Abstract
The tail suspension test (TST) has been widely used as a screening assay for antidepressant drugs. However, the neural substrates underlying the stress response and antidepressant-like effect during the TST remain largely unknown despite the prevalence of this test. In the present study, we used immunohistochemistry to examine alterations in c-Fos expression as a measure of neuronal activity in the mouse brain after acute administration of the antidepressant drugs nortriptyline or escitalopram (or saline as a control) with or without a subsequent TST session. We found that without the TST session, nortriptyline administration enhanced the density of c-Fos-immunoreactive cells in regions of the central extended amygdala, paraventricular hypothalamic nucleus, and relevant regions of the brain stem, whereas escitalopram did not change c-Fos expression in any region. Following the TST in the absence of antidepressant drugs, we observed a significant increase in c-Fos-positive cell density in a number of brain regions within the limbic telencephalon, hypothalamus, and brain stem. We detected a statistically significant interaction using an analysis of variance between the main effects of the drug and stress response in four regions: the infralimbic cortex, lateral septal nucleus (intermediate part), ventrolateral preoptic nucleus, and solitary nucleus. Following the TST, escitalopram but not nortriptyline increased c-Fos-positive cell density in the infralimbic cortex and ventrolateral preoptic nucleus, whereas nortriptyline but not escitalopram increased c-Fos expression in the solitary nucleus. Both antidepressants significantly increased c-Fos expression in the lateral septal nucleus (intermediate part). The present results indicate that neuronal activity increases in septo-hypothalamic regions and related structures, especially the lateral septal nucleus, following administration of drugs producing an antidepressant-like effect in mice subjected to the TST.
Keywords: Neuroscience
1. Introduction
Major depression is a common psychiatric disorder comprising emotional, homeostatic, and cognitive dysfunction. Owing to its immense impact on the quality of life of individuals and the economic burden upon society, major depression is a global health issue [1]. Most antidepressant drugs that are currently available reportedly act through modulation of monoaminergic transmission. However, their efficacies are considerably limited [2], and their precise mechanisms of action remain elusive.
The tail suspension test (TST) is an experimental procedure used in preclinical studies for screening potential antidepressant drugs [3]. It is a reliable behavioral test that assesses the antidepressant-like effect of a given agent as a decrease in the immobility time of a mouse suspended by its tail. However, the neuronal correlates of behavior during the TST have been rarely investigated, despite its widespread use. Characterization of the neuronal population responsible for the antidepressant-like effect in the TST may offer important insights into the therapeutic mechanisms of antidepressants used to treat major depression.
Recently, we explored the neuronal correlates of behavior during the forced swim test in mice by analyzing alternations in c-Fos expression using immunohistochemical techniques [4]. Our results suggested the involvement of the lateral septum and the hypothalamus in the active coping behavior observed during the test. In the present study, we used a similar method to investigate the relationship between behavior during the TST and the associated pattern of neuronal activation. We used the antidepressants nortriptyline, which preferentially inhibits reuptake of noradrenaline, and escitalopram, a selective serotonin reuptake inhibitor. We used BALB/c mice for the study, as they have been increasingly selected for research as a stress-vulnerable inbred mouse strain [5]. To the best of our knowledge, this is the first study to systematically investigate the pattern of neuronal activation in mice during the TST.
2. Materials and methods
2.1. Animals
Male BALB/c (BALB/cByJJcl) mice 7–9 weeks old were purchased from Kyudo Co. (Saga, Japan), housed in groups of 2–4 per cage, and kept under standard controlled laboratory conditions (12-h light/dark cycle with lights on at 8:00 AM; 25 ± 2 °C; 50% humidity; pelleted food and water ad libitum). To facilitate their adaptation to a novel surrounding, the mice were transported to the testing room 18–24 h prior to the experiment. All experimental protocols were approved by the ethics committee of Kyushu University (permit number, A25-087-02). All procedures were performed in accordance with the ethical standards of the institution and the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
2.2. Drug administration
Nortriptyline hydrochloride (Sigma-Aldrich, St. Louis, MO; 30 mg/kg) and escitalopram oxalate (Sigma-Aldrich; 5 mg/kg) were dissolved in 0.9% saline on the test day, which took place 7–10 days after the arrival of the mice in the laboratory. The dose was determined based on previous reports [3, 6]. 10 mL/kg of nortriptyline or escitalopram solution was administered intraperitoneally 30 min prior to testing. The control animals received injections of 0.9% saline (10 mL/kg). After the drug treatment, the animals were held in their cages and then either remained undisturbed or underwent the TST. Thus, the following six experimental groups were generated: saline without the TST (saline-TST[−]); nortriptyline-TST(−); escitalopram-TST(−); saline-TST(+); nortriptyline-TST(+); and escitalopram-TST(+). Five animals were included in each TST(−) group, and 18–19 mice were included in each TST(+) group. The sample size of the TST(−) groups was comparable to that in previous Fos studies on antidepressants [7, 8], and the sample size of the TST(+) groups was comparable to that in previous TST studies [9].
2.3. The tail suspension test
The TST sessions were conducted between 10:00 and 13:00. We used the TST method modified by Tomida et al. [10]. Thirty minutes after injection, the mice were suspended by their tails using an elastic band attached to the tail by adhesive tape, and the elastic band was hooked on a horizontal rod. Each mouse was visually isolated from the surrounding room by dark-colored cardboard, which was positioned at least 150 mm away from the mouse. The distance between the head of the mouse and the floor was approximately 200 mm. Based on the results of a previous forced swim test study [11], we chose a test duration of 10 min for more consistent c-Fos induction, instead of the 6 min used in a standard protocol. Behavior was recorded with a digital camera, and the immobility time during the 10-min test was scored by trained observers blinded to the treatment. A mouse was judged to be immobile only when it hung passively and was completely motionless.
2.4. Fos immunohistochemistry
Two hours after the onset of the TST session (150 min after drug administration), the mice were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PB (pH 7.4). The brains were left in situ for 1 h at room temperature and then removed from the skull. After a 4 h postfixation period in 4% paraformaldehyde, the brains were saturated with 30% sucrose in PBS for 3 days. The entire brain, except the olfactory bulbs, was sectioned into 40 μm coronal slices with a cryostat (Leica CM3050 S; Leica Microsystems, Germany). Selected sections were rinsed in PBS and then preincubated for 30 min with 3% H2O2 in PBS to deplete endogenous peroxidase activity. After preincubation, the sections were rinsed with PBS and then incubated for 1 h in 1% bovine serum albumin in PBS containing 0.3% Triton X–100 and 0.1% sodium azide before being incubated in the c-Fos antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:50,000, rabbit polyclonal) for 3 to 4 days at room temperature. The sections were then rinsed in PBS and were incubated in biotinylated anti-rabbit antibody (1:200, goat anti-rabbit IgG, Vector Laboratories, Inc., Burlingame, CA) for 90 min. The sections were rinsed again and then were processed according to the ABC method using a Vector kit (Vectastain Elite ABC Standard Kit, Vector Laboratories; diluted 1:200 in PBS with 0.1% Triton X–100). After being rinsed, the sections were incubated with diaminobenzidine (DAB, Sigma Aldrich; 500 μg/mL) and nickel ammonium sulfate (Wako, Japan; 25 mg/ml, dissolved in 0.2 M acetate buffer) producing a black reaction product in cell nuclei. We terminated the reaction by rinsing the tissue in PBS. The sections were mounted on gelatin-coated slides, dehydrated in ascending concentrations of ethanol, counterstained with neutral red (Wako), and coverslipped with DPX.
2.5. Fos-immunoreactive cell counting
To select the brain regions for semi-quantitative analysis of c-Fos-immunoreactive cells, we processed and microscopically examined the sections at 120 μm intervals throughout the brain in two mice from each of the six treatment groups. Additional sections involving the preoptic areas were further examined for anatomical complexity. In addition to the examination of these sections, we also considered the findings of preceding studies on forced swimming [4, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21] and other emotional behaviors [22] when selecting brain regions for semi-quantification. We then standardized the anatomical level (i.e., anterior-posterior coordinates) at which the sections containing the regions of interest were selected using a brain atlas [23], and, following this, collected the sections from the remaining animals accordingly. One exception was the intermediate hypothalamic area, which is located ventrolateral to the anterior hypothalamic area, according to Roeling et al. [24]. Table 1 shows the 28 brain areas that were selected for analysis and the corresponding anterior-posterior coordinates. Brightfield images of the regions of interest were photographed with a digital camera (DP72; Olympus, Japan) attached to a light microscope (BX51; Olympus; 20 × objective). All photomicrographs were exported in TIFF format, processed in ImageJ (http://rsbweb.nih.gov/ij/, ver.1.44, National Institutes of Health, USA), converted to grayscale, and adjusted to have an appropriate contrast. We then counted the number of immunoreactive nuclei above a specific threshold. After comparing the results of the ImageJ counts with those from the microscopic examination, we established a threshold that we used uniformly for each region. In most regions, we used a square frame (250 × 250 μm2) to quantify the number of c-Fos-immunoreactive cells. A rectangular frame (125 × 250 μm2) was used in the bed nucleus of the stria terminalis (lateral dorsal part), dentate gyrus (granular layer), hippocampal CA1 subfield (pyramidal layer), and the locus coeruleus. In the raphe pallidus nucleus, a smaller square frame (125 × 125 μm2) was used. All areas were analyzed bilaterally in one section, except the midline structures (medial septal nucleus, dorsal raphe nucleus, raphe interpositus nucleus, and raphe pallidus nucleus). The resulting values were averaged for each individual, and these averages were compared across groups.
Table 1.
The 28 brain areas selected for analysis, and the densities of c-Fos-immunoreactive cells in the TST(−) and TST(+) conditions for the saline-, nortriptyline-, and escitalopram-treated groups. The distance from bregma for each region is presented based on the Franklin and Paxinos mouse brain atlas [20], except for the intermediate hypothalamic area, which was located based on Roeling et al. [21]. The letters D, S, or I following the name of the brain region indicate a main effect of the drug or of stress (TST), or of their interaction, respectively, as determined by ANOVA (p < 0.05; for bold letters, p < 0.01). The numerical values are the numbers of c-Fos-immunoreactive cells/0.01 mm2. *p < 0.05, **p < 0.01 versus saline control animals in the same condition; †p < 0.05 versus the corresponding TST(−) group. The data are presented as the mean ± S.E.M.; n = 5–6 per group for the TST(−) groups, and n = 18–19 per group for the TST(+) groups. a, area; n, nucleus.
| Brain regions (distance from Bregma, mm) | TST(−) | TST(+) | ||||
|---|---|---|---|---|---|---|
| saline | nortriptyline | escitalopram | saline | nortriptyline | escitalopram | |
| Infralimbic cortex (+1.70) D, S, I | 0.49 ± 0.1 | 0.76 ± 0.2 | 0.54 ± 0.1 | 2.15 ± 0.5† | 2.38 ± 0.4† | 4.17 ± 0.7†* |
| Accumbens n., core (+1.10) D | 0.13 ± 0.1 | 0.08 ± 0.1 | 0.41 ± 0.1 | 0.48 ± 0.2 | 0.08 ± 0.0 | 0.44 ± 0.1 |
| Accumbens n., shell (+1.10) | 1.23 ± 0.5 | 0.63 ± 0.2 | 0.55 ± 0.2 | 1.65 ± 0.6 | 1.03 ± 0.3 | 1.70 ± 0.7 |
| Lateral septal n., dorsal (+1.10) D, S | 0.80 ± 0.3 | 0.78 ± 0.3 | 1.18 ± 0.4 | 1.73 ± 0.3 | 5.10 ± 0.9†** | 3.93 ± 0.7† |
| Lateral septal n., intermediate (+1.10) D, S, I | 0.53 ± 0.2 | 0.33 ± 0.1 | 0.95 ± 0.2 | 0.48 ± 0.1 | 2.65 ± 0.9†** | 2.23 ± 0.4†** |
| Lateral septal n, ventral (+1.10) S | 0.58 ± 0.2 | 2.43 ± 0.6 | 2.03 ± 0.8 | 5.74 ± 1.2† | 9.61 ± 1.7† | 7.64 ± 1.2† |
| Medial septal n. (+1.10) S | 0.26 ± 0.2 | 0.00 ± 0.0 | 0.02 ± 0.0 | 0.74 ± 0.3 | 0.36 ± 0.1 | 0.44 ± 0.1† |
| Bed n. of stria terminalis, lateral, dorsal (+0.14) D | 0.90 ± 0.3 | 14.21 ± 2.4** | 1.46 ± 0.4 | 1.30 ± 0.3 | 13.04 ± 1.5** | 1.44 ± 0.4 |
| Bed n. of stria terminalis, lateral, vental (+0.14) D, S | 0.50 ± 0.1 | 4.60 ± 0.9** | 0.54 ± 0.2 | 2.96 ± 0.4† | 6.98 ± 0.7** | 2.22 ± 0.3† |
| Lateral preoptic a. (+0.02) S | 1.30 ± 0.4 | 2.37 ± 0.5 | 0.96 ± 0.2 | 3.23 ± 0.5† | 3.92 ± 0.3† | 4.45 ± 0.6† |
| Ventrolateral preoptic n. (+0.02) D, S, I | 0.96 ± 0.2 | 0.96 ± 0.2 | 1.36 ± 0.3 | 3.64 ± 0.5† | 2.85 ± 0.4† | 5.80 ± 0.5†** |
| Paraventricular hypothalamic n. (-0.94) D, S | 1.05 ± 0.3 | 8.33 ± 2.2** | 1.36 ± 0.4 | 11.23 ± 1.8† | 18.76 ± 2.8†* | 12.36 ± 2.0† |
| Intermediate hypothalamic a. (-0.94) S | 2.94 ± 0.9 | 2.78 ± 0.3 | 2.95 ± 0.6 | 6.42 ± 0.4† | 5.79 ± 0.2† | 7.35 ± 0.6† |
| Dentate gyrus (-1.58) S | 0.72 ± 0.2 | 0.41 ± 0.1 | 0.64 ± 0.1 | 1.24 ± 0.2 | 1.05 ± 0.2† | 0.73 ± 0.1 |
| Field CA1 of hippocampus (-1.58) D | 0.31 ± 0.2 | 0.41 ± 0.1 | 0.05 ± 0.0 | 0.43 ± 0.1 | 0.55 ± 0.1 | 0.25 ± 0.1 |
| Arcuate hypothalamic n. (-1.58) D, S | 1.15 ± 0.4 | 1.78 ± 0.7 | 1.04 ± 0.4 | 2.95 ± 0.4† | 5.00 ± 0.6†* | 2.68 ± 0.5† |
| Central amygdaloid n. (-1.58) D | 1.00 ± 0.4 | 10.75 ± 1.2** | 2.13 ± 0.7 | 1.01 ± 0.2 | 13.71 ± 2.0** | 1.72 ± 0.5 |
| Medial amygdaloid n. (-1.58) D, S | 1.06 ± 0.2 | 1.34 ± 0.3 | 0.75 ± 0.4 | 2.37 ± 0.4† | 4.43 ± 0.7†* | 2.46 ± 0.5† |
| Lateral habenular n. (-1.58) D, S | 0.85 ± 0.1 | 0.70 ± 0.1 | 1.89 ± 0.5 | 2.08 ± 0.5 | 3.06 ± 0.8 | 4.19 ± 0.7† |
| Dorsomedial hypothalamic n. (-1.82) S | 3.09 ± 1.0 | 3.34 ± 0.7 | 1.57 ± 0.6 | 4.50 ± 1.5 | 7.26 ± 0.9† | 6.13 ± 1.0† |
| Dorsal raphe n. (-4.36) | 1.24 ± 0.3 | 1.34 ± 0.2 | 0.96 ± 0.5 | 1.96 ± 0.4 | 1.49 ± 0.2 | 1.83 ± 0.2 |
| Lateral periaqueductal gray (-4.36) S | 2.43 ± 0.7 | 2.08 ± 0.1 | 1.86 ± 0.4 | 4.36 ± 0.4† | 3.50 ± 0.4 | 4.41 ± 0.5† |
| Ventrolateral periaqueductal gray (-4.36) S | 1.76 ± 0.5 | 2.35 ± 0.6 | 1.41 ± 0.3 | 9.48 ± 1.2† | 10.43 ± 1.4† | 9.87 ± 1.1† |
| Locus coeruleus (-5.34) S | 2.59 ± 0.4 | 4.00 ± 1.2 | 1.76 ± 0.3 | 8.04 ± 1.2† | 7.59 ± 0.8† | 9.13 ± 1.1† |
| Lateral parabrachial n. (-5.34) D | 3.40 ± 1.6 | 11.78 ± 2.0** | 2.01 ± 0.6 | 1.72 ± 0.7 | 15.07 ± 2.0** | 2.90 ± 0.9 |
| Raphe interpositus nucleus (-5.80) D, S | 0.19 ± 0.1 | 0.59 ± 0.2 | 0.64 ± 0.3 | 0.52 ± 0.2 | 1.28 ± 0.3 | 1.51 ± 0.3* |
| Raphe pallidus n. (-5.80) S | 1.28 ± 0.6 | 1.76 ± 0.9 | 0.72 ± 0.1 | 3.69 ± 0.5† | 2.16 ± 0.3 | 2.44 ± 0.6† |
| Solitary n. (-7.08) D, I | 1.77 ± 0.8 | 5.75 ± 1.2** | 0.96 ± 0.3 | 0.97 ± 0.2 | 10.49 ± 1.3** | 1.48 ± 0.4 |
2.6. Statistical analysis
All statistical analyses were performed with JMP version 11 software (SAS Institute Inc.). The mean differences in the immobility time among the three TST(+) groups were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett’s test. We analyzed the differences in the density of the c-Fos-immunoreactive cells in each selected brain region among the six treatment groups using a two-way ANOVA. When a main effect of drug (antidepressant) or a significant interaction was found, we further analyzed the differences among the TST(−) groups and among the TST(+) groups for that relative condition using Dunnett’s test. When a main effect of stress (TST) was found, we analyzed the differences between the corresponding TST(−) and TST(+) groups using an unpaired Student’s t-test (two-tailed). We calculated Spearman’s rank correlation coefficient for the immobility time and the densities of the c-Fos-immunoreactive cells in each brain region, for the combined population of the three TST(+) groups. The significance level was set at p < 0.05, and all values are expressed as the mean ± S.E.M.
3. Results
3.1. Antidepressant-like effects in the tail suspension test
The acute administration of nortriptyline or escitalopram to mice significantly reduced their immobility time (approximately 40%) in the TST compared with mice that received saline (182.9 ± 19.3 s in saline-TST(+); 112.6 ± 19.3 s in nortriptyline-TST(+); 111.5 ± 19.8 s in escitalopram-TST(+); F = 4.47, p < 0.05, Dunnett’s test, p < 0.05 for each antidepressant group versus the saline group).
3.2. Effects of antidepressants and the TST on c-Fos expression
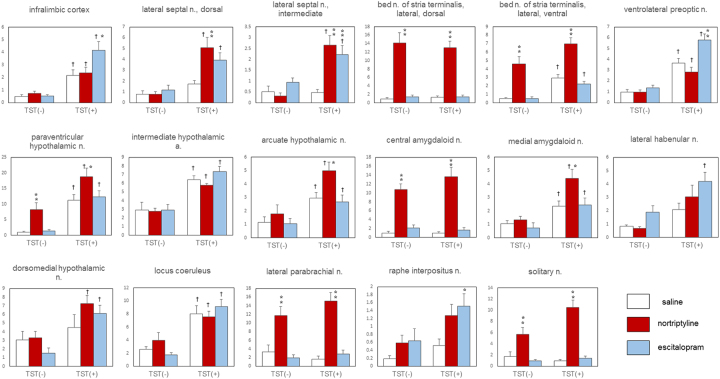
The mean (± S.E.M.) numbers of c-Fos-immunoreactive cells in 28 brain regions are shown in Table 1 (see also Fig. 1). The ANOVA revealed a main effect of drug (antidepressant) in 15 regions, and a main effect of stress (TST) in 20 regions. We found a significant interaction between drug and stress in four brain regions: the infralimbic cortex, lateral septal nucleus (intermediate part), ventrolateral preoptic nucleus, and solitary nucleus.
Fig. 1.
The effects of nortriptyline, escitalopram, and the TST on c-Fos expression in the mouse brain. Only representative regions are depicted. The data are presented as the mean ± S.E.M.; n = 5 for each TST(−) group and n = 18–19 for each TST(+) group. *p < 0.05, **p < 0.01 versus the saline control group; †p < 0.05 versus the corresponding basal group.
In the TST(−) groups, nortriptyline treatment significantly increased the number of c-Fos-immunoreactive cells in six brain regions: the bed nucleus of the stria terminalis (lateral division, dorsal part and lateral division, ventral part), the paraventricular hypothalamic nucleus, central amygdaloid nucleus, lateral parabrachial nucleus, and solitary nucleus. Escitalopram treatment alone did not induce a significant change in the density of c-Fos-positive cells in any region examined.
Among the 20 regions in which we found a main effect of stress, we detected a significant increase in the density of c-Fos-immunoreactive cells in 13 regions in the saline-TST(+) groups compared with the saline-TST(−) groups. Those regions included several nuclei in the preoptic, hypothalamic, and brainstem areas. The density of c-Fos-positive cells was significantly higher in the TST(+) group than that in the TST(−) group only in the antidepressant-pretreated animals in the following four regions: the lateral septal nucleus (dorsal part and intermediate part), lateral habenular nucleus, and dorsomedial hypothalamic nucleus.
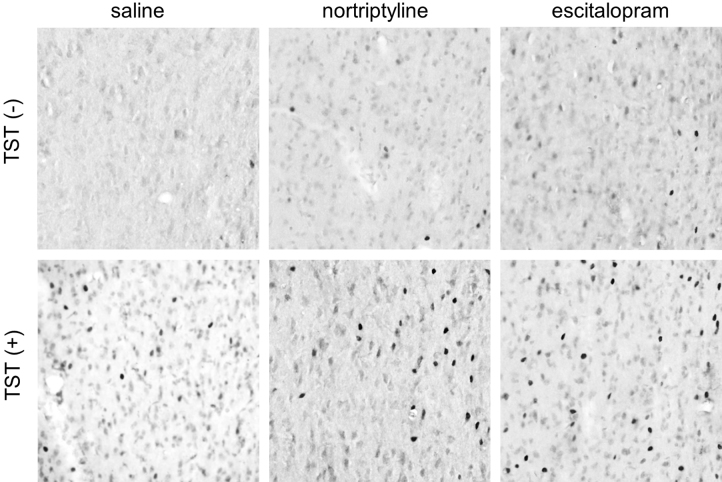
In the following seven regions, antidepressant administration induced a significant change in c-Fos expression in the TST(+) groups only: the infralimbic cortex, lateral septal nucleus (dorsal and intermediate parts), ventrolateral preoptic nucleus, arcuate hypothalamic nucleus, medial amygdaloid nucleus, and raphe interpositus nucleus. Nortriptyline, but not escitalopram, significantly increased the number of cells expressing c-Fos immunoreactivity in the lateral septal nucleus (dorsal part), arcuate hypothalamic nucleus, and medial amygdaloid nucleus. By contrast, only escitalopram elicited a change in the infralimbic cortex, ventrolateral preoptic nucleus, and raphe interpositus nucleus. In the intermediate part of the lateral septal nucleus, both antidepressants significantly increased the number of c-Fos-immunoreactive cells (Fig. 2).
Fig. 2.
Representative photomicrographs of c-Fos immunoreactivity in the intermediate part of the lateral septal nucleus obtained from the six experimental groups. The left side of the image is lateral, and the top is dorsal. Each photomicrograph covers an area of 250 × 250 μm2.
3.3. Correlation between immobility time and c-Fos expression
We analyzed correlations between the density of c-Fos immunoreactive cells and immobility time in the TST for the 28 brain regions. We found a significant negative correlation between immobility time and the density of c-Fos-immunoreactive cells in the infralimbic cortex (r = −0.46, p < 0.05), dorsal part of the lateral septal nucleus (r = −0.38, p < 0.05), intermediate part of the lateral septal nucleus (r = −0.38, p < 0.05), and lateral preoptic area (r = −0.37, p < 0.05).
4. Discussion
In our previous study, we examined the pattern of c-Fos expression in the brains of mice following their performance in a forced swim test [4]. We found a significant c-Fos response to swim stress in several hypothalamic and brainstem regions. In addition, the acute administration of the antidepressant imipramine increased the number of brain regions, scattered within the lateral septum and hypothalamus, involved in the response to forced swim stress. These findings are in sharp contrast to those obtained for rats in the forced swim test, in which repeated administration of an antidepressant attenuates the c-Fos response to swim stress in the limbic and hypothalamic regions [11, 12, 13, 14]. In the present study, we examined the pattern of c-Fos expression following acute administration of antidepressants to mice subsequently subjected to the TST. We found a synergistic effect of drug and stress within areas of the mouse brain comparable to the limbic and hypothalamic regions. In many of the regions that we investigated, antidepressant pretreatment significantly enhanced the c-Fos response to TST. Thus, although our results were similar to those obtained in our previous study examining c-Fos expression in the mouse brain following the forced swim test [4], the current data more clearly reflect the interaction between drug and stress. The forced swim test (for rats and mice) and the TST (for mice) are seemingly similar behavioral tests that are both commonly used to examine the antidepressant-like effects of drugs [25]. However, our results suggest that the effects of antidepressants on the patterns of brain activation during these tests are not uniform. As discussed in our previous report [4], the reduced c-Fos response to stress after repeated antidepressant treatment (in the forced swim test for rats) and enhanced response after acute treatment (in the forced swim test and TST for mice) might reflect different aspects of the multifaceted action of antidepressant drugs.
Administration of nortriptyline and escitalopram, without TST, had different effects on c-Fos expression in the brain. The effect of nortriptyline, which induced a significant c-Fos response within the central extended amygdala, hypothalamus, and brainstem, was in good accordance with previous studies [26, 27]. In contrast, escitalopram induced no significant changes in c-Fos density in all regions examined. This is unexpected, as many studies have reported that the effect of serotonin selective reuptake inhibitors on c-Fos expression within the central extended amygdala and other regions is comparable to that of tricyclic antidepressants (e.g. citalopram and escitalopram, [8, 28]; fluoxetine, [26, 29, 30, 31, 32]; fluvoxamine, [7]). While the small sample size prevents further interpretation regarding this discrepancy, the effect of animal strain may be a worthwhile topic for future research.
In our previous study [4], we reported that the forced swim test, without antidepressant treatment, enhanced c-Fos response in 5 of 40 brain regions examined. In the present study, the TST, without antidepressant treatment, induced a significant increase in c-Fos expression in 13 of 28 brain regions examined. While three regions (the ventrolateral preoptic nucleus, ventrolateral periaqueductal gray, and locus coeruleus) were activated by both tests, two regions (the dorsomedial hypothalamic nucleus and lateral parabrachial nucleus) were activated only by the forced swim test, and nine regions (including the infralimbic cortex) were activated only by the TST (note that we did not examine the lateral-ventral part of the bed nucleus of the stria terminalis in our previous study). The latter nine regions include the four regions that were significantly activated after the forced swim test only in animals that had received antidepressant treatment (the ventral part of the lateral septal nucleus, paraventricular hypothalamic nucleus, intermediate hypothalamic area, and arcuate hypothalamic nucleus). In our experimental conditions, the TST appeared to activate more widespread areas compared with the forced swim test, although a direct comparison is difficult due to differences in sample size.
In the intermediate part of the lateral septal nucleus, we found a significant interaction between drug and stress, and both antidepressants increased the density of c-Fos-immunoreactive cells in this region. A similar trend was found in the dorsal part of the lateral septal nucleus, although this interaction was not statistically significant (p = 0.07). In both the intermediate and dorsal parts of the lateral septal nucleus, the density of c-Fos-positive cells was significantly and negatively correlated with the duration of immobility. The lateral septal nucleus is located in a crucial position in the limbic telencephalon and hypothalamus network and presumably plays a regulatory role in emotional behaviors [33]. The results of a number of studies have implicated this structure as being important in the depression-like behavior observed in animal models. Rats with learned helplessness show selective reduction of stress-induced c-Fos expression in the lateral septum [34]. Performance of the forced swim stress test reportedly decreases the spontaneous firing rate of lateral septal neurons in rats [35]. Moreover, lesioning the lateral septum extends the immobility time [36, 37] and increases the response of the hypothalamic–pituitary–adrenal axis in the forced swim test in rats [37]. In contrast, acute [38] and chronic [39] administration of antidepressants increases the spontaneous firing rate of lateral septal neurons in rats. Furthermore, intraseptal injection of a serotonin 1A agonist reduces immobility time in the forced swim test in rats [37]. The present results, which suggest the involvement of the lateral septum in the antidepressant-like effect in the TST, are in good agreement with these findings. This region might be a common site of action of nortriptyline and escitalopram.
The infralimbic cortex is involved in the modulation of visceral functions related to emotional and other factors and has been regarded as an important node in the neural circuitry underlying depression [40]. Hamani et al. [41] reported that electrical stimulation of the medial prefrontal cortex (including its subdivision the infralimbic cortex) in rats induces an antidepressant-like effect in the forced swim test that is completely abolished by depletion of serotonin but not noradrenaline. In the present study, our ANOVA revealed a significant interaction between drug and stress and a significant negative correlation between the density of c-Fos-positive cells and the duration of immobility in the infralimbic cortex. In addition, the administration of escitalopram, but not nortriptyline, enhanced c-Fos expression in this region in the TST(+) group. Our results are consistent with those reported by Hamani et al. [41], supporting the notion that the infralimbic cortex is a site of action through which escitalopram, but not nortriptyline, exerts an antidepressant-like effect during the TST.
Declarations
Author contribution statement
Kentaro Hiraoka: Performed the experiments; Analyzed and interpreted the data.
Keisuke Motomura: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Satoru Yanagida, Ayako Ohashi: Performed the experiments.
Nozomi Ishisaka-Furuno: Analyzed and interpreted the data.
Shigenobu Kanba: Conceived and designed the experiments.
Funding statement
This work was supported by the Japan Society for the Promotion of Science, Grant-in Aid for Scientific Research (B), 25293252, 2013 (SK).
Competing interest statement
The authors declare the following conflicts of interest: Keisuke Motomura has received honoraria from Pfizer. Shigenobu Kanba has received grants for research from Pfizer, Ono, GlaxoSmithKline, Astellas, Janssen, Yoshitomiyakuhin, Eli Lilly Japan, Otsuka, Mochida, Daiichi-Sankyo, Dainippon Sumitomo, Meiji Seika Pharma, Shionogi, Eisai, and MSD, as well as honoraria or consultation fees from Pfizer, Janssen, GlaxoSmithKline, Eli Lilly Japan, Eisai, Meiji Seika Pharma, Taisho Toyama, Astellas, Ono, Mochida, Otsuka, Abott Japan, Shionogi, Dainippon Sumitomo, Nippon-Chemifa, Yoshitomiyakuhin, and MSD. The other authors declare no conflicts of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Ms. Yuka Matsushita for her technical and secretarial assistance.
References
- 1.Whiteford H.A., Degenhardt L., Rehm J., Baxter A.J., Ferrari A.J., Erskine H.E., Charlson F.J., Norman R.E., Flaxman A.D., Johns N., Burnstein R., Murray C.J., Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Rush A.J., Warden D., Wisniewski S.R., Fava M., Trivedi M.H., Gaynes B.N., Nierenberg A.A. STAR*D, Revising conventional wisdom. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- 3.Cryan J.F., Mombereau C., Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Yanagida S., Motomura K., Ohashi A., Hiraoka K., Miura T., Kanba S. Effect of acute imipramine administration on the pattern of forced swim-induced c-Fos expression in the mouse brain. Neuroscience Lett. 2016;629:119–124. doi: 10.1016/j.neulet.2016.06.059. [DOI] [PubMed] [Google Scholar]
- 5.Uchida S., Hara K., Kobayashi A., Otsuki K., Yamagata H., Hobara T., Suzuki T., Miyata N., Watanabe Y. Epigenetic status of gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Młniec K., Nowak G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. 2012;64:249–255. doi: 10.1016/s1734-1140(12)70762-4. [DOI] [PubMed] [Google Scholar]
- 7.Veening J.G., Coolen L.M., Spooren W.J.P.M., Joosten H., van Oorschot R., Mos J., Ronken E., Oliver B. Patterns of c-fos expression induced by fluvoxamine are different after acute vs. chronic oral administration. Eur. Neuropsychopharmacol. 1998;8:213–226. doi: 10.1016/s0924-977x(97)00072-2. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen C., Helboe L. Regional pattern of binding and c-Fos induction by (R)- and (S)-citalopram in rat brain. Neuroreport. 2003;14:2411–2414. doi: 10.1097/00001756-200312190-00024. [DOI] [PubMed] [Google Scholar]
- 9.Ripoll N., David D.J.P., Dally E., Hascoët M., Bourin M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav. Brain Res. 2003;143:193–200. doi: 10.1016/s0166-4328(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 10.Tomida S., Mamiya T., Sakamaki H., Miura M., Aosaki T., Masuda M., Niwa M., Kameyama T., Kobayashi J., Iwaki Y., Imai S., Ishikawa A., Abe K., Yoshimura T., Nabeshima T., Ebihara S. Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat. Gen. 2009;41:688–695. doi: 10.1038/ng.344. [DOI] [PubMed] [Google Scholar]
- 11.Muigg P., Hoelzl U., Palfrader K., Neumann I., Wigger A., Landgraf R., Singewald N. Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol. Psychiatry. 2007;61:782–796. doi: 10.1016/j.biopsych.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Duncan G.E., Johnson K.B., Breese G.R. Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake andexpression of Fos-like immunoreactivity. J. Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duncan G.E., Knapp D.J., Johnson K.B., Breese G.R. Functional classification of antidepressants based on antagonism of swim stress-induced Fos-like immunoreactivity. J. Pharmacol. Exp. Ther. 1996;277:1076–1089. [PubMed] [Google Scholar]
- 14.Silva M., Aguiar D.C., Diniz C.R.A., Guimarães F.S., Joca S.R.L. Neuronal NOS inhibitor and conventional antidepressant drugs attenuate stress-induced Fos expression in overlapping brain regions. Cell. Mol. Neurobiol. 2012;32:443–453. doi: 10.1007/s10571-011-9775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullinan W.E., Herman J.P., Battaglia D.F., Akil H., Watson S.J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 16.Cullinan W.E., Helmreich D., Watson S. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J. Comp. Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Bellchambers C.E., Chieng B., Keay K.A., Christie M.J. Swim-stress but not opioid withdrawal increases expression of c-Fos immunoreactivity in rat periaqueductal gray neurons which project to the rostral ventromedial medulla. Neuroscience. 1998;83:517–524. doi: 10.1016/s0306-4522(97)00399-0. [DOI] [PubMed] [Google Scholar]
- 18.Salchner P., Lubec G., Engelmann M., Orlando G.F., Wolf G., Sartori S.B., Hoeger H., Singewald N. Genetic functional inactivation of neuronal nitric oxide synthase affects stress-related Fos expression in specific brain regions. Cell. Mol. Life Sci. 2004;61:1498–1506. doi: 10.1007/s00018-004-4140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lino-de-Oliveira C., de Oliveira R.M.W., Pádua Carobrez A., de Lima T.C.M., Bel E.A.D., Guimarães F.S. Antidepressant treatment reduces Fos-like immunoreactivity induced by swim stress in different columns of the periaqueductal gray matter. Brain Res. Bull. 2006;70:414–421. doi: 10.1016/j.brainresbull.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Wulsin A.C., Herman J.P., Solomon M.B. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaszner B., Kormos V., Kozicz T., Hashimoto H., Reglodi D., Helyes Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expession in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience. 2012;202:283–299. doi: 10.1016/j.neuroscience.2011.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Veening J.G., Coolen L.M., de Jong T.R., Joosten H.W., de Boer S.F., Koolhaas J.M., Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur. J. Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Franklin K.B.J., Paxinos G. third ed. Elsevier Academic Press; San Diego: 2008. The Mouse Brain in Stereotaxic Coordinates, Compact. [Google Scholar]
- 24.Roeling T.A.P., Veening J.G., Kruk M.R., Peters J.P.W., Vermelis M.E.J., Nieuwenhuys R. Efferent connections of the hypothalamic "aggression area" in the rat. Neuroscience. 1994;59:1001–1024. doi: 10.1016/0306-4522(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 25.Cryan J.F., Holmes A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 26.Beck C.H.M. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J. Psychiatr. Neurosci. 1995;20:25–32. [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro M.G., de Oliveira I.R., Santana R.C., Costa D.T., Quarantini L.C., de Castro e Silva E., Fregoneze J.B. c-Fos expression identifies brain areas activated in response to nortriptyline. Mol. Psychiatry. 2007;12:613–615. doi: 10.1038/sj.mp.4001989. [DOI] [PubMed] [Google Scholar]
- 28.Morelli M., Pinna A., Ruiu S., Zompo M.D. Induction of Fos-like-immunoreactivity in the central extended amygdala by antidepressant drugs. Synapse. 1999;31:1–4. doi: 10.1002/(SICI)1098-2396(199901)31:1<1::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Lino-de-Oliveira C., Sales A.J., Bel E.A.D., Silveira M.C.L., Guimarães F.S. Effect of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res. Bull. 2001;55:747–754. doi: 10.1016/s0361-9230(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 30.Salchner P., Singewald N. Neuroanatomical substrates involved in the anxiogenic-like effect of acute fluoxetine treatment. Neuropharmacology. 2002;43:1238–1248. doi: 10.1016/s0028-3908(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 31.Fraga I.C., Fregoneze J.B., Carvalho F.L.Q., Dantas K.B., Azenvedo C.S., Pinho C.B., de Castro e Silva E. Acute fluoxetine administration differentially affects brain c-Fos expression in fasted and refed rats. Neuroscience. 2005;134:327–334. doi: 10.1016/j.neuroscience.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Miyata S., Hamamura T., Lee Y., Miki M., Habara T., Oka T., Endo S., Taoka H., Kuroda S. Contrasting Fos expression induced by acute reboxetine and fluoxetine in the rat forebrain: neuroanatomical substrates for the antidepressant effect. Psychopharmacology. 2005;177:289–295. doi: 10.1007/s00213-004-2072-7. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan T.P., Chambers R.A., Russell D.S. Regulation of affect by lateral septum: implications for neuropsychiatry. Brain. Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Steciuk M., Kram M., Kramer G.L., Petty F. Decrease in stress-induced c-Fos-like immunoreactivity in the lateral septal nucleus of learned helpless rats. Brain Res. 1999;822:256–259. doi: 10.1016/s0006-8993(99)01134-8. [DOI] [PubMed] [Google Scholar]
- 35.Contreras C.M., Chacón L., Rodríguez-Landa J.F., Bernal-Morales B., Guitiérrez-García A.G., Saavedra M. Spontaneous firing rate of lateral septal neurons decreases after forced swimming test in Wistar rat. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2004;28:343–348. doi: 10.1016/j.pnpbp.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Contreras C.M., Lara-Morales H., Molina-Hernández M., Saavedra M., Arrellín-Rosas G. An early lesion of the lateral septal nuclei produces changes in the forced swim test depending on gender. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1995;19:1277–1284. doi: 10.1016/0278-5846(95)00266-9. [DOI] [PubMed] [Google Scholar]
- 37.Singewald G.M., Rjabokon A., Singewald N., Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contreras C.M., Alcalá-Herrera V., Marván M.L. Action of antidepressants on the septal nuclei of the rat. Physiol. Behav. 1989;46:793–798. doi: 10.1016/0031-9384(89)90039-5. [DOI] [PubMed] [Google Scholar]
- 39.Contreras C.M., Marván M.L., Alcalá-Herrera V., Guzmán-Sáenz M.A. Chronic clomipramine increases firing rate in lateral septal nuclei of the rat. Physiol. Behav. 1990;48:551–554. doi: 10.1016/0031-9384(90)90298-i. [DOI] [PubMed] [Google Scholar]
- 40.Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends. Cogn. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Hamani C., Diwan M., Macedo C.E., Brandão M.L., Shumake J., Gonzalez-Lima F., Raymond R., Lozano A.M., Fletcher P.J., Nobrega J.N. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]