Abstract
Objective:
To provide a comprehensive description of abnormal behaviors in patients with Gaucher disease type 3 (GD3) and relate these behaviors to demographic, neurodevelopmental, and neurologic characteristics.
Methods:
Thirty-four Egyptian patients with GD3 (mean age of 7.9 years) were enrolled in the study. They were selected based on parent report and/or physician observation of one or more abnormal behaviors documented in 2 settings and by 2 different individuals and/or by video recording. Behaviors were grouped into 4 categories: Crying/Withdrawal, Impatience/Overactivity, Anger/Aggression, and Repetitive Acts. Baseline and follow-up 6–12 monthly neurologic evaluations included IQ assessment and an EEG. All patients were receiving enzyme replacement therapy (30–60 IU/kg every 2 weeks) and were followed for periods of 3–10 years.
Results:
Supranuclear palsy of horizontal gaze, and of both horizontal and vertical gaze, bulbar symptoms, seizures, convergent strabismus, abnormal gait, and neck retroflexion were present in 97.1%, 50%, 55.9%, 29.4%, 29.4%, 20.6%, and 4.4% of patients, respectively. The most abnormal behavioral features were excessive anger (88.2%) and aggression (64.7%), and both were significantly higher in males. Anger/Aggression scores were highly correlated with IQ but not with either EEG/Seizure status or neurologic signs.
Conclusions:
We describe behavioral problems with a unique pattern of excessive anger and aggression in patients with GD3. Defining these components using quantitative behavioral scoring methods holds promise to provide a marker of neurologic disease progression and severity.
Gaucher disease (GD) results from a glucocerebrosidase (GBA) mutation leading to glucocerebroside accumulation in multiple organs. Based on the genotype and/or the presence of neurologic involvement, 3 clinical subtypes are distinguished. Type 1 is nonneuronopathic; types 2 and 3 are neuronopathic. Type 3 (GD3) has a broad phenotypic spectrum1,2 with more than 50% of GD3 cases manifesting in infancy. The usual neurologic presentation includes developmental delay, supranuclear gaze palsy, bulbar symptoms, neurocognitive impairment, and seizures. Enzyme replacement therapy (ERT) has been reported to have no effect3,4 or lead to the stabilization5,6 or even improvement of neurologic function.7
Reports on GD3 describe the physical manifestations and neurologic spectrum, but none have referred to the behavioral aspects of the disease.8–11 However, more than half of parents of children in our GD3 cohort complain about a set of abnormal behaviors, including extreme anger and aggression, persistent crying, excessive motor activity, and bizarre repetitive acts. The treating physician (M.A.) has noted behavioral changes and tendency for aggressive behavior,2,12 but other than those preliminary reports, the behavioral phenotype of GD3 has not been systematically studied and is currently unknown.
The aims of this retrospective exploratory study were to characterize these behavioral patterns and relate them to neurodevelopmental and neurologic characteristics. Such foundational knowledge will permit the development of working hypotheses for a subsequent prospective study. Based on a detailed single clinical observation as well as neurologic and treatment-related response, we describe the abnormal behaviors occurring in the cohort of 34 Egyptian patients diagnosed with GD3.
METHODS
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the local Ethical Committee. All patients/parents/legal guardians of patients provided their written informed consent.
Patients.
Thirty-four Egyptian patients with GD3 whose parents reported and/or physician (M.A.) observed one or more abnormal behaviors were enrolled in the study, while those who did not show any behavioral abnormalities were excluded. The GD3 group showing behavioral problems represents 52% of the Cairo University Pediatric Hospital GD3 cohort containing 65 patients in total. Definitive diagnosis of GD was typically made between 9 and 15 months of age from reduced leukocyte β-GBA activity (<1 µmol/g of protein/h); type 3 diagnosis is determined by genotyping and/or the presence of neurologic manifestation and persistence beyond infancy. Genetic testing of 27 children was performed by full sequencing of the gene for common mutations associated with GD3. All were homozygous for L444P.
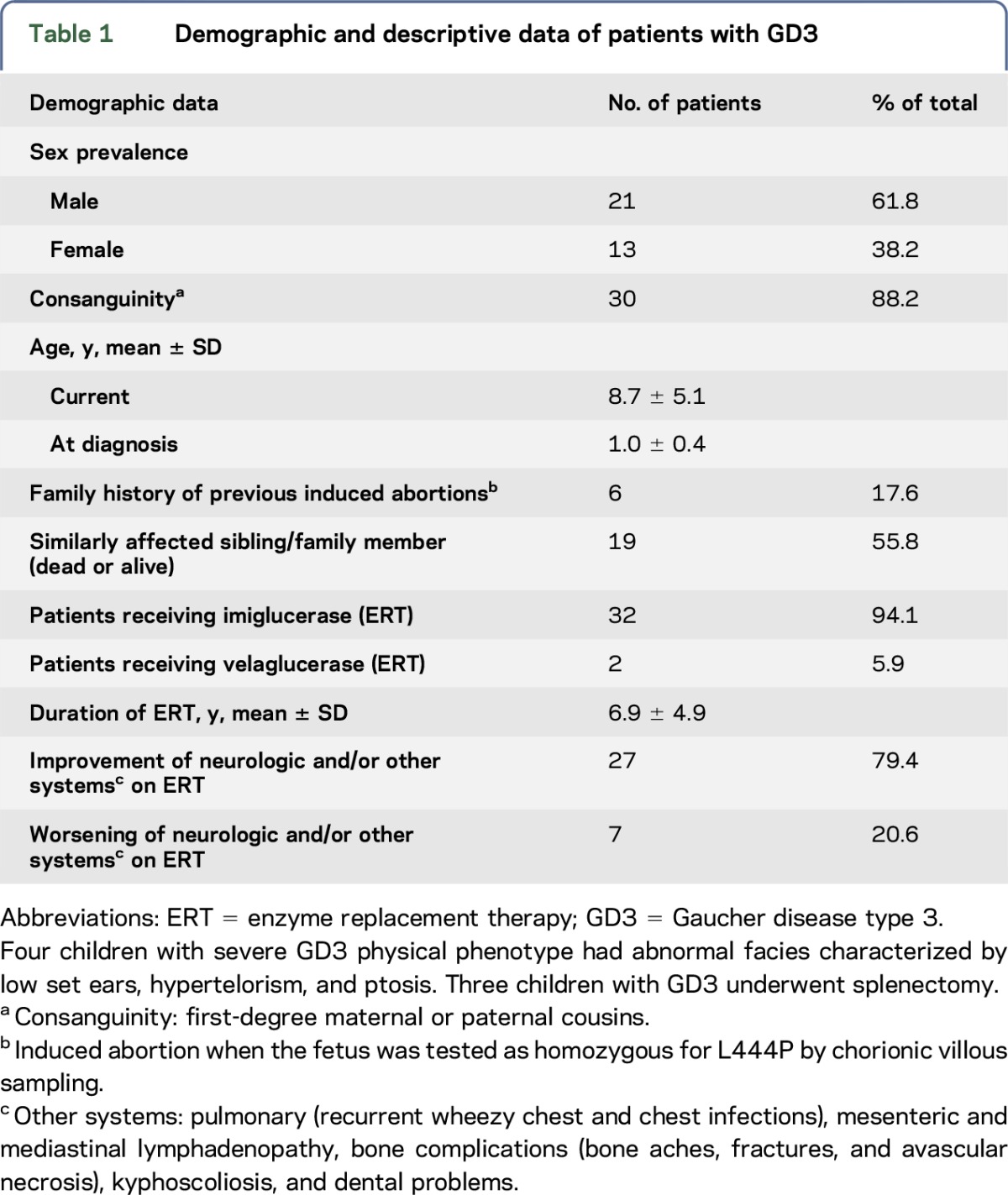
Since diagnosis, the patients have been followed for periods of 3–10 years. They underwent systematic neurologic re-evaluation at 6-month intervals. The data presented here were derived from the retrospective review of the records of a single evaluation of each child between 2006 and 2015. The patients' demographic and descriptive data are shown in table 1.
Table 1.
Demographic and descriptive data of patients with GD3
Procedures.
Abnormal behaviors.
Table 2 shows the list of abnormal behaviors, which was developed through observation of patients in the clinic and discussions with their parents about frequency and variation among these behaviors that occurred outside the clinic. Therefore, some descriptors and categories may be colloquial and culture specific. Behaviors of 18 patients were documented by video recording; 12 of these videos were reviewed with coauthors (E.G.S. and I.N.) to identify, confirm, and categorize behaviors. All behaviors included in this report were documented in at least 2 settings, including hospital (observation by the examining physician during a clinic visit), school, and/or home. All these behaviors were witnessed by more than one person, including parents, siblings, peers, treating physician, and sometimes strangers who reported it to parents.
Table 2.
Abnormal behaviors grouped by category: Descriptors, number, and percentages of patients with Gaucher disease type 3 showing abnormal behavior
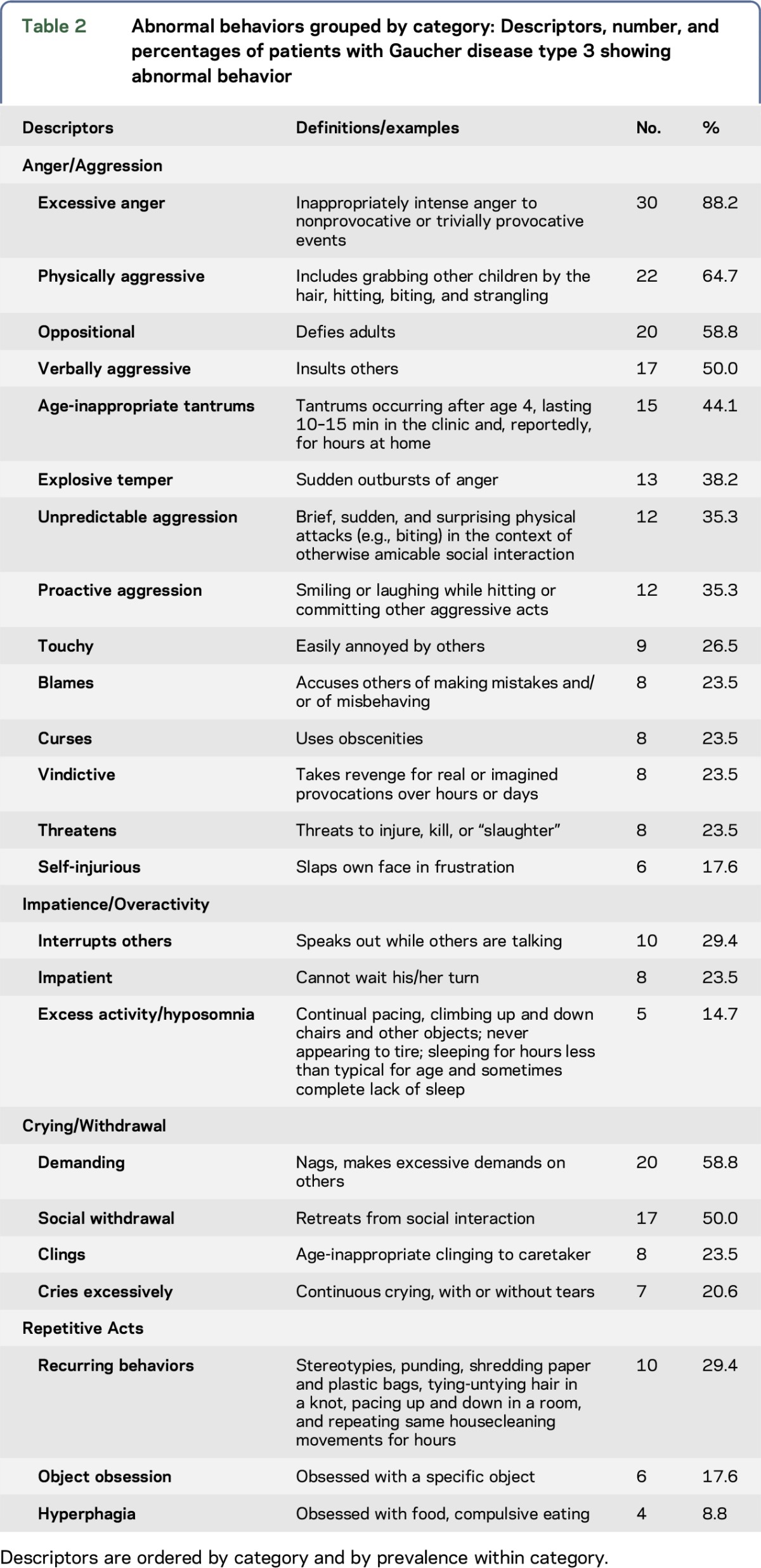
Behaviors were grouped into 4 categories based on similarity of form and/or function, co-occurrence, and/or sequential occurrence. Scales for each category consisted of 1 point for each of the behaviors assigned to that category: Anger/Aggression (0–14), Impatience/Overactivity (0–3), Crying/Withdrawal (0–4), and Repetitive Acts (0–3). The larger number of Anger/Aggression items represents both the diversity of behaviors in this category and their salience to observers.
Cognitive, EEG, and clinical neurologic assessment.
The same clinician (M.A.) conducted baseline and follow-up neurologic evaluations every 6–12 months, individualized according to the patient's condition. Other evaluations included IQ assessment and an EEG recorded under standard International 10–20 system. Wechsler scales for preschool, school age, and adult subjects were used to assess IQ. For children younger than 4 years, the Vineland Adaptive Behaviour Scales II was used to estimate IQ.13
EEGs were graded as follows: 0 = normal, 1 = nonepileptiform abnormality, and 2 = epileptiform abnormality.14 If clinical seizures were present, an additional point was given. The continuity of this 4-point scale was indicated by the observation that the percentage of patients with EEG scores of 0, 1, and 2 who were known to have seizures was 8%, 20%, and 50%, respectively. The neurologic signs listed in table e-1 at Neurology.org/ng were scored as absent or present (0, 1).
Statistical analysis.
Descriptive statistics were used to describe results by percentages, mean values, and SDs. To assess changes in behavior with age, the symptom profiles of the children were ordered by age and examined for patterns of occurrence. Nonparametric Spearman rank-order correlation was used to test for association between variables; the Mann-Whitney U test was used to assess male-female differences. Neurologic signs that were significantly associated with one or more behavior categories were identified by the Pratt measure15 in exploratory multiple regressions of behavior on the full set of signs. Significance values were adjusted for multiple tests using the false discovery rate protocol.16
RESULTS
All enrolled children presented initially with organomegaly, motor, and rarely global delay. Nineteen children had neurologic symptoms initially; the other 15 developed neurologic symptoms subsequently. Prevalence of neurologic manifestations noted in our type 3 GD cohort is shown in table e-1. Thirty-two patients were started on ERT, imiglucerase (30–60 IU/kg every 2 weeks) and 2 patients on velaglucerase alfa (60 IU/kg every 2 weeks). Patients received ERT regularly except during the 2-year worldwide enzyme shortage in 2009–2011.
IQ.
The IQ for the group was 71.1 ± 10.5 (mean ± SD); for females and males, it was 74.3 ± 8.9 and 68.9 ± 11.0, respectively. These results suggest borderline intellectual functioning overall for this group. Compared with population norms, they were 2 SDs below the mean. Only 1 patient had an IQ in the average range (>85).
EEG status/clinical seizures.
Five patients (14.7%) had EEG ratings of 1; 16 (47%) had EEG ratings of 2. Half of those with ratings of 2 had clinical seizures. Initial EEG recording showed a clear epileptic focus in 10 children, 8 of whom had clinical seizures that ranged from focal or focal with secondary generalization to generalized or myoclonic types. Seizure frequency varied from 1 to 2 per month to several per day. Patients were treated with valproic acid at doses up to 60 mg/kg/d with variable seizure control. EEG epileptic foci resolved in 4 children spontaneously in later childhood over a 3-year period.
Qualitative behavioral observations.
A wide range of abnormal behaviors was observed. The frequency varied from daily to monthly depending on the type and precipitating factor(s) such as a younger sibling smiling at the patient or touching him, but in many children, they have been going on for years. Table 3 displays the number (and percentage) of patients with GD3 showing each abnormal behavior, with examples, grouped by category.
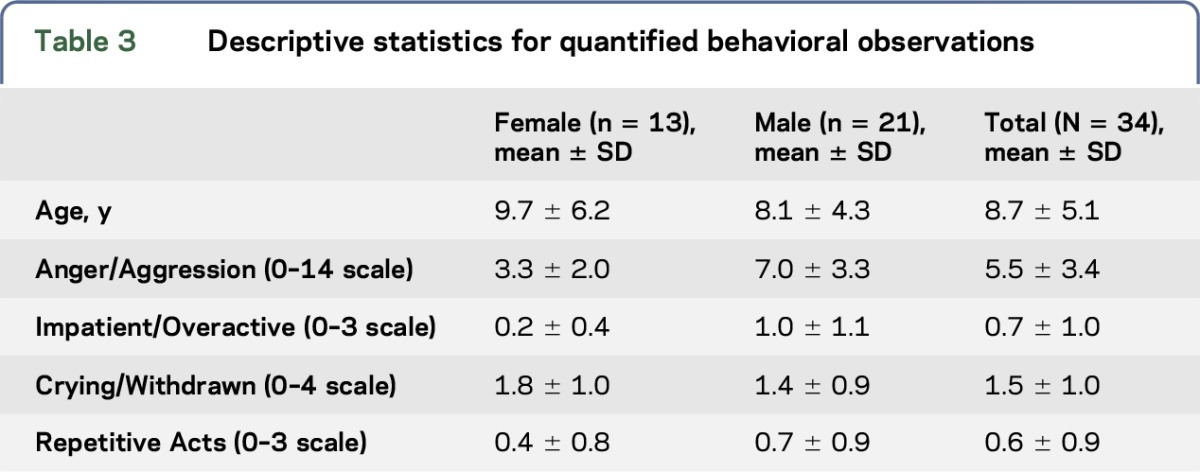
Table 3.
Descriptive statistics for quantified behavioral observations
Anger/Aggression.
These behaviors were directed to peers, siblings, parents, and other adults/authority figures including the treating physician. Angry and aggressive acts were the most common, prominent, and troublesome of the abnormal behaviors and were reported to have had a major negative effect on patients' and parents' quality of life. For 23 (69.7%) patients, these behaviors were the parents' main concern or sole complaint.
Based on the median age at which the various aggressive behaviors appeared, tantrums past toddler age, oppositional behavior, physical aggression, and angry self-injury appeared earliest to develop; these behaviors tended to be the most prevalent across the group. Unpredictable and proactive aggression, using obscenities and vindictive behavior, tended to appear subsequently and were less prevalent; being touchy/easily annoyed, explosive temper, blaming others, verbal aggression and insults, and homicidal threats were generally the last to appear and tended to be least prevalent.
Impatience/Overactivity.
Parents reported that a child's overactivity in social gatherings was disruptive, embarrassing, tiring, and sometimes impossible to deal with.
Crying/Withdrawal.
Children with these characteristics were wary of strangers, socially withdrawn, and clung to and were demanding of their parents.
Repetitive Acts.
These varied in type and complexity; they often could not be interrupted.
Quantitative behavioral results.
Anger/Aggression.
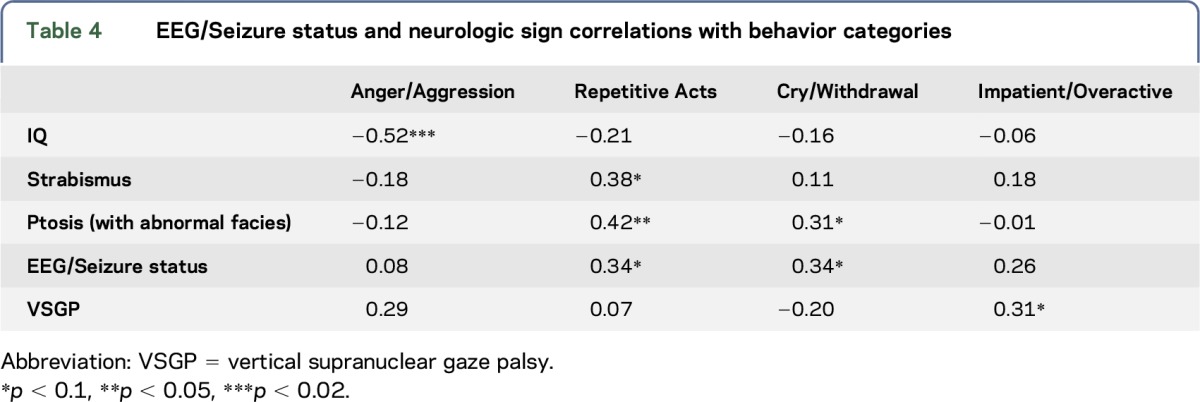
All but one of the 34 subjects had scores ≥3 on the Anger/Aggression measure. Mean values for this measure, calculated for successive 3-year periods, increased for females and almost doubled for males from value at 1–3 years of age to a peak at 4–6-year-olds, then fell back to near values observed at 1–3-year-olds by adolescence (figure). Males also showed higher Anger/Aggression than females (U = 162, p < 0.02, table 3). Anger/Aggression scores were highly negatively correlated with IQ but not with either the EEG/Seizure status or neurologic signs (table 4). Anger/Aggression scores were correlated with Impatient/Overactive scores (r = 0.45, p < 0.01).
Figure. Age trends for all 4 abnormal behavior categories.
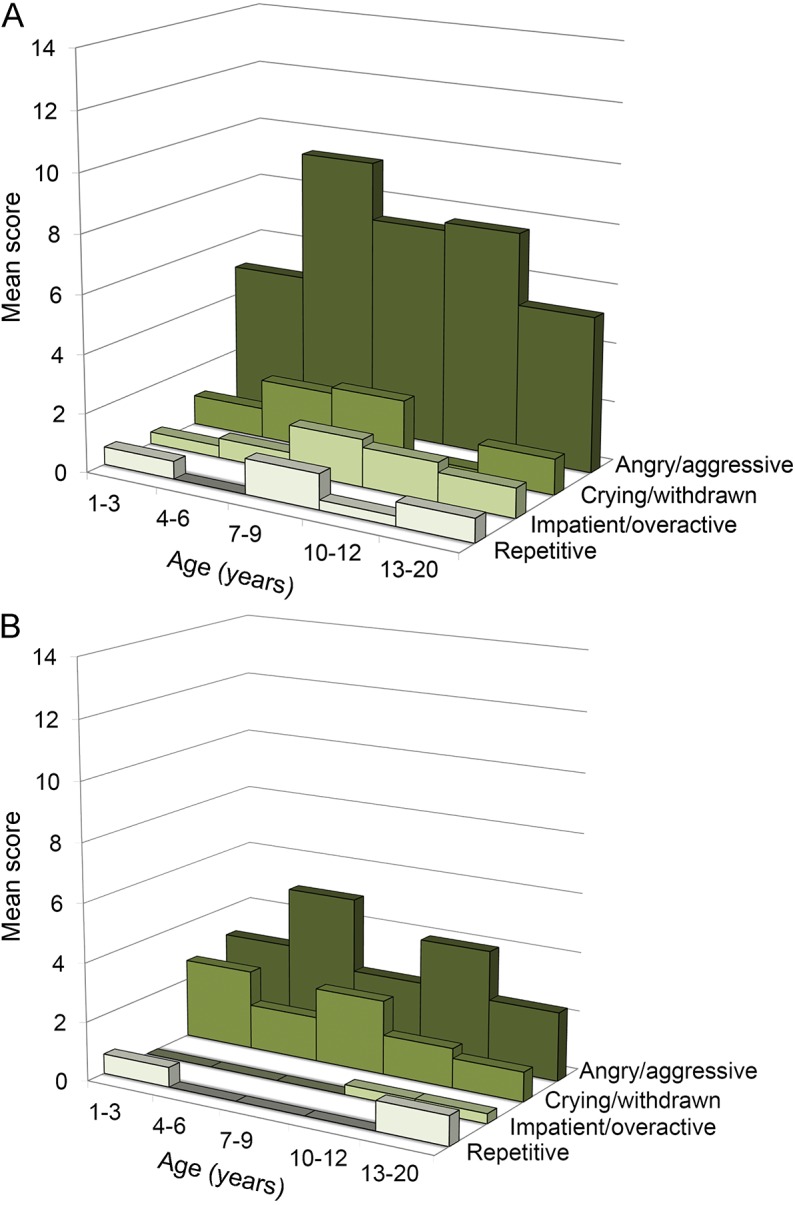
(A) Males. (B) Females.
Table 4.
EEG/Seizure status and neurologic sign correlations with behavior categories
Impatience/Overactivity.
Four of the seven 7–9-year-olds were reported to show one or more signs of Impatience/Overactivity, which was the age range for highest scores on this scale. Males were more Impatient/Overactive than females according to a Mann-Whitney test (U = 136.5, p < 0.02). Across all subjects, there was a near-significance for Impatience/Overactivity scores to be correlated with vertical supranuclear gaze palsy (VSGP) (table 4).
Crying/Withdrawal.
One or more Crying/Withdrawal behaviors were shown by 10 of the 12 youngest children (1–5 years old) in contrast to Impatient/Overactive and Repetitive Acts, which were each shown by only 2 of these 12 children. These scores appeared to decline after age 9 (figure). Crying/Withdrawn scores were nonsignificantly higher in females (figure). Over all subjects, these scores were associated with EEG/Seizure status (table 4). Crying/Withdrawal scores were not correlated with any of the other 3 behavior categories.
Repetitive Acts.
Repetitive Act scores were nonsignificantly higher for males. These scores were correlated with Impatient/Overactive scores (r = 0.47, p < 0.005). Repetitive Act scores were also correlated with ptosis (r = 0.42, p < 0.02); correlations with EEG status and with strabismus tended to approach significance as showed in table 4.
DISCUSSION
Abnormal behavioral features in the patients with GD3 have not been described in detail. The largest GD type 3 study analyzing the Neurological Outcomes Subregistry of the International Collaborative Gaucher Group, which included 40/131 Egyptian patients mostly homozygous L444P, contained no reference to abnormal behaviors.17 The prevalence of supranuclear gaze palsy and convergent strabismus in our study cohort resemble the registry data, but bulbar symptoms and seizures were more prevalent in our cohort, suggesting more severe CNS involvement.
Abnormal behaviors that occur in various genetic diseases and other clinical conditions include changes in attention and activity as well as simple and complex repetitive acts (e.g., motor stereotypies, object manipulation, and compulsive acts).18 However, the profile of behavioral abnormalities varies in different clinical conditions. Some of these behaviors appear in many different conditions, presumably due to a confluence of genetic/developmental dysfunction in commonly affected brain systems together with the shaping of behavior by responses from caregivers and others.19
Our cohort showed a wide spectrum of behavioral abnormalities. Angry and aggressive behaviors were the most prevalent and the greatest cause of concern. These included both reactive and proactive aggression. Reactive aggression is a response to perceived provocation or challenge to personal autonomy or social norms and is often accompanied by anger. Proactive aggression, such as deliberate harassment and bullying, without provocation, is committed to dominate others and/or for material gain, e.g., attention, control of toys etc.20 Children have been observed to smile or laugh while committing proactive aggression.21,22
The sequence in which tantrums, oppositional behavior, and physical aggression develop early; proactive aggression, the use of obscenities and vindictive behavior appear later, and blaming others, verbal aggression and insults, and homicidal threats are last to appear; reflects the children's increasing cognitive and verbal capacity. However, the progressive increases in angry and aggressive behaviors through age 4–6 differ from the normal trajectory. Very large scale studies with hundreds to thousands of children have determined that trajectories of physical aggression typically increase up through age 3, then decline.23 Although the greater aggression in males was to be expected,24 the overall anger intensity and aggression severity in these patients with GD3 appear to be considerably greater than normal and significantly affect quality of life for them and their parents. We identified a strong correlation between IQ and the Anger/Aggression scale, which replicates the relationship found in other neurodegenerative disease conditions.25,26 Whether other features of GD3, such as physical disability/discomfort, exacerbate anger and aggression remains to be determined.
Elevated rates of EEG abnormality and frank clinical seizures are well known in children with GD3. In general, EEG abnormalities and clinical seizures are associated with a range of behavioral problems, most often internalizing anxiety and depression, but also extending to externalizing inattention and acting out.27 Some forms of juvenile epilepsy remit spontaneously during development.28–30 At least 4 patients with GD3 seen in the Cairo Hospital have had apparently spontaneous normalization of EEG and/or remission of seizures in later childhood. These observations raise the question of whether changes in EEG might be associated with reductions in abnormal behavior.
Anger/Aggression and Repetitive Acts were both significantly associated with Impatient/Overactive behaviors. Such associations are common across clinical conditions.24 On the other hand, these 3 categories had different patterns of association with other independent variables: While Impatient/Overactive behaviors were just marginally associated with VSGP, higher Anger/Aggression was strongly associated with lower IQ as noted, while Repetitive Acts correlated with the EEG/Seizure score and with 2 neurologic signs. One possible interpretation of this pattern is that a primary dysfunction in corticobasal ganglia pathways regulating attention/activity reduces both inhibitory prefrontal control over several different neurologic subsystems such as temporal lobe/hypothalamic circuits that control anger and aggression31 and dopaminergic mesolimbic circuits that foster the repetition of behaviors that are reinforcing.32,33 Behaviors in GBA1 genotype D409V/null mice seem similar in that according to their handlers. Mice were hyperactive, jumpy, and aggressive toward other mice and humans, biting far more often than wild-type mice or mice with other GBA1 mutations.34
Crying/Withdrawal behaviors appeared early in development and were not associated with any of the other behavioral patterns. Crying and social withdrawal are internalizing behaviors to which females are more susceptible than males,35 and the mean Cry/Withdrawal score was the only one that was higher for females, albeit nonsignificantly. These behaviors were associated with the EEG/Seizure score. Sixty-two percent of patients had abnormal EEGs and/or seizures consistent with the neurologic involvement of GD3. This is far higher than the rate of EEG abnormalities in childhood populations.36 EEG abnormalities and clinical seizures are associated with a range of behavioral problems, most often internalizing anxiety and depression27 which may relate to the Cry/Withdrawal pattern of GD3. Spontaneous recovery from childhood seizures is well known.30 Such recovery may contribute to the reduction of the Crying/Withdrawal pattern after 7–9 years of age.
That these behaviors are intrinsic to the disease and not due to additional shared genes/inherited traits is suggested by the observation that these patients, while derived from consanguineous matings, are not from a tightly inbred population with a founder. They are from small, independent (at least for many generations) groups.
For this initial study, we assessed behaviors with descriptors developed in collaboration with some parents and therefore most likely to be recognized and understood by other parents within the Egyptian culture. This approach has produced statistically significant results but may limit their replicability. Behaviors were recorded as present/absent for simplicity, but frequency measures might provide data for more sensitive analyses of behavioral profiles and correlations with neurologic function. The use of cluster analyses to determine data-driven groupings of behavior was precluded by systematic shifts in behaviors with age. The abnormal development of anger and aggression in children with GD3 was highlighted by comparison with published data on normal developmental trajectories of these behaviors. As noted below, comparison of affected children's behaviors to that of their healthy siblings would provide a useful control for family-related variables in the future.
This retrospective study has raised questions about developmental changes with age, disease progression, and treatment effects in GD3 that can only be answered by longitudinal follow-up. This work should involve standardized scales of aggression and other behaviors that are available in Arabic translation, e.g., the Child Behavior Checklist37 to track behavior and neurologic function together over time. Standardized scales would also facilitate the application of The Diagnostic and Statistical Manual of Mental Disorders, 5th edition or the International Classification of Diseases, 10th revision diagnostic categories, e.g., the Impatient/Overactive category may well correspond to attention-deficit/hyperactivity disorder. Future studies should also include comparisons with healthy siblings or neurotypicals as well as with those with the nonneuronopathic GD type 1 who have equivalent medical problems.
Abnormal behaviors are a phenotypic feature of GD3 in the Egyptian cohort, the largest GD3 cohort reported worldwide. The behavior patterns are clearly complex. Our working hypothesis is that they may involve a Crying/Withdrawal component that manifests early, affects both males and females, and may relate to epileptiform activity in the brain. A second, apparently independent component of Impatience/Overactivity primarily involves males, can be associated with bizarre repetitive acts and/or highly salient intense anger and severe aggression. The repetitive acts may be associated with several neurologic signs. The Anger/Aggression component, which may start later and persist longer than Crying/Withdrawal, is clearly associated with lower cognitive ability.
These components defined by quantitative behavioral scoring methods might serve as markers of neurologic disease progression and severity. EEG recording together with structural and functional brain imaging methods can provide insight into the localization of the underlying pathophysiologic processes in Gaucher type 3, which would guide future treatments targeting the CNS disease.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to the patients and their families who participated in this study as well as the Center for Neurobehavioral Development (CNBD) for providing the spaces for the video assessments with coauthors (E.G.S. and I.N.). They thank Dr. Robin Rumsey for her help in distinguishing behavioral abnormalities during initial video assessments; Dr. Greg Grabowski for the observations of the genetically intrinsic nature of the behavioral abnormalities; Dr. Pramod Mistry, Yale University, for help with GBA1 sequencing; Dr. Khaled Eid for technical help; Dr. Mohamed Abd Elmonem, Cairo University, Egypt, for help with GBA1 genotyping; and Genzyme A Sanofi Company, Shire Plc, and the Hope project for providing ERT product.
GLOSSARY
- ERT
enzyme replacement therapy
- GBA
glucocerebrosidase
- GD3
Gaucher disease type 3
- VSGP
vertical supranuclear gaze palsy
Footnotes
Supplemental data at Neurology.org/ng
AUTHOR CONTRIBUTIONS
Magy Abdelwahab, study concept and design, recruited and studied the patients and gathered all data, interpreted the data, and edited the manuscript. Michael Potegal, analysis and interpretation of data and writing and editing the manuscript. Elsa Shapiro, analysis and interpretation of data and editing and critical revision of manuscript for intellectual content. Igor Nestrasil, analysis and interpretation of data and writing and editing the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
Dr. Magy Abdelwahab has received travel funding from a nonprofit entity. Dr. Michael Potegal reports no disclosures. Dr. Elsa Shapiro has served on scientific advisory boards for ReGenXBio, Armagen, Shire, SOBI, BioMarin, and Eloxx; has received travel funding/speaker honoraria from Shire and BioMarin; has been a consultant for ReGenXBio, Armagen, Shire, SOBI, BioMarin, Eloxx, Alexion, Chiesi, BluebirdBio, Sangamo, Aeglea, and Lysogene; and is a partner in Shapiro & Delaney, LLC. Dr. Igor Nestrasil has been a consultant for Armagen, BioMarin, Lysogene, ReGenXBio, and ICON and has received research support from Shire, Genzyme, BioMarin, NIH, The Penn Medicine Orphan Disease Center, and the University of Pennsylvania. Go to Neurology.org/ng for full disclosure forms.
REFERENCES
- 1.Benko W, Ries M, Wiggs EA, Brady RO, Schiffmann R, Fitzgibbon EJ. The saccadic and neurological deficits in type 3 Gaucher disease. PLoS One 2011;6:e22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelwahab M, Blankenship D, Schiffmann R. Long-term follow-up and sudden unexpected death in Gaucher disease type 3 in Egypt. Neurol Genet 2016;2:e55 doi: 10.1212/NXG.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erikson A, Bembi B, Schiffmann R. Neuronopathic forms of Gaucher's disease. Baillieres Clin Haematol 1997;10:711–723. [DOI] [PubMed] [Google Scholar]
- 4.Campbell PE, Harris CM, Vellodi A. Deterioration of the auditory brainstem response in children with type 3 Gaucher disease. Neurology 2004;63:385–387. [DOI] [PubMed] [Google Scholar]
- 5.Altarescu G, Hill S, Wiggs E, et al. The efficacy of enzyme replacement therapy in patients with chronic neuronopathic Gaucher's disease. J Pediatr 2001;138:539–547. [DOI] [PubMed] [Google Scholar]
- 6.Erikson A, Astrom M, Mansson JE. Enzyme infusion therapy of the Norrbottnian (type 3) Gaucher disease. Neuropediatrics 1995;26:203–207. [DOI] [PubMed] [Google Scholar]
- 7.Vellodi A, Bembi B, de Villemeur TB, et al. Management of neuronopathic Gaucher disease: a European consensus. J Inherit Metab Dis 2001;24:319–327. [DOI] [PubMed] [Google Scholar]
- 8.Choy FY, Zhang W, Shi HP, et al. Gaucher disease among Chinese patients: review on genotype/phenotype correlation from 29 patients and identification of novel and rare alleles. Blood Cells Mol Dis 2007;38:287–293. [DOI] [PubMed] [Google Scholar]
- 9.Goker-Alpan O, Wiggs EA, Eblan MJ, et al. Cognitive outcome in treated patients with chronic neuronopathic Gaucher disease. J Pediatr 2008;153:89–94. [DOI] [PubMed] [Google Scholar]
- 10.Davies EH, Erikson A, Collin-Histed T, Mengel E, Tylki-Szymanska A, Vellodi A. Outcome of type III Gaucher disease on enzyme replacement therapy: review of 55 cases. J Inherit Metab Dis 2007;30:935–942. [DOI] [PubMed] [Google Scholar]
- 11.Tajima A, Yokoi T, Ariga M, et al. Clinical and genetic study of Japanese patients with type 3 Gaucher disease. Mol Genet Metab 2009;97:272–277. [DOI] [PubMed] [Google Scholar]
- 12.Abdelwahab M. Management of type 3 Gaucher disease. Int J Clin Rev 2012;10:93–99. [Google Scholar]
- 13.Delaney KA, Rudser KR, Yund BD, Whitley CB, Haslett PA, Shapiro EG. Methods of neurodevelopmental assessment in children with neurodegenerative disease: Sanfilippo syndrome. JIMD Rep 2014;13:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noachtar S, Binnie C, Ebersole J, Mauguiere F, Sakamoto A, Westmoreland B. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 1999;52:21–41. [PubMed] [Google Scholar]
- 15.Thomas DR, Hughes E, Zumbo BD. On variable importance in linear regression. Soc Indic Res 1998;45:253–275. [Google Scholar]
- 16.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 17.Tylki-Szymanska A, Vellodi A, El-Beshlawy A, Cole JA, Kolodny E. Neuronopathic Gaucher disease: demographic and clinical features of 131 patients enrolled in the International Collaborative Gaucher Group Neurological Outcomes Subregistry. J Inherit Metab Dis 2010;33:339–346. [DOI] [PubMed] [Google Scholar]
- 18.Moss J, Oliver C, Arron K, Burbidge C, Berg K. The prevalence and phenomenology of repetitive behavior in genetic syndromes. J Autism Dev Disord 2009;39:572–588. [DOI] [PubMed] [Google Scholar]
- 19.Muehlmann AM, Lewis MH. Abnormal repetitive behaviours: shared phenomenology and pathophysiology. J Intellect Disabil Res 2012;56:427–440. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard JA, McAuliffe MD, Morrow MT, Romano LJ. Reactive and proactive aggression in childhood and adolescence: precursors, outcomes, processes, experiences, and measurement. J Pers 2010;78:95–118. [DOI] [PubMed] [Google Scholar]
- 21.Arsenio W, Cooperman S. Children's conflict-related emotions: implications for morality and autonomy. New Dir Child Dev 1996:25–39. [DOI] [PubMed] [Google Scholar]
- 22.Benenson JF, Carder HP, Geib-Cole SJ. The development of boys' preferential pleasure in physical aggression. Aggress Behav 2008;34:154–166. [DOI] [PubMed] [Google Scholar]
- 23.Fanti KA, Henrich CC. Trajectories of pure and co-occurring internalizing and externalizing problems from age 2 to age 12: findings from the National Institute of Child Health and Human Development Study of Early Child Care. Dev Psychol 2010;46:1159–1175. [DOI] [PubMed] [Google Scholar]
- 24.Potegal M, Archer J. Sex differences in childhood anger and aggression. Child Adolesc Psychiatr Clin N Am 2004;13:513–528, vi–vii. [DOI] [PubMed] [Google Scholar]
- 25.Oliver C, Petty J, Ruddick L, Bacarese-Hamilton M. The association between repetitive, self-injurious and aggressive behavior in children with severe intellectual disability. J Autism Dev Disord 2012;42:910–919. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder SR, Marquis JG, Reese RM, et al. Risk factors for self-injury, aggression, and stereotyped behavior among young children at risk for intellectual and developmental disabilities. Am J Intellect Dev Disabil 2014;119:351–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn DW, Austin JK, Perkins SM. Prevalence of psychopathology in childhood epilepsy: categorical and dimensional measures. Dev Med Child Neurol 2009;51:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavazzuti GB, Cappella L, Nalin A. Longitudinal study of epileptiform EEG patterns in normal children. Epilepsia 1980;21:43–55. [DOI] [PubMed] [Google Scholar]
- 29.Loiseau P, Pestre M, Dartigues JF, Commenges D, Barberger-Gateau C, Cohadon S. Long-term prognosis in two forms of childhood epilepsy: typical absence seizures and epilepsy with rolandic (centrotemporal) EEG foci. Ann Neurol 1983;13:642–648. [DOI] [PubMed] [Google Scholar]
- 30.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med 1998;338:1715–1722. [DOI] [PubMed] [Google Scholar]
- 31.Potegal M. Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behav Brain Res 2012;231:386–395. [DOI] [PubMed] [Google Scholar]
- 32.Fasano A, Petrovic I. Insights into pathophysiology of punding reveal possible treatment strategies. Mol Psychiatry 2010;15:560–573. [DOI] [PubMed] [Google Scholar]
- 33.McBride SD, Parker MO. The disrupted basal ganglia and behavioural control: an integrative cross-domain perspective of spontaneous stereotypy. Behav Brain Res 2015;276:45–58. [DOI] [PubMed] [Google Scholar]
- 34.Dai M, Liou B, Swope B, et al. Progression of behavioral and CNS deficits in a viable Murine model of chronic neuronopathic Gaucher disease. PLoS One 2016;11:e0162367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zahn-Waxler C, Crick NR, Shirtcliff EA, Woods KE. The origins and development of psychopathology in females and males. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Volume 1 Theory and Method. Hoboken: Wiley; 2006. [Google Scholar]
- 36.Shelley BP, Trimble MR. “All that spikes is not fits,” mistaking the woods for the trees: the interictal spikes—an “EEG chameleon” in the interface disorders of brain and mind: a critical review. Clin EEG Neurosci 2009;40:245–261. [DOI] [PubMed] [Google Scholar]
- 37.Achenbach TM, Ruffle TM. The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 2000;21:265–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


