Abstract
Guidelines for the treatment of non-small cell lung cancer adenocarcinoma positive in epidermal growth factor mutations indicate tyrosine kinase inhibitors. There are currently three tyrosine kinase inhibitors that can be used as first line treatment: gefitinib, erlotinib and afatinib. Regarding erlotinib and afatinib dosage can be modified in the case of severe adverse effects. In the case of disease relapse investigation for T790M mutation has to be made either with re-biopsy or liquid biopsy and osimertinib has to be administered when T790M is diagnosed. Based on a case series we indicate which is the best approach for T790M mutation.
Keywords: Gefitinb, Erlotinib and afatinib, Adenocarcinoma, NSCLC
1. Introduction
In the era of pharmacogenomics targeted treatments based on the genome of the tumor provide efficient treatments with less side effects when compared to classic treatment modalities. This is the case with non-small cell lung cancer and adenocarcinoma histological subtype [1], [2], [3]. In the case of adenocarcinoma we investigate whether there is epidermal growth factor mutation (EGFR), anaplastic lymphoma kinase mutation (ALK) and currently programmed death-ligand 1 (PD-L1) overexpression [4]. There are currently three agents that can be administered as first line treatment in EGFR mutation positive patients: erlotinib, afatinib and gefitinib. These three agents are considered tyrosine kinase inhibitors (TKIs) and their most common side effects are rash, pneumonitis and diarrhea. These adverse effects are considered dose dependent and in the case of erlotinib and afatinib there can be a dose variation. Tyrosine kinase inhibitors are very efficient, however; disease relapse is observed in a different timeline of the treatment for most patients. There are several reasons why resistance is observed in these agents, several pathways have been investigated and indicated that overexpression of vascular endothelial growth factor (VEGF), insulin growth factor 1 (IGF-1), fibrotic growth factor (FGF), Platelet-derived growth factor receptor (PDGFR), tyrosine-protein kinase erbB-2 (HER), V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS), loss of Phosphatase and tensin homolog (PTEN), Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic (PIK3CA), tyrosine-protein kinase Met (c-MET) [5]. An effort has been previously made to provide an agent which downregulates overexpression of VEGFR with success [6]. The most common reason for TKI relapse is the observation of the novel mutation on T790M [7]. In this case osimertinib is administered [8]. There are two methods of investigating T790M mutation either with re-biopsy in a site of disease relapse or with liquid biopsy [9], [10]. We will present four cases and our centers' opinion on this matter.
2. Case 1
A 59 year old woman was diagnosed with NSCLC adenocarcinoma from malignant pleural effusion and which was positive to EGFR exon 19 (Fig. 1). Upon diagnosis she had with bone scan metastasis to the vertebra. She was administered erlotinib 150mg and she presented skin rash grade III which retrieved within the 20 days. After nine months of erlotinib 150mg administration with a new CT thorax lymphagiomatosis is observed within the lung parenchyma (Fig. 2). Her therapy is switched to gefitinib 250mg. There is clear efficiency of the new drug with Ro of the Thorax (observation every month) and new bone scan. After six months of gefitinib 250mg again there is disease relapse with a new CT thorax and bone scan (Fig. 3). Liquid biopsy for T790M was performed as she refused medical thoracoscopy with negative results. Therapy was switched to chemotherapy with carboplatin/pemetrexed and zoledronic acid. She continued this treatment for ten months until death occurred from brain metastasis and meningeal spread.
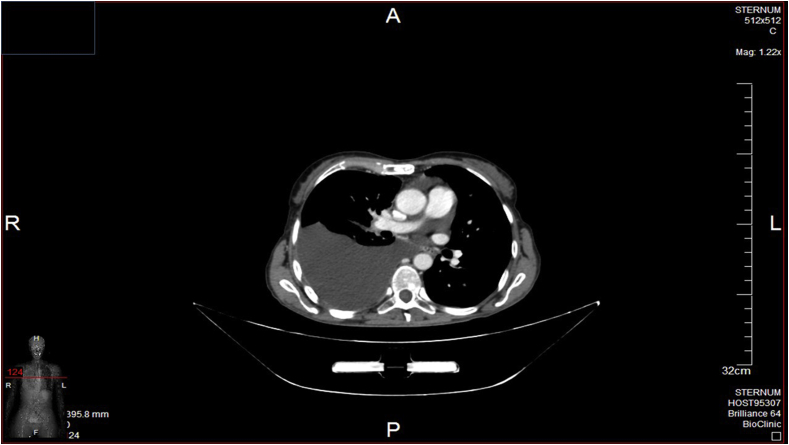
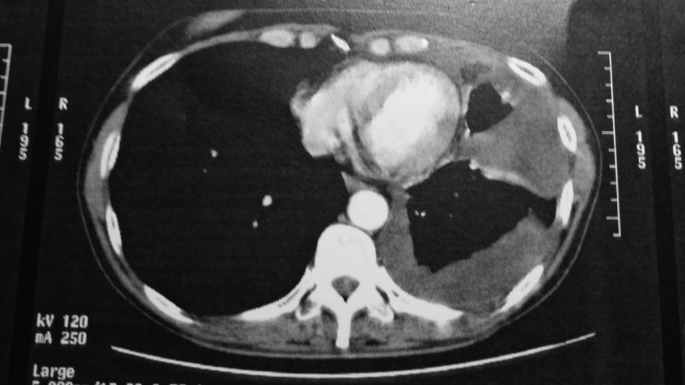
Fig. 1.
Pleural effusion.
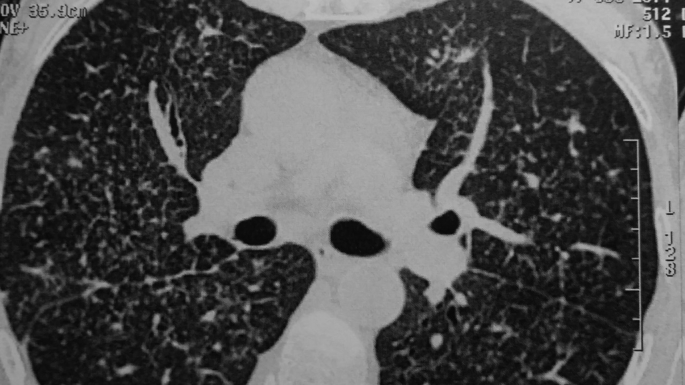
Fig. 2.
Lung lymphagiomatosis.
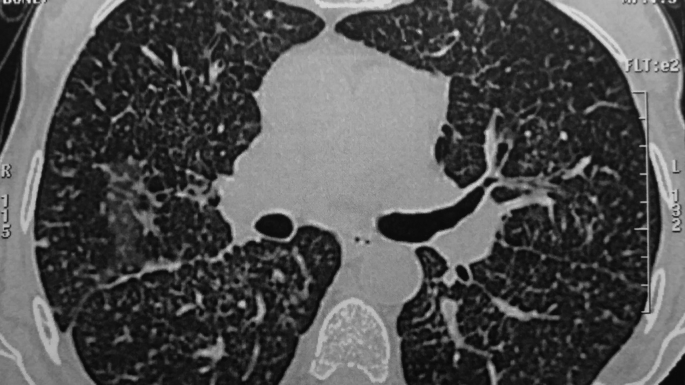
Fig. 3.
Lung lymphagiomatosis with mediastinal lymphnode enlargement.
3. Case 2
A 76 year old woman was diagnosed with NSCLC adenocarcinoma from a CT thorax guided biopsy, which was positive to EGFR exon 19 (Fig. 4). First line treatment was erlotinib 150mg with skin rash and diarrhea grade I for four months. Treatment dosage was changed to 100mg due to the diarrhea which was an issue for the patient in its everyday life. In total the patient received erlotinib for 17 months until disease progression with mediastinal lymphadenopathy. She continued for another five months erlotinib 100mg until clinical PD and she was switched to gefitinib 250mg. After seven months of gefitinib 250mg administration the primary site has enlarged, however; there is no mediastinal lymphadenopathy, and re-biopsy is performed in the primary site. T790M is positive in the new tissue specimen and the patient is receiving osimertinib 80mg to date (10 months).
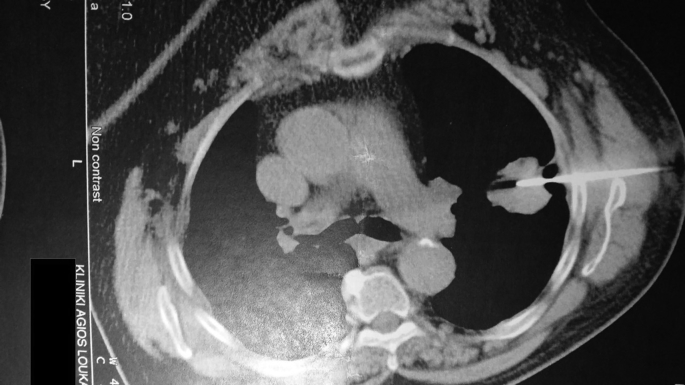
Fig. 4.
Left lung mass ct biopsy.
4. Case 3
A 56 year old woman was diagnosed from medical thoracoscopy (positive pleura infiltration) with adenocarcinoma and EGFR exon 19 (Fig. 5). She was stage IV due to bone metastasis. She was a passive smoker and her husband was diagnosed with squamous cell NSCLC Stage IIa five years before her diagnosis. She also has Crohn disease. Afatinib 40mg was initiated upon diagnosis. She had rash and diarrhea grade IV for 2 months until afatinib dosage was modified to 30mg. After one month again due to the adverse effects the dosage was modified to 20mg. After one month zoledronic acid was initiated for bone pain management. After 12 months of afatinib 20mg administration new bone metastasis occurred, however; the administration continued for another four months until mediastinal lymphadenopathy was observed along with pleural effusion (Fig. 6). Endobronchial ultrasound re-biopsy was performed and T790M was investigated with negative results (Fig. 7). Liquid biopsy was also performed however; again it was negative. Chemotherapy with carboplatin/pemetrexed was initiated while zoledronic acid continued until now.
Fig. 5.
Pleural effusion during medical thoracoscopy.
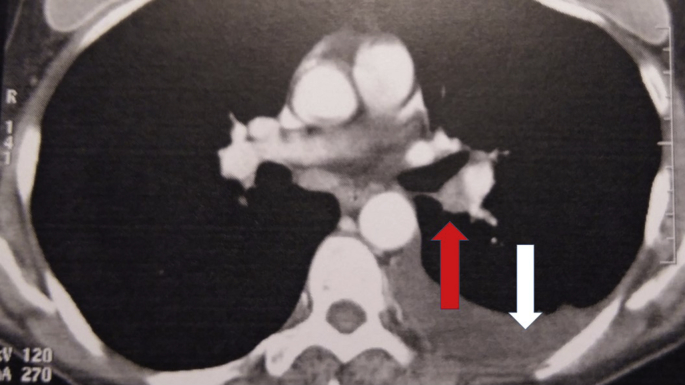
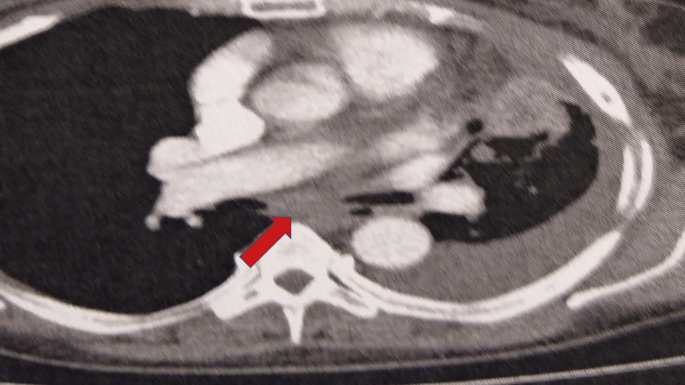
Fig. 6.
White arrow indicates pleural effusion, while red arrow lymph node.
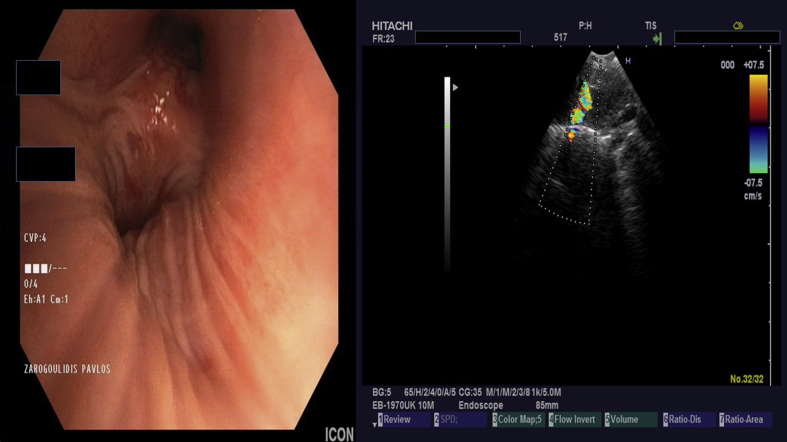
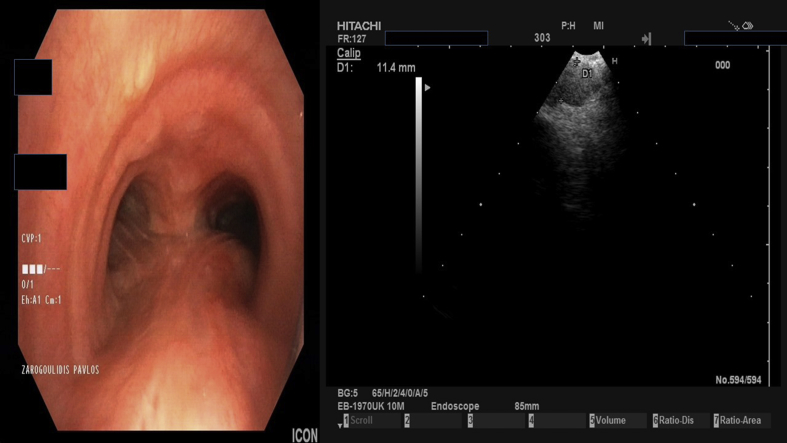
Fig. 7.
Endobronchial ultrasound performed by Dr. Paul Zarogoulidis with a Pentax EB-1970UK lymph node 11 RS biopsy.
5. Case 4
A 76 year old woman is diagnosed by CT thorax guided biopsy (left lung mass) with NSCLC adenocarcinoma EGFR exon 19 positive (Fig. 8). Upon diagnosis she has multiple bone metastasis. Afatinib 40 mg is initiated along with zoledronic acid for bone pain management. She has rash and diarrhea grade I which are well managed. After ten months the dosage is increased to 50mg and she continues this dosage for twelve months, until disease progression with mediastinal lymphadenopathy and eye metastasis (Fig. 9). Endobronchial ultrasound re-biopsy was performed and T790M was positive (Fig. 10). She receives to date osimertinib 80mg and the disease is well controlled after 10 months of osimertinib administration.
Fig. 8.
Left lung mass ct biopsy.
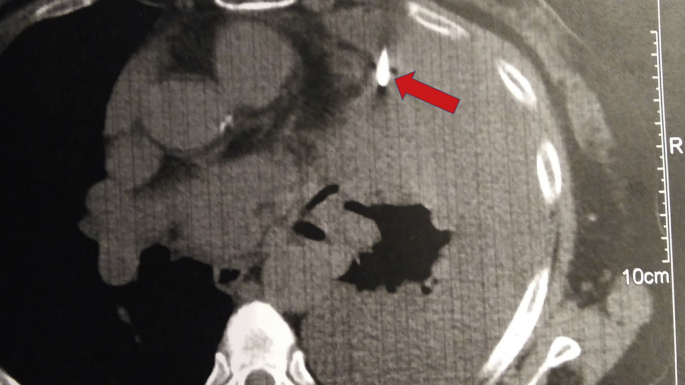
Fig. 9.
Disease relapse with lymph node number 7 enlargement (red arrow).
Fig. 10.
Endobronchial ultrasound performed by Dr. Paul Zarogoulidis with a Pentax EB-1970UK lymph node 7 biopsy.
6. Discussion
Currently there are additional therapies as second line treatment for adenocarcinoma with immunotherapies (nivolumab and pembrolizumab). In the case of pembrolizumab PD-L1 overexpression has to be at least >1%, while in the case on nivolumab this is not necessary. In the last months pembrolizumab was licensed as first line treatment for adenocarcinoma in the case of PD-L1 overexpression ≥50%. In pembrolizumab's SPC, as 1st line treatment, pembrolizumab is indicated in EGFR and ALK wild type patients pembrolizumab as monotherapy is indicated for the first-line treatment of metastatic non-small cell lung carcinoma (NSCLC) in adults whose tumors express PD-L1 with a ≥50% tumor proportion score (TPS) with no EGFR or ALK positive tumor mutations [11]. Liquid biopsy is excellent in the case where the patient does not agree to an interventional method for T790M investigation, however; the sensitivity of the method is lower than the tissue specimen. However; an issue remains whether the site of the re-biopsy contains T790M mutation and additional liquid biopsy has to be performed. There are publications where we have transformation of adenocarcinoma to small cell lung cancer (SCLC) after TKI treatment or immunotherapy treatment [12], [13]. There is also the case where different metastatic sites of the primary disease (adenocarcinoma) transformed to SCLC after TKI administration, while in the same patient other metastatic sites had T790M mutation [14]. As a conclusion re-biopsy should be performed in any site primary or metastatic that occurs during TKI therapy, not only to investigate T790M, but also to investigate whether the tumor has changed and chemotherapy is needed. Liquid biopsy can be a surrogate method of biopsy or an additional method. There is the case where the site that we chose to re-biospy is negative, however; another metastatic site might be positive for T790M. More trials are needed towards this very important daily issue that treating physicians have to manage.
Conflicts of interest
Paul Zarogoulidis, Aggeliki Rapti have received funding as members of the advisory board of Astra-Zeneca regarding osimertinib.
References
- 1.Domvri K., Zarogoulidis P., Darwiche K., Browning R.F., Li Q., Turner J.F., Kioumis I., Spyratos D., Porpodis K., Papaiwannou A., Tsiouda T., Freitag L., Zarogoulidis K. Molecular targeted drugs and biomarkers in NSCLC, the evolving role of individualized therapy. J. Cancer. 2013;4(9):736–754. doi: 10.7150/jca.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarogoulidis K., Zarogoulidis P., Darwiche K., Boutsikou E., Machairiotis N., Tsakiridis K., Katsikogiannis N., Kougioumtzi I., Karapantzos I., Huang H., Spyratos D. Treatment of non-small cell lung cancer (NSCLC) J. Thorac. Dis. 2013;5(Suppl 4):S389–S396. doi: 10.3978/j.issn.2072-1439.2013.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domvri K., Darwiche K., Zarogoulidis P., Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Transl. Lung Cancer Res. 2013;2(4):256–258. doi: 10.3978/j.issn.2218-6751.2012.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettinger D.S., Wood D.E., Aisner D.L., Akerley W., Bauman J., Chirieac L.R., D'Amico T.A., DeCamp M.M., Dilling T.J., Dobelbower M., Doebele R.C., Govindan R., Gubens M.A., Hennon M., Horn L., Komaki R., Lackner R.P., Lanuti M., Leal T.A., Leisch L.J., Lilenbaum R., Lin J., Loo B.W., Jr., Martins R., Otterson G.A., Reckamp K., Riely G.J., Schild S.E., Shapiro T.A., Stevenson J., Swanson S.J., Tauer K., Yang S.C., Gregory K., Hughes M. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. JNCCN. 2017;15(4):504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 5.Huang L., Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B. 2015;5(5):390–401. doi: 10.1016/j.apsb.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krawczyk P., Mlak R., Powrozek T., Nicos M., Kowalski D.M., Wojas-Krawczyk K., Milanowski J. Mechanisms of resistance to reversible inhibitors of EGFR tyrosine kinase in non-small cell lung cancer. Contemp. Oncol. 2012;16(5):401–406. doi: 10.5114/wo.2012.31768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M., Hatabu H. Osimertinib in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017;376(20):1992–1993. doi: 10.1056/NEJMc1703339. [DOI] [PubMed] [Google Scholar]
- 8.Mayor S. Osimertinib effective in EGFR T790M-positive lung cancer. Lancet. Oncol. 2017;18(1):e9. doi: 10.1016/S1470-2045(16)30654-4. [DOI] [PubMed] [Google Scholar]
- 9.Zarogoulidis P., Gaga M., Huang H., Darwiche K., Rapti A., Hohenforst-Schmidt W. Tissue is the issue and tissue competition. Re-biopsy for mutation T790: where and why? Clin. Transl. Med. 2017;6(1):6. doi: 10.1186/s40169-017-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remon J., Menis J., Hasan B., Peric A., De Maio E., Novello S., Reck M., Berghmans T., Wasag B., Besse B., Dziadziuszko R. The APPLE trial: feasibility and activity of AZD9291 (osimertinib) treatment on positive PLasma T790M in EGFR-mutant NSCLC patients. Clin. Lung Cancer. 2017 Mar 1 doi: 10.1016/j.cllc.2017.02.005. EORTC 1613. pii: S1525-7304(17)30048-7. [Epub ahead of print]. PMID: 28341106. [DOI] [PubMed] [Google Scholar]
- 11.Reck M., Rodriguez-Abreu D., Robinson A.G., Hui R., Csoszi T., Fulop A., Gottfried M., Peled N., Tafreshi A., Cuffe S., O'Brien M., Rao S., Hotta K., Leiby M.A., Lubiniecki G.M., Shentu Y., Rangwala R., Brahmer J.R., K.- Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.K., Lee J., Kim S., Kim S., Youk J., Park S., An Y., Keam B., Kim D.W., Heo D.S., Kim Y.T., Kim J.S., Kim S.H., Lee J.S., Lee S.H., Park K., Ku J.L., Jeon Y.K., Chung D.H., Park P.J., Kim J., Kim T.M., Ju Y.S. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017 doi: 10.1200/JCO.2016.71.9096. JCO2016719096. [DOI] [PubMed] [Google Scholar]
- 13.Imakita T., Fujita K., Kanai O., Terashima T., Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: a case report. Respir. Med. Case Rep. 2017;21:52–55. doi: 10.1016/j.rmcr.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suda K., Murakami I., Yu H., Kim J., Ellison K., Rivard C.J., Mitsudomi T., Hirsch F.R. Heterogeneity in immune marker expression after acquisition of resistance to EGFR kinase inhibitors: analysis of a case with small cell lung cancer transformation. J. Thorac. Oncol. 2017 Jun;12(6):1015–1020. doi: 10.1016/j.jtho.2017.02.002. [Epub 2017 Feb 11].PMID: 28193529. [DOI] [PubMed] [Google Scholar]