Abstract
Background
An 8 amino acid peptide sequence derived from proliferating cell nuclear antigen (PCNA) has been shown to effectively kill several breast cancer and neuroblastoma cell lines when added exogenously to cell cultures.
Methods
In this study, the expression of the 8 amino acid peptide sequence (caPeptide) was placed under control of a tetracycline responsive promoter in MDA-MB-231 cells.
Results
Endogenous expression of the peptide resulted in an increase in genomic DNA damage. CaPeptide induction combined with treatment of sublethal doses of cisplatin resulted in a marked increase in death of the cisplatin-resistant MDA-MB-231 cell line. CaPeptide was found to interact with POLD3, one of the subunits of DNA polymerase delta necessary for binding to PCNA.
Conclusion
These results suggest an important line of inquiry into the possible role that caPeptide might play in the reversal of cisplatin resistance in breast and other cancers. This is of particular interest in those cancers where cisplatin is the first line of chemotherapy and where the acquisition of resistance is a common malady.
Keywords: PCNA, caPCNA, caPeptide, Cisplatin, Triple-negative breast cancer, Drug resistance
Introduction
Proliferating cell nuclear antigen (PCNA) is remarkable for the many cellular activities in which it plays a central function. It is involved in DNA replication, DNA repair, translesion DNA synthesis, DNA methylation, chromatin remodeling, cell cycle regulation and protein degradation [1–8]. Key to PCNA’s multifaceted nature is its ability to form a homotrimer that encircles double helix DNA [9–11]. From this position, it serves as a central docking structure for the coordinated binding and organization of many different proteins [1, 4, 7, 12]. This ability to bind many partners suggests a malleability of the PCNA homotrimer that is essential to the execution of its many functions [11].
One important binding region on PCNA is the interdomain connecting loop (IDCL) so called because it spans the two major domains on a PCNA monomer. It is located on the outside of the PCNA trimeric ring and extends from amino acid 118–135. Polymerase δ (pol δ), p21, flap endonuclease 1, DNA (cytosine) methyltransferase and DNA ligase1 are some of the proteins that bind to this region [1].
Cancer-associated PCNA (caPCNA) is an isoform of PCNA that is highly expressed in cancer cells with little expression in non-malignant cells [13–15]. Given the central role that PCNA plays in repair and replication, caPCNA may be involved in the aberrant repair and replication present in cancer cells [16–20]. Investigations into the differences between caPCNA and PCNA led to the discovery of an 8 amino acid peptide sequence that could be used as an antigen to raise antibody specific to caPCNA [15]. This peptide was subsequently named caPeptide. It is homologous to amino acids 126–133 in the IDCL domain. Because it is part of a binding domain, caPeptide might also have the potential to function as an in vivo blocking peptide and be used to interfere with binding partners of caPCNA, possibly mitigating the aberrant functions due to caPCNA. Preliminary work has found that when the membrane penetrating D-Arg9 (R9) sequence is added to the N-termini of the caPeptide, the peptide is very toxic to breast cancer, lymphoma, pancreatic and neuroblastoma cell lines [21] [Smith et al. in press].
Cisplatin (CDDP) has been used clinically since 1978 for inducing tumor cell death. It is one of the most widely used chemotherapeutic drugs in a large part because of the broad spectrum of cancers that it effectively kills. However, the activity of CDDP is not specific to just tumors, and it also affects normal cells and often results in diverse and dangerous side effects. These side effects include nausea, vomiting, myelosuppression, immunosuppression, renal toxicity, neurotoxicity, cardio toxicity and hearing loss. In addition, tumor resistance to CDDP is a common occurrence and leads to aggressive secondary malignancies. For these reasons, clinical strategies often seek to reduce the dosage of CDDP used to treat patients, commonly by combining a lower dose of CDDP with as many as four other tumor fighting drugs [22].
In breast cancer, treatment options for many forms have improved in recent years with the advent of endocrine therapy [23]. Blocking cell surface receptors with drugs such as tamoxifen and trastuzumab results in relatively specific growth inhibition of estrogen receptor positive and Her2-amplified breast cancers with fewer side effects than traditional chemotherapy. However, for triple-negative type breast cancers which lack the targeted cell surface receptors, chemotherapy is still the only option for adjuvant therapy [23–25]. MDA-MB-231 cells are a triple-negative cell line derived from a metastatic adenocarcinoma of the breast. In addition, they are resistant to CDDP [26]. This has lead to their use as a model to study cisplatin resistance in general and, more specifically, cisplatin resistance in triple-negative breast cancers [27–29].
In this paper, we placed caPeptide under control of a tetracycline responsive promoter in stably transfected MDA-MB-231 cells. By endogenously expressing the caPeptide in MDA-MB-231 cells, we were able to promote genomic DNA damage and reverse cisplatin resistance.
Results
caPeptide is toxic to breast cancer cell lines
A panel of breast cancer cell lines treated with R9 linked to the N-termini of the caPeptide (R9-cc-caPeptide) or with an R9 linked scrambled version of the caPeptide (R9-cc-scrPeptide) resulted in loss of viability in R9-caPeptide-treated cells beyond that of R9-scrPeptide-treated cells in every breast cancer cell line tested except for the BT474 line (Fig. 1). The increased loss of viability ranged from 49 ± 1 % in the most sensitive cell line (HCC1428) to 5 ± 7 % in the least sensitive line (BT474). MDA-MB-231 cells were the most sensitive triple-negative breast cancer cell line with an increased loss of viability of 47 ± 2 %.
Fig. 1.
Loss of viability relative to scrambled control peptide in a panel of breast cancer cell lines treated with 60 μM R9-caPeptide for 72 h. Cell surface receptor status for each cell line is indicated below chart (N no, Y yes, “−” negative, “+” positive, “++” amplified)
Sensitivity to caPeptide generally trended with cell surface receptor status [30–32]. Cell lines that were Her2 amplified or the lone EGFR-amplified cell line (MDA-MB-468) were less sensitive to caPeptide treatment with the exception of the UACC-893 cell line. Triple-negative breast cancer cell lines trended toward being more sensitive to caPeptide with the exception of the EGFR-amplified MDA-MB-468 cell line. The resistant BT-474 cell line was the only line positive for all 4 cell surface receptors considered, being amplified for Her2 as well as positive for EGFR, ER and PR.
caPeptide can be endogenously expressed in MDA-MB-231 cells
MDA-MB-231 cells constitutively expressing the reverse tetracycline-controlled transactivator protein (rtTA) were stably transfected with pCaPeptide (Fig. 2). The resulting cell pool was named 231-caPep. Features of pCaPeptide include sequence coding for a caPeptide–nuclear localization signal repeat fusion peptide (caPeptide-3xNLS), followed by sequence for 2a peptide and a mCherry reporter gene; all under transcriptional control of the tetracycline regulated promoter, pTight. The transcript is translated in a single run with the 2a peptide serving to separate equimolar product of caPeptide-3xNLS from mCherry. Fluorescent microscopy was used to monitor caPeptide-3 × NLS expression via the mCherry reporter after induction with doxycycline (Dox; Fig. 2c). Reverse transcription PCR was used to confirm transcription of caPeptide (Fig. 2d). Two different PCRs were used to amplify the cDNA product of the reverse transcription reaction. One reaction used primers that annealed to the 5′ end and 3′ end of the mCherry sequence in the cDNA template. The product from this reaction was 770 bp reflecting the full length of the mCherry sequence. The other reaction used an upstream primer that annealed to the 5′ end of the caPeptide sequence and the same downstream primer as in the first reaction. The resulting 930 bp sequence reflects the full length of the mCherry plus the caPeptide-3 × NLS and 2a peptide sequence. A small amount of transcriptional “leakage” can be detected in the uninduced sample (Fig. 2d, reaction #2, “-”Dox lane).
Fig. 2.
a Nucleotide sequence of caPeptide placed into expression vector along with the corresponding amino acid translation. The amino acids corresponding to a.a. 126–133 of caPCNA are underlined. The peptide sequence was followed by a three nuclear localization signal repeat (3 × NLS). b Diagram representation of expression vector used for creation of stable inducible cell population. Expression vector features include the tetracycline inducible promoter, pTight, caPeptide-3 × NLS followed by the co-translational cleavage moderator, 2a peptide and the fluorescent reporter gene, mcherry. Also present is a puromycin resistance selectable marker. c Bright field and fluorescence composite microscopy image showing Dox induction of caPeptide and reporter protein mcherry in 231-caPeptide cell lines. Induction results in a single transcript containing the coding information for the respective peptide and mcherry reporter which is then translated in a single ribosomal event but cleaved into peptide and reporter gene through the activity of the 2a peptide sequence. This results in equimolar expression of peptide and reporter gene. d Reverse transcription PCR of mRNA from Dox-treated and untreated 231-caPeptide cell populations. Reaction #1 used primers that annealed to the 5′ and 3′ ends of the mcherry sequence in the cDNA template. Reaction #2 used primers that annealed to the 5′ end of caPeptide and same 3′ end primer as reaction #1. The expected products are 770 and 930 bp, respectively. e Lysates of 231-v.c cells (vector control) and 231-flg-caPeptide cells metabolically labeled with tritiated lysine in the presence and absence of doxycycline were immunoprecipitated with an antibody specific to the flag tag (Anti-Flag) and an antibody developed by using the caPeptide sequence as an immunogen (Anti-caPCNA). The samples were separated by gel electrophoresis and visualized by autoradiography
Metabolically labeling the stably transfected 231-fca-Pep cell line which has a flag-tagged version of the caPeptide construct enabled visualization of the peptide itself. 231-fcaPep cells and an “empty” vector control cell line (231-v.c.) were labeled with tritiated lysine without induction or in conjunction with Dox-induced expression. The lysates were immunoprecipitated with anti-flag M2 affinity beads purchased from Sigma or with anti-caPCNA antibody developed in previous work by using caPeptide as the immunizing peptide (15). The eluate from immunoprecipitation was separated by SDS-PAGE. The gel was fixed, treated with signal enhancer, dried and exposed to film. Only the Dox-induced samples in both the anti-flag and anti-caPCNA immunoprecipitations presented a signal at the correct size of 9 kd (Fig. 2e). The 231-caPep cell line was similarly labeled and immunoprecipitated with anti-caPCNA. The peptide expression from this cell line is shown in the top panel of the experiment in Fig. 3a.
Fig. 3.
a 231-caPeptide cells labeled with tritiated lysine and treated with or without Dox were fractionated to separate cytoplasm from nucleus. To identify the fractions containing caPeptide, lysates were immunoprecipitated with anti-caPCNA antibody, separated by gel electrophoresis and imaged by autoradiography. To determine the success of fractionation, lysates were subjected to immunoblotting, and antibodies to Histone H3 and GAPDH were used to verify nuclear and cytoplasmic fractions. b Tritiated 231-caPeptide lysates treated with or without Dox were immunoprecipitated with antibody specific to POLD3. After gel electrophoresis, tritiated caPeptide was detected by autoradiography and equal amounts of immunoprecipitated POLD3 in “−”and “+” Dox samples was verified by immunoblotting
caPeptide localizes to nucleus and interacts with POLD3
Nuclear fractionation of Dox-induced and metabolically labeled 231-caPep cells was performed to confirm that the nuclear localization signal fused to caPeptide served the function of routing the peptide to the nucleus. Following fractionation, the cytoplasmic and nuclear fractions were immunoprecipitated using the anti-caPCNA antibody and processed as described above to obtain a signal through autoradiography. As expected, the caPeptide was found to be predominantly in the Dox-induced nuclear fraction with some signal being present in the uninduced nuclear fraction, likely the result of “leaking” expression. Western blot analysis of fractions was used to determine the effectiveness of fractionation. Immunoblotting with anti-GAPDH provided verification of cytoplasmic fractions, and anti-Histone H3 was used to verify nuclear fractions (Fig. 3a).
As a partial sequence of the IDCL binding region of PCNA, caPeptide can potentially interact with PCNA-interacting proteins that bind the IDCL. In a Biacore assay, caPeptide prevented PCNA binding to the p66 subunit of pol δ (POLD3) [Smith SJ et al., in press]. To test whether caPeptide binds POLD3 within cells, tritiated lysate from uninduced and Dox-induced 231-caPep was immunoprecipitated with antibody against POLD3. After immunoprecipitation, eluates were processed through to obtaining a signal by autoradiography. An immunoprecipitation with anti-caPCNA served as a marker for caPeptide. This procedure resulted in a signal in Dox-induced lysates that co-migrated with the caPeptide marker indicating that POLD3 and caPeptide interact with each other (Fig. 3b).
caPeptide expression results in damage to genomic DNA
Inducing caPeptide expression resulted in an increase in DNA damage as measured by comet assay (Fig. 4). In addition, caPeptide expression in combination with treatment of low doses of CDDP resulted in more DNA damage than either induction or dosing alone. 231-caPep cells were left untreated, treated for 24 h with either 1 μg/ml Dox or 5 μM CDDP, or treated 24 h with both 1 μg/ml Dox and 5 μM CDDP. The comet assay was performed under alkaline conditions to reveal single- and double-strand DNA breaks, abasic sites and alkaline labile DNA adducts. Cells were photographed and scored as having no damage, low damage, medium damage or high damage. From 60 to 130, cells were scored per sample. The experiment was repeated three times. Thirteen percent of untreated cells had some degree of damage versus 25 % of CDDP alone, 32 % of Dox-treated cells and 52 % of cells treated with both CDDP and Dox. Cells treated with both CDDP and Dox had a fourfold increase in number of cells damaged compared to untreated and twice as much damage as CDDP-treated cells. This understates the full potency of the combination treatment as not only is the total percent of damaged cells increased but the degree of damage incurred increases as well.
Fig. 4.
Comet assay of 231-caPep cells that were left untreated, treated for 24 h with either 5 μM CDDP (CDDP) or 1 μg/ml Dox (CaPep), or treated 24 h with both 5 μM CDDP and 1 μg/ml Dox (CDDP + CaPep). Cells were scored as having no damage, low damage, medium damage or high damage. a Representative examples of cells in three categories of damage. b Compiled results of the different treatments and categories of damage (FC fold change, p p value of Student’s t test)
caPeptide expression coupled with CDDP treatment leads to cell death
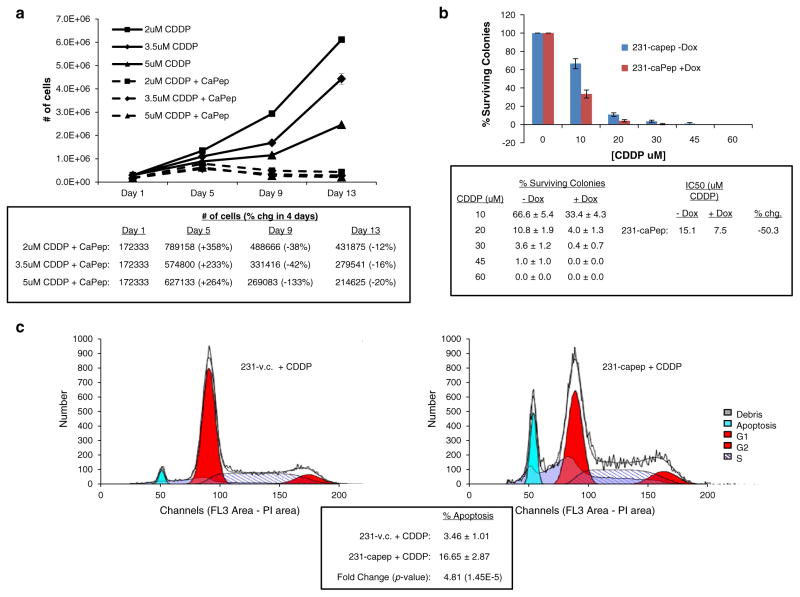
Combining caPeptide expression with low levels of CDDP causes cell death as measured by cellular growth assay (Fig. 5a). 231-caPep cells were plated at a starting concentration of 1 × 105 cells per 60-mm plate. Each time point and condition was plated in triplicate. Dox-treated samples were treated with 1 μg/ml Dox every other day starting on Day 1. Cisplatin at 2, 3.5 or 5 μM was added every fourth day starting on Day 1. The media was replenished every fourth day on schedule with the CDDP and every other Dox treatment. Triplicate plates were collected and counted on Day 1, 5, 9 and 13. Consistent with the CDDP resistance of the parental MDA-MB-231 cell line, 231-caPep cells treated with CDDP and without induction of caPeptide grew rapidly and entered exponential growth albeit at an increasingly delayed time as the CDDP burden increased from 2 to 5 μM. Cells treated with CDDP and expressing caPeptide initially entered a positive acceleration phase of growth, but with the replenishing of media, Dox and CDDP on Day 5, cells began dying at a dose-dependent rate.
Fig. 5.
a Growth curve on 231-caPep cells treated with CDDP, with and without Dox-induced caPeptide expression. 231-caPep cells were plated at 1 × 105 cells per 60-mm plate on Day 0 for each condition and time point. Each sample was plated in triplicate. On Day 1, Dox at 1 μg/ml and CDDP at 2, 3.5 or 5 μM was added to appropriate samples. Dox was replenished every other day and CDDP and media changed every fourth day. Samples were collected and counted on days 1, 5, 9 and 13. Cells treated with CDDP and expressing caPeptide began dying at a dose-dependent rate after replenishing media, Dox and CDDP on Day 5. b Clonogenic assay on 231-caPep cells with or without induced caPeptide expression treated with increasing doses of CDDP. 231-caPep was treated with or without 1 μg/ml of Dox and 0, 10, 20, 30, 45 and 60 μM of CDDP for 24 h. One thousand cells of each condition were plated in triplicate. Dox continued to be added to “+Dox” samples at a rate of 1 μg/ml Dox every other day. After 5 days, plates were stained and colonies counted. CaPeptide expressing cells were more susceptible to CDDP with the IC50 of caPeptide expressing cells dropping by 50 %. c Cell cycle analysis of 231-caPep cells expressing caPeptide and treated with a low level of CDDP. 231-caPep and 231-v.c. cells were treated overnight with 10 μM CDDP and 1 μg/ml Dox. Flow cytometry was used to analyze the DNA content of prepared samples. Cells expressing caPeptide had a fivefold increase in apoptotic cells
caPeptide expression coupled with CDDP treatment decreases clonogenicity
Treating caPeptide expressing 231-caPep cells with increasing doses of CDDP leads to a loss of clonogenicity compared to uninduced 231-caPep cells (Fig. 5b). 231-caPep cells were treated with or without 1 μg/ml of Dox and 0, 10, 20, 30, 45 and 60 μM of CDDP for 24 h. After treatment, 1,000 cells of each condition were plated in triplicate. Dox continued to be added to “+Dox” samples at a rate of 1 μg/ml Dox every other day. Colonies were allowed to grow for 7 days at which point plates were stained and colonies counted. Cells expressing caPeptide showed increased susceptibility to CDDP toxicity. Dosing with 10 μM CDDP resulted in 33 % survival rate of colonies expressing caPeptide versus 67 % for non-expressing cells. At 20 μM of CDDP, the respective percentages were 4 versus 11 % and by 30 μM, the percent of surviving caPeptide expressing colonies was 0.4 versus 4 % for non-expressing cells. The difference in survival between 231-caPep cells with and without Dox decreased the IC50 to CDDP by 50 % from 15.1 μM down to 7.5 μM.
caPeptide expression coupled with CDDP treatment increases apoptosis
Treating caPeptide expressing 231-caPep cells with a low level of CDDP increases the number of cells undergoing apoptosis as measured by cell cycle analysis (Fig. 5c). 231-caPep and 231-v.c. cells were seeded in 10-cm plates and allowed to attach for 5 h. The cells were then treated overnight with 10 μM CDDP and 1 μg/ml Dox. Cells were collected the next day, washed, fixed, treated with RNase and stained with propidium iodide. Flow cytometry was used to analyze the DNA content of the cells. Cells expressing caPeptide had 17 % of their population in apoptosis versus 3.5 % of control cells. This is a fivefold increase in apoptotic cells.
caPeptide-induced DNA damage is coincident with apoptosis
Treating MDA-MB-231 cells with R9-cc-caPeptide results in elevated levels of DNA damage as measured by an increase in phosphorylated H2AX (γH2AX). This increase in DNA damage is accompanied by progressively higher levels of apoptosis as measured by an increase in the 89-kDa-cleaved fragment of PARP (Fig. 6). Plated MDA-MB-231 cells were treated with 75 μM of R9-cc-caPeptide. The cells were collected at 0, 12, 24, 48 and 72 h. They were then fixed, permeabilized, and stained with fluorescent antibodies to γH2AX and the cleaved PARP fragment. Flow cytometry was used to analyze the samples. Background levels of γH2AX and cleaved PARP were determined from the 0 h time point. The population of cells staining for high levels of γH2AX rose over the first 24 h of treatment and remained steadily elevated through the remaining time points. The percentage of cells staining for high levels of the cleaved fragment of PARP increased throughout the 72 h time course. At 0 h, only .2 % of the cell population had cells that stained for high levels of cleaved PARP fragment, and by 72 h, over 70 % of cells had high levels of cleaved PARP.
Fig. 6.
MDA-MB-231 cells were treated with R9-cc-caPeptide for 0, 12, 24, 48 and 72 h. The cells were collected, fixed, permeabilized and stained with fluorescent antibodies to γH2AX and the 89-kDa-cleaved fragment of PARP. Flow cytometry was used to analyze the samples
Discussion
The discovery of a cancer-associated isoform of PCNA found in cancer cell lines with little expression in non-malignant cell lines provides both a unique marker for the diagnosis of disease and a unique target to attack in cancer cells [13–15]. Earlier work in our laboratory led to the development of an antibody that could detect caPCNA [15] [Smith SJ et al. in press]. This antibody was raised by using an 8 amino acid peptide fragment from the IDCL (aa118–135). This region is the binding site for many of PCNA’s partner proteins including Pol δ, p21, flap endonuclease 1, DNA (cytosine) methyltransferase and DNA ligase1 [1]. The antigenic peptide is homologous to amino acids 126–133 of the IDCL and was subsequently named caPeptide. Since the peptide sequence is in a binding domain, caPeptide also has the potential to be used as a competitor for caPCNA binding partners. CaPeptide might be able to interfere with critical caPCNA functions which in turn might lead to the initiation of apoptosis in cancer cells. If the role of caPCNA is similar to PCNA, then it is important in DNA replication and repair and may be involved in the error-prone DNA replication and repair that is often found in cancer cells [16–20].
In this and other work done in our laboratory, a nona-arginine was linked to caPeptide. The presence of the R9 sequence facilitates the destabilization of the cell membrane and the formation of transient pores through which a R9-peptide is transported into the cell [33]. The results of these experiments showed that treatment with micro-molar levels of R9-cc-caPeptide was cytotoxic to a panel of breast cancer and neuroblastoma cell lines [Smith SJ et al. in press] [21].
In this study, we placed caPeptide under control of a tetracycline responsive promoter in stably transfected MDA-MB-231 cells. Contrary to the experiments using exogenous caPeptide treatment, which showed striking cell toxicity and death in MDA-MB-231 cells (Fig. 1), we were unable to cause cell death simply by inducing endogenous caPeptide expression. There was, however, a significant increase in DNA damage as measured by comet assay (Fig. 4). The number of cells with low levels of DNA damage doubled. In addition, 6 % of the caPeptide expressing cells now had an intermediate level of DNA damage. But, this increase in DNA damage did not correlate with a measurable difference in growth rate, clonogenicity or apoptosis.
Possible explanations for the difference between exogenous and endogenous results are many and could include unequal quantities of peptide present, different target interactions and adaptive response to the peptide. In cells treated exogenously with 20 μM of R9-cc-caPeptide, about 25 ng of peptide was internalized after 24 h (data not shown) and could be detected by Western blotting with the caPCNA antibody. The amount of induced caPeptide in these experiments was apparently much lower as it was not detectable by Western blotting. Metabolically labeling the cells with tritium was necessary to achieve detection.
Besides the difference in effective peptide concentration, localization differences between an exogenously added peptide and a nuclearly expressed peptide could lead to a different set of target interactions. Like the induced peptide, R9-cc-caPeptide predominantly gravitates to the nucleus [21], but the possibility remains that as it passes from outside the cell to the nucleus, it may have a distinct subset of interactions from those of the induced peptide.
An additional consideration is the problem of “leaky” expression that is inherent to some degree in inducible systems. As a consequence, inducible systems typically measure the difference between a low level of expression (“leaking”) and a higher level of induced expression [34–36]. The selected cells in an inducible system are already tolerant of low levels of expression. This is different than exogenous treatment where no previous exposure has occurred.
Cisplatin resistance is a common problem in the treatment of many forms of cancer [22]. This along with CDDP’s many noxious side effects has led to a combinatorial approach in the adjuvant treatment of cancers [22]. MDA-MB-231 cells are a triple-negative breast cancer cell line that is one of the most resistant to CDDP. In a panel of 22 triple-negative breast cell lines, they were one of the 4 most resistant cell lines having an IC50 higher than the highest dosage of 30 μM for 72 h, versus an IC50 of 8 μM for the median cell line and 2 μM for the lowest cell line [26]. In our present study, we find that caPeptide expression reverses cisplatin resistance resulting in a significant reduction in the effective dose needed to kill MDA-MB-231 cells.
Repair of DNA damage caused by CDDP has been reported to involve PCNA [37–40]. DNA damage was increased in cells expressing caPeptide and treated with CDDP (Fig. 4). The number of cells with intermediate damage increased from 6 and 3 % in cells expressing caPeptide or treated with CDDP, respectively, compared to 12 % in cells both expressing caPeptide and treated with CDDP. In addition, 10 % of cells expressing caPeptide and treated with CDDP showed a high level of DNA damage versus 0 % in cells expressing caPeptide and 2 % in cells treated with CDDP.
The reversal of CDDP resistance in caPeptide expressing cells was evident by other measures as well. Cell death was seen in growth curves that followed cells for 2 week periods of low CDDP treatment coupled with caPeptide expression (Fig. 5a). Shorter exposures with higher dosages were also effective. Clonogenicity was decreased with 24 h treatment at increasing CDDP concentrations (Fig. 5b), and cell cycle analysis detected an increase in apoptosis in caPeptide expressing cells treated overnight with CDDP (Fig. 5c).
DNA damage resulting from caPeptide treatment was coincident with apoptosis (Fig. 6). Gu et al. [21] illustrated that γH2AX levels in irradiated neuroblastoma cells treated with R9-cc-caPeptide remained elevated for at least 48 h compared with cells treated with a control peptide where γH2AX levels reverted to baseline levels. They also showed that the prolonged elevation of γH2AX levels in caPeptide-treated cells correlated with impaired DNA repair by homologous recombination (HR) and a reduction in the recruitment of the HR repair protein Rad51 to sites of DNA damage. The findings of the present study are consistent with a hypothesis that DNA damage normally incurred during replication and cell division is left unrepaired in caPeptide-treated cells leading to elevated levels of γH2AX over the course of the experiment and increasing apoptosis as a result of the unrepaired DNA damage.
In this study, we show that the induced peptide interacts with POLD3 (Fig. 3b). Interestingly, immunofluorescence studies with the R9-cc-caPeptide showed that while neuroblastoma cells treated with R9-cc-caPeptide experienced a decrease in recruitment of Fen1 and DNA ligase1 to PCNA in replication foci, the association of POLD3 with PCNA in the foci was unaffected [21]. Another study found that in Biacore experiments where PCNA is flowed over POLD3 captured on the surface of the sensor chip, caPeptide is able to inhibit PCNA binding with POLD3 [Smith SJ et al. in press]. Together these results suggest that caPeptide may be able to interfere with POLD3 binding to PCNA but might not be able to dislodge POLD3 that is already bound in complex. This in turn suggests that the caPeptide that is co-immunoprecipitated with POLD3 in this study is associated with POLD3 that is not already in complex with PCNA. As such this may explain why caPeptide’s effect in this study seems to be fairly innocuous under normal replication conditions, perhaps merely delaying replication and repair by competing some POLD3 away from the pool available to enter complexes, but accentuated under replication stress provoked by CDDP. For instance, when replication forks encounter a DNA lesion such as a CDDP adduct, translesion synthesis (TLS) is initiated to bypass the lesion and a process of polymerase switching occurs where POLD is temporarily displaced from the replication complex and TLS polymerases are enjoined to carry replication past the lesion [41–44]. In such a situation, caPeptide’s interaction with POLD3 could aggravate the reinsertion of POLD into the replication complex at a time when prolonged replication fork stalling means an increased risk of fork collapse and subsequent apoptosis.
Initial investigations into caPeptide suggest that the study of this peptide and its interactions may lead to a greater understanding of caPCNA, error-prone replication and repair, and cellular acquisition and reversal of drug resistance. In addition, there is the potential for the development of a novel therapeutic treatment of cancer. To that end, future work could entail improving peptide stability, a problem commonly associated with peptide therapy. Alternatively, using caPeptide and its target interactions as the basis for small molecule design could lead to the development of a new therapy. The need for targeted cancer therapies makes caPeptide and caPCNA of great interest for future study.
Methods
Vectors
The pTet-DualOn vector was purchased from Clontech (631112). It constitutively expresses the tetracycline-controlled transcriptional activator, Tet-On Advanced and the green fluorescent reporter ZsGreen1.
The pCaPeptide vector was made through numerous cloning steps. The tetracycline responsive promoter, ptight (Clontech) and mCherry reporter gene were amplified from the vector pTRE-Dual2 (Clontech, PT5038-5). The upstream primer for ptight amplification contained a flanking BglII restriction site; the downstream primer had a HindIII restriction site. The upstream primer for mCherry contained an XbaI restriction site followed by the 2a peptide sequence (from Thosea asigna virus) and finally complementary mCherry sequence. The downstream primer was flanked with another XbaI site. The PCR products were inserted into pcDNA3.1+ (Invitrogen) with the BglII/HindIII digest removing the CMV promoter from the vector. The caPeptide coding sequence followed by 3× repeated nuclear localization signal (from simian virus large T-antigen) was then inserted into the vector. This fragment was made by making two oligomers. The sense oligomer contained an upstream HindIII site followed by caPeptide sequence and the first NLS repeat. The antisense fragment contained the three NLS and an outside XhoI site. The two oligos were annealed, extended, digested and inserted into the HindIII/XhoI site of the vector. Finally, a puromycin resistance gene was inserted in place of the neomycin resistance gene for more flexible selection options.
An additional version of the pCaPeptide (pfCaPeptide) was made containing a 3× flag sequence that is recognized by Sigma’s anti-flag M2 affinity beads. The sequence was cloned upstream of the caPeptide sequence to form a flag-tagged fusion peptide upon expression in cells.
Cell lines
All cells were either obtained from the American Type Culture Collection or were the kind gift of Dr. Susan Kane of the Beckman Research Institute at the City of Hope. MDA-MB-231, MDA-MB-436, MCF7, SKBR3 and MDA-MB-468 cells were cultured in DMEM (CellGro, 10-013-CV) with 10 % FBS (Gemini Bio-Products, 100-106) and 1 % Pen/Strep (Sigma, P4333). HCC1428, HCC1143, HCC1937, UACC893, HCC38, MDA-MB-361, HCC1569 and BT474 cells were cultured in RPMI 1640 (CellGro, 10-040-CV) with 10 % FBS and 1 % Pen/Strep.
Treatment with R9 peptides
Twenty thousand cells of each breast cancer cell line were plated in triplicate in opaque-walled 96-well plates (Corning, 3610) and cultured for 72 h with their regular growth media containing either 60 μM of a nona-arginine-linked caPeptide (R9-cc-caPeptide), amino acid sequence RRRRRRRRRCCLGIPEQEY, or with 60 μM of a nona-arginine linked scrambled version of the capeptide, amino acid sequence RRRRRRRRRCCEPGLIYEQ, or with equivalent volume of the peptide diluent, PBS. Both peptides were made by AnaSpec (Fremont, CA, USA). At the end of the 72-h incubation, the number of viable cells was determined using CellTiter-Glo assay (Promega, G7572) by measuring the resulting luminescence with a Beckman Coulter DTX880 multimode detector. For each cell line, cell death specific to caPeptide was determined by subtracting the percent of viable cells in the R9-caPeptide-treated cells from the percent of viable cells in the scrambled version of the peptide.
Inducible cell pools
Five million MDA-MB-231 cells growing in DMEM with 10 % FBS (Gemini Bio-Products, 100-106) and 1 % Pen/Strep (Sigma, P4333) were electroporated with 20 μg of pTet-DualOn vector in 800 μl of growth media using a cuvette with a 0.4-cm electrode gap (Bio-Rad, 165-2088) and Gene Pulser apparatus (Bio-Rad, 165-2075) set at 250 mV and 960 uFD. Cells were plated in a 100-mm tissue culture plate with fresh growth media. The cells were allowed to recover and expand and then sorted by flow cytometry for cells expressing the ZsGreen reporter protein. Sorted cells were cultured and re-sorted two more times over a period of 6 weeks. This cell pool was named 231-v.c. The 231-v.c cells were then electroporated with pCaPeptide and pfCaPeptide in the same way as described above. These cells were cultured in DMEM media with 10 % FBS (tested for the absence of tetracyclines) and 1 % Pen/Strep. After recovering, cells were initially selected with 1 μg/ml puromycin (Sigma, P8833). The resulting cell population was tested for inducibility by adding 1 μg/ml Dox (Clontech, 631311) and observing development of mCherry signal by fluorescent microscopy. Through experience, it was noted that only cells that had low but detectable background expression of mCherry were capable of being induced to higher levels of expression with the addition of Dox. Therefore, flow cytometry was used to sort cells that had both Zsgreen expression and low-level mCherry expression. Puromycin selection pressure was removed, and the cells were sorted two more times over a period of 6 weeks to obtain a stable population of cells. The resulting populations were named 231-caPep and 231-fcaPep.
Reverse transcription PCR
231-caPep cells were seeded in a 60-mm plate at 5 × 105 cells per sample and allowed to attach for 5 h. Dox was added to induce sample at a final concentration of 1 μg/ml. Cells were incubated overnight at 37 °C and 5 % CO2. The next morning, RNA was prepared using standard protocol for purifying total RNA from animal cells provided with RNeasy Mini kit (Qiagen, 74104). QIAshredder columns (Qiagen, 79654) were used for the homogenization step. Genomic DNA elimination and cDNA synthesis were performed using RT2 Easy First Strand kit (Qiagen, 330401). The amplification reactions were performed in MJ Mini Thermocycler (Bio-Rad, PTC-1148C) using KAPA HiFi Hotstart (KAPA Biosystems, KK2501). Reaction condition #1 used primers that annealed to the 5′ (5′GTGAGCAAGGGCGAGG AGG-3′) and 3′ (5′GTTCCACGATGGTGTAGTCC-3′) ends of the mcherry sequence in the cDNA template. Reaction condition #2 used primers that annealed to the 5′ end of caPeptide (5′GCGTGCTGCCTGGGCATCC-3′) and the same downstream primer as in reaction #1. Thirty cycles of PCR were performed. Samples were electrophoresed on a 1 % agarose gel and stained with ethidium bromide.
Metabolic labeling
231-caPep and 231-v.c. cells plated to be 80 % confluent on the day of the experiment were washed 3×s with PBS and incubated in depletion media. The depletion media consisted of DMEM lacking lysine (Life Technologies, A14431-01 supplemented with L-glutamine, sodium pyruvate and L-arginine). After 15 min, the depletion media was removed. The cells were then incubated for 5 h in fresh depletion media supplemented with 80 uCi of tritiated lysine (Perkin Elmer, NET376) per plate. The plates were washed with PBS, and lysates were collected in NP-40 buffer (50 mM Tris pH 7.4, 1 % NP-40, 150 mM NaCl, 2 mM EDTA).
Immunoprecipitations and Westerns
Antibodies used in this study included anti-caPCNA as described previously (15), anti-Flag (Sigma, A2220), anti-POLD3 (BioAcademia, 2A1C11), anti-GAPDH (SCBT, sc-25778) and anti-Histone H3 (Cell Signaling, 9717).
Immunoprecipitations were performed on 2 × 106 trichloroacetic acid precipitable counts per minute and 1 ml volume of NP-40 buffer. Antibody in the amount recommended by source was added and incubated overnight at 4 °C with rotation. Twenty microliters of packed Protein G agarose (SCBT, sc-2002) was added to the samples and incubated for another 2 h at 4 °C with rotation. Beads were pelleted and washed 3xs with PBS. After the final wash, sample buffer was added to the beads and the samples were separated by SDS-PAGE.
To visualize labeled capeptide, a Tricine SDS-PAGE system was used as described by the American Electrophoresis Society (www.aesociety.org). After electrophoresis, the gel was fixed and treated with Amplify (GE Healthcare, NAMP100) as described by the manufacturer. The gel was then dried and exposed to BioMax MR film (Kodak, 870-1302).
To visualize other proteins, Laemmli SDS-PAGE was used for separation. Proteins were then transferred to nitro-cellulose or PVDF. Membranes were blocked with TBS-T (20 mM Tris pH 7.4, 150 mM NaCl, 0.05 % Tween-20) containing 5 % dried milk. Primary antibody incubations were done using the supplier’s recommended dilution rate and diluting with either Odyssey blocking buffer (Li-Cor, 927-40000), TBS-T containing 5 % milk or TBS-T containing 5 % BSA. The primary antibody incubations were performed overnight at 4C. Secondary antibody incubations were performed with species-specific antibodies conjugated to fluorescent dyes specific for detection with the Odyssey Infrared Imaging System (Li-Cor) or with secondary antibodies conjugated to horse radish peroxidase for detection by chemiluminescence. Incubations were at room temperature for 1 h. The blots were then washed 3 times for 10 min in TBS-T and imaged by using the Odyssey system or by using ECL Prime detection reagent (GE Healthcare, RPN 2232) and exposing to film.
Nuclear fractionation
Metabolically labeled cells as described above were harvested with trypsin–EDTA and then centrifuged at 500×g for 5 min. Cytoplasmic and nuclear fractions were collected using Subcellular Protein Fractionation Kit (Thermo Scientific, 78840). In brief, the cell pellet was washed with ice-cold PBS and resuspended in 200 μl of cytoplasmic elution buffer containing protease inhibitors. The suspension was incubated on ice for 10 min with periodic shaking after which the samples were centrifuged for 5 min at 500×g. The supernatant (cytoplasmic extract) was collected, and the pellet was resuspended in 200 μl of membrane extraction buffer with protease inhibitors, vortexed for 5 s and incubated on ice for 10 min. After incubating, the samples were centrifuged at 3,000×g for 5 min. The supernatant was discarded, and the pellet was resuspended in 100 μl of nuclear extraction buffer containing 5 mM CaCl2 and 300 units of micrococcal nuclease. The samples were vortexed on the highest setting for 15 s and incubated for 15 min at room temperature with periodic shaking. After incubation, the samples were vortexed for another 15 s and centrifuged at 13,500×g for 5 min. The supernatant (nuclear extract) was collected.
Comet assay
231-caPep cells were seeded in a 24-well plate at 5 × 104 cells per sample. After attachment, the cells were either left untreated, treated for 24 h with 1 μg/ml Dox, treated for 24 h with 5 μM CDDP (Sigma, P4394), or treated for 24 h with both 1 μg/ml Dox and 5 μM CDDP. After treatment, the cells were trypsinized and assayed under alkaline conditions using the CometAssay kit (Trevigen, 4250-050-K) and following Trevigen’s protocol. The samples were stained with SYBR Green (Trevigen, 4250-050-05) and visualized by fluorescent microscopy. DNA damage to individual cells was categorized as having no damage, low damage, medium damage or high damage. Sixty to 130 cells for each sample condition were scored, and the assay was repeated three times.
Growth curves
231-caPep cells were plated at 1 × 105 cells per 5 ml in 60-mm dishes and allowed to attach overnight. Samples were plated in triplicate for each condition and time point. The next day (Day 1), samples were treated with 2, 3.5 or 5 μM CDDP, and half of the samples were treated with 1 μg/ml Dox. Dox was replenished every other day, and CDDP and media were changed every fourth day. Samples were collected and cells counted on days 1, 5, 9 and 13.
Clonogenic assay
231-caPep cells were treated with or without 1 μg/ml Dox and 0, 10, 20, 30, 45 and 60 μM CDDP for 24 h. After treatment, cells were trypsinized and counted. One thousand cells of each condition were plated in triplicate in 100-mm dishes. Dox continued to be added to Dox-treated samples at a rate of 1 μg/ml every other day. Colonies were allowed to grow for 5 days at which point plates were stained with 0.5 % methylene blue in 50 % ethanol. Colonies were counted for each condition.
Cell cycle analysis
231-caPep and 231-v.c. cells were seeded at 1 × 106 cells per 10 cm plate and allowed to attach for 5 h. The cells were then treated overnight with 10 μM CDDP and 1 μg/ml Dox. Cells were trypsinized and washed 2 times with ice-cold PBS without Ca+2 or Mg+2 (PBS) and centrifuged at 200×g for 5 min at 4 °C. After washing, the cell pellets were resuspended by slow addition of 100 % ethanol (stored at −20 °C) and constant vortexing. The cell suspensions were stored at −20 °C overnight. The next morning, the cells were pelleted by centrifuging at 200×g for 10 min at 4 °C. The supernatant was removed, and the cell pellets were washed 2 times with PBS and centrifugation at 200×g for 10 min at 4 °C. The resulting cell pellets were resuspended in 400 μl of 0.1 % Triton X-100 (Fisher Scientific, BP151), 20 μg/ml propidium iodide (Sigma, P4170) and .2 mg/ml DNAse-free RNAse (Roche, 11119915001). The cell suspension was incubated at room temperature for 1 h. The stained cells were analyzed by flow cytometry. ModFit LT v.3.2.1 (Verity Software House) was used to fit data to cell cycle model.
Immunofluorescent DNA damage and apoptosis assay
MDA-MB-231 cells were plated at 4 × 105 cells per 6-cm plate and allowed to attach overnight. The following day, the cells for the “0” time point were collected, fixed, permeabilized and frozen in FBS with 10 % DMSO as per protocol described in Apoptosis, DNA Damage and Cell Proliferation Kit (BD Biosciences, 562253). Also on this day, 75 μM of R9-cc-caPeptide in normal growth media was added to remaining plates of cells. These cells were collected at 12, 24, 48 and 72 h after addition of R9-cc-caPeptide. Upon collection, the cells were fixed, permeabilized, and frozen in same manner as the “0” time point. On the day of analysis, the cells were thawed and re-fixed/permeabilized and stained with fluorescent antibodies, Alexa Fluor 647 Mouse Anti-H2AX (pS139) (BD Biosciences, 51-9007683) and PE Mouse Anti-Cleaved PARP (Asp214) (BD Biosciences, 51-9007684). The cells were washed and analyzed by flow cytometry. All incubations and washes were done as described in the protocol of the Apoptosis, DNA Damage and Cell Proliferation Kit.
Acknowledgments
We would like to thank Dr. Susan Kane for the kind gift of the various vectors that were used to clone the constructs in this paper as well as several of the breast cancer cell lines included in the panel. This work was supported in part by research awards from the National Institutes of Health/National Cancer Institute (R01 CA121289), Department of Defense (W81XWH-11-1-0786) and the City of Hope Board of Governors to LHM. In addition, research reported in this publication was supported by National Cancer Institute of the National Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
None.
Contributor Information
Robert G. Lingeman, Department of Molecular Biology, Beckman Research Institute of the City of Hope, 1450 E. Duarte Rd., Duarte, CA 91010, USA
Robert J. Hickey, Department of Molecular Pharmacology, Beckman Research Institute of the City of Hope, 1450 E. Duarte Rd., Duarte, CA 91010, USA
Linda H. Malkas, Department of Molecular Biology, Beckman Research Institute of the City of Hope, 1450 E. Duarte Rd., Duarte, CA 91010, USA
References
- 1.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 2.Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot. 2011;107:1127–1140. doi: 10.1093/aob/mcq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K, Myung K. PCNA modifications for regulation of post-replication repair pathways. Mol Cells. 2008;26(1):5–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Naryzhny SN. Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci. 2008;65:3789–3808. doi: 10.1007/s00018-008-8305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson PL, Xu F, Xiao W. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 2008;18:162–173. doi: 10.1038/cr.2007.114. [DOI] [PubMed] [Google Scholar]
- 6.Moldovan G, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Cleaver JE, Laposa RR, Limoli CL. DNA replication in the face of (In)surmountable odds. Cell Cycle. 2003;2(4):310–315. [PubMed] [Google Scholar]
- 8.Lehmann AR. Replication of damaged DNA. Cell Cycle. 2003;2(4):300–302. [PubMed] [Google Scholar]
- 9.Barsky D, Venclovas C. DNA sliding clamps: just the right twist to load onto DNA. Curr Biol. 2005;15:R989–R992. doi: 10.1016/j.cub.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Majka J, Burgers P. The PCNA-RFC families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol Biol. 2004;78:227–260. doi: 10.1016/S0079-6603(04)78006-X. [DOI] [PubMed] [Google Scholar]
- 11.Adelman JL, Chodera JD, Kuo IW, Miller TF, Barsky D. The mechanical properties of PCNA: implications for the loading and function of a DNA sliding clamp. Biophys J. 2010;98:3062–3069. doi: 10.1016/j.bpj.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prives C, Gottifredi V. The p21 and PCNA partnership. Cell Cycle. 2008;7(24):3840–3846. doi: 10.4161/cc.7.24.7243. [DOI] [PubMed] [Google Scholar]
- 13.Bechtel PE, Hickey RJ, Schnaper L, Sekowski JW, Long BJ, Freund R, Liu N, Rodriguez-Valenzuela C, Malkas LH. A unique form of proliferating cell nuclear antigen is present in malignant breast cells. Cancer Res. 1998;58:3264–3269. [PubMed] [Google Scholar]
- 14.Wang X, Hickey RJ, Malkas LH, Koch MO, Li L, Zhang S, Sandusky GE, Grignon DJ, Eble JN, Cheng L. Elevated expression of cancer-associated proliferating cell nuclear antigen in high-grade prostatic intraepithelial neoplasia and prostate cancer. Prostate. 2011;71:748–754. doi: 10.1002/pros.21291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malkas LH, Herbert BS, Abdel-Aziz W, Dobrolecki LE, Liu Y, Agarwal B, Hoelz D, Badve S, Schnaper L, Arnold RJ, Mechref Y, Novotny MV, Loehrer P, Goulet RJ, Hickey RJ. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. PNAS. 2006;103:19472–19477. doi: 10.1073/pnas.0604614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekowski JW, Malkas LH, Schnaper L, Bechtel PE, Long BJ, Hickey RJ. Human breast cancer cells contain an error-prone DNA replication apparatus. Cancer Res. 1998;58:3259–3263. [PubMed] [Google Scholar]
- 17.Francisco DC, Peddi P, Hair JM, Flood BA, Cecil AM, Kalogerinis PT, Sigounas G, Georgakilas AG. Induction and processing of complex DNA damage in human breast cancer cells MCF-7 and nonmalignant MCF-10A cells. Free Radical Biol Med. 2008;44:558–569. doi: 10.1016/j.freeradbiomed.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 18.Rassool FV, Tomkinson AE. Targeting abnormal DNA double strand break repair in cancer. Cell Mol Life Sci. 2010;67:3699–3710. doi: 10.1007/s00018-010-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentley J, Diggle CP, Harnden P, Knowles MA, Kiltie AE. DNA double strand break repair in human bladder cancer is error prone and involves microhomology-associated end-joining. Nucleic Acids Res. 2004;32(17):5249–5259. doi: 10.1093/nar/gkh842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandoval JA, Grosfeld JL, Hickey RJ, Malkas LH. Structural analysis of the human neuroblastoma DNA replication complex: insights into faulty proliferation. J Pediatr Surg. 2006;41:266–270. doi: 10.1016/j.jpedsurg.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 21.Gu L, Smith SH, Li C, Hickey RJ, Stark JM, Fields GB, Lang WH, Sandoval JA, Malkas LH. A PCNA-derived cell permeable peptide selectively inhibits neuroblastoma cell growth. PLoS One. 2014;9(4):e94773. doi: 10.1371/journal.pone.0094773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florea A, Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ and Panel members. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Anals of Oncology. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodler E, Korde L, Gralow J. Current treatment options in triple negative breast cancer. Breast Dis. 2010;32:99–122. doi: 10.3233/BD-2010-0304. [DOI] [PubMed] [Google Scholar]
- 25.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer: current status and future directions. Ann Oncol. 2009;20:1913–1927. doi: 10.1093/annonc/mdp492. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack KM, Canada RG, Andrews PA. The effects of terbium on the cellular accumulation of cisplatin in MDA-MB-231 human breast tumor cells. Cancer Chemother Pharmacol. 1997;39:217–222. doi: 10.1007/s002800050563. [DOI] [PubMed] [Google Scholar]
- 28.Garand C, Guay D, Sereduk C, Chow D, Tsofack SP, Langlois EP, Hongwei HY, Lebel M. An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells. Cancer Sci. 2011;102:1410–1417. doi: 10.1111/j.1349-7006.2011.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi H, Yang J, Wu W, Wang W, Kong X, Wang Y, Yun X, Zong H, Wei Y, Zhang S, Gu J. Knockdown of BCL2L12 leads to cisplatin resistance in MDA-MB-231 breast cancer cells. Biochim Biophys Acta. 2008;1782:649–657. doi: 10.1016/j.bbadis.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Kao J, Salari K, Bocanegra M, Choi Y, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4(7):1–16. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She Q, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N. Breast tumor cells with PI3 K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One. 2008;3(8):1–10. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subik K, Lee J, Baxter L, Strzepek T, Costello D, Crowley P, Xing L, Hung M, Bonfiglio T, Hicks DG, Tang P. The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67, and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer. 2010;4:35–41. [PMC free article] [PubMed] [Google Scholar]
- 33.Herce HD, Garcia AE, Litt J, Kane RS, Martin P, Enrique N, Rebolledo A, Milesi V. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys J. 2009;97:1917–1925. doi: 10.1016/j.bpj.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Chen X, Xiao D. Tetracycline-inducible expression systems: new strategies and practices in the transgenic mouse modeling. Acta Biochim Biophys Sin. 2007;39:235–246. doi: 10.1111/j.1745-7270.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 35.Mills AA. Changing colors in mice: an inducible system that delivers. Genes Dev. 2001;15:1461–1467. doi: 10.1101/gad.909301. [DOI] [PubMed] [Google Scholar]
- 36.Rossi FMV, Blau HM. Recent advances in inducible gene expression systems. Curr Opin Biotechnol. 1998;9:451–456. doi: 10.1016/s0958-1669(98)80028-1. [DOI] [PubMed] [Google Scholar]
- 37.Haneda H, Katabami M, Miyamoto H, Isobe H, Shimizu T, Ishiguro A, Moriuti T, Takasaki Y, Kawakami Y. The relationship of the proliferating cell nuclear antigen protein to cis-diamminedichloroplatinum (II) resistance of a murine leukemia cell line P388/CDDP. Oncology. 1991;48:234–238. doi: 10.1159/000226934. [DOI] [PubMed] [Google Scholar]
- 38.Miura M, Domon M, Sasaki T, Kondo S, Takasaki Y. Restoration of proliferating cell nuclear antigen (PCNA) complex formation in xeroderma pigmentosum group A cells following cis-diamminedichloroplatinum (II)-treatment by cell fusion with normal cells. J Cell Physiol. 1992;152:639–645. doi: 10.1002/jcp.1041520324. [DOI] [PubMed] [Google Scholar]
- 39.Miyaji T, Kato A, Yasuda H, Fujigaki Y, Hishida A. Role of the increase in p21 in cisplatin-induced acute renal failure in rats. J Am Soc Nephrol. 2001;12:900–908. doi: 10.1681/ASN.V125900. [DOI] [PubMed] [Google Scholar]
- 40.Ando T, Kawabe T, Ohara H, Ducommun B, Itoh M, Okamoto T. Involvement of the interaction between p21 and proliferating cell nuclear antigen for the maintenance of G2/M arrest after DNA damage. J Biol Chem. 2001;276:42971–42977. doi: 10.1074/jbc.M106460200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Zhang S, Lin SHS, Wang X, Wu L, Lee EYC, Lee MYWT. Structure of monoubiquitinated PCNA. Implications for DNA polymerase switching and OkaZaki fragment maturation. Cell Cycle. 2012;11:2128–2136. doi: 10.4161/cc.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda Y, Piao J, Kamiya K. DNA replication-coupled PCNA mono-ubiquitination and polymerase switching in a human in vitro system. J Mol Biol. 2010;396:487–500. doi: 10.1016/j.jmb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Zhuang Z, Johnson RE, Haracska L, Prakash L, Prakash S, Benkovic SJ. Regulation of polymerase exchange between Polη and Polδ by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. PNAS. 2008;105:5361–5366. doi: 10.1073/pnas.0801310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freudenthal BD, Gakhar L, Ramaswarmy S, Washington MT. Structure of monoubiquitinated PCNA and implications for translesion synthesis and DNA polymerase exchange. Nat Struct Mol Biol. 2010;17:479–485. doi: 10.1038/nsmb.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]