Abstract
Objectives
To determine the incidence and 1-year outcomes of an elderly population with perioperative atrial arrhythmia (PAA) within 7 days of hip fracture surgery.
Design
Retrospective cohort study.
Setting
The Rochester Epidemiology Project (REP).
Participants
Elderly adults consecutive undergoing hip fracture repair from 1988 to 2002 in Olmsted County, Minnesota (N=1,088, mean age 84.0 ± 7.4, 80.2% female).
Measurements
Baseline clinical variables were analyzed in relation to survival using Cox proportional hazards methods for comparison.
Results
Sixty-one participants (5.6%) developed PAA within the first 7 days. During 1 year of follow-up, 239 (22%) participants died. PAA was associated with greater mortality (45% vs 21%; hazard ratio (HR)=2.8, 95% confidence interval (CI)=1.9–4.2). Other mortality risk factors were male sex (HR=2.0, 95% CI=1.5–2.6), congestive heart failure (HR=2.1, 95% CI=1.7–2.8), chronic renal insufficiency (HR=2.0, 95% CI=1.5–2.8), dementia (HR=2.9, 95% CI=2.2–3.7), and American Society of Anesthesiologists risk Class III, IV, or V (HR=3.3, 95% CI=1.9–5.9).
Conclusion
Elderly adults undergoing hip fracture surgery who develop PAA within 7 days have significantly higher 1-year mortality than those who do not. Further studies are indicated to determine whether prevention of PAA will reduce mortality in this population.
Keywords: aged, hip fractures, postoperative complication, cardiac arrhythmias
The incidence of perioperative atrial arrhythmia (PAA) ranges from 20% to 50%1,2 after cardiac surgery. Its predictors, cost, length of hospital stay, and long-term outcomes in that population are well known.3–8 Although short-term outcomes of PAA after noncardiac surgery have been evaluated in a few studies,9–11 long-term outcomes of PAA after noncardiac surgery are less well defined, especially in elderly adults. Furthermore, every noncardiac surgical procedure is not the same in terms of perioperative events, hospital length of stay, and short- and long-term outcomes. These outcomes may differ according to the age of the individual, underlying comorbidities, and types of surgical procedures. Hip fracture surgery is one of the most common noncardiac surgeries performed in elderly adults,12 but data on the incidence and outcomes of PAA in this population are limited because of significant underrepresentation of elderly adults in clinical studies.13
The incidence and long-term outcomes of PAA in the first 7 days after hip fracture repair in elderly adults have not been elucidated. Therefore, the current study sought to determine the incidence of PAA in first 7 days and the effect on 1-year mortality in elderly adults after hip fracture repair.
METHODS
Data source
Participants were obtained from the Rochester Epidemiology Project (REP),14 a linked computerized database of medical records that includes a dynamic cohort of 502,820 individuals who resided in Olmsted County at some point between 1966 and 2010 and received health care for any reason at a healthcare provider within the system. Information available electronically (electronic REP indexes) includes demographic characteristics, medical diagnostic codes, surgical procedure codes, and death information (including causes of death).
Study Population
A population-based, retrospective cohort study was conducted of individuals consecutively undergoing surgery for hip fracture repair from January 1, 1988, through December 31, 2002, in Olmsted County, Minnesota. All hip fracture surgery episodes were identified on the basis of International Classification of Disease, Ninth Revision, codes. Only individuals who sustained a first femoral neck or intertrochanteric fracture as the primary indication for surgery were included in the study. Individuals younger than 65 or who had surgery more than 72 hours after fracture (because of proven higher mortality due to delayed surgery),15 had had in-hospital fractures (because of poorer preoperative condition and known to have worse outcomes in terms of length of stay and mortality),16 were nonoperatively managed, or had incomplete data, secondary pathological fractures, or fractures due to high-energy trauma injury were excluded from this cohort. The Mayo Clinic institutional review board approved the study. All participants provided prior authorization to use their medical records for research.
Criteria for PAA
PAA was defined as new episodes of atrial arrhythmia (AA) in the 7 days after surgery. Diagnoses were made based on electrocardiographic diagnostic criteria for atrial fibrillation (AF), atrial flutter, paroxysmal atrial tachycardia, supraventricular tachycardia, and multifocal atrial tachycardia consistent with traditional definitions and consensus statements.17 Mortality was defined as death from any cause within the first year after hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analyses
The association between participant demographic characteristics, medical history, and baseline clinical data and the outcome of 7-day PAA was evaluated using chi-square tests or Fisher exact tests for categorical variables and two-sample t-tests for continuous variables. Survival was estimated using the Kaplan–Meier method, and survival time was calculated from 7 days after surgery to date of death or last follow-up to a maximum of 1 year after surgery. The association between participant demographic characteristics, medical history, baseline clinical data, and the development of PAA within 7 days after surgery and 1-year survival was evaluated using Cox proportional hazards regression analysis. In addition to univariate models, a multivariable model was developed using backward selection; all variables in the final model were significant at p < .05. Hazard ratios (HRs) are reported with 95% confidence intervals (CIs). All statistical tests were two-sided, and p<.05 was considered statistically significant. The analysis was performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The participant population consisted of 1,116 individuals with hip fracture between 1988 and 2002. Because the main focus of this study was on the development of PAA within 7 days after hip fracture repair and its effect on 1-year mortality, 28 individuals who died without at least 7 days of follow-up were excluded. Thus, the analysis cohort comprised 1,088 participants (Table 1) with a mean age of 84.0 ± 7.4; 80.2% were female. Mean body mass index (BMI) was 23.3 ± 4.9 kg/m2. One hundred sixty-two (14.9%) participants were American Society of Anesthesiology (ASA) Class 1 or 2, and 925 (85.1%) were ASA Class 3, 4, or 5. A history of AA was reported in 293 (26.9%) participants. Cardiovascular risk factors included hypertension (58%), coronary artery disease (23%), congestive heart failure (CHF) (26%), diabetes mellitus (12%), chronic renal insufficiency (CRI) (11%), AF (19%), obstructive sleep apnea (1.5%), cerebrovascular accident (27%), and dementia (34%).
Table 1.
Participant Characteristics and Preoperative Risk Factors, Overall and According to Perioperative Atrial Arrhythmia (PAA)
| Characteristic | Total, N=1,088 | PAA, n=61 | No PAA, n=1,027 | P-Value |
|---|---|---|---|---|
| Age at surgery, mean±SD | 84.0 (7.4) | 87.4 (6.4) | 83.8 (7.5) | <.001 |
| Age at Surgery≥85 years, n (%) | 531 (48.8) | 41 (67.2) | 490 (47.7) | .004 |
| Male, n (%) | 215 (19.8) | 17 (27.9) | 198 (19.3) | .10 |
| BMI, kg/m2, mean±SD | 23.3 (4.9) | 22.2 (4.5) | 23.4 (4.9) | .06 |
| BMI, kg/m2, n (%) | .08 | |||
| <18.5 | 157 (14.6) | 15 (25.9) | 142 (14.0) | |
| 18.5–25.9 | 592 (55.1) | 28 (48.3) | 564 (55.5) | |
| 26.0–29.9 | 228 (21.2) | 12 (20.7) | 216 (21.3) | |
| ≥30.0 | 97 (9.0) | 3 (5.2) | 94 (9.3) | |
| Preoperative clinical risk factors, n (%) | ||||
| Hypertension | 626 (57.5) | 29 (47.5) | 597 (58.1) | .11 |
| Diabetes mellitus | 131 (12.0) | 4 (6.6) | 127 (12.4) | .18 |
| Myocardial infarction | 248 (22.8) | 13 (21.3) | 235 (22.9) | .78 |
| Congestive heart failure | 282 (25.9) | 15 (24.6) | 267 (26.0) | .81 |
| Atrial fibrillation | 209 (19.2) | 10 (16.4) | 199 (19.4) | .57 |
| Atrial flutter | 49 (4.5) | 4 (6.6) | 45 (4.4) | .43 |
| Cerebrovascular accident | 297 (27.3) | 14 (23.0) | 283 (27.6) | .43 |
| Obstructive sleep apnea | 16 (1.5) | 0 (0.0) | 16 (1.6) | .64 |
| Chronic renal insufficiency | 119 (10.9) | 12 (19.7) | 107 (10.4) | .03 |
| Dementia | 367 (33.7) | 28 (45.9) | 339 (33.0) | .04 |
| American Society of Anesthesiologists Class 3, 4, or 5 | 925 (85.1) | 57 (93.4) | 868 (84.6) | .07 |
| Preoperative history of AA, n (%) | .70 | |||
| No history of AA | 795 (73.1) | 42 (68.9) | 753 (73.3) | |
| History of AA, normal sinus rhythm at surgery | 180 (16.5) | 11 (18.0) | 169 (16.5) | |
| History of AA, in AA at surgery | 113 (10.4) | 8 (13.1) | 105 (10.2) | |
| Length of stay, days, mean±SD | 10.2 (7.8) | 10.9 (4.3) | 10.2 (8.0) | |
SD=standard deviation; BMI=body mass index; AA=atrial arrhythmia.
Sixty-one (5.6%) participants developed PAA in the first 7 days. Participants with PAA are compared with those without PAA in Table 1. Participants with PAA were significantly older than those without (mean age 87.4 vs 83.8, p<.001). Incidence of PAA was higher in very elderly adults (≥85) (7.7% vs 3.6%, p=.004). Participants who developed PAA were more likely to have dementia (45.9% vs 33.0%, p=.04) and CRI (19.7% vs. 10.4%, p=.03) than those without. Participants with PAA were more likely to have lower BMI (mean 22.2 vs 23.4 kg/m2, p=.06) and higher ASA scores (ASA 3, 4, or 5, 93.4% vs 84.6%, p=.07) than those without, but these did not reach statistical significance.
All-cause mortality
During 1 year of follow-up, 239 participants died (23%). Mortality was significantly higher in participants with PAA (45%) than in those without (21%) (HR=2.8, 95% CI=1.9–4.2, p<.001) (Figure 1). Older age (HR for 10-year increase=1.7, 95% CI=1.4–2.0), male sex (HR=2.0, 95% CI=1.5–2.6), history of myocardial infarction (HR=1.9, 95% CI=1.5–2.5), history of CHF (HR=2.1, 95% CI=1.7–2.8), history of CRI (HR=2.0, 95% CI=1.5–2.8), history of dementia (HR=2.9, 95% CI=2.2–3.7), ASA Class 3, 4, or 5 (HR=3.0, 95% CI=1.9–5.9), and AA at the time of surgery (vs no history of AA) (HR=2.6, 95% CI=1.9–3.7) were also associated with mortality. Further analysis using multivariable Cox regression showed that older age (HR=1.4, 95% CI=1.2–1.7), male sex (HR=2.2, 95% CI=1.6–2.9), history of CHF (HR=1.7 (1.3–2.2), history of dementia (HR=2.4, 95% CI=1.8–3.1), ASA Class 3, 4, or 5 (HR=1.9, 95% CI=1.1–3.3), history of AA and in AA at surgery (HR=1.8, 95% CI=1.3–2.5), and development of AA within 7 days after surgery (HR=2.1, 95% CI=1.4–3.1) were independently associated with 1-year mortality. Details of the univariate analysis are described in Table 2. HRs were modestly attenuated in the multivariable model (Figure 2).
Figure 1.
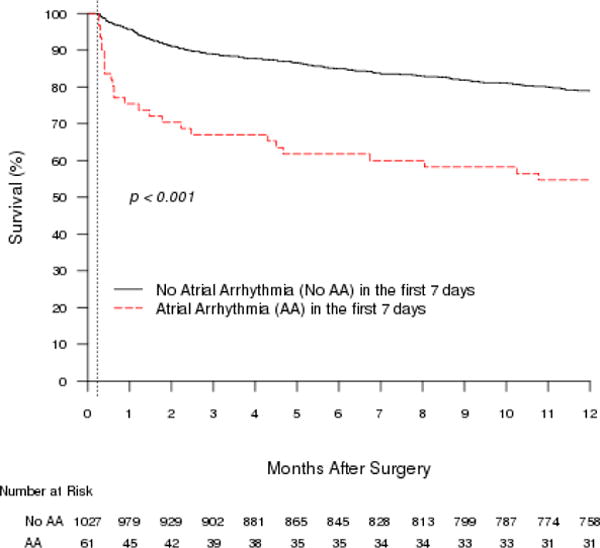
One-year Kaplan–Meier survival curves associated with atrial arrhythmia in the first 7 days after hip fracture surgery. The largest difference in mortality between the two groups occurred by 30 days, although the difference persisted and remained significant for up to 1 year after surgery.
Table 2.
Univariate Analysis of Risk Factors of 1-Year Mortality with Perioperative Atrial Arrhythmia (AA)
| Clinical Risk Factor | Hazard Ratio (95% Confidence Interval) | P-Value |
|---|---|---|
| Postoperative AA within 7 days | 2.8 (1.9–4.2) | <.001 |
| Age at surgery, per 10-year increase | 1.7 (1.4–2.0) | <.001 |
| Aged ≥ 85 at surgery | 1.9 (1.4–2.4) | <.001 |
| Male | 2.0 (1.5–2.6) | <.001 |
| Body mass index, kg/m2 | ||
| Mean±standard deviation | 0.97 (0.95–1.0) | .05 |
| <18.5 | 1.6 (1.1–2.2) | .007 |
| 26.0–29.9 | 0.9 (0.7–1.3) | .65 |
| ≥30.0 | 0.8 (0.5–1.3) | .35 |
| Preoperative risk factors | ||
| Hypertension | 0.8 (0.6–1.0) | .06 |
| Diabetes mellitus | 1.4 (1.0–2.0) | .05 |
| Myocardial infarction | 1.9 (1.5–2.5) | <.001 |
| Congestive heart failure | 2.1 (1.7–2.8) | <.001 |
| Obstructive sleep apnea | 1.2 (0.5–3.3) | .69 |
| Cerebrovascular accident | 1.2 (0.9–1.6) | .17 |
| Chronic renal insufficiency | 2.0 (1.5–2.8) | <.001 |
| Dementia | 2.9 (2.2–3.7) | <.001 |
| Atrial fibrillation | 1.7 (1.3–2.3) | <.001 |
| Atrial flutter | 2.0 (1.2–3.3) | .004 |
| American Society of Anesthesiologists Class 3, 4, or 5 | 3.3 (1.9–5.9) | <.001 |
| Preoperative AA | ||
| History of AA, normal sinus rhythm at surgery | 1.2 (0.9–1.7) | .28 |
| History of AA, in AA at surgery | 2.6 (1.9–3.7) | <.001 |
Figure 2.
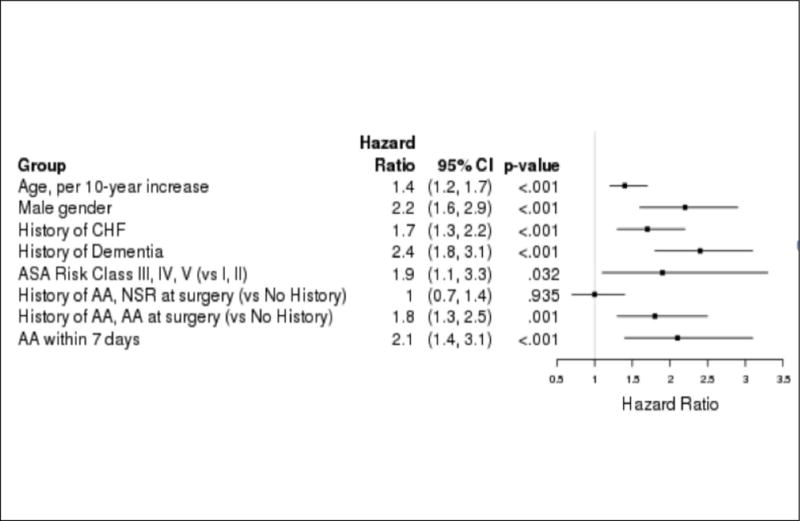
Multivariate Cox proportional hazards model for 1-year mortality. Multivariate analysis of significant variables found atrial arrhythmia (AA) within 7 days to be the third highest risk factor for 1-year mortality after hip surgery in elderly adults. Increasing age according to decade and male sex had greater risks. CHF=congestive heart failure; ASA=American Society of Anesthesiologists; AA=atrial arrhythmia; NSR=normal sinus rhythm.
DISCUSSION
This study demonstrated an association between PAA and long-term mortality in a large cohort of elderly adults undergoing hip fracture surgery for the first time. The findings of this study address important knowledge gaps regarding the incidence and outcomes of PAA specific to individuals aged 65 and older by studying a large cohort of individuals undergoing hip fracture surgery. PAA in the first 7 days was associated with almost three times as high a risk of mortality at 1 year.
In the preoperative setting in this elderly population, a higher prevalence of a history of AA (including AF) was found than in the general population (27% vs 8%)17. Individuals with preexisting AA are potentially at greater risk of hemodynamic instability resulting from hip fracture hospitalization. In addition, the study subjects were very elderly and had many cardiovascular risk factors. These risk factors, along with preoperative AA and advanced age, are known to be associated with a higher incidence of pre and postoperative AA.2,17–19
There are plausible explanations for the higher incidence of PAA in elderly adults; they have a greater propensity to develop AA after surgery because the collagen content of the atria is greater and because of other factors of aging.1 Arterial hypotension is the most common hemodynamic complication of general and regional anesthesia in elderly adults because of low autonomic hemostasis and carotid baroreceptor response. Fluid resuscitation is often used to reverse arterial hypotension. Elderly adults have impaired ability to physiologically withstand the wide fluctuation in the fluid balance because of low cardiopulmonary reserve compounded by a high incidence of heart failure and CRI. One study found positive perioperative fluid balance in 88% of participants undergoing noncardiac surgical procedures, ranging from 1.9 to 6.2 L.20 Volume overload in the setting of CRI and CHF causes significant tricuspid regurgitation, predisposing to AA.21
A previous study10 found a 6.9% incidence of PAA in elderly adults (>80) undergoing total hip or knee arthroplasty, similar to this study’s incidence and population, although another study21 found a higher incidence of supraventricular tachycardia (18%) after noncardiac thoracic surgical procedures; these individuals were felt to be at higher risk of PAA because they had more-extensive surgery. A 12.6% incidence of PAA was found in individuals undergoing noncardiac surgery admitted to a surgical intensive care unit with diagnoses of sepsis requiring treatment with sympathomimetic drugs.9
PAA is also known to increase short-term morbidity and mortality. Thirty-day mortality of 25%11 and 17%22 was found in individuals with PAA who underwent pneumonectomy, but no previous data are available regarding PAA and its association with long-term mortality in elderly adults after noncardiac surgery.
The current study found a significant association between PAA and long-term mortality in a large cohort of elderly adults undergoing hip fracture surgery. Because PAA may be a marker for a sicker elderly population rather than a primary cause of mortality, statistical methods known to delineate risk factors of disease processes in large population-based studies were used. Based on this Cox proportional hazards analysis, the current study provides strong evidence that the association between PAA and 1-year mortality persists after adjustment for known strong predictors of late mortality such as advanced age, hypertension, male sex, CHF, CRI, and dementia. Several plausible mechanisms could explain the association between PAA and long-term mortality. Individuals with PAA may develop exacerbations of underlying compensated CHF due to loss of atrial contraction or may have poor exercise tolerance, high fall risk, and thromboembolic events including stroke. They may experience proarrhythmic effects of antiarrhythmics or intolerance of rate control drugs, resulting in hemodynamic consequences. Finally, they may develop poorly controlled hypertension or hemorrhagic events with anticoagulation.
Current American College of Cardiology and American Heart Association (ACC/AHA) guidelines17 outline the preventive and treatment strategies of postoperative AF after cardiac surgery, including use of beta-blockers, calcium channel blockers, digoxin, sotalol, amiodarone, cardioversion, and pacing, but these management strategies did not affect cost or hospital LOS. Little has been published regarding morbidity and mortality outcomes after implementation of ACC/AHA guidelines to manage PAA after noncardiac surgery. In addition, no data are available regarding the prevention or treatment of PAA in elderly adults undergoing hip fracture surgery.
This study should be interpreted in the context of certain limitations inherent to its retrospective design and methodology. The study population was predominantly Caucasian and from a single center, possibly limiting study generalizability, although other studies from the REP database14,23 support generalizability to other geographical populations. All-cause mortality was used, so study participants may have died from a cause unrelated to arrhythmia such as cancer or other undocumented systemic conditions. There is a possibility that the participants may have developed a new arrhythmia, such as AF, after hospital discharge, contributing to mortality and morbidity. The underuse of systemic anticoagulation, which is known to reduce stroke, may have contributed to higher mortality, although advanced age, risk of fall after hip fracture surgery, and safety concerns may account for underuse of systemic anticoagulation in elderly adults.24 The data did not allow postdischarge management, long-term or recurrent stroke, recurrent AF, or rehospitalization to be addressed. Despite these limitations, this investigation highlights that PAA is associated with excess long-term mortality, even after adjustment for known comorbid conditions.
CONCLUSION
In elderly adults, PAA in the first 7 days after hip fracture surgery is associated with a risk of 1-year mortality that is almost three times as great. PAA is a potentially preventable complication after surgery. Further studies are indicated to determine whether prevention and treatment of PAA are associated with lower long-term mortality in elderly adults undergoing hip fracture surgery.
Acknowledgments
The authors wish to express their gratitude to Donna K. Lawson, LPN, and Chere Dolliver for their commitment to excellence in data abstraction and study management. In addition, we are grateful for the mentorship of L. Joseph Melton III and Veronique Roger.
Financial Disclosure: AHA Grant 03–30103N-04 funded this project. In addition, this study was made possible by the Rochester Epidemiology Project (Grant RO1-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases) and Grant 1 KL2 RR024151 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Sponsor’s Role: There was no sponsor involvement in data collection, analysis, interpretation, or manuscript preparation.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov <http://nihroadmap.nih.gov/. There are no other disclosures on the part of any of the individual authors.
Conflict of Interest: The authors have no conflicts of interest to declare.
Author Contributions: Gupta, Huddleston, Steckelberg, Gullerud: study concept and design; data acquisition, analysis, and interpretation. Huddleston, Wright: conceptualization, data interpretation. Kirkland: analysis input, manuscript editing. All authors: creation and revision of manuscript.
References
- 1.Andrews TC, Reimold SC, Berlin JA, et al. Prevention of supraventricular arrhythmias after coronary artery bypass surgery. A meta-analysis of randomized control trials. Circulation. 1991;84(5 Suppl):III 236–244. [PubMed] [Google Scholar]
- 2.Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 3.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. J Am Med Assoc. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 4.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery: Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 5.Caretta Q, Mercanti CA, De Nardo D, et al. Ventricular conduction defects and atrial fibrillation after coronary artery bypass grafting. Multivariate analysis of preoperative, intraoperative and postoperative variables. Eur Heart J. 1991;12:1107–1111. doi: 10.1093/oxfordjournals.eurheartj.a059845. [DOI] [PubMed] [Google Scholar]
- 6.Dixon FE, Genton E, Vacek JL, et al. Factors predisposing to supraventricular tachyarrhythmias after coronary artery bypass grafting. Am J Cardiol. 1986;58:476–478. doi: 10.1016/0002-9149(86)90018-4. [DOI] [PubMed] [Google Scholar]
- 7.Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821–825. [PubMed] [Google Scholar]
- 8.Leitch JW, Thomson D, Baird DK, et al. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100:338–342. [PubMed] [Google Scholar]
- 9.Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998;114:462–468. doi: 10.1378/chest.114.2.462. [DOI] [PubMed] [Google Scholar]
- 10.Kahn RL, Hargett MJ, Urquhart B, et al. Supraventricular tachyarrhythmias during total joint arthroplasty: Incidence and risk. Clin Orthop Relat Res. 1993:265–269. [PubMed] [Google Scholar]
- 11.Krowka MJ, Pairolero PC, Trastek VF, et al. Cardiac dysrhythmia following pneumonectomy: Clinical correlates and prognostic significance. Chest. 1987;91:490–495. doi: 10.1378/chest.91.4.490. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ., III Hip fractures: A worldwide problem today and tomorrow. Bone. 1993;14(Suppl 1):S1–S8. doi: 10.1016/8756-3282(93)90341-7. [DOI] [PubMed] [Google Scholar]
- 13.Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 15.Mullen JO, Mullen NL. Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992:214–222. [PubMed] [Google Scholar]
- 16.Foss NB, Palm H, Kehlet H. In-hospital hip fractures: Prevalence, risk factors and outcome. Age Ageing. 2005;34:642–645. doi: 10.1093/ageing/afi198. [DOI] [PubMed] [Google Scholar]
- 17.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: Predictors, outcomes, and resource utilization. J Am Med Assoc. 1996;276:300–306. [PubMed] [Google Scholar]
- 19.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circ Arrhythm Electrophysiol. 2011;4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christians KK, Wu B, Quebbeman EJ, et al. Postoperative atrial fibrillation in noncardiothoracic surgical patients. Am J Surg. 2001;182:713–715. doi: 10.1016/s0002-9610(01)00799-1. [DOI] [PubMed] [Google Scholar]
- 21.Amar D, Roistacher N, Burt M, et al. Clinical and echocardiographic correlates of symptomatic tachydysrhythmias after noncardiac thoracic surgery. Chest. 1995;108:349–354. doi: 10.1378/chest.108.2.349. [DOI] [PubMed] [Google Scholar]
- 22.Von Knorring J, Lepantalo M, Lindgren L, et al. Cardiac arrhythmias and myocardial ischemia after thoracotomy for lung cancer. Ann Thorac Surg. 1992;53:642–647. doi: 10.1016/0003-4975(92)90325-x. [DOI] [PubMed] [Google Scholar]
- 23.Melton LJ, Iii, Therneau TM, Larson DR. Long-term trends in hip fracture prevalence: The influence of hip fracture incidence and survival. Osteoporos Int. 1998;8:68–74. doi: 10.1007/s001980050050. [DOI] [PubMed] [Google Scholar]
- 24.Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]


