Abstract
Background and Aims The breadfruit genus (Artocarpus, Moraceae) includes valuable underutilized fruit tree crops with a centre of diversity in Southeast Asia. It belongs to the monophyletic tribe Artocarpeae, whose only other members include two small neotropical genera. This study aimed to reconstruct the phylogeny, estimate divergence dates and infer ancestral ranges of Artocarpeae, especially Artocarpus, to better understand spatial and temporal evolutionary relationships and dispersal patterns in a geologically complex region.
Methods To investigate the phylogeny and biogeography of Artocarpeae, this study used Bayesian and maximum likelihood approaches to analyze DNA sequences from six plastid and two nuclear regions from 75% of Artocarpus species, both neotropical Artocarpeae genera, and members of all other Moraceae tribes. Six fossil-based calibrations within the Moraceae family were used to infer divergence times. Ancestral areas and estimated dispersal events were also inferred.
Key Results Artocarpeae, Artocarpus and four monophyletic Artocarpus subgenera were well supported. A late Cretaceous origin of the Artocarpeae tribe in the Americas is inferred, followed by Eocene radiation of Artocarpus in Asia, with the greatest diversification occurring during the Miocene. Borneo is reconstructed as the ancestral range of Artocarpus, with dozens of independent in situ diversification events inferred there, as well as dispersal events to other regions of Southeast Asia. Dispersal pathways of Artocarpus and its ancestors are proposed.
Conclusions Borneo was central in the diversification of the genus Artocarpus and probably served as the centre from which species dispersed and diversified in several directions. The greatest amount of diversification is inferred to have occurred during the Miocene, when sea levels fluctuated and land connections frequently existed between Borneo, mainland Asia, Sumatra and Java. Many species found in these areas have extant overlapping ranges, suggesting that sympatric speciation may have occurred. By contrast, Artocarpus diversity east of Borneo (where many of the islands have no historical connections to the landmasses of the Sunda and Sahul shelves) is unique and probably the product of over water long-distance dispersal events and subsequent diversification in allopatry. This work represents the most comprehensive Artocarpus phylogeny and biogeography study to date and supports Borneo as an evolutionary biodiversity hotspot.
Keywords: Ancestral area reconstruction, Artocarpeae, Artocarpus, Borneo, dispersal, divergence date estimates, historical biogeography, Moraceae, phylogeny, Southeast Asia
INTRODUCTION
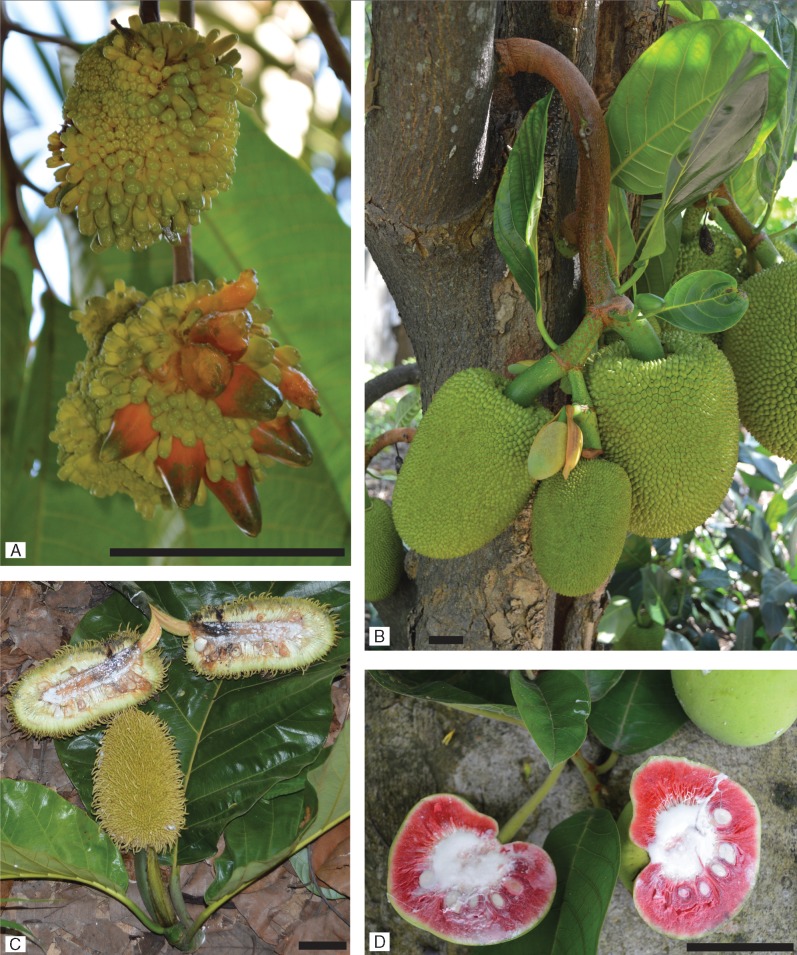
Artocarpus (Moraceae – mulberry family) is an economically and ecologically important genus of approx. 70 tree species native to South and Southeast Asia and Oceania (Jarrett, (1959a; Kochummen, 2000; Berg et al., 2006; Zerega et al., 2010). All members of the genus have fleshy compound infructescences (syncarps), which develop from inflorescences with up to thousands of tiny flowers tightly packed and condensed on a receptacle. Several species, including breadfruit [A. altilis (Parkinson) Fosberg], jackfruit (A. heterophyllus Lam.) and cempedak [A. integer (Thunb.) Merr.], produce large, edible syncarps and are valuable crops (Fig. 1). Many Artocarpus species also serve as important food sources for forest animals, such as elephants and orangutans (Campbell-Smith et al., 2011; Sekar et al., 2015). Much of the native Artocarpus range is in a geologically complex region of the world and encompasses large, biodiverse forests that are under threat due to development and agriculture (Wilcove et al., 2013). Some Artocarpus species are classified as vulnerable on the IUCN red list, although most species have not been assessed. Understanding the biogeography and evolutionary history of the genus will be important for advancing further research and for informing conservation efforts including of crop wild relatives.
Fig. 1.
Representative species from Artocarpus subgenera. (A) Subgenus Prainea: A. limpato. (B) Subgenus Cauliflori: A. heterophyllus. (C) Subgenus Artocarpus: A. sericicarpus. (D) Subgenus Pseudojaca: A. dadah. All scale bars are 5 cm.
Artocarpus is part of the tribe Artocarpeae, which also includes two small neotropical genera, Batocarpus and Clarisia (three species each). The tribe has a disjunct distribution, with Artocarpus diversity centred in Southeast Asia, and Batocarpus and Clarisia restricted to Central and South America (Zerega et al., 2010; Berg, 2001). It is unclear when, where and how the tribe diversified and dispersed into its current disjunct range. In a family-level study, Zerega et al. (2005) estimated the crown age of the Artocarpeae tribe at 65·1 (52·2–80·6) Mya. Based on locations of fossils, paleoclimate, and geological evidence, they proposed that a Eurasian origin of Moreaceae, followed by migration across the boreotropical North Atlantic Land Bridge during the Eocene, was at least as likely as a previously proposed Gondwana origin for the family. However, only five species of Artocarpus were included in the study and no ancestral range reconstructions were conducted, leaving the ancestral range and the influence of the complex biogeography of Southeast Asia on the spatial and temporal evolution of Artocarpus unexplored.
The geological (Hall, 2002, 2009; http://searg.rhul.ac.uk/) and floristic history (Morley, 2000, 2012; de Bruyn et al., 2014) of Southeast Asia have been described and summarized by several authors. Geologically, the region includes Sundaland (Sunda shelf including southern Indochina, peninsular Thailand and Malaysia, Sumatra, and parts of Borneo and Java), the Sahul shelf (including Australia and the island of New Guinea), the eastern Pacific Ocean and Philippine Sea plates, and Wallacea (the area of collision between the Sahul and Sunda shelves, including numerous islands such as Sulawesi, the Moluccas and the Lesser Sunda Islands). The Wallacean Islands have various origins in the West Pacific and Australia and have never been connected with Sundaland nor with the Sahul shelf. The landmasses of the Sahul shelf have never been connected to Sundaland nor to the Eurasian continent. In contrast, Sundaland has been part of the Eurasian continent since the Mesozoic and the islands of Sundaland are largely of continental origin. They formed a contiguous landmass with Eurasia during times of low sea levels, and during the middle Eocene the Sunda Shelf is thought to have experienced its greatest land area (de Bruyn et al., 2014). From the Oligocene into the early Quaternary, sea levels fluctuated frequently, with landmasses of Sundaland variously submerged and emergent. Throughout this time, central and north-western Borneo remained emergent and connected to the mainland (Hall, 2009; de Bruyn et al., 2014). It is only in relatively recent times that the continuous landscape disappeared and was replaced by island chains (Bendiksby et al., 2010), and the present-day geography is atypical of what it has looked like during most of the past tens of thousands of years.
The geological history of Southeast Asia has been described as being the result of more than 300 million years of ‘Colliding Worlds’ (van Oosterzee, 1997) due to its position at the interface of the Sunda and Sahul plates. Dramatic sea-level fluctuations throughout the past hundreds of millions of years have led to large variations in the amount of exposed land area and terrestrial connections among the islands and has had an impact on the evolution of the biota in the region (Hall, 2009, 2012; de Bruyn et al., 2014). Studying taxa that are centred in this region can contribute to a more complete understanding of how, when and where taxa diversified and dispersed, and may reveal common patterns. Within Southeast Asia, certain regions may have higher rates of endemism and diversification. For example, de Bruyn et al. (2014) recently identified Borneo and Indochina as ‘evolutionary hotspots’ in a phylogenetic meta-analysis of both flora and fauna, and several studies cite Borneo as the centre of diversification for multiple taxa (Nauheimer et al., 2012; Webb and Ree, 2012).
The most recent phylogeny of Artocarpus (Zerega et al., 2010) supports its monophyly and recognizes four subgenera: Artocarpus, Pseudojaca, Cauliflori and Prainea (Table 1; Zerega et al., 2010), but not all subgeneric sections and series (sensu Jarrett, 1959c, 1960) were included in that analysis. The subgenera are not geographically restricted, and taxa from all subgenera are found throughout the Sunda and Sahul shelves as well as in Wallacea and the Philippines. A well-resolved phylogeny will help understand the complicated biogeography of Artocarpus as well as help address species delineation for several species with broad geographical ranges. For example, the range of Artocarpus nitidus Trécul (sensu Jarrett, 1960) includes the Philippines, Borneo, Sumatra, mainland Southeast Asia and China, but it has been variously sunken or segregated into separate species, subspecies or varieties based on size and indumentum of the syncarp, and slight differences in the shape and venation of the leaves, as well as variations in geographical range. In a recent taxonomic treatment, Berg et al. (2006) treated A. nitidus as having several ‘informal entities’ that aligned to some degree with formerly treated subspecies, while previous authors treated them as five separate species as detailed in Jarrett (1960). Another example is A. lacucha Buch.-Ham. Jarrett (1960) recognized a well-defined A. lacucha restricted in its range from India into Indo-Burma and southern China, while Berg et al. (2006) sunk several morphologically diverse species into A. lacucha, extending its range into the Philippines, the Indo-Pacific Islands and New Guinea. Berg justified this approach based on shared features that indicate intermittent growth in combination with deciduousness; however, he also recognized informal ‘forms’ within A. lacucha based on variations in leaf shape and inflorescence morphology.
Table 1.
Taxa included in this study
| Genus/subgenus (taxa included/total no. of taxa in group) | Taxon | Geographical region(s) assigned for biogeographical analyses | No. of individuals in ‘Full’ dataset | No. of individuals in ‘Reduced’ dataset | No. of individuals in Exemplar dataset |
|---|---|---|---|---|---|
| Artocarpus/Artocarpus (24/31) | Artocarpus altilis | ES | 6 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus anisophyllus | B, Sum, TM | 7 | 6 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus blancoi | P | 1 | 1 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus brevipedunculatus | B | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus camansi | ES | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus chama | TM, IB, SC | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus elasticus | B, Sum, J, TM, IB | 3 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus excelsus | B | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus hirsutus | WG | 1 | 1 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus hispidus | TM | 1 | 1 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus kemando | B, Sum, TM, IB | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus lanceifolius | B, Sum, TM, IB | 4 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus lowii | Sum, TM | 3 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus maingayi | Sum, TM | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus mariannensis | ES | 1 | 1 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus obtusus | B | 1 | 1 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus odoratissimus | B, P | 3 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus rigidus | B, Sum, J, TM, IB | 3 | 3 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus scortechinii | Sum, TM | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus sepicanus | ES | 1 | 1 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus sericicarpus | B, P, ES, Sul | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus tamaran | B | 2 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus teijsmannii | B, Sum, TM, ES, Sul | 3 | 2 | 1 |
| Artocarpus/Artocarpus (24/31) | Artocarpus treculianus | P | 1 | 1 | 1 |
| Artocarpus/Cauliflori (3/3) | Artocarpus annulatus | B | 1 | 1 | 1 |
| Artocarpus/Cauliflori (3/3) | Artocarpus heterophyllus | WG | 6 | 2 | 1 |
| Artocarpus/Cauliflori (3/3) | Artocarpus integer | B, Sum, TM, IB, ES, Sul | 4 | 2 | 1 |
| Artocarpus/Prainea (2/4) | Artocarpus limpato | B, Sum, TM | 2 | 2 | 1 |
| Artocarpus/Prainea (2/4) | Artocarpus papuanus | ES | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus altissimus | B, Sum, TM, IB | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus dadah** | B, Sum, TM, IB | 10 | 8 | 2 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus fretessii** | B, ES, Sul | 3 | 3 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus fulvicortex | Sum, TM | 2 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus glaucus | B, Sum, J, TM | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus gomezianus subsp. gomezianus | Sum, J, TM, IB | 3 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus lacucha** | TM, IB, SC | 7 | 6 | 2 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus nitidus cf. subsp. humilis* | B | 2 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus nitidus subsp. borneensis* | B | 3 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus nitidus subsp. griffithii* | TM, IB, SC | 3 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus nitidus subsp. lingnanensis* | IB, SC | 5 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus ovatus** | P | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus primackii | B | 4 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus rubrovenius | P | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus styracifolius | IB, SC | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus thailandicus | IB | 3 | 3 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus tomentosulus | B | 2 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus tonkinensis | IB, SC | 2 | 2 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus vrieseanus var. vrieseanus | ES, Sul | 1 | 1 | 1 |
| Artocarpus/Pseudojaca (20/24) | Artocarpus xanthocarpus* | P, SC | 1 | 1 | 1 |
| Batocarpus | Batocarpus costaricensis | NCA, SA | 1 | 1 | 1 |
| Batocarpus | Batocarpus sp. | 1 | 1 | ||
| Clarisia | Clarisia biflora | NCA, SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Antiaris toxicaria subsp. madagascariensis | A | 1 | 1 | 1 |
| Outgroup (Moraceae) | Antiaropsis decipiens | ES | 1 | 1 | 1 |
| Outgroup (Moraceae) | Bagassa guiannensis | SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Brosimum lactescens | NCA, SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Broussonetia cf. kurzii | Sum, IB, SC | 1 | 1 | 1 |
| Outgroup (Moraceae) | Broussonetia greveana | A | 1 | 1 | 1 |
| Outgroup (Cannabaceae) | Cannabis sativa | E | 1 | 1 | 1 |
| Outgroup (Moraceae) | Castilla elastica | NCA, SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Dorstenia choconiana | NCA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Fatoua villosa | E | 1 | 1 | 1 |
| Outgroup (Moraceae) | Ficus carica | E | 1 | 1 | 1 |
| Outgroup (Moraceae) | Ficus insipida | NCA, SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Ficus pachyclada | A | 1 | 1 | |
| Outgroup (Rosaceae) | Fragaria vesca | NCA | 1 | ||
| Outgroup (Moraceae) | Hullettia dumosa | Sum, TM | 1 | 1 | 1 |
| Outgroup (Moraceae) | Hullettia griffithiana | TM | 1 | 1 | 1 |
| Outgroup (Cannabaceae) | Humulus lupulus | E | 1 | 1 | 1 |
| Outgroup (Moraceae) | Maclura africana | A | 1 | 1 | |
| Outgroup (Moraceae) | Maclura amboinensis | TM, IB, ES, SC | 1 | ||
| Outgroup (Moraceae) | Maclura andamanica | IB | 1 | ||
| Outgroup (Moraceae) | Maclura brasiliensis | NCA, SA | 1 | ||
| Outgroup (Moraceae) | Maclura cochinchinensis Asia | B, P, Sum, J, TM, IB, ES, Sul, SC, E | 1 | ||
| Outgroup (Moraceae) | Maclura cochinchinensis Borneo | B, P, Sum, J, TM, IB, ES, Sul, SC, E | 1 | ||
| Outgroup (Moraceae) | Maclura fruticosa | TM, IB, SC | 1 | ||
| Outgroup (Moraceae) | Maclura pomifera | NCA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Maclura spinosa | WG | 1 | ||
| Outgroup (Moraceae) | Maclura thorelii | IB | 1 | ||
| Outgroup (Moraceae) | Maclura tinctoria subsp. mora | SA | 1 | ||
| Outgroup (Moraceae) | Maclura tinctoria subsp. tinctoria | NCA, SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Maclura tricuspidata | SC | 1 | 1 | 1 |
| Outgroup (Moraceae) | Melicia excelsa | A | 1 | ||
| Outgroup (Moraceae) | Morus alba | E | 1 | 1 | 1 |
| Outgroup (Moraceae) | Morus notabilis | SC | 1 | ||
| Outgroup (Moraceae) | Parartocarpus bracteatus | B, Sum, P | 1 | 1 | 1 |
| Outgroup (Moraceae) | Parartocarpus venenosus | B | 1 | 1 | 1 |
| Outgroup (Urticaceae) | Pilea microphylla | NCA, SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Sorocea briquetii | SA | 1 | 1 | 1 |
| Outgroup (Moraceae) | Sorocea steinbachii | 1 | 1 | ||
| Outgroup (Moraceae) | Sparattosyce dioica | ES | 1 | 1 | 1 |
| Outgroup (Moraceae) | Treculia africana | A | 1 | 1 | 1 |
| Outgroup (Moraceae) | Treculia obovoidea | 1 | 1 |
Taxa are organized alphabetically, by genus and species. For ingroup Artocarpeae taxa, the genus, and within Artocarpus the subgenus, are indicated (number of species sampled in taxon group/total number of species in specified taxon group based on Zerega et al., 2010). Outgroup taxa are also listed and are shown in the phylogeny of the full dataset in Fig. S1. Range distributions used in biogeography analyses are shown for each taxon as follows: B = Borneo and Palawan, Sum = Sumatra, TM = Thai-Malay peninsula, IB = IndoBurma, P = Philippines, J = Java and the lesser Sunda Islands, WG = Western Ghats, Sul = Sulawesi, ES = East of Sulawesi, SC = southern China, A = Africa, NCA = North and Central America, SA = South America, E = Eurasia. The number of individuals included in the full, reduced and exemplar datasets for each taxon is indicated.
A. nitidus sensu Berg et al. (2006) and Jarrett (1960);
A. lacucha sensu Berg et al. (2006).
The aims of the present study were to employ data from eight loci (two nuclear and six chloroplast) and extensive taxon sampling to reconstruct the evolutionary history of Artocarpeae, especially Artocarpus, in order to test the monophyly of and relationships among Artocarpus subgenera, and to help inform the species boundaries of difficult to delineate Artocarpus species. In addition, we estimated divergence dates and inferred ancestral ranges within Artocarpeae to understand dispersal patterns in a highly complex biogeographical region. Specifically, we aimed to identify the ancestral range of tribe Artocarpeae and test if Borneo is an evolutionary hotspot for the genus. Investigating the evolutionary and biogeographical history of Artocarpus species is also of interest due to the economic importance of the genus. Understanding its origins, diversification and crop wild relatives will be important for conservation efforts.
MATERIALS AND METHODS
Taxon sampling
Outgroup sampling included taxa in the Cannabaceae, Urticaceae and Rosaceae as well as 26 taxa from 15 genera in Moraceae, encompassing all Moraceae tribes recognized by Clement and Weiblen (2009). Ingroup sampling represented all Artocarpeae genera, all four Artocarpus subgenera (Artocarpus, Prainea, Cauliflori, Pseudojaca; Zerega et al., 2010), as well as all of Jarrett’s (1959a, b, c, 1960) named sections and series (Table 1). In most cases, there were at least two exemplars for each taxon. For taxa with difficult to delineate species boundaries (A. nitidus and A. lacucha), we included multiple exemplars of the taxa that have been placed in these species (14 and 19, respectively, Table 1). We included a total of two Batocarpus, one Clarisia and 52 Artocarpus taxa (Table 1; Table S1). We used three different datasets for phylogenetic inference, dating and dispersal approximations: the full dataset used all accessions, the reduced dataset used up to two accessions per taxon, and the exemplar dataset used a single accession per taxon (Table 1, see Results for an explanation of the criteria for each dataset).
DNA extraction and sequencing
While some DNA sequences used in this study came from Zerega et al. (2010), most of the samples were generated for the present study as follows. We extracted DNA using a CTAB method (Zerega et al., 2002) or Qiagen DNeasy Mini Plant Kit (cat. no. 69104, Qiagen, Valencia, CA, USA). For recalcitrant herbarium samples we modified the Qiagen protocol and added 35 µL of proteinase K and 75 µL β-mercaptoethanol to each sample with the lysis buffer and incubated at 45 °C overnight. An additional 20 µL of proteinase K was added before incubation for 12 h at 45^°C. After the addition of buffer AP2 (Qiagen), samples were incubated in a − 20 °C freezer overnight, and then the recommended kit protocol was followed. CTAB DNA extractions from herbarium samples were cleaned using a QIAquick PCR Purification Kit (cat. no. 28104, Qiagen). We quantified DNA using a NanoDrop 2000 device (Thermo Scientific, Waltham, MA, USA) and visualized DNA by running samples out on a 1 % agarose gel.
We used the same PCR recipe for each region [5 µL of 2× MyTaq Mix (cat. no. BIO-25041, Bioline, London, UK), 3 µL of water, 0·5 µL of each 10 mm primer and 1 µL of DNA template] with the exception of G3pdh which included the addition of 0·4 % bovine serum albumin to each PCR. We developed internal primers for rbcL and matK (Supplementary Data Table S2). PCR conditions were as follows: ITS: 94 °C/5 min, then 30 cycles of [94 °C/30 s, 53 °C/30 s, 72 °C/2 min], then a final extension of 72 °C/10 min; G3pdh: 94 °C/3·5 min, then 36 cycles of [95 °C/1 min, 55 °C/1 min and 72 °C/min], then a final extension at 72 °C/7 min; matK: 94 °C/5 min, then 35 cycles of [94 °C/30 s, 52 °C/20 s and 72 °C/50 s], then a final extension at 72 °C/5 min; rbcL: 95 °C/4 min, then five cycles of [94 °C/30 s, 55 °C/1 min and 72 °C/1 min], then 30 cycles of [94 °C/30 s and 54 °C/1 min]; trnL-trnF: 94 °C/3 min, then 32 cycles of [94 °C/45 s, 52 °C/30 s and 72 °C/90 s], then a final extension of 74 °C/7 min; trnH-psbA: 80 °C/5 min, then 35 cycles of [94 °C/30 s, 58–48 °C (touchdown)/30 s, 72 °C/1 min], then a final extension at 72 °C/10 min; trnS-G: 80 °C/5 min, then 30 cycles of [95 °C/1 min, 66 °C/1 min], then a final extension at 66 °C/10 min; trnV-ndhC: 80 °C/5 min, then 35 cycles of [94 °C/30 s, 55 °C/30 s, 72 °C/2 min], then a final extension at 65 °C/5 min. We used gel electrophoresis to confirm that PCR was successful. We cleaned PCR products using the QIAquick PCR Purification Kit or an ethanol cleaning using a centrifuge spindown in 100 % ethanol for 30 min at 4 °C, followed by a wash in 70 % ethanol for 15 min at 4 °C.
To cycle sequence PCR products we used: 3 µL of water, 1 µL of ABI Big Dye (Applied Biosystems, Foster City, CA, USA), 3 µL of 100× Big Dye buffer, 1 µL of either the forward or reverse 10 mm primer and 2 µL of PCR product. Conditions for cycle sequencing were 95 °C/1 min, then 32 cycles of [96 °C/10 s, 50 °C/5 s and 60 °C/30 s]. We cleaned the product using the ethanol protocol described above with a 7 % addition of 125 mm EDTA to the preliminary 100 % ethanol. We added 10 µL of HiDi formamide before running plates on an Applied Biosystems 3730 sequencer.
We trimmed traces and edited contigs manually using CodonCode v.5.1. We checked each sequence against the NCBI Nucleotide database using BLAST (Altschul et al., 1990, 1997) to identify contamination. Sequences were aligned using MAFFT (Katoh and Standley, 2013) and we manually checked alignments in Mesquite (Maddison and Maddison, 2011).
Phylogenetic analyses
To determine the best model of evolution, we analysed each region in jmodeltest2 (Guindon and Gascuel, 2003; Darriba et al., 2012) with five substitution schemes, +F, +I and +G, ML optimized, and NNI tree search. We chose the best model based on likelihood scores (Table S2). We used Bayesian inference (BI) as implemented in MrBayes (Ronquist et al., 2012) (5^000^000 generations, 25 % burn-in, temperature set to allow >50 swapping frequency among chains, 4 chains, 2 runs) and maximum-likelihood (ML) in RAxML using default settings and data partitioned by locus. The full dataset was analysed using RAxML, while the reduced and exemplar datasets were analysed in RAxML and MrBayes. All analyses were performed on the CIPRES computing core (Miller et al., 2010). To investigate discordance between the two nuclear regions and the chloroplast, three ML trees were estimated in RAxML using ITS, G3pdh and the combined chloroplast regions, respectively. We then used these trees as input for ASTRAL, which estimates species trees by decomposing input trees into quartets and calculates the proportion of these quartets represented in the final species tree (Mirarab et al., 2014).
Dating
We used Beast v.1.8.1 (Heled and Drummond, 2010) to date species divergence using the exemplar (single accession per taxon) dataset, which also included outgroup sampling. We formatted datasets in Beauti v.1.8.1 with separate rate models for each locus, an uncorrelated relaxed clock (priors: ucld.stdev with exponential distribution, mean = 0·33; ucld.mean with gamma distribution shape and scale = 1), Yule process speciation (prior yule.birthRate with gamma distribution shape = 0·001, scale = 1000), and six lognormal fossil-based calibrations (see below) within Moraceae using the option ‘real space’. The mean was set to 20 and the log (Stdev) was set to 0·75 for all fossil priors. The root of the tree was Fragraria vesca (Rosaceae, Rosales) and was constrained to 110 Mya with a uniform prior based on estimates of the Rosales (Wang et al., 2009), the order to which Moraceae belongs.
Many of the fossils attributed to the family Moraceae are leaf impressions that are often poorly preserved and lack truly diagnostic characters, leaving limited numbers of definitive fossils that could be used for calibrations. Collinson (1989) reviewed fossils of Moraceae and related families and confirmed the identification of several fossils from reproductive and wood structures. The oldest fossil fruits with diagnostic features of modern Ficus are known from early Eocene formations in southern England (Chandler, 1962, 1963a, b). Broussonetia fossil fruits with diagnostic characters are recorded from the upper Eocene of southern England (Chandler, 1961; Collinson, 1989). Morus fruits have been recorded from the early Eocene or later in southern England (Chandler, 1963a), the USSR (Takhtajan, 1982) and Germany (Gregor, 1978). Fossil fruit from Chlorophora bicarinata [resembling the extant Milicia excelsa (Welw.) C.C.Berg] have been recorded from the middle Eocene of southern England (Chandler, 1961). The earliest reliable fossil with affinities to modern Artocarpus comes from fossil wood described as Artocarpoxylon deccanensis from the Deccan Intertrappean beds of the Mandla district, Madhya Pradesh, India. This formation has been dated to 54·4 ± 8·1 Mya (Srivastava et al., 1986). Fossil wood described as Cudrianoxylon engolismense from the Eocene of France is assigned to Maclura section Cudrania, and the formation from which it was reported is broadly dated to the Eocene (Dupéron-Laudoueneix, 1980). We used the above fossils for calibrations, and if they were assigned to a broad time range (i.e. Eocene), fossil offsets were selected at the youngest age within the range. We used this broad approach for Broussonetia (youngest date within the upper Eocene), Ficus (youngest date within the lower Eocene), Morus (youngest date in the lower Eocene), Milicia (youngest date within the mid-Eocene) and Maclura (Eocene). Based on the above information and using this approach, we placed the following fossil offsets at the stem node for the following clades: Artocarpus 54 Mya, Broussonetia 34 Mya, Ficus 48 Mya, Morus 48 Mya, Maclura section Cudrania 34 Mya and Milicia 38 Mya.
Biogeography
We used Artocarpus species distribution information from Jarrett (1959a, b, c, 1960, 1975), Kochummen (2000), Soepadmo and Saw (2000), Berg (2001, 2005), Zhekun and Gilbert (2003) and Berg et al. (2006, 2011) to assign Artocarpus taxa to the following areas, modified from Turner et al. (2001): Southern China, Western Ghats of India, Indo-Burma sensu Myers et al. (2000), Thai-Malay Peninsula, Borneo, Sumatra, Java, Philippines, Sulawesi and east of Sulawesi (Table 1). For other Artocarpeae and outgroup taxa we included Africa, Eurasia, North/Central America, and South America. For taxa found in more than one area, multiple assignments were allowed, with six areas being the highest for a single taxon. To reconstruct ancestral ranges and estimate dispersal events we used S-DIVA (Yu et al., 2010) and Lagrange (Ree and Smith, 2008) as implemented in RASP v.3.02 (Yu et al., 2015) using the single-accession exemplar dataset. In S-DIVA, we used 201 Beast output trees to test two to four areas per node with and without extinction, and no constraints on dispersal. In Lagrange we tested two to three areas per node, with and without dispersal constraints. Dispersal constraints followed those in Webb and Ree (2012), which we coded with the probability of 0·5 to allow dispersals between all areas. To visualize dispersal, the number and direction of dispersal events were calculated based on the most likely (>50 %) ancestral range reconstructions at each node. In cases where the most likely range reconstruction consisted of two areas, all permutations of dispersal were assigned with a value that was proportional such that each node was only counted once. For example, if a dispersal event was reconstructed from a node with one area (X) to a node with two areas (YZ), then X to Y was assigned a value of 0·5, and X to Z was assigned a value of 0·5.
RESULTS
The three datasets (exemplar, reduced, full) each had approx. 18 % missing data and 40·2–44·7 % variable and 20·9–25·1 % parsimony-informative characters. The regions G3pdh (27–36·3 % missing samples) and trnH-psbA (15–18·6 % missing samples) had the most missing taxa for each dataset. Only three samples did not have any nuclear regions: A. styracifolius Pierre and two accessions of A. kemando Miq. All taxa had at least one chloroplast region.
Phylogenetic analyses
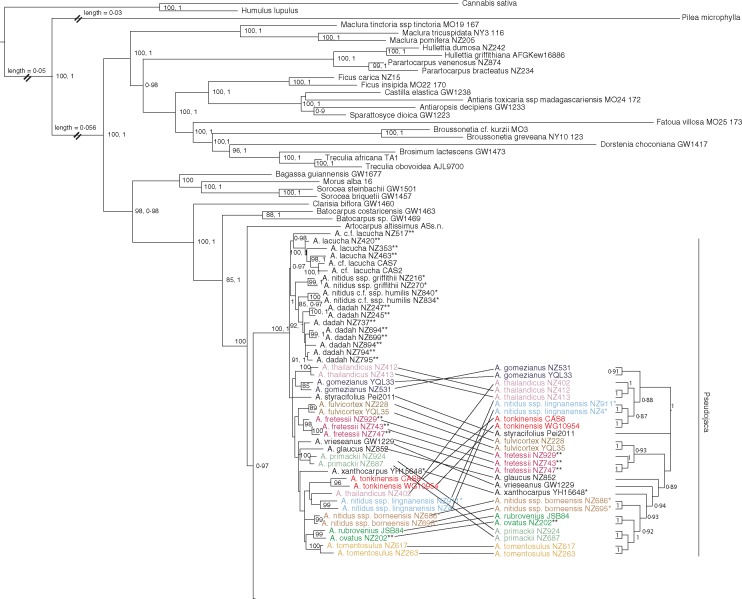
After analysis of the full dataset (Fig. S1) we reduced the number of individuals per species for the reduced and exemplar datasets based on well-supported (ML bootstrap >95 %) species clades. Within these clades, we randomly chose accession(s) that had sequences from all regions to form the reduced and exemplar datasets. In the reduced dataset, most species (or subspecies) with multiple samples were reduced to two samples, except if the species was paraphyletic in the full dataset. In the exemplar dataset, most species were reduced to a single individual for use in the dating analysis because the Yule prior used assumes each sequence represents a distinct species. Separate ML analyses of each nuclear region (ITS and G3pdh) were compared with the chloroplast phylogeny. No conflicts with bootstrap values above 70 % were found between the nuclear and chloroplast datasets. Similarly, the proportion of input quartet trees satisfied by the final ASTRAL species tree was 0·92. All regions were subsequently combined and analysed using ML and BI (Fig. 2).
Fig. 2.
Maximum likelihood tree of the genus Artocarpus based on eight loci (six plastid, two nuclear). Numbers after taxon names refer to specific collections (see Table S1 for details). Whole integer bootstrap values (80–100) are from ML analysis, while BI PP ranges from 0·8 to 1. Highly supported portions (>80, 0·8) of the topology agreed in both ML and BI analyses except in one part, where the corresponding BI tree clade is shown for comparison. Names and vertical lines to the right of the tree represent from left to right: series, sections, and subgenera (sensu Jarrett). *A. nitidus sense Berg et al. (2006) and Jarrett (1960). **A. lacucha sense Berg et al. (2006).
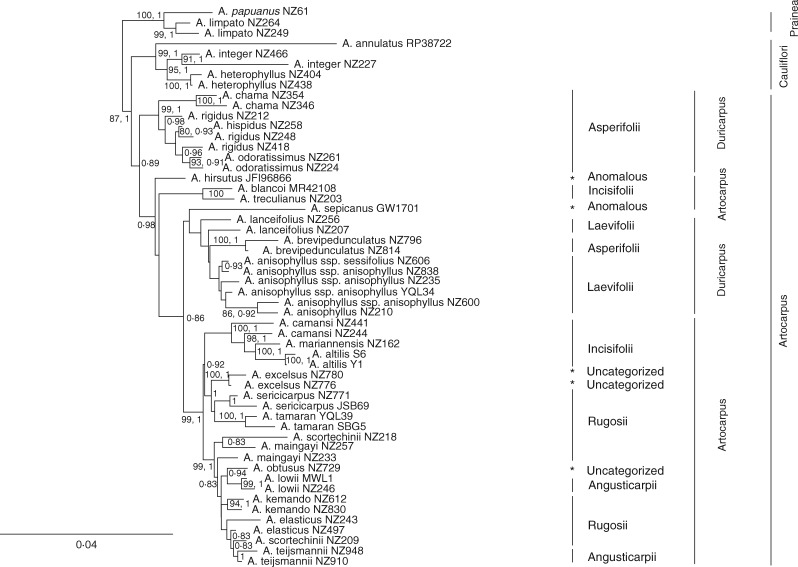
The genus Artocarpus as well as three (subgen. Artocarpus, Cauliflori and Prainea) of the four subgenera proposed by Zerega et al. (2010) had strong support (Fig. 2) as monophyletic. The fourth subgenus (Pseudojaca) was monophyletic if the anomalous A. altissimus (Miq.) J. J. Smith, which is sister to the entire genus, was excluded from it.
Within subgenus Artocarpus, Jarrett (1959) recognized two sections (section Artocarpus with four series, and section Duricarpus with two series, Table 1). Neither section was supported as monophyletic, nor were any of the series within them. The placement of a few species differed in the full vs. reduced dataset. In the analysis of the reduced dataset, exemplars of A. anisophyllus Miq. formed a clade, but in analysis of the full dataset, A. brevipedunculatus (F. M. Jarrett) C. C. Berg was nested within it. Also, exemplars of A. lanceifolius Roxb. formed a clade in the full dataset, but they formed a basal grade to A. anisophyllus and A. brevipedunculatus in the reduced dataset.
Within subgenus Pseudojaca, Jarrett (1960) delineated two sections: the monotypic section Glandulifolium (A. altissimus) and section Pseudojaca, which in the present analysis is monophyletic. While there was no strong support for many of the relationships among taxa in section Pseudojaca, there was strong support at the tips for nearly all of the species (excluding the difficult to delineate A. lacucha and A. nitidus) represented by more than one accession. With three accessions, the only species lacking support in the ML analysis was A. thailandicus C. C. Berg, although it was well supported in the BI analysis. Within section Pseudojaca, Jarrett (1960) recognized two series: Clavati (defined by the presence of interfloral bracts with clavate heads) and Peltati (defined by the presence of interfloral bracts with peltate heads). Series Clavati comprises three species, but only A. styracifolius was included in the present study, and it was nested within series Peltati. Within series Peltati, both A. lacucha (sensu Berg et al., 2006) and A. nitidus (sensu Berg et al., 2006; sensu Jarrett, 1960) were polyphyletic.
Dating
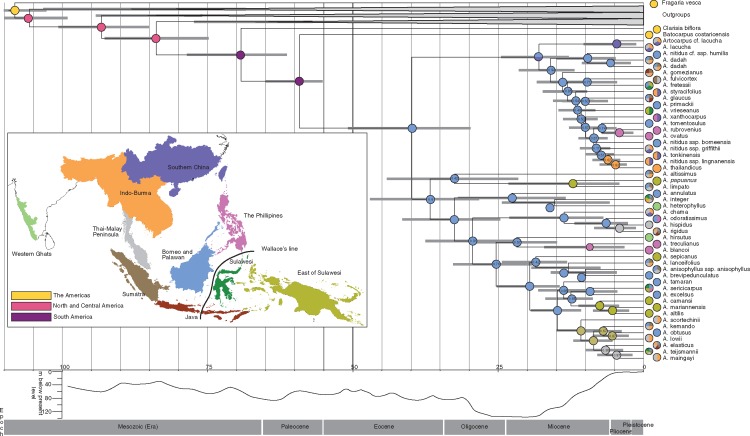
The tree topology resulting from the BEAST analysis of the single accession per taxon exemplar dataset was the same as the ML and BI topologies with one exception (Fig. 3). In the BEAST analysis A. altissimus was sister to subgenus Prainea with very low support [(posterior probability (PP) = 0·4), whereas in the ML and BI analyses A. altissimus was sister to all other Artocarpus taxa. Using the exemplar dataset in BEAST, the crown of Artocarpeae was estimated to be 69·61 Mya (61·39–78·47 Mya) and the crown of Artocarpus estimated at 40·07 Mya (29·8–50·81 Mya) (Fig. 3). The crown of Prainea + A. altissimus was estimated at 32·47 Mya (21·6–44·09 Mya). The crown of subgenus Cauliflori was estimated at 22·83 Mya (13·43–31·85 Mya). The crown of subgenus Artocarpus was estimated at 29·61 Mya (22·33–37·49 Mya) (Fig. 3). Subgenus Pseudojaca had the youngest crown estimate at 18·31 Mya (12·89–24·45 Mya). The ages of the outgroups will be further discussed in a forthcoming article.
Fig. 3.
Divergence date estimates and ancestral range reconstruction for Artocarpus. Dating, topology and node posterior support are from a BEAST analysis using six fossil calibrations and a constrained root. Error bars are 95 % confidence intervals. All nodes have posterior probability (PP) of 1·0 except where noted on the node. Coloured circles are the most likely state of a Lagrange ancestral area reconstruction allowing two areas per node. Pie charts at tips indicate the extent of the range for the indicated taxon. Sea level graph from Lambeck and Chappell (2001).
Biogeography
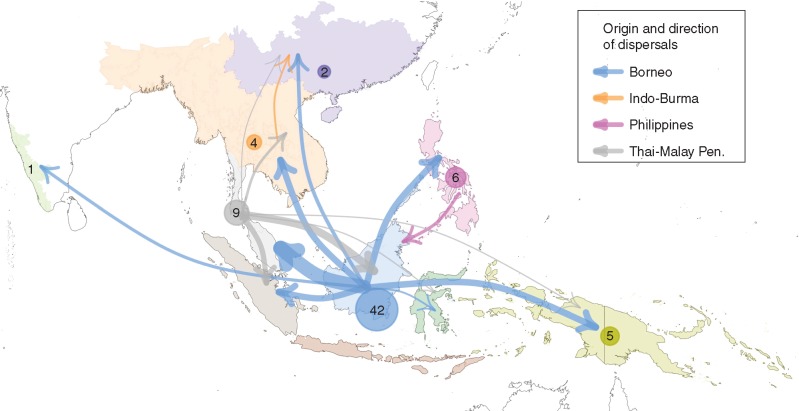
Analyses using Lagrange and SDIVA produced similar results (Supplementary Data Table S3, Fig. S2), with strong support for Borneo as the ancestral range for Artocarpus (Fig. 3). The results presented here are based on allowing two ancestral regions per node and no dispersal constraints. The root of the tribe Artocarpeae is reconstructed as North/Central America and South America. Range reconstruction of Artocarpus inferred the most cases of in situ diversification in Borneo (42 events) followed by the Thai Malay peninsula (eight events), the Philippines (six events) and East of Sulawesi (five events) (Fig. 4, Supplementary Data Table S4). Borneo was inferred as the source of the most dispersal events (32 events), followed by the Thai-Malay Peninsula (12 events) (Fig. 4, Table S4). Dispersal events from Borneo include movement eastward across Wallace’s line (i.e. A. fretessii), westward into the Thai-Malay Peninsula (i.e. A. lanceifolius), northward into Indo-Burma (A. dadah Miq.) and north-east into the Philippines (A. odoratissimus Blanco).
Fig. 4.
Dispersal and in situ speciation events in Artocarpus. Arrows indicate dispersal events, and are colour-coded to match the region from which the event originated. Line thickness is proportional to the number of events. The circled numbers in each area indicate the number of in situ diversification events in that region. Events with an occurrence of 1·0 or higher are displayed. See Table S4 for all events.
DISCUSSION
Phylogenetic analyses
This study comprises more than 75 % of the Artocarpus species recognized by the most recent treatments (Jarrett, 1959a, b, c, 1960; Kochummen, 2000; Zhekun and Gilbert, 2003; Berg et al., 2006), provides 50–100 % coverage of all subgeneric ranks, includes coverage of all previously defined sections and series (sensu Jarrett 1959a, b, c, 1960), and includes more than a dozen taxa that have not been included in previous phylogenetic analyses (Zerega et al., 2010). While it is not our aim here to redefine taxonomic divisions, this comprehensive analysis of a large and economically important genus will be important for future revisionary work and some taxonomic implications are briefly discussed.
ML and BI analysis support the division of the genus Artocarpus into the four subgenera proposed by Zerega et al. (2010), if A. altissimus is segregated into a new monotypic subgenus. Previous phylogenetic analyses did not include A. altissimus, but in the present study it was found to be sister to the rest of the genus (Figs S1 and S2) or sister to subgenus Prainea (Fig. 3). Jarrett (1960) placed A. altissimus in subgenus Pseudojaca as they share alternate, distichous leaves with non-amplexicaul stipule scars (compared to spirally alternate leaves with amplexicaul stipule scars found in subgenera Artocarpus and Cauliflori) and they also share apically fused adjacent carpellate perianths (compared to lack of apical fusion between adjacent carpellate perianths in subgenera Artocarpus, Cauliflori and Prainea). However, A. altissimus has long bifid styles, which is uncharacteristic of the subgenus Pseudojaca (but present in some members of subgenera Artocarpus and Prainea), and it also has several vegetative characters that are anomalous for the genus as a whole. These include palmately tri-nerved leaves, geniculate petioles and crenate-dentate leaf margins with glandular tissue evenly spaced along the edge. These anomalies led Jarrett (1960) to place A. altissimus in a monotypic section (Glandulifolium) within subgenus Pseudojaca. The affinities of A. altissimus have been difficult to determine ever since it was first described, having been previously placed in Grewia (Malvaceae) and Morus (Moraceae) (Jarrett, 1960). Morus belongs to the sister tribe (Moreae) of Artocarpeae, and A. altissimus shares the tri-nerved leaf base and crenate leaf margin present in some Morus species. These characters are absent in all other Artocarpus taxa. Further analyses will be necessary to determine the precise affinities of A. altissimus, but it does not appear to be part of a monophyletic subgenus Pseudojaca.
The defining characters of subgenus Artocarpus are described in detail in Zerega et al. (2010). Some of the most recognizable traits of this subgenus include fleshy perianths of individual flowers fused only medially (apices and proximal portions are typically free) to adjacent perianths on the syncarp, and inflorescences are never rami- or cauliflorous. Subgenus Artocarpus is a well-supported clade (ML, 87 %; BI, 1·0 PP, Fig. 2), but there is no support for previous classifications below the subgeneric level, and we make no recommendations for further divisions at that level. Several species in this subgenus were not included in previous phylogenetic analyses. Among these, A. brevipedunculatus has indurated perianth apices and falls within a clade that shares this character. Artocarpus obtusus Jarrett, with rugose male inflorescences, falls within a clade that largely shares this character. Found at much higher elevations than other species in the subgenus, A. excelsus Jarrett grows on Mt Kinabalu in Sabah, Malaysia. Jarrett (1959) discussed the yet to be described A. excelsus in her treatment of A. lowii King, a lowland species found in the Thai-Malay Peninsula. She noted several morphological affinities between the two species, although they are not sister species in the present analysis. Artocarpus teysmannii Miq. has also not been included in previous analyses and is placed in a clade with A. elasticus Reinw. ex Blume and A. scortechinii King. All three species share the character of having perianth apices of varying lengths, with A. elasticus and A. teijsmannii also having sterile perianth apices and quite pronounced length variation between sterile and fertile perianths. The two subspecies of A. anisophyllus (the only Artocarpus species with adult leaves incised all the way to the midrib, appearing compound) are included for the first time in a phylogenetic analysis here. They are differentiated based on the sessile (subsp. sessifolius) or petiolate (subsp. anisophyllus) nature of the leaflets (lobes). However, the evidence does not support these divisions. In this analysis, one subspecies was nested within the other. In addition, the character itself appears quite labile, and both sessile and petiolate leaflets can be found on the same individual (N. J. C. Zerega, pers. observ.). Finally, within subgenus Artocarpus there were three species that were not resolved as monophyletic. Artocarpus hispidus is nested within the morphologically similar A. rigidus, the main difference being the dense hispid pubescence found on the twigs of A. hispidus Jarrett compared to the sparser pubescence in A. rigidus Blume. With a much more restricted range (Malay Peninsula), A. hispidus may be better considered a subspecies of the widespread A. rigidus, which is found in Indo-Burma, the Thai-Malay Peninsula, Borneo, Sumatra and Java. Finally, neither of the two exemplars of A. scortechinii nor the two exemplars of A. maingayi King is monophyletic, and this will require further sampling and investigation.
The defining characters of subgenus Cauliflori are described in detail in Zerega et al. (2010). The most striking synapomorphy of the clade is the presence of rami- or cauliflorous istillate inflorescences. With only three species, subgenus Cauliflori included 100 % taxon sampling and is well supported.
The defining characters of subgenus Pseudojaca are described in detail in Jarrett (1959a, b, c, 1960) and Zerega et al. (2010). The most recognizable traits of this subgenus are the alternate distichous leaf arrangement with non-amplexicaul stipules, coupled with the medial and apical (and sometimes basal) fusion of fleshy perianth tissue of individual flowers to adjacent perianth tissue on the syncarp. Subgenus Pseudojaca (excluding A. altissimus) is well supported, but many of the shallower nodes are poorly supported. The much shorter branch lengths in this subgenus compared to the other subgenera could indicate a more recent radiation, supported in the dating analysis (Fig. 3). Alternatively, variation in the rates of evolution could lead to short branch lengths, which we did not test here. There is much less character variation in subgenus Pseudojaca compared to the other subgenera, and there is a great deal of character overlap among the leaves and inflorescences across the subgenus (Jarrett, 1960; Berg et al., 2006). These apparently reduced levels of variation could be indicative of a more recent radiation of subgenus Pseudojaca.
Several species in subgenus Pseudojaca have been variously reduced and expanded by different authors due to a paucity of variable characters and difficulties with species delineation. Several exemplars of two such species are included in the analysis: A. nitidus and A. lacucha. Artocarpus nitidus has been considered as five different species, a single species with five subspecies, and a single species with no intraspecific taxa but with four informal entities recognized (Jarrett, 1960; Berg et al., 2006). The present analysis includes four of the five putative subspecies sensu Jarrett (1960). Phylogenetic reconstruction indicates that they represent four distinct lineages (Fig. 2), and they should be elevated to species rank. Additionally, Berg et al. (2006) reduced A. xanthocarpus Teysm. and Binnend. into A. nitidus, but this is not supported here. Artocarpus lacucha has been treated as a species restricted to Indo-Burma (Jarrett, 1960), or as a highly variable species ranging from the western Ghats of India to east of Wallace’s line (Berg et al., 2006). Compared to Jarrett’s (1960) circumscription, Berg et al. (2006) reduced several taxa into A. lacucha (Fig. 2). The present analysis supports the treatment of Jarrett (1960) and the recognition of A. lacucha, A. dadah, A. fretessii Teysm. & Binnend. and A. ovatus Blanco as distinct taxa at the specific rank. Both the A. nitidus and the A. lacucha species complexes would benefit from additional phylogeographical study and detailed morphological studies.
Subgenus Prainea (2010) was recognized at the sectional level within the genus Artocarpus by Renner (1907), and subsequently raised to the generic level by Jarrett (1959). A readily recognizable synapomorphy of the clade is that the fleshy perianths of individual flowers are not fused at all to the perianths of adjacent flowers on the syncarp. Two of the four species in subgenus Prainea are included in the present analysis, and there is strong support for its monophyly. However its placement within the genus is uncertain. In the ML and BI analyses it is sister to subgenus Artocarpus + Cauliflori with no support for this placement (Fig. 2). In the Beast analysis it is placed sister to the anomalous A. altissimus, which are both in turn sister to subgenus Artocarpus + Cauliflori (Fig. 3). However, this is also not well supported. Prainea has historically been difficult to place as it shares leaf phyllotaxy and stipule characters with subgenus Pseudojaca but anatomical leaf glandular characters with subgenus Artocarpus (Renner, 1907; Zerega et al., 2010), while the lack of fusion of adjacent perianths sets it apart from both subgenera, leading Jarrett (1959a, b, c) to treat it as its own genus. However, studies have shown that in young pistillate inflorescences of species from both subgenera Artocarpus and Pseudojaca, the perianths are unfused and only fuse later due to rapid divisions and subsequent enlargement of the ground tissue (Jarrett, 1959a, b, c; Sharma, 1965; Moncur, 1985). Complete sampling of subgenus Prainea and more extensive data sampling from the nuclear and chloroplast genomes, combined with detailed developmental anatomical study, may help to resolve relationships.
Dating and biogeography
In the mid- to late Cretaceous (83·8 Mya, 74·85–92·65 Mya) the stem node of the tribe Artocarpeae diverged from the rest of the family Moraceae. The biogeographical reconstruction infers a likely origin of the tribe in the Americas. The split between American (Clarisia and Batocarpus) and Asian Artocarpeae (Artocarpus) occurred in the Palaeocene (59·67 Mya, 55·24–65·03 Mya) with a radiation of Artocarpus from Borneo in the Eocene to Oligocene (40·07 Mya, 29·8–50·81 Mya). The time frame in which Artocarpus radiated coincides with boreotropical flora and a North Atlantic Land Bridge that could have allowed for dispersal from the Americas into Eurasia (McLoughlin, 2001). We are unaware of any fossil evidence for Batocarpus and Clarisia, but there are several records of fossils of Artocarpus and related extinct taxa (Artocarpoxylon, Artocarpoxidium and Artocarpoides) in Austria, France, Texas, Colorado, Louisiana, Canada, Kansas and Greenland from the Cretaceous into the Eocene (Collinson, 1989). These fossils support the possible presence of Artocarpus ancestors in areas where they might be expected if there had been dispersal across a North Atlantic Land Bridge. However, they must be viewed with some caution, and this is why they were not used in divergence date estimates. The vast majority of the fossils are from deeply cleft fossil leaves, which share gross similarities with some extant Artocarpus species, but the fossils lack cuticles and have poorly preserved venation. In her review of Moraceae fossils, Collinson (1989) indicated that these leaf fossils may be correctly identified as Artocarpus ancestors, but they are not 100 % diagnostic. In summary, the data presented here suggest an ancestral range for Artocarpeae in the Americas and dispersal across a North Atlantic Land Bridge in the Eocene during a time of boreotropical flora. Further detailed examination of fossil data is warranted to determine how well they support this proposal.
Artocarpus ancestors probably spread throughout Eurasia in the Eocene and began to diversify during this period of higher global temperatures. There are limited fossils of Artocarpus and its ancestors in Asia, but there are well-characterized wood fossils from the Intertrappean Deccan Beds in India dated from the Palaeocene to the Miocene periods, suggesting the genus or its ancestors had reached India by then (Mehrotra et al., 1984). This is consistent with dispersal across land, as India collided with Asia sometime between 43 and 50 Mya (McLoughlin, 2001; Sanmartín and Ronquist, 2004). Also, the first ever Artocarpus fossil from China was recently described from a site that is considered to have strong phytogeographical connections with India (Jacques et al., 2015). The fossil is well preserved and comes from the middle Miocene Fotan flora of Zhangpu County, South Fujian, China, an area that has been considered to represent tropical rainforest based on the occurrence of distinctive winged fruit fossils (Jacques et al., 2015).
From southern Asia Artocarpus could have dispersed across land into what is now the island of Borneo. While Artocarpus may ultimately derive from extinct taxa in mainland Asia, Borneo is reconstructed as the greatest incubator of extant diversity and the ancestral range of Artocarpus as it exists today. Diversification in Borneo may have been followed by several separate dispersal events (and subsequent radiations) throughout Southeast Asia and Malesia (Figs 3 and 4). Radiation of Artocarpus is reconstructed as beginning in the Eocene and continuing through the Oligocene and into the Miocene and Pliocene, with the greatest diversification inferred to occur in the Miocene. Fluctuating sea levels during these periods may have allowed for isolation and diversification during times of high sea levels, followed by radiation and dispersal during periods of lower sea levels (Turner et al., 2001). During the Miocene, sea levels were generally lower than they are today, and frequent land connections existed between mainland Asia, the Thai/Malay peninsula and what is now northern and central Borneo, and parts of Sumatra and western Java (Hall, 2009, 2012; de Bruyn et al., 2014). However, the Philippines, Sulawesi, the Moluccas and other islands of Wallacea were frequently submerged until the mid-Miocene to Pliocene and did not share land connections to Borneo or to each other.
Considering the extant range for Artocarpus species included in this study, Borneo experienced higher levels of in situ diversification and emigration than any other area, especially during the Miocene. Borneo, the Thai-Malay Peninsula, mainland Asia, and parts of Sumatra and Java were frequently connected between 60 and 5 Mya (Hall, 2009; de Bruyn et al., 2014), and the species found in these areas have largely overlapping ranges today, suggesting that sympatric speciation may have occurred followed by dispersal (Fig. 4). The Thai-Malay Peninsula may have been a gateway for Artocarpus from Borneo into mainland Asia, Sumatra and Java. For example, half of the Thai-Malay taxa included in the study diversified in Borneo. All of the Artocarpus taxa in Java and Sumatra are a subset of what is found in the Thai-Malay Peninsula. Sumatra was connected to the Thai-Malay Peninsula and parts of Borneo (but closer to the former) from the Palaeocene into the Oligocene, and variously connected and disconnected due to fluctuating water levels in the Miocene. Several of the species in Sumatra are not found in Borneo and are only otherwise known from the Thai-Malay Peninsula. This, along with ancestral range reconstructions, suggests that dispersal into Sumatra came through the Thai-Malay Peninsula (Fig. 3). Java in turn houses a subset of the Sumatran taxa, suggesting taxa dispersed into Java from Sumatra, probably during the late Miocene and early Pliocene when dispersal would have been more likely. Moving northward, based on the taxa in this study, Indo-Burma houses 73 % of the taxa found in the Thai-Malay Peninsula (but only 40 % of Borneo taxa), and southern China in turn houses 83 % of the taxa found in Indo-Burma (but only 33 % of Thai-Malay Peninsula and 0 % of Borneo taxa) (Table S1). This suggests northward dispersal of taxa out of Borneo into the Thai-Malay Peninsula, Indo-Burma and southern China, with some in situ diversification of new species throughout the Miocene and into the Pliocene (Figs 3 and 4). An alternative, and not mutually exclusive, explanation is allopatric speciation on the variously isolated landmasses during times of sea-level fluctuations with secondary contact after dispersal to Borneo. Possible dispersal routes over water are discussed below.
An outlier in Artocarpus distribution is the Western Ghats of southern India. An Indo-Malayan influence in the flora and fauna of southern India has long been recognized (Hora, 1944, 1949), and there are many examples of extant plant taxa present in the wet tropical forests of the Western Ghats and north-eastern India, but absent from the more arid central Indian region (Bahulikar et al., 2004; Apte et al., 2006; Banu et al., 2009; Kuttapetty et al., 2014). Recent analysis of fossil flora from the Deccan Intertrappean beds in central India suggests that the wet tropical forests, similar to present-day forests of the Western Ghats and north-east India, were flourishing in central India during the late Cretaceous into the Oligocene (Kapgate, 2013). Artocarpus hirsutus is restricted to the Western Ghats and it is reconstructed with an ancestral area of Borneo. Its extant distribution may be the result of long-distance overwater dispersal from Borneo to the Indian peninsula, or it may be the result of overland dispersal of its ancestral lineage through the Thai-Malay Peninsula into the Asian mainland and into India during a period when central India would have been home to wet tropical forests, but it has subsequently gone extinct outside of the Western Ghats (Figs 3 and 4). Given that support for its position in the phylogeny is low and it has been considered a morphologically anomalous species difficult to place (Jarrett, 1959a, b, c; Zerega et al., 2010), further work is needed to elucidate the evolutionary and biogeographical history of this species. Another Western Ghats species (A. heterophyllus, jackfruit) is a complicated taxon, as it is an economically important crop that is widely cultivated throughout the tropics today. It exhibits high levels of morphological (Azad et al., 2007; Khan et al., 2010) and genetic diversity (Melhem, 2015) in the Western Ghats, and this area has been proposed as its area of origin. However, high levels of morphological and genetic diversity also exist in Indo-Burma (Bangladesh) (Khan et al., 2010; Witherup, 2013; Witherup et al., 2013), and its centre of diversity and wild relative(s) remain unclear. Given that it may also be native in Indo-Burma and that its sister species, A. integer (cempedak, an important crop in Malaysia), is native to the Thai-Malay Peninsula and Borneo, A. heterophyllus may have reached the Western Ghats via overland dispersal through Indo-Burma. Inclusion of the Sri Lankan endemic, A. nobilis Thwaites, in future phylogenetic analyses, as well as phylogeographical studies of Western Ghats species such as A. heterophyllus and A. hirsutus, could help further elucidate biogeographical patterns in Artocarpus between Indo-Malaya and India.
Apart from one very widely distributed taxon (A. teijsmannii), Sulawesi and islands eastwards harbour very different species diversity than mainland Asia, Sumatra and Java, but they share diversity with Borneo. Sulawesi taxa are largely a subset of Borneo taxa, and our results infer a dispersal event to Sulawesi from Borneo during the Miocene (Table S4). Taxa present eastward, in Wallacea and Oceania, are the same as those in Sulawesi plus three additional lineages that may have diversified in New Guinea and Oceania [A. papuanus (Becc.) Renner, A. sepicanus Diels, and the lineage containing A. altilis, A. camansi Blanco and A. mariannensis Trécul – breadfruit and its wild progenitors]. Because there were no land connections from Borneo into Sulawesi and Wallacea, this finding suggests overwater dispersal from Borneo, mostly during the Miocene. The Artocarpus taxa on the islands of the Philippines are quite distinct. Of the Philippine taxa included in this study, only 29 % of them have distributions overlapping with Borneo and there is no overlap with any other region apart from the widespread A. sericicarpus Jarrett. The Philippines is home to a high number of endemic taxa that diversified in the mid-Miocene to Pliocene, when the Philippine islands became emergent (Hall, 1998). There are additional endemic Philippine Artocarpus taxa that we were unable to include in the present study. They have morphological affinities to taxa included in the present study, suggesting that once taxa reached the islands, in situ species radiation was not uncommon.
With several inferred dispersal events across large expanses of water from Borneo into Sulawesi and eastward (Fig. 4, Table S4), it is important to consider how these may have occurred. Dispersal of syncarps and seeds in Artocarpus is not well studied. Large syncarps often drop and germinate near the mother tree (N. J. C. Zerega, pers. observ.) or are consumed by large mammals, such as elephants, orangutans and flying foxes (Campbell-Smith et al., 2011; Canale et al., 2013; Sekar et al., 2015; Sekar and Sukumar, 2015). However, whether the seeds survive passage through mammalian guts or if such passage increases germination rates is largely unknown. Recent studies examined whether Asian tapirs could facilitate long-distance seed dispersal in several species including A. integer (Thunb.) Merr. (Campos-Arceiz et al., 2012). They found that the tapirs consumed very few seeds, and of those that were consumed only 2·8 % of A. integer seeds survived passage through the gut and 0 % were able to germinate (Campos-Arceiz et al., 2012). Sekar et al. (2015) tested how well domestic bovids (Bos primigenius – cattle, and Bubalus bubalis – buffalo) and Asian elephants (Elephas maximus) in India could disperse A. chama seeds. They found that seeds passing through elephants are more likely to survive and germinate compared to seeds passing through bovids, and that elephants can act as dispersers of A. chama seeds. The ancestors of modern Asian elephants diverged from mastodons in the Oligocene and diverged from the African elephant (Loxodonta africana) in the late Miocene (Kappelman et al., 2003; Shoshani et al., 2006; Rohland et al., 2007). Asian elephants and their ancestors may have been important dispersers of several Artocarpus species (Sekar et al., 2015). This mode of dispersal may help to explain the expansion of Artocarpus from Borneo throughout parts of mainland Asia. As the range of the modern Asian elephant and other large mammals shrinks, so too may the dispersal of Artocarpus species.
With regard to dispersal across long distances of open water to the islands east of Borneo, a possible disperser may have been flying foxes (Pteropus ssp.). Much of the diversification within Pteropus occurred in the Miocene to Pliocene, coinciding with Artocarpus diversification (Almeida et al., 2014). Various Artocarpus species have been recorded as preferred roosting sites and a food source for several Pteropus species in the Caroline Islands, Philippines and elsewhere (Mildenstein et al., 2005; Buden et al., 2013). Pteropus species are predominantly insular species with restricted ranges and are capable of flying up to 50 km in a single night (Mickleburgh et al., 1992; Almeida et al., 2014). There is a great deal of diversity and endemism of Pteropus species, which can be explained by its being a specialized island taxon. Islands provide isolated areas (allopatry) where divergence can proceed relatively quickly by genetic drift without interference from frequent gene flow. Sympatry of Pteropus species most often results from multiple colonization events rather than in situ speciation (Almeida et al., 2014). This same pattern is observed in Artocarpus species in the islands east of Borneo; however, in situ diversification is also important (Fig. 4, Table S4). Further investigation of the ecological interactions between Artocarpus and Pteropus is warranted.
CONCLUSION
We present a much expanded Artocarpus phylogeny that will be useful for future revisionary work, and we infer the biogeographical history of this important genus. Borneo is reconstructed as being central in the diversification of the genus Artocarpus, and it probably served as the centre from which extant species dispersed and diversified in several directions. Much of this probably occurred during the Miocene, a period when sea levels were frequently low, providing land connections between Borneo, present-day mainland Asia, Sumatra and Java. The Thai-Malay Peninsula may have been a gateway for Artocarpus into mainland Asia, Sumatra and Java, and some of the species found in these areas have extant overlapping ranges, suggesting that sympatric speciation may have occurred. In contrast, Artocarpus diversity east of Borneo, including Sulawesi, Wallacea, Oceania and the Philippines, is markedly different from the Thai-Malay group, with Philippine diversity being particularly unique and home to several endemic taxa. Also in contrast to the Thai-Malay group, reaching these islands probably involved long-distance overwater dispersal as opposed to overland dispersal. While the barrier to crossing Wallace’s line has proved to be a hindrance for the dispersal in many faunal groups, it is less so among plants (Van Welzen et al., 2011). Other examples of an inferred origin of flora in Borneo and subsequent dispersal across Wallace’s line during the Miocene include Rhododendronsection Vireya (Ericaceae) (Brown et al., 2006; Webb and Ree, 2012), Alocasia (Araceae) (Nauheimer et al., 2012) and Begonia (Thomas et al., 2012). Finally, the dispersal of two taxa native to the Western Ghats of India may be the result of overland or overwater dispersal.
Borneo houses the highest levels of extant Artocarpus endemism, and our results support other studies showing Borneo to be a biodiversity and evolutionary hotspot (Myers et al., 2000; Mittermeier et al., 2005; de Bruyn et al., 2014). These findings offer further support for the critical importance of conservation of this area in the face of rapid rates of deforestation and development (Koh and Sodhi, 2010; Wilcove et al., 2013).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following: Table S1: a list of specimens sampled, their geographical origin and abbreviations used in the study, together with GenBank accession numbers for the resulting DNA sequences (provided as an Excel file). Table S2: primers used, primer sources and the model of evolution used for Bayesian analysis. Table S3: results from LaGrange and S-DIVA biogeography analyses, with nodes that correspond to Fig. S2. Table S4: number of dispersal and in situ speciation events in Artocarpus. Figure S1: maximum-likelihood tree of the full dataset. Figure S2: consensus tree from BEAST with labelled nodes corresponding to Table S3.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the following herbaria for use of material: BISH, CHIC, F, FRIM, GH, K, KKU, MIN, MO, NY, PTBG, S, SAN, US, WIS; the following people for field assistance and specimens: S. Brono, L. Aloyziuz, J. Jumian, P. Miun, T. Motley, K. Noikkotr, R. Sudomoon, G. Weiblen, D. Zerega; K. Ksiazek for lab and field assistance; and helpful comments from anonymous manuscript reviewers. This work was supported by the National Science Foundation (grant number NSF-DEB 0919119).
REFERENCES
- Almeida FC, Giannini NP, Simmons NB, Helgen KM.. 2014. Each flying fox on its own branch: a phylogenetic tree for Pteropus and related genera (Chiroptera: Pteropodidae). Molecular Phylogenetics and Evolution 77: 83–95. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–10. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25: 3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte GS, Bahulikar RA, Kulkarni RS, et al. 2006. Genetic diversity analysis in Gaultheria fragrantissima Wall. (Ericaceae) from the two biodiversity hotspots in India using ISSR markers. Current Science 91: 1634–1640. [Google Scholar]
- Azad AK, Jones JG, Haq N.. 2007. Assessing morphological and isozyme variation of jackfruit (Artocarpus heterophyllus Lam.) in Bangladesh. Agroforestry Systems 71: 109–125. [Google Scholar]
- Bahulikar RA, Lagu MD, Kulkarni BG, et al. 2004. Genetic diversity among spatially isolated populations of Eurya nitida Korth. (Theaceae) based on inter-simple sequence repeats. Current Science 86: 824–831. [Google Scholar]
- Banu S, Lagu MD, Gupta VS.. 2009. Phylogeographical studies in disjunct populations of Symplocos laurina Wall. using cytoplasmic PCR-RFLP approach. Tree Genetics & Genomes 6: 13–23. [Google Scholar]
- Bendiksby M, Schumacher T, Gussarova G, et al. 2010. Elucidating the evolutionary history of the Southeast Asian, holoparasitic, giant-flowered Rafflesiaceae: Pliocene vicariance, morphological convergence and character displacement. Molecular Phylogenetics and Evolution 57: 620–633. [DOI] [PubMed] [Google Scholar]
- Berg CC. 2001. Moreae, Artocarpeae, and Dorstenia (Moraceae) with introductions to the family and Ficus and with additions and corrections to Flora Neotropica Monograph 7. New York: New York Botanical Garden. [Google Scholar]
- Berg CC. 2005. A new species of Artocarpus (Moraceae) from Thailand. Blumea - Biodiversity, Evolution and Biogeography of Plants 50: 531–533. [Google Scholar]
- Berg CC, Corner EJH, Jarrett FM.. 2006. Moraceae - genera other than Ficus. Leiden: National Herbarium Nederland. [Google Scholar]
- Berg CC, Pattharahirantricin N, Chantarasuwan B, Santisuk T.. 2011. Flora of Thailand, Vol. 10, Pt. 4: Cecropiaceae and Moraceae Bangkok: Forest Herbarium, Royal Forest Department. [Google Scholar]
- Brown GK, Nelson G, Ladiges PY.. 2006. Historical biogeography of Rhododendron section Vireya and the Malesian Archipelago. Journal of Biogeography 33: 1929–1944. [Google Scholar]
- Buden D, Helgen KM, Wiles G.. 2013. Taxonomy, distribution, and natural history of flying foxes (Chiroptera, Pteropodidae) in the Mortlock Islands and Chuuk State, Caroline Islands. ZooKeys 345: 97–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Smith G, Campbell-Smith M, Singleton I, Linkie M.. 2011. Raiders of the Lost Bark: orangutan foraging strategies in a degraded landscape. PLoS One 6: e20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Arceiz A, Traeholt C, Jaffar R, Santamaria L, Corlett RT.. 2012. Asian tapirs are no elephants when it comes to seed dispersal. Biotropica 44: 220–227. [Google Scholar]
- Canale GR, Kierulff MCM, Chivers DJ.. 2013. A critically endangered capuchin monkey (Sapajus xanthosternos) living in a highly fragmented hotspot In: Marsh KL, Chapman AC, eds. Primates in fragments: complexity and resilience. New York, NY: Springer USA, 299–311. [Google Scholar]
- Chandler MEJ. 1961. Flora of the Lower Headon Beds of Hampshire and the Isle of Wight. Bulletin of the British Museum (Natural History, Geology) 5: 91–158. [Google Scholar]
- Chandler MEJ. 1962. The Lower Tertiary floras of Southern England, II, Flora of the pipe-clay series of Dorset (Lower Bagshot). London: British Museum (Natural History). [Google Scholar]
- Chandler MEJ. 1963a. The Lower Tertiary floras of Southern England III, Flora of the Bournemouth Beds; the Boscombe, and the Highcliff Sands. London: British Museum (Natural History). [Google Scholar]
- Chandler MEJ. 1963b. Revision of the Oligocene floras of the Isle of Wight. Bulletin of the British Museum (Natural History), Geology 6: 321–384. [Google Scholar]
- Clement WL, Weiblen GD.. 2009. Morphological evolution in the mulberry family (Moraceae). Systematic Botany 34: 530–552. [Google Scholar]
- Collinson ME. 1989. The fossil history of the Moraceae, Urticaceae (including Cecropiaceae), and Cannabaceae In: Crane PR, Blackmore S, eds. Evolution, systematics, and fossil history of the Hamamelidae, vol.2; ‘Higher’ Hammelidae. Oxford: Clarendon Press, 319–339. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D.. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn M, Stelbrink B, Morley RJ, et al. 2014. Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Systematic Biology 63: 879–901. [DOI] [PubMed] [Google Scholar]
- Duperon Laudoueneix M. 1980. Presence d'un bois fossile de Moraceae dans l'Eocene de la Charente. Cr 105e Congr. nat. Soc. sav., Caen, Sci, 1: 117–29. [Google Scholar]
- Gregor HJ. 1978. Die miozänen Frucht- und Samenfloren der Oberpfälzer Braunkohle. I. Funde aus den sandigen Zwischenmitteln. Palaeontographica, Abt. B 167: 8–103. [Google Scholar]
- Guindon S, Gascuel O.. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Hall R. 1998. The plate tectonics of Cenozoic SE Asia and the distribution of land and sea In: Hall R, Holloway JD, eds. Biogeography and Geological Evolution of South East Asia. Leiden: Backhuys Publishers, 66–131. [Google Scholar]
- Hall R. 2002. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. Journal of Asian Earth Sciences 20: 353–431. [Google Scholar]
- Hall R. 2009. Southeast Asia’s changing palaeogeography. Blumea - Biodiversity, Evolution and Biogeography of Plants 54: 148–161. [Google Scholar]
- Hall R. 2012. Sundaland and Wallacea: geology, plate tectonics and palaeogeography In: Gower DJ, Johnson KG, Richardson JE, Rosen BR, Rüber L, Williams ST, eds. Biotic evolution and environmental change in Southeast Asia. Cambridge: Cambridge University Press, 32–78. [Google Scholar]
- Heled J, Drummond AJ.. 2010. Bayesian inference of species trees from multilocus data. Molecular Biology and Evolution 27: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hora SL. 1944. On the Malayan affinities of the fresh-water fish fauna of peninsular India, and its bearing on the probable age of the Garo-Rajmahal gap. Proceedings of the National Institute of Sciences of India 15: 362–364. [Google Scholar]
- Hora SL. 1949. Symposium on Satpura hypothesis of the distribution of the Malayan fauna and flora in peninsular. Proceedings of the National Institute of Sciences of India 15: 309–314. [Google Scholar]
- Jacques FMB, Shi G, Su T, Zhou Z.. 2015. A tropical forest of the middle Miocene of Fujian (SE China) reveals Sino-Indian biogeographic affinities. Review of Palaeobotany and Palynology 216: 76–91. [Google Scholar]
- Jarrett FM. 1959a. Studies in Artocarpus and allied genera, I. General considerations. Journal of the Arnold Arboretum 40: 1–29. [Google Scholar]
- Jarrett FM. 1959b. Studies in Artocarpus and allied genera, II. A revision of Prainea. Journal of the Arnold Arboretum 40: 30–37. [Google Scholar]
- Jarrett FM. 1959c. Studies in Artocarpus and allied genera, III. A revision of Artocarpus subgenus Artocarpus. Journal of the Arnold Arboretum 40: 113–155, 298–368. [Google Scholar]
- Jarrett FM. 1960. Studies in Artocarpus and allied genera, IV. A revision of Artocarpus subgenus Pseudojaca. Journal of the Arnold Arboretum 41: 73–139. [Google Scholar]
- Jarrett FM. 1975. Four new Artocarpus species from Indo-Malesia (Moraceae). Blumea 22: 409–410. [Google Scholar]
- Kapgate DK. 2013. Vegetation succession and environmental changes in Central India during Early Cenozoic. Chinese Science Bulletin 58: 97–103. [Google Scholar]
- Kappelman J, Tab Rasmussen D, Sanders WJ, et al. 2003. Oligocene mammals from Ethiopia and faunal exchange between Afro-Arabia and Eurasia. Nature 426: 549–552. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Zerega NJC, Hossain S, Zuberi MI.. 2010. Jackfruit (Artocarpus heterophyllus Lam.) diversity in Bangladesh: land use and artificial selection. Economic Botany 64: 124–136. [Google Scholar]
- Kochummen KM. 2000. Artocarpus J. R. & G. Forster. nom. conserv In: Soepadmo E, Saw LG, eds. Tree flora of Sabah and Sarawak, Malaysia. Kuala Lumpur: Sabah Forestry Department, Forest Research Institute Malaysia, and Sarawak Forestry Department, 187–212. [Google Scholar]
- Koh LP, Sodhi NS.. 2010. Conserving Southeast Asia’s imperiled biodiversity: scientific, management, and policy challenges. Biodiversity and Conservation 19: 913–917. [Google Scholar]
- Kuttapetty M, Pillai PP, Varghese RJ, Seeni S.. 2014. Genetic diversity analysis in disjunct populations of Rhododendron arboreum from the temperate and tropical forests of Indian subcontinent corroborate Satpura hypothesis of species migration. Biologia 69: 311–322. [Google Scholar]
- Lambeck K, Chappell J.. 2001. Sea level change through the last glacial cycle. Science 292: 679–686. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR.. 2011. Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org.
- McLoughlin S. 2001. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany 49: 271–300. [Google Scholar]
- Mehrotra RC, Prakash U, Bande MB.. 1984. Fossil woods of Lophopetalum and Artocarpus from the Deccan Intertrappean Beds of Mandla district, Madhya Pradesh, India. Palaeobotanist 32: 310–320. [Google Scholar]
- Melhem T. 2015. Diversity of jackfruit (Artocarpus heterophyllus Lam.) in the Western Ghats of south India. Master’s thesis, Northwestern University, Evanston.
- Mickleburgh SP, Hutson AM, Racey PA.. 1992. Old World fruit bats: an action plan for their conservation. Gland, Switzerland: IUCN. [Google Scholar]
- Mildenstein TL, Stier SC, Nuevo-Diego CE, Mills LS.. 2005. Habitat selection of endangered and endemic large flying-foxes in Subic Bay, Philippines. Biological Conservation 126: 93–102. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T.. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees Gateway Computing Environments Workshop (GCE). New Orleans. [Google Scholar]
- Mirarab S, Reaz R, Bayzid MS, et al. 2014. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics 30: i541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittermeier RA, Robles Gil P, Hoffman M, et al. 2005. Hotspots revisited: Earth’s biologically richest and most endangered ecoregions. Chicago: Conservation International. [Google Scholar]
- Moncur MW. 1985. Floral ontogeny of the jackfruit, Artocarpus heterophyllus Lam. (Moraceae). Australian Journal of Botany 33: 585–593. [Google Scholar]
- Morley RJ. 2000. Origin and evolution of tropical rain forests. Chichester: John Wiley & Sons. [Google Scholar]
- Morley RJ. 2012. A review of the Cenozoic palaeoclimate history of Southeast Asia In: Gower D, Johnson KG, Rosen BR, Richardson J, Rüber L, Williams ST, eds. Biotic evolution and environmental change in Southeast Asia. Cambridge: Cambridge University Press, 79–114. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J.. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Nauheimer L, Boyce PC, Renner SS.. 2012. Giant taro and its relatives: a phylogeny of the large genus Alocasia (Araceae) sheds light on Miocene floristic exchange in the Malesian region. Molecular Phylogenetics and Evolution 63: 43–51. [DOI] [PubMed] [Google Scholar]
- Ree RH, Smith SA.. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- Renner O. 1907. Beiträge zur Anatomie und Systematik der Artocarpeen und Conocephaleen, insbesondere der Gattung Ficus. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 39: 319–448. [Google Scholar]
- Rohland N, Malaspinas A-S, Pollack JL, et al. 2007. Proboscidean mitogenomics: chronology and mode of elephant evolution using mastodon as outgroup. PLoS Biol 5: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. 2012. MrBayes 3·2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín I, Ronquist F.. 2004. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Systematic Biology 53: 216–243. [DOI] [PubMed] [Google Scholar]
- Sekar N, Sukumar R.. 2015. The Asian elephant is amongst the top three frugivores of two tree species with easily edible fruit. Journal of Tropical Ecology 31: 385–394. [Google Scholar]
- Sekar N, Lee C-L, Sukumar R.. 2015. In the elephant’s seed shadow: the prospects of domestic bovids as replacement dispersers of three tropical Asian trees. Ecology 96: 2093–2105. [DOI] [PubMed] [Google Scholar]
- Sharma MR. 1965. Morphological and anatomical investigations on Artocarpus Forst. III. the flower. Phytomorphology 15: 185–201. [Google Scholar]
- Shoshani J, Walter RC, Abraha M, et al. 2006. A proboscidean from the late Oligocene of Eritrea, a ‘missing link’ between early Elephantiformes and Elephantimorpha, and biogeographic implications. Proceedings of the National Academy of Sciences USA 103: 17296–17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soepadmo E, Saw LG.. 2000. Tree flora of Sabah and Sarawak, vol. 3. Sabah Forestry Department, Forest Research Institute, Malaysia, Sawarak Forestry Department. [Google Scholar]
- Srivastava AP, Rafagopalan G, Ambwani K.. 1986. Fission-track dating of fossil palm wood from Shahpura, Mandla District, Madhya Pradesh. Geophytology, 16. [Google Scholar]
- Takhtajan A. 1982. Fossil flowering plants of the USSR, vol. 2, Ulmaceae – Betulaceae. Leningrad: NAUKA. [Google Scholar]
- Thomas DC, Hughes M, Phutthai T, et al. 2012. West to east dispersal and subsequent rapid diversification of the mega-diverse genus Begonia (Begoniaceae) in the Malesian archipelago. Journal of Biogeography 39: 98–113. [Google Scholar]
- Turner H, Hovenkamp P, van Welzen PC.. 2001. Biogeography of Southeast Asia and the West Pacific. Journal of Biogeography 28: 217–230. [Google Scholar]
- van Oosterzee P. 1997. Where worlds collide: The Wallace Line. Ithaca, NY: Cornell University Press. [Google Scholar]
- Van Welzen PC, Parnell JAN, Slik JWF.. 2011. Wallace’s Line and plant distributions: two or three phytogeographical areas and where to group Java? Biological Journal of the Linnean Society 103: 531–545. [Google Scholar]
- Wang H, Moore MJ, Soltis PS, et al. 2009. Rosid radiation and the rapid rise of angiosperm-dominated forests. Proceedings of the National Academy of Sciences USA 106: 3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CO, Ree R.. 2012. Historical biogeography inference in Malesia In: Gower D, Johnson K, Richardson J, Rosen B, Ruber L, Williams S, eds. Biotic evolution and environmental change in Southeast Asia. Cambridge: Cambridge University Press, 191–215. [Google Scholar]
- Wilcove DS, Giam X, Edwards DP, Fisher B, Koh LP.. 2013. Navjot’s nightmare revisited: logging, agriculture, and biodiversity in Southeast Asia. Trends in Ecology & Evolution 28: 531–540. [DOI] [PubMed] [Google Scholar]
- Witherup C. 2013. Genetic diversity of Bangladeshi jackfruit (Artocarpus heterophyllus, Moraceae). Masters thesis, Northwestern University.
- Witherup C, Ragone D, Wiesner-Hanks T, et al. 2013. Development of microsatellite loci in Artocarpus altilis (Moraceae) and cross-amplification in congeneric species. Applications in Plant Sciences 1: 1200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, He X-J. 2010. S-DIVA (Statistical Dispersal-Vicariance Analysis): a tool for inferring biogeographic histories. Molecular Phylogenetics and Evolution 56: 848–850. [DOI] [PubMed] [Google Scholar]
- Yu Y, Harris AJ, Blair C, He X.. 2015. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Molecular Phylogenetics and Evolution 87: 46–49. [DOI] [PubMed] [Google Scholar]
- Zerega NJC, Mori S, Lindqvist C, Zheng Q, Motley TJ.. 2002. Using amplified fragment length polymorphisms (AFLP) to identify Black Cohosh (Actaea racemosa). Economic Botany 56: 154–164. [Google Scholar]
- Zerega NJC, Clement WL, Datwyler SL, Weiblen GD.. 2005. Biogeography and divergence times in the mulberry family (Moraceae). Molecular Phylogenetics and Evolution 37: 402–416. [DOI] [PubMed] [Google Scholar]
- Zerega NJC, Nur Supardi MN, Motley TJ.. 2010. Phylogeny and recircumscription of Artocarpeae (Moraceae) with a focus on Artocarpus. Systematic Botany 35: 766–782. [Google Scholar]
- Zhekun Z, Gilbert MG.. 2003. Moraceae In: Wu ZY, Raven PH, Hong DY, eds. Flora of China volume 5 (Ulmaceae through Basellaceae). St. Louis, MO: Missouri Botanical Garden Press; Beijing: Science Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.