Abstract
Background The origin of the Equisetum strobilus has long been debated and the fossil record has played an important role in these discussions. The paradigm underlying these debates has been the perspective of the shoot as node–internode alternation, with sporangiophores attached at nodes. However, fossils historically excluded from these discussions (e.g. Cruciaetheca and Peltotheca) exhibit reproductive morphologies that suggest attachment of sporangiophores along internodes, challenging traditional views. This has rekindled discussions around the evolution of the Equisetum strobilus, but lack of mechanistic explanations has led discussions to a stalemate.
Scope A shift of focus from the node–internode view to a perspective emphasizing the phytomer as a modular unit of the shoot, frees the debate of homology constraints on the nature of the sporangiophore and inspires a mechanism-based hypothesis for the evolution of the strobilus. The hypothesis, drawing on data from developmental anatomy, regulatory mechanisms and the fossil record, rests on two tenets: (1) the equisetalean shoot grows by combined activity of the apical meristem, laying down the phytomer pattern, and intercalary meristems responsible for internode elongation; and (2) activation of reproductive growth programmes in the intercalary meristem produces sporangiophore whorls along internodes.
Conclusions Hierarchical expression of regulatory modules responsible for (1) transition to reproductive growth; (2) determinacy of apical growth; and (3) node–internode differentiation within phytomers, can explain reproductive morphologies illustrated by Cruciaetheca (module 1 only), Peltotheca (modules 1 and 2) and Equisetum (all three modules). This model has implications – testable by studies of the fossil record, phylogeny and development – for directionality in the evolution of reproductive morphology (Cruciaetheca–Peltotheca–Equisetum) and for the homology of the Equisetum stobilus. Furthermore, this model implies that sporangiophore development is independent of node–internode identity, suggesting that the sporangiophore represents the expression of an ancestral euphyllophyte developmental module that pre-dates the evolution of leaves.
Keywords: Cruciaetheca, development, Equisetum, Equisetales, evolution, fossil, hierarchy, modularity, Peltotheca, phytomer, sphenopsid, strobilus
EQUISETALEAN REPRODUCTIVE MORPHOLOGY: A COLLECTION OF EVOLUTIONARY PUZZLES
Equisetum is the sole living representative of a large clade with a rich fossil record – the sphenopsids – that includes two major lineages, the Sphenophyllales and the Equisetales (the latter including the Calamitaceae and Equisetaceae, among others). The origin of the reproductive structures of Equisetum has challenged plant morphologists for many years (Zimmermann, 1930, 1961; Eames, 1936; Boureau, 1964; Stewart, 1964, 1983; Riggs and Rothwell, 1985; Kenrick and Crane, 1997). Lack of consensus on this issue has led to competing hypotheses of homology (e.g. Page, 1972; Naugolnykh, 2004) and has generated debate on phylogenetic relationships (e.g. Rothwell, 1999; Pryer et al., 2001; Schneider, 2013). Concurrently, steadily accumulating data from the fossil record have added to this puzzle, by broadening the range of reproductive morphological diversity documented for the equisetalean lineage (Good, 1975; Naugolnykh, 2004; Escapa and Cuneo, 2005; Cuneo and Escapa, 2006). While this rich fossil record has inspired hypotheses of morphological evolution (Zimmermann, 1930; Stewart, 1964; Naugolnykh, 2004; Cuneo and Escapa, 2006), none of those hypotheses has suggested developmental mechanisms responsible for such evolutionary changes.
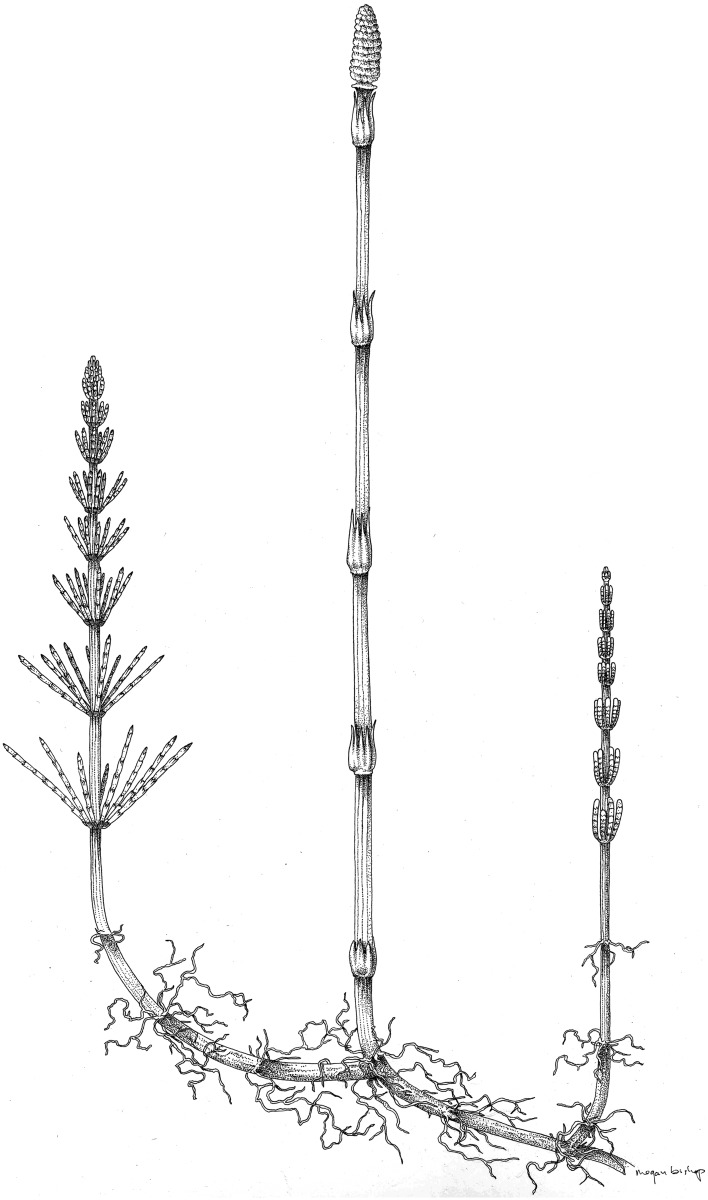
The morphology of Equisetum is highly canalized and unique among living vascular plants. The sporophyte consists of an underground rhizome from which upright aerial shoots arise. Stems bear leaves, branches in whorls at conspicuous nodes that typically are separated by relatively long internodes, giving the plants a jointed or articulated appearance. Leaves are linear with a single vein, and are almost completely fused together, forming a collar at each node; adventitious roots are also borne at nodes. Fertile organs consist of peltate sporangium-bearing structures known as sporangiophores that are also borne in whorls at the stem tip, forming terminal strobili (Milde, 1867; Eames, 1936; Bierhorst, 1971; Stewart and Rothwell, 1993) (Fig. 1).
Fig. 1.
Growth habit of Equisetum emphasizing the below- and above-ground stems with long internodes, nodes bearing whorled appendages, adventitious roots and terminal strobilus consisting of whorls of sporangiophores. Drawing by Megan Bishop.
While all Equisetum species, living and extinct, share the morphological features listed above, when fossil equisetaleans are added to the equation they reveal a much wider range of reproductive morphological diversity than is found among living species. Within this context, a striking feature is the pattern of modularity that overprints the overall organization of shoots, independent of the distribution of fertile parts. For instance, in living Equisetum, the internodes that make up the shoots are exclusively vegetative, whereas fertile appendages (sporangiophores) are found only on specialized shoot tips with determinate growth (strobili) (Fig. 2A). In contrast, in the extinct genus Cruciaetheca (as well as in species of the extinct genera Equisetinostachys, Tschernovia and Paracalamitina; Meyen, 1971; Naugolnykh, 2002; Cuneo and Escapa, 2006) some internodes bear whorls of sporangiophores along part or all of their length; such internodes are usually aggregated in fertile regions along stems (Fig. 2B). In yet another group of fossils, such as Peltotheca (as well as in species of the extinct genera Pothocites and Archaeocalamites; Paterson, 1841; Stur, 1887; Escapa and Cuneo, 2005), vegetative internodes are replaced in the terminal region of specialized fertile shoots by internodes covered in sporangiophores (Fig. 2C, D). The morphological diversity encompassed by these fossils raises fundamental questions about the origins, homology, and evolution of equisetacean reproductive structures.
Fig. 2.
Diversity of reproductive morphology in Equisetaceae and allied groups. (A) In Equisetum, strobili consisting of whorls of sporangiophores and characterized by determinate growth top shoots with otherwise typical vegetative structure; Equisetum hyemale; scale bar = 10 mm. (B) In Cruciaetheca, successive internodes bearing whorls of sporangiophores form fertile regions along the shoots; Cruciaetheca patagonica (MPEF Pb 1154); scale bar = 5 mm. (C, D) In Peltotheca, the terminal regions of fertile shoots consist of successive internodes that bear whorls of sporangiophores and exhibit an apoxogenetic pattern typical of determinate growth; Peltotheca furcata (C, MPEF Pb 1440a, scale bar = 10 mm; D, CIRGEO Pb 610, scale bar = 5 mm).
Modern studies of fossil and living organisms have emphasized that evolution at the organismal level is the result of changes in development (Cronk et al., 2002; Sanders et al., 2009; Rothwell et al., 2014). In the case of equisetacean reproductive morphology, attempts to understand the developmental changes that underlie documented patterns of diversity have been frustrated by the fact that, except for one of them (Equisetum), all the different morphologies are known only in a fossil state, which is a major hurdle for studies of development. This is not unlike the case of several major features of plant structure, whose evolutionary origins are buried deep in the fossil record and can only be resolved by combining an understanding of development in extant taxa with the recognition of anatomical or morphological ‘fingerprints’ for diagnostic developmental features, in the fossil record (Rothwell and Lev-Yadun, 2005; Rothwell et al., 2014).
Understanding of the mode of shoot development in Equisetum provides a model that reveals potential morphological fingerprints that could be used to uncover features of development from the fossil record. Here we apply this approach to detect such developmental features in the fossil record and use those features, in concert with data on the reproductive development of Equisetum, to propose a hierarchical modularity hypothesis for the evolution of reproductive morphology in equisetaceans. According to this hypothesis, the evolution and diversity of equisetacean reproductive morphology are the result of additive changes in a hierarchy of modular programmes that together regulate reproductive development.
VEGETATIVE GROWTH IN EQUISETUM: A DRAMATIC ILLUSTRATION OF DEVELOPMENTAL MODULARITY
An alternate view of shoot modularity circumvents homology issues
Our hypothesis hinges on a shift of emphasis in the characterization of shoot structure. The modular structure of plant shoots can be described as a sequence of alternating nodes and internodes, but also as a stack of phytomers, each of which includes a node and an internode. Seemingly trivial, these differences in perspectives on shoot structure that are not mutually exclusive leads, in the case of Equisetaceae, to two models with different explanatory power. Traditional adherence to the ‘node–internode alternation’ perspective has led to a stalemate in understanding of the evolution of equisetacean reproductive structures. Because of emphasis on the node as the point of attachment of lateral appendages and implied equivalence of these appendages, irrespective of their nature, attempts to explain different equisetacean reproductive structures within this paradigm have become mired in homology issues, with no resolution in sight. In the hypothesis we propose here, we swap this perspective for the equally traditional ‘shoot as a stack of phytomers’ perspective. While reflecting the same structural realities, this perspective circumvents long-standing homology issues and provides a framework for explaining the diversity and evolution of equisetacean reproductive structures in an internally consistent, testable system of developmental mechanisms.
A phytomer-centred developmental model
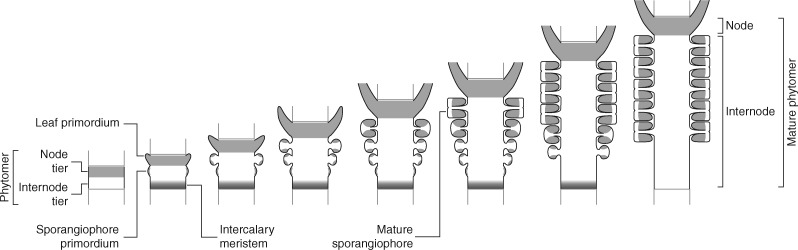
The pattern of stacked phytomers that form the Equisetum shoot, each consisting of a node bearing leaves and a subtending internode, is laid down at the shoot apical meristem. Developmental studies (Golub and Wetmore, 1948a, b) have documented in detail how this modular pattern is laid down during vegetative growth (Fig. 3). Each phytomer develops from a set of adjacent merophytes that are sectorially contiguous and synchronized developmentally (merophytes are groups of clonally related cells resulting from a single derivative of the apical cell). The set of merophytes that forms a phytomer represents three successive apical cell derivatives produced by a complete cycle of divisions of the tetrahedral apical cell (along each of its three cutting faces) (Golub and Wetmore, 1948a). This set of merophytes has been referred to as a segment ring (Reess, 1867) or a primordial ring (Golub and Wetmore, 1948a).
Fig. 3.
Pattern and process in the growth and structure of the vegetative Equisetum shoot. The tetrahedral apical cell produces derivatives by dividing successively along each of its three cutting faces. Further divisions in each apical derivative produce lineages of clonally related cells – merophytes. Developmental synchronization of the merophytes representing three successive apical cell derivatives results in a developmental unit that forms a phytomer. An upper node tier and a lower internode tier differentiate within each phytomer. The internode tier segregates a basal meristematic zone – an intercalary meristem – from which growth of the internode occurs. The resulting shoot structure is a stack of phytomers, each of which consists of a node and the subtending internode, whose growth is the result of the combined activity of the apical meristem and the intercalary meristems of each internode.
Developmental synchronization between the merophytes that form a phytomer is at the origin of the whorled organization of Equisetum shoots. Although successive divisions of the apical cell produce derivatives in a helical pattern, the merophytes of a primordial ring become horizontally aligned to form a unitary tier of the shoot – a phytomer – at a very short distance behind the apical cell (Golub and Wetmore, 1948a). This is probably the result of cross-talk between the merophytes within each phytomer which establishes them as a unitary developmental domain; repression of this synchronization programme may be responsible for the Equisetum teratologies that display helical taxis (e.g. Bierhorst, 1971).
Early divisions in the development of each phytomer differentiate an upper tier, which produces a nodal region, and a lower tier that generates an internode. Whereas cell division and differentiation in the nodal tier leads to development of leaf primordia but produces no elongation, the internodal tier forms an intercalary meristem that is responsible for the elongation of the internode (Golub and Wetmore, 1948a, b).
The result of this mode of development is that overall shoot growth represents the combined effect of two spatially separated types of processes: (1) growth at the shoot apical meristem, which leads to the general longitudinal patterning of the shoot into successive phytomers; and (2) growth within each phytomer wherein leaves and branches (and roots) develop at the node, whereas the internode elongates from an intercalary meristem; overall shoot elongation hence results primarily from local elongation in individual phytomers. Specifically, internode elongation from the intercalary meristem proceeds by addition of new cells acroscopically with respect to the meristem (and subsequent elongation of those cells). Consequently, meristematic tissue is maintained at the base of each internode and tissues are progressively more mature distally (apically) within the internode (Fig. 3), e.g. the stomatal development patterns documented in Equisetum by Cullen and Rudall (2016).
THE DIVERSITY OF EQUISETACEAN REPRODUCTIVE MORPHOLOGY: A HIERARCHICAL MODULARITY HYPOTHESIS
Close morphological and anatomical similarity in vegetative structure with extant Equisetum (e.g. Stanich et al., 2009; Channing et al., 2011), as well as evidence for intercalary meristems in calamitacean and sphenophyllalean sphenopsids (Good, 1971; Schabilion, 1975), indicate that the same mode of development combining apical and intercalary growth was present in extinct Equisetaceae and other equisetaleans. Within the constraints of this mode of development, evolution of the structural diversity of reproductive morphology can be explained by additive changes in a hierarchy of regulatory modules responsible for the development of reproductive structures. These regulatory modules are: (1) a module regulating the transition to reproductive growth in the shoot apical meristem (RM1); (2) a module regulating determinacy of growth in the apical meristem (RM2); (3) a module regulating node–internode differentiation within phytomers (RM3); and (4) a module responsible for the development of fertile appendages (sporangiophores) (RM4). Our hypothesis explains differences in equisetacean reproductive morphology based on changes in the expression of the first three of these modules and has implications for the evolutionary origins of the fourth.
As a first tenet of this hypothesis, we propose that a switch to reproductive growth (RM1) in the shoot apical meristem leads to the production of fertile phytomers. Specifically, this transition to reproductive growth activates RM4 in the intercalary meristem of phytomers laid down by the apical meristem from that point onwards. As a result, sporangiophores are produced in whorls along the shoot segment that develops from the intercalary meristem (internode). In the developing Equisetum strobilus, sporangiophore primordia arise as a result of periclinal divisions of superficial cells (Bower, 1935) (Fig. 8C). We hypothesize that expression, in the internode protoderm layer produced by the intercalary meristem, of the same developmental programme responsible for sporangiophore development in the strobilus (RM4), would produce whorls of sporangiophores attached along that internode.
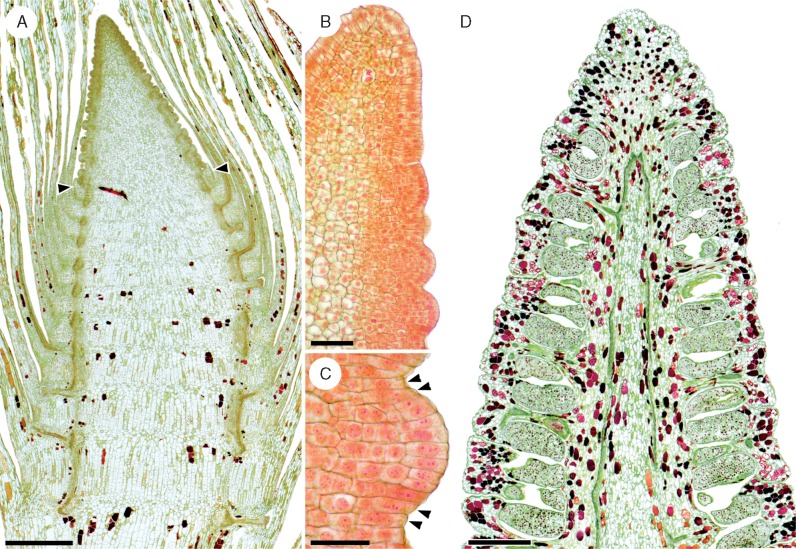
Fig. 8.
The anatomy and development of the Equisetum strobilus (E. telmateia illustrated here) corroborates the interpretation that the regulatory programme responsible for node–internode differentiation is repressed in the fertile phytomers that develop into sporangiophore whorls. (A) Tip of a developing fertile shoot; the two arrowheads indicate the limit between vegetative phytomers (below) and the strobilus (above). This limit coincides with a sharp transition between the vegetative phytomers, which exhibit marked alternation of nodes and elongated internodes, and fertile phytomers, in which such an alternation is conspicuously missing. The latter is consistent with the absence of node–internode differentiation in fertile phytomers. Additionally, acropetal maturation of tissues in the strobilus is consistent with development of successive phytomers from an apical meristem and rejects interpretation of the strobilus as a single fertile internode (which would grow from a meristem at its base and, thus, exhibit a basipetal maturation pattern). Scale bar = 1 mm. (B) Tip of a developing strobilus; the origin of sporangiophores can be traced to vertically adjacent bulges that are visible 8–10 phytomers away from the apical cell. Scale bar = 100 μm. (C) A sporangiophore primordium is initiated by periclinal divisions of superficial cells throughout the entire thickness of the phytomer, except for the topmost and basalmost layers of the phytomer (arrowheads). This indicates that a sporangiophore whorl develops from the entire thickness of a strobilar phytomer and not just from its upper or lower section (i.e. node or internode), consistent with the absence of node–internode differentiation in these phytomers. Scale bar = 50 μm. (D) Longitudinal cell patterning in the mature strobilus lacks the node–internode differentiation that is conspicuous in vegetative shoots, another feature consistent with absence of node–internode differentiation in strobilar phytomers. Scale bar = 1 mm.
If sporangiophore whorls were produced along the internode by activity of the intercalary meristem, they could not correspond to nodes in the classical sense and would not represent nodes in the Equisetum model, wherein node and internode develop from distinct developmental domains of the phytomer. This implication eliminates the equivalence between sporangiophore whorl and node, and it rejects the positional homology of leaves and sporangiophores. Further, if multiple sporangiophore whorls can be produced along a single internode, then direct equivalence between a sporangiophore whorl and a phytomer is implicitly rejected. The premise of direct equivalence between sporangiophore whorl, node and phytomer is the foundation for hypotheses of homology in the node–internode view that has failed to explain the diversity of equisetacean reproductive morphology. In contrast, the alternative view of the Equisetum shoot as a stack of phytomers, together with the potential for production of sporangiophores along internodes, allow for developmental explanations for that diversity.
The basic transition to a reproductive programme (i.e. activation of RM1) leading to production of sporangiophores (RM4) along internodes will generate, in the absence of other developmental constraints, the Cruciaetheca-type morphology (Fig. 2B): a succession of fertile phytomers with leaf-bearing nodes and internodes that bear whorls of sporangiophores. Reversal to a vegetative growth programme in the apical meristem (RM1 turned off) will resume production of vegetative phytomers (in which RM4 is turned off). Multiple such reversals will result in shoots along which regions consisting of fertile phytomers alternate with vegetative regions. Based on the parameters of intercalary meristematic growth, we can predict the occurrence of patterns of maturation whereby (1) sporangiophores are increasingly more mature distally (apically) within individual internodes (a pattern becoming less conspicuous in fully mature phytomers) (Fig. 4); and (2) in shoots tips that are still growing, the internode segments that bear sporangiophores (or the number of sporangiophore whorls) are longer in successively more basal phytomers. Furthermore, within this model, heterochronic change, i.e. change in the duration of expression of RM4 with respect to the overall duration of intercalary meristematic growth, will determine the length of the sporangiophore-bearing region of the internode (or the number of sporangiophore whorls produced along the internode) (Fig. 6A).
Fig. 4.
Developmental sequence of a fertile internode growing from an intercalary meristem that expresses a reproductive programme. Divisions in the intercalary meristem at the base of the internode add new cells acroscopically, so tissue maturity increases acropetally within the internode. Because of this pattern of maturation, in growing internodes sporangiophores are larger toward the top of the internode and their size decreases (with maturity) basipetally; these size differences disappear in mature internodes (phytomers).
Fig. 6.
Heterochronic change in the expression of a reproductive programme in the intercalary meristem result in different lengths of the internode portions covered in sporangiophores, depending on the duration of expression of the reproductive programme; these are illustrated by several extinct equisetaleans. At one end of the spectrum (1), Cruciaetheca genoensis exemplifies a condition in which the reproductive programme, turned on at the beginning of internode development, is expressed for a short fraction of the entire interval of internode growth, following which the internode completes growth in vegetative mode; the result is an internode that bears only two sporangiophore whorls at its apical end. Extension of the duration of expression of the reproductive programme over a longer portion of the interval of internode growth (2) produces internodes covered in a higher number of sporangiophore whorls (3–5 in Cruciaetheca patagonica) and with a shorter sterile portion at the base. At the other end of the spectrum (3), Peltotheca furcata exemplifies a condition in which the reproductive program is expressed throughout the entire interval of internode growth, producing internodes covered in sporangiophore whorls from top to base. Cruciaetheca genoensis, MPEF Pb 1406; Cruciaetheca patagonica, MPEF Pb 1154; Peltotheca furcata, MPEF Pb 1441b.
Secondly, a switch to determinate growth (RM2) in an apical meristem expressing the reproductive programme (RM1) will result in determinate reproductive structures consisting of a sequence of fertile phytomers that exhibits an apoxogenetic pattern, as seen in the Peltotheca type (Fig. 2C, D). Thirdly, we propose that repression of node–internode differentiation (RM3) will result in phytomers that lack leaves and intercalary meristems, and do not undergo internodal elongation. Combined with a determinate pattern of reproductive growth (RM2), this will produce the Equisetum-type morphology in which sporangiophores form a terminal strobilus (Fig. 2A).
In the following sections, a discussion of evidence supporting this hypothesis will be presented. We first evaluate the basic equivalence between the equisetacean intercalary meristem and other plant meristems, and implications for the capacity of the intercalary meristem to execute reproductive developmental programmes. We then discuss evidence from the fossil record and from teratologies and development of extant Equisetum, for separate and combined activity of the regulatory modules proposed. In the process we address the implications of these hypotheses for the homology of the Equisetum strobilus. Finally, we bring together all data and observations in a unified explanation of the origins of morphological diversity of equisetacean reproductive structures.
MERISTEMS AND THE TRANSITION TO REPRODUCTIVE GROWTH: A SURVEY OF GENETIC REQUISITES
Currently available evidence (Supplementary Data S1) suggests that: (1) the basic components of genetic switches responsible for the transition from vegetative to reproductive development are shared among most (if not all) land plants; and (2) all plant meristems share common regulatory mechanisms underlying homeostasis and functioning, and are, therefore, equivalent in terms of fundamental competences, including the competence to run a reproductive developmental programme. It is, therefore, reasonable to consider the possibility of the equisetacean intercalary meristem sharing these fundamental competences of plant meristems, including the capacity to respond to the same genetic switches for the vegetative to reproductive transition. This provides the basic elements for the developmental regulatory changes that we propose to explain the diversity and evolution of equisetacean reproductive morphology. This developmental framework also suggests potential tests for the hypotheses advanced here.
VARIATIONS ON ONE MODULE: THE REPRODUCTIVE GROWTH PROGRAMME IN THE INTERCALARY MERISTEM
Evidence from the fossil record
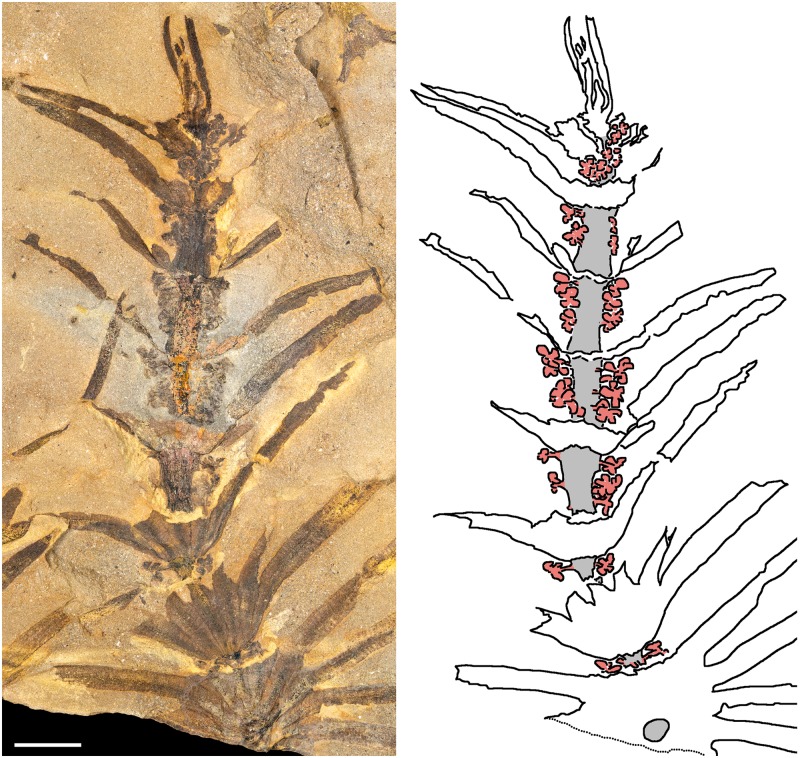
In a description of Lower Carboniferous archaeocalamitaleans, Bateman (1991) documents appendages arranged along internodes, interpreted as adventitious roots, and discusses circumstantial evidence for sporangiophores inserted along internodes. The extinct equisetalean Cruciaetheca patagonica, known from the Early Permian of Argentina (Cuneo and Escapa, 2006), features fertile zones consisting of successive internodes that bear multiple whorls of sporangiophores, intercalated between vegetative shoot portions (Fig. 2B). This is consistent with development from an apical meristem in which RM1 is successively expressed (leading to production of reproductive phytomers) and repressed (vegetative phytomers). Furthermore, C. patagonica provides evidence corroborating the proposition that production of sporangiophores on the fertile phytomers is the result of expression of a reproductive programme (RM4) in the intercalary meristem. First, developing shoot tips show increasingly longer internodes basally, bearing increasingly longer fertile zones (Fig. 5). Secondly, within more developed internodes, the largest sporangiophores are located apically, closest to the node above (Figs 4 and 5), and sporangiophore size decreases conspicuously toward the base of the internode. Additionally, these sporangiophore-bearing internodes also exhibit a vegetative segment at the base. These observations are consistent with a pattern of development wherein (1) an intercalary meristem produces tissues which are displaced apically as they mature; (2) RM4 is turned on in the intercalary meristem from the beginning of phytomer development and internode growth; and (3) RM4 is switched off before the conclusion of intercalary growth so the basal portion of the internode develops in vegetative mode.
Fig. 5.
Apical region of a growing Cruciaetheca patagonica shoot in reproductive mode. Patterns of sporangiophore (shown in red at right, on the explanatory tracing of the photograph) size and distribution corroborate the proposition that sporangiophores are produced along internodes as the result of expression of a reproductive programme in the intercalary meristem. The extent of fertile zones of successive internodes increases with the increase in internode length toward the base of the shoot (due to bending of the stem – shown in grey – leaf bases mask significant parts of internodes in the basal portion of this shoot). Furthermore, within internodes, the largest sporangiophores are located apically and sporangiophore size decreases toward the base of the internode. These observations are consistent with a pattern of development wherein an intercalary meristem produces tissues and sporangiophores which are displaced apically as they mature. MPEF Pb 1359b; scale bar = 10 mm.
A decrease in sporangiophore size basally within individual internodes, conspicuous in Late Permian Phyllotheca verbitskae (Verbitskaja and Radczenko, 1968; Doweld, 2002), provides further support for the hypothesis proposed here. The same pattern is probably present in Early Permian Cruciaetheca genoensis (Cuneo and Escapa, 2006) but is less conspicuous because this species produces only two whorls of sporangiophores per internode. Occurrences that may be illustrating the same developmental pattern include specimens reported as Tschernovia sp. and Equisetinostachys sp. by Meyen (1971) and Naugolnykh (2002, 2004), respectively, from the Middle and Late Permian.
Species of Cruciaetheca, as well as Peltotheca (Escapa and Cuneo, 2005), another equisetalean decribed from the same Permian rock unit as Cruciaetheca, illustrate heterochronic variation in the expression of RM4 in the intercalary meristem (Fig. 6). Thus, C. patagonica produced 3–5 sporangiophore whorls per phytomer, consistent with a longer extent of RM4 expression than in C. genoensis, which produced only two sporangiophore whorls per phytomer (Cuneo and Escapa, 2006). Conversely, Peltotheca furcata illustrates an instance in which RM4 is expressed throughout the entire duration of intercalary growth of the phytomers and, as a result, internodes are covered in sporangiophore whorls all the way from top to base (Escapa and Cuneo, 2005). A similar condition is present in the Mississippian species Pothocites grantonii and Archaeocalamites radiatus (Paterson, 1841; Stur, 1887), the Late Mississippian–Early Permian Tschernovia ? velizensis (Duran et al., 1997) and possibly in the Permian fossils Koretrophyllites grandis (Gorelova, 1960), Equisetinostachys grandis (Rasskazova, 1961) and the Triassic species Equisetites bracteatus (Kon’no, 1962). These fossil occurrences demonstrate that a regulatory module for sporangiophore production (RM4) acting in intercalary meristems had evolved by early Carboniferous time.
In some of the cases referenced here, the extent of fertile zones is difficult to assess due to imperfect fossil preservation or poor quality of published illustrations. For the same reasons, it is unclear whether any equisetaleans provide evidence for variations in the timing of the onset of RM4 with respect to the beginning of intercalary meristematic growth. Such variations would be reflected in fertile regions positioned along the internode at different distances below the node, but evidence from the fossil record is equivocal, i.e. in two species of Koretrophyllites from the Pennsylvanian and Permian of Siberia (Radczenko, 1956; Gorelova and Radczenko, 1962) sporangiophores form fertile zones that seem to be positioned at the base of internodes.
Evidence from Equisetum teratologies
The diversity of teratological forms encountered in various species of Equisetum has fascinated plant morphologists for many years and inspired hypotheses for the homologies of equisetalean reproductive morphology (reviews in Page, 1972; Naugolnykh, 2004). Taken together, the different teratologies demonstrate tremendous variability in reproductive morphology. This variability spans the whole range between terminal strobili and internodes lined with sporangiophores, including transitional forms that bridge the morphospace between regular sporangiophores and sporangium-bearing leaves (e.g. Page, 1972). These reflect a great deal of plasticity in the location of expression of the regulatory programmes responsible for development of the sporangiophore, as a whole, and of individual sporangia, demonstrating that virtually everything is developmentally possible in the reproductive morphology of Equisetum. This very broad heterotopic and heterochronic potential cautions against indiscriminate use of individual teratologies as evidence in support of specific interpretations, when assessing competing hypotheses on evolutionary change in equisetacean reproductive morphology.
Nevertheless, some Equisetum teratologies show close similarity to reproductive morphologies documented in the fossil record, corroborating our developmental hypothesis. Cases in which up to four sporangiophore whorls are borne along the top part of Equisetum internodes (e.g. Boureau, 1964; Naugolnykh, 2004) correspond to the Cruciaetheca-type morphology. In other cases, the sporangiophore whorls (up to> 10) extend along the entire internode (e.g. Tschudy, 1939; Page, 1972; Naugolnykh, 2004), like those of Peltotheca. These Equisetum teratologies are important because they provide parallels between the reproductive morphology of extinct equisetaceans for which the anatomy of development has yet to be documented, and a living species in which the development is well understood. For this reason, developmental studies of such teratological Equisetum morphologies are needed as critical tests for the hypothesis presented here.
WHEN THREE REGULATORY MODULES COALESCE: REPRODUCTIVE DEVELOPMENT IN EQUISETUM
Two hypotheses on the nature of the Equisetum strobilus
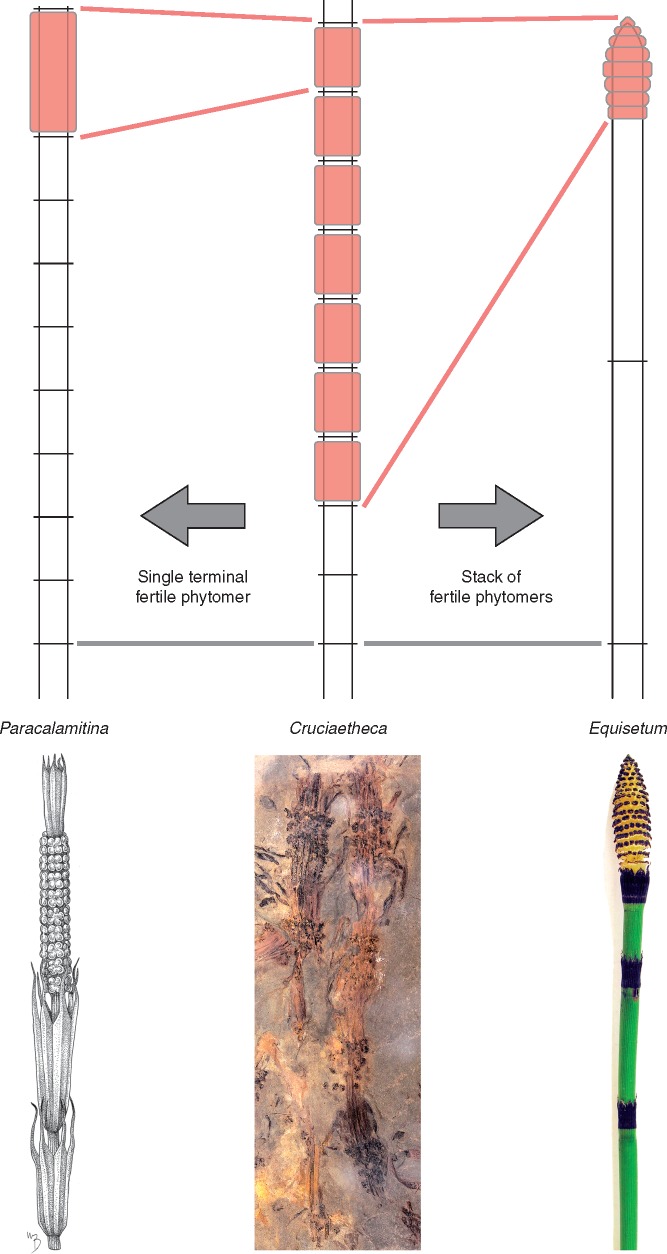
Fossil equisetaceans that feature sporangiophores borne along part or the entire length of internodes, such as Cruciaetheca or Peltotheca, have engendered discussions on homology that revolve around the nature of the Equisetum strobilus. The traditional hypothesis, implicit in many anatomical treatments of this structure (e.g. Browne, 1912, 1915, 1920), has been that the strobilus of Equisetum represents a condensed succession of nodes and internodes (Stewart, 1964; Stewart and Rothwell, 1993) (Fig. 7). An alternative hypothesis has been proposed by Naugolnykh (2004). In this hypothesis, the morphological divide between the Cruciaetheca-type reproductive morphology (sequence of fertile internodes; illustrated by Equisetinostachys in Naugolnykh’s original discussion) and the Equisetum strobilus is bridged by Paracalamitina striata, a Permian sphenopsid that is interpreted as having only one fertile internode in a terminal position (Fig. 7). Although neither of the two hypotheses proposes explicit mechanisms of developmental change leading to the morphology of the Equisetum strobilus, their implications for homology are clear: in the traditional hypothesis the strobilus is a stack of fertile phytomers, whereas in Naugolnykh’s (2004) hypothesis the strobilus is equivalent to a single fertile phytomer. While basic plant developmental patterns, as constrained by meristematic growth, do not corroborate this latter hypothesis (Supplementary Data S2), the view of the Equisetum strobilus as a stack of phytomers is supported by both developmental and morphological data.
Fig. 7.
Two hypotheses for the homology of the Equisetum strobilus. The traditional hypothesis proposes that the strobilus is a condensed succession of nodes and internodes or a stack of fertile phytomers. In an alternative hypothesis, the Equisetum strobilus is regarded as a single fertile internode of a terminal phytomer, as interpreted in Paracalamitina striata (Naugolnykh, 2002, 2004). Paracalamitina striata redrawn from Naugolnykh (2002); drawing by Megan Bishop. Cruciaetheca patagonica, MPEF Pb 633.
The Equisetum strobilus as a stack of phytomers
In contrast to the terminal fertile phytomer hypothesis, the traditional view has been that the Equisetum strobilus is a node–internode succession. This view is supported by the acropetal maturation of tissues in the strobilus (Fig. 8A), which is consistent with development of successive phytomers from an apical meristem. If the strobilus were a single internode and developed (as Equisetum internodes do) from an intercalary meristem positioned at its base, a basipetal maturation pattern would be expected instead. Furthermore, studying several Equisetum species, Page (1972) has shown that for any population of the same species the number of nodes on vegetative shoots is equal to the sum of nodes plus sporangiophore whorls on fertile shoots. Based on these results, combined with observations of teratological specimens, Page proposed that the units of the fertile and vegetative axes are equivalent to one another and are serially homologous. From a developmental perspective, Page’s observations support the interpretation of the strobilus as a stack of phytomers and indicate that each sporangiophore whorl corresponds to a phytomer.
In our hypothesis, reproductive development in Equisetum involves all three major regulatory modules discussed above (plus RM4). Production of a terminal strobilus is due fundamentally to the combined activity of RM1 (transition to reproductive growth turning on RM4 in each phytomer) and RM2 (apical meristem determinacy). In addition to these two, the anatomy of the strobilus indicates the presence of a third set of developmental controls on the patterning of fertile structures. This is clearly apparent in the sharp transition marked by a conspicuous difference in anatomy between the strobilus and the vegetative shoot subtending it: whereas the vegetative shoot exhibits an alternation of nodes and elongated internodes, in the strobilus this alternation is conspicuously missing (Fig. 8A). Nevertheless, Page’s (1972) results are a strong indication that the transition to reproductive growth in the apical meristem maintains, in the strobilus, the basic phytomer patterning of the shoot. Together, these indicate the activity of an additional regulatory module that alters patterning within strobilar phytomers (RM3). This module may be responsible for either shutting down internodal elongation or shutting off node–internode differentiation altogether (as suggested by the studies of Barratt, 1920) and, with that, the specification of an intercalary meristem at the base of the phytomer.
In developing Equisetum strobili, the origin of sporangiophores can be traced to bulges that are visible on the flanks of the strobilus apex approx. 8–10 phytomers away from the apical cell (in E. telmateia). The bulges are initiated by periclinal divisions of superficial cells of the phytomer, which add to the volume of tissue beneath this layer (Fig. 8B). Importantly, with the conspicuous exception of cells in the topmost and basalmost layers of the phytomer, the superficial cells throughout the entire thickness of the phytomer undergo periclinal divisions to form a sporangiophore (Fig. 8C). This indicates that a sporangiophore whorl develops from the entire thickness of a strobilar phytomer and not just from its upper or lower section (i.e. node or internode). Furthermore, like the developing strobilus, the mature anatomy of the strobilus (Fig. 8D) completely lacks the node–internode differentiation that is so conspicuous in vegetative shoots. Together, these corroborate the interpretation that, rather than just shutting down internodal elongation, RM3 shuts off node–internode differentiation in the strobilar phytomers.
In the ‘strobilus as a stack of phytomers’ view, the traditional assumption has been that the whorls of sporangiophores correspond to nodes (e.g. Page, 1972). However, the developmental anatomy of the strobilus implies that the question of whether whorls of sporangiophores mark the position of nodes is irrelevant. If each sporangiophore whorl (1) corresponds to a strobilar phytomer in which there is no node–internode differentiation, and (2) develops from the entire thickness of that phytomer, then expression of the sporangiophore development programme (RM4) is independent of node–internode identity. This is consistent with the potential for production of sporangiophores at various locations (for various durations) along internodes, proposed in our hypothesis to explain Cruciaetheca and Peltotheca-type reproductive morphologies.
PELTOTHECA, AN EVOLUTIONARY INTERMEDIATE IN TWO MODULES
The reproductive morphologies of Cruciaetheca and Equisetum illustrate two ends of the spectrum of reproductive developmental trajectories for equisetacean shoot phytomers. In Cruciaetheca, fertile phytomers follow the same developmental trajectory as the vegetative phytomers, i.e. node–internode differentiation and internode elongation, with the addition of only the most basic reproductive regulatory module, RM1, which leads to production of sporangiophores (RM4) from tissues originating in the intercalary meristems. In contrast, in Equisetum, the co-expression of all three major regulatory modules leads to (1) determinate growth and (2) repression of node–internode differentiation and of intercalary growth in the fertile phytomers; activity of RM4 leads to development of a single whorl of sporangiophores on each phytomer. Clues to the changes involved in the evolutionary transition between the former and the latter developmental trajectory may be provided by fossils such as Peltotheca, which we hypothesize as representing an intermediate morphology (Fig. 9).
Fig. 9.
Hypothesis for the evolution of reproductive morphology in the Equisetaceae, reflecting evolution of the complexity of reproductive developmental mechanisms by successive addition of non-overlapping developmental modules. The most basic morphology (Cruciaetheca type) is the result of the expression of a single reproductive regulatory module (RM1), which controls the transition to reproductive growth by promoting the regulatory pathway responsible for sporangiophore development in the intercalary meristem of internodes. The result is a series of fertile phytomers bearing whorls of sporangiophores along internodes, part of a shoot with indeterminate growth. Expression, against this background, of a second regulatory module that induces determinacy in development (RM2), results in a series of fertile phytomers (bearing sporangiophores along the internodes) that exhibit an apoxogenetic pattern of determinate growth, as seen in the Peltotheca type. A third regulatory module (RM3) is responsible for the repression of node–internode differentiation within phytomers. If expressed in concert with RM1 and RM2, this produces a terminal strobilus of the Equisetum type that is a stack of phytomers, but not a node–internode sequence; each of these phytomers lacks an intercalary meristem and internodal elongation, and produces a single whorl of sporangiophores that develop from the entire thickness of the phytomer. In this hypothesis, corroborated by fossil morphologies, RM1, 2 and 3 are uncoupled and hierarchical: RM2 and RM3 act downstream of RM1, and RM3 is probably only expressed downstream of RM2.
In Peltotheca, internodes entirely covered in several whorls of sporangiophores form a terminal fertile zone on specialized reproductive shoots (Escapa and Cuneo, 2005). Like Equisetum, Peltotheca produced fertile shoots with determinate growth. This is demonstrated by specimens in which all sporangiophores along the internodes are mature – even those on apical internodes that show an apoxogenetic pattern – indicating that the sequence of fertile phytomers occupied a terminal position on the fertile shoot (Fig. 2C, D). However, unlike Equisetum but similar to the structure of Cruciaetheca, the fertile phytomers of Peltotheca exhibit node–internode differentiation – as indicated by the presence of leaf scars on the nodes of the fertile zone – and intercalary growth – as demonstrated by the elongated internodes. Together, these suggest that in Peltotheca only RM1 (+RM4) and RM2 are active, leading to transition to reproductive growth and determinacy of the apical meristem, but not RM3; hence the presence of node–internode differentiation and intercalary growth.
THE DISTINCTIVE MODE OF SHOOT GROWTH OF EQUISETACEAE: MODULARITY AND HIERARCHY
Assessment of the rich equisetacean fossil record in light of the distinctive mode of development of Equisetum suggests a model of growth for Equisetaceae that explains the diversity of reproductive morphologies documented in the group. In this model, the equisetacean shoot grows by the combined action of apical and intercalary meristems. The apical meristem lays down a sequence of phytomers (along with the radial patterning of shoot tissues), whereas the intercalary meristem at the base of each phytomer increases the length of that phytomer. Overlain on these patterns of development, expression of one or several reproductive regulatory modules leads to different reproductive morphologies. All these observations and ideas can be integrated into a hypothesis on the evolution of the complexity of reproductive developmental mechanisms in the Equisetaceae by successive addition of non-overlapping developmental modules (Fig. 9).
The most basic of these morphologies, seen in the Cruciaetheca type, is the result of the expression of a single reproductive regulatory module, RM1. This is the minimum requirement for the transition to reproductive growth and promotes the activity of RM4, the regulatory pathway responsible for sporangiophore development, in the intercalary meristem region of internodes. The result is what we hypothesize as the plesiomorphic condition for equisetacean reproductive structures, consisting of series of fertile phytomers bearing whorls of sporangiophores along internodes, that alternate with series of vegetative phytomers along shoots with indeterminate growth. Heterochronic variations in the expression of RM4 result in different lengths of the internode portions covered in sporangiophores (Fig. 6).
The second regulatory module (RM2) induces determinacy in the development of reproductive structures. The result is a series of fertile phytomers (bearing sporangiophores along the internodes) that exhibit apically the apoxogenetic morphological pattern characteristic of determinate growth, as seen in the Peltotheca type. The third regulatory module (RM3) is responsible for the repression of node–internode differentiation within phytomers. In combination with the activity of RM1 and RM2, this produces a terminal strobilus which is a stack of phytomers, but not a node–internode sequence – as seen in the Equisetum type. Each of these phytomers lacks an intercalary meristem and internodal elongation, and produces a single whorl of sporangiophores that develop from the entire thickness of the phytomer.
Our hypothesis uses modularity of regulatory programs to explain the different types of reproductive structures produced across the Equisetaceae by the evolution of development. If this hypothesis is a valid explanation for the range of morphologies reviewed here, then the very existence of these morphologies in tangible organisms points to two basic features of the developmental system of this group of plants. On the one hand, because they can act in concert and yet their concerted expression is not required for the production of each type of reproductive structure, RM1, RM2, and RM3 are uncoupled (Fig. 9). On the other hand, the proposed interactions of regulatory modules required for the production of different morphologies are consistent with hierarchical patterns. Thus, RM4 acts downstream of RM1; in the same way, RM2 and RM3 have to act downstream of RM1. At the same time, RM2 and RM3 seem to be independent, e.g. Peltotheca vs. Equisetum morphologies. Nevertheless, the range of morphologies observed, as reflections of the realized morphospace of the potential patterns of co-expression of these modules, indicates that RM3 is only expressed downstream of RM2.
MISSING PIECES OF THE PUZZLE
The origins of intercalary growth
Irrespective of its implications for the diversity of equisetacean reproductive morphologies, growth of Equisetum shoots from two types of meristems (apical and intercalary) is a well-established fact. Whereas growth by internode elongation is a feature of all vegetative shoots articulated into nodes and internodes (Esau, 1965), in most plants internode elongation arises from diffuse cell elongation throughout most of the internode, in the immediate vicinity of the shoot apex, and not from well circumscribed intercalary meristems. Such intercalary meristematic areas, located at the base of internodes and characterized by prolonged activity, have evolved independently in the sphenopsid clade (Equisetales and their close relatives, the Sphenophyllales; Esau, 1965; Good, 1971; Schabilion, 1975) and in some monocotyledons, particularly grasses (Lehmann, 1906; Prat, 1935).
These two instances of intercalary growth share a morphological correlate – long internodes – and an anatomical one – rhexigenous protoxylem lacunae. Termed carinal canals in Equisetum, the rhexigenous lacunae are longitudinal spaces generated by mechanical failure, under excessive tension due to elongation, of the earliest tracheary elements to mature. Whereas internode length is relative and not readily comparable across plants of different sizes and lineages, rhexigenous protoxylem lacunae are comparatively rare among tracheophytes and could serve as a potential anatomical fingerprint for internode elongation from well-circumscribed intercalary meristems. Because of their mode of formation, the sides of these cavities are lined with the remains of secondary wall thickenings of the torn tracheary elements, which also help in their recognition. In fact, the presence of rhexigenous protoxylem lacunae in several Middle Devonian plants (Ibyka and other cladoxylaleans) was one of the features that led Skog and Banks (1973) to propose that they represented a plexus of taxa at the base of the sphenopsid lineage. While Scheckler (1974) has asked for caution in the use of this anatomical feature to recognize sphenopsids deep in the fossil record, the improvements in our knowledge of the Devonian fossil record during the intervening time period could allow for a modern re-evaluation of these views.
In considering the deepest origins of growth from intercalary meristems, it is interesting to note that growth from basally located meristems characterizes the sporophyte axes of mosses and hornworts. Whereas for hornworts it is unclear whether the sporophyte undergoes any organized apical growth, in mosses the sporophyte grows in a first phase from an apical cell. Following this early phase, intercalary growth leading to elongation of the seta is initiated from sub-apical tissues as the sporophyte apex transitions to a reproductive growth programme, losing meristematic competence and developing into a sporangium (Tomescu et al., 2014). In light of these features of bryophyte development, and considering the close relationships between bryophytes and the earliest polysporangiophytes–tracheophytes, Niklas (2000) has proposed a combination of growth from apical and intercalary meristems as a hypothetical developmental system for the branched sporophytes of the earliest polysporangiophytes. In his model, elongation in the earliest branched sporophytes arose principally from intercalary meristems located at the base of each of the sporophyte branches, which terminated in sporangia.
The fourth module: what about the sporangiophore?
A direct implication of the hypothesis presented here is that sporangiophore development is fundamentally independent of node–internode identity: sporangiophores are produced either on phytomers that lack node–internode differentiation (in the Equisetum strobilus) or in various positions along internodes. In turn, this is relevant to discussions of sporangiophore homology. These discussions have a rich history and have been complicated by the diversity of morphologies of fertile appendages documented among the Equisetales and the Sphenophyllales, as well as by the lack of resolution in the phylogeny of sphenopsids as a group. Without attempting to review or even list all the hypotheses that have been proposed, it will suffice to say that discussions of the Equisetum sporangiophore (reviewed in some detail by Page, 1972) have proposed everything from total or partial foliar homologies to origin as a sui generis structure.
If sporangiophore development is independent of node–internode identity, this is inconsistent with leaf homology – leaf development is associated with nodal identity in vegetative phytomers that express node–internode differentiation. Thus, when considered in the narrow context provided by the canalized organography of extant Equisetum, the sporangiophore appears to represent a sui generis structure, as proposed by Barratt (1920). Conversely, when considered within a broader evolutionary context and from the perspective of an upward outlook at plant evolution (Bower, 1935; Stewart, 1964), these observations imply that sporangiophores evolved independently of leaves. It is possible that the equisetacean sporangiophore represents the expression of a regulatory module that evolved for the production, in basal euphyllophytes (trimerophytes), of structures that pre-date the evolution of node–internode differentiation in the sphenopsid clade and the evolution of leaves. This would be the regulatory module responsible for the development of lateral (determinate) fertile branches on the otherwise undifferentiated axes of trimerophytes. These ideas are not inconsistent with the view of the sporangiophore as originating by sequential modification of fertile lateral branches in early tracheophytes whose sporophytes consisted of undifferentiated branching axes, a perspective taken in one of the most frequently conjured applications of the telome theory (Zimmermann, 1952; Stewart, 1964, 1983).
Additional diversity of sphenopsid reproductive structures
Sitting at the tip of one of the deepest branches of vascular plant phylogeny (the sphenopsids), Equisetum has a highly distinctive morphology. Virtually every aspect of its morphology, be it vegetative or reproductive, has generated discussions and hypotheses, many of which have relied heavily upon data from the fossil record. Here we have proposed a hypothesis that addresses one of these aspects: the morphology of fertile zones and strobili. Furthermore, we have focused only on a sub-set of sphenopsid reproductive morphologies for which the distinctive aspects of Equisetum development are directly relevant; these structures consist of successions of sporangiophore whorls and characterize members of the Equisetaceae.
The sphenopsids have produced at least two other major types of fertile structures (usually strobili) not discussed here. One of these, characteristic of many Calamitaceae, consists of regularly alternating fertile (sporangiophore) whorls and vegetative (bract) whorls (Good, 1975), whereas the other, characteristic of the Sphenophyllales (Riggs and Rothwell, 1985), features sporangium-bearing units with foliar morphology. Hypotheses of homology for all these different types of reproductive structures will be discussed in a forthcoming paper (I. H. Escapa et al., in preparation) that will undertake a broader survey of the sphenopsid fossil record and will expand on the hypotheses presented here. Nevertheless, it is worth pointing out that while calamitacean and sphenophyllalean reproductive structures are usually regarded as consisting of strobili (determinate growth), notes buried in the older literature (Hoskins and Cross, 1943) and new fossils from the Early Permian of Argentina (N. R. Cuneo et al., unpubl. data) reveal the presence of reproductive structures with indeterminate growth at least in the Sphenophyllales.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Data S1: a survey of the genetic underpinnings of meristem functioning and the transition to reproductive growth: implications for the capacity of the intercalary meristem to execute reproductive developmental programmes. Data S2: evaluation of the terminal fertile phytomer hypothesis for the origin of the Equisetum strobilus.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dennis K. Walker for preparing sections of Equisetum telmateia strobili, Megan Bishop for the drawings of Equisetum and Paracalamitina, Maria Tekleva and Aleksandra Sokolova for help with the Russian literature, and Eduardo Ruigómez for assistance with fossil specimens. Richard Bateman, an anonymous reviewer, and the handling editor, Silvia Pressel, are thanked for comments and suggestions that improved the manuscript. This work was supported by the US National Science Foundation [IIA-1322504] and American Philosophical Society grants to A.M.F.T.
GLOSSARY
Acropetal (maturation): pattern of tissue maturation along a structure or organ, in which the basal region is the earliest to mature and tissue maturation progresses toward the tip (distal region) of the structure or organ. Antonym: basipetal.
Acroscopic: refers to position along an axial plant organ relative to a reference location; specifically, positioning closer to the apex, as compared with the reference location. Antonym: basiscopic.
Apoxogenetic development (n., apoxogenesis): developmental pattern that results in a progressively smaller and generally simpler primary construction apically (Eggert, 1961), e.g. a tapering off of a shoot. The opposite pattern is termed epidogenesis.
Archaeocalamitales (adj., archaeocalamitalean): major group of extinct sphenopsids characterized by linear, dichotomous leaves, secondary growth and compact strobili composed of whorls of sporangium-bearing apendages; the occurrence of foliar appendages (bracts) between whorls of sporangium-bearing appendages is equivocal. Archaeocalamitales grew in the paleotropics primarily during the Mississippian and Pennsylvanian Periods, and have frequently been hypothesized to have given rise to the Calamitaceae.
Basipetal (maturation): pattern of tissue maturation along a structure or organ, in which the apical region is the earliest to mature and tissue maturation progresses toward the base (proximal region) of the structure or organ. Antonym: acropetal.
Basiscopic: refers to position along an axial plant organ relative to a reference location; specifically, positioning closer to the base, as compared with the reference location. Antonym: acroscopic.
Calamitaceae (adj., calamitacean): major group of extinct sphenopsids, sometimes recognized as the Calamitales but traditionally included in Order Equisetales, characterized by simple linear leaves, secondary growth and arborescent habit, compact terminal fertile regions in which whorls of sporangiophores alternate with whorls of foliar appendages (bracts), and spores bearing elaters. Calamitales grew in the paleotropics primarily during the Pennsylvanian and Permian Periods, and have been hypothesized by some to have given rise to the Equisetaceae by a reduction in plant size, loss of woody tissues and loss of bract whorls within the cones.
Derivative of an apical cell (apical cell derivative): cell produced directly by the division of an apical cell.
Determinate growth (n., determinacy): growth pattern in which growth ceases once a set developmental checkpoint is reached. Antonym: indeterminate growth.
Ectopic expression (of a gene): expression of a gene in a location (or at a point in developmental time) where it is not normally expressed in the regular developmental pattern of an organism.
Equisetaceae (adj., equisetacean): crown group family of Order Equisetales, characterized by compact determinate fertile regions consisting of whorls of (peltate) sporangiophores; secondary growth is absent in extant species and equivocal in extinct ones. The Equisetaceae include living species of Equisetum, as well extinct species of Equisetum and similar plants that extend back through time to the Triassic Period.
Equisetales (adj., equisetalean): major group of sphenopsids that traditionally includes Equisetum and other taxa of the Equisetaceae, as well as the extinct Calamitaceae and, sometimes, the Archaeocalamitaceae. Equisetales are characterized by whorled, usually single-veined leaves that may be basally fused forming a sheath, and fertile regions consisting of one or more whorls of sporangium-bearing appendages that may alternate with foliar appendages (leaves or bracts).
Euphyllophytes: informal name for Sub-division Euphyllophytina (Kenrick and Crane, 1997), a clade that is the sister group of lycophytes (Sub-division Lycophytina). Eupyllophytes include a basal grade of extinct plants with simple organography consisting of undifferentiated branching axes (referred to here as trimerophytes), several other extinct lineages, as well as all living non-lycophyte tracheophytes.
Fertile zone: sequence of successive fertile phytomers along a shoot. Fertile zones usually alternate with vegetative (sterile) zones along shoots.
Indeterminate growth: growth pattern in which growth continues indefinitely throughout the life span of the organism. Antonym: determinate growth.
Intercalary meristem: the meristematic region at the base of each internode, found in some plant groups, such as the sphenopsids and grasses (Poaceae).
Merophyte: a group of clonally related cells resulting from sequential cell divisions that originate in a single derivative of the apical cell of a meristem.
Orthologs, orthologous genes: genes in different species that originated by vertical descent from a single gene of the last common ancestor (https://en.wikipedia.org/wiki/Homology_( biology)#Orthology).
Periclinal division: orientation of the plane of cell division parallel with the outer surface of an organ.
Phytomer: modular unit of the shoot consisting of one node (with the attached leaf) and the subtending internode.
Polysporangiophytes: the clade of plants that share the branched sporophyte as a synapomorphy (Super-division Polysporangiomorpha of Kenrick and Crane, 1997).
Primordial ring: set of sectorially contiguous merophytes that form a phytomer (Golub and Wetmore, 1948a). Synonym: segment ring.
Promeristem: classic anatomy term for the stem cell niche of an apical meristem. Divisions of promeristem cells produce derivatives which go on to differentiate into primary meristems (procambium, ground meristem, protoderm); select cells of procambial lineage (residual procambium), sometimes in combination with select cells in the ground meristem lineage (i.e. pith ray cells in seed plant stems), form the vascular cambium.
Regulatory module: system of developmental regulators (transcription factors, etc.) deployed as a unit of a regulatory programme and responsible for a well-circumscribed developmental or morphological outcome.
Rhexigenous protoxylem lacuna: elongated, irregularly tubular cavity that occupies the position of a protoxylem strand and is generated by the failure of protoxylem tracheary elements due to tensional stress; it can be recognized by its position and by the presence of secondary wall thickening remnants (annular, helical) attached to the walls of the lacuna.
Sectorially contiguous merophytes: merophytes that are adjacent to each other laterally, i.e. are derived from successive derivatives of an apical cell.
Segment ring: set of sectorially contiguous merophytes that form a phytomer (Reess, 1867). Synonym: primordial ring.
Sphenophyllales (adj., sphenophyllalean): major group of extinct sphenopsids characterized by whorls of cuneate to broadly ovate leaves with dichotomizing veins, exarch protosteles, secondary growth and fertile regions consisting of whorls of sporangium-bearing appendages with foliar parts. Sphenophyllales were small shrubs or vines that had worldwide distribution, primarily from the Mississippian through the Triassic Periods.
Sphenopsida (adj., sphenopsid): major clade of euphyllophytes characterized by jointed stems bearing whorled appendages. The Sphenopsida extend from the Late Devonian through the recent and include the Archaeocalamitales, Sphenophyllales and Equisetales, along with several other extinct lineages; of these, only about 15 species of the genus Equisetum are alive today.
Sporangiophore: sporangium-bearing appendage of the sphenopsids. The homology of sporangiophores in different sphenopsid lineages is disputed.
Strobilus (pl. strobili): aggregation of sporangium-bearing appendages (e.g. leaves, sporangiophores) at the tip of a shoot with determinate growth.
Taxis: mode or geometry of arrangement, e.g. of lateral appendages on a main axis.
Telome theory: system of hypotheses proposed by Zimmermann (1952) to explain the evolution of complex plant sporophyte body plans and organs from plants with simple body plans (i.e. undifferentiated branching axes like those characteristic of many Late Silurian and Early Devonian land plants). At the core of the telome theory are the telome truss, a basic branching unit of the archetypal early land plant body plan, and a set of hypothetical basic processes of morphological evolution; under the telome theory, combinations of several of these processes in different sequences and proportions were hypothesized to have generated different plant body plans and organs. See Stewart (1964) for a comprehensive summary.
Teratological form (n., teratology): morphology generated by a significant deviation from the regular pattern of development in an organ or organism; the study of these morphologies.
Trimerophytes: basal grade of the euphyllophytes from which sphenopsids and other major clades of living euphyllophytes are derived. Includes extinct plants with simple organography consisting of undifferentiated branching axes bearing sporangia at the tips of branches in specialized fertile regions. Sphenopsids, seed plants, several groups of ferns and the psilopsids (i.e. Psilotum and Tmesipteris) all are derived from trimerophyte-grade euphyllophytes.
Upward outlook (in morphological evolution): a perspective on evolution that emphasizes explanations of complex structures of derived groups as modifications of simpler structures of more basal (less derived) groups.
LITERATURE CITED
- Barratt K. 1920. A contribution to our knowledge of the vascular system of the genus Equisetum. Annals of Botany 34: 201–235. [Google Scholar]
- Bateman RM. 1991. Palaeobiological and phylogenetic implications of anatomically-preserved archaeocalamites from the Dinantian of Oxroad Bay and Loch Humphrey Burn, southern Scotland. Palaeontographica B 223: 1–59. [Google Scholar]
- Bierhorst DW. 1971. Morphology of vascular plants. Toronto: Macmillan. [Google Scholar]
- Boureau E. 1964. Traité de paléobotanique. III. Sphenophyta, Noeggerathiophyta. Paris: Masson et Cie. [Google Scholar]
- Bower FO. 1935. Primitive land plants. London: Macmillan. [Google Scholar]
- Browne IMP. 1912. Contributions to our knowledge of the anatomy of the cone and fertile stem of Equisetum. Annals of Botany 26: 663–703. [Google Scholar]
- Browne IMP. 1915. A second contribution to our knowledge of the anatomy of the cone and fertile stem of Equisetum. Annals of Botany 29: 231–264. [Google Scholar]
- Browne IMP. 1920. A third contribution to our knowledge of the anatomy of the cone and fertile stem of Equisetum. Annals of Botany 34: 237–263. [Google Scholar]
- Channing A, Zamuner A, Edwards D, Guido D.. 2011. Equisetum thermale sp. nov. (Equisetales) from the Jurassic San Agustin hot spring deposit, Patagonia: anatomy, paleoecology, and inferred paleoecophysiology. American Journal of Botany 98: 680–697. [DOI] [PubMed] [Google Scholar]
- Cronk QBC, Bateman RM, Hawkins JA.. 2002. Developmental genetics and plant evolution. London: Taylor & Francis. [Google Scholar]
- Cullen E, Rudall PJ.. 2016. The remarkable stomata of horsetails (Equisetum): patterning, ultrastructure and development. Annals of Botany 119: 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuneo NR, Escapa IH.. 2006. The equisetalean genus Cruciaetheca nov. from the Lower Permian of Patagonia, Argentina. International Journal of Plant Sciences 167: 167–177. [Google Scholar]
- Doweld AB. 2002. Annulina M.F. Neuburg: illegitimate generic name replaced by Umbellaphyllites Rasskazova, 1961 (Equisetophyta). Paleontological Journal 36: 219–221. [Google Scholar]
- Duran M, Hunicken MA, Anton AM.. 1997. Novedosos hallazgos de Sphenopsida en la Formacion Bajo de Veliz, Provincia de San Luis, Argentina. Ameghiniana 34: 259–264. [Google Scholar]
- Eames AJ. 1936. Morphology of vascular plants, lower groups. New York: McGraw-Hill. [Google Scholar]
- Esau K. 1965. Plant anatomy, 2nd edn. New York: Wiley & Sons. [Google Scholar]
- Escapa IH, Cuneo NR.. 2005. A new equisetalean plant from the early Permian of Patagonia, Argentina. Review of Palaeobotany and Palynology 137: 1–14. [Google Scholar]
- Golub SJ, Wetmore RH.. 1948a. Studies of development in the vegetative shoot of Equisetum arvense L. I. The shoot apex. American Journal of Botany 35: 755–767. [Google Scholar]
- Golub SJ, Wetmore RH.. 1948b. Studies of development in the vegetative shoot of Equisetum arvense L. II. The mature shoot. American Journal of Botany 35: 767–781. [Google Scholar]
- Good CW. 1971. The ontogeny of Carboniferous articulates: calamite leaves and twigs. Palaeontographica B 133: 137–158. [Google Scholar]
- Good CW. 1975. Pennsylvanian-age calamitean cones, elater-bearing spores, and associated vegetative organs. Palaeontographica B 153: 28–99. [Google Scholar]
- Gorelova SG. 1960. New Late Permian Koretrophyllites from Kuzbass In: Markovsky BP, ed. Novye vidy drevnikh rastenij i bespozvonochnykh SSSR (New species of older plants and invertebrates of the USSR). Moskow: Gosgeoltekhizdat, 29. [Google Scholar]
- Gorelova SG, Radczenko GP.. 1962. Most common plants in the Upper Permian deposits of the mountain district at Altai-Saiansk (in Russian). VSEGEI (Leningrad) 79: 39–243. [Google Scholar]
- Hoskins JH, Cross AT.. 1943. Monograph of the paleozoic cone genus Bowmanites (Sphenophyllales). American Midland Naturalist 30: 113–163. [Google Scholar]
- Kenrick P, Crane PR.. 1997. The origin and early diversification of land plants. Washington: Smithsonian Institution Press. [Google Scholar]
- Kon’no E. 1962. Some species of Neocalamites and Equisetites in Japan and Korea. Tohoku University Science Reports. Series 2,Geology 5: 21–47. [Google Scholar]
- Lehmann E. 1906. Zur Kenntnis der Grassgelenke. Berichte der Deutschen Botanischen Gesellschaft 24: 185–189. [Google Scholar]
- Meyen SV. 1971. Phyllotheca-like plants from the Upper Paleozoic flora of Angaraland. Palaeontographica B 133: 1–33. [Google Scholar]
- Milde J. 1867. Monographia Equisetorum. Verhandlungen der Kaiserlichen Leopoldino-Carolinischen deutschen Akademie der Naturforscher 32: 1–605. [Google Scholar]
- Naugolnykh SV. 2002. Paracalamitina striata: a newly reconstructed equisetophyte from the Permian of Angaraland. Journal of Paleontology 76: 377–385. [Google Scholar]
- Naugolnykh SV. 2004. On some aberrations of extant horsetails (Equisetum L.) and the origin of the family Equisetaceae. Paleontological Journal 38: 335–342. [Google Scholar]
- Niklas KJ. 2000. The evolution of plant body plans – a biomechanical perspective. Annals of Botany 85: 411–438. [Google Scholar]
- Page CN. 1972. An interpretation of the morphology and evolution of the cone and shoot of Equisetum. Botanical Journal of the Linnean Society 65: 359–397. [Google Scholar]
- Paterson R. 1841. Description of Pothocites grantonii, a new fossil vegetable. Transactions of the Botanical Society of Edinburgh 1: 45. [Google Scholar]
- Prat H. 1935. Recherches sur la structure et le mode de croissance des chaumes. Annales des Sciences Naturelles, Botanique, serie 10 17: 81–145. [Google Scholar]
- Pryer K, Schneider H, Smith AR. et al. 2001. Horsetails and ferns are a monophyletyic group and the closest living relatives to seed plants. Nature 409: 618–621. [DOI] [PubMed] [Google Scholar]
- Radczenko GP. 1956. Family Sorocaulaceae fam. nov. (in Russian). Materialy Vsesoyuznogo Nauchno-Issledovatel’skogo Geologicheskogo Instituta Series: Novaja Serija 12: 206–219. [Google Scholar]
- Rasskazova ES. 1961. Sphenophytes from the Upper Paleozoic of the Tunguska Basin, parts 1–2. Annals of the Scientific Institute of Arctic Geology 23–24: 1–73. [Google Scholar]
- Reess M. 1867. Zur Entwicklungsgeschichte der Stammspitze von Equisetum. Jahrbücher für Wissenschaftliche Botanik 6: 209–236. [Google Scholar]
- Riggs SD, Rothwell GW.. 1985. Sentistrobus goodii n. gen. and sp., a permineralized sphenophyllalean cone from the Upper Pennsylvanian of the Appalachian Basin. Journal of Paleontology 59: 1194–1202. [Google Scholar]
- Rothwell GW. 1999. Fossils and ferns in the resolution of land plant phylogeny. Botanical Review 65: 189–218. [Google Scholar]
- Rothwell GW, Yev-Ladun S.. 2005. Evidence of polar auxin flow in 375 million-year old fossil wood. American Journal of Botany 92: 903–906. [DOI] [PubMed] [Google Scholar]
- Rothwell GW, Wyatt SE, Tomescu AMF.. 2014. Plant evolution at the interface of paleontology and developmental biology: an organism-centered paradigm. American Journal of Botany 101: 899–913. [DOI] [PubMed] [Google Scholar]
- Sanders H, Rothwell GW, Wyatt SE.. 2009. Key morphological alterations in the evolution of leaves. International Journal of Plant Sciences 170: 860–868. [Google Scholar]
- Schabilion JT. 1975. Intercalary growth in the fossil arthrophyte, Sphenophyllum. Review of Palaeobotany and Palynology 20: 103–108. [Google Scholar]
- Scheckler SE. 1974. Systematic characters of Devonian ferns. Annals of the Missouri Botanical Garden 61: 462–473. [Google Scholar]
- Schneider H. 2013. Evolutionary morphology of ferns (monilophytes) In: Ambrose BA, Purugganan MD, eds. Annual Plant Reviews Volume 45: The evolution of plant form Chichester: Wiley-Blackwell, 115–140. [Google Scholar]
- Skog JE, Banks HP.. 1973. Ibyka amphikoma, gen. at sp. n., a new protoarticulate precursor from the Late Middle Devonian of New York State. American Journal of Botany 60: 366–380. [Google Scholar]
- Stanich NA, Rothwell GW, Stockey RA.. 2009. Phylogenetic diversification of Equisetum (Equisetales) as inferred from Lower Cretaceous species of British Columbia, Canada. American Journal of Botany 96: 1–12. [DOI] [PubMed] [Google Scholar]
- Stewart WN. 1964. An upward outlook in plant morphology. Phytomorphology 14: 120–134. [Google Scholar]
- Stewart WN. 1983. Paleobotany and the evolution of plants. New York: Cambridge University Press. [Google Scholar]
- Stewart WN, Rothwell GW.. 1993. Paleobotany and the evolution of plants, 2nd edn. Cambridge: Cambridge University Press. [Google Scholar]
- Stur D. 1887. Beiträge zur Kenntnis der Flora der Vorwelt 2. Die Karbon-Flora der Schatzlarer Schichten. Abhandlungen der Kaiserlich-Königlichen Geologischen Reichsanstalt (Wien) 11: 228–235. [Google Scholar]
- Tomescu AMF, Wyatt SE, Hasebe M, Rothwell GW.. 2014. Early evolution of the vascular plant body plan – the missing mechanisms. Current Opinion in Plant Biology 17: 126–136. [DOI] [PubMed] [Google Scholar]
- Tschudy RH. 1939. The significance of certain abnormalities in Equisetum. American Journal of Botany 26: 744–749. [Google Scholar]
- Verbitskaja NG, Radczenko GP.. 1968. New Siberian articulates (in Russian). In: Novye vidy drevnikh rastenii i bespozvonochnykh SSSR, vol. 2, part 1. Moscow: Nedra, 13–18. [Google Scholar]
- Zimmermann W. 1930. Die Phylogenie der Pflanzen. Jena: Fischer. [Google Scholar]
- Zimmermann W. 1952. Main results of the telome theory. Palaeobotanist 1: 456–470. [Google Scholar]
- Zimmermann W. 1961. Phylogenetic shifting of organs, tissues, and phases in pteridophytes. Canadian Journal of Botany 39: 1547–1553. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.