Abstract
The usefulness of fluorescence in studying protein motions derives from its sensitivity, kinetic resolution, and compatibility with both live cells and physiological assays. Recent advances in microscopy and membrane protein purification have permitted the observation of fluorescence changes that accompany the functional transitions of complex eukaryotic membrane proteins. These techniques rely on probes that can clearly report the environmental changes of specific residues, but most commonly available side-chain-reactive probes are not well suited for this purpose. Here, we introduce a red Cys-reactive probe, aminophenoxazone maleimide (APM), designed with improved chemical and spectral properties for reporting protein conformational change. APM is compact, uncharged, and has a short linker between probe and protein, all of which ensure that it can closely track the motions of the side chain to which it is attached. It undergoes large polarity-dependent changes in Stokes shift, as well as large bathochromic shifts in both excitation maximum (from 521 nm in toluene to 598 nm in water) and emission maximum (580 nm to 633 nm). These polarity-dependent spectral changes offer a potentially simple means of relating fluorescence to local structure and motion, although they are partially offset by some complicating factors in APM fluorescence. We find that, like a rhodamine maleimide, APM senses the conformational changes underlying voltage sensing in the Shaker potassium channel, and it is superior at a site that shows limited reactivity to the rhodamine. The spectral characteristics of APM can also report subtle differences between aqueous positions in purified preparations of the β2 adrenergic receptor.
Keywords: fluorescene, membrane proteins, protein dynamics
Fluorescence has long been used to characterize the conformational changes that underlie protein function. The intrinsic fluorescence of tryptophan is sensitive to its surroundings, a characteristic that has made it an invaluable and popular tool for studying protein motions in vitro. Side-chain-reactive environment-sensitive fluorophores, such as the dansyl group, have similarly enjoyed widespread use, but, like tryptophan, their excitation and emission spectra overlap significantly with cellular autofluorescence, dramatically limiting their usefulness in live cells. This overlap poses a problem for many eukaryotic membrane proteins, which cannot be easily assayed outside of their native environments.
Recent advances in microscopy technology have permitted the observation of fluorescence changes (ΔF) that accompany the functional transitions of Cys-labeled membrane proteins in live cells (1). This technology has proved particularly useful with voltage-gated ion channels, and, with concurrent electrophysiological recording, it has allowed analysis of the motions of specific residues that accompany voltage sensing. Similarly, advances in eukaryotic membrane protein purification and labeling have made fluorescence attractive for in vitro study of the agonist-induced signaling of G-protein coupled receptors (2). Progress in these techniques has extended from initial simple ensemble measurements to polarization studies (3), lifetimes (4), resonance energy transfer (5, 6), and single-molecule studies of functional membrane proteins (7, 8).
An important limitation of these techniques is that available side-chain-reactive probes commonly used in these studies (xanthenes such as rhodamines, fluoresceins, and Alexa dyes) are poorly suited to report protein conformational change. Xanthenes have the advantages of being bright (i.e., high extinction coefficient, ε, and quantum yield, Qf) and of being readily available as Cys-reactive derivatives, but they have significant drawbacks, including (i) relatively long, flexible linkers between the probe and protein, which raise questions about whether the probe motions faithfully mirror the motions of the residue to which it is attached; (ii) multiple charges and relatively large surface areas, which can perturb local structure and motion, as well as inhibit labeling at certain positions; (iii) small Stokes shifts, which may lead to complicating probe-probe interactions in multimeric proteins; and, most important, (iv) complex and poorly defined sets of environmental factors that give rise to their ΔF values during conformational change. Studies of fluorescein have shown that the factors affecting its excitation and emission spectra are complex and all but uninterpretable in terms of protein motion (9). The environmental shifts of other xanthenes have not been characterized as rigorously, although some studies suggest they are similarly intractable (10–12).
Correlations of xanthene ΔF measurements with concurrently recorded physiological properties, such as gating charge displacement in ion channels, have convincingly established that xanthene fluorescence can report both the positions and the kinetics of conformational changes associated with functional transitions (1, 10). However, the chemical and spectral properties of the xanthenes have hindered attempts to characterize the physical nature of these protein motions. A fluorophore with simple and well characterized excitation and emission shifts (and, in particular, one whose fluorescence is attuned to a single environmental property) would permit analog readouts of local structure and dynamics, and could significantly increase the capacity of this fluorescence methodology to describe conformational change.
Pioneering work by Weber and others (e.g., ref. 13; reviewed in ref. 14) demonstrated that exceptionally environment-sensitive fluorophores can be engineered by placement of electron donating and accepting groups on opposite ends of an aromatic ring system. This approach has been used for a number of environment-sensitive probes, such as dansyl, ANS, and Aladan, an amino acid that we recently described (15). Here, we exploit this donor-acceptor strategy in a ring system with an extended π-conjugation, creating a Cys-reactive probe whose fluorescence falls well outside the range of most cellular autofluorescence. This fluorophore, aminophenoxazone maleimide (APM), has advantages over the commonly used xanthenes: it has a shorter linker between probe and Cys, it is uncharged in the ground state and has a smaller surface area, and it shows significantly larger Stokes shifts. APM also shows large polarity-dependent bathochromic (or red) shifts in its excitation and emission spectra that may simplify the interpretation of protein ΔF values. We find that APM can label a residue in the Shaker potassium channel that shows a poor signal when labeled with tetramethylrhodamine maleimide (TMRM), and that it can report subtle differences between positions in purified preparations of the β2 adrenergic receptor (β2AR).
Materials and Methods
APM Synthesis. AP methylamine (4). Resorcinol 2 (110 mg, 0.46 mmol, prepared from 1 as in Fig. 1) and 4-nitroso-N,N-dimethylaniline (3, 110 mg, 0.73 mmol) were heated to 80°C in 5 ml of dry isopropanol (iPrOH). ZnCl2 (64 mg, 0.47 mmol) was added in two parts over 1 h, and the reaction heated for 2 h more. It was quenched with 10 ml of 0.5 M EDTA (pH 8) and 50 ml of ethyl acetate. The organic layer was washed with brine and then dried and concentrated. It was taken up in 2 ml of trifluoroacetic acid (TFA) with 100 μl of thioanisole, stirred for 30 min, and precipitated with 50 ml of cold ether. The precipitate was washed twice with ether and dried under N2 to give 60 mg of purple solid 4 as its TFA salt (34% over two steps). For 1H NMR (CD3OD, 400 MHz), shifts were: δ 7.89 (d, 1 H), 7.68 (dd, 1 H), 7.23 (s, 1 H), 7.15 (d, 1 H), 6.97 (s, 1 H), 4.57 (s, 2 H), and 3.56 (s, 6 H). MS for C15H16N3O2 (MH)+ calculated and found: 270.3.
Fig. 1.
Synthesis of APM. Reaction details and characterizations are given in Materials and Methods.
APM (5). The TFA salt of 4 (85 mg, 0.22 mmol) and maleic anhydride (23 mg, 0.23 mmol) were stirred in 5 ml of dry AcOH for 3 h. The solvent was removed under high vacuum, and the residue was resuspended in 2 ml of dimethylformamide (DMF). Precipitation with 50 ml of cold ether was followed by ether wash until no more AcOH remained gave 75 mg (93%) of the amic acid as a purple solid. Of this acid, 60 mg (0.16 mmol) was dissolved in 2 ml of dry DMF. ZnCl2 (44 mg, 0.32 mmol) was added, followed by 18 ml of dry benzene, and the reaction was heated to 75°C. (SiMe3)2NH (132 μl, 0.64 mmol) was added via syringe, and the reaction was heated to reflux for 3 h. It was cooled and quenched with 5 ml of EDTA solution, 10 ml of brine, and 100 ml of ethyl acetate. The organic layer was separated and washed in 2 × 10 ml of 200 mM Mes (pH 6) then brine. It was dried and concentrated and then resuspended in 50 ml of dry acetone and filtered to give 47 mg (84%) of purple oil. Further purification by HPLC on a C18 column, eluting with a linear gradient of 30–45% CH3CN over 30 min with 0.1% TFA, gave APM as a purple powder after lyophilization. For 1H NMR (DMSO-d6, 400 MHz), the shifts were: δ 7.63 (d, 1 H), 7.13 (s, 2 H), 7.09 (dd, 1 H), 6.79 (s, 1 H), 6.32 (s, 1 H), 6.26 (s, 1H), 4.85 (s, 2 H), and 3.22 (s, 6 H). MS for C19H16N3O4 (MH)+ calculated and found: 350.3.
Steady-State Fluorescence. Emission and excitation spectra were recorded from 100 nM solutions of HPLC-purified APM-SEt on a Fluoromax-3 (Jobin Yvon, Longjumeau, France) instrument. Spectra were corrected for variations in the detector efficiency and lamp intensity with files provided by the manufacturer. Quantum yields were determined relative to sulforhodamine 101 in EtOH (Qf = 1.0) (16), with corrections for optical density at the exciting wavelength (515 nm) and differences in refractive index. For Stokes shifts, λ2 corrections to the intensity were used in wavelength/wavenumber conversion (14).
Lifetime Measurements. Lifetimes were measured on a time-correlated single-photon counting instrument built around a LSM 510 laser-scanning microscope (Zeiss). Air-exposed APM-SEt solutions (5 μM) were excited by means of two-photon excitation by using a mode-locked Ti:Sapphire laser tuned to 850 nm and detected by using ultra-fast R3809U MCP-PMTs (Hamamatsu, Middlesex, NJ), with an IR-blocking filter to remove excitation light. Decay rates were fitted with origin software.
Voltage-Clamp Fluorometry. Molecular biology and two electrode voltage-clamp fluorometry were performed as described (1). Oocytes were labeled with 5 μM APM in a high-potassium solution (92 mM KCl/0.75 mM CaCl2/1 mM MgCl2/10 mM Hepes, pH 7.5) for 30 min on ice in the dark. For APM, light was filtered with an HQ 535/50 exciter and a 565LP dichroic (both from Chroma Technology, Rockingham, VT), as well as a 3RD/600–620 emitter (Omega Engineering, Stamford, CT).
β2AR Fluorescence. Modified β2AR was expressed in Sf9 cells, solubilized, and purified by using methods described in ref. 17. To specifically label Cys-265 in the cytoplasmic end of transmembrane segment 6 (TM6), we used a modified β2AR in which Cys-378 and Cys-406 were both mutated to Ser (β2ARC378S,C406S). Cys-265 has been shown to be the most reactive Cys in β2ARC378S,C406S, and it is readily labeled with maleimide probes (18). To specifically label position Ala-271 in TM6, we generated a modified receptor with A271C and C265A mutations in the β2ARC378S,C406S background. Purified receptor (1 μM) was labeled with 1 μM APM for 1 h on ice, quenched with 1 mM Cys, and separated from unincorporated dye by Sephadex G50 chromatography. Spectra were recorded from 50 nM β2AR in dodecylmaltoside micelles on a Jobin Yvon Fluoromax-3 instrument.
Results
Synthesis and Fluorescence Characterization of APM. APM was synthesized by the zinc-mediated condensation of resorcinol 2 and nitrosoaniline 3 (Fig. 1), followed by maleimide formation with a mild two-step protocol (19). This route is amenable to synthesis of other AP derivatives, such as the amine-reactive succinimidyl ester (data not shown).
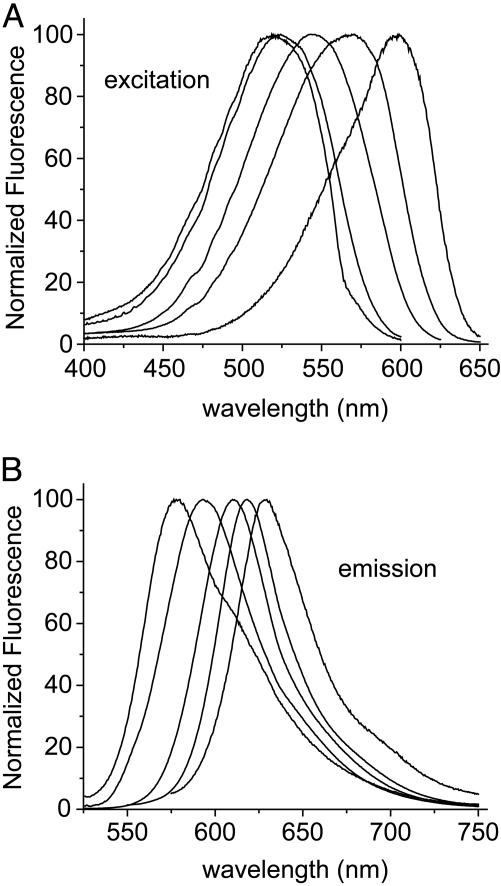
APM-SEt, the ethanethiol adduct of APM, was used to characterize its spectral environment sensitivity in organic solvents (Table 1 and Fig. 2). APM-SEt is soluble in solvents ranging from toluene to water, although it is only marginally soluble in the most polar or nonpolar solvents. Excitation spectra do not differ appreciably from absorption spectra (data not shown) and comprise single broad peaks with maxima shifting from 520 nm to 598 nm with increasing solvent polarity. The molar extinction coefficient ε does not vary significantly by solvent.
Table 1. Spectral properties of the APM-ethanethiol adduct in solvents of increasing polarity.
| Solvent | Extinction coefficient (ε) M-1·cm-1 | Excitation maximum, nm (λexc) | Emission maximum, nm (λem) | Quantum yield* (Qf) | Lifetime†, ns (τ) | Stokes shift‡, cm-1 (nm) |
|---|---|---|---|---|---|---|
| Toluene | — | 521 | 580 | 0.10 | 0.43, 1.72 a2/a1 = 0.082 | -2,986 (95) |
| Dioxane | — | 523 | 583 | 0.17 | 1.47 | -2,972 (94) |
| Ethyl acetate | 47,000 | 526 | 596 | 0.49 | 2.31 | -2,892 (94) |
| Tetrahydrofuran | — | 535 | 602 | 0.44 | 2.20 | -2,810 (92) |
| Acetone | 51,000 | 540 | 609 | 0.91 | 4.24 | -2,727 (91) |
| CH3CN | 47,000 | 553 | 614 | 0.78 | 3.53 | -2,627 (90) |
| DMSO | 48,000 | 563 | 630 | 0.45 | 2.05 | -2,546 (92) |
| EtOH | 52,000 | 565 | 621 | 0.59 | 2.14 | -2,191 (78) |
| MeOH | — | 572 | 622 | 0.34 | 1.58 | -2,152 (77) |
| H2O | — | 598 | 633 | 0.05 | 0.41, 2.01 a2/a1 = 0.67 | -1,858 (72) |
Fluorescence quantum yields determined relative to sulforhodamine 101 in EtOH (16).
a2/a1 is the amplitude ratio of decay rates for solvents with lifetimes fit by two exponentials.
Stokes shift calculated as difference in average frequency, v̄em - v̄exc, with equivalent wavelength values given in parentheses.
Fig. 2.
Corrected and normalized steady-state fluorescence excitation (A) and emission (B) spectra of the ethanethiol adduct of APM in (from left to right) toluene, ethyl acetate, acetonitrile, ethanol, and water.
Emission maxima fall well into the red region of the spectrum, varying with increasing polarity from 580 nm to 633 nm. These kinds of bathochromic shifts are typical of probes in which the solvent interacts more strongly with the excited state than with the ground state (20). They are also consistent with semiempirical calculations on the parent AP compound, showing an increase in dipole moment accompanying the S0→S1 transition (21). The increase in Stokes shift correlates well with increasing dielectric constant of the medium, as would be expected for a dipolar molecule such as APM (20). As with other carbonyl-containing fluorophores, there is a complicating effect in water and alcohols due to hydroxyl hydrogen bonding to the carbonyl oxygen, which leads to excitation and emission at lower energies (13, 15). None of the excitation or emission properties vary in water between pH 3 and 10 (data not shown); this insensitivity to pH may be particularly important at the membrane, where changes in membrane potential cause transient changes in ionic composition and pH (22).
Consistent with studies of the AP parent compound (23), lifetimes and quantum yields vary together with solvent polarity, with longest lifetimes and largest yields in solvents of intermediate polarity, such as acetone (Table 1). Lifetimes can be fit with single exponentials, except for toluene and water, which require two exponential terms. This complexity may reflect the presence of more than one emitting species, possibly due to aggregation or other effects arising from the limited solubility of APM-SEt in these solvents. Consistent with this idea, emission spectra in toluene and water also have more pronounced shoulders than in solvents of intermediate polarity, and these shoulders are not apparent in the aqueous environment surrounding the TM6 helix of β2AR (described below).
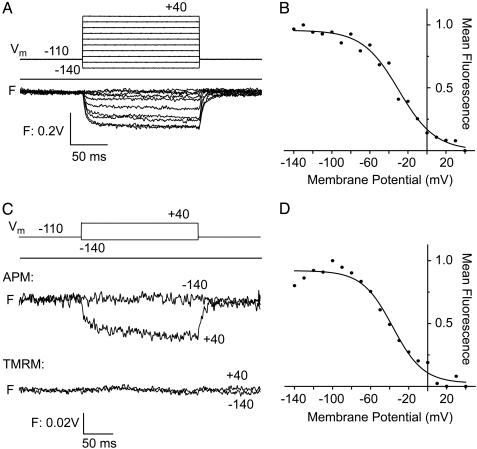
Fluorescence Measurement of APM-Labeled Membrane Proteins. APM was used to monitor changes in the environment surrounding the voltage-sensing S4 segment of the Shaker potassium channel expressed in Xenopus oocytes. Channels labeled at a Cys substituted at Ala-359, an accessible residue in the S3–S4 linker immediately before the N terminus of S4 (24), changed 600- to 620-nm bandpass emission over the voltage of activation (Fig. 3A), as shown earlier with TMRM-labeled channels (25, 26). Therefore, APM can report on a known membrane protein functional rearrangement in a live cell. Channels were also labeled at a Cys substituted at Leu-361, a residue at the N terminus of S4 that lies on its hydrophobic face. Mutations on this face have a low impact on channel function, suggesting that it faces lipid (27, 28), a model supported by structural work on the bacterial channel KvAP (29, 30). An APM label at position 361 showed a decrease in 600- to 620-nm bandpass emission with membrane depolarization (Fig. 3B) under conditions in which oocytes from the same batch labeled with TMRM showed no ΔF (Fig. 3B). The absence of a TMRM ΔF may be due to either low reactivity of the Cys at this position or to the fact that the environmental change at this site has only a weak influence on TMRM fluorescence. Given that both probes employ maleimides, either the smaller neutral APM has greater access to this site, or APM is more sensitive to the local environmental changes. Further experiments will be required to distinguish between these possibilities.
Fig. 3.
Voltage-clamp fluorometry of Xenopus oocytes expressing Shaker A359C or L361C channels. Positions of these residues in S4 are described in ref. 26. (A) Fluorescence emission (F) of Shaker A359C labeled with APM according to the voltage-step protocol shown above, with membrane potentials (Vm) in mV. (B) Fluorescence–voltage (F–V) relations of APM-labeled Shaker A359C, using normalized mean fluorescence of traces shown in A. Solid line is a Boltzmann curve fit, with a midpoint (Vh) of –29 mV. (C) Fluorescence emission of Shaker L361C labeled with APM (Upper) or TMRM (Lower) according to the voltage-step protocol shown above. (D) F–V relations of APM-labeled Shaker L361C with normalized mean fluorescence obtained with voltage protocol shown in A. For this mutant, Vh = –36 mV.
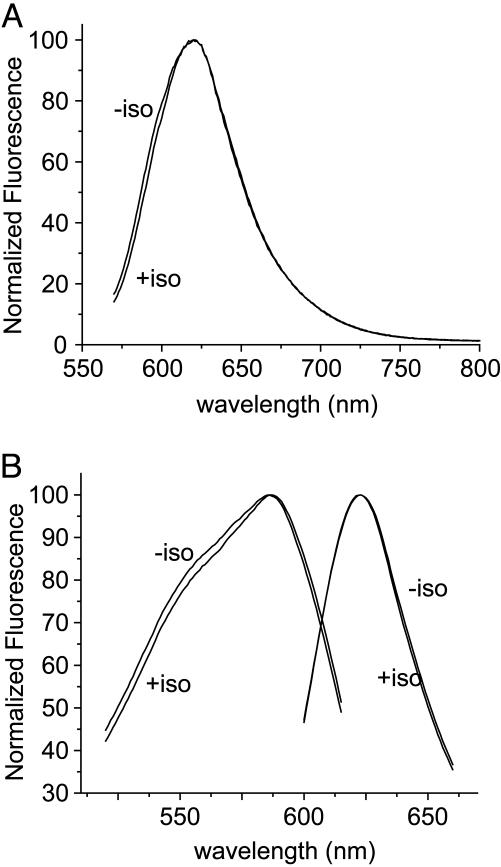
APM was also used to characterize the environments around two exposed positions near the cytoplasmic end of TM6 of β2AR, with and without addition of the agonist isoproterenol (Fig. 4). Receptors labeled with APM at Cys-265 have an emission maximum of 620 nm, whereas those labeled at Cys-271 are consistent with greater aqueous exposure, with an emission maximum of 623 nm. Addition of isoproterenol has little effect on the APM at Cys-265, whereas the Cys-271 probe undergoes a small (<2 nm) shift in excitation maximum. The cytoplasmic end of TM6 is known to undergo structural changes during activation by agonists (4, 18, 31, 32); agonist-induced changes in lifetime and bandpass intensity are observed for fluorescein-labeled Cys-265 (4, 18), and large agonist-induced increases in bandpass intensity are observed with TMRM (32). One interpretation of these observations is that the TMRM fluorescence increases as Cys-265 moves away from a quenching residue in another domain, whereas the immediate aqueous environment of Cys-265 remains unchanged, as determined by the APM spectra.
Fig. 4.
Fluorescence of purified APM-labeled β2AR in detergent micelles. (A) Corrected and normalized emission spectra of β2AR labeled with APM at Cys-265, with (+ iso) or without (– iso) the addition of 100 μM isoproterenol. λem = 620 nm. (B) Exicitation and emission spectra of β2AR labeled with APM at Cys-271, with or without the addition of 100 μM isoproterenol. λem = 623 nm.
Discussion
The usefulness of fluorescence in studying protein motions derives from its sensitivity, its kinetic resolution, and its compatibility with both live cells and concurrent physiological assays. Newer imaging techniques, such as voltage-clamp fluorometry, ultrafast methods, and single-molecule fluorescence, have opened areas of study of the functional transitions of proteins, by characterizing their underlying conformational changes. Although genetically encoded fluorescent proteins and quantum dots have proved to be extraordinarily useful as probes for many applications, they are much too large to report motions of individual side-chains or domains. Advances in fluorescent studies of protein motions have prompted the need for probes that can clearly report the environmental changes of specific residues.
There are two general objectives in designing an ideal probe for these applications: that the probe sample (and report) the same environments as the side chain to which it is attached, and that its fluorescence can be interpreted in terms of protein structure and motion. APM improves on the shortcomings of existing probes more clearly on the first objective than the second, because its design incorporates several favorable chemical characteristics. Its molecular volume is 231 Å3, making it more compact than other probes with red emission, such as TMRM (329 Å3), and less than one-half the size of the commercial maleimides of Texas red (474 Å3), Cy3 (479 Å3), and Alexa 546 (632 Å3); it has three atoms separating the point of Cys attachment from the nearest point of the fluorophore, whereas TMRM has seven and Alexa 546-C5 maleimide has 14; and it is uncharged, so that movements into the nonpolar part of the membrane will be unhindered by the energetic penalties of moving charges into low dielectric media (33). All of these properties should enable APM to track the motions of the attached side chain much more closely than possible with the available xanthenes, and its fluorescence should better reflect the environment of the native side chain. This improvement is most pronounced relative to the Alexa dyes, which were designed with long linkers and bulky charged substituents to have as little interaction with the attached protein as possible (34) and are inappropriate for studying motions of specific residues.
The size and neutrality of APM may also enhance its reactivity toward certain Cys residues. Probe reactivity with individual Cys residues is dictated by the chemical nature of both the probe and the Cys. For proteins in intact membranes, only Cys residues in at least partially aqueous environments will react with maleimides or other selective organic reagents; those in the membrane interior or buried within protein are unreactive (35). Of potentially reactive sites, steric clash or unfavorable electrostatic interactions with the probe itself may prevent labeling, which may be the cause of a poor TMRM signal from a Cys near the N terminus of the Shaker S4 segment (Fig. 3C). This site shows a significant ΔF with APM, possibly owing to its smaller size and increased accessibility. The APM maleimide adds some bulk and distance between probe and protein, but maleimides have been shown to have superior specificity and reactivity toward Cys compared with most other sulfhydryl-reactive groups. An exception is the disulfide-forming methanethiolsulfonate reagent; however, disulfides are excellent fluorescence quenchers via excited-state electron transfer (36), so that they are not generally useful for attaching fluorophores.
APM also makes some improvement on the second general objective of generating ΔF values that can easily be interpreted in terms of protein motion. Its dipole moment leads to excitation and emission red shifts with increasing solvent polarity, and because polarity can be related to the different protein environments (i.e., lipid interior is nonpolar, aqueous is polar, and protein is intermediate), these shifts provide a simple means of relating fluorescence to local structure and motion. In contrast, the xanthenes that have been used in these studies are symmetric and, therefore, do not have significant dipoles. Factors dominating their fluorescence shifts are more difficult to relate to proteins; for example, pH and hydrogen-bonding character dominate fluorescein environment sensitivity (9), and there is no simple way to predict local variations in these properties around a protein, even one of known structure. Other xanthenes have not been characterized as well; indeed, multicharged xanthenes are not soluble in nonpolar solvents, precluding their full characterization and making predictions about their fluorescence in the membrane or protein interior speculative.
APM Stokes shifts, measured from excitation to emission-peak maximum, vary from 35 to 70 nm, larger than the ≈20-nm Stokes shifts that are typically found in red probes. This increase may reduce complications from resonance energy homotransfer between subunits in homomultimeric proteins such as potassium channels, where as up to four probes are attached per protein.
The spectra of dyes such as APM are inherently complex, and its improved characteristics are partially offset by some complicating factors. The carbonyl group is an excellent electron acceptor, but it leads to spectral shifts as a hydrogen bond acceptor in hydroxylated solvents. Spectra move to the red with shifts on the order of 500 cm–1 (compare CH3CN and MeOH in Table 1), an effect that prohibits simple analog readouts of environment polarity from emission. APM also shows large changes in quantum yield, from 5% in bulk water to 91% in acetone, which precludes using bandpass intensity measurements, because the emission is likely to be changing in multiple ways. These changes in quantum yield can be overcome with acquisition of full excitation and emission spectra, as with the β2AR fluorescence presented here, in which subtle differences between positions can be measured. Future generations of fluorophores could address these spectral shortcomings, as well as the need to develop probes to label membrane proteins at sites deep in the membrane interior, where many of the most interesting conformational changes are likely to occur.
Acknowledgments
We thank Axel Brunger for comments on the manuscript. This work was supported by National Institutes of Health Grants R01MH653340 (to L.Y.J.), R01NS35549 (to E.Y.I.), and R01NS28471 (to B.K.K.).
Author contributions: B.C. designed research; B.C., A.P., X.Y., G.S., and C.S.G. performed research; B.C. contributed new reagents/analytic tools; B.C., A.P., and C.S.G. analyzed data; and B.C., Y.N.J., B.K.K., E.Y.I., and L.Y.J. wrote the paper.
Abbreviations: APM, aminophenoxazone maleimide; ΔF, fluorescence change; β2AR, β-2 adrenergic receptor; TMRM, tetramethylrhodamine maleimide; TFA, trifluoroacetic acid.
References
- 1.Mannuzzu, L. M., Moronne, M. M. & Isacoff, E. Y. (1996) Science 271, 213–216. [DOI] [PubMed] [Google Scholar]
- 2.Kobilka, B. K. & Gether, U. (2002) Methods Enzymol. 343, 170–182. [DOI] [PubMed] [Google Scholar]
- 3.Cha, A. & Bezanilla, F. (1998) J. Gen. Physiol. 112, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghanouni, P., Gryczynski, Z., Steenhuis, J. J., Lee, T. W., Farrens, D. L., Lakowicz, J. R. & Kobilka, B. K. (2001) J. Biol. Chem. 276, 24433–24436. [DOI] [PubMed] [Google Scholar]
- 5.Cha, A., Snyder, G. E., Selvin, P. R. & Bezanilla, F. (1999) Nature 402, 809–813. [DOI] [PubMed] [Google Scholar]
- 6.Glauner, K. S., Mannuzzu, L. M., Gandhi, C. S. & Isacoff, E. Y. (1999) Nature 402, 813–817. [DOI] [PubMed] [Google Scholar]
- 7.Peleg, G., Ghanouni, P., Kobilka, B. K. & Zare, R. N. (2001) Proc. Natl. Acad. Sci. USA 98, 8469–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnleitner, A., Mannuzzu, L. M., Terakawa, S. & Isacoff, E. Y. (2002) Proc. Natl. Acad. Sci. USA 99, 12759–12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klonis, N., Clayton, A. H., Voss, E. W., Jr. & Sawyer, W. H. (1998) Photochem. Photobiol. 67, 500–510. [PubMed] [Google Scholar]
- 10.Cha, A. & Bezanilla, F. (1997) Neuron 19, 1127–1140. [DOI] [PubMed] [Google Scholar]
- 11.Xu, Q. H., Scholes, G. D., Yang, M. & Fleming, G. R. (1999) J. Phys. Chem. A 103, 10348–10358. [Google Scholar]
- 12.Vogel, M., Rettig, W., Sens, R. & Drexhage, K. H. (1988) Chem. Phys. Lett. 147, 452–460. [Google Scholar]
- 13.Weber, G. & Farris, F. J. (1979) Biochemistry 18, 3075–3078. [DOI] [PubMed] [Google Scholar]
- 14.Lakowicz, J. R. (1999) Principles of Fluorescence Spectroscopy (Academic, New York).
- 15.Cohen, B. E., McAnaney, T. B., Park, E. S., Jan, Y. N., Boxer, S. G. & Jan, L. Y. (2002) Science 296, 1700–1703. [DOI] [PubMed] [Google Scholar]
- 16.Karstens, T. & Kobs, K. (1980) J. Phys. Chem. 84, 1871–1872. [Google Scholar]
- 17.Kobilka, B. K. (1995) Anal. Biochem. 231, 269–271. [DOI] [PubMed] [Google Scholar]
- 18.Ghanouni, P., Steenhuis, J. J., Farrens, D. L. & Kobilka, B. K. (2001) Proc. Natl. Acad. Sci. USA 98, 5997–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy, P. Y., Kondo, S., Fujita, S. & Toru, T. (1998) Synthesis 999–1002.
- 20.Reichardt, C. (1990) Solvent Effects in Organic Chemistry (Verlag Chemie, Weinheim, Germany).
- 21.Rasimas, J. P. & Blanchard, G. J. (1994) J. Phys. Chem. 98, 12949–12957. [Google Scholar]
- 22.Valkina, O. N., Vergun, O. V., Turovetsky, V. B. & Khodorov, B. I. (1995) FEBS Lett. 361, 145–148. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki, S. & Taguchi, T. (1997) J. Photochem. Photobiol. A 104, 189–195. [Google Scholar]
- 24.Baker, O. S., Larsson, H. P., Mannuzzu, L. M. & Isacoff, E. Y. (1998) Neuron 20, 1283–1294. [DOI] [PubMed] [Google Scholar]
- 25.Loots, E. & Isacoff, E. Y. (1998) J. Gen. Physiol. 112, 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi, C. S., Loots, E. & Isacoff, E. Y. (2000) Neuron 27, 585–595. [DOI] [PubMed] [Google Scholar]
- 27.Li-Smerin, Y. & Swartz, K. J. (2001) J. Gen. Physiol. 117, 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi, C. S. & Isacoff, E. Y. (2002) J. Gen. Physiol. 120, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang, Q. X., Wang, D. N. & MacKinnon, R. (2004) Nature 430, 806–810. [DOI] [PubMed] [Google Scholar]
- 30.Cuello, L. G., Cortes, D. M. & Perozo, E. (2004) Science 306, 491–495. [DOI] [PubMed] [Google Scholar]
- 31.Jensen, A. D., Guarnieri, F., Rasmussen, S. G., Asmar, F., Ballesteros, J. A. & Gether, U. (2001) J. Biol. Chem. 276, 9279–9290. [DOI] [PubMed] [Google Scholar]
- 32.Swaminath, G., Xiang, Y., Lee, T. W., Steenhuis, J., Parnot, C. & Kobilka, B. K. (2004) J. Biol. Chem. 279, 686–691. [DOI] [PubMed] [Google Scholar]
- 33.Grabe, M., Lecar, H., Jan, Y. N. & Jan, L. Y. (2004) Proc. Natl. Acad. Sci. USA 101, 17640–17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panchuk-Voloshina, N., Haugland, R. P., Bishop-Stewart, J., Bhalgat, M. K., Millard, P. J., Mao, F. & Leung, W. Y. (1999) J. Histochem. Cytochem. 47, 1179–1188. [DOI] [PubMed] [Google Scholar]
- 35.Li, J., Xu, Q., Cortes, D. M., Perozo, E., Laskey, A. & Karlin, A. (2002) Proc. Natl. Acad. Sci. USA 99, 11605–11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen, Y. & Barkley, M. D. (1998) Biochemistry 37, 9976–9982. [DOI] [PubMed] [Google Scholar]