Abstract
Proper allocation of limited healthcare resources is a challenging task for policymakers in developing countries. Allocation of and access to these resources typically varies based on how need is defined, thus determining how individuals access and acquire healthcare. Using the introduction of antiretroviral therapy in southern Mozambique as an example, we examine alternative definitions of need for rural populations and how they might impact the allocation of this vital health service. Our results show that how need is defined matters when allocating limited healthcare resources and the use of need-based metrics can help ensure more optimal distribution of services.
Keywords: Antiretroviral therapy, Healthcare resources, Location-allocation analysis, Rural populations, sub-Saharan Africa
1. Introduction
Health as human capital implies that investments in health will generate broader returns, and this link between health and economic performance has been established at both the micro and macro levels. This relationship can also work in reverse, as poor health can inhibit labor force participation and stunt economic growth. The negative implications of poor health in agriculturally-reliant areas have been documented (Canning, 2006; Walker et al., 2006), and illness can deepen and perpetuate poverty traps by stifling economic mobility among resource constrained households. A variety of policy levers have been applied to attempt to disrupt this negative health-wealth relationship in low-income countries (LICs). In LICs, health clinics are commonly used to improve healthcare access and reduce the frequency and severity of adverse health events. Factors such as location and services offered can both affect a clinic’s efficacy, as they can a regional clinic system’s efficacy, which can affect the value of this policy instrument.
Mozambique is a LIC that has been plagued by high rates of HIV, which disproportionately affects subsistence farmers and imperils their livelihoods (Dodson et al., 2016). We examine the initial deployment of antiretroviral therapy (ART) in a particularly hard-hit region of Mozambique, and how the optimal allocation of ART services among the existing network of clinics varies depending on how need is defined.
Access has been broadly defined in the healthcare literature as either non-spatial or spatial (Donabedian, 1973). Non-spatial access often refers to societal factors that contribute to or prevent access to healthcare (Yao et al., 2013). Ideally, all members of society should have equal opportunity to acquire the healthcare that they need (Ricketts, 1994). In practice, however, non-spatial access is often directly influenced by demographic and economic factors, setting up an environment that creates ‘winners’ and ‘losers.’ The political ecology of health suggests that these non-spatial factors contribute to this inequity, shaping how people access healthcare (Turshen, 1977; King, 2009). This inequity prevents true access to care—especially for impoverished rural populations such as those in Mozambique and many other parts of sub-Saharan Africa. Non-spatial processes have implications for spatial (physical) access. Spatial access often refers to the physical features that impede or facilitate access to healthcare (e.g., physical distance, rivers, forests, mountains, etc.) (Yao et al. 2013). This type of access is often couched in either the ability to use health services or actual utilization of them (Joseph and Bantock, 1982; Joseph and Phillips, 1984). This analysis is primarily concerned with the ability to use healthcare and we demonstrate that this ability is highly contingent on how ‘need’ is defined.
Because access to healthcare plays an integral part in shaping healthcare utilization, facility location and health services offered is a critical issue for urban and regional policy making (Higgs, 2009). The last few decades have seen the development and use of many facility location models for health services (Calvo and Marks, 1973; Bennett et al., 1982; Osleeb and McLafferty, 1992; Ratick et al., 2009; Yao and Murray, 2014). Additionally, allocation models are typically employed to assist with healthcare planning and describe clinic service areas or distribute health services (Cromley and McLafferty, 2011; Yao and Murray, 2014). Thus, facility location models can be combined with allocation models to optimize the distribution of key health services amongst health clinics. Our research adapts location-allocation modelling to understand how varying definitions of ‘need’ change the optimal distribution of a vital health service—ART.
Using health clinic data from 2009, i.e., when the massive scale-up of HIV services began, we evaluate village access to health clinics and correlate that access with population, economic status, and agricultural intensity. We consider multiple measures of access for this analysis, using distance to nearest clinic, distance to nearest clinic offering ART, and average quality of surrounding clinics. Cost is not considered a barrier to access in this study because of the commitment made by the Mozambican government to provide ART for free through state-run clinics (WHO, 2007; Yao et al., 2014). To examine the potential implications of limited resources, we construct a village-specific quality score comprised of available resources for each clinic that is then weighted by its distance from the village. We question whether the initial clinics selected to offer ART were optimally chosen to best serve surrounding communities. To examine this, we perform a location-allocation analysis to understand how the initial configuration of clinics chosen to offer ART may be underperforming and offer an optimal configuration solution. We construct multiple metrics for need and then model how access to ART varies as a function of need for initial and optimal configurations. We also evaluate how a reorganization of health services such as ART can improve access for the need-based groups.
This study contributes to the literature on access to care in spatially innovative ways. First, we take advantage of precise spatial information about both villages and clinics. Second, we integrate a comprehensive quality measure into the optimality analysis, considering all clinics within a predefined radius of each village rather than just the closest one. And third, we examine how improvements in access may correlate with changes in livelihood sustainability.
2. Study area
Mozambique, a sub-Saharan country with a population of 27 million and GNP per capita of 525 USD (World Bank, 2015), has long been striving to contain such diseases as malaria, cholera, and tuberculosis. Mozambique is also among the sub-Saharan countries most severely affected by the HIV/AIDS epidemic, with adult HIV prevalence estimated at 12% nationwide, and as high as 25% in southern Gaza province, where our data are collected (Ministry of Health, 2009). In 2004, ART was rolled out nationwide but through a very limited number of health clinics; less than 25% of the population with advanced HIV were actually enrolled in ART as of 2007 (Audet et al., 2010). Additionally, Mozambique, and its southern region in particular, is prone to natural disasters such as devastating flooding and severe drought (Klinman and Reason, 2008; Matyas and Silva, 2013). Thus, households in this region may be further disadvantaged by the occurrence of a natural disaster, forcing them to deal with many disease vectors, thereby eroding household human capital by creating unhealthy landscapes. Figure 1 depicts the location of health clinics surveyed and surveyed villages in the area.
Figure 1. Map of study area.
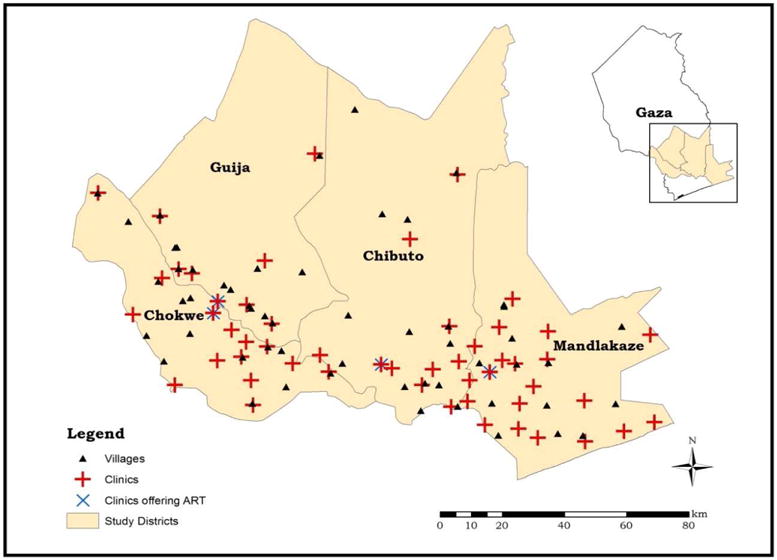
Source: map constructed by authors
Agriculture is a vital part of the Mozambican economy, where just over 85% of the population engages in this livelihood strategy (FAO, 2015). Most of those engaged are subsistence farmers and are more vulnerable to poor health as they often have a small or no social safety net to rely on. The government of Mozambique has identified cash cropping as a poverty alleviation strategy and has placed a growing emphasis on these types of crops (Silva, 2008; PARPA, 2007).
Subsistence agriculturalists are by definition highly vulnerable to shocks that affect their livelihood. They are also highly vulnerable to health shocks and, in turn, highly dependent on the clinical services that, if accessible and adequate, may help cushion these shocks. Chronic illnesses such as diabetes, cancer, and hypertension plague older generation subsistence agriculturalists, while communicable diseases such as HIV/AIDS, sexually transmitted diseases (STDs), and cholera, to name a few, typically affect the younger generations (Negin, 2005; Hawkes and Ruel, 2006). Additionally, subsistence agricultural households have limited economic mobility and are thus limited in livelihood diversification in the face of health declines (Ulrich et al., 2012). Poor health and disease are capable of diminishing household stocks of human capital, have far-reaching consequences for those reliant on physical labor, and are felt more acutely in households that are subsistence-reliant; these types of households will often resort to farming less land as a coping strategy for dealing with poor health, thus further jeopardizing their well-being (Obrist et al., 2007; Dodson et al., 2016). Therefore, access to vital healthcare services is essential to helping these households resume their livelihood activities.
As part of the government’s poverty alleviation strategy, investments in health infrastructure are cited as a top priority (PARPA, 2007). The government of Mozambique uses state-run health clinics as a platform to roll out high-quality services such as ART, prevention of mother to child transmission of HIV (PMTCT), maternal and child health (MCH) services, and immunizations (WHO, 2007; PARPA, 2007). Importantly, in an effort to support the poverty alleviation strategy, the government provides certain services to the general public for free; these services include HIV testing, ART, services for pregnant women (e.g. prenatal, delivery and counseling services), immunizations, and care to children under five (WHO, 2007). The use of state-run health clinics as a platform for extending vital health services is where this research seeks to make a meaningful contribution; it seeks to help inform policymakers in the approach needed to address equity in access to healthcare.
3. Data and methods
The data used in this analysis comes from wave two (2009) of a longitudinal household-based survey of rural women’s health, as well as a parallel survey of health clinics. The data collection was funded by NICHD [R21HD048257, R01HD058365]. These surveys were developed to better understand how Mozambique’s high rate of labor migration affects the spread of HIV and STDs.
The first survey wave was conducted in 2006 in 56 villages located in four districts (Chibuto, Chokwè, Guíjà and Mandlakaze) of Gaza province in southern Mozambique and included a sample of 1680 women aged 18–40. Fourteen villages per district were selected with probabilities proportional to their population size based on census data. In each village, 30 households with at least one married woman of target age were chosen through probability sampling; these households were split between those with women married to migrants and those married to non-migrants. In 2009, the second wave of the survey was carried out. The respondents who were not available for interview were replaced by randomly selected women from the same villages. In follow-up attempts conducted later in that year and early in the following year some of the original respondents who had not been interviewed were located and interviewed. Their substitutes were retained in the sample. As a result, the total sample size increased to 1,868. The survey instrument included a variety of demographic, economic, and health questions. Additionally, all health clinics (53) in the four districts were surveyed in 2009 about the MCH services offered. The clinic survey was based on interviews with key clinic personnel.
Oral consent was obtained from each household survey respondent and each key respondent from the health clinics. All data were de-identified to preserve the confidentiality of the respondents. The project was approved by Institutional Review Boards at [Arizona State University] in the USA and [Mozambique Ministry of Health Research Ethics Committee] in Mozambique.
We captured need using three different variables from the dataset, these include total population, household responses to asset ownership, and reported food insecurity. Our notion of access to health services was then based on survey data on clinic offerings as well as geographic location data. The clinic survey included 15 different attributes that address overall infrastructure, staffing, and actual services offered (e.g., ART, SRH, etc)1.
Our analytical strategy encompasses descriptive statistics, PCA, location-allocation analysis, and GIS. Descriptive statistics were used to explore the level of reliance on agriculture for households in the survey, frame the motivation for the primary research question, and explore the relationship between livelihood vulnerability (measured by food hardship) and access to healthcare. PCA was used to generate an asset score (which is used as the measure of economic well-being) for the households, as well as the HSQ index; specifically, PCA was used to derive the weights assigned to each asset or health clinic attribute that contributes to that respective index. Location-allocation analysis is used to examine how clinic placement may affect households that are potentially more vulnerable. Spatial methods are applied to examine optimal clinic placement based on the location-allocation analyses and explore potential improvements associated with locating health clinics more effectively.
Descriptive statistics are used to contextually understand the division of household labor dedicated to agriculture, as well as the potential vulnerability households face when distance and access to healthcare vary spatially. We assume that households residing in the same village will have roughly the same level of distance to overcome, as well as access opportunities such as access to high-quality services. Two measures of access are generated: one based on network connectivity of the current road system in the study districts (geographic access), and the other measured through a comprehensive HSQ index that considers the quality of care available (quality measure of access). While other studies have demonstrated that Euclidean distance can be a sufficient measure of spatial access in sub-Saharan Africa (Tanser et al., 2006; Yao et al., 2012), it is not useful for understanding distance and access barriers as they occur in reality. Euclidean distance fails to accommodate for geographic barriers and these need to be accounted for as individuals must travel around these impediments. The current road network is comprised of primary and secondary routes, as well as trails. Therefore, we take a hierarchical approach in weighting the roads by their respective speed limit to help minimize travel impedance. More specifically, the roads provide data on the ‘level’ for each road (e.g., primary, secondary, and trails) and we assume that these different levels correspond with the overall travel impedance. This means that individuals, whether driving or walking, can move more quickly along primary routes versus secondary and trail routes, and these differences are accounted for in our access measures.
3.1 Defining and measuring livelihood vulnerability
PCA is used to calculate a measure of potential economic status at the household level. Many studies have demonstrated the value of using wealth-based indicators to rank households based on asset or wealth (Filmer and Pritchett, 1999; Khan et al., 2006; Flimer and Kinnon, 2012; Giesbert and Schindler, 2012; Yao et al., 2014). We use detailed information on dwelling conditions, access to electricity and water, access to a latrine, and asset ownership to generate a composite measure of economic well-being using PCA, based on weights derived from the first component in the PCA. Livestock are often considered assets for subsistence agriculturalists in developing countries and these were included in the composite index (Thornton et al., 2007; Giesbert and Schindler, 2012). Livestock were converted to tropical livestock units (TLUs) using the Food and Agriculture Organization (FAO) conversion formula. We also generate a measure of food hardship. For this measure, we identify households that reported having fewer than two meals a day in the past week.
For all of the livelihood vulnerability measures, households are grouped based on terciles. Terciles were chosen based on the distribution of the underlying data, as well as the relatively small number of villages. They are often preferable for developing countries because dividing samples into quartiles or quintiles often results in arbitrary cutoff values in which a large proportion of households would be allocated to an adjacent category due to a clumping of wealth scores at the lower end of the distribution (Khan et al. 2006). For population, tercile 1 is equal to the least populated villages while tercile 3 represents the most populated. For asset score, tercile 1 is equal to those with the least number of assets (worst economic status) while tercile 3 represents those with the greatest number of assets. For food hardship, tercile 1 is equal to the least amount of food hardship while tercile 3 represents the greatest food hardship. Table 1 shows the ranges used for each tercile for defining the livelihood vulnerability measures. We tested for correlation between our metrics using a correlation matrix and found little to no correlation between the metrics (coefficients for all pairs < 0.40).
Table 1.
Tercile ranges for village population and livelihood vulnerability measures
| Access measure | Mean | Std. Dev. | Tercile 1 | Tercile 2 | Tercile 3 |
|---|---|---|---|---|---|
| Population N |
1750 | 1524.61 | 86–1000 (21) |
1001–1502 (17) |
1503–7069 (17) |
| Asset score N |
−0.02 | 0.89 | −1.87 – −0.40 (19) |
−0.39 – 0.29 (19) |
0.30 – 4.08 (17) |
| Food hardship (%) N |
8 | 7 | 0 – 3 (18) |
3.1 – 12 (18) |
12.1 – 31 (19) |
3.2 Minimizing distance to health clinics through optimal placement
Measuring optimal placement of health clinics in this study is modeled through location-allocation analysis. The purpose is to assess the initial spatial organization of ART and determine if it is sited in such a manner that provides best access to the study region villages based on their level of need. The measure of access used for this analysis is average distance, weighted by travel speeds on the roads in the region, and can be obtained using a location-allocation model implemented in a GIS.
3.2.1 Defining access to high-quality health clinics
Measuring access to high quality healthcare is a multi-step process. First, the HSQ index for each health clinic in the study area is constructed. We modelled the HSQ index after that of Yao et al. (2013) and have expanded it to include additional measures of quality pertaining to health clinics. The HSQ index is the weighted sum of health clinic attributes:
where i is the index of attribute i = 1,2,…15, j is the index of the health clinic, j = 1,2,…N; vi is the ith attribute; wi is the weight for the ith attribute; and, aj is the HSQ index of the jth clinic. The attributes included in the HSQ are listed in Table 2.
Table 2.
Health clinic attributes
| Resources available | 2009 (N=53) | |
|---|---|---|
| Mean | Std. Dev. | |
| Number or rooms in clinic | 2.17 | 1.30 |
| Clinic has access to piped water* | 0.26 | 0.45 |
| Clinic has access to electricity* | 0.57 | 0.50 |
| Clinic receives NGO assistance* | 0.47 | 0.50 |
|
| ||
| Services offered | ||
|
| ||
| Prenatal consultations* | 0.98 | 0.14 |
| Counseling and testing* | 0.55 | 0.50 |
| PMTCT* | 0.49 | 0.50 |
| ART for general public* | 0.07 | 0.26 |
| ART for pregnant women* | 0.19 | 0.39 |
| Delivery assistance* | 0.68 | 0.47 |
| Postpartum consultations* | 0.98 | 0.14 |
| Child consultations* | 0.96 | 0.19 |
| Child at risk care* | 0.51 | 0.50 |
| Family planning* | 0.98 | 0.14 |
| Child vaccinations* | 0.92 | 0.27 |
Note:
these attributes are binary
A hedonic model was used to understand the contributory value of each specific health clinic attribute. Higher values of the HSQ index are indicative of better health services. The weights, wi, for health clinic attributes were estimated using PCA.
The second step was to use the HSQ for each clinic to create an access metric for each village that includes both distance and quality of nearby health clinics. Specifically, this metric is the weighted sum of the HSQs for all clinics within a ten kilometer radius of a village, with weights corresponding to the inverse of the distance of the clinic from the specific village.
The services included in the HSQ are primarily MCH-related, and thus do not reflect the broader array of services available to the population. However, these MCH services are used as a proxy for the capacity of a facility to offer high-quality services in general. The use of these services as a proxy is justified by the high fertility rates observed across villages in the study (Yao et al., 2012), which suggest that the quality of services offered is most likely to be distributed based upon maternal need. It is important to note that while the majority of services examined in the quality measure are MCH-specific, the health clinics do have the ability to offer general services to the public (i.e., males may also be seen at these clinics) as these health clinics represent the only type of health facility immediately available to rural residents (Yao et al., 2014).
3.2.2 Location-allocation analysis
Location-allocation is modeled using the p-median model which seeks to minimize the average total distance between demand points and their closest facilities (Hakimi, 1964, 1965; ReVelle and Swain, 1970; Kumar, 2004). Essentially, the p-median model is used to select the best configuration of health clinics offering ART to the general public. This is done by choosing where a defined number of clinics offering ART should be sited given the initial configuration of 53 clinics. Formally stated, the p-median model takes the following form:
Facing the following restraints:
A clinic has to be allotted with a separate demand site (village): xij ≤ xij for all (i,j)
An open clinic must be allotted a demand:
Only the p clinics are to be located:
All villages assigned to them equal the number of clinics to be located.
Total demand from a separate village: xij = (0,1) for all (I,j) is allotted to only one facility) When:
Z = the objective function.
I = all of the villages where the nodes on network along the subscript i are an index signifying a specific demand area.
J = the collection of candidate clinics when the nodes on the network along the subscript j are an index which signifies a particular clinic location.
ai = the village-weight based on defined need for village i.
dij = denotes the distance or time in terms of the travel cost and separates place i from candidate clinic j.
HSQj = the HSQ score for clinic j
xij = equal to 1 when demand at place i is allotted to a facility opened at site j, or equal to 0 when demand at place i is not allotted to the location.
p = the number of clinics to be located.
Because the p-median problem is a combinatorial problem of type “N choose P”, the solution frontier can grow extremely large. This can be solved efficiently in a GIS through the use of heuristics while generating a near-optimal solution. A heuristic in this sense is used to overcome computationally expensive processes by solving a problem more quickly when classic methods are too slow or fail to find an optimal solution. The location-allocation model is implemented in ArcGIS 10.4; ArcGIS use a heuristic that allows for an optimal solution to be obtained by first generating an origin-destination (OD) matrix of shortest-path costs between all clinics and villages along the network; it then uses Hillsman editing to produce edited versions of the OD matrix which can then be used to develop a set of semirandomized solutions that applies a Teitz and Bart substitution to generate a full set of solutions. When no additional improvement is found, the best solution is returned (ESRI, 2016).
The analysis weights the villages by population for the first iteration, by asset score for the second, and by food hardship for the final iteration. For the asset score-weighted version, the inverse of the asset score is used to give more priority to those with the lowest economic status. For each of the need-based metrics, we establish: the baseline assessment (the initial configuration of clinics offering ART); the optimal, “from scratch” allocation (assumes optimal configuration chosen from all possible health clinic locations); the initial configuration plus one additional clinic; the initial configuration plus five additional clinics; the initial configuration plus ten additional clinics. The three configurations that examine “additional” clinics reflect the ‘best’ way to expand ART given the initial configuration already in place. A one-way analysis of variance was used to test for significance between categories.
4. Results
We descriptively explored the role agriculture plays for households in the study area. Migration is quite common in this region given the close proximity to South Africa and neighboring countries, as male members of the household typically seek work elsewhere; this often leaves majority of agricultural responsibility to women within the household (Agadjanian et al., 2011). This is confirmed in Table 3.A and 3.B, as stark differences emerge between the percentage of women and men engaging in agriculture for the study sites. Based on the survey respondents’ reports, just fewer than 30% of male household heads usually participate in agricultural activities, compared to nearly 100% of women.
Table 3.
Division of household agricultural labor and women’s agricultural effort
| 3.A Participation in agriculture | 3.B Women’s effort in agricultural tasks | ||||
|---|---|---|---|---|---|
|
| |||||
| A lot | A little | Not at all | |||
| Wife | 98.75% | ||||
| Husband | 29.59% | Field preparation | 86.63% | 11.46% | 1.91% |
| Children | 11.25% | Planting | 92.41% | 6.60% | 0.98% |
| Relatives | 43.67% | Weeding | 86.79% | 11.24% | 1.97% |
| Non-relatives | 16.73% | Harvesting | 86.52% | 12.39% | 1.09% |
4.1 Livelihood vulnerability
Descriptive statistics for the livelihood vulnerability metrics were used in an exploratory manner to better understand how the initial distribution of health clinics may impact different groups within the population differently. Table 4 reports the results from the descriptive statistics under the initial allocation of clinics offering ART. The table shows how distance to the nearest health clinic, nearest ART clinic, and the overall quality of services offered varies by our need-based metrics for the defined terciles. While distance to the nearest clinic is not significantly different for the terciles based on any of the metrics, there is considerable variation—suggesting room for improvement in minimizing the distance traveled to access ART. However, there are significant differences between terciles for the food hardship metric when examining distance to the nearest clinic offering ART to the general public. We chose to examine the role of clinic placement further with respect to population, asset score, and food hardship.
Table 4.
Distance and access to health clinics by village population and livelihood vulnerability under current allocation of ART
| Measures | Population concentration | Asset Score | Food Hardship | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Tercile | 1 | 2 | 3 | Sig | 1 | 2 | 3 | Sig | 1 | 2 | 3 | Sig |
| Distance to nearest clinic (km) | 4.71 | 5.91 | 5.64 | 4.44 | 6.41 | 5.13 | 4.99 | 4.68 | 6.37 | |||
| Distance to nearest clinic offering ART treatment (km) | 24.77 | 23.56 | 23.61 | 29.73 | 23.09 | 18.73 | 28.72 | 16.30 | 26.93 | ** | ||
| Mean HSQ | 0.53 | 0.47 | −0.53 | −1.09 | 0.80 | 0.76 | 0.02 | 0.73 | −0.18 | |||
|
| ||||||||||||
| Number of villages | 21 | 17 | 17 | 19 | 19 | 17 | 18 | 18 | 19 | |||
Note: Significance defined as:
p<0.05
4.2 Optimal solutions for the placement of health clinics
As of 2009, only 4 clinics in the study area were providing ART. We compared the initial configuration of clinics providing ARTs with the optimal configuration as indicated by each of our defined need metrics. Figure 3 shows the initial and optimal configurations of clinics offering ART. Under the initial configuration, we can easily see that residents of some villages have to travel disproportionately further to access this service than others. Additionally, two villages in the Chibuto district are surrounded by lakes and thus are not able to easily access health clinics offering ART, suggesting a sub-optimal configuration. To find the optimal solution, we assumed that none of the 53 clinics offered ART and modeled clinic configuration by choosing the optimal location for 4 clinics to offer ART from the initial set of clinic locations; this was done for all metrics analyzed. Each clinic is weighted by its HSQ and identifies those clinics with the best potential for offering ART. There is variation in the optimal solutions, suggesting that the way in which need is defined matters.
Figure 3. Initial versus optimal configurations for clinics offering ART when weighted by total population, asset score, and food hardship.
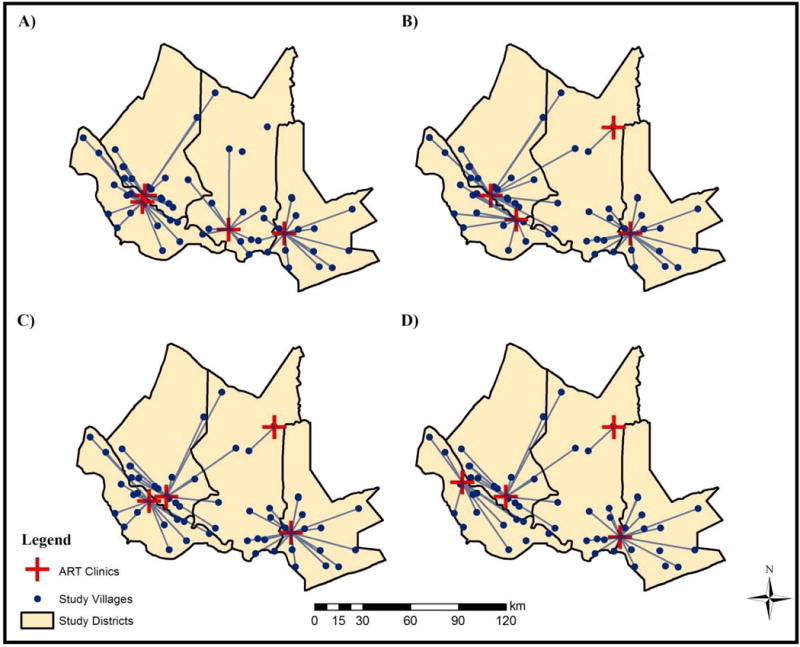
Note: Maps constructed by authors. A) Initial configuration of clinics offering ART. B) Optimal configuration when weighted by total population. C) Optimal configuration when weighted by asset score. D) Optimal configuration when weighted by food hardship.
When villages are weighted by population, only half of the clinics overlap between the initial configuration and the optimal solution. The optimal solution accounts for geographic barriers and allows for all villages to be served while reducing the average distance travelled by roughly 3.2km (see Table 5).
Table 5.
Recalculation of access to health clinics when villages are weighted by population
| Measures | Population concentration | Asset score | Food hardship | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Terciles | 1 | 2 | 3 | Sig. | 1 | 2 | 3 | Sig. | 1 | 2 | 3 | Sig. |
| Initial (km) | 24.77 | 23.56 | 23.61 | 29.73 | 23.09 | 18.73 | 28.72 | 16.30 | 26.93 | ** | ||
| Optimal (km) | 19.20 | 19.12 | 23.94 | 19.40 | 23.65 | 18.66 | 22.11 | 15.69 | 23.94 | * | ||
| Initial + 1 (km) | 20.48 | 18.85 | 23.61 | 20.78 | 23.09 | 18.73 | 24.28 | 16.30 | 22.18 | * | ||
| Initial + 5 (km) | 20.01 | 13.87 | 15.02 | 16.99 | 17.25 | 15.33 | 18.69 | 15.83 | 15.26 | |||
| Initial + 10 (km) | 12.55 | 12.90 | 9.51 | 14.30 | 12.00 | 8.53 | * | 11.80 | 9.93 | 13.34 | ||
We also performed the analysis for villages weighted by asset score. Figure 3 compares the initial configuration with the optimal configuration for clinics offering ART. The optimal solution results in a decline of average distance travelled by roughly 1.8km when the initial and optimal configurations are compared (see Table 6).
Table 6.
Recalculation of access to health clinics when villages are weighted by asset score
| Measures | Population concentration | Asset score | Food hardship | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Terciles | 1 | 2 | 3 | Sig. | 1 | 2 | 3 | Sig. | 1 | 2 | 3 | Sig. |
| Initial (km) | 24.77 | 23.56 | 23.61 | 29.73 | 23.09 | 18.73 | 28.72 | 16.30 | 26.93 | ** | ||
| Optimal (km) | 20.07 | 20.15 | 26.36 | 19.00 | 24.34 | 22.87 | 24.81 | 15.72 | 25.40 | ** | ||
| Initial + 1 (km) | 20.48 | 18.85 | 23.61 | 20.78 | 23.09 | 18.73 | 24.28 | 16.30 | 22.18 | * | ||
| Initial + 5 (km) | 17.78 | 13.45 | 15.60 | 14.53 | 16.82 | 15.97 | 17.42 | 15.21 | 14.73 | |||
| Initial + 10 (km) | 9.72 | 11.01 | 13.10 | 9.88 | 12.45 | 11.16 | 12.35 | 9.76 | 11.37 | |||
Additionally, we sought to understand how access to ART varied for villages that were more vulnerable to food hardship, and this is also shown in Figure 3. The solution set for optimal clinics suggest that a complete reconfiguration is needed. The optimal solution results in a decline of average distance travelled by roughly 3.6km when we compare the initial and optimal configurations (see Table 7).
Table 7.
Recalculation of access to health clinics when villages are weighted by food hardship
| Measures | Population concentration | Asset score | Food hardship | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Terciles | 1 | 2 | 3 | Sig. | 1 | 2 | 3 | Sig. | 1 | 2 | 3 | Sig. |
| Initial (km) | 24.77 | 23.56 | 23.61 | 29.73 | 23.09 | 18.73 | 28.72 | 16.30 | 26.93 | ** | ||
| Optimal (km) | 17.57 | 19.47 | 24.89 | 20.00 | 22.30 | 18.79 | 25.92 | 13.66 | 21.62 | *** | ||
| Initial + 1 (km) | 20.48 | 18.85 | 23.61 | 20.78 | 23.09 | 18.73 | 24.28 | 16.30 | 22.18 | * | ||
| Initial + 5 (km) | 18.41 | 16.07 | 15.70 | 17.52 | 16.33 | 16.70 | 22.34 | 15.29 | 13.14 | ** | ||
| Initial + 10 (km) | 12.08 | 9.98 | 13.91 | 11.21 | 14.33 | 10.27 | 15.83 | 10.87 | 9.43 | ** | ||
Not only were the initial and optimal configurations for clinics offering ART compared, but we also examined how to expand the initial configuration and offer ART in additional clinics. We examined what effect 1, 5, and 10 additional clinics offering ART would have on access. Table 5 shows the overall improvement in access as ART is extended to additional clinics for all of the metrics. For example, providing 1 more clinic reduces the trip to the nearest clinic offering ART by 3km. Furthermore, prioritizing by population also improves access for villages experiencing greater food hardship.
Figure 4 illustrates the optimal spatial solution for expanding ART to additional clinics, based on the initial configuration as a baseline. This configuration minimizes overall impedance and results in a savings of roughly 12.3km when 10 more clinics are added to the initial configuration of ART clinics. Table 6 highlights that when minimizing overall impedance and weighting villages by economic status, adding an additional 10 clinics to the initial configuration would result in a 12.8km average reduction. However, what is more telling is that when prioritizing villages with low economic status, adding 10 clinics offering ART would result in a nearly 20km savings. Table 7 highlights that when minimizing overall impedance, adding an additional 10 clinics to the initial configuration would result in an average reduction of nearly 12km. Interestingly, when prioritizing villages with a low asset score, 10 additional clinics offering ART would result in a savings of just over 17km travelled.
Figure 4. Optimal placement of the next 1, 5, and 10 clinics to offer ART for the defined livelihood vulnerability metrics.
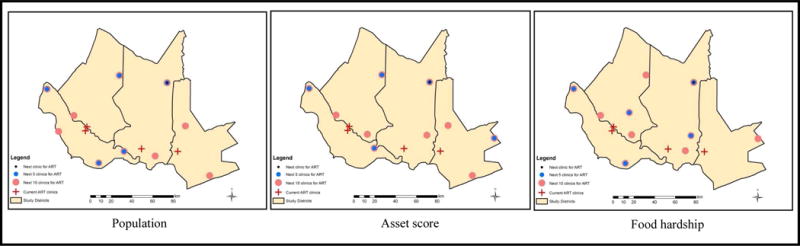
Source: Maps constructed by authors
5. Discussion
As part of its poverty alleviation strategy, the government of Mozambique has identified investments in human capital as one of its three main pillars for its development strategy; the need to invest in health and healthcare is nested within the human capital pillar (PARPA, 2007). Therefore, access to healthcare becomes an important precondition for investing in human capital. Extension of vital health services such as ART then represents a policy mechanism by which the government can achieve its overarching goal of poverty eradication. This analysis demonstrates that there are multiple ways to define need and the way need is defined affects how health services should be allocated moving forward.
Multiple measures of livelihood vulnerability were examined for distance and access effects for these villages. Results from the descriptive statistics revealed that households in this study are highly dependent on agriculture, with women being responsible for the majority of farming. Additionally, results from the descriptive statistics showed that, across terciles, most villages were located less than 6.5km from the nearest health clinic. While the average distances were not significantly different between terciles for each need-based metric, some interesting patterns emerged. Villages with the highest asset score were closer to health clinics than those with the lowest asset score. Additionally, villages that experienced the greatest food hardship were located significantly furthest from health clinics. We also examined distance to the nearest clinic offering ART, finding that there was no significant difference between terciles for the population and asset score measures. However, there was a significant difference between terciles for the food hardship measure.
We extended the analysis to examine the initial configuration of health clinics offering ART and compared that with the optimal solution. As of 2009, only 4 clinics in the study area were offering ART. Additionally, we examined how an additional 1, 5, and 10 clinics offering ART could provide better access for society. We then re-estimated the improvements in distance and access for all of the livelihood vulnerability measures. The location-allocation analyses focused on population, asset score, and food hardship. The population weighted location-allocation analysis first compared the initial configuration of clinics offering ART with what an optimal solution would be. Across terciles for population, the average distance travelled was just under 24km to the nearest clinic providing ART. Additionally, some villages are not serviced by the nearest clinic offering ART as significant geographic barriers are present. The optimal solution chose 4 clinics from all 53 possibilities and resulted in just over a 3km improvement in distance needed to travel. Because a complete re-organization is highly unlikely, we examined what the optimal locations for adding additional clinics would be given the initial configuration of those offering ART. Adding one more clinic further improved access by 3km. An additional 10 clinics in the study region would provide access to the surrounding villages and result in an average distance of roughly 10km. It has been established that most individuals are not willing to travel beyond 10km, one way, for healthcare (Yao et al., 2013). Thus, providing access by which individuals can travel less than 10km is a key step in providing equitable access to all.
It appears that those with the least number of assets are more disadvantaged in terms of access to ART than those who have higher socioeconomic standing. The most interesting results come from siting clinics offering ART when weighted by food hardship. Because much of the population of Mozambique are subsistence agriculturalists, changes in the ability to secure daily meals are of great importance for the overall well-being of the population. The initial configuration of clinics offering ART is not set up to help villages experiencing high levels of food hardship, and a complete reconfiguration is needed to help. Expanding to an additional 5 clinics would help villages with the highest levels of food hardship and bring the average travel distance closer to 10km (13.14km), and an additional 10 clinics would bring the average travel distance below 10km. While significant differences remain, there is still an overall reduction in distance travelled for the groups. This finding highlights the complexity associated with high rates of food insecurity and the ability to access healthcare.
Health clinics are one policy lever that can be used to help disrupt the negative health-wealth relationship. In this study, unhealthy households saw a larger reduction in the distance to nearest clinic, while healthier households gained in terms of access to high quality care. However, these differences were not statistically significant. There are number of possible explanations for this to be the case. First, clinic placement may be based on factors other than general healthcare needs, and may be located to deal directly with specific diseases or priority conditions. Second, the health measure is subjective and relies on perceived changes in health status. Finally, the lack of significance also underscores the fact that access is only one issue, and once access is improved, improvements in awareness and confidence in the health system itself needs to be fostered.
Whereas we generated need-based metrics to examine the allocation of key health services, one limitation we faced was not knowing whether the initial configuration of clinics offering ART was based on perceived need or some other defined metrics. Incorporating such metrics as a known baseline could have increased confidence in the results of our location-allocation analysis. Additionally, having detailed information on the number of ART visits for HIV-infected individuals could have improved the robustness of our results.
6. Concluding remarks
This research improves our understanding of how distance and access to high quality healthcare varies depending on how need is defined. The goal is to provide informed decisions of how healthcare should be allocated to vulnerable groups within society, using the initial deployment of ART as an example and this study offers a lesson on how to best allocate limited healthcare resources for future interventions. Few studies consider the inherent geography and solely rely on Euclidean distance when considering how vital resources should be allocated. However, this does not provide an optimal solution as it does not accurately represent reality. This analysis made use of the existing road network by analyzing how people travel to their nearest health clinic for care. This analysis is meant to provide policymakers with a framework for resource allocation and can be considered a lesson in modeling access to healthcare.
Health clinics are a primary policy mechanism that are supposed to provide valuable resources and care to those who need it most. Therefore, access to these facilities is vital for health-affected households to mitigate the potential negative effects of illness and disease. Because agriculture is such a laborious endeavor, access to ART and other similarly complex services is vital for allowing affected individuals to maintain their livelihoods and provide for their household. In the context of universalization of ART services that increasingly characterize rural Mozambique and similar settings, future work should capture and analyze the frequency and duration of ART stock-outs, as well as the number of patients initiating and continuing ART. It would also be interesting to examine whether or not the allocation of ART and other high-quality services are related to political priorities and varies across areas that are seen more or less loyal to the ruling regime. HIV services may have a history of suboptimal deployment and occasional current failures, but they are almost fully universal at this point. While this study examined the location-allocation issues in the context of early rollout of ART, it is highly relevant to other health challenges as its results can be extrapolated to other vital health care services applications. Thus, our study offers policymakers valuable guidance for distributing these services in resource-constrained settings.
Although equality in access to healthcare is a crucial policy goal, this goal is rarely achieved in practice—not only because resources are scarce but also because of how resources are allocated. The needs of disadvantaged populations should be a focus when striving for equity in health, and thus any improvements in the current healthcare system that provide these populations with opportunities to invest in their health should be the top priority for policymakers. By emphasizing the surrounding geography, accounting for the level of quality, and identifying vulnerable groups within society, this analysis presents a framework for deciding how to expand health services, while providing a more nuanced blueprint for future planning.
Highlights.
The way ‘need’ is defined matters for allocating limited healthcare resources.
The initial allocation of antiretroviral therapy is sub-optimally distributed.
Proper allocation of health services is an integral part of investing in human capital.
Acknowledgments
We are grateful for the generous support from the Eunice Kennedy Shriver Institute of Child Health & Human Development (R21HD048257, R01HD058365), as well as for the helpful comments from anonymous reviewers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The 2009 Mozambican AIDS Indicator Survey (AIS) was explored for potential assessment of HIV burden in this analysis. However, the AIS was not available to inform the initial allocation of ART in Mozambique, in general, and in the study area, in particular. Likewise, the AIS data could not be taken into account in the selection of villages for the initial wave (2006) of the women’s health survey. Moreover, the AIS was based on a national sample, which could not provide sufficient information on variation in the HIV burden throughout the study area. Yet, because the study area is relatively small and economically and culturally homogenous, this variation is believed to be minimal.
Contributor Information
Zan M. Dodson, University of Maryland, College Park, Department of Geographical Sciences, 2181 Samuel J. LeFrak Hall, College Park, MD 20742, United States.
Victor Agadjanian, University of Kansas, Department of Sociology, Lawrence, KS 66045, United States, (785) 864-9482.
Julia Driessen, University of Pittsburgh, Graduate School of Public Health, 130 De Soto Street, A614 Crabtree, Pittsburgh, PA 15261, United States, (412) 624-2475.
References
- Agadjanian V, Arnaldo C, Cau B. Health costs of wealth gains: labor migration and perceptions of HIV/AIDS risks in Mozambique. Social forces. 2011;89(4):1097–1117. doi: 10.1093/sf/89.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuedo-Dorantes C, Pozo S. Migration, remittances, and male and female employment patterns. The American economic review. 2006:222–226. [Google Scholar]
- Audet CM, Burlison J, Moon TD, Sidat M, Vergara AE, Vermund SH. Sociocultural and epidemiological aspects of HIV/AIDS in Mozambique. BMC international health and human rights. 2010;10(1):15. doi: 10.1186/1472-698X-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett VL, Eaton DJ, Church RL. Selecting sites for rural health workers. Social Science & Medicine. 1982;16(1):63–72. doi: 10.1016/0277-9536(82)90424-5. [DOI] [PubMed] [Google Scholar]
- Calvo AB, Marks DH. Location of health care facilities: an analytical approach. Socio-Economic Planning Sciences. 1973;7(5):407–422. [Google Scholar]
- Canning D. The economics of HIV/AIDS in low-income countries: the case for prevention. The Journal of Economic Perspectives. 2006;20(3):121–142. doi: 10.1257/jep.20.3.121. [DOI] [PubMed] [Google Scholar]
- Cromley EK, McLafferty SL. GIS and public health. Guilford Press; 2011. [Google Scholar]
- Donabedian A. Aspects of medical care administration. Harvard University Press; Cambridge, MA: 1973. [Google Scholar]
- de Sherbinin A, VanWey LK, McSweeney K, Aggarwal R, Barbieri A, Henry S, Hunter LM, Twine W, Walker R. Rural household demographics, livelihoods and the environment. Global Environmental Change. 2008;18(1):38–53. doi: 10.1016/j.gloenvcha.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHS. ICF Marco, Measure DHS. Mozambique AIS 2009. 2009 http://www.measuredhs.com/pubs/pdf/HF33/HF33.pdf. Last accessed, February 22, 2016.
- Dodson ZM, Dempewolf J, Silva JA. Does prolonged illness contribute to adaptive land use practices among subsistence agricultural households in rural Mozambique? Applied Geography. 2016;67:109–118. [Google Scholar]
- ESRI. Environmental Systems Research Institute. Algorithms used by the ArcGIS Network Analyst Extension. 2016 http://desktop.arcgis.com/en/arcmap/latest/extensions/network-analyst/algorithms-used-by-network-analyst.htm#ESRI_SECTION1_6FFC9C48F24746E182082F5DEBDBAA92. Last accessed: November 12, 2016/
- FAO. Food and Agriculture Organization of the United Nations. Rome based agencies work together to ensure food security in Mozambique. FAO In Action. 2015 http://www.fao.org/in-action/rome-based-agencies-work-together-to-ensure-food-security-in-mozambique/en/. Last accessed: February 19, 2016.
- Filmer D, Pritchett L. The effect of household wealth on educational attainment: evidence from 35 countries. Population and development review. 1999;25(1):85–120. [Google Scholar]
- Filmer D, Kinnon S. Assessing asset indices. Demography. 2012;49(1):359–392. doi: 10.1007/s13524-011-0077-5. [DOI] [PubMed] [Google Scholar]
- Giesbert L, Schindler K. Assets, shocks, and poverty traps in rural Mozambique. World Development. 2012;40(8):1594–1609. [Google Scholar]
- Hakimi SL. Optimum locations of switching centers and the absolute centers and medians of a graph. Operations research. 1964;12(3):450–459. [Google Scholar]
- Hakimi SL. Optimum distribution of switching centers in a communication network and some related graph theoretic problems. Operations Research. 1965;13(3):462–475. [Google Scholar]
- Hawkes C, Ruel M. The links between agriculture and health: an intersectoral opportunity to improve the health and livelihoods of the poor. Bulletin of the World Health Organization. 2006;84(12):984–990. doi: 10.2471/blt.05.025650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AE, Bantock PR. Measuring potential physical accessibility to general practitioners in rural areas: a method and case study. Social science & medicine. 1982;16(1):85–90. doi: 10.1016/0277-9536(82)90428-2. [DOI] [PubMed] [Google Scholar]
- Joseph AE, Phillips DR. Accessibility and utilization: geographical perspectives on health care delivery. Sage; 1984. [Google Scholar]
- Khan MM, Hotchkiss DR, Berruti AA, Hutchinson PL. Geographic aspects of poverty and health in Tanzania: does living in a poor area matter? Health policy and planning. 2006;21(2):110–122. doi: 10.1093/heapol/czj008. [DOI] [PubMed] [Google Scholar]
- King B. Political ecologies of health. Progress in Human Geography 2009 [Google Scholar]
- Klinman MG, Reason CJC. On the peculiar storm track of TC Favio during the 2006–2007 Southwest Indian Ocean tropical cyclone season and relationships to ENSO. Meteorology and Atmospheric Physics. 2008;100(1–4):233–242. [Google Scholar]
- Kumar N. Changing geographic access to and locational efficiency of health services in two Indian districts between 1981 and 1996. Social science & medicine. 2004;58(10):2045–2067. doi: 10.1016/j.socscimed.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Lokshin M, Glinskaya E. The World Bank Economic Review. 2009. The effect of male migration on employment patterns of women in Nepal; p. lhp011. [Google Scholar]
- Mabunda S, Aponte JJ, Tiago A, Alonso P. A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malaria Journal. 2009;8:74. doi: 10.1186/1475-2875-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin J. Assessing the impact of HIV/AIDS on economic growth and rural agriculture in Africa. Journal of International Affairs. 2005;58(2):267. [Google Scholar]
- Obrist B, Iteba N, Lengeler C, Makemba A, Mshana C, Nathan R, Alba S, Dillip A, Hetzel MW, Mayumana I, Schulze A, Mshinda H. Access to health care in contexts of livelihood insecurity: a framework for analysis and action. PLoS Medicine. 2007;4(10):e308. doi: 10.1371/journal.pmed.0040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osleeb JP, McLafferty S. A weighted covering model to aid in dracunculiasis eradication. Papers in Regional Science. 1992;71(3):243–257. [Google Scholar]
- PARPA. International Monetary Fund (IMF) IMF; Washington D.C.: 2007. Republic of Mozambique: Action Plan for the Reduction of Absolute Poverty 2006–2009 (PARPA II) (IMF Country Report 07/37). [Google Scholar]
- Ratick SJ, Osleeb JP, Hozumi D. Application and extension of the Moore and ReVelle hierarchical maximal covering model. Socio-Economic Planning Sciences. 2009;43(2):92–101. [Google Scholar]
- ReVelle CS, Swain RW. Central facilities location. Geographical analysis. 1970;2(1):30–42. [Google Scholar]
- Ricketts TC. Geographic methods for health services research: A focus on the rural-urban continuum 1994 [Google Scholar]
- Sicuri E, Bardají A, Nhampossa T, Maixenchs M, Nhacolo A, Nhalungo D, Alonso PL, Menéndez C. Cost-effectiveness of intermittent preventive treatment of malaria in pregnancy in southern Mozambique. PLoS One. 2010;5(10):e13407. doi: 10.1371/journal.pone.0013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JA. A multilevel analysis of agricultural trade and socioeconomic inequality in rural Mozambique. The Professional Geographer. 2008;60:174–189. [Google Scholar]
- Tanser F, Gijsbertsen B, Herbst K. Modelling and understanding primary health care accessibility and utilization in rural South Africa: an exploration using a geographical information system. Social Science & Medicine. 2006;63(3):691–705. doi: 10.1016/j.socscimed.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Thornton PK, Boone RB, Galvin KA, BurnSilver SB, Waithaka MM, Kuyiah J, Karanja S, Gonzalez-Estrada E, Herrero M. Coping strategies in livestock-dependent households in East and southern Africa: a synthesis of four case studies. Human Ecology. 2007;35(4):461–476. [Google Scholar]
- Turshen M. The political ecology of disease. Review of Radical Political Economics. 1977;9(1):45–60. doi: 10.1177/048661347700900104. [DOI] [PubMed] [Google Scholar]
- Ulrich A, Speranza CI, Roden P, Kiteme B, Wiesmann U, Nüsser M. Small-scale farming in semi-arid areas: Livelihood dynamics between 1997 and 2010 in Laikipia, Kenya. Journal of Rural Studies. 2012;28(3):241–251. [Google Scholar]
- Walker TS, Pitoro Raul, Tomo Alda, Sitoe Isabel, Salencia Celestino, Mahanzule Rosalina, Donovan Cynthia, Mazuze Feliciano M. Priority setting for public-sector agricultural research in Mozambique with the National Agricultural Survey Data 2006 [Google Scholar]
- Wang F. Quantitative methods and applications in GIS. CRC Press; 2006. [Google Scholar]
- WHO. World Health Organization. Mozambique progress report: High level Global Partners Forum PMTCT. Presented at Global Partners Forum 2007; Johannesburgh, South Africa. November 26–27, 2007.2007. [Google Scholar]
- World Bank. Development Indicators Mozambique. The World Bank. 2015 http://data.worldbank.org/country/mozambique.
- Yao J, Murray AT, Agadjanian V, Hayford SR. Geographic influences on sexual and reproductive health service utilization in rural Mozambique. Applied Geography. 2012;32(2):601–607. doi: 10.1016/j.apgeog.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Murray AT, Agadjanian V. A geographical perspective on access to sexual and reproductive health care for women in rural Africa. Social Science & Medicine. 2013;96:60–68. doi: 10.1016/j.socscimed.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Agadjanian V, Murray AT. Spatial and social inequities in HIV testing utilization in the context of rapid scale-up of HIV/AIDS services in rural Mozambique. Health & place. 2014;28:133–141. doi: 10.1016/j.healthplace.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Murray AT. Locational effectiveness of clinics providing sexual and reproductive health services to women in rural Mozambique. International Regional Science Review. 2014;37(2):172–193. [Google Scholar]


