Abstract
Background
The aim of this study was to investigate the antimicrobial property of peptide LL-37 sequences.
Material/Methods
Humanized antibacterial peptide LL-37 and the mutant were prepared by chemical synthesis. The physicochemical properties of antibacterial peptide LL-37 were analyzed by SWISS-MODEL online prediction tool. Molecular docking between antibacterial peptide LL-37 fragments and palmitoyl transferase PagP was made with Lamarckian genetic algorithm by AutoDock1.5.6.
Results
The systems contacted each other at 8.75 picosec. After 20 picsec, the system had no trend of dissociation, and the bond energy of weak bond -C-O-H…NH2-CH2- was calculated. The hydrophobic groups were important factors that led to contact and merged the two parts. The contacted weak bond -C-O-H…NH2-CH2- was the bridge for contacting LL-37 with palmitoyl transferase PagP. The binding sites of antibacterial peptide LL-37 and palmitoyl transferase PagP mainly included LYS8, GLU11, LEU28, LYS12, PHE27, ILE13, and PHE6 of antibacterial peptide LL-37 and ARG94, TRP89, ASN65, SER3, GLU90, GLU90, ASN100, HIS102, and THR92 of palmitoyl transferase PagP.
Conclusions
Antibacterial peptide LL-37 had stronger antibacterial effect via inhibition of activity of PagP.
MeSH Keywords: Acyl-Carrier Protein S-Acetyltransferase, Ambulatory Care Facilities, Anti-Bacterial Agents, Computer-Aided Design
Background
Antibacterial peptides are important effector molecules of the natural immune system [1,2]. Antibacterial peptide LL-37 is a cationic antimicrobial peptide found in the human body, and has broad-spectrum antibacterial activity, especially for some clinical drug-resistant bacteria. In addition, it has biological functions such as promoting wound healing, endotoxin binding, induction of angiogenesis, and inhibiting tumor cell growth [3,4].
Reports have indicated that the gram-negative bacterial cell outer membrane of palmitoyl transferase PagP can repair permeability of the outer membrane through activation of lipid A acylation, producing resistance to antibacterial peptides [5,6]. Therefore, new antibacterial drugs may be found by investigating the pathway of inhibition of acylation of lipid A [7,8].
Hydrolytic fragments of antibacterial peptide LL-37 with different lengths had good bioactivity, which laid a foundation for studies of the redesign of the antibacterial peptide LL-37 molecular and its activities [9–11]. This study analyzed the antimicrobial peptide LL-37 sequences, and simulated the interaction with palmitoyl transferase PagP through bioinformatics methods, based on which mutation of antibacterial peptide LL-37 molecular was created and which in vitro activity was studied.
Material and Methods
Materials
The following strains were purchased from the American Type Culture Collection (ATCC): Escherichia coli ATCC2592 (E. coli standard strain) and E. coli ATCC35218 (E. coli resistance standard strain). Strains of E. coli, isolated from clinical condition, were No. 1-11 including No. 5 the extended spectrum beta-lactamases (ESBLs)-producing E. coli. Data of polypeptide and protein are presented in Table 1).
Table 1.
Data resources of polypeptides and proteins for this study.
| Polypeptides and proteins | Data resources |
|---|---|
| Antibacterial peptide LL-37 | PDB ID: 2K6O |
| Polymyxin B | APD ID: AP02204 |
| Transferase PagP | UniProtKB – P37001 (PAGP_ECOLI) |
Data simulation
Sequence analysis
The physicochemical properties of antibacterial peptide LL-37 were analyzed using the SWISS-MODEL online prediction tool; comparative analysis was made using the amino acid sequence with SWISS-MODEL and BLAST on NCBI. ANTHEPROT6.6.6 software was used to analyze the structure of antibacterial peptide LL-37.
Dynamics simulation
ADF2014 (Amsterdam Density Functional) was used to set ReaxFF simulation parameters and conditions; four antibacterial peptide LL-37 molecules and one palmitoyl transferase PagP molecule were placed in the periodic space of 100×100×100 A3 by a random, uniform method at room temperature 298 K; analysis of internal stress relaxation within 0 picosec was made with the Velocity Verlet (NVT) system, in order to make the temperature and pressure fluctuate near the preset value, and analyze the system conformation through different time points. In the discriminatory analysis of products, the intermolecular bonding energy was calculated with density functional theory (using PBE-D3 (BJ) functional, TZ2P base group), in order to study the properties of the molecules [12].
Molecular docking
Molecular docking between antibacterial peptide LL-37 fragments and palmitoyl transferase PagP was made with Lamarckian genetic algorithm by AutoDock1.5.6, with Lattice size of 60×60×60 A3 and coordinates of 7.063, 32.452, and 49.199.
Preparation of humanized antimicrobial peptide LL-37 and the mutant
Humanized antibacterial peptide LL-37 and the mutant were prepared by chemical synthesis (Hefei Cellmano Biotech Limited) and separated and characterized by high performance liquid chromatography and mass spectrum; we dissolved 5 mg antibacterial peptide sample in 1 mL water-solvent containing 0.01% acetic acid and 0.2% BSA, then perform double-dilution in order to obtain concentrations of 500, 160, 80, 40, 20, 10, 5, 2.5, 1.25, and 0.625 μg/mL, each placed in an EP tube.
Determination of bacteriostatic activity of humanized antibacterial peptide LL-37 and mutant
First, we poured 5 mL of MH nutrient broth in the tube, inoculated with the bacteria to be tested, and then placed the test tube in a shaker for enrichment under conditions of 37°C, 180 rpm for 16–18 hours. Then, 10 μL of each of the diluted modified antibacterial peptides and unmodified antibacterial peptides with different concentrations were added into each well of the 96-well plate (with vice well), and the 13 kinds of E. coli added accordingly. The group with only E. coli without antimicrobial peptide served as a positive control; and the group with only medium served as a negative control. The enriched bacterium solution was diluted into 5×105 CFU/mL, then a transfer of 90 μL was made to each well with antimicrobial peptide in sequence. The 96-well plates were covered and sealed with medical tape, then cultured at 37°C with shaking for 24 hours; then observed under 600 nm wavelength by Microplate reader; the experimental results were recorded and the averages calculated and the data analyzed.
Effects of humanized antibacterial peptide LL-37 and mutant on the expression of bacteria PagP enzyme
Primers (upstream of PagP: AAGGAGATATAATGAACGTGAGT AAATATGT; downstream of PagP: TCAAAACTGAAA GCGCATCC) were synthesized by Sangon Biological Engineering Co., Ltd., Shanghai). The expression of PagP enzyme was identified via RT-PCR. The reaction conditions were as follows: pre-degenerate for 5 minutes under 94°C, degenerate for 30 seconds under 94°C, annealing for 30 seconds under 55°C, extending for 30 seconds under 72°C, for a total of 35 cycles; re-extending for 7 minutes under 72°C.
Statistical treatment
SPSS 17.0 data processing software was used for analysis. The measurement data was presented as mean and standard deviation (x±s) and compared with the t-test. Chi-square test was used for the count data. The difference was statistically significant when p<0.05.
Results
Results of molecular dynamics simulation between antimicrobial peptide LL-37 and palmitoyl transferase PagP
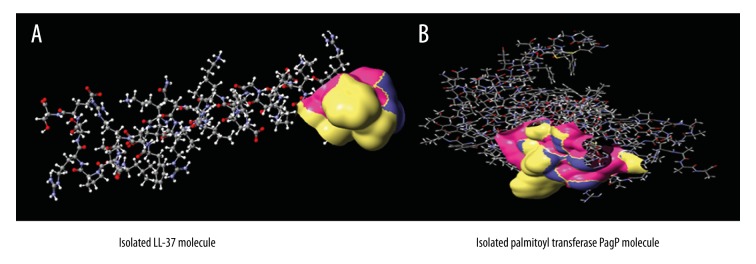
ReaxFF method of simulation of the stable structure obtained after energy minimization of antibacterial peptide LL-37 and palmitoyl transferase PagP by molecular mechanics showed that: the systems contact each other at 8.75 picosec due to the mutual merge of hydrophobic groups of LL-37 and palmitoyl transferase PagP (Figure 1) of which the spherical atoms were contact atoms of LL-37 and PagP; atoms in red, blue, white, and gray represent O, N, H, and C atoms, respectively; the left comes from LL-37 and the right comes from PagP. After 20 picsec, the system had no trend of dissociation, and weak bond energy, bone -C-O-H…NH2-CH2- was calculated as-14.97 Kcal/mol. The contact position of antimicrobial peptide LL-37 and palmitoyl transferase PagP are shown in Figure 2; yellow represents the region of hydrophobic groups. Our analysis showed that the hydrophobic groups were the important factors which lead to contact and merging of the two parts. It was important to note that the contacted weak bond -C-O-H…NH2-CH2- in Figure 1 was the bridge for contacting LL-37 with palmitoyl transferase PagP. Among them, -NH2 came from the middle part of the LL-37 molecule, but not the yellow hydrophobic region shown in Figure 2A. -NH2 group was taken to the contact region and formed a weak bond structure -C-O-H4H2-CH2- after antibacterial peptide LL-37 arrived at the surface of palmitoyl transferase PagP through bending and climbing.
Figure 1.
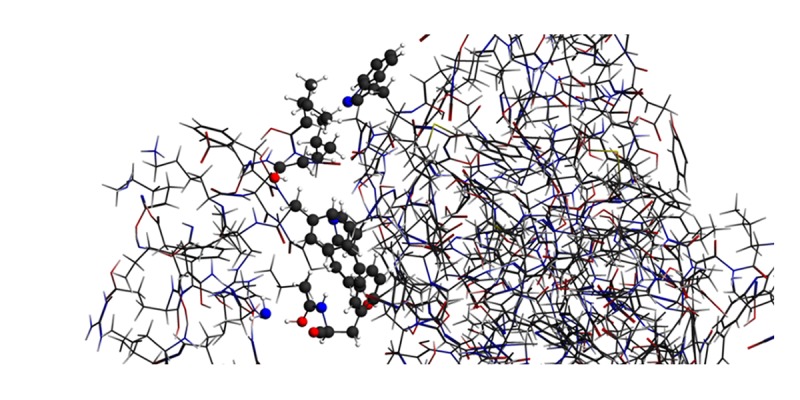
“Entangled” structure of antibacterial peptide LL-37 and palmitoyl transferase PagP after 8.75 picosec.
Figure 2.
(A, B) Hydrophobicity of antibacterial peptide LL-37 and transferase PagP.
Docking simulation between antimicrobial peptide LL-37 fragment and palmitoyl transferase Pagp
Molecular docking showed that the binding sites of antibacterial peptide LL-37 and palmitoyl transferase PagP mainly included LYS8, GLU11, LEU28, LYS12, PHE27, ILE13, and PHE6 of antibacterial peptide LL-37 and ARG94, TRP89, ASN65, SER3, GLU90, GLU90, ASN100, HIS102 and THR92 of palmitoyl transferase PagP. The best docking conformation was determined after multiple docking, and the corresponding ligand efficiency are shown in Table 2.
Table 2.
The ligand efficiency of docking between antimicrobial peptide LL-37 fragment and palmitoyl transferase PagP.
| Docking ligands | Ligand efficiency (Kcal/mol) |
|---|---|
| LLGDFFRKS | −1.6100 |
| RKSKEKIGK | −17358 |
| KEKIGKEFKR | −1.7547 |
| KEFKRIVQRIK | −2.2389 |
| FKRIVQRIKDF | −2.2358 |
| VQRIKDFLRNL | −2.2167 |
| KDFLRNLVPR | −1.7358 |
| FLRNLVPRTES | −1.6130 |
Humanized antimicrobial peptide LL-37 and the mutant
The mutant was to replace leucine (No. 1), glycine (No. 3), glutamic acid (No. 16), aspartic acid (No. 26), arginine (No. 34), threonine (No. 35) to glycine, leucine, glutamine, lysine, threonine, and arginine on the basis of LL-37 and other amino acid residues that remained changeless (Figure 3).
Figure 3.
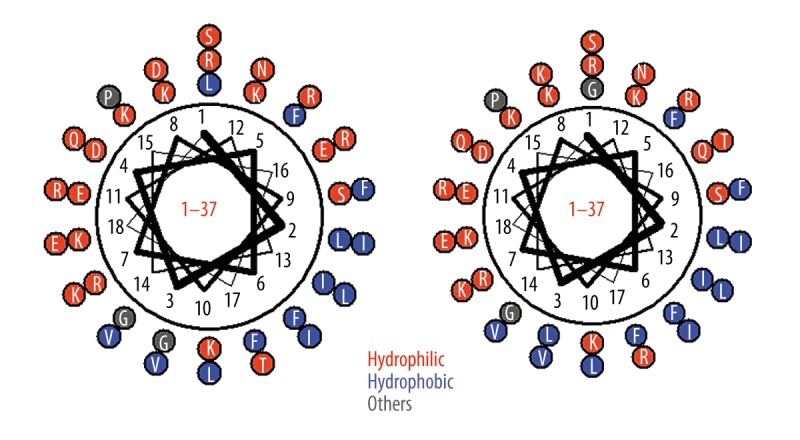
Distribution of hydrophilic amino acid and hydrophobic amino acid and spiral pattern of antibacterial peptide LL-37 and mutant (left: antibacterial peptide LL-37; right: mutant).
Determination of antimicrobial activity of humanized antimicrobial peptide LL-37 and the mutant
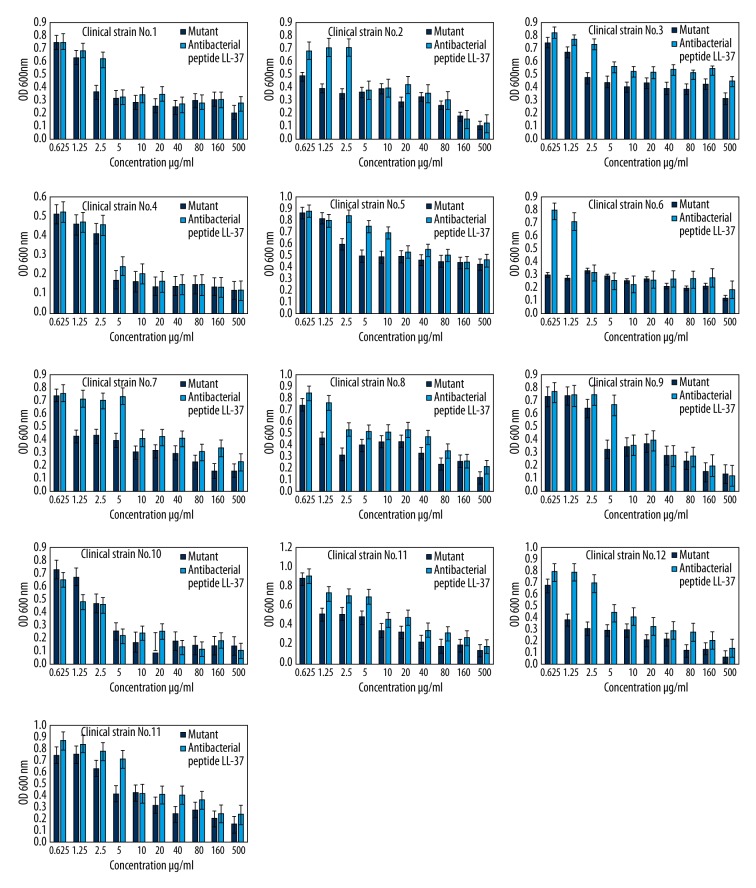
The change of the OD value of the 13 kinds of E. coli after being treated by mutants and non-mutants with different concentrations can be seen in Figure 4. The minimum inhibitory concentration of both antibacterial peptide LL-37 and mutants on 13 kinds of E. coli were within 20 μg/mL, and the minimum inhibitory concentration of mutant on E. coli was significantly lower than that of antibacterial peptide LL-37 (p<0.05).
Figure 4.
The mean OD value of 13 kinds of E. coli after treated by antimicrobial peptide LL-37 and mutant with different concentrations.
The change of expression of bacteria PagP enzyme after treated by humanized antibacterial peptide LL-37 and mutant
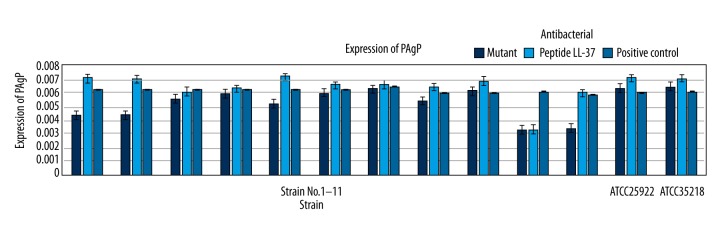
Figure 5 shows that the expression of PagP enzyme on each different E. coli after adding mutant, LL-37, and no antibacterial peptide was different, the expression of enzyme with mutant was significantly lower (p<0.05), while the expression of enzyme with LL-37 significantly increased (p<0.05).
Figure 5.
Effects of humanized antibacterial peptide LL-37 and mutant on the expression of 13 E. coli PagP enzymes.
Discussion
Antibacterial peptide LL-37 has the potential to become a new antibacterial drug [13,14]. A study showed that it can increase the permeability of the outer membrane through destroying the outer membrane of gram-negative bacteria, and can be inhibited by protein palmitoyl transferase PagP from the bacterial outer membrane [15].
The analysis of bioinformatics method showed that when antibacterial peptide LL-37 united with protein palmitoyl transferase PagP of the bacterial outer membrane, more -C-O-H…NH2-CH2- formed in the middle of the antimicrobial peptide LL-37 were good for the stability of the bond. In addition, the hydrophobicity and molecular flexibility of antibacterial peptide LL-37 were important for the form of -C-O-H…NH2-CH2- through molecular folding and climbing. Docking results showed that the main binding sites of antibacterial peptide LL-37 and protein palmitoyl transferase PagP of the bacterial outer membrane were mainly concentrated in the middle of the antimicrobial peptide LL-37. Therefore, this study was performed as a de novo design of antibacterial peptide LL-37, and obtained antibacterial peptide LL-37 and its mutant through artificial chemical synthesis. Results of antibacterial experiments indicated that the minimum inhibitory concentration of both antibacterial peptide LL-37 and mutant on the 13 E. coli was within 20 μg/mL, and the antibacterial peptide LL-37 and non-mutant had good antibacterial activity; and the mutant had significantly increased antibacterial effect on 13 E. coli at low concentration when compared with LL-37, and its MIC also showed significant decrease. Therefore, the results suggested that the antimicrobial activity of LL-37 mutant on most of E. coli was higher than LL-37.
In addition, the expression of E. coli PagP with mutant was significantly decreased compared to that of the group with antibacterial peptide LL-37 (p<0.05), while the expression of E. coli PagP with antibacterial peptide LL-37 was significantly increased compared to that of the group without antibacterial peptide (p<0.05). The results showed that for most of the E. coli, the expression of PagP enzyme after adding mutant was lower than with antibacterial peptide LL-37, while the expression of PagP enzyme after adding antibacterial peptide LL-37 was higher than without antimicrobial peptides. Reports showed that the alkyl acyltransferase PagP of the outer membrane of gram-negative bacteria cells can transfer a fatty acid chain with 16 carbons from phospholipid to β2 position of lipoid A, producing 7 lipoid A with the structure of fatty acid chain, which could disturb the recognition of host immune cell TLR-4 on lipid A, at the same time, lead to its resistance to antimicrobial peptide [16,17]. Therefore, antibacterial peptide LL-37 could stimulate the production of PagP, and increase the expression of PagP. While the mutant of LL-37 designed by us was intended to inhibit the activity of PagP, and as such the expression decreases. We concluded that the cause for enhanced antibacterial effect of mutant of antibacterial peptide was to decrease the trans membrane effect of PagP on antibacterial peptides through inhibiting the activity of PagP enzyme, thereby increasing the antibacterial activity. The further study in the future should look at the mechanism from the protein level.
Conclusions
The obtained mutant of humanized antibacterial peptide LL-37 had stronger antibacterial effect; the reason for the enhancement of the antibacterial effect was that the activity of PagP was inhibited, which provided a better condition for the future application in the field of medicine and health care, and agriculture production.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Source of support: This project was funded by The Natural Science Foundation of Hubei Province (No: 2015CFB627) and Science and Technology Project of Huangshi city (No: 2013A077-4)
References
- 1.Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: Key components of the innate immune system. Crit Rev Biotechnol. 2012;32:143–71. doi: 10.3109/07388551.2011.594423. [DOI] [PubMed] [Google Scholar]
- 2.Iyer BR, Mahalakshmi R. Residue-dependent thermodynamic cost and barrel plasticity balances activity in the PhoPQ-activated enzyme PagPof Salmonella typhimurium. Biochemistry. 2015;54(37):5712–22. doi: 10.1021/acs.biochem.5b00543. [DOI] [PubMed] [Google Scholar]
- 3.Girnita A, Zheng H, Grönberg A, et al. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene. 2012;31(3):352–65. doi: 10.1038/onc.2011.239. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Xhindoli D, Pacor S, Benincasa M, et al. The human cathelicidin LL-37 – A pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta. 2016;1858(3):546–66. doi: 10.1016/j.bbamem.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Sørensen OE, Clemmensen SN, Dahl SL, et al. Papillon-Lefèvre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest. 2014;124(10):4539–48. doi: 10.1172/JCI76009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop RE. The lipid A palmitoyltransferase PagP: Molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol. 2005;57(4):900–12. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 7.Chalabaev S, Chauhan A, Novikov A, et al. Biofilms formed by gram-negative bacteria undergo increased lipid a palmitoylation, enhancing in vivo survival. MBio. 2014;5(4) doi: 10.1128/mBio.01116-14. pii: e01116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shprung T, Peleg A, Rosenfeld Y, et al. Effect of PhoP-PhoQ activation by broad repertoire of antimicrobial peptides on bacterial resistance. J Biol Chem. 2012;287(7):4544–51. doi: 10.1074/jbc.M111.278523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannehl C, Gutsmann T, Brezesinski G. Surface activity and structures of two fragments of the human antimicrobial LL-37. Colloids Surf B Biointerfaces. 2013;109:129–35. doi: 10.1016/j.colsurfb.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Wang G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem. 2008;283:32637–43. doi: 10.1074/jbc.M805533200. [DOI] [PubMed] [Google Scholar]
- 11.Ren SX, Shen J, Cheng AS, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. Plos One. 2013;8(5):e63641. doi: 10.1371/journal.pone.0063641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ADF2014, SCM. Theoretical Chemistry. Vrije Universiteit; Amsterdam, The Netherlands: http://www.scm.com. [Google Scholar]
- 13.Fox MA, Thwaite JE, Ulaeto DO, et al. Design and characterization of novel hybrid antimicrobial peptides based on cecropin A, LL-37 and magainin II. Peptides. 2012;33(2):197–205. doi: 10.1016/j.peptides.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Midura-Nowaczek K, Markowska A. Antimicrobial peptides and their analogs: Searching for new potential therapeutics. Perspect Medicin Chem. 2014;6:73–80. doi: 10.4137/PMC.S13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop RE. The lipid A palmitoyltransferase PagP: Molecular mechanisms and role in bacterial pathogenesis. Mol Microbiol. 2005;57(4):900–12. doi: 10.1111/j.1365-2958.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 16.Otzen D. Membrane protein folding and stability. Arch Biochem Biophys. 2014;564(564):262–64. doi: 10.1016/j.abb.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Bishop RE, Gibbons HS, Guina T, et al. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 2000;19(19):5071–80. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]