998; Belleh et al. 2000; Gupta et al. 2002; Callewaert et al. 2009) |
| FBN3 (Q75N90) | 1/44 | --- |
| HABP2 (Q14520) | 1/3 | --- |
| HGFAC (Q04756) | 2/2 | --- |
| JAG1 (P78504) | 11/16 | Alagille syndrome 1; Tetralogy of Fallot (Oda et al. 1997; Krantz et al. 1998; Eldadah et al. 2001) |
| JAG2 (Q9Y219) | 9/16 | --- |
| LRP1 (Q07954) | 5/22 | --- |
| LRP1B (Q9NZR2) | 4/14 | --- |
| LTBP2 (Q14767) | 1/20 | Glaucoma 3, primary congenital, D; Microspherophakia and/or megalocornea, with ectopialentis and with or without secondary glaucoma; Weill-Marchesani syndrome 3 (Ali et al. 2009; Kumar et al. 2010; Haji-Seyed-Javadi et al. 2012) |
| MEGF6 (O75095) | 1/27 | --- |
| MEGF8 (Q7Z7M0) | 2/5 | Carpenter syndrome 2 (Twigg et al. 2012) |
| MEGF10 (Q96KG7) | 2/15 | Myopathy, early-onset, areflexia, respiratory distress, and dysphagia (Logan et al. 2011; Boyden et al. 2012) |
| MEGF11 (A6BM72) | 2/14 | --- |
| MMRN1 (Q13201) | 1/1 | Factor V Quebec (Hayward et al. 1996) |
| NCAN (O14594) | 2/2 | --- |
| NELL1 (Q92832) | 1/5 | --- |
| NID2 (Q14112) | 1/5 | --- |
| NOTCH1 (P46531) | 20/36 | Aortic valve disease 1 (Garg et al. 2005) |
| NOTCH2 (Q04721) | 20/36 | Alagille syndrome 2; Hajdu-Cheney syndrome (McDaniell et al. 2006; Isidor et al. 2011; Simpson et al. 2011) |
| NOTCH2NL (Q7Z3S9) | 5/6 | --- |
| NOTCH3 (Q9UM47) | 14/34 | Cerebral arteriopathy with subcortical infarcts and leukoencephalopathy; Myofibromatosis, infantile 2 (Joutel et al. 1997; Dichgans et al. 1999; Fouillade et al. 2008; Martignetti et al. 2013) |
| NOTCH4 (Q99466) | 18/29 | --- |
| PAMR1 (Q6UXH9) | 1/1 | --- |
| PEAR1 (Q5VY43) | 1/9 | --- |
| PGBM (P98160) | 3/4 | Schwartz-Jampel syndrome; Dyssegmental dysplasia Silverman-Handmaker type (Nicole et al. 2000; Arikawa-Hirasawa et al. 2001) |
| PGCB (Q96GW7) | 1/1 | --- |
| PROC (P04070) | 1/2 | Thrombophilia due to protein C deficiency, autosomal dominant and autosomal recessive (Romeo et al. 1987; Miyata et al. 1995; Couture et al. 1998) |
| PROZ (P22891) | 1/2 | --- |
| RELN (P78509) | 2/8 | Lissencephaly 2 (Hong et al. 2000) |
| SLIT1 (O75093) | 2/9 | --- |
| SLIT2 (O94813) | 3/7 | --- |
| SLIT3 (O75094) | 3/9 | --- |
| SNED1 (Q8TER0) | 10/15 | --- |
| SREC2 (Q96GP6) | 1/7 | Van den Ende-Gupta syndrome (Anastasio et al. 2010) |
| STAB1 (Q9NY15) | 3/16 | --- |
| STAB2 (Q8WWQ8) | 6/17 | --- |
| SUSD1 (Q6UWL2) | 2/3 | --- |
| SVEP1 (Q4LDE5) | 4/9 | --- |
| TEN1 (Q9UKZ4) | 1/8 | --- |
| TEN2 (Q9NT68) | 2/8 | --- |
| TEN4 (Q6N022) | 2/8 | --- |
| TIE1 (P35590) | 1/3 | --- |
| TPA (P00750) | 1/1 | Increased activity results in excessive bleeding; Defective release results in thrombosis or embolism (Degen et al. 1986). |
| TSP3 (P49746) | 1/3 | --- |
| UMOD (P07911) | 3/3 | Familial juvenile hyperuricemic nephropathy 1; Medullary cystic kidney disease 2; Glomerulocystic kidney disease with hyperuricemia and isosthenuria (Hart et al. 2002; Rampoldi et al. 2003) |
| UMODL1 (Q5DID0) | 1/3 | --- |
| UROK (P00749) | 1/1 | Quebec platelet disorder (Paterson et al. 2010) |
| VASN (Q6EMK4) | 1/1 | --- |
| VWA2 (Q5GFL6) | 2/2 | --- |
| VWDE (Q8N2E2) | 3/7 | --- |
| WIF1 (Q9Y5W5) | 2/5 | --- |
Potential targets of POFUT1 are listed based on a ScanProsite database search of all human proteins containing EGF repeats that contain the C2XXXX(S/T)C3 consensus sequence for O-fucosylation cross-referenced with the Uniprot database. Splice variants were not considered. The number of EGF repeats containing the consensus sequence/total number of EGF domains is listed, as well as any known human pathologies associated with the putative targets. Confirmed POFUT1 targets are listed in boldface.
Table II.
List of putative human gene targets of POFUT2
| Name and UNIPROT ID | Consensus/total | Known human pathology (if any) |
|---|---|---|
| ADAMTS1 (Q9UHI8) | 3/3 | --- |
| ADAMTS2 (O95450) | 2/4 | Ehlers-Danlos syndrome 7 C (Colige et al. 1999) |
| ADAMTS3 (O15072) | 2/4 | --- |
| ADAMTS4 (O75173) | 1/1 | --- |
| ADAMTS5 (Q9UNA0) | 2/2 | --- |
| ADAMTS6 (Q9UKP5) | 3/5 | --- |
| ADAMTS7 (Q9UKP4) | 5/8 | --- |
| ADAMTS8 (Q9UP79) | 2/2 | --- |
| ADAMTS9 (Q9P2N4) | 12/15 | --- |
| ADAMTS10 (Q9H324) | 3/5 | Weill-Marchesani syndrome 1 (Dagoneau et al. 2004; Kutz et al. 2008) |
| ADAMTS12 (P58397) | 6/8 | --- |
| ADAMTS13 (Q76LX8) | 7/8 | TTP, congenital (Levy et al. 2001; Kokame et al. 2002; Antoine et al. 2003; Schneppenheim et al. 2003; Ricketts et al. 2007) |
| ADAMTS14 (Q8WXS8) | 2/4 | --- |
| ADAMTS15 (Q8TE58) | 3/3 | --- |
| ADAMTS16 (Q8TE57) | 6/6 | --- |
| ADAMTS17 (Q8TE56) | 4/5 | Weill-Marchesani-like syndrome (Morales et al. 2009) |
| ADAMTS18 (Q8TE60) | 4/5 | Microcornea, myopic chorioretinal atrophy, and telecanthus (Aldahmesh et al. 2013) |
| ADAMTS19 (Q8TE59) | 4/5 | --- |
| ADAMTS20 (P59510) | 11/15 | --- |
| ADAMTSL1 (Q8N6G6) | 8/9 | --- |
| ADAMTSL2 (Q86TH1) | 6/7 | Geleophysic dysplasia 1 (Le Goff et al. 2008) |
| ADAMTSL3 (P82987) | 8/10 | --- |
| ADAMTSL4 (Q6UY14) | 2/6 | Ectopialentis 2, isolated (Ahram et al. 2009); Ectopialentis et pupillae (Christensen et al. 2010) |
| ADAMTSL5 (Q6ZMM2) | 1/1 | --- |
| BAI1 (O14514) | 4/5 | --- |
| BAI2 (O60241) | 4/4 | --- |
| BAI3 (O60242) | 4/4 | --- |
| C-6 (P13671) | 1/3 | Complement component 6 deficiency (Ikinciogullari et al. 2005) |
| CILP2 (Q8IUL8) | 1/1 | --- |
| CTGF (P29279) | 1/1 | --- |
| CYR61 (O0062) | 1/1 | --- |
| HMCN1 (Q96RW7) | 6/6 | Age-related macular degeneration 1 (Schultz et al. 2003) |
| ISM1 (B1AKI9) | 1/1 | --- |
| NOV (P48745) | 1/1 | --- |
| PPN (O95428) | 4/5 | --- |
| PROP (P27918) | 4/7 | Properdin deficiency (Fredrikson et al. 1996; Fredrikson et al. 1998; van den Bogaard et al. 2000) |
| SEM5A (Q13591) | 2/7 | --- |
| SEM5B (Q9P283) | 2/5 | --- |
| SPON1 (Q9HCB6) | 5/6 | --- |
| SSPO (A2VEC9) | 10/24 | --- |
| THS7A (Q9UPZ6) | 4/15 | --- |
| THS7B (Q9C0I4) | 4/18 | --- |
| THSD1 (Q9NS62) | 1/1 | --- |
| THSD4 (Q6ZMP0) | 3/6 | --- |
| TSP1 (P07996) | 3/3 | --- |
| TSP2 (P35442) | 3/3 | Intervertebral disc disease (Hirose et al. 2008) |
| WISP1 (O95388) | 1/1 | --- |
| WISP2 (O76076) | 1/1 | --- |
| WISP3 (O95389) | 1/1 | Progressive pseudorheumatoidarthropathy of childhood (Hurvitz et al. 1999) |
Potential targets of POFUT2 are listed based on a ScanProsite database search of all human proteins containing TSRs that also contain the CX2−3(S/T)C consensus sequence for O-fucosylation cross-referenced with the Uniprot database. Splice variants were not considered. The number of TSRs containing the consensus sequence/total number of TSR domains is listed, as well as any known human pathologies associated with the putative targets. Confirmed POFUT2 targets are indicated in boldface.
O-Fucosylation of EGF repeats
The Notch family of receptors has more predicted O-fucose sites than any other protein (see Table I) (Moloney et al. 2000b). Pofut1 knockout is embryonic lethal in mice (Shi and Stanley 2003; Okamura and Saga 2008). These knockout mice show severe growth retardation during early embryogenesis, particularly in somite formation. Neural tube, cardiac and blood vessel defects are also evident in these mice—all phenotypes associated with defects in Notch signaling. POFUT1 also plays a critical role in mediating other Notch dependent processes including promotion of T-cell differentiation during lymphopoiesis (Yao et al. 2011). Results from many groups reveal that POFUT1 is essential for normal Notch-ligand binding and Notch signaling (Shi and Stanley 2003; Okajima et al. 2005; Rampal et al. 2005; Stahl et al. 2008; Kakuda and Haltiwanger 2017). Recently reported Notch1-DLL4 (Luca et al. 2015) and Notch1-JAG1 (Luca et al. 2017) cocrystal structures have additionally shown that the fucose on EGF repeat 12 of the extracellular domain of Notch1 directly interacts with the Notch activating ligand DLL4, and the fucose on EGF repeats 8 and 12 interact with JAG1, demonstrating the potential importance of these fucose residues at the interface of protein-protein interactions. In addition to its fucosyltransferase activity, the Drosophila homolog of POFUT1, Ofut1, also acts as a chaperone for Notch protein folding (Okajima et al. 2005), although it is not clear that this function is conserved in mammalian systems (Stahl et al. 2008).
Fringe enzymes can elongate O-fucose residues with a GlcNAc to further regulate Notch signaling (Figure 4A) (Moloney et al. 2000a). Fringe was originally described in Drosophila, where it was recognized that mutations in fringe caused a Notching phenotype in wings (Irvine and Wieschaus 1994). Further work demonstrated that Fringe is an important regulator of Notch signaling (Panin et al. 1997; Klein and Arias 1998). While Drosophila expresses only one Fringe enzyme, there are three mammalian homologs (Lunatic Fringe, Manic Fringe and Radical Fringe) (Johnston et al. 1997). Fucose elongation by any of the three Fringes causes an increase in Notch signaling mediated by members of the Delta-like ligand (DLL) family, but can have variable effects on signaling initiated by the Jagged (JAG) family of ligands in mammals (LeBon et al. 2014; Kakuda and Haltiwanger 2017). These enzymes play extremely important roles in regulating Notch signaling throughout development. For instance, Lunatic Fringe is required for normal somitogenesis (Evrard et al. 1998; Zhang and Gridley 1998). Recent work has demonstrated that addition of GlcNAc by Fringe to Notch's extracellular domain creates a “Fringe-mediated Notch code,” where modifications at specific EGF repeats can either enhance DLL-mediated signaling or inhibit JAG-mediated Notch signaling (Harvey et al. 2016; Kakuda and Haltiwanger 2017).
While POFUT1 is predicted to modify many other proteins based on consensus sequences, modification of most of these proteins has not been confirmed (Table I). Dysregulation of POFUT1 activity has, however, been shown to play an important role in several disorders and processes involving other proteins. Heterozygous mutations in POFUT1 have been associated with a rare dermatologic condition, Dowling-Degos disease, characterized by pigmentation abnormalities (Li et al. 2013; Chen et al. 2014). O-Fucosylation of EGF repeats also appears to play an important role in regulating the clustering of acetylcholine receptors by agrin (Kim et al. 2008). Amplification of POFUT1 has also been implicated as a prognostic marker and potential drug target for several cancer types including breast cancer (Milde-Langosch et al. 2014), oral squamous cell carcinoma (Yokota et al. 2013) and hepatocellular carcinoma (Sawey et al. 2011; Ma et al. 2016).
O-Fucosylation of TSRs
Like Pofut1, knockout of Pofut2 in mice is embryonic lethal with severe defects in gastrulation, indicating its importance in development (Du et al. 2010). A recent report strongly suggests that ADAMTS9 is the target protein responsible for these defects, as knockout of Adamts9 resulted in a phenotype essentially identical to Pofut2 knockout (Benz et al. 2016). Other target proteins play an important role in regulating cell proliferation, migration and differentiation. O-Fucosylation of CCN1, which is required for its secretion, has been shown to be vital to these processes (Perbal 2004; Niwa et al. 2015). Additionally, members of the A Disintegrin and Metalloproteinase with ThromboSpondin motifs (ADAMTS) family of metalloproteinases play critical roles in mediating angiogenesis, extracellular structuring, inflammation and other developmental processes (Dubail and Apte 2015). Several proteins in this family also depend on O-fucosylation for their secretion (Ricketts et al. 2007; Wang et al. 2007; Vasudevan et al. 2015; Benz et al. 2016; Dubail et al. 2016). One of the affected proteins, ADAMTS13, is particularly noteworthy as its deficiency results in thrombotic thrombocytopenic purpura (TTP), a life threatening hematologic disorder (Ricketts et al. 2007). More work will be needed to determine the importance of O-fucosylation for processes mediated by other specific proteins.
O-Fucose residues on TSRs can be elongated with glucose by β3-glucosyltransferase (B3GLCT) (Figure 4B) further promoting secretion of target proteins. Mutations in this enzyme cause the human disease Peters plus syndrome, characterized by a number of defects in the eye chambers, limbs and intellectual development (Oberstein et al. 2006). Elimination of B3GLCT activity results in reduced secretion of some, but not all of the proteins regulated by POFUT2 modification (Vasudevan et al. 2015). A recent report from our lab suggests that the carbohydrate modifications added by POFUT2 and B3GLCT serve as a novel quality control system that recognizes and stabilizes properly folded TSRs. POFUT2 recognizes and sequentially fucosylates properly folded TSRs in the ER allowing B3GLCT to bind and add glucose to these TSRs. The data suggest that addition of these sugars stabilizes the folded form of the TSR, removing it from a folding cycle in the ER. Once all TSRs on a protein have been processed the protein can exit the ER (Vasudevan et al. 2015).
Fucose analogs
The development of chemically modified fucose analogs has revolutionized the study of fucose and fucosyltransferases by providing a valuable tool for modifying, tracking and inhibiting fucosylation of proteins. As early as 1992, it was recognized that the Lewis fucosyltransferase could tolerate GDP-fucose modified at the C-6 position by even a large addition, and that this could be used as a powerful tool for labeling lipids and proteins that incorporated this modified form of fucose (Srivastava et al. 1992). Taking advantage of this enzymatic promiscuity, researchers have developed strategies to use fucose analogs with an azide or alkyne group at the C-6 position to metabolically incorporate fucose analogs producing labeled fucosylated glycoproteins. Once incorporated into target proteins, “click”-chemistry can be used to attach fluorophores or other groups to the fucose analog. This strategy has allowed for successful in vivo imaging of fucose in several model organisms (Laughlin et al. 2008; Laughlin and Bertozzi 2009), plants (Anderson et al. 2012) and cell cultures (Sawa et al. 2006; Hsu et al. 2007). Others have used this strategy to tag fucosylated proteins with biotin allowing for their identification using anti-biotin or streptavidin probes for detection by Western blot or isolation using a streptavidin pulldown (Liu et al. 2012; Al-Shareffi et al. 2013), potentially allowing for the identification of unknown fucosylated glycoproteins. These tools continue to develop, as one group recently showed that 7-alkynyl fucose is more efficiently utilized by FUT8 than 6-alkynyl fucose (Kizuka et al. 2016). This type of development could ultimately allow for more efficient and/or targeted labeling of glycoproteins.
In addition to their utility for identifying and tracking fucosylated proteins, fucose analogs have also been investigated as potential inhibitors of fucosyltransferases. Monosaccharide analogs have already been approved for the treatment of lysosomal storage disorders, diabetes and are being developed for potential use in other diseases (Gloster and Vocadlo 2012). As discussed above, fucose plays an important role in many cancer types and other disorders, so the development of fucosyltransferase inhibitors might serve as a valuable clinical tool. Several groups have begun screening and developing inhibitors toward this end (Fuster and Esko 2005; Hosoguchi et al. 2010; Rillahan et al. 2011; Dalziel et al. 2014). One group used click chemistry to generate fucose analogs with a variety of different groups and screened them as potential fucosyltransferase inhibitors, identifying several candidates (Lee et al. 2003). Fucose analogs that inhibit transfer of fucose by several fucosyltransferases including FUT4, FUT7 and FUT8 can be used to prevent selectin-mediated cell migration, a process that plays an important role in cancer metastasis (Rillahan et al. 2012; Villalobos et al. 2015). These fucose analog inhibitors are orally active and slow tumor cell proliferation in mice (Okeley et al. 2013). Additionally, taking advantage of the role fucosylation plays in regulating learning and memory, fucose analogs have been used to cause reversible amnesia and inhibition of long-term memory formation (Rose and Jork 1987; Krug et al. 1991; Lorenzini et al. 1997).
In addition to their use as fucosyltransferase inhibitors, fucose analogs that are tolerated by fucosyltransferases can be incorporated into target proteins and potentially alter protein behavior. For example, fucose analogs that are efficiently utilized by POFUT1 (Al-Shareffi et al. 2013) can be used to alter Notch signaling. Notch receptors are a major POFUT1 target, as discussed above, and can be activated by two different ligand families: DLL and JAG ligands. Modification of attached fucose residues at the 6-carbon with alkyne or alkene groups causes an inhibition of Notch activation that preferentially affects DLLs (Schneider et al. In Review). This ability to discriminate between ligands provides a potentially very useful tool for evaluating Notch signaling. Similar strategies might be developed into targeted therapeutics for disease.
Acknowledgements
We would like to thank Drs Pamela Stanley, Hideyuki Takeuchi and Shinako Kakuda for their helpful comments and suggestions.
Funding
Original work was supported by NIH grant GM061126.
Conflict of interest statement
None declared.
Abbreviations
- ADAMTS
A Disintegrin and Metalloproteinase with ThromboSpondin motifs
- ADCC
Antibody dependent cellular cytotoxicity
- AFP
α-Fetoprotein
- B3GLCT
β3-glucosyltransferase
- BPH
Benign prostatic hyperplasia
- CLL
Chronic lymphocytic leukemia
- DLL
Delta-like ligand
- EGF
Epidermal growth factor-like
- FUT
Fucosyltransferase
- FX protein
GDP-keto-6-deoxymannose 3,5 epimerase
- GalNAc
N-acetylgalactosamine
- GlcNAc
N-acetylglucosamine
- JAG
Jagged
- LAD2
Leukocyte adhesion deficiency II
- LTP
Long-term potentiation
- mAb
Monoclonal antibody
- POFUT
Protein O-fucosyltransferase
- PPS
Peters Plus Syndrome
- PSA
Prostate-specific antigen
- RBC
Red blood cell
- TSR
Thrombospondin type 1 repeat
- TTP
Thrombotic thrombocytopenic purpura
References
- Abd El-Aziz MM, Barragan I, O'Driscoll CA, Goodstadt L, Prigmore E, Borrego S, Mena M, Pieras JI, El-Ashry MF, Safieh LA et al. 2008. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 40:1285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczyk B, Tharmalingam T, Rudd PM. 2012. Glycans as cancer biomarkers. Biochim Biophys Acta (BBA)-Gen Subjects. 1820:1347–1353. [DOI] [PubMed] [Google Scholar]
- Ahram D, Sato TS, Kohilan A, Tayeh M, Chen S, Leal S, Al-Salem M, El-Shanti H. 2009. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Human Genetics. 84:274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shareffi E, Chaubard JL, Leonhard-Melief C, Wang SK, Wong CH, Haltiwanger RS. 2013. 6-Alkynyl fucose is a bioorthogonal analog for O-fucosylation of epidermal growth factor-like repeats and thrombospondin type-1 repeats by protein O-fucosyltransferases 1 and 2. Glycobiology. 23:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Alshammari MJ, Khan AO, Mohamed JY, Alhabib FA, Alkuraya FS. 2013. The syndrome of microcornea, myopic chorioretinal atrophy, and telecanthus (MMCAT) is caused by mutations in ADAMTS18. Hum Mutat. 34:1195–1199. [DOI] [PubMed] [Google Scholar]
- Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M et al. 2009. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet. 84:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff M, Carter JE, Chadwick RB, Johnson C, Grasbeck R, Abdelaal MA, Broch H, Jenner LB, Verroust PJ, Moestrup SK et al. 1999. Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat Genet. 21:309–313. [DOI] [PubMed] [Google Scholar]
- Anastasio N, Ben-Omran T, Teebi A, Ha KC, Lalonde E, Ali R, Almureikhi M, Der Kaloustian VM, Liu J, Rosenblatt DS et al. 2010. Mutations in SCARF2 are responsible for Van Den Ende-Gupta syndrome. Am J Hum Genet. 87:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Wallace IS, Somerville CR. 2012. Metabolic click-labeling with a fucose analog reveals pectin delivery, architecture, and dynamics in Arabidopsis cell walls. Proc Natl Acad Sci USA. 109:1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine G, Zimmermann K, Plaimauer B, Grillowitzer M, Studt JD, Lammle B, Scheiflinger F. 2003. ADAMTS13 gene defects in two brothers with constitutional thrombotic thrombocytopenic purpura and normalization of von Willebrand factor-cleaving protease activity by recombinant human ADAMTS13. Br J Haematol. 120:821–824. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Isokawa O, Suda T, Watanabe M, Suzuki Y, Asakura H. 1998. The fucosylation index of α‐fetoprotein as a possible prognostic indicator for patients with hepatocellular carcinoma. Cancer. 83:2076–2082. [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, Yamada Y. 2001. Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet. 27:431–434. [DOI] [PubMed] [Google Scholar]
- Ashikov A, Routier F, Fuhlrott J, Helmus Y, Wild M, Gerardy-Schahn R, Bakker H. 2005. The human solute carrier gene SLC35B4 encodes a bifunctional nucleotide sugar transporter with specificity for UDP-xylose and UDP-N-acetylglucosamine. J Biol Chem. 280:27230–27235. [DOI] [PubMed] [Google Scholar]
- Audo I, Sahel JA, Mohand-Said S, Lancelot ME, Antonio A, Moskova-Doumanova V, Nandrot EF, Doumanov J, Barragan I, Antinolo G et al. 2010. EYS is a major gene for rod-cone dystrophies in France. Hum Mutat. 31:E1406–E1435. [DOI] [PubMed] [Google Scholar]
- Babcock D, Gasner C, Francke U, Maslen C. 1998. A single mutation that results in an Asp to His substitution and partial exon skipping in a family with congenital contractural arachnodactyly. Hum Genet. 103:22–28. [DOI] [PubMed] [Google Scholar]
- Bamford RN, Roessler E, Burdine RD, Saplakoglu U, dela Cruz J, Splitt M, Goodship JA, Towbin J, Bowers P, Ferrero GB et al. 2000. Loss-of-function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat Genet. 26:365–369. [DOI] [PubMed] [Google Scholar]
- Beck A, Reichert JM. 2012. Marketing approval of mogamulizumab: A triumph for glyco-engineering In: MAbs. Taylor & Francis; p. 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker DJ, Lowe JB. 2003. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 13:41R–53R. [DOI] [PubMed] [Google Scholar]
- Belleh S, Zhou G, Wang M, Der Kaloustian VM, Pagon RA, Godfrey M. 2000. Two novel fibrillin-2 mutations in congenital contractural arachnodactyly. Am J Med Genet. 92:7–12. [PubMed] [Google Scholar]
- Benz BA, Nandadasa S, Takeuchi M, Grady RC, Takeuchi H, LoPilato RK, Kakuda S, Somerville RP, Apte SS, Haltiwanger RS. 2016. Genetic and biochemical evidence that gastrulation defects in Pofut2 mutants result from defects in ADAMTS9 secretion. Dev Biol. 416:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi F, Liney DL, Patracchini P, Gemmati D, Legnani C, Arcieri P, Pinotti M, Redaelli R, Ballerini G, Pemberton S et al. 1994. Molecular defects in CRM+ factor VII deficiencies: Modelling of missense mutations in the catalytic domain of FVII. Br J Haematol. 86:610–618. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Marchetti G, Patracchini P, del Senno L, Tripodi M, Fantoni A, Bartolai S, Vannini F, Felloni L, Rossi L et al. 1987. Factor XII gene alteration in Hageman trait detected by TaqI restriction enzyme. Blood. 69:1421–1424. [PubMed] [Google Scholar]
- Bird J, Kimber S. 1984. Oligosaccharides containing fucose linked α(1–3) and α(1–4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev Biol. 104:449–460. [DOI] [PubMed] [Google Scholar]
- Boyden SE, Mahoney LJ, Kawahara G, Myers JA, Mitsuhashi S, Estrella EA, Duncan AR, Dey F, DeChene ET, Blasko-Goehringer JM et al. 2012. Mutations in the satellite cell gene MEGF10 cause a recessive congenital myopathy with minicores. Neurogenetics. 13:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD. 2000. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 24:438–441. [DOI] [PubMed] [Google Scholar]
- Callewaert BL, Loeys BL, Ficcadenti A, Vermeer S, Landgren M, Kroes HY, Yaron Y, Pope M, Foulds N, Boute O et al. 2009. Comprehensive clinical and molecular assessment of 32 probands with congenital contractural arachnodactyly: Report of 14 novel mutations and review of the literature. Hum Mutat. 30:334–341. [DOI] [PubMed] [Google Scholar]
- Cappello S, Gray MJ, Badouel C, Lange S, Einsiedler M, Srour M, Chitayat D, Hamdan FF, Jenkins ZA, Morgan T et al. 2013. Mutations in genes encoding the cadherin receptor-ligand pair DCHS1 and FAT4 disrupt cerebral cortical development. Nat Genet. 45:1300–1308. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Jan Y-H, Juan Y-H, Yang C-J, Huang M-S, Yu C-J, Yang P-C, Hsiao M, Hsu T-L, Wong C-H. 2013. Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proc Natl Acad Sci USA. 110:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Li Y, Liu H, XA Fu, Yu Y, Yu G, Wang C, Bao F, Liany H, Wang Z. 2014. Analysis of POFUT1 gene mutation in a Chinese family with Dowling-Degos disease. PLoS One. 9:e104496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T-C, Tu S-H, Chen L-C, Chen M-Y, Chen W-Y, Lin Y-K, Ho C-T, Lin S-Y, Wu C-H, Ho Y-S. 2015. Down-regulation of α-L-fucosidase 1 expression confers inferior survival for triple-negative breast cancer patients by modulating the glycosylation status of the tumor cell surface. Oncotarget. 6:21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Fiskerstrand T, Knappskog PM, Boman H, Rodahl E. 2010. A novel ADAMTSL4 mutation in autosomal recessive ectopia lentis et pupillae. Investig Ophthalmol Vis SciJ. 51:6369–6373. [DOI] [PubMed] [Google Scholar]
- Cichon S, Martin L, Hennies HC, Muller F, Van Driessche K, Karpushova A, Stevens W, Colombo R, Renne T, Drouet C et al. 2006. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 79:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J, Miller ON, Sellinger OZ. 1964. The metabolism of L-fucose in the rat. J Biol Chem. 239:4011–4017. [PubMed] [Google Scholar]
- Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W et al. 1999. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 65:308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin RW, Littink KW, Klevering BJ, van den Born LI, Koenekoop RK, Zonneveld MN, Blokland EA, Strom TM, Hoyng CB, den Hollander AI et al. 2008. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet. 83:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture P, Demers C, Morissette J, Delage R, Jomphe M, Couture L, Simard J. 1998. Type I protein C deficiency in French Canadians: Evidence of a founder effect and association of specific protein C gene mutations with plasma protein C levels. Thromb Haemost. 80:551–556. [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Megarbane A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L et al. 2004. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 75:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. 2014. Emerging principles for the therapeutic exploitation of glycosylation. Science. 343:1235681. [DOI] [PubMed] [Google Scholar]
- Davey R, Tourault M, Holland P. 1978. The clinical significance of anti‐H in an individual with the Oh (Bombay) phenotype. Transfusion. 18:738–742. [DOI] [PubMed] [Google Scholar]
- de la Salle C, Charmantier JL, Ravanat C, Ohlmann P, Hartmann ML, Schuhler S, Bischoff R, Ebel C, Roecklin D, Balland A et al. 1993. The Arg-4 mutant factor IX Strasbourg 2 shows a delayed activation by factor XIa. Nouvelle Revue Francaise D'hematol. 35:473–480. [PubMed] [Google Scholar]
- de Peredo AG, Klein D, Macek B, Hess D, Peter-Katalinic J, Hofsteenge J. 2002. C-mannosylation and o-fucosylation of thrombospondin type 1 repeats. Mol Cell Proteom. 1:11–18. [DOI] [PubMed] [Google Scholar]
- Debeer P, Schoenmakers EF, Twal WO, Argraves WS, De Smet L, Fryns JP, Van De Ven WJ. 2002. The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J Med Genet. 39:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen SJ, Rajput B, Reich E. 1986. The human tissue plasminogen activator gene. J Biol Chem. 261:6972–6985. [PubMed] [Google Scholar]
- den Hollander AI, Heckenlively JR, van den Born LI, de Kok YJ, van der Velde-Visser SD, Kellner U, Jurklies B, van Schooneveld MJ, Blankenagel A, Rohrschneider K et al. 2001. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 69:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, ten Brink JB, de Kok YJ, van Soest S, van den Born LI, van Driel MA, van de Pol DJ, Payne AM, Bhattacharya SS, Kellner U et al. 1999. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet. 23:217–221. [DOI] [PubMed] [Google Scholar]
- Dewald G, Bork K. 2006. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun. 343:1286–1289. [DOI] [PubMed] [Google Scholar]
- Dichgans M, Filippi M, Bruning R, Iannucci G, Berchtenbreiter C, Minicucci L, Uttner I, Crispin A, Ludwig H, Gasser T et al. 1999. Quantitative MRI in CADASIL: Correlation with disability and cognitive performance. Neurology. 52:1361–1367. [DOI] [PubMed] [Google Scholar]
- Dipta T, Hossain A. 2011. The bombay blood group: Are we out of risk. Mymensingh Med J. 20:536–540. [PubMed] [Google Scholar]
- Du J, Takeuchi H, Leonhard-Melief C, Shroyer KR, Dlugosz M, Haltiwanger RS, Holdener BC. 2010. O-fucosylation of thrombospondin type 1 repeats restricts epithelial to mesenchymal transition (EMT) and maintains epiblast pluripotency during mouse gastrulation. Dev Biol. 346:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubail J, Apte SS. 2015. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 44:24–37. [DOI] [PubMed] [Google Scholar]
- Dubail J, Vasudevan D, Wang LW, Earp SE, Jenkins MW, Haltiwanger RS, Apte SS. 2016. Impaired ADAMTS9 secretion: A potential mechanism for eye defects in Peters Plus Syndrome. Sci Rep. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, Nyrén O. 2010. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: A cohort study. Am J Epidemiol. 172:1280–1285. [DOI] [PubMed] [Google Scholar]
- Eldadah ZA, Hamosh A, Biery NJ, Montgomery RA, Duke M, Elkins R, Dietz HC. 2001. Familial Tetralogy of Fallot caused by mutation in the jagged1 gene. Hum Mol Genet. 10:163–169. [DOI] [PubMed] [Google Scholar]
- Etzioni A, Sturla L, Antonellis A, Green ED, Gershoni‐Baruch R, Berninsone PM, Hirschberg CB, Tonetti M. 2002. Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am J Med Genet. 110:131–135. [DOI] [PubMed] [Google Scholar]
- Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. 1998. Lunatic fringe is an essential mediator of somite segmentation and patterning. Nature. 394:377–381. [DOI] [PubMed] [Google Scholar]
- Ezawa I, Sawai Y, Kawase T, Okabe A, Tsutsumi S, Ichikawa H, Kobayashi Y, Tashiro F, Namiki H, Kondo T. 2016. Novel p53 target gene FUCA1 encodes a fucosidase and regulates growth and survival of cancer cells. Cancer Sci. 107:734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M. 2011. Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci USA. 108:12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Marrero JA. 2014. Emerging trends in hepatocellular carcinoma: Focus on diagnosis and therapeutics. Clin Med Insights Oncol. 8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouillade C, Chabriat H, Riant F, Mine M, Arnoud M, Magy L, Bousser MG, Tournier-Lasserve E, Joutel A. 2008. Activating NOTCH3 mutation in a patient with small-vessel-disease of the brain. Hum Mutat. 29:452. [DOI] [PubMed] [Google Scholar]
- Fredrikson GN, Gullstrand B, Westberg J, Sjoholm AG, Uhlen M, Truedsson L. 1998. Expression of properdin in complete and incomplete deficiency: Normal in vitro synthesis by monocytes in two cases with properdin deficiency type II due to distinct mutations. J Clin Immunol. 18:272–282. [DOI] [PubMed] [Google Scholar]
- Fredrikson GN, Westberg J, Kuijper EJ, Tijssen CC, Sjoholm AG, Uhlen M, Truedsson L. 1996. Molecular characterization of properdin deficiency type III: Dysfunction produced by a single point mutation in exon 9 of the structural gene causing a tyrosine to aspartic acid interchange. J Immunol (Baltimore, MD: 1950). 157:3666–3671. [PubMed] [Google Scholar]
- Fuster MM, Esko JD. 2005. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat Rev Cancer. 5:526–542. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Epp A, Linares G, Westendorf L, Sutherland MK, Neff-LaFord H, Drachman JG, Peng S, Law C-L. 2015. A sugar engineered non-fucosylated anti-CD40 antibody, SEA-CD40, with enhanced immune stimulatory activity alone and in combination with immune checkpoint inhibitors. ASCO Annual Meeting Proceedings. p. 3074.
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. 2005. Mutations in NOTCH1 cause aortic valve disease. Nature. 437:270–274. [DOI] [PubMed] [Google Scholar]
- Ginsburg V. 1960. Formation of guanosine diphosphate L-fucose from guanosine diphosphate D-mannose. J Biol Chem. 235:2196–2201. [PubMed] [Google Scholar]
- Girelli D, Russo C, Ferraresi P, Olivieri O, Pinotti M, Friso S, Manzato F, Mazzucco A, Bernardi F, Corrocher R. 2000. Polymorphisms in the factor VII gene and the risk of myocardial infarction in patients with coronary artery disease. N Engl J Med. 343:774–780. [DOI] [PubMed] [Google Scholar]
- Gloster TM, Vocadlo DJ. 2012. Developing inhibitors of glycan processing enzymes as tools for enabling glycobiology. Nat Chem Biol. 8:683–694. [DOI] [PubMed] [Google Scholar]
- Goggins M. 2005. Molecular markers of early pancreatic cancer. J Clin Oncol. 23:4524–4531. [DOI] [PubMed] [Google Scholar]
- Goldmuntz E, Bamford R, Karkera JD, dela Cruz J, Roessler E, Muenke M. 2002. CFC1 mutations in patients with transposition of the great arteries and double-outlet right ventricle. Am J Hum Genet. 70:776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PM, Bentley DR, Mibashan RS, Nilsson IM, Giannelli F. 1989. Molecular pathology of haemophilia B. EMBO J. 8:1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LM, Twal WO, Duffy MJ, McDermott EW, Hill AD, O'Higgins NJ, McCann AH, Dervan PA, Argraves WS, Gallagher WM. 2003. Elevated expression and altered processing of fibulin-1 protein in human breast cancer. Br Jancer. 88:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenestege WM, Thebault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van Cutsem E, Hoenderop JG, Knoers NV et al. 2007. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Investig. 117:2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PA, Putnam EA, Carmical SG, Kaitila I, Steinmann B, Child A, Danesino C, Metcalfe K, Berry SA, Chen E et al. 2002. Ten novel FBN2 mutations in congenital contractural arachnodactyly: Delineation of the molecular pathogenesis and clinical phenotype. Hum Mutat. 19:39–48. [DOI] [PubMed] [Google Scholar]
- Haji-Seyed-Javadi R, Jelodari-Mamaghani S, Paylakhi SH, Yazdani S, Nilforushan N, Fan JB, Klotzle B, Mahmoudi MJ, Ebrahimian MJ, Chelich N et al. 2012. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum Mutat. 33:1182–1187. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS. 2004. Glycoprotein-Mediated Cell Interactions, O-linked. In: Lennarz WJ, Lane MD, editors. Encyclopedia of biological chemistry. Elsevier, Vol. 2, p. 277–282. [Google Scholar]
- Haltiwanger RS. 2009. Fucose is on the TRAIL of colon cancer. Gastroenterology. 137:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani M, Fanale MA, Bello CM, Kipps TJ, Offner F, Verhoef G, Federico M, Gregory SA, Sonet A, Assouline S. 2013. Safety profile and clinical response to MEDI-551, a humanized monoclonal anti-CD19, in a phase 1/2 study in adults with relapsed or refractory advanced B-cell malignancies. Blood. 122:1810–1810. [Google Scholar]
- Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM et al. 2002. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 39:882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BM, Rana NA, Moss H, Leonardi J, Jafar-Nejad H, Haltiwanger RS. 2016. Mapping sites of O-glycosylation and fringe elongation on Drosophila Notch. J Biol Chem. M116:732537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward CP, Rivard GE, Kane WH, Drouin J, Zheng S, Moore JC, Kelton JG. 1996. An autosomal dominant, qualitative platelet disorder associated with multimerin deficiency, abnormalities in platelet factor V, thrombospondin, von Willebrand factor, and fibrinogen and an epinephrine aggregation defect. Blood. 87:4967–4978. [PubMed] [Google Scholar]
- Hidalgo A, Ma S, Peired AJ, Weiss LA, Cunningham-Rundles C, Frenette PS. 2003. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood. 101:1705–1712. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Chiba K, Karasugi T, Nakajima M, Kawaguchi Y, Mikami Y, Furuichi T, Mio F, Miyake A, Miyamoto T et al. 2008. A functional polymorphism in THBS2 that affects alternative splicing and MMP binding is associated with lumbar-disc herniation. Am J Hum Genet. 82:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homeister JW, Thall AD, Petryniak B, Malý P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G. 2001. The α (1, 3) fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 15:115–126. [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA. 2000. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 26:93–96. [DOI] [PubMed] [Google Scholar]
- Hoskins LC. 1967. The ABO blood group antigens and their secretion by healthy and diseased gastric mucosa. Ann N Y Acad Sci. 140:848–860. [DOI] [PubMed] [Google Scholar]
- Hosoguchi K, Maeda T, Furukawa J-I, Shinohara Y, Hinou H, Sekiguchi M, Togame H, Takemoto H, Kondo H, Nishimura S-I. 2010. An efficient approach to the discovery of potent inhibitors against glycosyltransferases. J Med Chem. 53:5607–5619. [DOI] [PubMed] [Google Scholar]
- Hsu T-L, Hanson SR, Kishikawa K, Wang S-K, Sawa M, Wong C-H. 2007. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc Natl Acad Sci USA. 104:2614–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Farkas T, Zhong W, Tan M, Thornton S, Morrow AL, Jiang X. 2005. Norovirus and histo-blood group antigens: Demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol. 79:6714–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zhang J, Li C, Yang G, Liu M, Wang QK, Tang Z. 2010. Identification of a novel homozygous nonsense mutation in EYS in a Chinese family with autosomal recessive retinitis pigmentosa. BMC Med Genet. 11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, Holderbaum D, Pauli RM, Herd JK, Van Hul EV et al. 1999. Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet. 23:94–98. [DOI] [PubMed] [Google Scholar]
- Hutson AM, Atmar RL, Graham DY, Estes MK. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 185:1335–1337. [DOI] [PubMed] [Google Scholar]
- Huze C, Bauche S, Richard P, Chevessier F, Goillot E, Gaudon K, Ben Ammar A, Chaboud A, Grosjean I, Lecuyer HA et al. 2009. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 85:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara Y, Nishihara S, Yasutomi H, Kitamura T, Matsuo K, Shimizu N, Inada K-I, Kodera Y, Yamamura Y, Narimatsu H. 2001. Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidemiol Biomark Prevent. 10:971–977. [PubMed] [Google Scholar]
- Ikinciogullari A, Tekin M, Dogu F, Reisli I, Tanir G, Yi Z, Garrison N, Brilliant MH, Babacan E. 2005. Meningococccal meningitis and complement component 6 deficiency associated with oculocutaneous albinism. Eur J Pediatr. 164:177–179. [DOI] [PubMed] [Google Scholar]
- Illidge T, Klein C, Sehn LH, Davies A, Salles G, Cartron G. 2015. Obinutuzumab in hematologic malignancies: Lessons learned to date. Cancer Treatment Rev. 41:784–792. [DOI] [PubMed] [Google Scholar]
- Ilver D, Arnqvist A, Ögren J, Frick I-M, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 279:373–377. [DOI] [PubMed] [Google Scholar]
- Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. 1991. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 113:1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KD, Wieschaus E. 1994. fringe, a Boundary-specific signaling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell. 79:595–606. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Massaro DJ, Heath EC. 1968. The metabolism of l-fucose III. The enzymatic synthesis of β-l-fucose 1-phosphate. J Biol Chem. 243:1103–1109. [PubMed] [Google Scholar]
- Ishii T, Ishida T, Utsunomiya A, Inagaki A, Yano H, Komatsu H, Iida S, Imada K, Uchiyama T, Akinaga S. 2010. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 16:1520–1531. [DOI] [PubMed] [Google Scholar]
- Ishikawa HO, Ayukawa T, Nakayama M, Higashi S, Kamiyama S, Nishihara S, Aoki K, Ishida N, Sanai Y, Matsuno K. 2010. Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila. J Biol Chem. 285:4122–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidor B, Lindenbaum P, Pichon O, Bezieau S, Dina C, Jacquemont S, Martin-Coignard D, Thauvin-Robinet C, Le Merrer M, Mandel JL et al. 2011. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nat Genet. 43:306–308. [DOI] [PubMed] [Google Scholar]
- Itai S, Nishikata J, Yoneda T, Ohmori K, Yamabe H, Arii S, Tobe T, Kannagi R. 1991. Tissue distribution of 2‐3 and 2‐6 sialyl lewis A antigens and significance of the ratio of two antigens for the differential diagnosis of malignant and benign disorders of the digestive tract. Cancer. 67:1576–1587. [DOI] [PubMed] [Google Scholar]
- Jeschke U, Mylonas I, Shabani N, Kunert-Keil C, Schindlbeck C, Gerber B, Friese K. 2005. Expression of sialyl lewis X, sialyl Lewis A, E-cadherin and cathepsin-D in human breast cancer: Immunohistochemical analysis in mammary carcinoma in situ, invasive carcinomas and their lymph node metastasis. Anticancer Res. 25:1615–1622. [PubMed] [Google Scholar]
- Jiang M, Wang Z, Yu Z, Bai X, Su J, Cao L, Zhang W, Ruan C. 2011. A novel missense mutation close to the charge-stabilizing system in a patient with congenital factor VII deficiency. Blood Coagulat Fibrinol. 22:264–270. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Wright WW, Shaper JH, Hokke CH, Van den Eijnden DH, Joziasse DH. 1998. Murine sperm-zona binding, a fucosyl residue is required for a high affinity sperm-binding ligand a second site on sperm binds a nonfucosylated, β-galactosyl-capped oligosaccharide. J Biol Chem. 273:1888–1895. [DOI] [PubMed] [Google Scholar]
- Johnston SH, Rauskolb C, Wilson R, Prabhakaran B, Irvine KD, Vogt TF. 1997. A family of mammalian Fringe genes implicated in boundary determination and the Notch pathway. Development. 124:2245–2254. [DOI] [PubMed] [Google Scholar]
- Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, Cruaud C, Maciazek J, Weissenbach J, Bousser MG et al. 1997. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 350:1511–1515. [DOI] [PubMed] [Google Scholar]
- Kakuda S, Haltiwanger R. 2017. Deciphering the Fringe-mediated Notch Code: Identification of Activating and Inhibiting Sites Allowing Discrimination between Ligands. Dev Cell. 40:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalovidouris SA, Gama CI, Lee LW, Hsieh-Wilson LC. 2005. A role for fucose α (1-2) galactose carbohydrates in neuronal growth. J Am Chem Soc. 127:1340–1341. [DOI] [PubMed] [Google Scholar]
- Kannagi R. 1997. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconjugate J. 14:577–584. [DOI] [PubMed] [Google Scholar]
- Kashyap PC, Marcobal A, Ursell LK, Smits SA, Sonnenburg ED, Costello EK, Higginbottom SK, Domino SE, Holmes SP, Relman DA. 2013. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc Natl Acad Sci USA. 110:17059–17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R, Ginsburg V. 1968. The metabolism of L-fucose by HeLa cells. Exp Cell Res. 50:127–132. [DOI] [PubMed] [Google Scholar]
- Kelly RJ, Ernst LK, Larsen RD, Bryant JG, Robinson JS, Lowe JB. 1994. Molecular basis for H blood group deficiency in Bombay (Oh) and para-Bombay individuals. Proc Natl Acad Sci USA. 91:5843–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-L, Chandrasekharan K, Glass M, Shi S, Stahl MC, Kaspar B, Stanley P, Martin PT. 2008. O-fucosylation of muscle agrin determines its ability to cluster acetylcholine receptors. Mol Cell Neurosci. 39:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizuka Y, Funayama S, Shogomori H, Nakano M, Nakajima K, Oka R, Kitazume S, Yamaguchi Y, Sano M, Korekane H. 2016. High-sensitivity and low-toxicity fucose probe for glycan imaging and biomarker discovery. Cell Chem Biol. 23:782–792. [DOI] [PubMed] [Google Scholar]
- Klein T, Arias AM. 1998. Interactions among Delta, Serrate and Fringe modulate Notch activity during Drosophila wing development. Development. 125:2951–2962. [DOI] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B, Neidhardt J, Magyar I, Plauchu H, Zech JC, Morle L, Palmer-Smith SM, Macdonald MJ, Nas V, Fry AE et al. 2013. Novel VCAN mutations and evidence for unbalanced alternative splicing in the pathogenesis of Wagner syndrome. Eur J Hum Genet. 21:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, Tamai H, Konno M, Kamide K, Kawano Y et al. 2002. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci USA. 99:11902–11907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz ID, Colliton RP, Genin A, Rand EB, Li L, Piccoli DA, Spinner NB. 1998. Spectrum and frequency of jagged1 (JAG1) mutations in Alagille syndrome patients and their families. Am J Hum Genet. 62:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. 1996. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 122:3537–3547. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Aminoff M, Jacobsen C, de La Chapelle A, Krahe R, Verroust PJ, Moestrup SK. 2000. Cubilin P1297L mutation associated with hereditary megaloblastic anemia 1 causes impaired recognition of intrinsic factor-vitamin B(12) by cubilin. Blood. 96:405–409. [PubMed] [Google Scholar]
- Krug M, Jork R, Reymann K, Wagner M, Matthies H. 1991. The amnesic substance 2-deoxy-D-galactose suppresses the maintenance of hippocampal LTP. Brain Res. 540:237–242. [DOI] [PubMed] [Google Scholar]
- Krug M, Wagner M, Staak S, Smalla K-H. 1994. Fucose and fucose-containing sugar epitopes enhance hippocampal long-term potentiation in the freely moving rat. Brain Res. 643:130–135. [DOI] [PubMed] [Google Scholar]
- Kudo T, Fujii T, Ikegami S, Inokuchi K, Takayama Y, Ikehara Y, Nishihara S, Togayachi A, Takahashi S, Tachibana K. 2007. Mice lacking α1, 3-fucosyltransferase IX demonstrate disappearance of Lewis x structure in brain and increased anxiety-like behaviors. Glycobiology. 17:1–9. [DOI] [PubMed] [Google Scholar]
- Kudo T, Ikehara Y, Togayachi A, Morozumi K, Watanabe M, Nakamura M, Nishihara S, Narimatsu H. 1998. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab Investig. 78:797–811. [PubMed] [Google Scholar]
- Kumar A, Duvvari MR, Prabhakaran VC, Shetty JS, Murthy GJ, Blanton SH. 2010. A homozygous mutation in LTBP2 causes isolated microspherophakia. Hum Genet. 128:365–371. [DOI] [PubMed] [Google Scholar]
- Kutz WE, Wang LW, Dagoneau N, Odrcic KJ, Cormier-Daire V, Traboulsi EI, Apte SS. 2008. Functional analysis of an ADAMTS10 signal peptide mutation in Weill-Marchesani syndrome demonstrates a long-range effect on secretion of the full-length enzyme. Hum Mutat. 29:1425–1434. [DOI] [PubMed] [Google Scholar]
- Kyselova Z, Mechref Y, Al Bataineh MM, Dobrolecki LE, Hickey RJ, Vinson J, Sweeney CJ, Novotny MV. 2007. Alterations in the serum glycome due to metastatic prostate cancer. J Proteome Res. 6:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau D, Rosenberg N, Zivelin A, Staretz-Chacham O, Kapelushnik J. 2009. Familial factor VII deficiency with foetal and neonatal fatal cerebral haemorrhage associated with homozygosis to Gly180Arg mutation. Haemophilia. 15:774–778. [DOI] [PubMed] [Google Scholar]
- Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. 2008. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 320:664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin ST, Bertozzi CR. 2009. In vivo imaging of Caenorhabditis elegans glycans. ACS Chem Biol. 4:1068–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D et al. 2008. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat Genet. 40:1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBon L, Lee TV, Sprinzak D, Jafar-Nejad H, Elowitz MB. 2014. Fringe proteins modulate Notch-ligand cis and trans interactions to specify signaling states. Elife. 3:e02950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LV, Mitchell ML, Huang S-J, Fokin VV, Sharpless KB, Wong C-H. 2003. A potent and highly selective inhibitor of human α-1, 3-fucosyltransferase via click chemistry. J Am Chem Soc. 125:9588–9589. [DOI] [PubMed] [Google Scholar]
- Lefebvre R, Lo MC, Suarez SS. 1997. Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol Reprod. 56:1198–1204. [DOI] [PubMed] [Google Scholar]
- Leonard BJ, Chen Q, Blajchman MA, Ofosu FA, Sridhara S, Yang D, Clarke BJ. 1998. Factor VII deficiency caused by a structural variant N57D of the first epidermal growth factor domain. Blood. 91:142–148. [PubMed] [Google Scholar]
- Leonhard-Melief C, Haltiwanger RS. 2010. Chapter Eighteen-O-Fucosylation of Thrombospondin Type 1 Repeats. Methods enzymol. 480:401–416. [DOI] [PubMed] [Google Scholar]
- Levy G, Ginsburg D. 2001. Getting at the variable expressivity of von Willebrand disease. Thrombosis Haemostasis. 86:144–148. [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R et al. 2001. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 413:488–494. [DOI] [PubMed] [Google Scholar]
- Li M, Cheng R, Liang J, Yan H, Zhang H, Yang L, Li C, Jiao Q, Lu Z, He J. 2013. Mutations in POFUT1, encoding protein O-fucosyltransferase 1, cause generalized Dowling-Degos disease. Am J Hum Genet. 92:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke S, Geyer H, Wuhrer M, Geyer R, Frank G, Gerardy-Schahn R, Zähringer U, Schachner M. 2001. Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology. 11:373–384. [DOI] [PubMed] [Google Scholar]
- Lin‐Chu M, Broadberry R. 1990. Blood transfusion in the para‐Bombay phenotype. Br J Haematol. 75:568–572. [DOI] [PubMed] [Google Scholar]
- Lipscomb BW, Treloar HB, Klenoff J, Greer CA. 2003. Cell surface carbohydrates and glomerular targeting of olfactory sensory neuron axons in the mouse. J Comp Neurol. 467:22–31. [DOI] [PubMed] [Google Scholar]
- Liu T-W, Ho C-W, Huang H-H, Chang S-M, Popat SD, Wang Y-T, Wu M-S, Chen Y-J, Lin C-H. 2009. Role for α-l-fucosidase in the control of Helicobacter pylori-infected gastric cancer cells. Proc Natl Acad Sci USA. 106:14581–14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-W, Kaji H, Togayachi A, Ito H, Sato T, Narimatsu H. 2012. A chemoenzymatic approach toward the identification of fucosylated glycoproteins and mapping of N-glycan sites. Glycobiology. 22:630–637. [DOI] [PubMed] [Google Scholar]
- Logan CV, Lucke B, Pottinger C, Abdelhamed ZA, Parry DA, Szymanska K, Diggle CP, van Riesen A, Morgan JE, Markham G et al. 2011. Mutations in MEGF10, a regulator of satellite cell myogenesis, cause early onset myopathy, areflexia, respiratory distress and dysphagia (EMARDD). Nat Genet. 43:1189–1192. [DOI] [PubMed] [Google Scholar]
- Lorenzini CGA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. 1997. 2-Deoxy-D-galactose effects on passive avoidance memorization in the rat. Neurobiol Learn Memory. 68:317–324. [DOI] [PubMed] [Google Scholar]
- Lowe JB. 1993. 7 The blood group-specific human glycosyltransferases. Bailliere's Clin Haematol. 6:465–492. [DOI] [PubMed] [Google Scholar]
- Lowe JB. 1997. Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney Int. 51:1418–1426. [DOI] [PubMed] [Google Scholar]
- Lu L, Hou X, Shi S, Körner C, Stanley P. 2010. Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J Biol Chem. 285:36,245–36,254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca VC, Jude KM, Pierce NW, Nachury MV, Fischer S, Garcia KC. 2015. Structural basis for Notch1 engagement of Delta-like 4. Science. 347:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca VC, Kim BC, Ge C, Kakuda S, Wu D, Roein-Peikar M, Haltiwanger RS, Zhu C, Ha T, Garcia KC. 2017. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science. 355:1320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lühn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D. 2001. The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet. 28:69–72. [DOI] [PubMed] [Google Scholar]
- Luo Y, Haltiwanger RS. 2005. O-fucosylation of notch occurs in the endoplasmic reticulum. J Biol Chem. 280:11289–11294. [DOI] [PubMed] [Google Scholar]
- Luo Y, Koles K, Vorndam W, Haltiwanger RS, Panin VM. 2006. a. Protein O-fucosyltransferase 2 adds O-fucose to thrombospondin type 1 repeats. J Biol Chem. 281:9393–9399. [DOI] [PubMed] [Google Scholar]
- Luo Y, Nita-Lazar A, Haltiwanger RS. 2006. b. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J Biol Chem. 281:9385–9392. [DOI] [PubMed] [Google Scholar]
- Ma L, Dong P, Liu L, Gao Q, Duan M, Zhang S, Chen S, Xue R, Wang X. 2016. Overexpression of protein O-fucosyltransferase 1 accelerates hepatocellular carcinoma progression via the Notch signaling pathway. Biochem Biophys Res Commun. 473:503–510. [DOI] [PubMed] [Google Scholar]
- Malý P, Thall AD, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL. 1996. The α (1, 3) fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 86:643–653. [DOI] [PubMed] [Google Scholar]
- Maroni L, Hohenester S, van de Graaf S, Tolenaars D, van Lienden K, Verheij J, Marzioni M, Karlsen T, Oude ER, Beuers U. 2017. Knockout of the primary sclerosing cholangitis-risk gene Fut2 causes liver disease in mice. Hepatology. doi: 10.1002/hep.29029 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Marquardt T, Lühn K, Srikrishna G, Freeze HH, Harms E, Vestweber D. 1999. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 94:3976–3985. [PubMed] [Google Scholar]
- Martignetti JA, Tian L, Li D, Ramirez MC, Camacho-Vanegas O, Camacho SC, Guo Y, Zand DJ, Bernstein AM, Masur SK et al. 2013. Mutations in PDGFRB cause autosomal-dominant infantile myofibromatosis. Am J Hum Genet. 92:1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselli RA, Fernandez JM, Arredondo J, Navarro C, Ngo M, Beeson D, Cagney O, Williams DC, Wollmann RL, Yarov-Yarovoy V et al. 2012. LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Hum Genet. 131:1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. 2006. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 79:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern DP, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, Ippoliti A, Vasiliauskas E, Berel D, Derkowski C. 2010. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet. 19:3468–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay GJ, Clarke S, Davis JA, Simpson DA, Silvestri G. 2005. Pigmented paravenous chorioretinal atrophy is associated with a mutation within the crumbs homolog 1 (CRB1) gene. Investig Ophthalmol Vis Sci. 46:322–328. [DOI] [PubMed] [Google Scholar]
- Milde-Langosch K, Karn T, Schmidt M, Zu Eulenburg C, Oliveira-Ferrer L, Wirtz RM, Schumacher U, Witzel I, Schütze D, Müller V. 2014. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res Treatment. 145:295–305. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Inoue H, Sakamoto Y, Kudo E, Naito T, Mikawa T, Mikawa Y, Isashiki Y, Osabe D, Shinohara S et al. 2005. Identification of a novel splice site mutation of the CSPG2 gene in a Japanese family with Wagner syndrome. Invest Ophthalmol Vis Sci. 46:2726–2735. [DOI] [PubMed] [Google Scholar]
- Miyata T, Zheng YZ, Sakata T, Kato H. 1995. Protein C Osaka 10 with aberrant propeptide processing: Loss of anticoagulant activity due to an amino acid substitution in the protein C precursor. Thromb Haemost. 74:1003–1008. [PubMed] [Google Scholar]
- Miyoshi E, Moriwaki K, Nakagawa T. 2008. Biological function of fucosylation in cancer biology. J Biochem. 143:725–729. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Nakano M. 2008. Fucosylated haptoglobin is a novel marker for pancreatic cancer: Detailed analyses of oligosaccharide structures. Proteomics. 8:3257–3262. [DOI] [PubMed] [Google Scholar]
- Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS. 2000. a. Fringe is a glycosyltransferase that modifies Notch. Nature. 406:369–375. [DOI] [PubMed] [Google Scholar]
- Moloney DJ, Shair LH, Lu FM, Xia J, Locke R, Matta KL, Haltiwanger RS. 2000. b. Mammalian Notch1 is modified with two unusual forms of O-linked glycosylation found on epidermal growth factor-like modules. J Biol Chem. 275:9604–9611. [DOI] [PubMed] [Google Scholar]
- Morales J, Al-Sharif L, Khalil DS, Shinwari JM, Bavi P, Al-Mahrouqi RA, Al-Rajhi A, Alkuraya FS, Meyer BF, Al Tassan N. 2009. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am J Hum Genet. 85:558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Noda K, Furukawa Y, Ohshima K, Uchiyama A, Nakagawa T, Taniguchi N, Daigo Y, Nakamura Y, Hayashi N. 2009. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology. 137:188–198. e182. [DOI] [PubMed] [Google Scholar]
- Müller J, Rana NA, Serth K, Kakuda S, Haltiwanger RS, Gossler A. 2014. O-fucosylation of the notch ligand mDLL1 by POFUT1 is dispensable for ligand function. PLoS One. 9:e88571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrey HE, Ficarro SB, Krishnamurthy C, Domino SE, Peters EC, Hsieh-Wilson LC. 2009. Identification of the Plasticity-Relevant Fucose-α(1− 2)-Galactose Proteome from the Mouse Olfactory Bulb. Biochemistry. 48:7261–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrey HE, Gama CI, Kalovidouris SA, Luo W-I, Driggers EM, Porton B, Hsieh-Wilson LC. 2006. Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci USA. 103:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole S, Davoine CS, Topaloglu H, Cattolico L, Barral D, Beighton P, Hamida CB, Hammouda H, Cruaud C, White PS et al. 2000. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia). Nat Genet. 26:480–483. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bochner BS, Tiemeyer M. 2008. E-selectin receptors on human leukocytes. Blood. 112:3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Suzuki T, Dohmae N, Simizu S. 2015. O‐fucosylation of CCN1 is required for its secretion. FEBS Lett. 589:3287–3293. [DOI] [PubMed] [Google Scholar]
- O'Brien DP, Gale KM, Anderson JS, McVey JH, Miller GJ, Meade TW, Tuddenham EG. 1991. Purification and characterization of factor VII 304-Gln: A variant molecule with reduced activity isolated from a clinically unaffected male. Blood. 78:132–140. [PubMed] [Google Scholar]
- Oberstein SAL, Kriek M, White SJ, Kalf ME, Szuhai K, den Dunnen JT, Breuning MH, Hennekam RC. 2006. Peters Plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Ame J Hum Genet. 79:562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS et al. 1997. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 16:235–242. [DOI] [PubMed] [Google Scholar]
- Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, Tsuruoka-Kinoshita S, Tsuboi T, Watabe S, Okamoto H. 2009. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development. 136:1653–1663. [DOI] [PubMed] [Google Scholar]
- Okajima T, Xu A, Lei L, Irvine KD. 2005. Chaperone activity of protein O-fucosyltransferase 1 promotes notch receptor folding. Science. 307:1599–1603. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Saga Y. 2008. Pofut1 is required for the proper localization of the Notch receptor during mouse development. Mech Dev. 125:663–673. [DOI] [PubMed] [Google Scholar]
- Okeley NM, Alley SC, Anderson ME, Boursalian TE, Burke PJ, Emmerton KM, Jeffrey SC, Klussman K, Law C-L, Sussman D. 2013. Development of orally active inhibitors of protein and cellular fucosylation. Proc Natl Acad Sci USA. 110:5404–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama N, Ide Y, Nakano M, Nakagawa T, Yamanaka K, Moriwaki K, Murata K, Ohigashi H, Yokoyama S, Eguchi H. 2006. Fucosylated haptoglobin is a novel marker for pancreatic cancer: A detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int J Cancer. 118:2803–2808. [DOI] [PubMed] [Google Scholar]
- Otero-Estévez O, Martínez-Fernández M, Vázquez-Iglesias L, Páez de la Cadena M, Rodríguez-Berrocal FJ, Martínez-Zorzano VS. 2013. Decreased expression of alpha-L-fucosidase gene FUCA1 in human colorectal tumors. Int J Mol Sci. 14:16986–16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco AR, Curtis MM, Ritchie JM, Munera D, Waldor MK, Moreira CG, Sperandio V. 2012. Fucose sensing regulates bacterial intestinal colonization. Nature. 492:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang P-C, Chiu PC, Lee C-L, Chang L-Y, Panico M, Morris HR, Haslam SM, Khoo K-H, Clark GF, Yeung WS. 2011. Human sperm binding is mediated by the sialyl-Lewisx oligosaccharide on the zona pellucida. Science. 333:1761–1764. [DOI] [PubMed] [Google Scholar]
- Panin VM, Papayannopoulos V, Wilson R, Irvine KD. 1997. Fringe modulates Notch-ligand interactions. Nature. 387:908–912. [DOI] [PubMed] [Google Scholar]
- Park ES, Putnam EA, Chitayat D, Child A, Milewicz DM. 1998. Clustering of FBN2 mutations in patients with congenital contractural arachnodactyly indicates an important role of the domains encoded by exons 24 through 34 during human development. Am J Med Genet. 78:350–355. [PubMed] [Google Scholar]
- Paterson AD, Rommens JM, Bharaj B, Blavignac J, Wong I, Diamandis M, Waye JS, Rivard GE, Hayward CP. 2010. Persons with Quebec platelet disorder have a tandem duplication of PLAU, the urokinase plasminogen activator gene. Blood. 115:1264–1266. [DOI] [PubMed] [Google Scholar]
- Perbal B. 2004. CCN proteins: Multifunctional signalling regulators. Lancet. 363:62–64. [DOI] [PubMed] [Google Scholar]
- Pestean A, Krizbai I, Böttcher H, Párducz Á, Joó F, Wolff JR. 1995. Identification of the Ulex europaeus agglutinin-I-binding protein as a unique glycoform of the neural cell adhesion molecule in the olfactory sensory axons of adult rats. Neurosci Lett. 195:117–120. [DOI] [PubMed] [Google Scholar]
- Pham TAN, Clare S, Goulding D, Arasteh JM, Stares MD, Browne HP, Keane JA, Page AJ, Kumasaka N, Kane L. 2014. Epithelial IL-22RA1-mediated fucosylation promotes intestinal colonization resistance to an opportunistic pathogen. Cell Host Microbe. 16:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ. 2014. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 514:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohle W, Acosta L, Ru H, Krug M. 1987. Incorporation of [3 H] fucose in rat hippocampal structures after conditioning by perforanth path stimulation and after LTP-producing tetanization. Brain Res. 410:245–256. [DOI] [PubMed] [Google Scholar]
- Putnam EA, Zhang H, Ramirez F, Milewicz DM. 1995. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 11:456–458. [DOI] [PubMed] [Google Scholar]
- Rampal R, Arboleda-Velasquez JF, Nita-Lazar A, Kosik KS, Haltiwanger RS. 2005. Highly conserved O-fucose sites have distinct effects on Notch1 function. J Biol Chem. 280:32133–32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampal R, Luther KB, Haltiwanger RS. 2007. Notch signaling in normal and disease States: Possible therapies related to glycosylation. Curr Mol Med. 7:427–445. [DOI] [PubMed] [Google Scholar]
- Rampoldi L, Caridi G, Santon D, Boaretto F, Bernascone I, Lamorte G, Tardanico R, Dagnino M, Colussi G, Scolari F et al. 2003. Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet. 12:3369–3384. [DOI] [PubMed] [Google Scholar]
- Rana N, Haltiwanger RS. 2011. Fringe benefits: Functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr Opin Struc Biol. 21:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch P, Rehman A, Künzel S, Häsler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF. 2011. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci USA. 108:19030–19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho J-h, Wright DP, Christie DL, Clinch K, Furneaux RH, Roberton AM. 2005. A novel mechanism for desulfation of mucin: Identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J Bacteriol. 187:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts LM, Dlugosz M, Luther KB, Haltiwanger RS, Majerus EM. 2007. O-fucosylation is required for ADAMTS13 secretion. J Biol Chem. 282:17014–17023. [DOI] [PubMed] [Google Scholar]
- Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, Dell A, Haslam SM, Paulson JC. 2012. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. 8:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillahan CD, Brown SJ, Register AC, Rosen H, Paulson JC. 2011. High‐throughput screening for inhibitors of sialyl‐and fucosyltransferases. Angew Chem Int Ed. 50:12534–12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A, Escuin S, Doudney K, Vekemans M, Stevenson RE, Greene ND, Copp AJ, Stanier P. 2012. Mutations in the planar cell polarity genes CELSR1 and SCRIB are associated with the severe neural tube defect craniorachischisis. Hum Mutat. 33:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G, Hassan HJ, Staempfli S, Roncuzzi L, Cianetti L, Leonardi A, Vicente V, Mannucci PM, Bertina R, Peschle C et al. 1987. Hereditary thrombophilia: Identification of nonsense and missense mutations in the protein C gene. Proc Natl Acad Sci USA. 84:2829–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SP, Jork R. 1987. Long-term memory formation in chicks is blocked by 2-deoxygalactose, a fucose analog. Behav Neural Biol. 48:246–258. [DOI] [PubMed] [Google Scholar]
- Saldova R, Fan Y, Fitzpatrick JM, Watson RWG, Rudd PM. 2011. Core fucosylation and α2-3 sialylation in serum N-glycome is significantly increased in prostate cancer comparing to benign prostate hyperplasia. Glycobiology. 21:195–205. [DOI] [PubMed] [Google Scholar]
- Sathish J. 2014. Checkpoint Modulators in Cancer Immunotherapy. Forum on Immunopathological Diseases and Therapeutics: Begel House Inc.
- Sawa M, Hsu T-L, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong C-H. 2006. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA . 103:12371–12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L et al. 2011. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell. 19:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser M, Hofferbert S, Bartz U, Lutze G, Lammle B, Engel W. 1995. The novel acceptor splice site mutation 11396(G-->A) in the factor XII gene causes a truncated transcript in cross-reacting material negative patients. Hum Mol Genet. 4:1235–1237. [DOI] [PubMed] [Google Scholar]
- Schneider M, Kumar V, Nordstrom LU, Feng L, Takeuchi H, Stanley P, Wu P, Haltiwanger RS In Review. Inhibition of Delta-induced Notch signaling using fucose analogs. [DOI] [PMC free article] [PubMed]
- Schneppenheim R, Budde U, Oyen F, Angerhaus D, Aumann V, Drewke E, Hassenpflug W, Haberle J, Kentouche K, Kohne E et al. 2003. von Willebrand factor cleaving protease and ADAMTS13 mutations in childhood TTP. Blood. 101:1845–1850. [DOI] [PubMed] [Google Scholar]
- Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, Mahan A, Runckel C, Cassera M, Vittal V et al. 2003. Analysis of the ARMD1 locus: Evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 12:3315–3323. [DOI] [PubMed] [Google Scholar]
- Serpa J, Mendes N, Silva LFS, Almeida R, Le Pendu J, David L. 2004. Two new FUT2 (fucosyltransferase 2 gene) missense polymorphisms, 739 G→ A and 839 T→ C, are partly responsible for non-secretor status in a Caucasian population from Northern Portugal. Biochem J. 383:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Moloney DJ, Haltiwanger R. 2003. Fringe modifies O-fucose on mouse Notch1 at epidermal growth factor-like repeats within the ligand-binding site and the Abruptex region. J Biol Chem. 278:7775–7782. [DOI] [PubMed] [Google Scholar]
- Shi S, Stanley P. 2003. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci USA. 100:5234–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. 2002. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem. 277:26,733–26,740. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M. 2003. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 278:3466–3473. [DOI] [PubMed] [Google Scholar]
- Simioni P, Tormene D, Tognin G, Gavasso S, Bulato C, Iacobelli NP, Finn JD, Spiezia L, Radu C, Arruda VR. 2009. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 361:1671–1675. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK et al. 2011. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 43:303–305. [DOI] [PubMed] [Google Scholar]
- Slater G, Itzkowitz S, Azar S, Aufses AH Jr. 1993. Clinicopathologic correlations of ABO and Rhesus blood type in colorectal cancer. Dis Colon Rectum. 36:5–7. [DOI] [PubMed] [Google Scholar]
- Srivastava G, Kaur K, Hindsgaul O, Palcic M. 1992. Enzymatic transfer of a preassembled trisaccharide antigen to cell surfaces using a fucosyltransferase. J Biol Chem. 267:22356–22361. [PubMed] [Google Scholar]
- St John JA, Claxton C, Robinson MW, Yamamoto F, Domino SE, Key B. 2006. Genetic manipulation of blood group carbohydrates alters development and pathfinding of primary sensory axons of the olfactory systems. Dev Biol. 298:470–484. [DOI] [PubMed] [Google Scholar]
- Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. 2008. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem. 283:13638–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Schachter H, Taniguchi N. 2009. N-glycans In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Suehiro K, Kawabata S, Miyata T, Takeya H, Takamatsu J, Ogata K, Kamiya T, Saito H, Niho Y, Iwanaga S. 1989. Blood clotting factor IX BM Nagoya. Substitution of arginine 180 by tryptophan and its activation by alpha-chymotrypsin and rat mast cell chymase. J Biol Chem. 264:21257–21265. [PubMed] [Google Scholar]
- Tonetti M, Sturla L, Bisso A, Benatti U, De Flora A. 1996. Synthesis of GDP-L-fucose by the human FX protein. J Biol Chem. 271:27274–27279. [DOI] [PubMed] [Google Scholar]
- Twigg SR, Lloyd D, Jenkins D, Elcioglu NE, Cooper CD, Al-Sannaa N, Annagur A, Gillessen-Kaesbach G, Huning I, Knight SJ et al. 2012. Mutations in multidomain protein MEGF8 identify a Carpenter syndrome subtype associated with defective lateralization. Am J Hum Genet. 91:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-González J, Leonhard-Melief C, Lira-Navarrete E, Jiménez-Osés G, Hernández-Ruiz C, Pallarés MC, Yruela I, Vasudevan D, Lostao A, Corzana F. 2016. A proactive role of water molecules in acceptor recognition by protein O-fucosyltransferase 2. Nat Chem Biol. 12:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard R, Fijen CA, Schipper MG, de Galan L, Kuijper EJ, Mannens MM. 2000. Molecular characterisation of 10 Dutch properdin type I deficient families: Mutation analysis and X-inactivation studies. Eur J Hum Genet. 8:513–518. [DOI] [PubMed] [Google Scholar]
- van Heel DA, Fisher SA, Kirby A, Daly MJ, Rioux JD, Lewis CM, Consortium GSM-AGotIIG . 2004. Inflammatory bowel disease susceptibility loci defined by genome scan meta-analysis of 1952 affected relative pairs. Hum Mol Genet. 13:763–770. [DOI] [PubMed] [Google Scholar]
- Vasudevan D, Haltiwanger RS. 2014. Novel roles for O-linked glycans in protein folding. Glycoconjugate J. 31:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan D, Takeuchi H, Johar SS, Majerus E, Haltiwanger RS. 2015. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr Biol. 25:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infectious Dis. 181:2–9. [DOI] [PubMed] [Google Scholar]
- Villalobos JA, Bo RY, Wallace IS. 2015. 2-fluoro-L-fucose is a metabolically incorporated inhibitor of plant cell wall polysaccharide fucosylation. PLoS One. 10:e0139091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacklin P, Mäkivuokko H, Alakulppi N, Nikkilä J, Tenkanen H, Räbinä J, Partanen J, Aranko K, Mättö J. 2011. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One. 6:e20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands AM, Fujita A, McCombs JE, Cervin J, Dedic B, Rodriguez AC, Nischan N, Bond MR, Mettlen M, Trudgian DC. 2015. Fucosylation and protein glycosylation create functional receptors for cholera toxin. Elife. 4:e09545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Koda Y, Soejima M, Kimura H. 1997. Two missense mutations of H Type α(1, 2) fucosyltransferase gene (FUT1) responsible for para‐Bombay phenotype. Vox Sanguinis. 72:31–35. [DOI] [PubMed] [Google Scholar]
- Wang LW, Dlugosz M, Somerville RP, Raed M, Haltiwanger RS, Apte SS. 2007. O-Fucosylation of thrombospondin type 1 repeats in ADAMTS-like-1/Punctin-1 regulates secretion implications for the adamts superfamily. J Biol Chem. 282:17024–17031. [DOI] [PubMed] [Google Scholar]
- Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, Pinsky BA, Chokephaibulkit K, Onlamoon N, Pattanapanyasat K. 2017. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science. 355:395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fukuda T, Li W, Gao C-X, Kondo A, Matsumoto A, Miyoshi E, Taniguchi N, Gu J. 2009. Requirement of Fut8 for the expression of vascular endothelial growth factor receptor-2: A new mechanism for the emphysema-like changes observed in Fut8-deficient mice. J Biochem. 145:643–651. [DOI] [PubMed] [Google Scholar]
- Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N. 2006. a. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem. 281:2572–2577. [DOI] [PubMed] [Google Scholar]
- Wang X, Gu J, Miyoshi E, Honke K, Taniguchi N. 2006. b. Phenotype changes of Fut8 knockout mouse: Core fucosylation is crucial for the function of growth factor receptor (s). Methods Enzymol. 417:11–22. [DOI] [PubMed] [Google Scholar]
- Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M. 2005. Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc Natl Acad Sci USA. 102:15791–15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Spellman MW. 1998. Purification and characterization of a GDP-fucose: Polypeptide fucosyltransferase from Chinese hamster ovary cells. J Biol Chem. 273:8112–8118. [DOI] [PubMed] [Google Scholar]
- Wei AH, Durrant ST, Hertzberg MS, Swords RT, Lewis ID, Jonas B, Cortes JE, Yarranton G, Walling JM. 2013. A phase I study of KB004, a novel non-fucosylated humaneered® antibody, targeted against the receptor tyrosine kinase EphA3, in advanced hematologic malignancies. Blood. 122:3838–3838. [Google Scholar]
- Werz DB, Ranzinger R, Herget S, Adibekian A, von der Lieth C-W, Seeberger PH. 2007. Exploring the structural diversity of mammalian carbohydrates (“glycospace”) by statistical databank analysis. ACS Chem Biol. 2:685–691. [DOI] [PubMed] [Google Scholar]
- Wiese TJ, Dunlap JA, Yorek MA. 1994. L-fucose is accumulated via a specific transport system in eukaryotic cells. J Biol Chem. 269:22705–22711. [PubMed] [Google Scholar]
- Wolpin BM, Kraft P, Gross M, Helzlsouer K, Bueno-de-Mesquita HB, Steplowski E, Stolzenberg-Solomon RZ, Arslan AA, Jacobs EJ, LaCroix A. 2010. Pancreatic cancer risk and ABO blood group alleles: Results from the pancreatic cancer cohort consortium. Cancer Res. 70:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier R, Podolsky D. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 448:427–434. [DOI] [PubMed] [Google Scholar]
- Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 299:2074–2076. [DOI] [PubMed] [Google Scholar]
- Yakubenia S, Frommhold D, Schölch D, Hellbusch CC, Körner C, Petri B, Jones C, Ipe U, Bixel MG, Krempien R. 2008. Leukocyte trafficking in a mouse model for leukocyte adhesion deficiency II/congenital disorder of glycosylation IIc. Blood. 112:1472–1481. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Satoh M. 2009. Production of therapeutic antibodies with controlled fucosylation MAbs. 1:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L-M, Lin B, Zhu L-C, Hao Y-Y, Qi Y, Wang C-Z, Gao S, Liu S-C, Zhang S-L, Iwamori M. 2010. Enhancement of the adhesive and spreading potentials of ovarian carcinoma RMG-1 cells due to increased expression of integrin α5β1 with the Lewis Y-structure on transfection of the α1, 2-fucosyltransferase gene. Biochimie. 92:852–857. [DOI] [PubMed] [Google Scholar]
- Yao D, Huang Y, Huang X, Wang W, Yan Q, Wei L, Xin W, Gerson S, Stanley P, Lowe JB. 2011. Protein O-fucosyltransferase 1 (Pofut1) regulates lymphoid and myeloid homeostasis through modulation of Notch receptor ligand interactions. Blood. 117:5652–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Ogawara K, Kimura R, Shimizu F, Baba T, Minakawa Y, Higo M, Kasamatsu A, Endo-Sakamoto Y, Shiiba M. 2013. Protein O-fucosyltransferase 1: A potential diagnostic marker and therapeutic target for human oral cancer. Int J Oncol. 43:1864–1870. [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Mitsui S, Kudo K, Nakano M. 1997. Identification of an N-glycosylated region of pig zona pellucida glycoprotein ZPB that is involved in sperm binding. Eur J Biochem. 248:86–92. [DOI] [PubMed] [Google Scholar]
- Yuan K, Kucik D, Singh RK, Listinsky CM, Listinsky JJ, Siegal GP. 2008. Alterations in human breast cancer adhesion-motility in response to changes in cell surface glycoproteins displaying alpha-L-fucose moieties. Int J Oncol. 32:797. [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Atkinson PH. 1975. Fucosyl-glycoprotein and precursor pools in HeLa cells. Biochemistry. 14:3107–3114. [DOI] [PubMed] [Google Scholar]
- Zenita K, Kirihata Y, Kitahara A, Shigeta K, Higuchi K, Hirashima K, Murachi T, Miyake M, Takeda T, Kannagi R. 1988. Fucosylated type‐2 chain polylactosamine antigens in human lung cancer. Int J Cancer. 41:344–349. [DOI] [PubMed] [Google Scholar]
- Zhang N, Gridley T. 1998. Defects in somite formation in lunatic fringe-deficient mice. Nature. 394:374–377. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun P, Liu J, Fu L, Yan J, Liu Y, Yu L, Wang X, Yan Q. 2008. Suppression of FUT1/FUT4 expression by siRNA inhibits tumor growth. Biochim Biophys Acta (BBA)-Mol Cell Res. 1783:287–296. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. 2006. Deletion of core fucosylation on α3β1 integrin down-regulates its functions. J Biol Chem. 281:38343–38350. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, Gu J, Taniguchi N. 2008. Branched N‐glycans regulate the biological functions of integrins and cadherins. FEBS J. 275:1939–1948. [DOI] [PubMed] [Google Scholar]
- Zipin A, Israeli-Amit M, Meshel T, Sagi-Assif O, Yron I, Lifshitz V, Bacharach E, Smorodinsky NI, Many A, Czernilofsky PA. 2004. Tumor-Microenvironment Interactions The Fucose-Generating FX Enzyme Controls Adhesive Properties of Colorectal Cancer Cells. Cancer Res. 64:6571–6578. [DOI] [PubMed] [Google Scholar]