Summary
The circadian (~ 24 hour) system has a central role in regulating the timing and coordination of photosynthesis – the clock controlled photosynthesis and photosynthetic products feedback to affect the circadian oscillator that generates rhythms. However, little is known about the mechanism(s) by which this feedback occurs. One group of likely candidates for signal transduction to the circadian clock are the PHYTOCHROME INTERACTING FACTOR (PIF) family of transcription factors which have been shown to be involved in numerous signaling pathways in Arabidopsis. Yet despite evidence that some PIFs are under circadian control and bind promoter motifs present in circadian genes, until now PIFs have not been shown to affect the circadian system.
Using a range of techniques, we have examined how circadian rhythms are affected in higher order pif mutants and the mechanisms by which PIFs regulate signaling to the circadian clock.
We show that PIFs mediate metabolic signals to the circadian oscillator and that sucrose directly affects PIF binding to the promoters of key circadian oscillator genes in vivo that may entrain the oscillator.
Our results provide a basis for understanding the mechanism for metabolic signaling to the circadian system in Arabidopsis.
Keywords: Arabidopsis, circadian clock, light signaling, metabolism, PHYTCHROME INTERACTING FACTORS (PIFs), photosynthesis, sucrose
Introduction
As the earth rotates around its axis, almost all organisms live with daily oscillations in their environment and have developed endogenous mechanisms, called circadian rhythms, to anticipate these changes and adapt accordingly. Circadian (~24 hour) regulated biological rhythms have been identified in a wide range of organisms from prokaryotic unicellular cyanobacteria to higher plants and mammals (Jolma et al., 2010). Conceptually, a circadian system can be divided into three parts: the oscillator mechanism, input pathways and output pathways. The oscillator that generates the rhythms has been widely studied in Arabidopsis thaliana, and shown to be comprised of a series of interlocking feedback loops. A central loop is based on CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), LATE ELONGATED HYPOCOTYL (LHY) and TIMING OF CAB EXPRESSION 1 (TOC1; a member of the PSEUDO RESPONSE REGULATOR, PRR family) regulating each other’s expression (Alabadi et al., 2001; Yakir et al., 2009). The input pathways serve to transmit various environmental signals such as light, perceived by the phytochrome (phy) and cryptochrome (cry) photoreceptors, and temperature to entrain the phase and waveform of the oscillator (Gould et al., 2006; Boikoglou et al., 2011; Greenham & McClung, 2015). Output pathways regulate such diverse processes as hormone production, reproductive development, defense responses and the expression of at least 30% of the genome (Greenham & McClung, 2015). The value of circadian systems can be seen in the poor performance of organisms that do not have functional, environment-matching circadian oscillators (Ouyang et al., 1998; Green et al., 2002; Dodd et al., 2005; Yerushalmi et al., 2011).
Increasingly, studies are also showing an important role for the circadian system in regulating the timing and co-ordination of metabolism. In a range of different organisms from mammals to plants (Eckel-Mahan & Sassone-Corsi, 2013) the circadian system controls the metabolic state of the cell and in many cases metabolic products are able to feedback to entrain the oscillator. In plants the circadian system controls photosynthesis and sugars produced by photosynthesis regulate the oscillator (Haydon et al., 2013). However, surprisingly little is known about the mechanism(s) by which sugars feedback to entrain the oscillator.
In Arabidopsis, the PIF (PHYTOCHROME INTERACTING FACTOR) family comprises seven members (PIF1, PIF3-8). PIFs were originally identified as basic helix-loop-helix (bHLH) transcription factors that interacted with the light-activated red/far-red photoreceptor phytochromes, but they are now known to be involved in numerous signaling pathways including temperature responses, hormone and sucrose signaling (Toledo-Ortiz et al., 2003; Castillon et al., 2007; Leivar & Quail, 2011; Leivar & Monte, 2014). Although PIFs are highly homologous proteins and display overlapping functions, monogenic pif mutants also display distinct phenotypes (Huq et al., 2004; Oh et al., 2004; Koini et al., 2009; Toledo-Ortíz et al., 2010). For example, pif1, pif3-pif5 and pif7 single mutants have short hypocotyls under red and/or far-red light conditions. pif1 mutants affect seed germination, chlorophyll and carotenoid accumulation in response to light (Huq et al., 2004; Oh et al., 2004; Oh et al., 2009; Toledo-Ortíz et al., 2010; Zhang et al., 2013) and pif4, but not pif3 or pif5, mutants show defective temperature sensing (Koini et al., 2009). In addition, PIFs have been shown to bind to central clock gene promoters both in vitro and in vivo (Martinez-Garcia et al., 2000; Oh et al., 2009; Oh et al., 2012), and a number of studies have shown that some PIFs are controlled by the circadian system (Yamashino et al., 2003; Nozue et al., 2007; Shin et al., 2013). Thus, PIFs are excellent candidates for transducing environmental signals to the clock. However, until now, studies on single and double pif mutants have failed to reveal a role for PIFs in the circadian system (Viczian et al., 2005; Nusinow et al., 2011).
Here we demonstrate that PIFs control metabolic signaling to the oscillator in plants; higher order pif mutants are significantly defective in sucrose regulation of circadian function. We also start to examine the mechanisms by which PIFs regulate sucrose signals to the oscillator. Our results show that sucrose affects PIF levels and activity and that sucrose-mediated PIF binding to the promoters of circadian oscillator genes alters their expression to affect circadian timing.
Materials and Methods
Plant Materials and Growth Conditions
The pifQ CCA1:LUC, wt CCA1:LUC and myc-tagged PIF4 transgenic lines were generated in the Col-0 background of Arabidopsis thaliana as described below. PIF1-HA (Zhu et al., 2015), PIF1-TAP (Bu et al., 2011), PIF3-MYC (Park et al., 2004), PIF5-MYC (Sakuraba et al., 2014) have been previously published. PIF overexpressor lines used are 35spro: PIF1-HA (Zhu et al., 2015), 35spro: PIF3-myc (Park et al., 2004), 35spro: PIF4-myc, 35spro: PIF5-myc (Sakuraba et al., 2014). Unless otherwise stated, seeds were imbibed and cold treated at 4°C for 4 days and sown onto Petri dishes on Murashige and Skoog medium supplemented with 0–3% (0–90mM) sucrose or 90mM mannitol (luciferase assay), or 2% sucrose (w/v) (leaf movement assay). For all the circadian experiments plants were entrained for 1 week in 14:10 light:dark (100 μmol m−2 s−1 white light, Philips fluorescent lights TLD 18W/840) for LL or 10 days under the same conditions for DD experiments, before being transferred to free running conditions. All experiments were done at a constant 23°C.
Construction of Vectors and Generation of Transgenic Plants
To generate the pPZP121 pAtCCA1::LUC construct, pFAMIR pAtCCA1::LUC was PCR amplified and cloned into pPZP121 vector with EcoRI 5′ and SacI 3′. The construct was then transformed into wt (Col-0) and pifQ by Agrobacterium- mediated floral dip. Transformants were selected with gentamycin resistance. To construct the myc-tagged PIF4 overexpression line, the full-length PIF4 open reading frame was cloned into pENTRY vector (Invitrogen Inc., Carlsbad, CA) and recombined with pGWB17 (for overexpression) (Nakagawa et al., 2007). The resulting binary construct was then transformed into pif4-2 using the Agrobacterium mediated transformation protocol as described (Clough & Bent, 1998). Single locus transgenic plants were selected based on antibiotic resistance and several homozygous lines were produced for analyses.
Bioluminescence Assays
For each assay, 3–7 seedlings from each of 6–8 independent pifQ CCA1:LUC and wt CCA1:LUC lines were imaged. Plants were sprayed with 2.5mM luciferin (D-Luciferin, Potassium salt, Gold Biotechnology, St Louis, MO, USA) in 0.01% Triton X-100 before being transferred to a growth chamber mounted with a Hamamatsu ORCA II ER CCD camera (C4742-98 ERG; Hamamatsu Photonics, Hamamatsu City, Japan). Light was provided by 620 light emitting diodes of different fluence rates (from 5 to 50 μE m−2 s−1). Luciferase activity was imaged for 25 minutes every two hours for at least four days. Images were analyzed with ImagePro software (Media Cybernetics, Inc., Bethesda, MD, USA). Data were imported into the Biological Rhythms Analysis Software System (BRASS; available from http://www.amillar.org) and analyzed with the FFT-NLLS suite of the program, as previously described (Plautz et al., 1997). Rhythms with a period between 14 and 34 hours were taken to be within the circadian range. The relative amplitude error (R.A.E.; range 0 to 1) was determined from FFT-NNLS analysis and used to assess individual rhythm robustness, with values close to 0 indicating robust cycling and values at or near 1 indicating a rhythm with an error value as large as the amplitude itself (not statistically significant).
Leaf Movement Assays
Plants were grown on MS medium supplemented with or without 2% sucrose in 14 L:10 D 100 μmol m−2s−1 (LD) for 7 days at 23°C before being transferred to 24-well cell culture plates (Greiner Labortechnik, Kremsmünster, Oberösterreich, Austria), one plant per well. The plates were put into continuous white light (LL, 60 μmol m−2s−1 provided by white LEDs for the sucrose experiments and red+green+blue LEDs for the no-sucrose experiments) at 23°C. Leaf movements were recorded every 20 minutes for seven days by Panasonic CCTV cameras, model WV-BP120 (Matsushita Communications Industrial, Yokohama, Japan). Post-run analysis was performed using the ImagePro Plus software (Media Cybernetics, Inc., Bethesda, MD, USA) and traces were analyzed by FFT-NLLS.
Sucrose pulses
Sucrose pulse experiments were carried out essentially as described (Haydon & Webb, 2016). pifQ CCA1:LUC plants and wt CCA1:LUC plants were grown for 10 days in 14 L:10 D 100 μmol m−2s−1 provided by white LEDs on MS media without sucrose on 0.8 μm pore nylon mesh filters to prevent the roots from penetrating into the media. The plants were transferred at dawn to continuous 5μE red light and luciferase activity imaged at 1 hour intervals. After 24 hours in continuous red light, the membranes with plants were transferred, for three hours onto MS media supplemented with 3% sucrose and irrigated with liquid MS + 3% sucrose. After the pulse, plants were washed with liquid MS media and transferred back onto MS plates for subsequent imaging. For the controls, the transfers were made using MS without sucrose. All the manipulations were done under green safe light. The time of the first peak after the sucrose pulse was determined using the BRASS “Peak time analysis” option.
Chromatin Immunoprecipitation (ChIP) Assays
PIF1, PIF3, PIF4 and PIF5 transgenic lines have been described previously (Oh et al., 2004; Park et al., 2004; Bu et al., 2011; Sakuraba et al., 2014). PIF1 is expressed from the native promoter and PIF3/4/5 are expressed from the 35S promoter. ChIP assays were performed essentially as described in (Moon et al., 2008). Seven day-old 12 L:12 D grown seedlings were transferred to dark/light for additional two days before vacuum infiltration with 1% formaldehyde for 15 minutes at RT. Cross-linking was quenched by 0.125M glycine for 5 minutes. Samples were washed using large amount of water, dried on filter papers and ground into powder in liquid nitrogen. One ml of nuclei isolation buffer (0.25M Sucrose, 15mM PIPES pH6.8, 5mM MgCl2, 60mM KCl, 15mM NaCl, 0.9% Triton X-100, 1mM PMSF and 1X Protease inhibitor cocktail [P9599, Sigma Aldrich, St. Louis, Missouri, USA]) was added to the powder and the samples were centrifuged at 16,000g for 10 minutes at 4°C. The pellets were resuspended with 1ml lysis buffer (50mM HEPES pH7.5, 150mM NaCl, 10mM EDTA, 1% Triton X-100, 0.1% Na Deoxycholate, 0.1% SDS, 1mM PMSF and 1X Protease inhibitor cocktail) prior to sonication. Sonicated samples were clarified by centrifuge at 16,000g at 4°C for 5 minutes. Two μl of c-MYC tag antibody (C3956, Sigma Aldrich, St. Louis, Missouri, USA) were used for immunoprecipitation at 4°C for overnight. 30Zl of salmon sperm DNA coated Dynabead protein A (10002D, Life technology, Carlsbad, California, USA) was then added into each sample for another two hours at 4°C. Immunoprecipitated samples were sequentially washed and eluted with the elution buffer (1% SDS, 0.1 M NaHCO3). 250Zl of eluted sample was incubated with 10Zl 5M NaCl at 65°C overnight. DNA was purified using QIAEX II Gel Extraction Kit (20051, Qiagen, Hilden, Germany) and analyzed by qPCR with the primers described in the Table S1.
To determine PIF protein concentrations for each time point, tissue was ground in liquid nitrogen and solubilized in same volume of urea extraction buffer (8M urea, 20mM Tris 7.5, 1mM PMSF, 1X protease inhibitor cocktail). After centrifugation at 10min 4°C the supernatant was collected and boiled with SDS sample buffer. Proteins were separated by SDS PAGE, transferred to PVDF membrane and analysed by immunoblotting.
Quantitative RT-PCR
Fifteen to eighteen seedlings of each genotype were grown on MS medium with or without 3% sucrose in 14:10 light:dark for 10 days before being transferred to free running conditions. Plants were harvested and total RNA as previously described (Green & Tobin, 1999). RNA samples were treated with DNase (PerfeCTa DNAse from Quanta bio) according to the manufacturer’s instructions. From each DNA-free RNA sample, 7 μl aliquots were used as a template to produce cDNA, using the qScript cDNA SuperMix (Quanta bio). The cDNA was diluted 4 or 5-fold and 1.5ul of template was used for RT-PCR reaction with SYBR green reagent (KAPA SYBR FAST qPCR kit Master Mix, Kapa Biosystems) according to the supplier’s protocol. Three technical repeats were made for each sample. Fluorescence was detected using the QuantStudio 12K Flex system (ThermoFisher Scientific). PROTEIN PHOSPHATASE 2A (PP2A) and TUBULIN (TUB) were used as controls for normalization. Quantitation calculations were carried out using the 2−ΔΔCT formula as described (Nozue et al., 2007). The primers are shown in Supplementary Table S1.
Results
Higher order pif mutants affect circadian rhythms
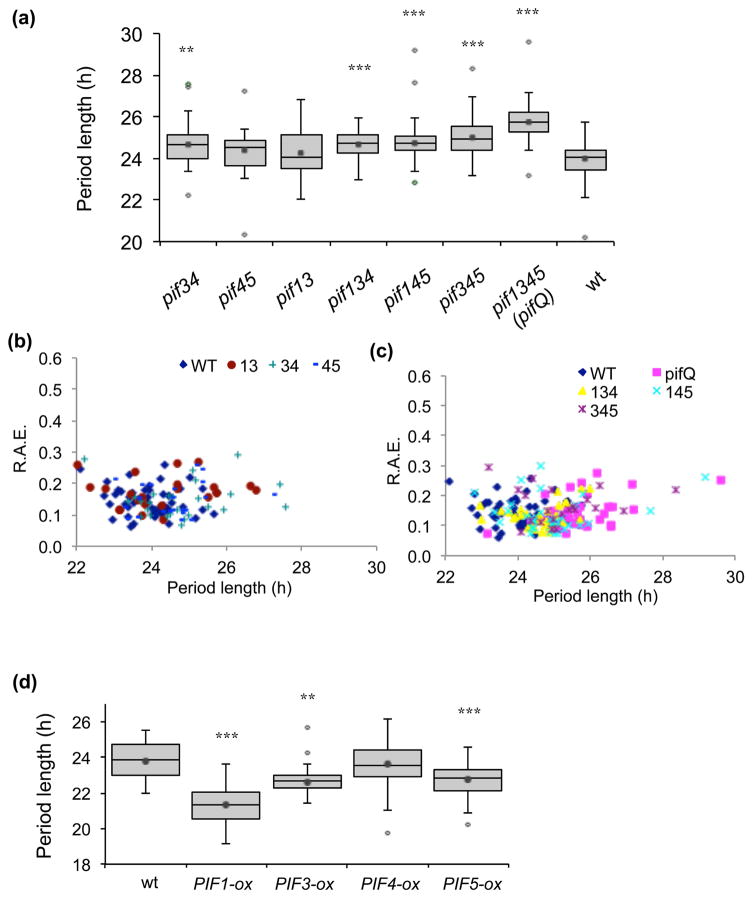
Our first goal was to determine, using higher order pif mutants and PIF-overexpression lines, whether PIFs may have a role in regulating the circadian system. Fig. 1(a) shows that, in continuous white light (LL) on 2% sucrose, pif13 (24.26 h ± 0.27 SEM) and pif45 (24.37 h ± 0.16 SEM) did not significantly affect rhythms compared with wild type (wt; 24.00 h ± 0.11 SEM). However, pif34 (24.66 h ± 0.17 SEM and the three triple mutants, pif345 (25.02 h ± 0.20 SEM), pif145 (24.73 h ± 0.12 SEM) and pif134 (24.65 h ± 0.13 SEM) had longer periods and the quadruple, pif1pif3pif4pif5 (pifQ), mutant was ~1.7 hours longer (25.71 h ± 0.12 SEM) than wt. p values for each pif mutant compared with wt by ANOVA single factor analysis and by Student’s t-test are shown in Supplementary Table S2 and S3. Relative amplitude error (R.A.E.) is used to assess the precision of a circadian rhythm, values close to 0 indicating robust cycling and values at or near 1 indicating a rhythm with an error value as large as the amplitude itself (not statistically significant) (Plautz et al., 1997). All of the pif mutant and wt plants had an R.A.E. below 0.3, suggesting that under these conditions, although they had longer periods, the pif mutants were still robustly rhythmic (Fig. 1b, c). Not only leaf movements were affected, we also observed that expression of circadian oscillator and output genes, LHY, PRR7, PRR9, TOC1 and CHLOROPHYLL A-B BINDING PROTEIN (CAB1) were altered in the pifQ mutant (Supplementary Fig. 1a–e). Consistent with the longer period phenotypes of the higher order pif mutants, overexpression of PIF1 (21.32 h ± 0.19 SEM), PIF3 (22.61 h ± 0.12 SEM) and PIF5 (22.76 h ± 0.17 SEM) significantly shortened leaf movement period length compared with wt (23.80 h ± 0.23 SEM) under the same conditions (Fig. 1d). Overexpression of PIF4 did not affect rhythms (23.66 h ± 0.27 SEM, p=0.44); however, among all the PIF overexpression lines, PIF4 displayed the lowest expression (Supplementary Fig. 1f). Taken together, our results demonstrate that PIFs regulate circadian rhythms.
Fig. 1.
Circadian rhythms are altered by mis-expression of PIF genes in Arabidopsis. The pif mutants and PIF-ox plants, together with a wild-type (wt) control, were entrained in LD before being transferred to LL and leaf movements imaged for a week. (a and d) The period lengths for each genotype. The interquartile range with whiskers for variability are shown. The average is shown as a dot inside the box. Outliers, defined as values outside the range [(Q1−1.5(Q3−Q1)), (Q3+1.5(Q3−Q1))] where Q1 and Q3 are first and third quartiles, are depicted as open diamonds. Average periods and SEM calculated with and without outliers are shown in Supplementary Table S3. Data from two independent experiments. (b and c) The R.A.E. for pif mutants and wt plants plotted against period length. n =20–30 for each line. * p<0.05, ** p<0.01, *** p<0.001 (Student two-tailed t-test).
PIFs regulate metabolic signaling to the circadian oscillator
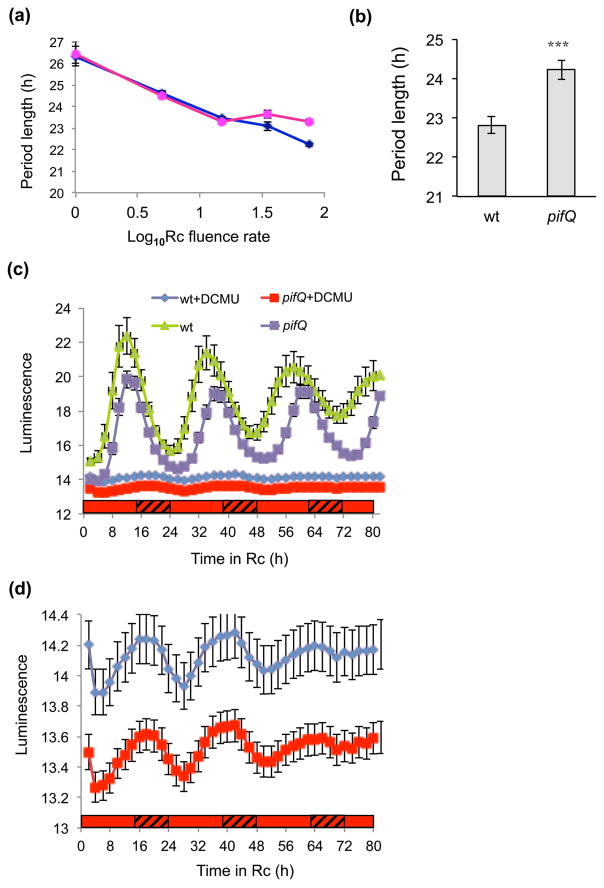
Our next aim was to identify which circadian pathways are controlled by PIFs. PIFs have been shown to regulate both metabolite and light regulation of diverse processes – they were first identified as phytochrome-interacting factors and shown to influence photomorphogenesis (Castillon et al., 2007; Xu et al., 2015). We started by examining whether PIFs are involved in red light signaling. Since the highest order pif mutant had the strongest phenotype (Fig. 1a–c), we generated transgenic pifQ and wt plants harboring the promoter of a key oscillator gene, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) fused to the LUCIFERASE reporter (CCA1:LUC). Fig. 2(a) and Supplementary Fig. 2 (a and b) show that in the absence of exogenous sucrose in low intensity red light (Rc) (at or below 15 μmol m−2s−1), pifQ CCA1:LUC plants had similar periods to wt CCA1:LUC (p=0.87, two-way ANOVA genotype/light intensity), However, in high Rc (35–75 uE) pifQ CCA1:LUC lines showed significantly longer periods LUC (p<0.001, two-way ANOVA genotype/light intensity). Consistent with its phenotype in high Rc, in high white light the pifQ mutant maintained a longer period of leaf movements (24.23 h ± 0.25 SEM) than wt (22.82 h ± 0.22 SEM) even in the absence of exogenous sucrose (Fig. 2b). Our findings that we only see differences between pifQ and wt plants under higher light intensities suggest that PIFs may be regulating metabolic signals from photosynthesis; if PIFs were mediating photoreceptor signals we might expect to also see differences at low and intermediate red light fluences.
Fig. 2.
PIFs regulate metabolic signaling in the circadian system in Arabidopsis. (a–b) High light effects on PIF regulation of circadian rhythms. pifQ CCA1:LUC and wt CCA1:LUC lines were entrained on medium without sucrose before being transferred to (a) Rc of different fluences or (b) 60 μmol m−2s−1 constant white light. (a) Luciferase activity and (b) leaf movements were plotted. The average of 3–4 independent experiments (a) n ≥66, (b) n ≥28. (b) The SEM was plotted, *** p<0.0001 (Student two-tailed t-test). (c–d) The effects of inhibiting photosynthesis on pifQ regulation of circadian rhythms. pifQ CCA1:LUC and wt CCA1:LUC lines were entrained on medium without sucrose before being transferred to 75 μmol m−2s−1 continuous red light (Rc). Two days before transfer to Rc, two groups of seedlings were replanted on medium containing 20ZM DCMU (filled and open green triangles) or without DCMU (filled and open blue circles). d, shows wt and pifQ with DCMU plotted on a larger Y axis scale. The average of 2 independent experiments (n ≥40). *** p<0.001 (Student two-tailed t-test). The red and hatched bars represent subjective light and respectively.
To confirm the requirement of photosynthesis for the pifQ phenotype, we grew plants with and without the photosynthesis inhibitor, DCMU. In high light conditions in the absence of DCMU, pifQ CCA1:LUC plants (22.98 h ± 0.15 SEM) showed longer period rhythms than wt (21.71 h ± 0.10 SEM; p>0.0001 Student two-tailed t-test) (Fig. 2c). Both pifQ CCA1:LUC and wt CCA1:LUC plants had similar amplitudes (p=0.76 two-tail Student t-test; Supplementary Fig. 3). By contrast, in the presence of DCMU, the period of the pifQ CCA1:LUC plants (22.49 h ± 0.10 SEM) was similar to wt CCA1:LUC lines (22.63 h ± 0.11SEM; p=0.32) (Fig. 2c and d).
Sucrose signaling to the circadian oscillator is controlled by PIFs
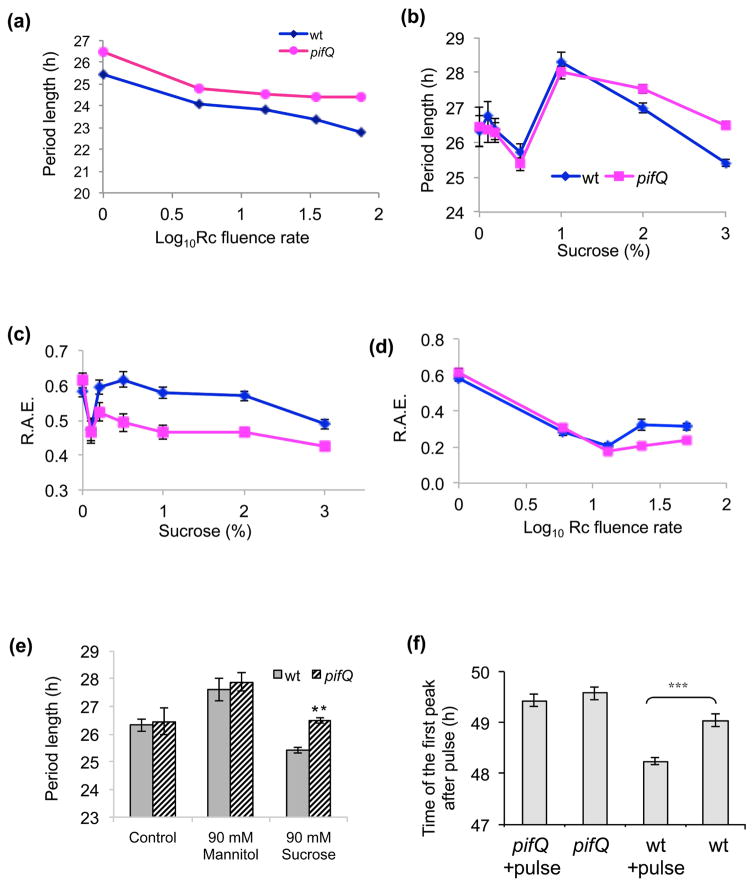
In Arabidopsis CO2 fixed during photosynthesis is partitioned into soluble and insoluble compounds with sucrose being generally the most abundant of the soluble compounds (Zeeman & Rees, 1999). In the presence of sucrose, pifQ CCA1:LUC plants had longer periods than wt (p<0.001, two-way ANOVA genotype/light intensity) at all fluences of Rc (Fig. 3a and Supplementary Fig. 2c). To confirm that PIFs are involved in sucrose signaling to the circadian oscillator, we generated a sucrose response curve in low fluence Rc (1 μmol m−2s−2; Fig. 3b). For wt CCA1:LUC plants the effects of sucrose on circadian rhythms were complex; consistent with previously published results (Knight et al., 2008; Haydon et al., 2013), high concentrations of sucrose slightly reduced the period length of CCA1:LUC rhythmicity compared with controls grown without sucrose (0% sucrose 26.32 h ± 0.4 SEM; 3% sucrose 25.41 h ± 0.10 SEM) as did the addition of low concentrations of sucrose (0.5% sucrose 25.7 h ± 0.24 SEM; Fig. 3b and Supplementary Fig. 4). However, with intermediate sucrose concentrations there was an increase in period length (1% sucrose 28.29 h ± 0.3 SEM). This complex picture may be a result of sucrose affecting the circadian system via more than one sucrose-sensing pathway including osmotic effects (Dalchau et al., 2011). pifQ CCA1:LUC (26.5 h ± 0.11 SEM) showed longer periods than wt CCA1:LUC plants (25.41 h ± 0.10 SEM) on high sucrose (p<0.001 for interaction of genotype and sucrose factors by two-way ANOVA).
Fig. 3.
PIFs are involved in directly regulating signals from sucrose to the oscillator. (a) PIF affects circadian rhythms in all light fluences in plants growing on sucrose. pifQ CCA1:LUC and wt CCA1:LUC lines were entrained on medium with 3% (90mM) sucrose before being transferred to Rc of different fluences. Luciferase activity was plotted together with the SEM. The average of 3–4 independent experiments (n ≥46). (b) Sucrose response curve for PIF regulation of the circadian oscillator. pifQ CCA1:LUC and wt CCA1:LUC lines were entrained on medium supplemented with 0, 0.1, 0.2, 0.5, 1, 2 or 3% sucrose, and transferred to 1Zmol red light luciferase activity was plotted together with the SEM. The average of 2–3 independent experiments, n ≥77. At the lowest (0.2% and below) sucrose concentrations, amplitudes of luciferase activity were very low which made it difficult to accurately measure circadian period and three biological repeats with n ≥120 plants were taken. (c–d) R.A.E. is affected by sucrose. The R.A.E. were plotted for (c) data shown in Fig. 3b and (d) data shown in Fig. 2a. (e) The effects of mannitol on PIF control of circadian rhythms. pifQ CCA1:LUC and wt CCA1:LUC lines were entrained on medium supplemented with or without 3% (90mM) sucrose or 90mM mannitol before being transferred to 1 μmol m−2s−2 continuous red light (Rc) at 23°C. Luciferase activity was plotted together with the SEM. The average of 2–3 independent experiments, n≥126. (f) pifQ plants are less sensitive to sucrose pulses. Sucrose pulse experiments were performed as described in Materials and Methods. n≥43. ** p<0.01, *** p<0.001 (Student two-tailed t-test).
The precision of circadian rhythms in response to growth on sucrose was also affected by the pifQ mutations. Above 0.1% sucrose, pifQ CCA1:LUC plants had significantly lower R.A.E.s than wt CCA1:LUC ((P<0.001, two-way ANOVA genotype/sucrose concentration; Fig. 3c). Similarly, when plants were grown in the absence of sucrose, at low light intensities (1–15 μE; Fig. 3d) both pifQ CCA1:LUC and wt CCA1:LUC show a light-dependent decrease in R.A.E. (p<0.001, two-way ANOVA genotype/light intensity) but R.A.E. was not affected by the mutation (p=0.64, two-way ANOVA genotype/light intensity). However, in high light (35–75 uE), wt CCA1:LUC plants show a significant increase in R.A.E. (Fig. 3d) compared to pifQ CCA1:LUC plants (p<0.001, two-way ANOVA genotype/light intensity). It is possible that the loss of an input pathway in the pifQ mutant enhances circadian precision.
To confirm that PIFs are regulating sucrose signals and not osmotic changes in the cell, we replaced sucrose with the non-metabolizable sugar mannitol in low light. We observed that pifQ CCA1:LUC plants no longer showed a longer period phenotype than wt CCA1:LUC (p=0.6; Fig. 3e). The 1.3 hour for wt (p<0.05, Student two-tailed t-test) and 1.4 hour for pifQ (p<0.05, Student two-tailed t-test) period differences between plants growing with mannitol and the minus mannitol controls indicate that mannitol may affect circadian rhythms but not via PIFs. Taken together, our results suggest that PIF effects on the circadian oscillator are sucrose-dependent.
If PIFs are involved in sucrose entrainment of the oscillator, we predicted that the circadian system in pifQ plants should be less sensitive not only to growth on sucrose but also to sucrose pulses. Sucrose effects on the circadian oscillator have been reported to be ‘gated’ and the application of sucrose during the subjective morning induces CCA1 expression but has little, or the opposite, effect at other times of day (Haydon et al., 2013). Fig. 3(f) and Supplementary Figure 5 show that a three hour pulse of sucrose given at subjective dawn advanced the timing of the next peak of CCA1:LUC by 0.8 hour in wt CCA1:LUC (p<0.0001 Student two-tailed t-test) but had no significant effect on the phase of CCA1:LUC in pifQ CCA1:LUC plants (p=0.4, Student two-tailed t-test). These results are consistent with PIFs regulating sucrose entrainment of the plant circadian oscillator.
Sucrose affects PIF expression and binding to directly control oscillator gene expression
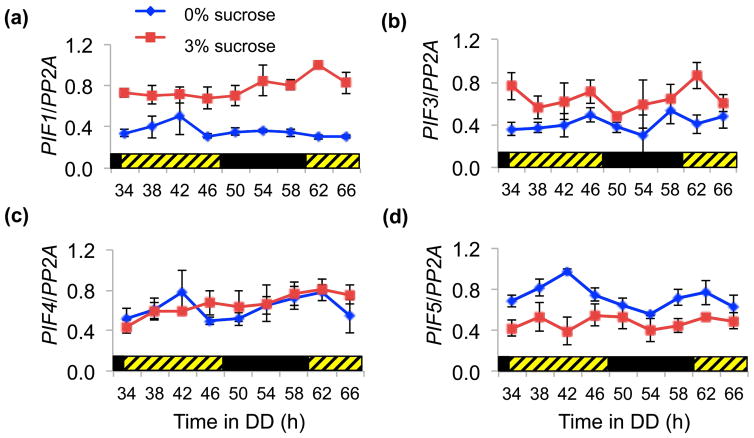
We then asked how PIFs may be regulating sucrose signals to the oscillator. We examined whether sucrose affected PIF expression. Fig. 4 shows that for PIF1, PIF3 and PIF5 there is a significant difference in expression in presence vs absence of sucrose; PIF1 and PIF3 were up-regulated by sucrose while PIF5 was down-regulated (p<0.001, two-way ANOVA time/sucrose). PIF4 levels were not significantly affected (p=0.53, two-way ANOVA time/sucrose).
Fig. 4.
Sucrose alters PIF gene expression in DD. Arabidopsis thaliana wt (Col-0) plants were entrained for 10 days in 14 L:10 D 100 μmol m−2s−1 before being transferred to DD. Shown is the average expression of (a) PIF1, (b) PIF3, (c) PIF4 and (d) PIF5 with SEM from three independent biological repeats. The black and hatched bars represent dark and subjective light respectively.
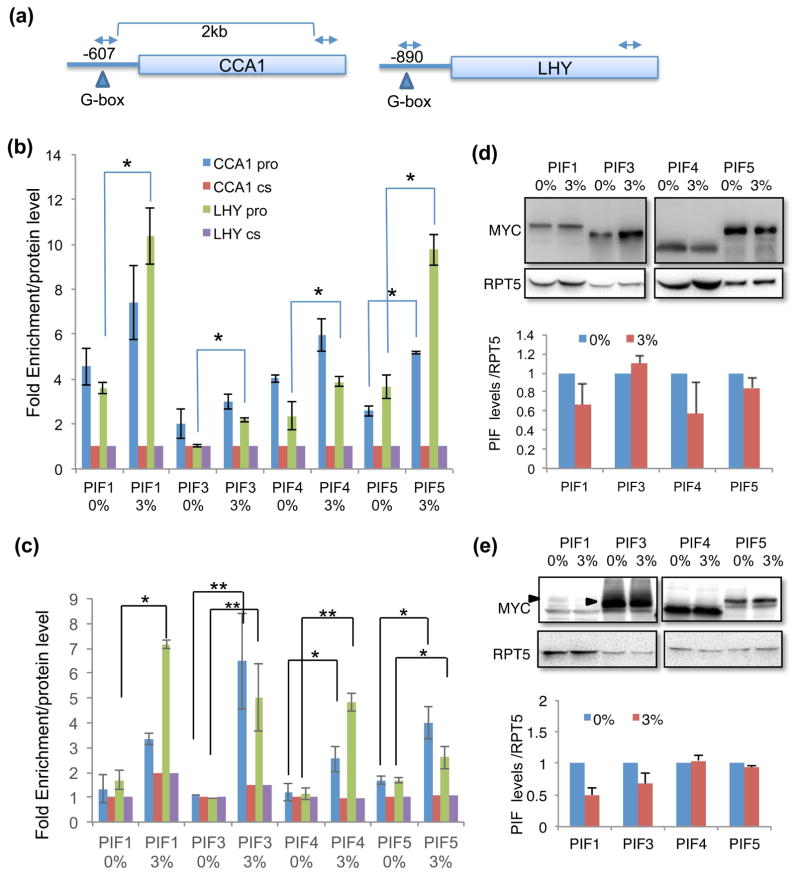
To test whether and how PIF protein activity was affected by sucrose, we carried out chromatin immunoprecipitation (ChIP) assays using tagged PIF1, PIF3, PIF4 and PIF5 transgenic lines for the promoters of CCA1 and the closely related, LATE ELONGATED HYPOCOTYL (LHY) genes (Fig. 5a). Since sucrose affects CCA1 expression early in the day (Haydon et al., 2013), we examined PIF binding at subjective dawn. We used plants harvested at ZT48 in DD and LL conditions. The DD condition was to ensure that endogenous levels of sucrose were minimal. We have also normalized ChIP enrichment with the levels of each PIF protein shown (Fig. 5d, e). Fig. 5(b) shows that, even in the absence of sucrose, the occupancy of all four PIFs was enriched at locations where G-boxes were present in the CCA1 and LHY promoters compared with the locations in the coding sequences where no G-box was present. In the presence of sucrose, the binding of each of the PIFs to the LHY promoter was significantly enhanced as was the binding of PIF5 to the CCA1 promoter (Fig. 5b). Strikingly, the sucrose enhanced binding was more significant in the light (LL) than dark (DD) conditions (Fig. 5c). PIFs showed little enrichment in the continuous light (LL) to CCA1 and LHY promoters in the absence of sucrose. The addition of sucrose, however, dramatically increased the PIF binding to the CCA1 and LHY promoters (Fig. 5c).
Fig. 5.
Sucrose enhances PIF binding to the promoters of CCA1 and LHY. (a) Schematic diagram of the CCA1/LHY genes in Arabidopsis. Arrow heads indicate the PIF binding site, G-box (CACGTG). (b and c) ChIP assays on CCA1 and LHY genes. Seedlings were grown with (3%) and without sucrose in 12L:12 D for seven days and then transferred to DD (b) or LL (c). Samples were collected at subjective dawn (after 48 hours in DD or LL immediately after 7 days of light dark cycles) for the ChIP assays. A coding region sequence was used as control for normalization and the results were standardized to PIF protein levels assayed by immunoblotting. cs, coding sequence; pro, promoter. Four (b) and three (c) independent biological ChIP assays were carried out and the average is shown with SEM (*, p<0.05, **, p<0.01). (d and e) The effect of sucrose on PIF protein levels under DD (d) and LL (e) conditions. Plants were grown as described above and samples were harvested after 48 hours in DD or LL, and the levels of proteins were assayed by Western blotting. Quantification of Western blots is shown at the bottom panels. The Western blots were repeated 3 times with independent biological repeats and PIF levels were normalized by RPT5 levels. Average is shown with SEM.
Since the oscillator component PRR7 has been shown to be involved in sucrose signaling to the clock (Haydon et al., 2013), we also examined whether PIFs could directly regulate the expression of PRR7 to mediate sucrose signaling. Supplementary Figure 6 shows that none of the PIFs were enriched on the G-box of PRR7 indicating that PRR7 may not be a direct target of PIFs in sucrose signaling under these conditions.
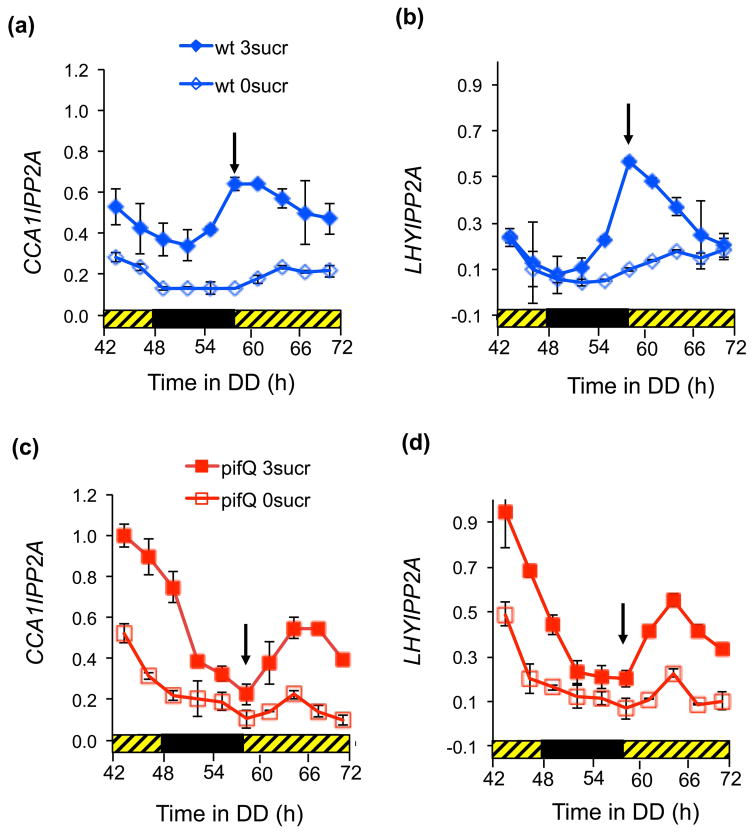
Finally, we examined how PIF binding might affect LHY and CCA1 expression. Fig. 6(a and b) and Supplementary Figure 7 show that the increased binding of PIFs to CCA1 and LHY promoters in the presence of sucrose at subjective dawn (Fig. 5) correlated with a peak of gene expression in wt plants. By comparison, in the absence of sucrose the peaks of CCA1 and LHY occurred significantly later. In the pifQ plants there was no peak of CCA1 or LHY expression at subjective dawn and sucrose did not affect the timing of the peaks of gene expression (Fig. 6c and d). Our results suggest that PIFs may be required for sucrose mediated LHY and CCA1 induction at subjective dawn. It is possible that sucrose signals to the circadian oscillator at certain times of day by changing PIF expression and activity to directly regulate oscillator gene expression.
Fig. 6.
PIF binding is associated with changes in CCA1 and LHY expression. Arabidopsis pifQ and wt plants were entrained for 10 days in 14 L:10 D 100 μmol m−2s−1 before being transferred to DD. Plants were harvested at indicated times for RT-qPCR assays for (a and c) CCA1, (b and d) LHY. Expression of CCA1 and LHY was normalized with PP2A expression and then to the maximum for all the samples in the experiment. The arrows indicate subjective dawn. The average of two independent biological repeats. Error bars indicate +/− SE. The black and hatched bars represent dark and subjective light respectively.
Discussion
PIFs are regulators of plant circadian rhythms
PIFs have key roles as integrators of multiple environmental and developmental signals (Leivar & Monte, 2014) making them strong candidates for regulators of signals to the circadian oscillator; however, until now evidence for whether and how PIFs may regulate the circadian oscillator was conflicting. The promoters of CCA1 and LHY have G-box elements that are bound by PIF3 in vitro and light-induced expression of CCA1 and LHY is reduced in PIF3 antisense plants (Martinez-Garcia et al., 2000). By contrast, quadruple pifQ mutants have elevated levels of CCA1/LHY expression both in dark and after short exposure to red light (Leivar et al., 2009). Nevertheless, circadian phenotypes have been reported to be unaffected in pif3, pif4, and pif5 monogenic mutants or the pif45 double mutant (Viczian et al., 2005; Nusinow et al., 2011). However, two recent reports showed that TOC1 in association with PIF3 and PIF4 mediate the circadian gating of growth responses under light/dark cycle or in response to elevated temperature, respectively (Soy et al., 2016; Zhu et al., 2016). In this paper we show that while the pif45 mutant did not significantly affect circadian rhythms of leaf movements, the pif34, pif345, pif145 and pif134 triple mutants and the pifQ quadruple mutant all affect circadian period, with higher order mutants showing stronger phenotypes (Fig. 1). These data suggest that PIFs act redundantly in the circadian system. Similar redundancy has previously been reported for PIF regulation of growth; while monogenic pif mutants show little effect on seedling morphology in DD, higher order mutant pif combinations demonstrate increasingly severe mutant phenotypes (Leivar et al., 2008; Shin et al., 2009; Leivar et al., 2012). It is possible that not all the PIFs affected in the pifQ mutant have an equal function in signaling to the circadian oscillator; overexpression of PIF4 had least effect on circadian rhythms, although pif34 was the only double mutant we examined that affected rhythms. Further studies are necessary to explore in more depth the contributions and interactions of each PIF in regulating the circadian clock.
PIFs directly mediate metabolic signaling to the oscillator
Light is crucial for plants; low light intensities can regulate photomorphogenesis and photoperiodism while higher intensities are required for photosynthesis (Webb & Satake, 2015). In the natural world plants are subject to different light qualities and quantities throughout the day. For example, low levels of light may occur at dawn several hours before light levels are high enough (“metabolic dawn”) for photosynthesis (Dodd et al., 2015). Moreover, photosynthetic capacity, photomorphogenesis and photoperiodism are all, at least in part, under circadian control. Thus, to ensure that photosynthetic capacity is optimized at the same time as other circadian-controlled processes are correctly regulated it is important that plants are able to perceive and integrate photosynthetic and photoreceptor signals to set the period, phase and amplitude of the oscillator. Consistent with this idea, photosynthetic products, especially sucrose, feedback and entrain the Arabidopsis oscillator (Devlin & Kay, 2001; Knight et al., 2008; Dalchau et al., 2011; Haydon et al., 2013). Suppressing photosynthesis causes an increase in circadian period that can be reversed by the addition of sucrose (Haydon et al., 2013).
We have demonstrated here that PIFs regulate sucrose signaling but are probably not directly involved in phytochrome-mediated light signals to the oscillator. These findings are in keeping with previous reports that light and sugar zeitgebers may function discretely (Haydon et al., 2013). Previous experiments have shown that in the morning, sucrose represses PRR7 and induces CCA1 expression and that pulses of sucrose around “dawn” in low continuous light shifts the phase of the subsequent circadian rhythm (Haydon et al., 2013). Our results suggest that phase setting by sucrose pulses at dawn requires PIFs and are consistent with PIFs acting as regulators of sucrose entrainment of the oscillator.
How do PIFs mediate sucrose entrainment of the circadian clock? The levels of PIFs may be important; transcription of PIF1, PIF3 and PIF5, but not PIF4, is affected by sucrose (Fig. 4) and overexpression of these three PIFs affect circadian period (Fig. 1d). PIF activity may also be important, PIFs are basic helix-loop-helix (bHLH) transcription factors that may directly regulate oscillator gene expression. PRR7 has been shown to be necessary for sucrose regulation of the clock (Haydon et al., 2013). However, PIFs do not directly control PRR7 expression (Supplementary Fig. 5) indicating that if PIFs are acting through PRR7 it is indirectly or at other times of the day. We show that at subjective dawn PIF binding to the promoters of CCA1 and LHY is enhanced by sucrose. The enhanced PIF binding at this time-point is correlated with increased CCA1 and LHY transcript levels resulting in an earlier peak of expression of both genes; it is possible that either PIF binding or the effects of PIF activity are different at other times of the circadian cycle. However, our results are consistent with the observation that exogenous sucrose shortens the circadian period in low light (Haydon et al., 2013) and suggest a mechanism for PIF mediation of signaling to the clock.
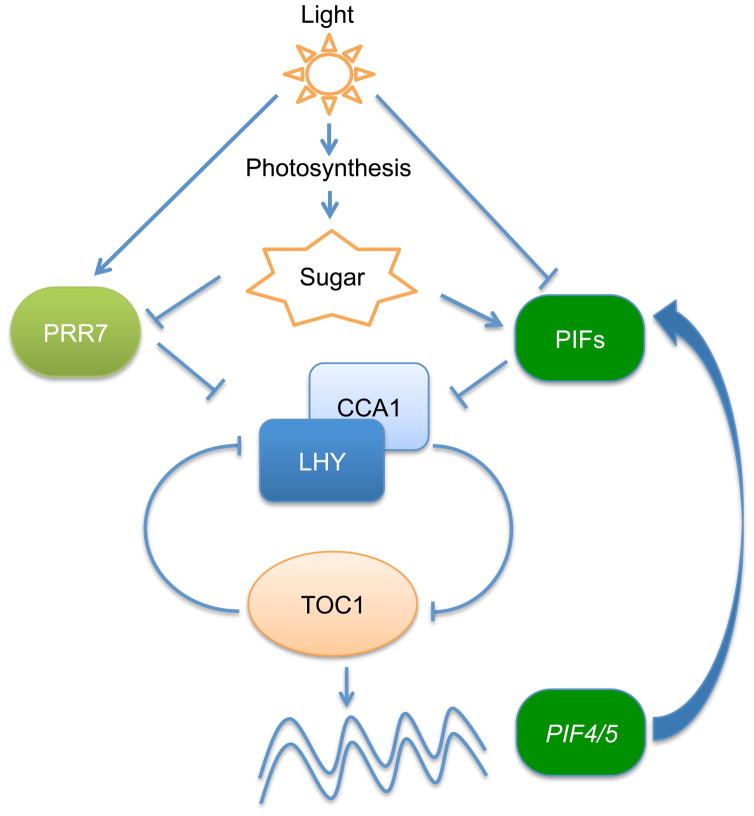
In conclusion, PIFs act as a signaling hub regulating multiple pathways, including environmental (light and temperature), hormonal (auxin, GA, ABA, BR, ethylene) and metabolic (ROS, chlorophyll, carotenoid, sucrose), to optimize plant growth and development (Liu et al., 2011; Stewart et al., 2011; Shin et al., 2013; Leivar & Monte, 2014). All of these pathways are also regulated by circadian clock (Shin et al., 2013; Greenham & McClung, 2015). Therefore, it is not surprising that PIFs are acting both in the input and output pathways of circadian clock to fine-tune plant growth and development (Fig. 7).
Fig. 7.
A model showing light and photosynthetic sugar input to the circadian clock in Arabidopsis. Light triggers photosynthesis as well as acting as an environmental signal to regulate PRR7 expression and inhibit PIFs by inducing degradation. Endogenous sugars produced by photosynthesis suppress the expression of PRR7. PIFs, the central negative regulators of the phytochrome signaling pathways, contribute to the metabolic sugar input to the circadian clock by directly regulating the CCA1/LHY expressions.
Supplementary Material
Fig. S1 Circadian oscillator and output gene expression in pifQ and wt plants.
Fig. S2 PIFs are involved in directly regulating signals from sucrose to the oscillator.
Fig. S3 The amplitude of CCA1:LUC oscillations in wt and pifQ plants is similar.
Fig. S4 Sucrose affects rhythms in wt and pifQ plants.
Fig. S5 Sucrose pulses change the phase of wt but not pifQ plants.
Fig. S6 PIFs do not directly regulate PRR7 expression.
Fig. S7 Sucrose affects the timing of CCA1 and LHY expression peaks in DD in wt plants.
Supplementary Table S1: Primer sequences used.
Supplementary Table S2: p values for pif mutants, compared with wt by ANOVA single factor analysis and by Student’s t-test.
Supplementary Table S3: Average period for pif mutant leaf movements calculated with and without outliers.
Acknowledgments
We thank members of the Huq and Green laboratories for critical reading of the manuscript, G. Choi for sharing myc-PIF3 and myc-PIF5 seeds and David Shlomo Shor for his critical technical assistance with the imaging systems. This work was supported by grants from National Institute of Health (NIH) (1R01 GM-114297), National Science Foundation (MCB-1543813) and a research grant from UT Austin to E.H., Ministry of Absorption to E.S., Israel Science Foundation (ISF 649/12) and Deutsche Forschungsgemeinschaft (DFG) to R.G. and United States Israel Bilateral Science Foundation (2015316) to R.G and E. H.
Footnotes
Author Contributions
E.S., I.P. and S.K. carried out the experiments, contributed to the experimental design. E.S. and I.P, contributed to the interpretation of the results and edited the manuscript, R.G. and E.H. devised the experiments and prepared the manuscript.
Competing interests
The authors declare no competing financial interests.
References
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293(5531):880–883. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- Boikoglou E, Ma Z, von Korff M, Davis AM, Nagy F, Davis SJ. Environmental memory from a circadian oscillator: the Arabidopsis thaliana clock differentially integrates perception of photic vs. thermal entrainment. Genetics. 2011;189(2):655–664. doi: 10.1534/genetics.111.131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q, Zhu L, Yu L, Dennis M, Lu X, Person M, Tobin E, Browning K, Huq E. Phosphorylation by CK2 enhances the rapid light-induced degradation of PIF1. J Biol Chem. 2011;286:12066–12074. doi: 10.1074/jbc.M110.186882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon A, Shen H, Huq E. Phytochrome Interacting Factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007;12:514–521. doi: 10.1016/j.tplants.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Dalchau N, Baek SJ, Briggs HM, Robertson FC, Dodd AN, Gardner MJ, Stancombe MA, Haydon MJ, Stan G-B, Gonçalves JM. The circadian oscillator gene GIGANTEA mediates a long-term response of the Arabidopsis thaliana circadian clock to sucrose. Proceedings of the National Academy of Sciences. 2011;108(12):5104–5109. doi: 10.1073/pnas.1015452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Kay SA. Circadian photoperception. Annu Rev Physiol. 2001;63:677–694. doi: 10.1146/annurev.physiol.63.1.677. [DOI] [PubMed] [Google Scholar]
- Dodd AN, Belbin FE, Frank A, Webb AA. Interactions between circadian clocks and photosynthesis for the temporal and spatial coordination of metabolism. Front Plant Sci. 2015;6:245. doi: 10.3389/fpls.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AA. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309(5734):630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–135. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould PD, Locke JC, Larue C, Southern MM, Davis SJ, Hanano S, Moyle R, Milich R, Putterill J, Millar AJ, Hall A. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell. 2006;18(5):1177–1187. doi: 10.1105/tpc.105.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Tingay S, Wang ZY, Tobin EM. Circadian rhythms confer a higher level of fitness to Arabidopsis plants. Plant Physiol. 2002;129(2):576–584. doi: 10.1104/pp.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Tobin EM. Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc Natl Acad Sci U S A. 1999;96(7):4176–4179. doi: 10.1073/pnas.96.7.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenham K, McClung CR. Integrating circadian dynamics with physiological processes in plants. Nature Reviews Genetics. 2015;16(10):598–610. doi: 10.1038/nrg3976. [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AA. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature. 2013;502(7473):689–692. doi: 10.1038/nature12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon MJ, Webb AA. Assessing the Impact of Photosynthetic Sugars on the Arabidopsis Circadian Clock. Environmental Responses in Plants: Methods and Protocols. 2016;1398:133–140. doi: 10.1007/978-1-4939-3356-3_12. [DOI] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305(5692):1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- Jolma IW, Laerum OD, Lillo C, Ruoff P. Circadian oscillators in eukaryotes. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2010;2(5):533–549. doi: 10.1002/wsbm.81. [DOI] [PubMed] [Google Scholar]
- Knight H, Thomson AJ, McWatters HG. Sensitive to freezing6 integrates cellular and environmental inputs to the plant circadian clock. Plant Physiol. 2008;148(1):293–303. doi: 10.1104/pp.108.123901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19(5):408–413. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Leivar P, Monte E. PIFs: systems integrators in plant development. Plant Cell. 2014;26(1):56–78. doi: 10.1105/tpc.113.120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Cohn MM, Quail PH. Phytochrome signaling in green Arabidopsis seedlings: impact assessment of a mutually negative phyB-PIF feedback loop. Mol Plant. 2012;5(3):734–749. doi: 10.1093/mp/sss031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, Quail PH. Definition of Early Transcriptional Circuitry Involved in Light-Induced Reversal of PIF-Imposed Repression of Photomorphogenesis in Young Arabidopsis Seedlings. Plant Cell. 2009;21(11):3535–3553. doi: 10.1105/tpc.109.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang Y, Liu R, Hao H, Wang Z, Bi Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. Journal of Plant Physiology. 2011;168(15):1771–1779. doi: 10.1016/j.jplph.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia JF, Huq E, Quail PH. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288(5467):859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- Moon J, Zhu L, Shen H, Huq E. PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc Natl Acad Sci U S A. 2008;105(27):9433–9438. doi: 10.1073/pnas.0803611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. Journal of bioscience and bioengineering. 2007;104(1):34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN. Rhythmic growth explained by coincidence between internal and external cues. Nature. 2007;448(7151):358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- Nusinow DA, Helfer A, Hamilton EE, King JJ, Imaizumi T, Schultz TF, Farre EM, Kay SA. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature. 2011;475(7356):398–402. doi: 10.1038/nature10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21(2):403–419. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell. 2004;16(11):3045–3058. doi: 10.1105/tpc.104.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Wang ZY. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nature Cell Biology. 2012;14(8):802–U864. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95(15):8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Kim J, Lee Y, Shin J, Oh E, Chung WI, Liu JR, Choi G. Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol. 2004;45(8):968–975. doi: 10.1093/pcp/pch125. [DOI] [PubMed] [Google Scholar]
- Plautz JD, Straume M, Stanewsky R, Jamison CF, Brandes C, Dowse HB, Hall JC, Kay SA. Quantitative analysis of Drosophila period gene transcription in living animals. J Biol Rhythms. 1997;12(3):204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Jeong J, Kang MY, Kim J, Paek NC, Choi G. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nature communications. 2014;5:4636. doi: 10.1038/ncomms5636. [DOI] [PubMed] [Google Scholar]
- Shin J, Anwer MU, Davis SJ. Phytochrome-interacting factors (PIFs) as bridges between environmental signals and the circadian clock: diurnal regulation of growth and development. Mol Plant. 2013;6(3):592–595. doi: 10.1093/mp/sst060. [DOI] [PubMed] [Google Scholar]
- Shin J, Kim K, Kang H, Zulfugarov IS, Bae G, Lee CH, Lee D, Choi G. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci U S A. 2009;106(18):7660–7665. doi: 10.1073/pnas.0812219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy J, Leivar P, González-Schain N, Martín G, Diaz C, Sentandreu M, Al-Sady B, Quail PH, Monte E. Molecular convergence of clock and photosensory pathways through PIF3–TOC1 interaction and co-occupancy of target promoters. Proceedings of the National Academy of Sciences. 2016;113(17):4870–4875. doi: 10.1073/pnas.1603745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Maloof JN, Nemhauser JL. PIF genes mediate the effect of sucrose on seedling growth dynamics. PLoS ONE. 2011;6(5):e19894. doi: 10.1371/journal.pone.0019894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15(8):1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Ortíz G, Huq E, Rodríguez-Concepción M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by Phytochrome-Interacting Factors. Proc Natl Acad Sci U S A. 2010;107:11626–11631. doi: 10.1073/pnas.0914428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viczian A, Kircher S, Fejes E, Millar AJ, Schafer E, Kozma-Bognar L, Nagy F. Functional characterization of phytochrome interacting factor 3 for the Arabidopsis thaliana circadian clockwork. Plant Cell Physiol. 2005;46(10):1591–1602. doi: 10.1093/pcp/pci175. [DOI] [PubMed] [Google Scholar]
- Webb AA, Satake A. Understanding circadian regulation of carbohydrate metabolism in Arabidopsis using mathematical models. Plant Cell Physiol. 2015;56(4):586–593. doi: 10.1093/pcp/pcv033. [DOI] [PubMed] [Google Scholar]
- Xu X, Paik I, Zhu L, Huq E. Illuminating Progress in Phytochrome-Mediated Light Signaling Pathways. Trends in plant science. 2015;20(10):641–650. doi: 10.1016/j.tplants.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Yakir E, Hilman D, Kron I, Hassidim M, Melamed-Book N, Green RM. Posttranslational regulation of CIRCADIAN CLOCK ASSOCIATED1 in the circadian oscillator of Arabidopsis. Plant Physiol. 2009;150(2):844–857. doi: 10.1104/pp.109.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashino T, Matsushika A, Fujimori T, Sato S, Kato T, Tabata S, Mizuno T. A Link between circadian-controlled bHLH factors and the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol. 2003;44(6):619–629. doi: 10.1093/pcp/pcg078. [DOI] [PubMed] [Google Scholar]
- Yerushalmi S, Yakir E, Green RM. Circadian clocks and adaptation in Arabidopsis. Mol Ecol. 2011;20(6):1155–1165. doi: 10.1111/j.1365-294X.2010.04962.x. [DOI] [PubMed] [Google Scholar]
- Zeeman S, Rees TA. Changes in carbohydrate metabolism and assimilate export in starch-excess mutants of Arabidopsis. Plant, Cell & Environment. 1999;22(11):1445–1453. [Google Scholar]
- Zhang Y, Mayba O, Pfeiffer A, Shi H, Tepperman JM, Speed TP, Quail PH. A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in Arabidopsis. Plos Genetics. 2013;9(1):e1003244. doi: 10.1371/journal.pgen.1003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-Y, Oh E, Wang T, Wang Z-Y. TOC1–PIF4 interaction mediates the circadian gating of thermoresponsive growth in Arabidopsis. Nature communications. 2016;7:13692. doi: 10.1038/ncomms13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Bu Q, Xu X, Paik I, Huang X, Hoecker U, Deng XW, Huq E. CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nature communications. 2015;6:7245. doi: 10.1038/ncomms8245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Circadian oscillator and output gene expression in pifQ and wt plants.
Fig. S2 PIFs are involved in directly regulating signals from sucrose to the oscillator.
Fig. S3 The amplitude of CCA1:LUC oscillations in wt and pifQ plants is similar.
Fig. S4 Sucrose affects rhythms in wt and pifQ plants.
Fig. S5 Sucrose pulses change the phase of wt but not pifQ plants.
Fig. S6 PIFs do not directly regulate PRR7 expression.
Fig. S7 Sucrose affects the timing of CCA1 and LHY expression peaks in DD in wt plants.
Supplementary Table S1: Primer sequences used.
Supplementary Table S2: p values for pif mutants, compared with wt by ANOVA single factor analysis and by Student’s t-test.
Supplementary Table S3: Average period for pif mutant leaf movements calculated with and without outliers.