Abstract
We used event-related functional MRI to study awareness of prior episodes during memory retrieval and its relationship to the intention to retrieve memories. Participants completed cues with words from a prior list (intentional test) or with the first words that came to mind (incidental test). During both tests, explicit memory was separated from priming in the absence of explicit memory. Priming was associated with hemodynamic decreases in left fusiform gyrus and bilateral frontal and occipital brain regions; explicit memory was associated with bilateral parietal and temporal and left frontal increases. Retrieval intention did not change these patterns but was associated with activity in right prefrontal cortex. Our results provide firm evidence that implicit and explicit memory have distinct functional neuroanatomies, and that strategic control of retrieval engages brain structures distinct from those involved in both implicit and explicit memory. They have critical implications for theories of memory and consciousness, which often equate consciousness with control.
The neuroscience of human memory has been dominated by distinctions between forms of memory that involve different kinds of consciousness. Foremost is the distinction between explicit and implicit memory (1). Explicit memory involves conscious remembering of prior episodes, often by means of intentional retrieval of those episodes, whereas implicit memory involves influences of prior episodes on current behavior without intentional retrieval, and sometimes without conscious remembering of those prior episodes. Many studies of implicit memory have focused on priming, the facilitated processing of stimuli as a function of prior exposure, an important mechanism by which memory facilitates perception.
Tulving and Schacter (2) proposed that priming and explicit memory depend on distinct neural systems. Although there is support for this view (3–7), a separation at the level of functional neuroanatomy has not yet been firmly established, owing to conceptual and methodological ambiguities in prior neuroimaging studies (6, 8). These studies have either compared incidental tests (in which participants respond with the first item coming to mind) with intentional tests (in which participants try to retrieve studied items) or have only used incidental tests (6). Brain activity in incidental tests can, however, reflect not only priming but also unintentional conscious remembering of prior episodes (unintentional or involuntary explicit memory) (4, 8–13), and sometimes contamination by intentional retrieval of prior episodes. Moreover, brain activity in intentional tests reflects not only explicit memory for specific episodes but also the general intention to retrieve prior episodes, or retrieval mode (14–18). Addressing these ambiguities has awaited a theoretical approach (8, 12) that distinguishes (i) implicit and explicit memory for specific episodes from retrieval intention, and, more specifically, (ii) unintentional implicit memory from unintentional and intentional explicit memory. The approach prescribes a behavioral paradigm that permits this separation (4, 8–11), which we here implemented for functional MRI. Our results provide firm evidence that priming and explicit memory are neuroanatomically separable, and that retrieval intention engages brain structures distinct from those involved in both priming and explicit memory.
Participants completed three-letter word stems, either with words from a prior study list (intentional test), or with the first word that came to mind (incidental test). In both cases, they indicated whether the completion was from the prior study list. They completed stems covertly, and responded orally with their completions between trials. Stems of studied words completed with studied words judged not to be from the study list were defined as primed items, and stems of studied words completed with studied words judged to be from the study list were defined as remembered items. Stems of unstudied words completed with unstudied words judged not to be from the study list were defined as correct rejections (CRs), thus providing comparison items for which there was no memory. The primed items gave a measure of priming that was far less likely to be contaminated by either unintentional or intentional explicit memory, as compared with priming measures used in prior neuroimaging studies.
Experiment 1 used only an intentional test (4, 10) to gain large numbers of primed, remembered, and CR observations. Prior studies (6, 19, 20) suggest that priming is associated with hemodynamic response decreases in occipital (extrastriate), inferior temporal, and prefrontal cortices, perhaps reflecting improved perception and identification processes, whereas explicit memory is associated with response increases in medial temporal, parietal, and prefrontal brain regions (21, 22). We used these data to guide our imaging contrasts. Experiment 2 compared intentional and incidental tests to assess whether the neural correlates of priming and explicit memory interacted with retrieval intention, and whether retrieval intention and explicit memory for specific episodes engaged similar brain regions. Experiment 2 also used fixation periods that permitted neural responses to CRs alone to be contrasted across the intentional and incidental tests, allowing the purest assessment possible of the correlates of retrieval intention (11, 18, 23).
Methods
Experiment 1. Participants. Twenty-five healthy right-handed native German speakers (18–36 years of age, with 19 being female) volunteered for paid participation with written informed consent. Scanning occurred at the Magdeburg University Faculty of Medicine, in accordance with its ethics committee guidelines.
Paradigm. The experiment consisted of two parts, each comprising a study phase of 160 trials, and a test phase of 240 trials. The materials were 480 German three-letter word stems and corresponding target words with a mean probability of target completion of 0.40 in the absence of prior study of the target word (A.R.-K., unpublished normative data). Within the set, each stem was unique. The complete words were presented at study, and the stems were presented at test. Of 240 stems in each test phase, 160 could be completed with the corresponding words from the study list. Studied/unstudied status of words was counterbalanced across participants. In the study phases, participants counted the syllables of each word appearing on the screen (10, 11), and responded by pressing a button with either their right or left index finger for one or two syllables or more than two syllables, respectively (hand counterbalanced across participants). Participants were told not to try to memorize the words. Each study trial consisted of a ready cue (“?”) for 400 ms, a central fixation cross for 150 ms, a word for 1,000 ms, and a further fixation cross for 1,200 ms.
In the test phases, participants were instructed to complete each stem with a word from the preceding study list, if possible, but with the first word that came to mind if they could not remember a studied word. Participants indicated by pressing a button if they had completed each stem with a studied or an unstudied word (left and right index fingers for studied and unstudied, respectively, counterbalanced across participants), but did not respond orally until a speech cue appeared. Making false-positive study-list membership judgments was discouraged. Each test trial consisted of a central fixation cross for 1,000 ms, a word stem for 300 ms, an asterisk for 3,200 ms, and a cue of three exclamation marks (“!!!”) for 2,000 ms, which prompted participants to respond orally with the word they had used to complete the stem. Oral responses were recorded with a microphone at the bottom end of the head coil and scored as target vs. nontarget words. Fig. 5, which is published as supporting information on the PNAS web site, shows the test trial structure and data categories.
MRI scanning. Echo-planar images were acquired on a GE Medical Systems Signa 1.5-T MRI scanner (repetition time = 2.0 s; echo time = 35 ms). Images consisted of 23 axial slices [64 × 64, voxel size = 3.13 × 3.13 × 6 mm (slice thickness = 5 mm with 1 mm gap)] and were acquired in an interleaved manner (1–23 in steps of two, 2–22 in steps of two, from bottom to top). A total of 788 volumes were acquired during each of two test sessions (i.e., the stem-completion test phases). The first three volumes of each session were discarded.
Data processing and analysis. Data analysis was performed by using statistical parametric mapping (spm2, Wellcome Department of Imaging Neuroscience, London). Echo-planar images were corrected for acquisition delay, realigned, normalized (voxel size: 3 × 3 × 3 mm), and smoothed (Gaussian kernel: 8 × 8 × 8 mm). A high-pass filter (cutoff period = 128 s) was applied to the data and model. Statistical analysis was performed in two stages of a mixed-effects model. In the first stage, neural activity was modeled by a delta function at stimulus onset. The ensuing hemodynamic response was modeled by convolving these delta functions with a canonical hemodynamic response function (HRF) (24). The resulting time courses were down-sampled each scan to form covariates in a general linear model. Separate covariates were modeled for the conditions of interest, one time-locked to each speech event, six for the rigid-body movement parameters determined from realignment, and a single covariate representing the mean (constant) over scans. Contrasts of the parameter estimates for each covariate comprised the data for the second-stage analyses, which treated participants as a random effect. Specifically, images of each contrast of interest for the canonical HRF were entered into one-sample Student's t tests.
The critical events at test were the onsets of (i) stems of studied words completed with studied target words judged to be from the study list (remembered items), (ii) stems of studied words completed with studied target words judged not to be from the study list (primed items), and (iii) stems of unstudied words completed with unstudied words judged not to be from the study list (CRs). For imaging purposes, the CRs included stems completed with nontarget as well as target words. The one-tailed Student t test planned comparisons were as follows: to isolate the neural correlates of priming, responses to primed items were subtracted from responses to CRs; to isolate the neural correlates of explicit memory, responses to primed items were subtracted from responses to remembered items. The significance level was set to 0.001 (uncorrected), with a minimum of five adjacent voxels. Coordinates of local maxima of activation were converted into the Talairach reference frame (25).
Experiment 2. Participants. Sixteen healthy right-handed native German speakers (20–41 years of age, with eight being female) volunteered for paid participation, with written informed consent. Scanning occurred at the Wellcome Department of Imaging Neuroscience, London, in accordance with local ethics approval.
Paradigm. The experiment resembled experiment 1 (see Fig. 5), including the use of the same counterbalancing procedures and German materials; only the differences from experiment 1 are described. The main difference was that the two test phases had different instructions: One test phase used intentional test instructions as in experiment 1; the other test phase used incidental test instructions, that is, participants completed each stem with the first word that came to mind, then indicated by pressing a button if they happened to remember it from the study phase (9, 10). Order of intentional and incidental tests was counterbalanced across participants. This procedure, together with the counterbalancing procedure of experiment 1, ensured that all words and stems appeared in both the intentional and the incidental tests across participants. The test trial timing differed from experiment 1, so that speech occurred only during a 1-s gap between volume acquisitions, reducing movement artifacts in the data owing to oral responses (26). Following the central fixation cross for 1,000 ms and word stem for 300 ms, the asterisk was presented for 3,700 ms, and then the speech cue (“!!!”) was presented for 1,000 ms during the gap between image acquisitions. A final difference was the insertion of 18 s of fixation every eight trials.
MRI scanning. Echo-planar images were acquired on a Siemens 1.5-T Sonata scanner with an echo time of 50 ms. Images consisted of 22 axial slices [64 × 64, voxel size = 3 × 3 × 5 mm (slice thickness = 3 mm with 2 mm gap)] and were acquired in a sequential, descending manner (excluding cerebellum). The volume acquisition time was 2.0 s and the volume repetition time was 3.0 s, resulting in a 1.0-s gap between acquisitions to accommodate the speech (26). A total of 605 volumes were acquired during each of two test sessions (i.e., the stem-completion test phases). The first five volumes of each session were discarded.
Data processing and analysis. These resembled experiment 1, except that interactions of the planned comparisons used in experiment 1 with the new factor of retrieval intention were tested (ANOVA). Also, the fixation periods in experiment 2 allowed more efficient estimation of the main effect of events vs. baseline (27), permitting a contrast (two-tailed Student's t test) of the hemodynamic response for CRs alone across the intentional and incidental tests.
Results
Behavioral Results. Table 1 shows, for test stems corresponding to studied and unstudied words, the proportions completed with target and nontarget words, broken down by study-list membership judgment. Table 2, which is published as supporting information on the PNAS web site, shows reaction times for the study-list membership judgments. In experiment 1, collapsing across list-membership judgment, stems of studied words yielded a higher mean proportion of target completions than did stems of unstudied words (studied words: 0.64, SD = 0.06; unstudied words: 0.41, SD = 0.07), t(24) = 16.0, P < 0.001. In experiment 2, collapsing across list-membership judgment, stems of studied words also yielded higher mean proportions of target completions (incidental test: 0.61, SD = 0.08; intentional test: 0.64, SD = 0.06) than did stems of unstudied words (incidental test: 0.36, SD = 0.08; intentional test: 0.37, SD = 0.07), and two-way (incidental vs. intentional × studied vs. unstudied) ANOVA revealed only a main effect of prior study, F(1, 15) = 367.4, P < 0.001. To examine priming in the absence of explicit memory, we restricted analysis to stems completed with words judged as unstudied, and computed, separately for stems of studied and unstudied words, the proportions of each stem type completed with target words. In experiment 1, stems of studied words yielded a higher mean proportion of target completions than did stems of unstudied words (studied words: 0.57, SD = 0.09; unstudied words: 0.47, SD = 0.07), t(24) = 6.3, P < 0.001. In experiment 2, stems of studied words also yielded higher mean proportions of target completions (incidental test: 0.59, SD = 0.12; intentional test: 0.58, SD = 0.12) than did stems of unstudied words (incidental test: 0.43, SD = 0.13; intentional test: 0.43, SD = 0.10), and two-way (incidental vs. intentional × studied vs. unstudied) ANOVA revealed only a main effect of prior study, F(1, 15) = 76.1, P < 0.001. The results for both experiments demonstrate priming in the absence of explicit memory in both intentional and incidental tests. In experiment 2, intentional test instructions resulted in a higher proportion of remembered words than did incidental test instructions (see Table 1, second column), t(15) = 3.4, P < 0.01, which is consistent with prior behavioral data (10, 11).
Table 1. Mean word-stem completion proportions at test (relative to total number of word stems; with SDs in parentheses) in experiments 1 and 2.
| Stems of studied words
|
Stems of unstudied words
|
||||||
|---|---|---|---|---|---|---|---|
| Experiment | Overall | Remembered target | Primed target | Forgotten (nontarget) | Overall | CR target | CR nontarget |
| Experiment 1 | 0.93 (0.05) | 0.32 (0.11) | 0.32 (0.11) | 0.23 (0.05) | 0.87 (0.07) | 0.36 (0.08) | 0.39 (0.06) |
| International | |||||||
| Experiment 2 | |||||||
| International | 0.92 (0.04) | 0.31 (0.10) | 0.33 (0.13) | 0.22 (0.05) | 0.85 (0.07) | 0.30 (0.10) | 0.40 (0.10) |
| Incidental | 0.92 (0.05) | 0.20 (0.11) | 0.41 (0.12) | 0.28 (0.07) | 0.86 (0.08) | 0.36 (0.10) | 0.45 (0.11) |
Overall, total proportion of completed stems; remembered, stem completed with studied target word judged as studied; primed, stem completed with studied target word judged as unstudied; forgotten, stem completed with unstudied (i.e., nontarget) word; CR target, stem completed with unstudied target word judged as unstudied; CR nontarget, stem completed with unstudied nontarget word judged as unstudied. Differences of overall completion proportions from 1.0 are due to unscorable (e.g., inaudible) responses and uncompleted stems. Differences of overall completion proportions from the sum of the individual response categories (remembered plus primed plus nontarget for studied; CR target plus CR nontarget for unstudied) are because of small proportions of false alarms (stems completed with unstudied words judged as studied).
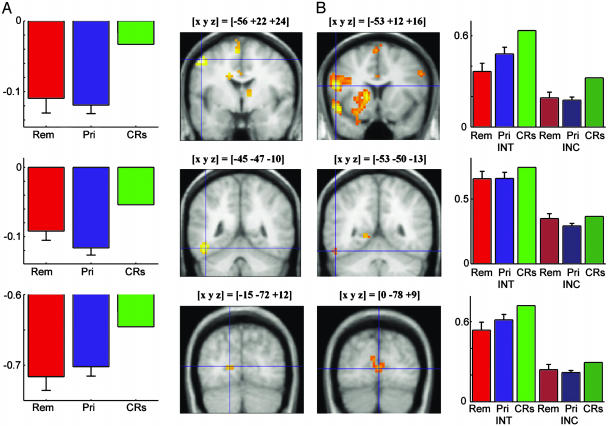
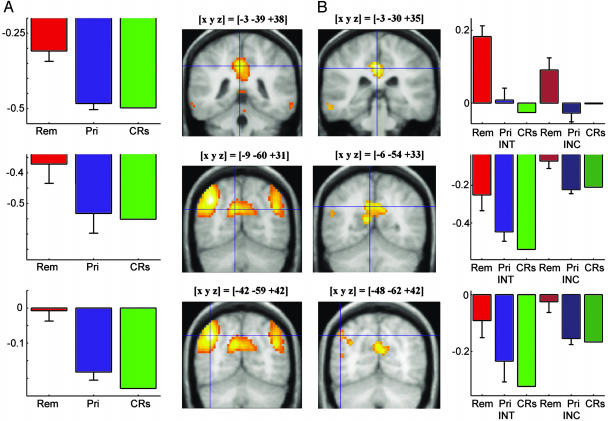
Experiment 1 fMRI Results. Compared with CRs, primed items elicited significantly decreased hemodynamic responses in several brain regions, including the left inferior temporooccipital junction (fusiform gyrus and inferior temporal gyrus), parts of the medial occipital cortex (bilateral cuneus and bilateral lingual gyrus), and the left and right inferior frontal gyrus (Fig. 1A). There was also a decreased hemodynamic response for primed items in the left medial temporal lobe (MTL) (Fig. 3). Table 3, which is published as supporting information on the PNAS web site, summarizes local maxima of activation in the CRs minus primed contrast in experiments 1 and 2. Fig. 2A displays significant increases in hemodynamic responses for remembered items in comparison with primed items. Remembered items were associated with extensive activation of bilateral parietal regions, including the precuneus, the superior and inferior parietal lobule, and the posterior cingulate; other regions that showed activations included the left superior and middle frontal gyri, bilateral temporal cortices, and the left MTL. Table 4, which is published as supporting information on the PNAS web site, shows the local maxima of activation in the remembered minus primed contrasts of experiments 1 and 2. A decreased hemodynamic response for remembered items compared with CRs was observed in several areas that also showed a decreased response for primed items compared with CRs, including prefrontal and occipital brain regions, and the left fusiform and inferior temporal gyri (data not shown).
Fig. 1.
Brain activity differences related to priming (implicit memory) in experiments 1 (A) and 2 (B). Compared with primed items, CRs showed greater activation in the left prefrontal cortex (Top), left fusiform gyrus (Middle), and right and left extrastriate cortex (Bottom). Activation threshold of P < 0.001 uncorrected, with a minimum of five adjacent voxels. Bar plots display peak percentage signal change of best-fitting canonical hemodynamic response function relative to mean signal over all voxels and scans; error bars show SE of the difference between remembered/primed items and CRs. Int, intentional test, Inc, incidental test, Rem, remembered, Pri, primed. Experiment 1 did not allow efficient estimation of baseline activity, and thus the absolute values of the parameter estimates are poorly estimated (i.e., the value of 0 on the y axis is somewhat arbitrary), although the differences between parameters are well estimated (27). Experiment 2 included periods of fixation to measure baseline, thus positive values are more accurate indications of activations relative to baseline (i.e., only the relative, not the absolute, values of parameter estimates can be compared across the experiments).
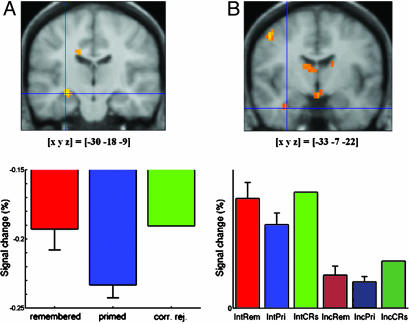
Fig. 3.
MTL activations in experiments 1 (A) and 2 (B). In both experiments, a region in the MTL showed lower activation for primed items compared with CRs. See the Fig. 1 legend for more details.
Fig. 2.
Brain activity differences related to conscious remembering (explicit memory) in experiments 1 (A) and 2 (B). Compared with primed items (and CRs), remembered items showed greater activation in the posterior cingulate (Top), precuneus (Middle), and inferior parietal lobule (Bottom) bilaterally. See the Fig. 1 legend for more details.
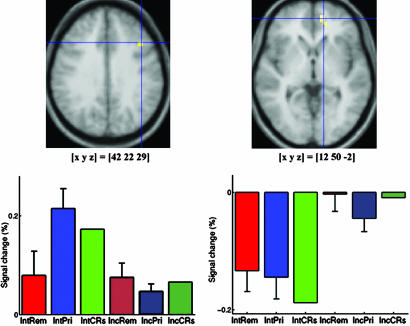
Experiment 2 fMRI Results. The comparison of primed items with CRs, collapsing across incidental and intentional tests, revealed a pattern very similar to that in experiment 1 (Figs. 1B and 3). None of the regions that were revealed showed a significant interaction with retrieval intention, with the exception of lingual gyrus [–18, –90, –2; Brodmann area (BA) 17], which showed a priming-related deactivation in the incidental, but not in the intentional, test. The comparison of remembered and primed items, collapsing across incidental and intentional tests, also revealed a pattern of lateral and medial parietal activations similar to that in experiment 1 (Fig. 2B). Tables 3 and 4 show voxels with local activation maxima that were also activated significantly in experiment 1 (P < 0.001, uncorrected). None of these regions showed a significant interaction with retrieval intention. However, four other regions showed a significant interaction: Right middle frontal gyrus (42, 22, 29; BA 9), left middle occipital gyrus (–15, –87, 18; BA 18), right anterior superior temporal gyrus (45, –17, 1; BA 22), and right posterior superior temporal gyrus (50, –55, 14; BA 39). All four regions showed greater activation for primed than remembered items in the intentional test, and little evidence of a difference in the incidental test (Fig. 4 Left).
Fig. 4.
Brain activity differences related to retrieval intention in experiment 2. (Left) A right middle frontal gyrus region showed an increased response for primed items (and CRs), but not for remembered items during intentional compared with incidental retrieval. (Right) A right anterior prefrontal region showed a decreased response for CRs (and primed and remembered items) during intentional compared with incidental retrieval. See the Fig. 1 legend for more details.
The comparison of incidental and intentional tests restricted to CRs only revealed three regions: Left and right posterior superior frontal gyrus (–18, 11, 55, and 15, 3, 63; BA 6), and right medial anterior prefrontal cortex (12, 50, –2; BA 10). The former two regions showed greater activation for CRs in the intentional compared with the incidental test, whereas the latter region showed greater activation for CRs in the incidental compared with the intentional test (Fig. 4 Right).
Discussion
Our results provide firm evidence that priming and explicit memory have distinct neuroanatomical correlates, by comparing the hemodynamic activity correlates of priming in the absence of explicit memory with those of explicit memory, a behavioral approach that has proven fruitful in electrophysiological studies (3–5). Moreover, the results show that retrieval intention has little influence on these distinct activation patterns. Instead, differences between intentional and incidental retrieval occur in other brain regions. Consequently, neurocognitive theories of memory retrieval must distinguish levels of theoretical description relating to awareness of memory (i.e., implicit vs. explicit memory for specific study episodes) and to strategic control of retrieval (i.e., intentional vs. incidental retrieval). Current theories and models of memory retrieval (e.g., refs. 28 and 29) often conflate these levels of description. Prior studies of the functional neuroanatomy of priming and explicit memory have also suffered from this conflation (6), because they simply compared incidental and intentional memory tests, or used only incidental tests. Consequently, brain activity attributed to priming could have reflected unintentional (or involuntary) explicit memory (4, 6, 8, 13), or even contamination by intentional retrieval, and brain activity attributed to explicit memory could have reflected both explicit memory for specific episodes and the general intention to retrieve prior episodes (18). Our conceptual and methodological approach (4, 8–12) overcomes these ambiguities.
Priming was evident in higher proportions of target completions for stems of studied words as compared with stems of unstudied words, even when those words were not judged as studied, and was associated with decreased hemodynamic responses in several brain regions (Fig. 1 and Table 3). The robustness of these findings is indicated by their replication across two experiments performed in different laboratories and using different scanners and acquisition parameters. As in previous studies (6, 20), priming-related response decreases were found in bilateral occipital and inferior temporal regions. The current results show that response decreases in these brain regions occur in the absence of awareness of the previous study episode, and thus reflect true implicit memory, rather than unintentional or intentional explicit memory. Response decreases in these areas also occurred for remembered items (data not shown), as well as primed items, demonstrating that priming-related response decreases occur irrespective of awareness of the study episode, which is consistent with the notion that priming sometimes or always accompanies explicit memory. Prefrontal regions (BA 9, 44, and 46 bilaterally, and BA 6, 8, and 47 on the left) also showed response decreases for primed items (and for remembered items). Left frontal decreases have been related to conceptual priming (30). The current frontal deactivations may also reflect phonological priming (31), in view of behavioral evidence that word-stem completion priming involves perceptual and lexical processing, but not conceptual processing (10, 11, 32).
A further region that showed a greater response to CRs than to primed items was the left MTL (hippocampus in experiment 1 and hippocampus/perirhinal cortex in experiment 2; Fig. 3 and Table 3). Whereas left hippocampal activation during conscious remembering has been reported previously (33), and was observed in experiment 1 (Table 4), the MTL has not typically been implicated in priming. However, some argue that MTL pathology produces implicit memory impairment that often goes undetected (34–36), so that the present result might implicate the MTL in priming. An alternative explanation, accommodating the more common view that priming does not involve the MTL (6), is that this result reflects novelty detection without awareness. The MTL is known to play a role in novelty detection (37, 38), and parahippocampal regions can respond differentially to novel vs. repeated stimuli in absence of conscious awareness (39).
Regions that showed greater responses to remembered items than to primed items (Fig. 2 and Table 4) included bilateral parietal, posterior cingulate, and anterior prefrontal brain regions that have been previously linked with explicit memory (21, 22). However, the current results extend prior findings by showing that activations in these regions occur even when remembered items are compared with primed items, and not just with unstudied items. This comparison allows for the possibility that priming may accompany explicit memory, and thus links the activations more closely with explicit memory, rather than with priming.
In experiment 2, only one region implicated in priming showed an interaction with retrieval intention (i.e., lingual gyrus, BA 17), and no region implicated in explicit memory showed such an interaction, despite the fact that robust activity differences related to retrieval intention were observed in right frontal regions. These data are inconsistent with a simple two-fold distinction between an intentional or controlled retrieval process that is necessary for explicit memory, and an automatic retrieval process, responsible for priming, that cannot result in explicit memory (28, 29). Such models would imply that brain activity related to explicit memory should be modulated by retrieval intention, and that the brain regions involved in explicit memory and in intentional retrieval should not be dissociable. Instead, our data are consistent with the view that explicit memory can occur both automatically, such as during incidental retrieval, and as a result of deliberate retrieval attempts (7, 8, 12, 13). The latter view makes a distinction between processes of strategic control that may be directed toward retrieval of prior episodes or simply toward performance of a current task, and underlying retrieval processes that may or may not result in explicit memory for a prior episode, regardless of the retrieval orientation adopted (7, 8, 12).
Activity differences related to retrieval intention were observed in the response to CRs (vs. fixation baseline) in left and right posterior superior frontal gyrus (BA 6), and right medial anterior prefrontal cortex, BA 10 (Fig. 4 Right). Memory for specific study episodes is unlikely to be involved for CRs, so the current results allow these differences to be clearly attributed to retrieval intention (18). Activation of BA 10 has previously been shown to be sustained rather than stimulus-related, and has thus been regarded as a correlate of an intentional retrieval mode (15–17, 40). Our event-related analysis involved referencing of stimulus-related hemodynamic responses to interstimulus fixation periods, such that the size of the stimulus-related response is relative to any such sustained activity. Therefore, a relatively smaller stimulus-related response of BA 10 in the intentional than in the incidental test is compatible with a more sustained response of this region in the intentional test, which is plausible, given that the intentional test involved a general orientation toward the past. Recent evidence also suggests a role for BA 10 in orientation toward the future (i.e., preparation for processing of upcoming stimuli) (41), providing an alternative explanation. Activity in BA 6, on the other hand, has not been linked with a sustained intentional retrieval mode, and might index stimulus-related control processes, such as the use of each stem as a cue to retrieve a specific study episode, which might also involve an increased working memory load (42).
An interaction between retrieval intention and awareness of prior study (remembered vs. primed) was also observed in experiment 2 in four brain regions that were different to those implicated in explicit memory. In these regions, including the right middle frontal gyrus (BA 9), a response increase was observed for primed items (and CRs) relative to remembered items, but only in the intentional test (Fig. 4 Left). This pattern mirrors that of the reaction times for the study-list-membership judgments in the intentional test (Table 2). Intentional test instructions resulted in a larger increase in reaction times for primed items and CRs than for remembered items. The most likely explanation is that in an intentional test there is greater generation and rejection of candidate completions for primed items and CRs than for remembered items. Activity in these regions, therefore, may be related to postretrieval monitoring (18). Such a role has been previously hypothesized for the right dorsolateral prefrontal region (17, 22, 26). This account again suggests that the processes and brain regions involved in strategic control of retrieval are separable from those involved in priming and explicit memory.
In summary, we show that priming-related hemodynamic response decreases in occipital, inferior temporal, and prefrontal cortices occur in both the absence and presence of explicit memory, and independent of attempts to retrieve studied items. Parietal, temporal, and prefrontal activations associated with explicit memory are also observed regardless of retrieval intention. Retrieval intention and postretrieval monitoring are associated with responses in right prefrontal regions distinct from those implicated in priming and explicit memory. Neurocognitive theories of memory must therefore distinguish the processes involved in the strategic control of retrieval from the processes involved in explicit or implicit memory for specific prior episodes, which is in contrast to many current theoretical approaches that conflate these levels of description. Consciousness of memory in the sense of intended vs. unintended retrieval of prior episodes should not be conflated with consciousness of memory in the sense of awareness vs. absence of awareness of specific prior episodes.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 426, TP D3, the International Leibniz Program of Magdeburg University (to B.H.S and A.R-K.), and the Wellcome Trust.
Author contributions: B.H.S., R.N.H., A.R.-K., and E.D. designed research; B.H.S., R.N.H., C.B., and V.T. performed research; B.H.S., R.N.H., A.R.-K., and C.B. analyzed data; and B.H.S., R.N.H., A.R.-K., H.-J.H., and E.D. wrote the paper.
Abbreviations: CR, correct rejection; BA, Brodmann area; MTL, medial temporal lobe.
References
- 1.Graf, P. & Schacter, D. L. (1985) J. Exp. Psychol. Learn. Mem. Cognit. 11, 501–518. [DOI] [PubMed] [Google Scholar]
- 2.Tulving, E. & Schacter, D. L. (1990) Science 247, 301–306. [DOI] [PubMed] [Google Scholar]
- 3.Rugg, M. D., Mark, R. E., Walla, P., Schloerscheidt, A. M., Birch, C. S. & Allan, K. (1998) Nature 392, 595–598. [DOI] [PubMed] [Google Scholar]
- 4.Schott, B., Richardson-Klavehn, A., Heinze, H. J. & Duzel, E. (2002) J. Cognit. Neurosci. 14, 578–592. [DOI] [PubMed] [Google Scholar]
- 5.Paller, K. A., Hutson, C. A., Miller, B. B. & Boehm, S. G. (2003) Neuron 38, 507–516. [DOI] [PubMed] [Google Scholar]
- 6.Henson, R. N. (2003) Prog. Neurobiol. 70, 53–81. [DOI] [PubMed] [Google Scholar]
- 7.Moscovitch, M. (2000) in Oxford Handbook of Memory, eds. Tulving, E. & Craik, F. I. M. (Oxford Univ. Press, New York), pp. 609–626.
- 8.Richardson-Klavehn, A., Gardiner, J. M. & Java, R. I. (1996) in Implicit Cognition, ed. Underwood, G. (Oxford Univ. Press, Oxford), pp. 85–158.
- 9.Richardson-Klavehn, A. & Gardiner, J. M. (1995) Psychol. Res. 57, 166–178. [DOI] [PubMed] [Google Scholar]
- 10.Richardson-Klavehn, A. & Gardiner, J. M. (1996) Psychon. Bull. Rev. 3, 238–244. [DOI] [PubMed] [Google Scholar]
- 11.Richardson-Klavehn, A. & Gardiner, J. M. (1998) J. Exp. Psychol. Learn. Mem. Cognit. 24, 593–609. [DOI] [PubMed] [Google Scholar]
- 12.Richardson-Klavehn, A., Gardiner, J. M. & Ramponi, C. (2002) Memory 10, 349–364. [DOI] [PubMed] [Google Scholar]
- 13.Schacter, D. L. (1987) J. Exp. Psychol. Learn. Mem. Cognit. 13, 501–518. [DOI] [PubMed] [Google Scholar]
- 14.Tulving, E. (1983) Elements of Episodic Memory (Oxford Univ. Press, New York).
- 15.Duzel, E., Cabeza, R., Picton, T. W., Yonelinas, A. P., Scheich, H., Heinze, H. J. & Tulving, E. (1999) Proc. Natl. Acad. Sci. USA 96, 1794–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepage, M., Ghaffar, O., Nyberg, L. & Tulving, E. (2000) Proc. Natl. Acad. Sci. USA 97, 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugg, M. D., Fletcher, P. C., Frith, C. D., Frackowiak, R. S. & Dolan, R. J. (1997) NeuroReport 8, 1283–1287. [DOI] [PubMed] [Google Scholar]
- 18.Rugg, M. D. & Wilding, E. L. (2000) Trends Cogn. Sci. 4, 108–115. [DOI] [PubMed] [Google Scholar]
- 19.Schacter, D. L., Alpert, N. M., Savage, C. R., Rauch, S. L. & Albert, M. S. (1996) Proc. Natl. Acad. Sci. USA 93, 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schacter, D. L. & Buckner, R. L. (1998) Neuron 20, 185–195. [DOI] [PubMed] [Google Scholar]
- 21.Buckner, R. L. & Wheeler, M. E. (2001) Nat. Rev. Neurosci. 2, 624–634. [DOI] [PubMed] [Google Scholar]
- 22.Rugg, M. D., Otten, L. J. & Henson, R. N. (2002) Philos. Trans. R. Soc. London B 357, 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugg, M. D., Fletcher, P. C., Allan, K., Frith, C. D., Frackowiak, R. S. & Dolan, R. J. (1998) NeuroImage 8, 262–273. [DOI] [PubMed] [Google Scholar]
- 24.Friston, K. J., Fletcher, P., Josephs, O., Holmes, A., Rugg, M. D. & Turner, R. (1998) NeuroImage 7, 30–40. [DOI] [PubMed] [Google Scholar]
- 25.Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of The Human Brain Thieme, Stuttgart).
- 26.Henson, R. N., Shallice, T., Josephs, O. & Dolan, R. J. (2002) NeuroImage 17, 543–558. [PubMed] [Google Scholar]
- 27.Josephs, O. & Henson, R. N. (1999) Philos. Trans. R. Soc. London B 354, 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacoby, L. L. (1998) J. Exp. Psychol. Learn. Mem. Cognit. 24, 3–26. [DOI] [PubMed] [Google Scholar]
- 29.Jacoby, L. L., Toth, J. P. & Yonelinas, A. P. (1993) J. Exp. Psychol. Gen. 122, 139–154. [Google Scholar]
- 30.Buckner, R. L., Goodman, J., Burock, M., Rotte, M., Koutstaal, W., Schacter, D., Rosen, B. & Dale, A. M. (1998) Neuron 20, 285–296. [DOI] [PubMed] [Google Scholar]
- 31.Poldrack, R. A., Prabhakaran, V., Seger, C. A. & Gabrieli, J. D. (1999) Neuropsychology 13, 564–574. [DOI] [PubMed] [Google Scholar]
- 32.Craik, F. I., Moscovitch, M. & McDowd, J. M. (1994) J. Exp. Psychol. Learn. Mem. Cognit. 20, 864–875. [DOI] [PubMed] [Google Scholar]
- 33.Eldridge, L. L., Knowlton, B. J., Furmanski, C. S., Bookheimer, S. Y. & Engel, S. A. (2000) Nat. Neurosci. 3, 1149–1152. [DOI] [PubMed] [Google Scholar]
- 34.Jernigan, T. L., Ostergaard, A. L. & Fennema-Notestine, C. (2001) J. Int. Neuropsychol. Soc. 7, 63–78. [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard, A. L. (1999) J. Int. Neuropsychol. Soc. 5, 175–190. [DOI] [PubMed] [Google Scholar]
- 36.Ostergaard, A. L. & Jernigan, T. L. (1993) in Implicit Memory, eds. Graf, P. & Masson, M. E. J. (Erlbaum, Hillsdale, NJ), pp. 327–349.
- 37.Duzel, E., Habib, R., Rotte, M., Guderian, S., Tulving, E. & Heinze, H. J. (2003) J. Neurosci. 23, 9439–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunwald, T., Lehnertz, K., Heinze, H. J., Helmstaedter, C. & Elger, C. E. (1998) Proc. Natl. Acad. Sci. USA 95, 3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grunwald, T., Pezer, N., Munte, T. F., Kurthen, M., Lehnertz, K., Van Roost, D., Fernandez, G., Kutas, M. & Elger, C. E. (2003) NeuroImage 20, Suppl. 1, S139–S145. [DOI] [PubMed] [Google Scholar]
- 40.Velanova, K., Jacoby, L. L., Wheeler, M. E., McAvoy, M. P., Petersen, S. E. & Buckner, R. L. (2003) J. Neurosci. 23, 8460–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakai, K. & Passingham, R. E. (2003) Nat. Neurosci. 6, 75–81. [DOI] [PubMed] [Google Scholar]
- 42.Wager, T. D. & Smith, E. E. (2003) Cogn. Affect. Behav. Neurosci. 3, 255–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.