Abstract
We compare the elongation behavior of native Escherichia coli RNA polymerase holoenzyme assembled in vivo, holoenzyme reconstituted from σ70 and RNA polymerase in vitro, and holoenzyme with a specific alteration in the interface between σ70 and RNA polymerase. Elongating RNA polymerase from each holoenzyme has distinguishable properties, some of which cannot be explained by differential retention or rebinding of σ70 during elongation, or by differential presence of elongation factors. We suggest that interactions between RNA polymerase and σ70 may influence the ensemble of conformational states adopted by RNA polymerase during initiation. These states, in turn, may affect the conformational states adopted by the elongating enzyme, thereby physically and functionally imprinting RNA polymerase.
All multisubunit RNA polymerases use initiation factors to recognize their promoters, a strategy that allows tight base-specific binding during the initiation phase of transcription and nonspecific binding during elongation, after release of the initiation factor. This function is performed by σ in eubacterial cells (1). Almost all bacteria contain multiple σ factors, one directing transcription to housekeeping genes and the remainder directing transcription to genes encoding specialized functions (1).
Genetic, biochemical, and structural characterization of the interaction between σ and RNA polymerase (E) (2–4) reveals that the interface between the two proteins is both extensive, having at least four regions of interaction (5–9), and dynamic, with some interactions depending on the formation of the preceding ones (5). Conformational changes in both partners result from this interaction. The changes in σ unmask and reposition its DNA-binding domains to allow promoter recognition (6, 7, 10–14). Conformational changes in RNA polymerase may reposition portions of RNA polymerase in close contact with the nucleic acids, but the functional consequences of such changes are unknown.
Usually, σ factors dissociate from elongating RNA polymerase shortly after RNA polymerase leaves the promoter (15–18) but remain associated with RNA polymerase longer than normal at a special class of promoters (19–21). The predominant eubacterial promoter has two conserved recognition sequences, centered at –10 and –35 bp upstream of the starting point of transcription (+1). Promoters with a reiterated –10 motif in the initially transcribed region exhibit prolonged σ association. This motif was discovered first in promoters directing lambdoid phage late transcription. The σ recognition of the reiterated –10 region induces a transcription pause (19–23) that is required to load the λQ elongation factor that antiterminates transcription (24). Reiterated –10 regions were identified recently (21–23) in a subset of bacterial promoters, including lacUV5. It is thought that σ dissociates shortly after passing the reiterated –10 region (23). Eσ70 from stationary cells may be refractory to dissociation as a significant fraction (≈30%) of RNA polymerase purified from such cells retained σ during elongation (25).
In Escherichia coli, the average rate of elongation is 50 nt/s (ref. 26 and references therein); however, this speed is not constant. First, the template encodes two known types of pauses. Class I pauses (like his) are mediated by a stem–loop structure that interacts with RNA polymerase (27, 28). Class II pauses (like ops) involve backtracking due to a weak DNA/RNA hybrid (29, 30). Single-molecule studies reveal additional diversity during elongation. First, RNA polymerase often hesitates for a few seconds, which is a behavior that has been attributed to the transient assumption of a RNA polymerase conformation refractory to elongation (31, 32). Second, RNA polymerase can pause for a longer time and then backtrack (33), which is a behavior that may be part of the proofreading mechanism of the enzyme (34). Last, the diversity in transcription rates (≥5-fold) of single molecules far exceeds that expected for a single transcribing species (35). Because each molecule maintains its rate for the duration of the measurements (≈5 min), this variation is not due to rapidly interconverting conformers. It is unclear whether these are relatively stable conformational states or a consequence of posttranslational modification (35).
Given the extensive and dynamic interface between RNA polymerase and σ, we wondered whether subtle alterations in these interactions could influence the range of conformational states adopted by RNA polymerase and, thereby, alter its pausing and elongation behavior. We examined this issue with RNA polymerase holoenzyme containing σ70, the E. coli housekeeping σ. We reasoned that holoenzyme reconstituted in vitro from RNA polymerase and σ70 (r-Eσ70) might have slightly different contacts than that of native RNA polymerase holoenzyme (n-Eσ70), assembled in vivo. Therefore, we compared the pausing and elongation properties of these two holoenzymes. Also, we compared the pausing and elongation properties of WT holoenyzme with those of holoenzyme with a σ70 mutant (E407K) that partially disrupts the strongest interaction between RNA polymerase and σ70 (5, 6, 8). We find reproducible differences in the elongation behavior of these populations. We suggest that altering the contacts between RNA polymerase and σ70 can alter the elongation properties of polymerase.
Materials and Methods
Proteins and DNA. Native Eσ70 or Eσ70(E407K) were purified from E. coli strain BL21(DE3) that was transformed with pET15rpoD or pET15rpoD-E407K, encoding N-terminal HIS-tagged σ70, and induced with 1 mM isopropyl β-d-thiogalactoside for 4 h. Native holoenzyme was purified as described (36, 37) with the following modifications. After polyethyleneimine precipitation and Ni2+-nitrilotriacetic acid (NTA) chromatography, the eluate was precipitated with 40% (NH4)2(SO4), and the pellet was resuspended in 0.5 ml of TGED (10 mM Tris, pH 7.9/5% glycerol/0.1 mM EDTA/1 mM DTT) and fractionated first on a Superdex 200 10/30 column (Pharmacia) and then on a MonoQ 5/5 column. For the experiments shown in Fig. 1, RNA polymerase core was obtained from BL21(DE3) culture, transformed with pGEMABC (S. Darst, The Rockefeller University, New York), purified as described above, except gel filtration was performed on Sephacryl 300 and Ni2+–NTA chromatography was omitted. For all other experiments using reconstituted enzyme, native Eσ70 was depleted of σ70 on a BioRex70 column, 100–200 mesh (Bio-Rad); eluted core subunits were concentrated on MonoQ (38). We purified σ70 and σ70-E407K on Ni2+–NTA resin. Templates for in vitro transcription were generated by PCR from pIA171 (his), pIA273 (ops) (29), or pIA146 (for elongation-rate determination; ref. 39) and gel-purified. For immobilized transcription, templates were 5′-labeled with Biotin-16-dUTP by Klenow fill-in and bound to Dynabeads (Dynal, Oslo) before transcription. Stalled A29-TECs were washed three or four times with TGED plus 300 mM KCl/0.1% Sarkosyl/0.1 mg/ml heparin/0.05 mg/ml BSA and two times with 1× transcription buffer (see below) before transcription was resumed.
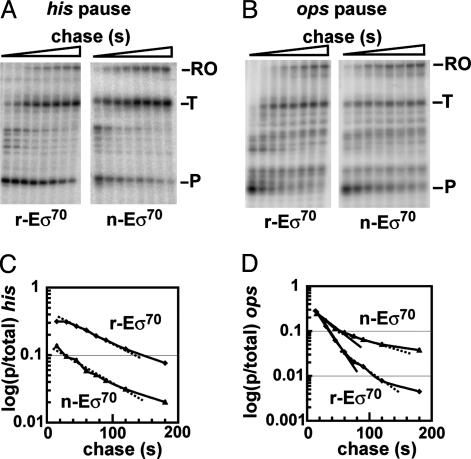
Fig. 1.
Pausing of σ70-dependent RNA polymerase holoenzyme complexes at the his and the ops pause sites. (A and B) Preformed [α-32P]CMP-labeled TECs were chased with 10 μM GTP and 150 μM each of ATP, CTP, and UTP. Samples were removed at the following time points: 15, 30, 45, 60, 75, 90, 120, and 180 s, and they were then run on a 10% denaturing polyacrylamide gel. The autoradiogram shows the time-dependent escape of TECs at his (A) and at ops (B). The pause site (P), terminated transcripts (T) and runoff transcription (RO) are indicated. (C and D) Reaction profiles, quantitated by plotting the fraction of paused RNA vs. time. The calculated pause half-life at his (C) was 73 s for r-Eσ70 (♦) and 41 s for n-Eσ70 (▴). Pause efficiencies, determined by backextrapolation of the exponentials to time point 0, were 40% for r-Eσ70 and 18% for n-Eσ70. At ops (D) solid and dashed straight lines indicate the two exponential phases for each holoenzyme complex. Half-lives for r-Eσ70 (♦) were 17 and 51 s, and for n-Eσ70 (▴), they were 27 and 106 s. Pause efficiencies, determined from the fast phase, were 40% for r-Eσ70 and 32% for n-Eσ70.
In Vitro Transcription. Core RNAP and σ70 were reconstituted on ice for 10 min; and reconstituted or native holoenzyme was added to their respective templates and shifted to 30°C for 15 min to form A29-halted complexes. The final transcription conditions were as follows: 20–50 nM template, 20–50 nM RNAP, 1× transcription buffer (20 mM Tris·HCl, pH 8.0/20 mM NaCl/10 mM MgCl2/5% glycerol/0.1 mM DTT/0.1 mM Na2·EDTA/0.05 mg/ml BSA), 150 μM ApU dinucleotide 2.5 μM each ATP and GTP, 1 μM CTP, and 1 μCi of [α-32P]-CTP (1 Ci = 37 GBq; 3,000 Ci/mmol, 10 mCi/ml). Elongation was resumed by adding 150 μM each ATP, CTP, and GTP each; 5–10 μM GTP; and 0.1 mg/ml heparin (final concentrations). Elongation rate was measured by using 80 μM NTPs. Elongation rate under limiting NTP concentrations was measured similarly, except that UTP alone was added to halted A29-TECs to resume elongation. Samples (10 μl) were removed at defined time intervals and added to 5 μl of formamide-loading dye. All samples were heated for 3 min at 90°C and run on 6–10% sequencing gels. Transcripts were quantitated by using a PhosphorImager scan and imagequant software (Molecular Dynamics). Retention of σ70His in stalled A29-TECs was determined by an indirect method (25).
Results
Reconstituted and Native RNA Polymerase Holoenzyme Complexes Have Distinct Pausing Properties. We tested whether r-Eσ70 and n-Eσ70 holoenzymes were functionally equivalent in their behavior at a Class I (his) and Class II (ops) pause. Strikingly, their behavior was distinct. Quantification of a representative pulse–chase experiment at his (Fig. 1 A) revealed a pause half-life of 73 s for r-Eσ70 and 41 s for n-Eσ70 (Fig. 1C). The two preparations also differed in pause efficiency (40% for r-Eσ70 and 18% for n-Eσ70). Quantification of a representative pulse–chase experiment at ops (Fig. 1B) revealed that the two enzyme preparations had similar pausing efficiencies (≈40%) but different kinetics (Fig. 1D). Escape from the ops pause is biphasic. n-Eσ70 showed a longer-pause half-life than r-Eσ70 in both phases, exactly opposite of their behavior at his. In the fast phase, n-Eσ70 showed a T1/2 = 27 s and r-Eσ70 showed a T1/2 = 17 s. In the slow phase, n-Eσ70 showed a T1/2 of 106 s and r-Eσ70 showed a T1/2 of 51 s. Results of three or more independent experiments at these two pause sites are given in summary in Table 1 (top two rows). We conclude that r-Eσ70 and n-Eσ70 possess distinct pausing behaviors at Class I and Class II pause sites.
Table 1. Pause half-lives and pause efficiencies of two different native and reconstituted RNAP holoenzymes at the his and ops pause sites.
|
his
|
ops
|
|||
|---|---|---|---|---|
| Enzyme | T1/2, s | p.e., % | T1/2, s | p.e., % |
| Native | 40 ± 3 | 26 ± 6 | 27 ± 0.4 | 31 ± 2 |
| Reconstituted* | 70 ± 4 | 39 ± 2 | 17 ± 1 | 38 ± 3 |
| Native† | 38 ± 6 | 23 ± 5 | 33 ± 2 | 28 ± 3 |
| Reconstituted† | 64 ± 4 | 40 ± 2 | 18‡ | 26‡ |
SD was obtained from at least three independent experiments. p.e., Pause efficiency.
Preparation of reconstituted enzyme from overexpressed RNAP core subunits.
Preparation of native enzyme used for σ70 depletion and a new reconstituted enzyme.
Single measurement.
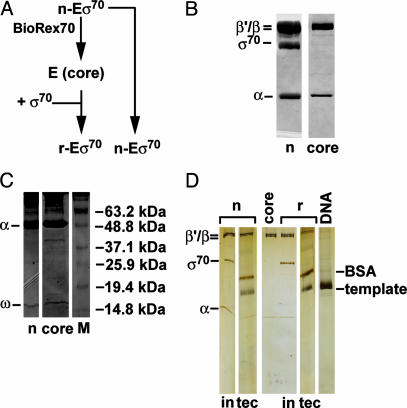
The n-Eσ70 and r-Eσ70 preparations used in the above experiments differed in several respects. They were purified at different times with slightly different procedures; n-Eσ70 was purified from RNA polymerase specified by its chromosomal genes, whereas r-Eσ70 was made from overexpressed RNA polymerase (see core RNA polymerase preparation 1 in Materials and Methods); and r-Eσ70 was reconstituted from components that had been stored separately in high glycerol. Therefore, we repurified n-Eσ70 to apparent homogeneity by using multiple columns, starting with extracts made from exponentially growing cells; separated a portion of the preparation into E and σ70; and immediately reconstituted it with purified σ70 (Fig. 2A). Purified n-Eσ70 and the core enzyme derived from it are shown in Fig. 2B. An overloaded 15% gel demonstrates that both preparations retain the ω-subunit and that n-Eσ70 does not have visible contamination with other low-molecular-weight proteins (Fig. 2C). We compared the pausing properties of r-Eσ70 and n-Eσ70. Newly purified r-Eσ70 and n-Eσ70 holoenzymes (Table 1, bottom two rows) showed properties identical to the original enzymes (Table 1, top two rows). Thus, transient separation of σ70 and core are sufficient to alter pausing behavior.
Fig. 2.
SDS/PAGE of purified native holoenzyme Eσ70, and RNA polymerase core. (A) Diagram of purification of core enzyme (E) and reconstituted enzyme (r) from native enzyme (n). (B) Coomassie blue staining of 1 μg of n-enzyme and of 1 μg of core RNA polymerase separated on a 10% SDS/PAGE gel shows the purity of holoenzyme preparation and efficient removal of σ70 (core). (C) Coomassie blue staining of 5 μg of native (n) or core separated on a 15% SDS/PAGE gel shows that both preparations have the ω subunit of RNA polymerase. The band above 37 kDa in the core preparation must be a degradation product of one of the core subunits. (D) The silver-stained 7–15% gradient SDS/PAGE gel shows input (in) of n-enzyme or r-enzyme and the washed TECs (tec), used for immobilized transcription.
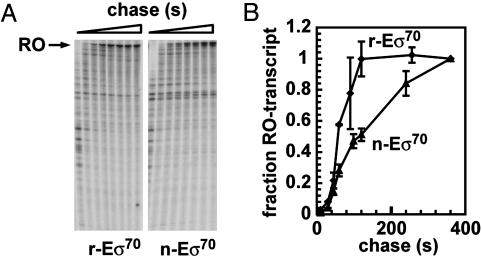
r-Eσ70 and n-Eσ70 Differ in Their Elongation Rates. We examined elongation rates by using a template in which the T7A1 promoter drives transcription of rpoB, which has no known terminators or regulatory pauses (32, 39). When stalled radiolabeled complexes of 29 nt in length (A29) were chased with 80 μM NTPs, r-Eσ70 and n-Eσ70 showed distinct elongation rates (Fig. 3A), Of the r-Eσ70 transcription-elongation complexes (TECs), 50% produced full-length transcripts after 50 s, whereas it took 100 s for 50% of the n-Eσ70 TECs to complete the runoff transcript (Fig. 3B), indicating that the two complexes differ significantly in their elongation rates.
Fig. 3.
Elongation rates of r-Eσ70 and n-Eσ70. (A) Preformed [32P]-CMP-labeled TECs were elongated on a template without strong pause sites by using 80 μM NTPs. Samples were taken at 10, 30, 45, 60, 90, 120, 240, and 360 s and analyzed on a 6% denaturing PAGE. (B) Reaction profiles of runoff transcription. The ratio of the runoff (RO) transcript to total transcripts was plotted against time. The calculated elongation rates were 50 s for r-Eσ70 and 100 s for n-Eσ70.
Very Low Amounts of σ70 Remain in the TECs. The simplest explanation for these differences is that r-Eσ70 and n-Eσ70 differentially retain σ70 in the TEC and that the presence of σ70 alters the pausing and elongation properties of the enzyme. Therefore, we determined the amounts of σ70 present in each TEC halted upstream of the pause site by using a procedure described by Bar-Nahum and Nudler (25). Paused ternary complexes with short radiolabeled transcripts were immobilized on Ni–NTA resin by virtue of their σ70His (25), and the fraction of the radiolabeled transcript retained on Ni–NTA was determined. This fraction is a measure of the fraction of ternary complexes that retain σ70His. This experiment indicated that ≤5% of the TECs retain σ70His (Fig. 4), which is similar to the previous determination for RNA polymerase purified from exponentially growing cells (25). Thus, the amount of σ70 in the TECs was too low to account for the differences in the pausing and elongation properties of r-Eσ70 and n-Eσ70. Also, these reactions contain sufficient heparin to disrupt the σ70 function during elongation (21, 39, 40).
Fig. 4.
Indirect quantitation of σ70His retention in TECs. WT native Eσ70 (n, lanes 1, 4, 7, 10, and 13), mutant n-Eσ70 (m; lanes 2, 5, 8, 11, and 14), and WT reconstituted Eσ70 (r; lanes 3, 6, 9, 12, and 15) were used to generate 32P-labeled TECs at transcript position +29 (A29). The TECs were incubated with Ni–NTA resin, washed with transcription buffer, and eluted from the resin by using formamide loading dye. We ran 5% of the original input reactions, 2.5% of supernatants of wash steps, and 4% of bound TECs (resin) on a 6% denaturing gel. The final calculated amounts of A29-TECs bound to the resin were 2.6% (lane 13), 0.4% (lane 14), and 3.5% (lane 15). The following transcripts were detected in the supernatants: after the first washing step, 8% (lane 4), 10% (lane 5), and 11% (lane 6); after the second washing step, 4% (lane 7), 4% (lane 8), and 5% (lane 9); and after the fifth step, 0.5% (lane 10), 0.2% (lane11), and 0.7% (lane 12).
The Difference in r-Eσ70 and n-Eσ70 Behavior at the his Pause Is Maintained After a Stringent Wash Procedure. In a second protocol, we stringently washed the TECs to remove free σ70 and potential nonpolymerase contaminants from elongating complexes and examined transcription at his and during elongation. Stalled TECs of n-Eσ70 and r-Eσ70 were formed and templates then immobilized by means of a terminal biotinylated nucleotide to a streptavidin matrix. A fraction of the stalled, immobilized TECs was then washed three or four times with a high-salt buffer containing 0.1% sarkosyl to remove free components, and subsequently reequilibrated to normal salt conditions before resumption of elongation (see Materials and Methods). A silver-stained gradient gel of stalled n-Eσ70 and r-Eσ70 A29-TECs demonstrated that σ70 was removed by this procedure and that no other contaminating bands were present in n-Eσ70 (Fig. 2D). Behavior at his was determined for both washed and unwashed TECs. Importantly, r-Eσ70 still paused ≈50% longer than n-Eσ70 even after extensive washing (Table 2, column 1), as was found for reactions carried out in solution (Table 1, column 1), although pause and efficiency were affected by this protocol (compare Tables 1 and 2). In contrast, washing eliminated the difference between the elongation rate of r-Eσ70 and n-Eσ70. Untreated n-Eσ70 elongated more slowly than r-Eσ70 on the immobilized template, whereas extensively washed n-Eσ70 elongated as rapidly as r-Eσ70 (Table 3). We consider the implications of these results in Discussion.
Table 2. Pause half-lives and pause efficiencies of native and reconstituted RNAP holoenzymes at the immobilized his pause template.
| RNAP holoenzymes | T1/2, s (before/after wash) | p.e., % (before/after wash) |
|---|---|---|
| Native* | 25/22 | 37/74 |
| Reconstituted* | 33/36 | 48/70 |
| Native† | 20/23 | 34/42 |
| Reconstituted† | 30/31 | 34/49 |
T1/2 and pause efficiency (p.e.) were determined before and after washing A29-stalled TECs (see Materials and Methods).
TECs washed three times with high-salt buffer and one time with transcription buffer.
TECs washed four times with high-salt buffer and one time with transcription buffer.
Table 3. Elongation rates of native and reconstituted RNAP holoenzymes at the immobilized rpoB template.
| RNAP holoenzyme | Without wash | With wash |
|---|---|---|
| Native | 90 ± 14 | 53 ± 1.4 |
| Reconstituted | 40 ± 0 | 40 ± 0 |
Stalled TECs were washed four times with high-salt buffer and two times with transcription buffer (see Materials and Methods). Data are the mean of two experiments.
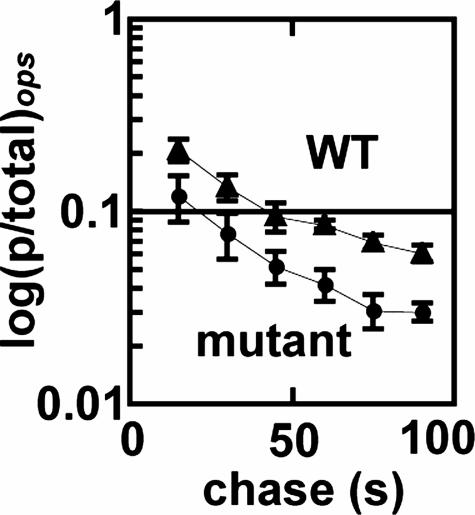
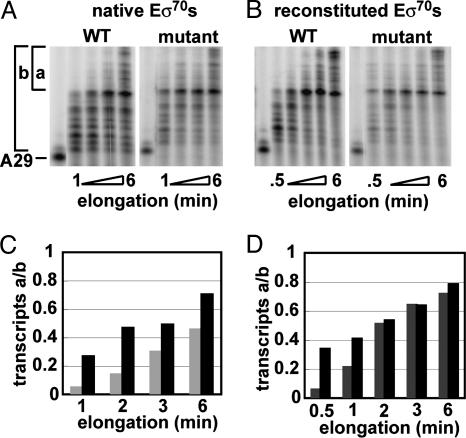
A Single Mutation in σ70 (E407K) Alters Elongation Properties of Native Eσ70. We used the σ70 (E407K) mutant to test whether altering a contact between σ70 and core RNA polymerase altered elongation properties. Mutant n-Eσ70 showed subtle, but reproducible, defects in elongation behavior. First, the pausing efficiency (fast phase) of mutant n-Eσ70 at the ops pause was significantly (≈2-fold) reduced compared with WT n-Eσ70 (Fig. 5). Second, although elongation rates of the two enzymes were the same at 80 μM NTPs (data not shown), they showed clear differences when assayed at very low NTPs (Fig. 6), a condition known to promote pausing (40, 41). At every examined time point, mutant n-Eσ70 had a significantly higher proportion of full-length transcripts than WT n-Eσ70 (shown as transcript ratio a/b; Fig. 6 A and C), and the same was true when comparing the early time points for mutant r-Eσ70and WT r-Eσ70 (Fig. 6 B and D). As expected from the elongation phenotypes presented in Fig. 3, WT r-Eσ70 also exhibited a higher proportion of full-length transcripts than WT n-Eσ70 (Fig. 6 A and B, compare light bars in Fig. 6D to gray bars in Fig. 6C). The most striking result was achieved with mutant r-Eσ70, which had 40% long transcripts after a 30-s incubation, a fraction not achieved by any other enzyme until 2 min (Fig. 6D, black bars).
Fig. 5.
Mutant n-Eσ70 has pausing properties distinct from WT n-Eσ70 at the ops pause site. Reaction profiles of WT and mutant n-Eσ (fast phase) are shown. Fractions of paused RNA generated by mutant n-Eσ and WT n-Eσ70 holoenzymes at ops were plotted against time. Back-extrapolation of the reactions to time 0 yielded pause efficiencies of 26% for WT n-Eσ70 and 16% for mutant n-Eσ70. Data are the average of four independent experiments.
Fig. 6.
Transcription elongation by WT and mutant Eσ70 under NTP limitation. (A and B) Autoradiograms of continuously labeled transcripts. A29-[32P]-CMP-labeled TECs were generated by using a subset of 2.5 μM NTPs. Elongation was resumed by addition of 2.5 μM UTP and continued for the indicated times. (C and D) Distribution of transcripts. As a relative measurement of the synthesis rate, the ratio of transcripts longer than a prominent transcript (a) to the total number of transcripts (b) is plotted vs. time. (C) Transcript ratio a/b of WT n-Eσ70 (gray bars) and mutant n-Eσ70 (black bars). (D) Transcript ratio a/b of WT r-Eσ70 (gray bars) and mutant r-Eσ70 (black bars).
Discussion
The principal contribution of this article is the demonstration that altering the interactions between RNA polymerase and σ70 alters behavior of RNA polymerase during the elongation phase. We demonstrate this alteration in two ways, (i) by comparing the elongation properties of n-Eσ70 assembled in vivo with r-Eσ70 assembled in vitro, and (ii) by comparing elongation properties of transcription initiated by WT σ70 with that initiated by a mutant σ70 defective in a major contact with RNA polymerase. We argue below that these effects are not due to either differential retention or reassociation of σ70 with elongating RNA polymerase, and they are unlikely to result from chemical heterogeneity of the enzyme. Some differences cannot be due to the differential presence of elongation factors. We suggest that the interactions between σ70 and RNA polymerase influence the ensemble of conformational states adopted by RNA polymerase during initiation. These states, in turn, affect the conformational states adopted by the elongating enzyme, thereby physically and functionally imprinting RNA polymerase.
Several considerations suggest that neither differential σ70 retention nor rebinding explain the different elongation properties. First, an affinity-purification method showed that very little σ70 (<5%) was present in our TECs (Fig. 4). Bar-Nahum and Nudler (25) used both this method and an independent assay to show that very little σ70 remains with RNA polymerase purified from exponentially growing cells. Second, after stringent washing of TECs that eliminated σ70, n-Eσ70 and r-Eσ70 still showed differential pausing at his (Fig. 2). Most importantly, even if σ70 were present, it is unlikely that it would act on our TECs. Our washing and transcription buffer contained heparin at a final concentration of 0.1 mg/ml. Brodolin (21), Ederth (39), and Neff (40) have shown that, at this concentration, heparin prevents σ70-mediated pausing at the lacUV5 reiterated –10 region, dissociates σ70 from elongating complexes, and prevents σ70 rebinding. Although this same concentration of heparin does not inhibit the very strong λpR′ pause (42), it is likely to inhibit any weak interactions fortuitously found in our templates. Heparin dissociation of σ70 from elongating complexes is completely consistent with recent structural and biochemical studies positing that σ70 dissociates in several steps. Removal of σ70 from the RNA exit channel is thought to trigger release of σ70 from its interaction with the β flap, which in turn, results in destabilization of other σ70–RNA polymerase contacts. Because heparin antagonizes the interaction between σ70 and the β flap domain (21, 39, 40), loss of this contact is probably sufficient to promote dissociation of weakly associated σ70 remaining in the elongation complex and to prevent its rebinding. Together, we propose that these considerations rule out differential σ70 action as a cause for the observed elongation differences.
Chemical damage to core RNA polymerase during handling could introduce heterogeneity that would result in elongation differences (35). Although it is difficult to conclusively disprove this possibility, we think it is unlikely to account for all differences observed because (i) to minimize oxidation damage, all steps were carried out in the presence of DTT or β-mercaptoethanol; and (ii) the same core RNA polymerase preparation reconstituted with WT or mutant σ70 showed elongation differences, indicating that any putative damage would have to occur after the reconstitution step.
Last, we consider whether these elongation phenotypes could result from differential presence of elongation factors. This explanation can be ruled out for the differences observed between WT and mutant σ70. Mutant n-Eσ70 subjected to the same purification protocol as WT n-Eσ70 still showed decreased pausing at ops and increased elongation rate at a very low NTP concentration. Likewise, mutant r-Eσ70 subjected to the same purification protocol as WT r-Eσ70 elongated more rapidly at low NTPs. The situation is more complex when comparing WT r-Eσ70 and WT n-Eσ70. Here, the BioRex chromatography step used to generate core RNA polymerase by removing σ70 might remove elongation proteins as well. Importantly, n-Eσ70, the starting material for purification of core RNA polymerase, was itself very pure. Before BioRex chromatography, n-Eσ70 had been purified by using Ni–NTA affinity chromotography, high-resolution gel filtration, and anion-exchange chromatography. We also used high-salt treatment, which is known to remove identified elongation factors. The purity of the preparation is demonstrated by Coomassie blue-stained gels as well as by silver staining (Fig. 2 B and D). An overloaded 15% Coomassie blue-stained gel showed that both r-Eσ70 and n-Eσ70 have the small 9-kDa ω-subunit of RNA polymerase and failed to identify any proteins unique to n-Eσ70 (Fig. 2C). To deplete any potential contaminants further, we used immobilized templates, which enabled us to treat TECs with a stringent buffer containing high salt (300 mM KCl), detergent (0.1% Sarkosyl), and 0.1 mg/ml heparin. A silver-stained gel indicated that σ70 was removed by this procedure and that no proteins other than polymerase subunits were visible (Fig. 2D). Because silver staining may overrepresent proteins present in trace amounts, this result is our most critical test of the idea that r-Eσ70 and n-Eσ70 maintain their elongation differences in the absence of other proteins. Differential pause duration at his was maintained after washing (compare Tables 1, column 1, and 2), leading us to argue that this parameter truly depends on properties of the elongating polymerase. However, the elongation-rate differential disappeared (Table 3), which could mean that washing removed a protein contaminant from n-Eσ70 that was not present in r-Eσ70 because of the additional BioRex chromatography step. However, the fact that pause efficiencies of both r-Eσ70 and n-Eσ70 at his were altered by washing suggests that the washing procedure may cause conformational changes in elongating polymerase (Table 2, column 2). This could eliminate the elongation differences between r-Eσ70 and n-Eσ70. In summary, we demonstrate that the elongation differences between mutant and WT Eσ70, and the pause duration difference of WT r-Eσ70 and WT n-Eσ70 at his arise neither from contaminating elongation factors nor from differential σ70 binding or reassociation. These differences are likely to reflect different, metastable states in the enzyme that result from altered interactions during initiation.
How might the distinct elongation behavior of mutant Eσ70 be generated? All alterations in elongation can be explained by a single functional change: a decreased propensity of mutant Eσ70 to backtrack. Thus, mutant n-Eσ70 has a decreased efficiency of pausing at ops (Fig. 2, Table 1) and at λPR' (8), where backtracking is central to the pause (29, 43, 44), but not at his (data not shown), in which backtracking is not important for pause behavior. Likewise, mutant Eσ70 gives an increase in elongation at very low NTP concentrations, which are believed to induce RNA polymerase to adopt unactivated states that are prone to backtracking and arrest (45), but not at high NTP concentrations (data not shown), at which backtracking is minimized.
What structural alteration in RNA polymerase could explain the decreased propensity of mutant Eσ70 to backtrack? σ70 (E407K) weakens the interaction between σ70 region 2.2 and a coiled-coil at the N terminus of the β′-subunit (5, 46, 47). This interaction is essential for σ70 recognition of the nontemplate strand of –10 region in both standard promoters and the reiterated –10 element that defines the λPR′ pause site (20, 48–53). However, the effect of this interaction on RNA polymerase has not been determined. The coiled-coil supports the rudder, which interacts with nascent RNA at the upstream edge of the DNA/RNA hybrid (54), an interaction believed to stabilize the elongation complex (55). The altered interaction between the coiled-coil and region 2.2 of σ70(E407K) could change either the initial positioning or conformation of the rudder. When set, this parameter might be maintained during elongation. Such a change could affect backtracking by elongating RNA polymerase, thereby resulting in the spectrum of functional changes observed when the mutant Eσ70 enzyme enters the elongation phase. σ70(E407K) and other similar σ70 mutants in region 2.2 confer a defect in Q-mediated antitermination. This defect has been attributed solely to poor recognition of the reiterated –10 region at the λPR′ pause site (48). However, pausing is not sufficient for Q function in (19, 56). Might the alteration in the rudder position postulated for mutant Eσ70 partially underlie this defect in Q mediated antitermination? This idea is consistent with the postulated role of Q-proteins to alter elongating RNA polymerase in the vicinity of the NTP-binding site so that it maintains its active conformation (43, 57, 58).
Regardless of the particular mechanism(s) involved, our data suggest that RNA polymerase has a memory of its extensive and dynamic interactions with its initiation factors and that altered interactions can result in altered elongation behavior. These studies are consistent with recent single-molecule studies of diverse enzymes, which reveal memory landscapes consistent with more than one reaction path (59, 60). The idea that interactions during the initiation can be propagated during elongation suggests an interesting mechanism for altering elongation. In eubacteria, using different σ factors as well as other initiation proteins may alter the spectrum of states of elongating polymerase, thereby modulating its intrinsic behavior as well as its response to various elongation factors. In eukaryotes, in which the initiation of RNA polymerase PolII serves as a platform to coordinate all subsequent processing and transport of mRNA, the ability to influence the repertoire of RNA polymerase states may be crucial to promoter-specific directions to the elongating polymerase.
Acknowledgments
We thank K. Severinov (Rutgers, The State University of New Jersey, Piscataway) for pET15rpoD; R. Landick (University of Wisconsin, Madison) for pIA171, pIA273, and pIA146; all members of the C.A.G. laboratory for technical advice and helpful discussions; and A. Hochheimer, R. Landick, and J. Roberts for critical reading of the manuscript. This work was supported by National Institutes of Health Grant GM057755.
Author contributions: C.A.G. designed research; Y.B.-H. and C.Z.L. performed research; and Y.B.-H. and C.A.G. wrote the paper.
Abbreviations: NTA, nitrilotriacetic acid; TEC, transcription-elongation complex.
References
- 1.Gruber, T. M. & Gross, C. A. (2003) Annu. Rev. Microbiol. 57, 441–466. [DOI] [PubMed] [Google Scholar]
- 2.Borukhov, S. & Nudler, E. (2003) Curr. Opin. Microbiol. 6, 93–100. [DOI] [PubMed] [Google Scholar]
- 3.Darst, S. A. (2001) Curr. Opin. Struct. Biol. 11, 155–162. [DOI] [PubMed] [Google Scholar]
- 4.Gross, C. A., Chan, C., Dombroski, A., Gruber, T., Sharp, M., Tupy, J. & Young, B. (1998) Cold Spring Harbor Symp. Quant. Biol. 63, 141–155. [DOI] [PubMed] [Google Scholar]
- 5.Gruber, T. M., Markov, D., Sharp, M. M., Young, B. A., Lu, C. Z., Zhong, H. J., Artsimovitch, I., Geszvain, K. M., Arthur, T. M., Burgess, R. R., et al. (2001) Mol. Cell 8, 21–31. [DOI] [PubMed] [Google Scholar]
- 6.Murakami, K. S., Masuda, S. & Darst, S. A. (2002) Science 296, 1280–1284. [DOI] [PubMed] [Google Scholar]
- 7.Murakami, K. S., Masuda, S., Campbell, E. A., Muzzin, O. & Darst, S. A. (2002) Science 296, 1285–1290. [DOI] [PubMed] [Google Scholar]
- 8.Sharp, M. M., Chan, C. L., Lu, C. Z., Marr, M. T., Nechaev, S., Merritt, E. W., Severinov, K., Roberts, J. W. & Gross, C. A. (1999) Genes Dev. 13, 3015–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassylyev, D. G., Sekine, S., Laptenko, O., Lee, J., Vassylyeva, M. N., Borukhov, S. & Yokoyama, S. (2002) Nature 417, 712–719. [DOI] [PubMed] [Google Scholar]
- 10.Dombroski, A. J., Walter, W. A., Record, M. T., Jr., Siegele, D. A. & Gross, C. A. (1992) Cell 70, 501–512. [DOI] [PubMed] [Google Scholar]
- 11.Callaci, S., Heyduk, E. & Heyduk, T. (1999) Mol. Cell 3, 229–238. [DOI] [PubMed] [Google Scholar]
- 12.Campbell, E. A., Muzzin, O., Chlenov, M., Sun, J. L., Olson, C. A., Weinman, O., Trester-Zedlitz, M. L. & Darst, S. A. (2002) Mol. Cell 9, 527–539. [DOI] [PubMed] [Google Scholar]
- 13.Mekler, V., Kortkhonjia, E., Mukhopadhyay, J., Knight, J., Revyakin, A., Kapanidis, A. N., Niu, W., Ebright, Y. W., Levy, R. & Ebright, R. H. (2002) Cell 108, 599–614. [DOI] [PubMed] [Google Scholar]
- 14.Sorenson, M. K., Ray, S. S. & Darst, S. A. (2004) Mol. Cell 14, 127–138. [DOI] [PubMed] [Google Scholar]
- 15.Burgess, R. R. (1971) Annu. Rev. Biochem. 40, 711–740. [DOI] [PubMed] [Google Scholar]
- 16.Hansen, U. M. & McClure, W. R. (1980) J. Biol. Chem. 255, 9564–9570. [PubMed] [Google Scholar]
- 17.Shimamoto, N., Kamigochi, T. & Utiyama, H. (1986) J. Biol. Chem. 261, 11859–11865. [PubMed] [Google Scholar]
- 18.Stackhouse, T. M., Telesnitsky, A. P. & Meares, C. F. (1989) Biochemistry 28, 7781–7788. [DOI] [PubMed] [Google Scholar]
- 19.Ring, B. Z., Yarnell, W. S. & Roberts, J. W. (1996) Cell 86, 485–493. [DOI] [PubMed] [Google Scholar]
- 20.Marr, M. T. & Roberts, J. W. (1997) Science 276, 1258–1260. [DOI] [PubMed] [Google Scholar]
- 21.Brodolin, K., Zenkin, N., Mustaev, A., Mamaeva, D. & Heumann, H. (2004) Nat. Struct. Mol Biol., 11, 551–557. [DOI] [PubMed] [Google Scholar]
- 22.Ring, B. Z. & Roberts, J. W. (1994) Cell 78, 317–324. [DOI] [PubMed] [Google Scholar]
- 23.Nickels, B. E., Mukhopadhyay, J., Garrity, S. J., Ebright, R. H. & Hochschild, A. (2004) Nat. Struct. Mol Biol., 11, 544–550. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, J. W., Yarnell, W., Bartlett, E., Guo, J., Marr, M., Ko, D. C., Sun, H. & Roberts, C. W. (1998) Cold Spring Harbor Symp. Quant. Biol. 63, 319–325. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Nahum, G. & Nudler, E. (2001) Cell 106, 443–451. [DOI] [PubMed] [Google Scholar]
- 26.Uptain, S. M., Kane, C. M. & Chamberlin, M. J. (1997) Annu. Rev. Biochem. 66, 117–172. [DOI] [PubMed] [Google Scholar]
- 27.Landick, R., Wang, D. & Chan, C. L. (1996) Methods Enzymol. 274, 334–353. [DOI] [PubMed] [Google Scholar]
- 28.Toulokhonov, I., Artsimovitch, I. & Landick, R. (2001) Science 292, 730–733. [DOI] [PubMed] [Google Scholar]
- 29.Artsimovitch, I. & Landick, R. (2000) Proc. Natl. Acad. Sci. USA 97, 7090–7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artsimovitch, I. & Landick, R. (2002) Cell 109, 193–203. [DOI] [PubMed] [Google Scholar]
- 31.Davenport, R. J., Wuite, G. J., Landick, R. & Bustamante, C. (2000) Science 287, 2497–2500. [DOI] [PubMed] [Google Scholar]
- 32.Neuman, K. C., Abbondanzieri, E. A., Landick, R., Gelles, J. & Block, S. M. (2003) Cell 115, 437–47. [DOI] [PubMed] [Google Scholar]
- 33.Shaevitz, J. W., Abbondanzieri, E. A., Landick, R. & Block, S. M. (2003) Nature 426, 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erie, D. A. (2002) Biochim. Biophys. Acta 1577, 224–239. [DOI] [PubMed] [Google Scholar]
- 35.Tolic-Norrelykke, S. F., Engh, A. M., Landick, R. & Gelles, J. (2004) J. Biol. Chem. 279, 3292–3299. [DOI] [PubMed] [Google Scholar]
- 36.Burgess, R. R. & Jendrisak, J. J. (1975) Biochemistry 14, 4634–4638. [DOI] [PubMed] [Google Scholar]
- 37.Nudler, E., Avetissova, E., Markovtsov, V. & Goldfarb, A. (1996) Science 273, 211–217. [DOI] [PubMed] [Google Scholar]
- 38.Lowe, P. A., Hager, D. A. & Burgess, R. R. (1979) Biochemistry 18, 1344–1352. [DOI] [PubMed] [Google Scholar]
- 39.Ederth, J., Artsimovitch, I., Isaksson, L. A. & Landick, R. (2002) J. Biol. Chem. 277, 37456–37463. [DOI] [PubMed] [Google Scholar]
- 40.Neff, N. F. & Chamberlin, M. J. (1980) Biochemistry 19, 3005–3015. [DOI] [PubMed] [Google Scholar]
- 41.Levin, J. R. & Chamberlin, M. J. (1987) J. Mol. Biol. 196, 61–84. [DOI] [PubMed] [Google Scholar]
- 42.Roberts, C. W. & Roberts, J. W. (1996) Cell 86, 495–501. [DOI] [PubMed] [Google Scholar]
- 43.Marr, M. T. & Roberts, J. W. (2000) Mol. Cell 6, 1275–1285. [DOI] [PubMed] [Google Scholar]
- 44.Marr, M. T., Datwyler, S. A., Meares, C. F. & Roberts, J. W. (2001) Proc. Natl. Acad. Sci. USA 98, 8972–8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster, J. E., Holmes, S. F. & Erie, D. A. (2001) Cell 106, 243–252. [DOI] [PubMed] [Google Scholar]
- 46.Arthur, T. M., Anthony, L. C. & Burgess, R. R. (2000) J. Biol. Chem. 275, 23113–23119. [DOI] [PubMed] [Google Scholar]
- 47.Arthur, T. M. & Burgess, R. R. (1998) J. Biol. Chem. 273, 31381–31387. [DOI] [PubMed] [Google Scholar]
- 48.Ko, D. C., Marr, M. T., Guo, J. & Roberts, J. W. (1998) Genes Dev. 12, 3276–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young, B. A., Anthony, L. C., Gruber, T. M., Arthur, T. M., Heyduk, E., Lu, C. Z., Sharp, M. M., Heyduk, T., Burgess, R. R. & Gross, C. A. (2001) Cell 105, 935–944. [DOI] [PubMed] [Google Scholar]
- 50.Anthony, L. C., Dombkowski, A. A. & Burgess, R. R. (2002) J. Bacteriol. 184, 2634–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulbachinskiy, A., Mustaev, A., Goldfarb, A. & Nikiforov, V. (1999) FEBS Lett. 454, 71–74. [DOI] [PubMed] [Google Scholar]
- 52.Malhotra, A., Severinova, E. & Darst, S. A. (1996) Cell 87, 127–136. [DOI] [PubMed] [Google Scholar]
- 53.Severinova, E., Severinov, K., Fenyo, D., Marr, M., Brody, E. N., Roberts, J. W., Chait, B. T. & Darst, S. A. (1996) J. Mol. Biol. 263, 637–647. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, G., Campbell, E. A., Minakhin, L., Richter, C., Severinov, K. & Darst, S. A. (1999) Cell 98, 811–824. [DOI] [PubMed] [Google Scholar]
- 55.Kuznedelov, K., Korzheva, N., Mustaev, A. & Severinov, K. (2002) EMBO J. 21, 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarnell, W. S. & Roberts, J. W. (1992) Cell 69, 1181–1189. [DOI] [PubMed] [Google Scholar]
- 57.Yarnell, W. S. & Roberts, J. W. (1999) Science 284, 611–615. [DOI] [PubMed] [Google Scholar]
- 58.Santangelo, T. J., Mooney, R. A., Landick, R. & Roberts, J. W. (2003) Genes Dev. 17, 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edman, L. & Rigler, R. (2000) Proc. Natl. Acad. Sci. USA 97, 8266–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lerch, H. P., Mikhailov, A. S. & Hess, B. (2002) Proc. Natl. Acad. Sci. USA 99, 15410–15415. [DOI] [PMC free article] [PubMed] [Google Scholar]