Abstract
Prostate specific membrane antigen (PSMA) scanning is a sensitive method of prostate cancer detection. In a 71 y.o. man with a PSA of 49 (6%F), 4 negative MRI studies and 6 negative biopsies over an 8 year interval, a 68Ga-PSMA PET/CT scan showed a PSMA-avid spot in the prostate. Using image fusion technology, the lesion was target-biopsied and Gleason 3 + 4 = 7 (cancer core length of 12 mm) was identified. This case may herald a new application for PSMA scanning and prostate cancer imaging.
Keywords: Prostate cancer, PSMA, mpMRI, Targeted biopsy
Abbreviations: mpMRI, multiparametric MRI; PSMA, prostate specific membrane antigen; 68Ga, 68Gallium; ROI, region of interest; CaP, prostate cancer
Introduction
68Ga-PSMA PET/CT was introduced for prostate cancer (CaP) scanning by Eder et al in 2012.1 For the detection of metastatic disease, the accuracy of PSMA scans compares favorably with all other imaging modalities.2 Sensitivity and specificity of 80% and 97%, respectively, have been reported for detection of nodal metastases or post-prostatectomy recurrences in the prostatic bed. Herein, we present a case where 68Ga-PSMA scanning provided the only clue to the location of a hard-to-find, serious cancer within the prostate.
Case presentation
A 71 year old Caucasian male was found on systematic biopsy to exhibit a 0.5 mm focus of Gleason 3 + 3 = 6 CaP (left lateral apex) in 2013, after 2 prior negative biopsies (2009, 2012) and a TURP (2012). PSA had risen from 2.6 ng/mL in 2009 to 8.5 ng/mL in May 2013. As part of an active surveillance cohort, he underwent repeat 3.0 T multiparametric MRI (Siemens MAGNETOM, Trio, Siemens Medical Solutions, Malvern, Pennsylvania) demonstrating no lesions and confirmatory systematic biopsies in late 2013. Negative MRI was obtained again in February 2016 and again in January 2017. Further negative biopsies were obtained in November 2014 and May 2016. At this point, a total of 74 cores of tissue had been removed from the patient's 25 cc prostate during 6 separate biopsy sessions. PSA increased sequentially to a maximum of 49.0 ng/mL in January 2017, at which point a 68Ga-HBED-CC PSMA PET/CT scan was performed, in the method described by Eder et al.1
The scan demonstrated radiotracer uptake (a PSMA ‘avid’ spot) in the midline prostate anterior to the urethra near a prior TURP defect (Fig. 1 A, B); no evidence of local or distant metastasis was noted. Using ProFuse imaging software and the Artemis biopsy system (Eigen, Grass Valley, CA), the 68Ga-PSMA region of interest was transposed onto bi-planar MRI images (Fig. 1 C, D). The MRI, with region of interest delineated, was then loaded into the Artemis device; while imaging the prostate via real-time ultrasound, a fusion with the stored MRI was performed. A 3-Dimensional model of the prostate, incorporating the PSMA-derived region of interest (ROI), was created and target biopsy performed (Fig. 2). Biopsy cores (N = 6) taken from the target demonstrated Gleason 3 + 4 = 7 CaP, with cancer core lengths of 4.5–12 mm with focal mucinous features. Radical robotic prostatectomy was then performed with histopathology demonstrating an index tumor of 3.5 cm diameter (12 cc), Gleason 3 + 4 CaP (40% pattern 4) and extensive mucinous features (70%). The dominant tumor on whole mount sectioning corresponded with the PSMA region of interest on biopsy (Fig. 3).
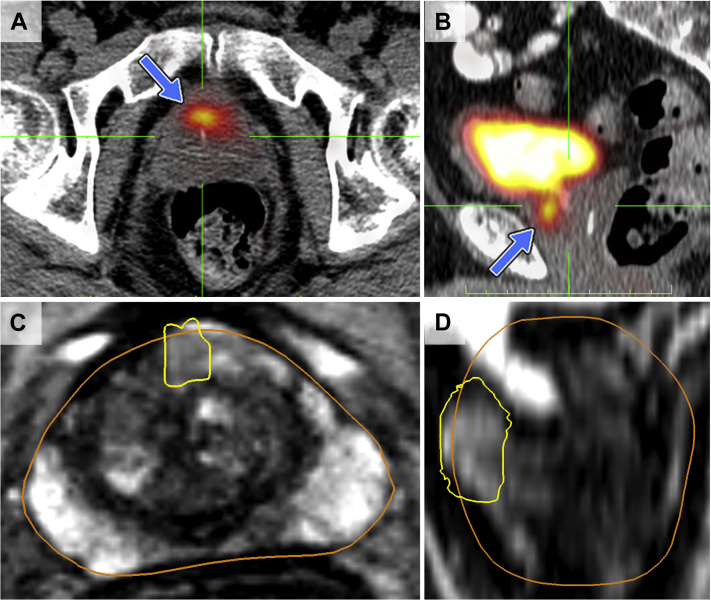
Figure 1.
PSMA radiotracer uptake (‘avidity’) on axial (A) and sagittal (B) images from PET/CT (blue arrow) compared to the corresponding MRI axial (C) and sagittal (D) regions. Note high concentration of 68Ga-PSMA radiotracer in the bladder. In C and D, PSMA regions of interest (yellow outlines) have been transposed onto the MRI using ProFuse software.
Figure 2.
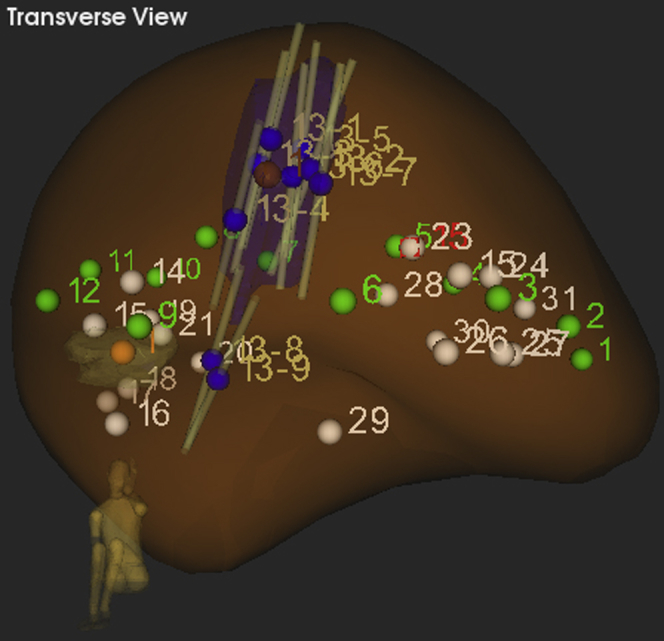
Artemis 3-D reconstruction of the prostate as viewed by the operator during biopsy. The PSMA target volume (purple) is now a 3-D region of interest (ROI) for a targeted biopsy. Biopsy cores (grey) targeted into the ROI revealed clinically significant prostate cancer. White dots represent previous negative biopsies as recorded by the Artemis system and green dots represent the systematic biopsy template.
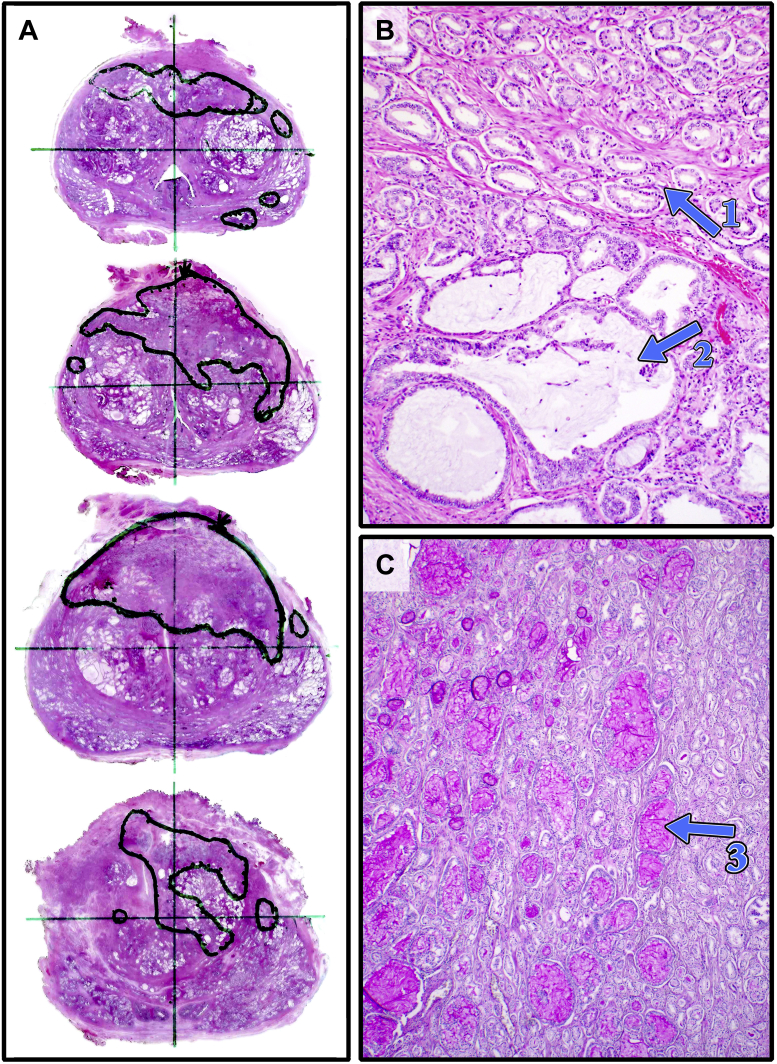
Figure 3.
Whole mount histopathology sliced axially from apex (top) to base (bottom). Note areas of mucinous Gleason 3 + 4 prostate cancer correlating with the PSMA “avid” spot on scanning and the region of interest (ROI) (A). In B, 100× magnification demonstrates Gleason 3 (arrow 1) and cribriform pattern with large mucinous lakes (arrow 2). The mucin is seen clearly on 40× PAS staining (C, arrow 3).
Discussion
Zettinig et al (2015) used PSMA-PET/MRI to perform targeted biopsies on men with positive PSMA or MRI scans of the prostate and demonstrated that the technique is specific for detecting prostate cancer.3 In this initial study, 4 of 5 men were reported to have positive targeted biopsies using PSMA-PET/MRI. This technique, however, is expensive and not readily available at most institutions, whereas PET/CT is widely available.
In this case report, we were able to fuse PET/CT with the Artemis MRI/US fusion biopsy system and create a PSMA ROI for a targeted biopsy. The ability to combine these two separate imaging techniques and perform a targeted biopsy has not been reported previously.
MRI-invisible prostate cancer is not rare, as in the present case. When whole-mount correlations are used, as many as 28% of clinically significant prostate cancers may be invisible to mpMRI.4 For such patients, improved imaging of the prostate is needed when mpMRI is normal and clinical suspicion for cancer remains. Nuclear imaging with novel ligands may be an option to increase cancer detection by providing valid targets, when other imaging modalities have failed.
Mucinous components, which are relatively uncommon in prostate cancers, may contribute to the difficulty of identifying cancers on mpMRI. In cases where pre-prostatectomy MRI has been correlated with surgical specimens, mucin-containing tumors did not follow the classic pattern of T2 hypo-intensity in the peripheral zone, thus obviating diagnosis by mpMRI.5 The present case showed a marked mucinous component of 70% (Fig. 3C). The degree to which various mucin components may contribute to a falsely negative MRI is a subject for further investigation.
Conclusion
We report a case where prostate cancer detection was aided by fusion of a 68Ga-PSMA PET/CT with real-time US to guide biopsy in the Artemis device. The clinically-significant cancer was not detected by multiple prior mpMRIs and systematic biopsies. An extensive mucinous component of the cancer may have contributed to invisibility on mpMRI.
Consent
On file.
Funding sources
None.
Conflict of interest
None.
Acknowledgments
Alan Priester, PhD and Jason Wu provided help with graphics. Richard Vanlangendonck, Jr., M.D. of Crescent City Physicians/Touro, New Orleans, LA performed the prostatectomy.
References
- 1.Eder M., Schäfer M., Bauder-Wüst U. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012 Apr 18;23(4):688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 2.Perera M., Papa N., Christidis D. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016 Dec;70(6):926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Zettinig O., Shah A., Hennersperger C. Multimodal image-guided prostate fusion biopsy based on automatic deformable registration. Int J Comput Assist Radiol Surg. 2015 Dec;10(12):1997–2007. doi: 10.1007/s11548-015-1233-y. [DOI] [PubMed] [Google Scholar]
- 4.Le J.D., Tan N., Shkolyar E. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: Correlation with whole-mount histopathology. Eur Urol. 2015 Mar;67(3):569–576. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 5.Westphalen A.C., Coakley F.V., Kurhanewicz J. Mucinous adenocarcinoma of the prostate: MRI and MR spectroscopy features. AJR Am J Roentgenol. 2009 Sep;193(3):W238–W243. doi: 10.2214/AJR.08.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]