Abstract
IMPORTANCE
Pictorial warnings on cigarette packs draw attention and increase quit intentions, but their effect on smoking behavior remains uncertain.
OBJECTIVE
To assess the effect of adding pictorial warnings to the front and back of cigarette packs.
DESIGN, SETTING, AND PARTICIPANTS
This 4-week between-participant randomized clinical trial was carried out in California and North Carolina. We recruited a convenience sample of adult cigarette smokers from the general population beginning September 2014 through August 2015. Of 2149 smokers who enrolled, 88% completed the trial. No participants withdrew owing to adverse events.
INTERVENTIONS
We randomly assigned participants to receive on their cigarette packs for 4 weeks either text-only warnings (one of the Surgeon General’s warnings currently in use in the United States on the side of the cigarette packs) or pictorial warnings (one of the Family Smoking Prevention and Tobacco Control Act’s required text warnings and pictures that showed harms of smoking on the top half of the front and back of the cigarette packs).
MAIN OUTCOMES AND MEASURES
The primary trial outcome was attempting to quit smoking during the study. We hypothesized that smokers randomized to receive pictorial warnings would be more likely to report a quit attempt during the study than smokers randomized to receive a text-only Surgeon General’s warning.
RESULTS
Of the 2149 participants who began the trial (1039 men, 1060 women, and 34 transgender people; mean [SD] age, 39.7 [13.4] years for text-only warning, 39.8 [13.7] for pictorial warnings), 1901 completed it. In intent-to-treat analyses (n = 2149), smokers whose packs had pictorial warnings were more likely than those whose packs had text-only warnings to attempt to quit smoking during the 4-week trial (40% vs 34%; odds ratio [OR], 1.29; 95% CI, 1.09–1.54). The findings did not differ across any demographic groups. Having quit smoking for at least the 7 days prior to the end of the trial was more common among smokers who received pictorial than those who received text-only warnings (5.7% vs 3.8%; OR, 1.53; 95% CI, 1.02–2.29). Pictorial warnings also increased forgoing a cigarette, intentions to quit smoking, negative emotional reactions, thinking about the harms of smoking, and conversations about quitting.
CONCLUSIONS AND RELEVANCE
Pictorial warnings effectively increased intentions to quit, forgoing cigarettes, quit attempts, and successfully quitting smoking over 4 weeks. Our trial findings suggest that implementing pictorial warnings on cigarette packs in the United States would discourage smoking.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT02247908
Reducing smoking prevalence is a top public health priority because smoking is the leading cause of preventable death globally.1 Clinical approaches to smoking cessation, including nicotine replacement2 and pharmacotherapy,3 and behavioral approaches, including counseling and financial incentives,4 are effective for the smokers that they reach.5 Policy approaches are also useful because even those with a small effect on a single person can have a large effect across the population.6 Policy approaches to discourage smoking initiation and encourage cessation include raising prices, clean indoor air laws, restricting tobacco marketing, and requiring warnings on cigarette packs.7
In 1966, the United States led the world by becoming the first to require warnings on cigarette packs.8 Since then, the United States has fallen far behind other countries by allowing pack warnings to become stale and ineffective.9 The World Health Organization Framework Convention on Tobacco Control, an international treaty, recommends that cigarette packs have pictorial warnings to communicate the harms of smoking; the United States has not ratified this treaty.7 Currently, 77 countries and jurisdictions require pictorial cigarette pack warnings.10 Through the 2009 Family Smoking Prevention and Tobacco Control Act, US law now requires these warnings.11 However, implementation of pictorial warnings in the United States has been stalled by a 2012 lawsuit by the tobacco industry, in which the US Court of Appeals for the District of Columbia Circuit ruled against the 9 pictorial warnings proposed by US Food and Drug Administration (FDA). The court dismissively stated that FDA had “not provided a shred of evidence” that pictorial warnings reduce smoking.12
A large body of research indicates that pictorial warnings are more effective than text-only warnings.13–19 Experimental research shows that pictorial warnings draw attention, elicit cognitive and affective reactions, and increase intentions to quit smoking,13 but few experiments have included longitudinal assessments of smoking behavior. Some observational studies have found increases in cessation-related behaviors,16–19 but the absence of randomization prevents these studies from ruling out the influence of confounders. To address these gaps, we conducted a large randomized clinical trial to assess the effect on smoking behavior of adding pictorial cigarette pack warnings to the front and back of cigarette packs.
Methods
Our research group has previously published a detailed description of the study protocol (NCT02247908) that we pilot tested in July and August 2014 with 56 adult smokers in North Carolina (Supplement 1).20,21 The University of North Carolina institutional review board approved all study procedures, and all participants provided their written informed consent.
Participants
We conducted a between-participant (or parallel-group) randomized clinical trial with adult smokers in North Carolina and California. We chose these 2 areas because they have populations that are diverse with respect to many of the disparities associated with smoking. Participants were 18 years or older, English speakers, and current smokers (defined as having smoked at least 100 cigarettes during their lifetime and now smoking every day or some days). We excluded pregnant women, people who smoked only roll-your-own cigarettes, people concurrently enrolled in a smoking cessation trial, people who smoked fewer than 7 cigarettes per week, and people who at baseline reported living in the same household as another study participant. We chose the cutoff of 7 cigarettes per week to exclude very light smokers who might not purchase their own packs. We recruited participants from September 2014 to August 2015 through Facebook, Craigs list, email lists, in-person recruitment, referrals from local retailers, flyers, yard signs, and bus and newspaper advertisements.
Key Points.
Question
Does adding pictorial warnings to the front and back of cigarette packs increase quit attempts?
Findings
In this randomized clinical trial that included 2149 adult smokers, 40% of smokers in the pictorial arm made a quit attempt compared with 34% in the text-only arm, a significant difference.
Meaning
Implementing pictorial warnings on cigarette packs could increase quit attempts among smokers.
Procedures
We screened smokers for eligibility online or by phone. We used responses to screening questions to estimate eligible smokers’ usual cigarette consumption and instructed them to bring an 8-day supply of cigarettes to the baseline visit, at which smokers enrolled and provided written informed consent. At this time, we informed participants that the study was examining how smokers understand the labels on their cigarette packs. At this visit, we assigned participants to receive 1 of 8 warnings using simple randomization based on a single allocation ratio. Using a random number generator, we created a randomly ordered, pre-populated list of study conditions and, as participants enrolled in the study, we assigned them to the next study condition on the list. Four pictorial warnings contained text required by the Tobacco Control Act and a picture to illustrate a health harm of smoking selected from the FDA’s originally proposed set of images22 (Figure 1). We chose these 4 warning images because they performed well in a previous internet study and avoided many of the criticisms in the lawsuits (eg, using a cartoon or a rare health harm of smoking).23 We removed the quit line number from the images, which was a source of contention in litigation against the warnings.12 Four text-only control warnings used the US Surgeon General’s warning statements that have been required on the side of cigarette packs since 1985. Participants attended 4 follow-up visits spaced 1week apart, bringing an 8-day supply of cigarettes to all but the final visit.
Figure 1.

Warnings Used in the Trial
Participants completed 2 computer surveys at the baseline visit and 1 survey at each visit thereafter. While participants completed the surveys at these appointments, research staff placed the assigned warnings on participants’ cigarette packs. Participants who missed visits completed the computer survey remotely and did not have their packs labeled that week. For smokers assigned to receive pictorial warnings, research staff removed the package cellophane and applied the self-adhesive labels to the top half of the front and back panels of participants’ cigarette packs, in accordance with the proposed FDA requirements.11 For participants with flip top packs, research staff cut through the label to allow the top to open freely. For smokers assigned to receive text-only warnings, research staff removed the package cellophane and applied the self-adhesive labels on the side of the packs covering the existing US Surgeon General’s warnings. We applied the new warning labels on top of the existing warnings to control for the effect of putting a label on smokers’ packs. Participants received an incorrect label at 0.1% (11 of 7384) of visits during which packs were labeled, but in all cases remained within their assigned trial arm (eg, one pictorial warning instead of another).
Participants received a cash incentive at the end of each visit, up to a total of $185 in North Carolina and $200 in California, depending on the number of surveys completed. Participation incentives were higher in California owing to the higher cost of living there. At the end of the final follow-up appointment, participants received information about local smoking cessation programs.
Outcome Measures
We used validated items and cognitively tested24 newly developed survey items with 15 adult smokers prior to finalizing the survey instrument. The baseline prelabeling survey assessed quit attempts in the last month and most secondary outcomes (eTable 1 in Supplement 2), and the baseline post labeling survey assessed demographic characteristics.
The primary trial outcome was attempting to quit smoking during the study. At week 1, week 2, week 3, and week 4 follow-up visits, we asked participants “During the last week, did you stop smoking for 1 day or longer because you were trying to quit smoking?” At week 4 follow-up, we also asked “Since you started the study, did you stop smoking for 1 day or longer because you were trying to quit smoking?” We considered participants to have made a quit attempt if they answered “yes” to any of the quit attempt questions.
We used the message impact framework (eFigure 1 in Supplement 2), a taxonomy of variables that pictorial warnings may affect,13 to guide the selection of secondary outcomes. Secondary outcomes were measured at week 4 follow-up: cognitive elaboration (thinking about the warning message and thinking about the harms of smoking); fear elicited by the warning and negative affect (eg, disgust, anger); perceived likelihood of harm from smoking; positive and negative smoking reinforcement attitudes; quit intentions; number of conversations in the past week about the warning, health risks of smoking, and quitting smoking; number of times forgoing a cigarette in the past week; and quitting smoking (defined as not smoking cigarettes in the 7 days before the week 4 follow-up visit). Participants who had quit smoking did not answer questions about quit intentions and forgoing a cigarette.
Statistical Analysis
Power analyses indicated that the target enrollment of 2250 smokers would provide 80% power to detect a 3% or larger difference in quit attempts, assuming an α = .05. We examined baseline differences between trial arms using χ2 tests for categorical variables and independent-samples t tests for continuous variables. We examined differential attrition using logistic regression models.
Intent-to-treat analyses of trial outcomes included all participants randomized.25 Analysis of the primary trial outcome of quit attempts used logistic regression to examine the association with trial arm, controlling for any variables that differed at baseline between arms or for which we found differential attrition. To examine if warning effects differed by participant characteristics, we added participant characteristics and their interaction with trial arm to a separate logistic regression model for each characteristic. To understand whether any effects of warnings emerged over time, exploratory analyses examined differences in quit attempts during the trial by each follow-up visit. For continuous secondary outcomes, we used independent samples t tests, examining whether the outcomes differed by trial arm. Analyses of continuous outcomes using nonparametric tests yielded an identical pattern of statistical significance. Analyses of secondary outcomes used the last observation available. We did not plan or conduct interim analyses. Analyses used SAS version 9.4 (Cary, NC). We set critical α = .05 and used 2-tailed statistical tests.
Results
Participant Characteristics
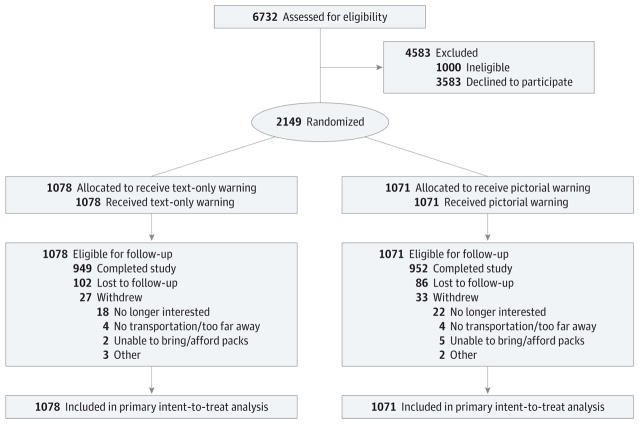
From October 2014 to September 2015, we enrolled 2149 adult current smokers, 101 smokers fewer than the enrollment target owing to recruitment challenges. The study flow diagram shows the number of people who underwent eligibility screening, enrolled in the trial, and completed the study (Figure 2). Briefly, of the 2149 participants who began the trial (1039 men, 1060 women, and 34 transgender people; mean [SD] age, 39.7 [13.4] years for text-only warning, 39.8[13.7] for pictorial warnings), 1901 completed it. Trial participants were diverse, including a substantial number of African American, sexual minority, low-education, and low-income smokers (Table 1). Randomization successfully created 2 trial arms (1078 smokers received text-only warnings, and 1071 received pictorial warnings) that did not differ with respect to participant characteristics (Table 1). Participants had their cigarette packs labeled for an average of 85% of their time in the trial (some participants missed study visits).
Figure 2.
Trial Enrollment, Randomization, and Retention
Table 1.
Participant Characteristicsa
| Characteristic | Text-Only Warnings (n = 1078) | Pictorial Warnings (n = 1071) |
|---|---|---|
| Study site | ||
| California | 594 (55.1) | 592 (55.3) |
| North Carolina | 484 (44.9) | 479 (44.7) |
| Age, y | ||
| 18–24 | 171 (16.1) | 152 (14.5) |
| 25–39 | 377 (35.5) | 398 (37.9) |
| 40–54 | 338 (31.8) | 304 (29.0) |
| ≥55 | 176 (16.6) | 195 (18.6) |
| Mean (SD) | 39.7 (13.4) | 39.8 (13.7) |
| Gender | ||
| Male | 507 (47.4) | 532 (50.0) |
| Female | 548 (51.2) | 512 (48.2) |
| Transgender | 15 (1.4) | 19 (1.8) |
| Gay, lesbian, or bisexual | 173 (16.3) | 195 (18.8) |
| Hispanic | 92 (8.6) | 89 (8.5) |
| Race | ||
| American Indian or Alaska Native | 7 (0.6) | 11 (1.0) |
| Asian | 28 (2.7) | 42 (4.0) |
| Black or African American | 484 (45.8) | 510 (48.9) |
| Native Hawaiian or other Pacific Islander | 11 (1.0) | 6 (0.6) |
| White | 393 (37.2) | 358 (34.3) |
| Other/multiracial | 134 (12.7) | 117 (11.2) |
| Education | ||
| High school degree or less | 333 (31.1) | 344 (32.5) |
| Some college | 519 (48.5) | 502 (47.4) |
| College graduate | 156 (14.6) | 156 (14.7) |
| Graduate degree | 63 (5.9) | 58 (5.5) |
| Household income, annual $ | ||
| 0–24 999 | 566 (53.3) | 589 (55.8) |
| 25 000–49 999 | 272 (25.6) | 266 (25.2) |
| 50 000–74 999 | 110 (10.3) | 92 (8.7) |
| ≥75 000 | 115 (10.8) | 109 (10.3) |
| Low income, <150% of federal poverty level | ||
| No | 506 (47.0) | 477 (44.8) |
| Yes | 570 (53.0) | 589 (55.2) |
| Cigarettes smoked per day, mean (SD) | 8.8 (6.6) | 8.7 (7.3) |
| Smoking frequency | ||
| Daily | 866 (80.4) | 864 (80.7) |
| Nondaily | 211 (19.6) | 207 (19.3) |
| Primary trial outcome at baseline | ||
| Made quit attempt in last month | 270 (26.2) | 275 (26.8) |
| Secondary trial outcomes at baseline, mean (SD)b | ||
| Perceived likelihood of harm from smokingc | 3.3 (0.9) | 3.3 (0.9) |
| Positive smoking attitudesc | 3.3 (1.0) | 3.3 (1.0) |
| Negative smoking attitudesc | 4.0 (0.8) | 4.0 (0.9) |
| Quit intentionsd | 2.2 (0.9) | 2.3 (0.9) |
| No. of conversations about warning in past week | 0.3 (1.1) | 0.4 (1.2) |
| No. of conversations about health risks of smoking in past week | 1.0 (1.7) | 0.9 (1.6) |
| No. of conversations about quitting smoking in past week | 1.2 (1.7) | 1.2 (1.7) |
| No. of times forgoing a cigarette in past week | 1.9 (2.3) | 2.1 (2.3) |
Unless otherwise noted, data are reported as number (percentage) of participants. Study characteristics and outcomes at baseline did not differ by trial arm.
The baseline surveys did not assess the following secondary outcomes: thinking about warning message, fear elicited by the warning, negative affect, and thinking about the harms of smoking. Missing demographic data range from 0.7% to 2.2%.
Response scale for perceived likelihood of harm from smoking, positive smoking attitudes, and negative smoking attitudes ranged from 1 to 5, with 5 indicating higher quantity or stronger endorsement.
Response scale for quit intentions ranged from 1 to 4, with 4 indicating higher intentions.
Analyses of attrition showed that 12% of participants did not complete the week 4 follow-up survey. A total of 188 participants were lost to follow up, and 60 participants withdrew from the study, mostly owing to lack of interest. Attrition did not differ by trial arm (12.0% in the text-only arm vs 11.1% in the pictorial arm; P = .25), and we did not find any evidence of differential attrition by demographic characteristics across trial arms. Participants were less likely to complete the week 4 survey if they enrolled at the California site (13.1% vs 9.7%; P = .01), smoked more cigarettes per day (10.4 vs 8.9 cigarettes per day; P = .01), or were younger (35.2 vs 40.3 years old; P = .01). During the trial, 28 participants reported living in the same household as another study participant, a finding that did not differ by trial arm (11 in the text-only arm vs 17 in the pictorial arm; P = .25); our intent-to-treat analyses included these participants.
Primary Outcome: Quit Attempts
Smokers who received pictorial warnings were more likely to report a quit attempt lasting 1 day or longer during the trial than were smokers who received text-only warnings (40% vs 34%; odds ratio [OR], 1.29 [95% CI, 1.09–1.54]) (Table 2). The effect of pictorial warnings on quit attempts did not differ among demographic subgroups we examined or cigarettes smoked per day (P > .12 for interaction for all comparisons; eTable 2 in Supplement 2). In exploratory analyses, the effect of warnings appeared by week 2 follow-up (eFigure 2 in Supplement 2).
Table 2.
Smokers Who Made at Least 1 Attempt to Quit Lasting 1 Day or Longer During the Trial, Intent-to-Treat Analysisa
| Trial Arm | Smokers Reporting a Quit Attempt, No. | Percentage (95% CI) | OR (95% CI) |
|---|---|---|---|
| Text-only | 366 of 1078 | 34.0 (31.1–36.8) | 1 [Reference] |
| Pictorial | 428 of 1071 | 40.0 (37.0–42.9) | 1.29 (1.09–1.54) |
Abbreviation: OR, odds ratio.
A quit attempt of 1 day or longer during the trial was the primary outcome.
Secondary Outcomes
Analyses of secondary outcomes indicated that having quit smoking for at least the 7 days immediately prior to their last visit was more common among smokers with pictorial than text-only warnings (5.7% vs 3.8%; OR, 1.53 [95% CI, 1.02–2.29]). Pictorial warnings also more often led to forgoing a cigarette (P = .04) and greater intentions to quit smoking (P < .001) than text-only warnings (Table 3). Pictorial warnings led to more thinking about the warning message (P < .001) and harms of smoking (P = .01); fear (P < .001) and negative affect (P < .001); and conversations about the warnings (P < .001), health risks of smoking (P < .001), and quitting (P = .01). Perceived likelihood of harm from smoking, positive smoking reinforcement attitudes, and negative smoking reinforcement attitudes did not differ by trial arm. Participants reported no adverse events during the trial.
Table 3.
Secondary Trial Outcomes at 4-Week Follow-up, Intent-to-Treat Analysis
| Outcome | Text-Only Warnings (n = 1078) | Pictorial Warnings (n = 1071) | Difference (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | |||
| Psychosocial outcomesa | ||||||
| Thinking about warning messageb | 1002 | 2.3 (1.2) | 995 | 3.0 (1.1) | 0.63 (0.53 to 0.73) | <.001 |
| Fear elicited by warning | 1078 | 1.8 (1.1) | 1070 | 2.4 (1.3) | 0.63 (0.53 to 0.74) | <.001 |
| Negative affect | 1078 | 1.8 (1.0) | 1070 | 2.4 (1.2) | 0.64 (0.55 to 0.73) | <.001 |
| Thinking about the harms of smokingb | 1003 | 2.9 (1.1) | 995 | 3.1 (1.1) | 0.14 (0.04 to 0.24) | .01 |
| Perceived likelihood of harm from smoking | 1077 | 3.4 (0.9) | 1071 | 3.4 (0.9) | 0.03 (−0.05 to 0.10) | .49 |
| Positive smoking reinforcement attitudes | 1078 | 3.0 (1.2) | 1070 | 3.0 (1.1) | −0.02 (−0.12 to 0.08) | .71 |
| Negative smoking reinforcement attitudes | 1078 | 3.7 (1.0) | 1070 | 3.7 (1.0) | −0.03 (−0.12 to 0.05) | .48 |
| Quit intentionsa | 1078 | 2.5 (1.1) | 1071 | 2.7 (1.1) | 0.17 (0.08 to 0.26) | <.001 |
| Behavioral outcomes | ||||||
| No. of conversations about warning in past week | 1077 | 1.2 (2.1) | 1071 | 1.8 (2.5) | 0.63 (0.43 to 0.83) | <.001 |
| No. of conversations about the health risks of smoking in past week | 1077 | 1.2 (2.0) | 1071 | 1.6 (2.4) | 0.40 (0.22 to 0.59) | <.001 |
| No. of conversations about quitting smoking in past week | 1077 | 1.4 (2.1) | 1071 | 1.6 (2.4) | 0.26 (0.07 to 0.45) | .01 |
| No. of times forgoing a cigarette in past week | 1077 | 2.5 (2.8) | 1070 | 2.7 (2.9) | 0.25 (0.01 to 0.49) | .04 |
| Quit smoking for ≥7 days, % (SE) | 1078 | 3.8 (0.6) | 1071 | 5.7 (0.7) | 1.9 (0.1 to 3.7) | .04 |
Response scale for all psychosocial outcomes except quit intentions ranged from 1 to 5, with 5 indicating higher quantity or stronger endorsement; response scale for quit intentions ranged from 1 to 4, with 4 indicating highest intentions.
Thinking about warning message and thinking about the harms of smoking were not assessed until week 1 follow-up, resulting in more missing data than for other variables.
Discussion
Our randomized clinical trial with a diverse sample of 2149 adult smokers found that pictorial cigarette pack warnings increased quit attempts from 34% to 40%, an absolute increase of 6%. In relative terms, this is an 18% increase. Pictorial warnings were equally effective for diverse population subgroups, including lower-education, lower-income, racial-minority, and sexual-minority smokers, supporting prior research suggesting that pictorial warnings would be unlikely to exacerbate smoking disparities.26,27 The warnings also increased intentions to quit smoking and forgoing cigarettes, both of which are predictors of subsequent quit attempts.16 Despite the relatively short duration of the trial, 5.7% of smokers exposed to pictorial warnings had quit smoking for at least 1 week by the end of the trial compared with 3.8% of those exposed to text-only warnings, translating to an absolute increase of 1.9%. In relative terms, this is a 50% increase.
The effects we observed appear modest, but they could have a substantial benefit across the population of US smokers. The recent Tips from Former Smokers campaign generated only an absolute increase of 3.7% in quit attempts, and yet this small change translated to an estimated 1.64 million quit attempts and 220000 smokers who quit smoking.28 Unlike national campaigns such as Tips, that cost $54 million for a 3-month flight of ads,29 pictorial warnings cost little to sustain once the work to develop and defend them against tobacco industry litigation is complete. Moreover, supplementing the introduction of pictorial warnings with a synergistic media campaign to reinforce their message may further increase their effect.30
These findings fill an important gap in the empirical literature on pictorial warnings. Our group’s recent meta analysis of controlled experiments,13 which typically presented warnings on a computer screen, found pictorial warnings were more effective than text-only warnings in improving 20 of 25 psychosocial outcomes. However, the meta analysis identified only 1 experiment that examined smoking behavior as an outcome. Among 56 adult smokers exposed to a single pictorial or text-only warning a week for 4 weeks, no change in smoking behavior was found, likely owing to inadequate power.31 Another experiment with 202 smokers, published after the meta-analysis search, also showed no effect on behavior.32 Most large observational studies evaluating smoking behavior before and after warning exposure have found increases in quit attempts and reductions in smoking prevalence in countries that implemented pictorial warnings,18,19 although some studies have not33; other studies indicate that some of the effects of warnings may partially wear out over time.34,35 Such studies support only limited inferences about effect, however, because countries often change several policies at the same time; secular trends and historical events are difficult to account for; and potential comparison countries may differ in other important ways.36 Experimental studies such as ours have the benefit of providing controlled conditions that in many ways mimic what smokers might experience after the adoption of a national policy. Smokers potentially see the warnings every time they smoke, which would equate to around 600 views in a month for a pack-a-day smoker.
The study of warning effects has generated many ideas, but few solid answers, about psychological processes by which pictorial warnings may change behavior. The message reactions hypothesis suggests that pictorial warnings elicit stronger initial affective and cognitive reactions, such as fear and thinking about the harms of smoking, more effectively motivating quitting.13,37,38 Support for this hypothesis comes from the findings of the present trial as well as our group’s earlier meta-analysis13 of the pictorial warnings literature. These findings suggest that negative emotional reactions were more generalized than just fear. The warnings may have had an even larger effect had we included the quit line number39 because research has shown that fear dissipates quickly, so providing a concrete way to take immediate action would prompt the healthy fear to motivate beneficial behavior.40,41 Future studies could quantify the added value of including the quit line number on pictorial warnings.
Other theories on the mechanisms by which warnings trigger behavior change include the social interaction hypothesis, which suggests that the warnings encourage conversations that reinforce the warnings’ effect.21,38,42 This is consistent with our trial findings and a promising area for future research; no studies in our group’s meta-analysis examined the dynamics of social interactions about pictorial warnings.13 The risk reappraisal hypothesis suggests that pictorial warnings may make smoking seem more dangerous, which motivates quitting.43,44 This hypothesis was not supported in our trial, nor in our group’s recent meta-analysis,13 which may be unsurprising— many interventions fail to change risk appraisals.44 Finally, the tarnishing hypothesis suggests that unpleasant images in the warnings may make smoking less enjoyable,45,46 a hypothesis that received some support in our group’s meta-analysis13 but not in the present main trial outcomes: smoking reinforcement attitudes did not change.
Study strengths include a large and diverse sample of smokers who received the warnings on the cigarette packs they used every day. The generalizability of the findings across the many diverse subgroups is encouraging.
A limitation is that the trial examined the potential effect of adding pictorial warnings to cigarette packs as well as implementing other label formatting changes required by the 2009 Tobacco Control Act compared with the present text-only warnings in the United States. Examining these changes together leaves open the possibility that the differences in smoking behaviors we observed may be owing to the combination of adding pictures and changing the warning format. Also, we do not know what effects pictorial warnings would have over a longer period of time, when applied universally to cigarette packs without necessitating removal of the cellophane, or when used in a different context (eg, in rural areas or replacing existing pictorial warnings with new ones).
Other limitations include the following: The pictorial warnings in this trial obscured the logo of certain cigarette brands (eg, Newport), and manufacturers might choose to move the branding elements should pictorial warnings be implemented as a national policy. The self-reported main trial outcome of quit attempts and secondary behavioral outcomes may have been subject to motivated participant responses if participants inferred the purpose of the study. Studies of longer duration may be able to confirm smoking cessation as a primary end point by cotinine testing.47 Participant self-selection could have led to a study population with greater interest in quitting smoking than the general population. Assessments as part of our study may have led participants to think more about their smoking, something that appeared to also happen in the text-only warning arm. Despite these limitations of our experimental pack-labeling protocol,20 we believe it allowed us to gather important evidence of the effect of pictorial warnings and offers a useful way to evaluate new warnings as they are developed.
Conclusions
Implementation of pictorial cigarette pack warnings in the United States is on hiatus.48 Our trial findings provide timely and important information as the United States and other countries consider requiring pictorial cigarette pack warnings. The World Health Organization Framework Convention on Tobacco Control now recommends pictorial warnings but stops short of requiring them.7 Our trial findings support strengthening the treaty to require pictorial warnings on cigarette packs.
Supplementary Material
Acknowledgments
Funding/Support: Research reported in this publication was supported by The National Cancer Institute and the FDA CTP under award No. P30CA016086-38S2.
Footnotes
Conflict of Interest Disclosures: Dr Ribisl has served as an expert consultant in litigation against cigarette manufacturers and internet tobacco vendors; he was also a member of the Tobacco Products Scientific Advisory Committee for the FDA Center for Tobacco Products (CTP) during part of this study. No other conflicts are reported.
Additional Contributions: We thank the research participants for taking part in our trial. We also thank staff at the Pacific Institute for Research and Evaluation and the staff at Ewald and Wasserman Research Consultants LLC; finally we thank Trent Johnson, MPH, Jennifer MacKinnon, MPH, and Marcy Boynton, PhD, for their assistance with the trial. They received no compensation for their contributions beyond that received in the normal course of their employment.
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the FDA.
Author Contributions: Dr Brewer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Brewer, Hall, Noar, Bach, Ribisl.
Acquisition, analysis, or interpretation of data: Brewer, Hall, Parada, Stein-Seroussi, Bach, Hanley, Ribisl.
Drafting of the manuscript: Brewer, Parada.
Critical revision of the manuscript for important intellectual content: Brewer, Hall, Noar, Stein-Seroussi, Bach, Hanley, Ribisl.
Statistical analysis: Brewer, Hall, Parada.
Obtained funding: Brewer, Noar, Ribisl.
Administrative, technical, or material support: Brewer, Hall, Stein-Seroussi, Bach, Hanley, Ribisl.
Study supervision: Brewer, Stein-Seroussi, Hanley.
Role of the Funder/Sponsor: The funding institutions had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2015: Raising Taxes on Tobacco. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 3.West R, Zatonski W, Cedzynska M, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med. 2011;365(13):1193–1200. doi: 10.1056/NEJMoa1102035. [DOI] [PubMed] [Google Scholar]
- 4.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- 5.Zhu S, Melcer T, Sun J, Rosbrook B, Pierce JP. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med. 2000;18(4):305–311. doi: 10.1016/s0749-3797(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 6.Frieden TR. A framework for public health action: the health impact pyramid. AmJ Public Health. 2010;100(4):590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO Framework Convention on Tobacco Control. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 8.United States Public Laws. Federal Cigarette Labeling and Advertising Act of 1966. 89th Congress. Public Law 89–92.
- 9.Institute of Medicine. Ending the Tobacco Problem: A Blueprint for the Nation. Washington, DC: Institute of Medicine; 2007. [Google Scholar]
- 10.Canadian Cancer Society. Cigarette Package Health Warnings: International Status Report. Ontario, Canada: Canadian Cancer Society; 2014. [Google Scholar]
- 11.United States Public Laws. Family Smoking Prevention and Tobacco Control Act of 2009. 111th Congress, 1st Session. Public Law 111-31 [H.R. 1256]. 2009.
- 12.R.J. Reynolds Tobacco Co vs United States Food and Drug Administration. Civil Case No. 11-1482 (RJL). United States District Court for the District of Columbia; 2011.
- 13.Noar SM, Hall MG, Francis D, Ribisl KM, Pepper J, Brewer NT. Pictorial cigarette pack warnings: a meta-analysis of experimental studies. Tob Control. 2016;25(3):341–354. doi: 10.1136/tobaccocontrol-2014-051978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noar SM, Hall MG, Brewer NT. Pictorial cigarette pack warnings have important effects. Am J Public Health. 2015;105(3):e1. doi: 10.2105/AJPH.2014.302510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond D. Health warning messages on tobacco products: a review. Tob Control. 2011;20(5):327–337. doi: 10.1136/tc.2010.037630. [DOI] [PubMed] [Google Scholar]
- 16.Yong HH, Borland R, Thrasher JF, et al. Mediational pathways of the impact of cigarette warning labels on quit attempts. Health Psychol. 2014;33(11):1410–1420. doi: 10.1037/hea0000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong HH, Fong GT, Driezen P, et al. Adult smokers’ reactions to pictorial health warning labels on cigarette packs in Thailand and moderating effects of type of cigarette smoked: findings from the international tobacco control southeast Asia survey. Nicotine Tob Res. 2013;15(8):1339–1347. doi: 10.1093/ntr/nts241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azagba S, Sharaf MF. The effect of graphic cigarette warning labels on smoking behavior: Evidence from the Canadian experience. Nicotine Tob Res. 2013;15(3):708–717. doi: 10.1093/ntr/nts194. [DOI] [PubMed] [Google Scholar]
- 19.Chang FC, Sung HY, Zhu SH, Chiou ST. Impact of the 2009 Taiwan tobacco hazards prevention act on smoking cessation. Addiction. 2014;109(1):140–146. doi: 10.1111/add.12344. [DOI] [PubMed] [Google Scholar]
- 20.Brewer NT, Hall MG, Lee JG, Peebles K, Noar SM, Ribisl KM. Testing warning messages on smokers’ cigarette packages: a standardised protocol. Tob Control. 2016;25(2):153–159. doi: 10.1136/tobaccocontrol-2014-051661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall MG, Peebles K, Bach LE, Noar SM, Ribisl KM, Brewer NT. Social interactions sparked by pictorial warnings on cigarette packs. Int J Environ Res Public Health. 2015;12(10):13195–13208. doi: 10.3390/ijerph121013195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nonnemaker FM, Kamyab K, Busey A, Mann N. Experimental Study of Graphic Cigarette Warning Labels: Final Results Report. Research Triangle Park, NC: RTI International; 2010. [Google Scholar]
- 23.Cameron LD, Pepper JK, Brewer NT. Responses of young adults to graphic warning labels for cigarette packages. Tob Control. 2015;24(e1):e14–e22. doi: 10.1136/tobaccocontrol-2012-050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications, Inc; 2004. [Google Scholar]
- 25.Brody T. Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines. London, England: Academic Press; 2011. [Google Scholar]
- 26.Gibson L, Brennan E, Momjian A, Shapiro-Luft D, Seitz H, Cappella JN. Assessing the consequences of implementing graphic warning labels on cigarette packs for tobacco-related health disparities. Nicotine Tob Res. 2015;17(8):898–907. doi: 10.1093/ntr/ntv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantrell J, Vallone DM, Thrasher JF, et al. Impact of tobacco-related health warning labels across socioeconomic, race and ethnic groups: results from a randomized web-based experiment. PLoS One. 2013;8(1):e52206. doi: 10.1371/journal.pone.0052206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAfee T, Davis KC, Alexander RL, Jr, Pechacek TF, Bunnell R. Effect of the first federally funded US antismoking national media campaign. Lancet. 2013;382(9909):2003–2011. doi: 10.1016/S0140-6736(13)61686-4. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Increases in quitline calls and smoking cessation website visitors during a national tobacco education campaign—March 19–June 10, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(34):667–670. [PubMed] [Google Scholar]
- 30.Brennan E, Durkin SJ, Cotter T, Harper T, Wakefield MA. Mass media campaigns designed to support new pictorial health warnings on cigarette packets: evidence of a complementary relationship. Tob Control. 2011;20(6):412–418. doi: 10.1136/tc.2010.039321. [DOI] [PubMed] [Google Scholar]
- 31.Malouff JM, Schutte NS, Rooke SE, MacDonell G. Effects on smokers of exposure to graphic warning images. Am J Addict. 2012;21(6):555–557. doi: 10.1111/j.1521-0391.2012.00284.x. [DOI] [PubMed] [Google Scholar]
- 32.McQueen A, Kreuter MW, Boyum S, et al. Reactions to FDA-proposed graphic warning labels affixed to U.S. smokers’ cigarette packs. Nicotine Tob Res. 2015;17(7):784–795. doi: 10.1093/ntr/ntu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardle H, Pickup D, Lee L, et al. Evaluating the Impact of Picture HealthWarnings on Cigarette Packets. London, England: Public Health Research Consortium; 2010. [Google Scholar]
- 34.Li L, Borland R, Yong H, et al. Longer term impact of cigarette package warnings in Australia compared with the United Kingdom and Canada. Health Educ Res. 2015;30(1):67–80. doi: 10.1093/her/cyu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borland R, Wilson N, Fong GT, et al. Impact of graphic and text warnings on cigarette packs: findings from four countries over five years. Tob Control. 2009;18(5):358–364. doi: 10.1136/tc.2008.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston, MA: Cengage Learning; 2002. [Google Scholar]
- 37.Witte K. Putting the fear back into fear appeals: The extended parallel process model. Commun Monogr. 1992;59(4):329–349. [Google Scholar]
- 38.Petty RE, Cacioppo JT. The elaboration likelihood model of persuasion. Adv Exp Soc Psychol. 1986;19:123–205. [Google Scholar]
- 39.Hammond D, Reid JL, Driezen P, Boudreau C. Pictorial health warnings on cigarette packs in the United States: an experimental evaluation of the proposed FDA warnings. Nicotine Tob Res. 2013;15(1):93–102. doi: 10.1093/ntr/nts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tannenbaum MB, Hepler J, Zimmerman RS, et al. Appealing to fear: Ameta-analysis of fear appeal effectiveness and theories. Psychol Bull. 2015;141(6):1178–1204. doi: 10.1037/a0039729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leventhal H, Cleary PD. The smoking problem: a review of the research and theory in behavioral risk modification. Psychol Bull. 1980;88(2):370–405. doi: 10.1037/0033-2909.88.2.370. [DOI] [PubMed] [Google Scholar]
- 42.Thrasher JF, Abad-Vivero EN, Huang L, et al. Interpersonal communication about pictorial health warnings on cigarette packages: Policy-related influences and relationships with smoking cessation attempts [published online May 31, 2015] Soc Sci Med. 2015 doi: 10.1016/j.socscimed.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27(2):125–130. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- 44.Sheeran P, Harris PR, Epton T. Does heightening risk appraisals change people’s intentions and behavior? Ameta-analysis of experimental studies. Psychol Bull. 2014;140(2):511–543. doi: 10.1037/a0033065. [DOI] [PubMed] [Google Scholar]
- 45.Nonnemaker JM, Choiniere CJ, Farrelly MC, Kamyab K, Davis KC. Reactions to graphic health warnings in the United States. Health Educ Res. 2015;30(1):46–56. doi: 10.1093/her/cyu036. [DOI] [PubMed] [Google Scholar]
- 46.Romer D, Peters E, Strasser AA, Langleben D. Desire versus efficacy in smokers’ paradoxical reactions to pictorial health warnings for cigarettes. PLoS One. 2013;8(1):e54937. doi: 10.1371/journal.pone.0054937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. 2005;100(3):299–303. doi: 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 48.Tobacco Control Legal Consortium. Cigarette Graphic Warnings and the Divided Federal Courts. St Paul, MN: Public Health Law Center; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.