Abstract
Background
The Fontan operation results in a circulation that is dependent on low pulmonary vascular resistance to maintain an adequate cardiac output. Medical therapies that lower pulmonary vascular resistance may augment cardiac output and improve long-term outcomes.
Objectives
This phase I/II clinical trial conducted by the Pediatric Heart Network was designed to evaluate short-term safety, pharmacokinetics (PK), and preliminary efficacy of udenafil in adolescents following Fontan.
Methods
A 5-day dose-escalation trial was conducted in five study cohorts of six subjects each (37.5, 87.5, and 125 mg daily, 37.5 and 87.5 mg by mouth twice daily). A control cohort with 6 subjects underwent exercise testing only. Adverse events (AEs) were recorded, PK samples were collected on study days six through eight, and clinical testing was performed at baseline and day five.
Results
The trial enrolled 36 subjects; mean age 15.8 years (58% male). There were no significant differences in subject characteristics between cohorts. No drug-related serious AEs were reported during the study period; 24 subjects had AEs possibly or probably related to study drug. Headache was the most common AE, occurring in 20 of 30 subjects. The 87.5 mg bid cohort was well tolerated, achieved the highest maximal concentration (506 ng/mL) and the highest average concentration over the dosing interval (279 ng/mL), and was associated with a suggestion of improvement in myocardial performance. Exercise performance did not improve in any of the dosing cohorts.
Conclusions
Udenafil was well-tolerated at all dosing levels. The 87.5 mg bid cohort achieved the highest plasma drug level and was associated with a suggestion of improvement in myocardial performance. These data suggest that the 87.5 mg bid regimen may be the most appropriate for a Phase III clinical trial.
Keywords: Fontan procedure, pharmacokinetics, heart failure
Introduction
The Fontan operation is a palliative procedure for children born with functional single ventricle heart disease1,2. This operation, which creates a total cavopulmonary connection, separates the systemic and pulmonary circuits and mitigates pre-existing chronic hypoxemia and ventricular volume overload. However, following the Fontan operation there is no ventricular pump to propel blood into the pulmonary arteries. Instead, blood returns to the lungs via passive flow from the systemic veins. The resulting physiology confers a degree of chronic heart failure, characterized by diastolic dysfunction, and a circulation dependent on low pulmonary vascular resistance (PVR) in order to maintain an adequate cardiac output with a modestly elevated central venous pressure3,4.
Phosphodiesterase Type 5 (PDE5) inhibitors are a unique class of medications that have demonstrated utility in reducing PVR and improving ventricular performance in patients with pulmonary hypertension and myocardial dysfunction5–10. These characteristics make this class of drug an appealing therapy to consider for patients with the Fontan circulation, in which the maintenance of low PVR and normal myocardial function are crucial determinants of long-term clinical outcomes. Although preliminary studies have demonstrated a modest short-term benefit, there are no data regarding the long-term use of this class of medication in the Fontan population10–14.
Udenafil is a novel PDE5 inhibitor that has an established record of safety and efficacy in adult males for the indication of erectile dysfunction15–17. In that population, the pharmacokinetics (PK) of udenafil (molecular weight 516 g/mole) and the major circulating metabolite, DA-8164 (molecular weight 405 g/mole), have been well characterized. Udenafil is rapidly absorbed following oral administration, followed by a log-linear decline in plasma concentration with a terminal half-life in the range of 16 hours. Both udenafil and DA-8164, which is equipotent, are cleared predominantly by hepatic metabolism and then excreted in the feces and urine. Udenafil bioavailability is known to increase greater than proportionally with increasing dose following single- and multiple-dose administration. Although udenafil has been studied in adult males, the safety, PK, and tolerability of udenafil have not been evaluated in children or adolescents, in females, or in those who have undergone the Fontan operation.
The aims of this study were to: 1) evaluate the short-term safety and tolerability of udenafil in male and female adolescents with single ventricle physiology who have undergone the Fontan operation, 2) determine the PK of udenafil in this population, and 3) evaluate the short-term impact of udenafil on clinical outcome measures affected by this unique form of chronic heart failure. The results of this phase I/II clinical trial provide preliminary data to aid in dose selection for a phase III clinical trial.
Methods
This multi-center, open-label, dose escalation, safety, PK, and pharmacodynamic study was conducted in adolescents with single ventricle physiology after Fontan palliation. Dosing cohort size was determined a priori to provide a sample-size sufficient to evaluate short-term safety and allow characterization of the PK endpoints in this patient population. The study was not powered to detect statistically significant differences in clinical testing. Subjects were enrolled in five cohorts of six subjects each at udenafil doses of 37.5 mg, 87.5 mg, and 125 mg daily, as well as 37.5 mg and 87.5 mg by mouth twice daily (bid) for five days. Serum electrolytes and liver enzyme levels were measured, and a pregnancy test was obtained and confirmed as negative prior to drug administration. Blood samples for udenafil and DA-8164 PK analysis were collected for 48 hours following the last udenafil dose. Clinical testing (echocardiography, exercise stress test, and endothelial function assessment) was performed at baseline and again on day five. A control cohort of six subjects underwent exercise testing without drug administration.
Subjects for this study were recruited from six Pediatric Heart Network (PHN) clinical centers in the United States and Canada. Subjects were identified from a review of the medical records at each center, and consent with or without assent was obtained as appropriate. The study was approved by the Institutional Review Board for the Protection of Human Subjects at each of the five sites in the Unites States, and by the Research Ethics Board for the site in Canada. Udenafil was used under an Investigational New Drug application with the Food and Drug Administration (IND #121,648). A Health Canada No Objection Letter was obtained for the use of udenafil in Canada. The study was overseen by the PHN’s Data and Safety Monitoring Board, and adverse events were reviewed by the PHN’s independent Medical Monitor. Funding for this project was provided by the National Heart, Lung, and Blood Institute and by Mezzion Pharma Co. Ltd. (Seoul, South Korea). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Safety / tolerability
Adverse events (AEs) were recorded throughout the eight-day study period and for three months thereafter. AEs were classified and reported based on seriousness (serious vs. non-serious), relation to the study drug (definitely, probably, possibly, or not related), and by expectedness (expected vs. unexpected).
PK analysis
On study day six, subjects were admitted to an observation unit where a blood-drawing IV was placed. Blood samples for PK analysis were drawn in a non-fasting state at time 0 (just prior to final dose) and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, and 48 hours post-dose. Plasma udenafil and DA-8164 concentrations were determined using a validated liquid chromatography with tandem mass spectrometry detection (LC/MS-MS) method by Nuvisan-GmbH, New Ulm Germany. The lower and upper limits of detection for udenafil and DA-8164 were 1.0 ng/mL and 1000 ng/mL. Samples below the limit of quantification were to be reported as 0.00 ng/mL for the purpose of calculating descriptive statistics. The precision of quality control samples during sample analysis was expressed as the percent coefficient of variation (%CV). For plasma udenafil, the mean %CV ranged from 3.3% to 5.8%. For plasma DA-8146, the mean %CV ranged from 4.7% to 8.7%. Accuracy during sample analysis was expressed as percent difference from theoretical (bias). For plasma udenafil, the mean bias ranged from −6.6% to 4.8% for calibration standards and −4.3% to 2.6% for quality control samples. For plasma DA-8146, the mean bias ranged from −1.1% to 2.2% for calibration standards and −2.4% to −1.1% for quality control samples. PK analysis of udenafil and DA-8164 were provided by R. Guttendorf (Aclairo Pharmaceutical Development Group, Inc, Vienna, Virginia).
The PK evaluation was based on non-compartmental PK parameters. These parameters included maximum plasma concentration (Cmax), time to maximum plasma concentration (tmax), area under the plasma concentration versus time curve from time zero through the dosing interval (AUC0−τ), average concentration over the dosing interval (Cavg) defined as AUC0−τ/τ, the terminal phase rate constant (kel), the terminal half-life (t½) defined as 0.693/kel, and oral clearance (CL/F). All PK parameters were calculated using WinNonlin (Pharsight Corporation, Version 6.4).
Clinical testing
On study day 5 (after eight doses for those randomized to twice daily dosing and four doses for those randomized to daily dosing), each subject underwent repeat clinical testing. Subjects were instructed to take the scheduled dose of study drug at home and arrive such that follow-up testing could start within an hour of the start time of the baseline PD tests ensuring that PD parameters were evaluated near peak drug exposure time. Subjects were asked to arrive in a fasting, non-caffeinated state, and underwent vascular function assessment as their first clinical test. A light snack was provided prior to echocardiographic assessment and exercise testing.
Exercise stress test
Subjects underwent maximal exercise testing using a standard ramp cycle protocol. Expired gases were collected and electrocardiographic data were recorded as described previously in Fontan protocols published by the Pediatric Heart Network18. The primary outcome of interest was maximal oxygen consumption at peak effort. Subjects were judged to achieve a maximum exercise performance if the respiratory exchange ratio was ≥ 1.1.
Echocardiography
Echocardiograms were performed by sonographers with specific training for this protocol. The primary outcome of interest was the myocardial performance index (MPI) using Doppler-based measures of inflow and outflow duration. The duration of inflow into the dominant ventricle and outflow across the dominant semilunar valve were measured and used to calculate MPI using the standard formula19. Additional tissue Doppler images were obtained and used to calculate the tissue Doppler based MPI as previously described20. Whenever possible, three measurements were made and the mean duration was used for the calculation. All measurements were made by a single reader at the echocardiography core lab (The Children’s Hospital of Philadelphia).
Vascular Function
Vascular function was assessed using peripheral arterial tonometry (PAT). Testing was performed in a quiet, darkened, temperature-controlled room (maintained at 70° to 75°F), and prior to other testing procedures (e.g., stress testing). Subjects were instructed to fast and to avoid exposure to caffeine and tobacco for 12 hours prior to testing. The testing protocol was performed using the Endo-PAT 2000 device (Itamar Medical, Caesarea, Israel) as instructed by the manufacturer and previously reported21,22. Measures of vascular function included the natural log transformed reactive hyperemia index (InRHI, the primary vascular outcome measure) and augmentation index (AI, a measure of arterial stiffness, the secondary outcome measure) normalized to a heart rate of 75 beats per minute. Studies were reviewed by the vascular core laboratory (Cincinnati Children’s Hospital Medical Center) for purposes of data quality assurance and quality control (QC). A QC score ranging from 1 (lowest) to 3 (highest) was used to judge the quality of the Endo-PAT measurements. Only those studies that had a score of 2 or 3 were used for analysis.
Statistical Methods
Key patient characteristics were compared using ANOVA at baseline among all six cohorts to detect potential imbalances. Descriptive statistics (arithmetic mean, standard deviation, coefficient of variation, and harmonic mean half-life (0.693/mean ke) values were calculated for udenafil and DA-8164 PK parameters. The relationship between udenafil and DA-8164 Cavg concentration values were inspected for trends likely to be of clinical relevance. The relationship between udenafil exposure (Cavg and Cmax values) and age, weight, and gender was also examined.
For clinical outcome measures (exercise stress test, echocardiography and vascular function), change scores between baseline and follow-up testing were compared using ANOVA and visual trend lines. Longitudinal changes within individual cohorts between baseline (day 1) and follow-up (day 5) studies were assessed using a paired t-test. Significance was set at p<0.05 for all t-test comparisons. As this was a phase I/II trial and not meant to confirm efficacy, p-values were not adjusted for multiple comparisons.
Results
A total of 36 subjects participated in this clinical trial, including 30 subjects who received study drug and six additional non-treated subjects that served as controls for the exercise stress test. All subjects who provided informed consent successfully completed the trial. Subject characteristics are listed in Table 1. The mean subject age was 15.8±1.3 years and 58% were male. There were no significant differences in key subject characteristics across the treatment groups with the exception of ventricular morphology. Overall, slightly more than one third of subjects had a dominant right ventricle (36%, n=13). The majority of subjects (58%, n=21) did not have a patent fenestration and the baseline mean arterial oxygen saturation was 93.8±3.1%.
Table 1.
Baseline characteristics for enrolled subjects by cohort.
| Characteristic | Overall (N=36) | Exercise testing only (N=6) | 37.5 mg daily (N=6) | 37.5 mg twice (N=6) | 87.5 mg daily (N=6) | 125 mg daily (N=6) | 87.5 mg twice (N=6) | P-value* |
|---|---|---|---|---|---|---|---|---|
| Age, year | 15.8±1.3 | 16.2±1.2 | 15.5±1.0 | 16.2±0.8 | 15.0±0.9 | 16.5±1.5 | 15.5±1.8 | 0.319 |
| Median (Interquartile Range) | 16 (15, 17) | 16 (15, 17) | 16 (15, 16) | 16 (16, 17) | 15 (14, 16) | 17 (16, 18) | 15 (14, 17) | 0.309 |
| Range, Min - Max | 14 – 18 | 15 – 18 | 14 – 17 | 15 – 17 | 14 – 16 | 14 – 18 | 14 – 18 | |
| Male | 21 (58%) | 4 (67%) | 4 (67%) | 3 (50%) | 3 (50%) | 4 (67%) | 3 (50%) | 1.000 |
| Race | 0.453 | |||||||
| White/Caucasian | 28 (78%) | 5 (83%) | 3 (50%) | 4 (67%) | 6 (100%) | 4 (67%) | 6 (100%) | |
| Black/African American | 3 (8%) | 0 (0%) | 2 (33%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Other/Unknown | 4 (11%) | 1 (17%) | 1 (17%) | 1 (17%) | 0 (0%) | 1 (17%) | 0 (0%) | |
| Height, cm | 165.7±10.1 | 163.6±5.6 | 161.0±12.9 | 164.9±10.9 | 168.6±7.8 | 171.3±7.8 | 164.7±14.2 | 0.585 |
| Weight, kg | 62.4±15.9 | 54.9±16.8 | 72.5±27.1 | 56.3±7.7 | 66.8±11.0 | 61.9±9.0 | 62.0±15.5 | 0.425 |
| Body mass index, kg/m2 | 22.6±5.0 | 20.3±4.8 | 27.2±8.1 | 20.8±3.2 | 23.4±3.2 | 21.0±2.0 | 22.6±4.8 | 0.157 |
| Right ventricle as dominant ventricle** | 13 (36%) | 1 (17%) | 3 (50%) | 3 (50%) | 0 (0%) | 5 (83%) | 1 (17%) | 0.032 |
| Oxygen saturation | 93.8±3.1 | 94.7±2.5 | 96.0±3.0 | 92.3±2.7 | 92.8±1.9 | 93.2±3.7 | 93.5±4.2 | 0.363 |
| Ventricular function | 0.677 | |||||||
| Normal | 30 (83%) | 6 (100%) | 4 (67%) | 4 (67%) | 5 (83%) | 5 (83%) | 6 (100%) | |
| Mild dysfunction | 6 (17%) | 0 (0%) | 2 (33%) | 2 (33%) | 1 (17%) | 1 (17%) | 0 (0%) | |
| Presence of fenestration | 15 (42%) | 1 (17%) | 1 (17%) | 3 (50%) | 4 (67%) | 2 (33%) | 4 (67%) | 0.378 |
P-values for continuous variables were calculated by ANOVA for parametric analysis or Kruskal-Wallis test for non-parametric analysis.
P-values for categorical variables were calculated by Fisher’s exact test.
There are 13 subjects with dominant RV, 22 subjects with dominant LV, and one subject with co-dominant ventricles (37.5 mg twice daily cohort).
Udenafil was well tolerated by all subjects at all dosing levels. A single serious unexpected AE was reported in this study, which was altered mental status unrelated to the study drug with onset nine days after drug discontinuation. This subject was observed in the hospital for 24 hours and subsequently discharged with no sequelae. No other serious AEs or AEs definitely related to the drug were reported. AEs thought to have a possible or probable relationship to study drug that occurred in more than one subject were limited to those known to be side effects of PDE5 inhibitors (Table 2). In total, 28 subjects (93%) reported at least one AE and 24 subjects (80%) experienced AEs determined by the investigators to have a possible or probable relationship to study drug. Of these, headache was the most common, occurring in 19 (63%) of the 30 subjects. Other AEs possibly or probably related to study medication that occurred in more than one subject included: facial flushing (33%), spontaneous penile erection (35% of males), nasal congestion (20%), nausea (10%), and abdominal discomfort (7%). Reported AEs did not increase with increasing dose.
Table 2.
Subjects (n (%)) who experienced drug-related* adverse events by preferred term
| Udenafil Treatment Groups | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 37.5 mg daily (N=6) | 37.5 mg twice daily (N=6) | 87.5 mg daily (N=6) | 125 mg daily (N=6) | 87.5 mg twice daily (N=6) | Total udenafil (N=30) | |
| Headache | 3 (50) | 4 (67) | 4 (67) | 4 (67) | 4 (67) | 19 (63) |
| Flushing | 1 (17) | 2 (33) | 4 (67) | 1 (17) | 2 (33) | 10 (33) |
| Nasal congestion | 1 (17) | 2 (33) | 1 (17) | 1 (17) | 1 (17) | 6 (20) |
| Spontaneous penile erection^ | 0 (0) | 1 (17) | 1 (17) | 2 (33) | 2 (33) | 6 (35) |
| Nausea | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 1 (17) | 3 (10) |
| Abdominal discomfort | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (17) | 2 (7) |
| Back pain | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (3) |
Probably or possibly; definitely related adverse events were not reported;
includes only male subjects exposed to udenafil (N=17).
All 13 scheduled blood samples for the PK analysis of udenafil and DA-8146 were successfully collected from each of the 30 subjects in the dosing cohorts at each pre-specified time point and all serum samples were determined to have a concentration that fell within the limits of quantification. Following oral administration of udenafil for five days, plasma udenafil concentrations increased rapidly until 1.3 to 2.3 hours followed by a log-linear decrease over the remainder of the sampling period (Figure 1a). Mean profiles were representative of individual subject profiles. Udenafil Cmax and AUC0−τ values increased out of proportion to increasing dose following multiple-dose oral administration. After once-daily dosing, mean udenafil Cmax values increased 4.6-fold and mean AUC0−τ values increased 4-fold when dose increased 3.3 times. After twice-daily dosing, mean udenafil Cmax values increased 3.3-fold and mean AUC0−τ values increased 3.2-fold when dose increased 2.3 times. Oral clearance (CL/F) decreased with increasing dose. Udenafil terminal phase half-life was approximately 10 to 13 hours for all 5 dosing regimens (Table 3). Analysis of PK by gender demonstrated that dose-normalized Cmax and Cavg values were not different for the male and female patients with Fontan physiology (Figure 2).
Figure 1.
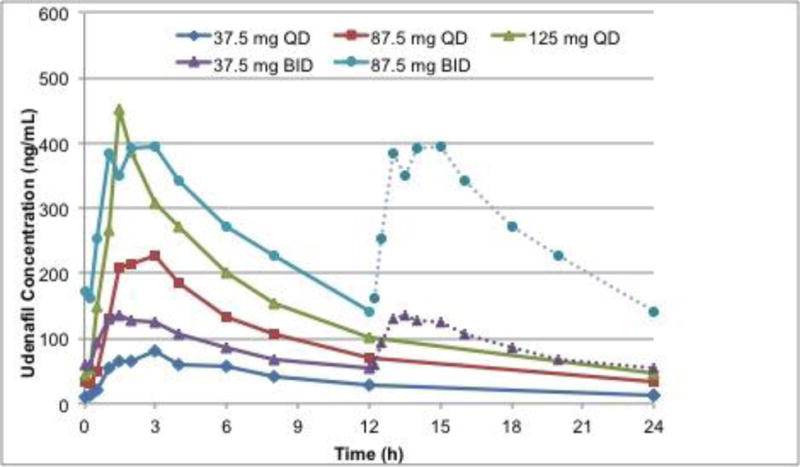
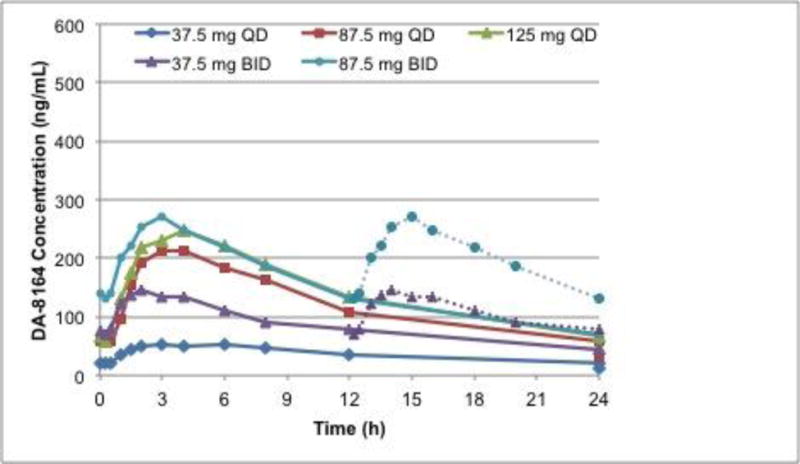
Mean concentration-time profile for udenafil (a) and DA-8164 (b) following oral administration of 37.5, 87.5, and 125 mg daily, and 37.5 and 87.5 mg twice daily. For twice daily dosing, the second twelve hours is imputed from the first twelve hours.
Table 3.
Summary comparison of udenafil pharmacokinetic parameters following oral administration for five days
| Mean (%CV) by Dosing Cohort | |||||
|---|---|---|---|---|---|
|
| |||||
| Parameter (CV%) | 37.5 mg daily (N=6) |
37.5 mg bid (N=6) |
87.5 mg daily (N=6) |
125 mg daily (N=6) |
87.5 mg bid (N=6) |
| Cmax (ng/mL) | 95 (49) | 152 (30) | 277 (39) | 438 (40) | 506 (37) |
| Tmax (hours) | 2.3 (39) | 1.6 (42) | 2.1 (35) | 1.6 (42) | 1.3 (22) |
| AUC0−τ (ng*h/mL) | 834 (48) | 1050 (37) | 2200 (21) | 3420 (53) | 3350 (24) |
| Cavg (ng/mL) | 34.7 (48) | 87.5 (37) | 91.7 (21) | 142.5 (53) | 279.2 (24) |
| CL/F (L/h) | 53.3 (40) | 39.9 (36) | 41.1 (19) | 43.3 (39) | 27.2 (20) |
| Kel (1/h) | 0.0562 (22) | 0.0525 (11) | 0.0553 (7) | 0.0662 (28) | 0.0672 (12) |
|
| |||||
| T½ (h) | 12.3 | 13.2 | 12.5 | 10.5 | 10.3 |
CV: coefficient of variation; bid: twice daily; Cmax = maximum plasma concentration; Tmax = time to Cmax; AUC0−τ = area under the concentration from time 0 to τ where τ = dosing interval (12 or 24 hours); Cavg = AUC0−τ/τ; CL/F = Oral Clearance (Dose/AUC0−τ/bioavailability); T½ = harmonic mean half-life (0.693/mean kel)
Figure 2.
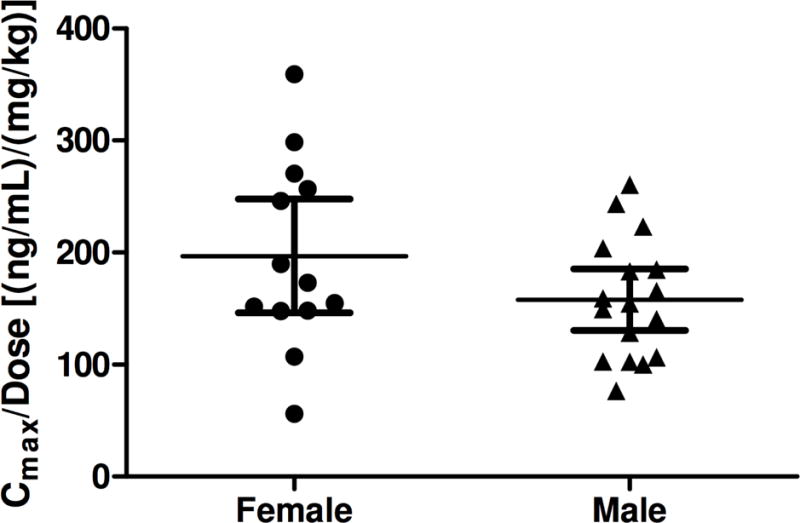
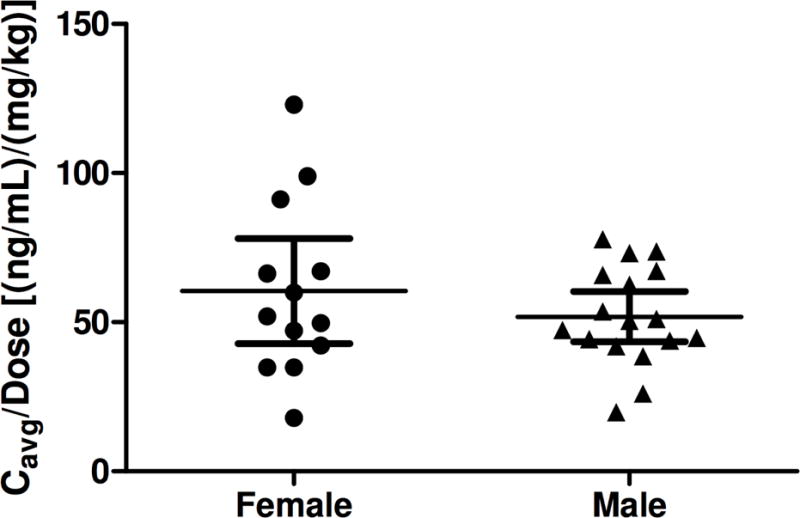
Individual subject and mean (95% confidence interval) dose-normalized udenafil Cmax (a) and Cavg (b) values following oral administration of udenafil for 5 days to male (N=17) and female (N=13).
The PK for DA-8164 was similar to that of udenafil. Plasma concentrations increased rapidly over 2 to 4 hours followed by a log-linear decrease over the remainder of the sampling period (Figure 1b). Mean profiles were again representative of individual subject profiles. After once-daily dosing, mean DA-8164 Cmax values increased 4.2-fold, and mean AUC0−τ values increased 4-fold when dose increased 3.3 times. After twice-daily dosing, mean DA-8164 Cmax and AUC0−τ values increased approximately proportionally with increase in dose; Cmax values increased 1.8-fold and mean AUC0−τ values increased 1.9-fold when dose increased 2.3 times. DA-8164 terminal phase half-life was approximately 12 to 15 hours (Table 4).
Table 4.
Summary comparison of DA-8164 pharmacokinetic parameters following oral administration of udenafil for five days
| Mean (%CV) by Treatment | |||||
|---|---|---|---|---|---|
|
| |||||
| 37.5 mg daily (N=6) |
37.5 mg bid (N=6) |
87.5 mg daily (N=6) |
125 mg daily (N=6) |
87.5 mg bid (N=6) |
|
| Cmax (ng/mL) | 61.3 (46) | 159 (52) | 227 (66) | 256 (13) | 293 (34) |
| Tmax (h) | 3.1 (51) | 2.1 (78) | 3.2 (30) | 3.8 (35) | 2.6 (36) |
| AUC0−τ (ng*h/mL) | 881 (43) | 1290 (55) | 2910 (65) | 3470 (14) | 2410 (24) |
| Cavg (ng/mL) | 36.7 (43) | 108 (55) | 121 (65) | 145 (14) | 201 (24) |
| kel (h) | 0.0474 (34) | 0.0491 (16) | 0.0464 (16) | 0.0480 (17) | 0.0564 (17) |
|
| |||||
| T½ (h) | 14.6 | 14.1 | 14.9 | 14.5 | 12.3 |
bid: twice daily; Cmax = maximum plasma concentration; Tmax = time to Cmax; AUC0−τ = area under the concentration from time 0 to τ where τ = dosing interval (12 or 24 hours); Cavg = AUC0−τ/τ; T½ = harmonic mean half-life (0.693/mean kel)
Of the 36 subjects who underwent exercise testing, 32 (89%) were able to reach maximal effort at both baseline and follow-up exercise testing (Table 5, Figure 3). In the short period of study there were no differences in the change in any exercise parameter from baseline to follow-up testing across dosing cohorts. Similarly there was no difference from baseline to follow-up testing within any individual cohort.
Table 5.
Comparison of exercise test results at baseline and follow-up
| Mean ± SD VO2 (mL/kg/min) by Dose Level | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Maximal VO2 at Maximum Exercise Efforta | N | All Subjects | N | 37.5 mg QD | N | 37.5 mg BID | N | 87.5 mg QD | N | 125 mg QD | N | 87.5 mg BID | N | Exercise Only | P-valueb |
| Baseline measurement | 34 | 28.5±5.8 | 6 | 24.6±6.9 | 6 | 30.4±6.2 | 6 | 28.4±6.2 | 5 | 28.6±3.0 | 5 | 28.0±5.2 | 6 | 30.6±6.2 | 0.542 |
| Follow-up measurement | 33 | 28.2±5.8 | 5 | 24.7±6.7 | 6 | 28.8±8.1 | 6 | 27.1±5.0 | 6 | 28.6±3.8 | 5 | 28.2±6.0 | 5 | 31.8±5.0 | 0.570 |
| Difference, FU – BL | 32 | −0.6±3.3 | 5 | −0.8±1.7 | 6 | −1.6±5.1 | 6 | −1.4±2.5 | 5 | 0.9±2.6 | 5 | 0.2±5.0 | 5 | −0.3±1.8 | 0.851 |
| p-value, paired T-test | 0.34 | 0.32 | 0.48 | 0.25 | 0.49 | 0.95 | 0.76 | ||||||||
|
| |||||||||||||||
| VO2 at Anaerobic Threshold | N | All Subjects | N | 37.5 mg QD | N | 37.5 mg BID | N | 87.5 mg QD | N | 125 mg QD | N | 87.5 mg BID | N | Exercise Only | P-valueb |
| Baseline measurement | 36 | 18.6±4.5 | 6 | 17.2±5.0 | 6 | 18.0±3.0 | 6 | 17.6±5.3 | 6 | 18.8±1.8 | 6 | 18.3±4.6 | 6 | 21.7±6.2 | 0.570 |
| Follow-up measurement | 34 | 18.2±4.4 | 5 | 16.3±2.0 | 6 | 18.1±3.0 | 6 | 16.5±4.8 | 6 | 20.0±3.2 | 6 | 16.5±5.2 | 5 | 22.4±5.5 | 0.135 |
| Difference, FU - BL | 34 | −0.3±2.6 | 5 | −0.5±4.1 | 6 | 0.1±1.0 | 6 | −1.1±1.9 | 6 | 1.2±1.9 | 6 | −1.7±2.1 | 5 | 0.1±3.8 | 0.469 |
| p-value, paired T-test | 0.47 | 0.81 | 0.79 | 0.21 | 0.18 | 0.11 | 0.95 | ||||||||
BL = baseline; FU = post-treatment follow-up (Day 5); Difference = FU-BL
BID = twice daily; QD = once daily; SD = standard deviation; VO2 = oxygen consumption
The maximum effort was achieved when the respiratory quotient at peak ≥ 1.1.
Parametric p-values were calculated by ANOVA. Non-parametric p-values were calculated by Kruskal-Wallis test.
Figure 3.
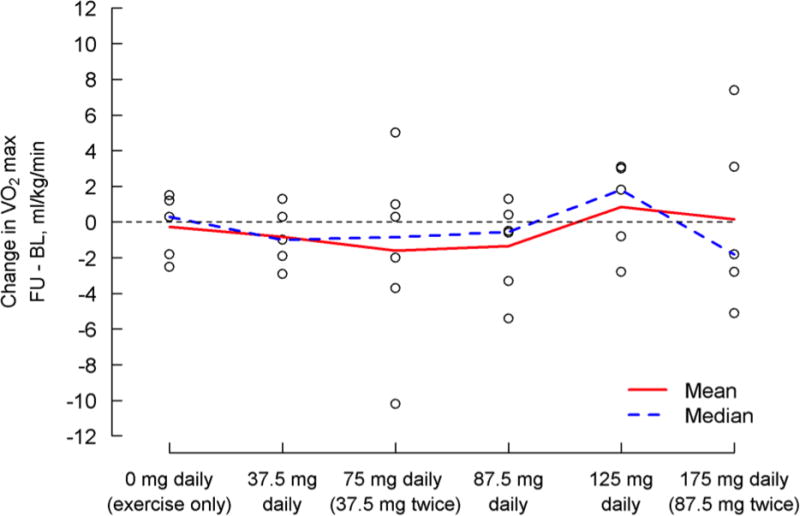
Change in maximal oxygen consumption (mL/kg/min) baseline to day five by treatment group. A positive change indicates improvement. Individual subject values are represented by the “o” on the figure.
Paired blood pool Doppler data were available for 27 of the 30 subjects, while paired tissue Doppler data were available for 26 subjects (Table 6, Figure 4). There were no significant differences in change scores across dosing cohorts for either blood pool or tissue Doppler based measures. However, there was a significant improvement from baseline to follow-up testing in blood pool MPI for those receiving 87.5 mg bid (0.55±0.14 to 0.41±0.08; p=0.04). This was not seen with tissue Doppler evaluation.
Table 6.
Comparison of ventricular performance at baseline and follow-up
| Blood Pool MPI | N | All Subjects | N | 37.5 mg QD | N | 37.5 mg BID | N | 87.5 mg QD | N | 125 mg QD | N | 87.5 mg BID | P-valueb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline measurement | 29 | 0.58±0.20 | 6 | 0.54±0.30 | 6 | 0.50±0.15 | 6 | 0.59±0.16 | 6 | 0.73±0.16 | 5 | 0.55±0.14 | 0.542 |
| Follow-up measurement | 28 | 0.52±0.17 | 5 | 0.50±0.19 | 6 | 0.49±0.087 | 6 | 0.51±0.16 | 5 | 0.70±0.20 | 6 | 0.41±0.078 | 0.570 |
| Difference, FU – BL | 27 | −0.059±0.13 | 5 | −0.052±0.17 | 6 | −0.003±0.10 | 6 | −0.076±0.14 | 5 | −0.054±0.18 | 5 | −0.12±0.090 | 0.851 |
| p-value, paired T-test | 0.031 | 0.52 | 0.95 | 0.25 | 0.54 | 0.044 | |||||||
|
| |||||||||||||
| Tissue Doppler MPI | N | All Subjects | N | 37.5 mg QD | N | 37.5 mg BID | N | 87.5 mg QD | N | 125 mg QD | N | 87.5 mg BID | P-valueb |
| Baseline measurement | 28 | 0.76±0.30 | 6 | 0.70±0.42 | 6 | 0.82±0.36 | 6 | 0.80±0.12 | 6 | 0.86±0.32 | 4 | 0.54±0.019 | 0.570 |
| Follow-up measurement | 28 | 0.71±0.20 | 5 | 0.64±0.21 | 6 | 0.73±0.23 | 6 | 0.72±0.14 | 5 | 0.82±0.27 | 6 | 0.64±0.17 | 0.135 |
| Difference, FU - BL | 26 | −0.056±0.19 | 5 | −0.052±0.40 | 6 | −0.089±0.14 | 6 | −0.089±0.13 | 5 | −0.074±0.14 | 4 | 0.060±0.068 | 0.469 |
| p-value, paired T-test | 0.16 | 0.78 | 0.18 | 0.16 | 0.31 | 0.18 | |||||||
MPI = myocardial performance index
BL = baseline; FU = post-treatment follow-up (Day 5); Difference = FU-BL
BID = twice daily; QD = once daily; SD = standard deviation; VO2 = oxygen consumption
The maximum effort was achieved when the respiratory quotient at peak ≥ 1.1.
Parametric p-values were calculated by ANOVA. Non-parametric p-values were calculated by Kruskal-Wallis test.
Figure 4.
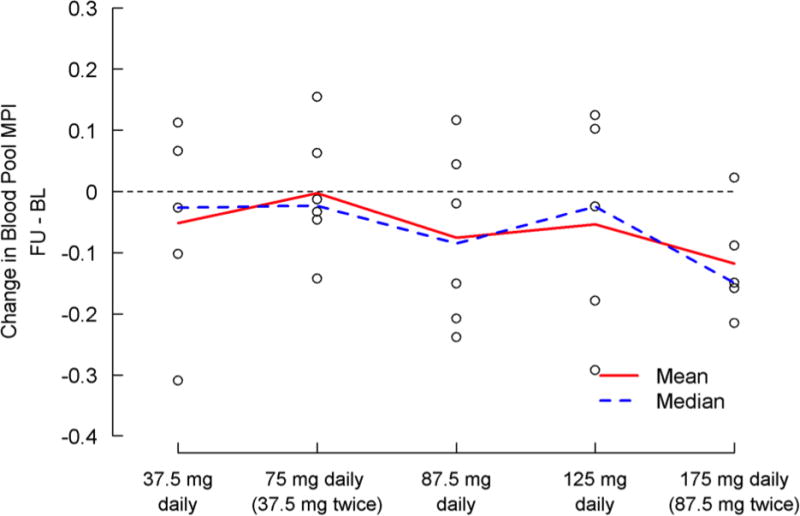
Change in blood pool Myocardial Performance Index baseline to day five by treatment group. A negative change indicates improvement. Individual subject values are represented by the “o” on the figure.
Paired PAT-derived vascular function data were available in 27 of the 30 subjects (Table 7, Figure 5). There was no evidence of significant differences in lnRHI, the primary vascular outcome measure, or AI among treatment groups. Similarly, there were no discernible differences between paired baseline and follow-up measures of lnRHI or AI in any dosing cohort.
Table 7.
Comparison of peripheral vascular function at baseline and follow-up
| Natural log of Reactive Hyperemic Index | N | All Subjects | N | 37.5 mg QD | N | 37.5 mg BID | N | 87.5 mg QD | N | 125 mg QD | N | 87.5 mg BID | P-valueb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline measurement | 28 | 0.52±0.28 | 6 | 0.48±0.31 | 6 | 0.60±0.30 | 6 | 0.41±0.25 | 6 | 0.63±0.37 | 4 | 0.49±0.12 | 0.704 |
| Follow-up measurement | 28 | 0.49±0.28 | 6 | 0.51±0.26 | 6 | 0.53±0.29 | 5 | 0.45±0.31 | 6 | 0.52±0.34 | 5 | 0.40±0.29 | 0.945 |
| Difference, FU – BL | 27 | −0.02±0.30 | 6 | 0.03±0.47 | 6 | −0.07±0.17 | 5 | 0.07±0.22 | 6 | −0.10±0.38 | 4 | −0.03±0.20 | 0.902 |
| p-value, paired T-test | 0.72 | 0.89 | 0.40 | 0.52 | 0.55 | 0.82 | |||||||
|
| |||||||||||||
| Augmentation Index adjusted to HR 75 | N | All Subjects | N | 37.5 mg QD | N | 37.5 mg BID | N | 87.5 mg QD | N | 125 mg QD | N | 87.5 mg BID | P-valueb |
| Baseline measurement | 28 | −1.62±12.60 | 6 | −3.16±17.40 | 6 | 6.12±9.38 | 6 | −6.59±9.50 | 6 | 2.03±8.74 | 4 | −8.97±15.09 | 0.280 |
| Follow-up measurement | 28 | −2.18±13.91 | 6 | −0.62±23.82 | 6 | 2.89±7.11 | 5 | −12.71±9.11 | 6 | 1.90±8.65 | 5 | −4.49±12.02 | 0.373 |
| Difference, FU - BL | 27 | −0.12±7.32 | 6 | 2.54±11.98 | 6 | −3.23±5.84 | 5 | −3.04±4.66 | 6 | −0.13±5.95 | 4 | 4.23±2.67 | 0.416 |
| p-value, paired T-test | 0.64 | 0.63 | 0.23 | 0.22 | 0.96 | 0.05 | |||||||
BL = baseline; FU = post-treatment follow-up (Day 5); Difference = FU-BL
BID = twice daily; QD = once daily; SD = standard deviation; VO2 = oxygen consumption
The maximum effort was achieved when the respiratory quotient at peak ≥ 1.1.
Parametric p-values were calculated by ANOVA. Non-parametric p-values were calculated by Kruskal-Wallis test.
Figure 5.
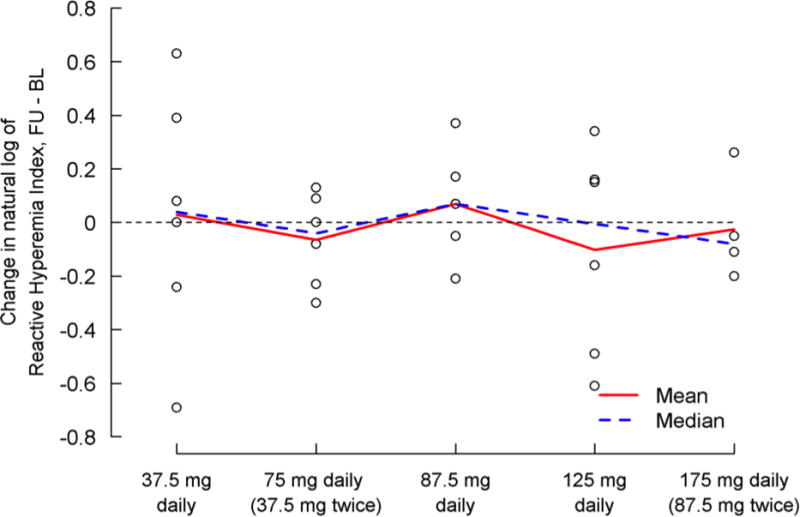
Change in the natural log of Reactive Hyperemia Index baseline to day five by treatment group. A positive change indicates an improvement. Individual subject values are represented by the “o” on the figure.
Discussion
We report the first phase I/II study of a PDE5 inhibitor in subjects with Fontan physiology. In this study we found that udenafil was well-tolerated at all trialed doses, and that the dosing regimen of 87.5 mg bid (175 mg/day) provided the highest plasma drug concentration and a suggestion of improvement in myocardial performance. Plasma udenafil concentration was noted to increase rapidly until Tmax followed by a log-linear decrease in concentration over time. While no statistically significant improvements were noted in other clinical testing, the duration of the trial was short and the primary purpose of this study was to assess safety. These short-term data suggest that udenafil would be suitable for testing in a phase III clinical trial, and that the dose of 87.5 mg bid would be the most appropriate.
This study adds to the growing list of studies investigating the potential benefit of modulators of PVR for treatment of those with single ventricle (Fontan) circulation-related heart failure10–14,23–26. PDE5 inhibitors have been the most frequently studied of the PVR modulators, and the results from those studies have been mixed. A number of studies have demonstrated acute improvements in exercise performance, ejection fraction, and cardiac output, while the only reported randomized clinical trial showed improvements in some sub-groups during sub-maximal exercise, but did not detect an improvement in maximal oxygen consumption10–14. More recently, investigators have evaluated the effect of Bosentan and inhaled prostacyclin on measures of exercise performance23–25. The results of these studies were also mixed; one demonstrating a possible benefit and the other demonstrating no evidence of benefit. Interestingly, while the improvement noted in the MPI in this study was modest, it is in keeping with results from other studies evaluating of PDE5 inhibitors in patients with Fontan physiology10,11,26.
The impact of occult liver dysfunction is an important factor when considering drug dosing in those who have undergone the Fontan operation. A recent paper evaluated the differences between udenafil metabolism in healthy subjects as compared to those with mild or moderate liver dysfunction as characterized by Child-Pugh class A and class B respectively27. While there was no need for a dose adjustment in those with mild impairment, there is suggestion that a 25% dose reduction might be appropriate in those with moderate hepatic impairment (class B). However, it should be noted that class B hepatic impairment, designated as “moderate” in this study, corresponds to significantly more advanced hepatic impairment than that which is typically seen in those with Fontan-associated liver disease. Further, we excluded potential subjects with known liver cirrhosis from this study so the impact of hepatic impairment on our results was likely minimal.
Although the literature regarding modulators of PVR in the Fontan population has continued to grow, there has yet to be an adequately powered long-term study of pulmonary vasodilators in this cohort. Of the classes of pulmonary vasodilators available, PDE5 inhibitors have the most acceptable safety profile and are therefore the most appropriate for a large-scale clinical trial. Udenafil has an established record of safety in the adult population with erectile dysfunction and was very well tolerated in this phase I/II clinical trial15,16. In this study 24 of the 30 subjects exposed to study drug reported adverse events thought to have a plausible connection to study drug, but these events were well tolerated and did not require the cessation of study drug. These results are similar to those reported in in a cohort of 40 healthy adult male subjects who were part of a previous safety, tolerability, and pharmacokinetic study17. Importantly, no drug-related serious adverse events were reported. The PK profile demonstrated in this study is similar to what has been seen in the adult population and suggests that twice daily dosing may be most appropriate to achieve the highest steady state drug concentration.
While the results of this study are adequate for dose selection for a phase III clinical trial, this phase I/II study was, by design, not powered to detect differences in the results of clinical testing from baseline to follow-up. Therefore, it is not possible to discern the potential clinical benefit of udenafil in the Fontan population from these data. Additionally, although the 87.5 mg twice daily dose achieved the highest area under the curve and the highest peak plasma concentration, higher doses were purposefully not tested. Dose selection was based in part on data from adult populations suggesting that higher doses were not well tolerated due to side effects, and therefore would not be practical for use as a daily dose for chronic administration.
Conclusion
This study demonstrates that udenafil was safe and well tolerated at all studied doses in male and female adolescents who had undergone Fontan palliation. Following oral administration, udenafil was rapidly absorbed with time to maximum concentration ranging from 1.3 to 2.3 hours. The measured half-life of udenafil was 10 to 13 hours, while that for DA-8164 was 12 to 15 hours. The dosing cohort that received 87.5 mg bid achieved the highest udenafil and DA-8164 plasma levels. With a 5-day treatment period and small sample size, the only suggestion of improvement in clinical measures was that noted in the myocardial performance index for those in the cohort that received 87.5 mg bid. These data provide support for pursuing a phase III trial and suggest that the 87.5 mg bid regimen may be the most appropriate dose to study.
Highlights.
Udenafil is a novel PDE-5 inhibitor with pharmacokinetics suggesting twice daily dosing.
There were no drug-related serious adverse events in a cohort of adolescent’s who had undergone the Fontan operation and were exposed to a five-day course of udenafil.
A dose of 87.5 mg twice daily achieved the highest maximal and average serum concentration and was associated with a suggestion of improvement in myocardial performance.
Acknowledgments
Funding for this project was by the National Heart, Lung, and Blood Institute and by Mezzion Pharma Co. Ltd. (Seoul, South Korea)
Abbreviations
- AE
adverse event
- AI
augmentation index
- AUC
area under the curve
- BID
twice daily
- Cavg
average concentration
- Cmax
maximal concentration
- mg
milligrams
- mL
milliliters
- MPI
myocardial performance index
- ng
nanograms
- PAT
peripheral arterial tonometry
- PDE5
phosphodiesterase type 5
- PHN
Pediatric Heart Network
- PK
pharmacokinetic
- PVR
pulmonary vascular resistance
- QC
quality control
- RHI
reactive hyperemia index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration:
ClinicalTrials.gov Identifier: NCT02201342
ClinicalTrials.gov URL: (https://clinicaltrials.gov/ct2/show/NCT02201342?term=udenafil&rank=12)
Disclosure: DJG, VZ, BHG, SC, MSH, EAR, EM, SM, SCM, KRS, RMP, MS, JRK, SMP report no relevant disclosures. TMD and JLY are consultants for Mezzion Pharma Co. Ltd. All authors have approved the final version of this manuscript.
Disclosures:
The views expressed are those of the authors and do not necessarily reflect official positions of the National Heart, Lung, and Blood Institute or the NIH.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971 May;26(3):240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreutzer G, Galindez E, Bono H, De Palma C, Laura JP. An operation for the correction of tricuspid atresia. The Journal of thoracic and cardiovascular surgery. 1973 Oct;66(4):613–621. [PubMed] [Google Scholar]
- 3.Averin K, Hirsch R, Seckeler MD, Whiteside W, Beekman RH, 3rd, Goldstein BH. Diagnosis of occult diastolic dysfunction late after the Fontan procedure using a rapid volume expansion technique. Heart. 2016 Feb 25; doi: 10.1136/heartjnl-2015-309042. [DOI] [PubMed] [Google Scholar]
- 4.Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart failure clinics. 2014 Jan;10(1):105–116. doi: 10.1016/j.hfc.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. The New England journal of medicine. 2005 Nov 17;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 6.Humpl T, Reyes JT, Holtby H, Stephens D, Adatia I. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005 Jun 21;111(24):3274–3280. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 7.Behling A, Rohde LE, Colombo FC, Goldraich LA, Stein R, Clausell N. Effects of 5′-phosphodiesterase four-week long inhibition with sildenafil in patients with chronic heart failure: a double-blind, placebo-controlled clinical trial. Journal of cardiac failure. 2008 Apr;14(3):189–197. doi: 10.1016/j.cardfail.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circulation. Heart failure. 2011 Jan;4(1):8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 9.Nagayama T, Hsu S, Zhang M, et al. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with preexisting advanced hypertrophy caused by pressure overload. Journal of the American College of Cardiology. 2009 Jan 13;53(2):207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg DJ, French B, Szwast AL, et al. Impact of sildenafil on echocardiographic indices of myocardial performance after the Fontan operation. Pediatric cardiology. 2012 Jun;33(5):689–696. doi: 10.1007/s00246-012-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circulation. Cardiovascular imaging. 2014 Mar;7(2):265–273. doi: 10.1161/CIRCIMAGING.113.001243. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg DJ, French B, McBride MG, et al. Impact of oral sildenafil on exercise performance in children and young adults after the fontan operation: a randomized, double-blind, placebo-controlled, crossover trial. Circulation. 2011 Mar 22;123(11):1185–1193. doi: 10.1161/CIRCULATIONAHA.110.981746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giardini A, Balducci A, Specchia S, Gargiulo G, Bonvicini M, Picchio FM. Effect of sildenafil on haemodynamic response to exercise and exercise capacity in Fontan patients. European heart journal. 2008 Jul;29(13):1681–1687. doi: 10.1093/eurheartj/ehn215. [DOI] [PubMed] [Google Scholar]
- 14.Tunks RD, Barker PC, Benjamin DK, Jr, et al. Sildenafil exposure and hemodynamic effect after Fontan surgery. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014 Jan;15(1):28–34. doi: 10.1097/PCC.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, Kim SW, Yang DY, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. European urology. 2011 Aug;60(2):380–387. doi: 10.1016/j.eururo.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Paick JS, Kim SW, Yang DY, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. The journal of sexual medicine. 2008 Apr;5(4):946–953. doi: 10.1111/j.1743-6109.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim BH, Lim HS, Chung JY, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. British journal of clinical pharmacology. 2008 Jun;65(6):848–854. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. Journal of the American College of Cardiology. 2008 Jul 8;52(2):99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 19.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function–a study in normals and dilated cardiomyopathy. Journal of cardiology. 1995 Dec;26(6):357–366. [PubMed] [Google Scholar]
- 20.Harada K, Tamura M, Toyono M, Yasuoka K. Comparison of the right ventricular Tei index by tissue Doppler imaging to that obtained by pulsed Doppler in children without heart disease. The American journal of cardiology. 2002 Sep 1;90(5):566–569. doi: 10.1016/s0002-9149(02)02541-9. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein BH, Golbus JR, Sandelin AM, et al. Usefulness of peripheral vascular function to predict functional health status in patients with Fontan circulation. The American journal of cardiology. 2011 Aug 1;108(3):428–434. doi: 10.1016/j.amjcard.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 22.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008 May 13;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (Treatment With Endothelin Receptor Antagonist in Fontan Patients, a Randomized, Placebo-Controlled, Double-Blind Study Measuring Peak Oxygen Consumption) study. Circulation. 2014 Dec 2;130(23):2021–2030. doi: 10.1161/CIRCULATIONAHA.113.008441. [DOI] [PubMed] [Google Scholar]
- 24.Schuuring MJ, Vis JC, van Dijk AP, et al. Impact of bosentan on exercise capacity in adults after the Fontan procedure: a randomized controlled trial. European journal of heart failure. 2013 Jun;15(6):690–698. doi: 10.1093/eurjhf/hft017. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes J, Ubeda-Tikkanen A, Clair M, et al. Effect of inhaled iloprost on the exercise function of Fontan patients: a demonstration of concept. International journal of cardiology. 2013 Oct 3;168(3):2435–2440. doi: 10.1016/j.ijcard.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabri MR, Zolfi-Gol A, Ahmadi A, Haghjooy-Javanmard S. Effect of Tadalafil on Myocardial and Endothelial Function and Exercise Performance After Modified Fontan Operation. Pediatric cardiology. 2016 Jan;37(1):55–61. doi: 10.1007/s00246-015-1238-x. [DOI] [PubMed] [Google Scholar]
- 27.Kim A, Lee J, Shin D, et al. Population pharmacokinetic analysis to recommend the optimal dose of udenafil in patients with mild and moderate hepatic impairment. British journal of clinical pharmacology. 2016 Aug;82(2):389–398. doi: 10.1111/bcp.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]


