Abstract
Oncolytic viruses have proven their therapeutic potential against a variety of different tumor entities both in vitro and in vivo. Their ability to selectively infect and lyse tumor cells, while sparing healthy tissues, makes them favorable agents for tumor-specific treatment approaches. Particularly, the addition of virotherapeutics to already established chemotherapy protocols (so-called chemovirotherapy) is of major interest. Here we investigated the in vitro cytotoxic effect of the oncolytic vaccinia virus GLV-1h68 combined with dual chemotherapy with nab-paclitaxel plus gemcitabine in four human pancreatic adenocarcinoma cell lines (AsPc-1, BxPc-3, MIA-PaCa-2, and Panc-1). This chemovirotherapeutic protocol resulted in enhanced tumor cell killing in two tumor cell lines compared to the respective monotherapies. We were thereby able to show that the combination of oncolytic vaccinia virus GLV-1h68 with nab-paclitaxel and gemcitabine has great potential in the chemovirotherapeutic treatment of advanced pancreatic adenocarcinoma. However, the key to a successful combinatorial chemovirotherapeutic treatment seems to be a profound viral replication, as tumor cell lines that were non-responsive to the combination therapy exhibited a reduced viral replication in the presence of the chemotherapeutics. This finding is of special significance when aiming to achieve a virus-mediated induction of a profound and long-lasting antitumor immunity.
Keywords: pancreatic cancer, vaccinia virus, chemovirotherapy, virotherapy, immunotherapy
Introduction
Pancreatic cancer still represents the tumor disease with the worst prognosis in terms of patient survival.1 With a 5-year survival rate of only 6% in the United States,2 current treatment regimens strongly fail in effectively stabilizing this tumor disease. Accordingly, novel therapeutic principles adapted to the distinct pancreatic cancer tumor biology3 are desperately needed.
Oncolytic viruses are able to specifically infect and lyse tumor cells; this virus-mediated oncolysis then results in induction of a profound antitumor immunity.4, 5, 6 Many years ago, reports on tumor patients which occasionally responded to natural virus infections gave the impulse for a systematic development of virotherapeutic agents. Since then, many naturally occurring viruses have been utilized and genetically optimized for work on a virotherapeutic cure and more efficient palliation for cancer patients.7 However, the clinical success of this new therapeutic regimen mostly remained small and thus far only single cases of a monovirotherapeutic cancer cure have been reported.8
The granulocyte-macrophage colony-stimulating factor (GM-CSF) expressing herpes simplex virus mutant talimogene laherparepvec (T-VEC) (Imlygic; Amgen) was approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2015 for treatment of metastatic melanoma,9, 10 which ultimately may pave the way for establishing even more virotherapeutic agents in clinical practice. Additionally, results of a phase Ib trial have already demonstrated an even stronger response of patients with melanoma to the combination of T-VEC with the checkpoint blockade inhibitor ipilimumab;11 accordingly, results from the phase II part of this immuno(viro)therapeutic trial (NCT01740297) are eagerly awaited.
Despite these promising developments, it is still believed that oncolytic virotherapy might find its way into widespread clinical use only in combination with already approved treatment modalities (e.g., chemotherapy), especially in tumor entities that are not as responsive to immunotherapy as melanoma. Recently, preliminary results of a phase III trial in which patients with head and neck cancer were treated with a combination of Reolysin (reovirus type 3 Dearing [RT3D]) with dual chemotherapy with carboplatin plus paclitaxel showed a statistically significant improvement in patient survival compared to the control arm.12
The vaccinia (Lister strain)-derived oncolytic virus GLV-1h68 (referred to as GL-ONC1 when used in clinical studies) was constructed previously by inserting three expression cassettes encoding for β-glucuronidase, β-galactosidase, and GFP (Ruc-GFP) at different gene loci of the virus.13 As a result, GLV-1h68 was found to be attenuated compared to wild-type vaccinia virus.14 In addition, expression of diagnostic markers also enabled real-time monitoring of virus infection, spread, and oncolysis,15 thus providing real-time insight on the success of tumor treatment.16
Here, we investigated the cytotoxic effect of the prototypic combination of GLV-1h68 with clinically approved dual chemotherapy with nab-paclitaxel plus gemcitabine on four well-characterized cell lines of human pancreatic adenocarcinoma in vitro. The remaining tumor cell masses and cell viabilities were analyzed at 72 hr after chemovirotherapeutic treatment. In addition, we also examined the effect of the respective chemotherapeutic agents on replication of GLV-1h68 as determined by viral growth curves. Potential interferences of the chemotherapeutic compounds with vaccinia virus-based virotherapeutics were also investigated.
Results
Response of Human Pancreatic Adenocarcinoma Cells to Chemo- or Virotherapeutic Treatment in Monotherapy
Prior to any chemovirotherapeutic combination treatment, suitable doses of each single agent had to be determined. In our consideration, proper concentrations of each single agent should result in a remaining tumor cell mass of ≈75% at 72 hr post initiation of treatment (defined here as a so-called 25% lethal dose [LD25]). Otherwise, higher doses/concentrations might result in even more enhanced tumor cell killing (>25%) by each of the compounds tested, which then might disguise any additional antitumoral effect when used in combination.
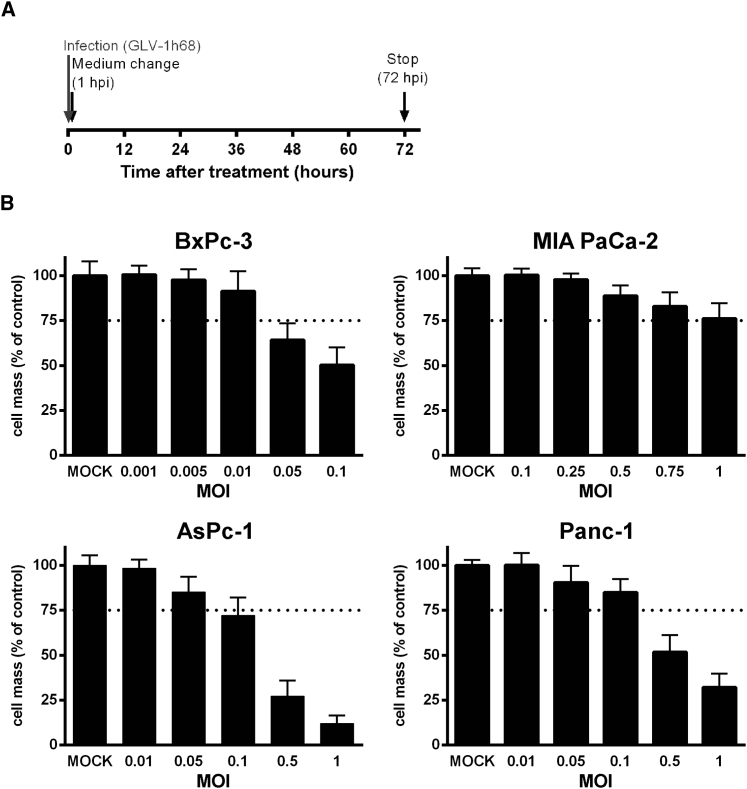
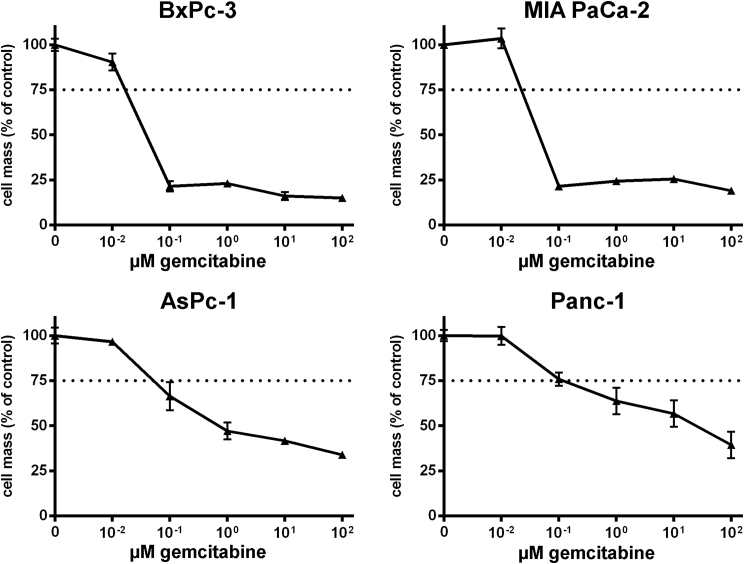
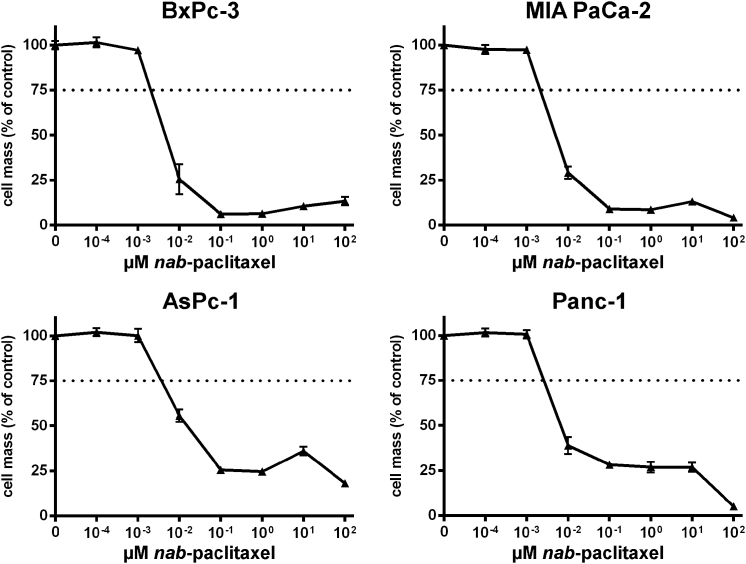
According to this setup, four human pancreatic adenocarcinoma cell lines (AsPc-1, BxPc-3, MIA PaCa-2, and Panc-1) were first treated in monotherapeutic approaches (1) with ascending doses of vaccinia virus GLV-1h68 (MOIs, multiplicity of infection), (2) with the chemotherapeutic agent nab-paclitaxel (nab-PTX), or (3) with the chemotherapeutic compound gemcitabine. In each case, the remaining tumor cell masses were analyzed by sulforhodamine B (SRB) viability assays 72 hr after the initiation of treatment (Figures 1, 2, and 3). As a result, all four tumor cell lines were found to respond to any of the applied agents in a dose-dependent manner, even though different levels of susceptibility to the oncolytic/chemotoxic treatments were seen in the respective tumor cell lines. In contrast to the other three tumor cell lines, BxPc-3 tumor cells responded to an MOI of GLV-1h68 of only 0.05 (Figure 1). In AsPc-1 and Panc-1 cells, increasing concentrations of gemcitabine resulted in dose-dependent tumor cell killing over the whole range of concentrations employed (Figure 2, lower panels), whereas gemcitabine concentrations exceeding a critical threshold of 10−1 μM in BxPc-3 and MIA PaCa-2 cells killed nearly all tumor cells (Figure 2, upper panels). Treatment with nab-paclitaxel resulted in an almost uniform response in all four tumor cell lines, likewise demonstrating a critical concentration threshold in the range of 10−2 to 10−3 μM, resulting in highly potent tumor cell killing (Figure 3). On the basis of these data, LD25 doses of each single agent could be determined for each of the respective tumor cell lines (Table 1).
Figure 1.
Treatment of Four Different Human Pancreatic Tumor Cell Lines with Oncolytic Vaccinia Virus GLV-1h68
(A) Virotherapy setting. Tumor cells were infected with the oncolytic vaccinia virus GLV-1h68. 1 hr post-infection (hpi), the inoculum was removed and normal growth medium was added. (B) Cytotoxicity was determined after 72 hr of treatment with increasing doses (MOIs) of GLV-1h68. The remaining tumor cell masses were analyzed by sulforhodamine B assays (n = 3, mean and SD are shown). Dotted lines indicate a tumor cell mass of 75% being remnant at 72 hpi. MOCK, untreated control; MOI, multiplicity of infection.
Figure 2.
Treatment of Four Different Human Pancreatic Tumor Cell Lines with Increasing Concentrations of Gemcitabine
Remaining tumor cell masses were analyzed at 72 hr after initiation of treatment with gemcitabine by sulforhodamine B assays (n = 3, mean and SD are shown). Dotted lines indicate a tumor cell mass of 75% being remnant at 72 hr post-initiation of gemcitabine treatment.
Figure 3.
Treatment of Four Different Pancreatic Tumor Cell Lines with Increasing Concentrations of nab-Paclitaxel
Remaining tumor cell masses were analyzed at 72 hr after initiation of treatment with nab-paclitaxel by sulforhodamine B assays (n = 3, mean and SD are shown). Dotted lines indicate a tumor cell mass of 75% being remnant at 72 hr post-initiation of nab-paclitaxel treatment.
Table 1.
LD25 Monotherapeutic Doses of GLV-1h68, Gemcitabine, and nab-Paclitaxel in Human Pancreatic Carcinoma Cell Lines: Further Employed for Chemovirotherapy
| Tumor Cell Line | Virus Dose of GLV-1h68 (MOI) | Gem Concentration (μM) | nab-PTX Concentration (μM) |
|---|---|---|---|
| MIA PaCa-2 | 0.5 | 3 × 10−2 | 5 × 10−3 |
| AsPc-1 | 0.1 | 1 × 10−1 | 1 × 10−2 |
| Panc-1 | 0.1 | 7.5 × 10−2 | 5 × 10−3 |
| BxPc-3 | 0.01 | 2 × 10−2 | 1 × 10−2 |
Gem, gemcitabine; LD25, 25% lethal dose (determined as the respective concentration of agents used in monotherapy resulting in a remaining tumor cell mass of ∼75% at 72 hr post-treatment); MOI, multiplicity of infection; nab-PTX, nab-paclitaxel.
Dual Chemovirotherapy with GLV-1h68 and Gemcitabine
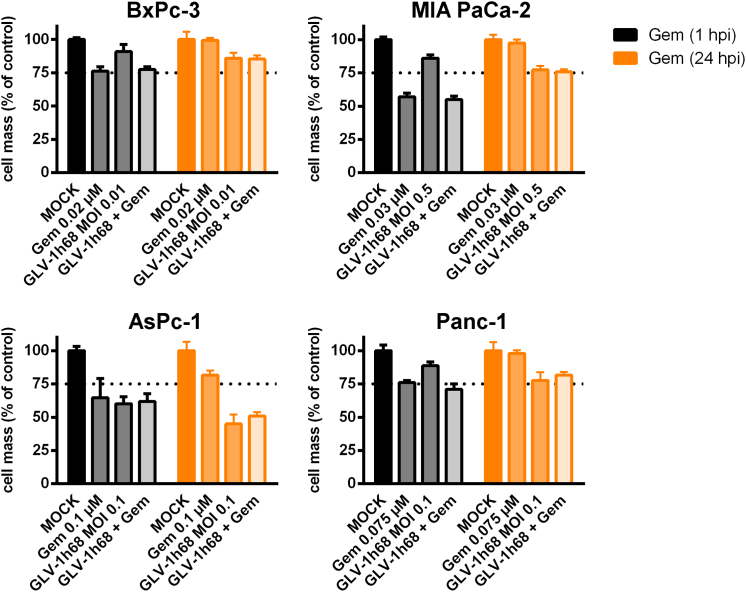
Next, a chemovirotherapeutic protocol combining GLV-1h68 and gemcitabine (gemcitabine-based dual chemovirotherapy) was designed. Accordingly, AsPc-1, BxPc-3, MIA PaCa-2, and Panc-1 tumor cells were treated with the previously determined LD25 doses of the two agents either in monotherapy or in combination. Gemcitabine was administered at either 1 or 24 hr post-infection (hpi) (Figure S1), while the remaining tumor cell masses were analyzed at 72 hpi by SRB assays (Figure 4); accordingly, durations of chemotherapy were either 71 hr (“early” addition of gemcitabine at 1 hpi) or 48 hr (“late” addition of gemcitabine at 24 hpi), respectively. When comparing the results of the remaining tumor cell masses using these distinct patterns of chemovirotherapy with both of the respective monotherapies (early versus late addition of gemcitabine), no considerably enhanced cytotoxicity was seen in any of the four tumor cell lines. Hence, this specific chemovirotherapy protocol (GLV-1h68 combined with gemcitabine) was not found to be superior to either of the two monotherapeutic approaches.
Figure 4.
Combinatorial Chemovirotherapeutic Treatment of Four Different Pancreatic Tumor Cell Lines with GLV-1h68 and Gemcitabine
Gemcitabine was added after infection with GLV-1h68 at either 1 or 24 hpi. The remaining tumor cell masses were analyzed by sulforhodamine B assays (n = 1, mean and SD are shown). The additional effect of the combination treatment was compared with the effect of both monotherapeutic treatment regimens, respectively. Dotted lines indicate a tumor cell mass of 75% being remnant at 72 hpi. Gem, gemcitabine; hpi, hour(s) post-infection; MOCK, untreated control; MOI, multiplicity of infection.
Dual Chemovirotherapy with GLV-1h68 and nab-Paclitaxel
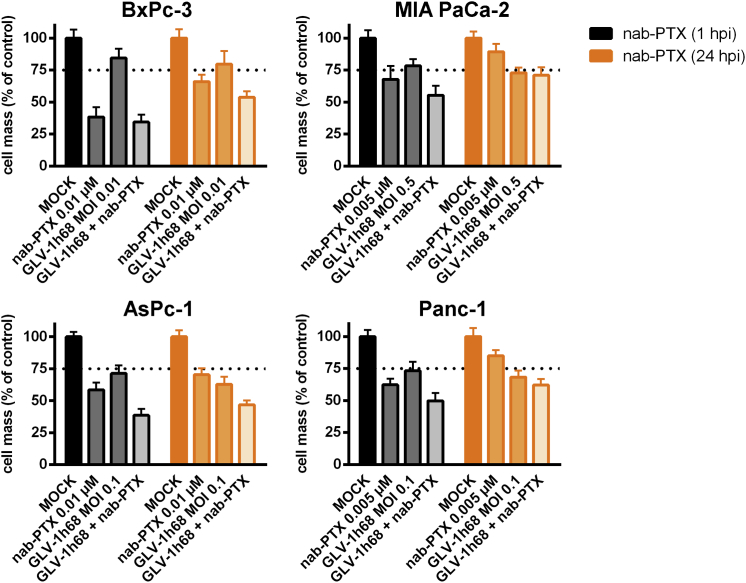
In the next setting, GLV-1h68 was combined with nab-paclitaxel (nab-PTX-based dual chemovirotherapy). This combination resulted only in slightly enhanced tumor cell killing compared to the respective monotherapies (Figure 5). Beyond this finding, the lag time between the initiation of viro- and chemotherapy (early versus late addition of nab-PTX at 1 hpi and 24 hpi, respectively) was not found to influence the efficacy of the chemovirotherapeutic combination.
Figure 5.
Combinatorial Chemovirotherapeutic Treatment of Four Different Pancreatic Tumor Cell Lines with GLV-1h68 and nab-Paclitaxel
nab-Paclitaxel (nab-PTX) was added after infection with GLV-1h68 at either 1 or 24 hpi (Figure 4). The remaining tumor cell masses were analyzed by sulforhodamine B assays (n = 3, mean and SD are shown). The additional effect of the combination treatment was compared with the effect of both monotherapeutic treatment regimens, respectively. Dotted lines indicate a tumor cell mass of 75% being remnant at 72 hpi. hpi, hour(s) post-infection; MOCK, untreated control; MOI, multiplicity of infection.
Taking the results of both dual-therapy approaches together, GLV-1h68 can be combined with chemotherapeutic compounds (e.g., gemcitabine and nab-paclitaxel) in highly flexible time patterns.
Triple Chemovirotherapy with GLV-1h68 Plus nab-Paclitaxel Plus Gemcitabine
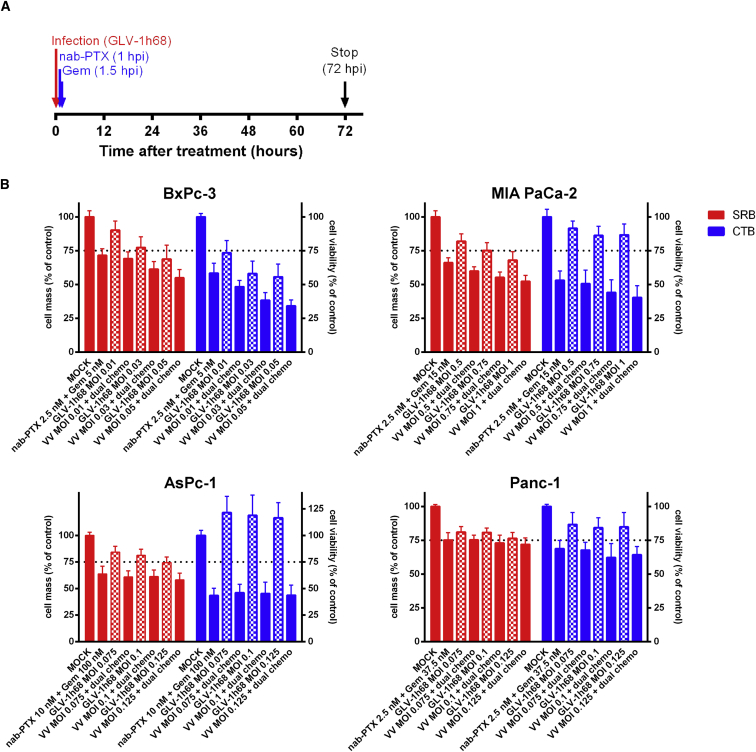
Based on the previously obtained results and their evaluation of clinical feasibility, a “final” chemovirotherapeutic triple protocol combining GLV-1h68 with the clinically approved dual chemotherapy of nab-paclitaxel plus gemcitabine was devised. In line with the prescribing information of nab-paclitaxel (Abraxane), nab-paclitaxel was applied first (at 1 hpi), whereas gemcitabine was added 30 min later (at 1.5 hpi) (Figure 6). Since the LD25 doses of both chemotherapeutic agents were determined previously in monotherapy, their concentrations in combination had to be adjusted slightly to the new triple-therapy setting (data not shown). Moreover, the MOIs of GLV-1h68 were varied based on their previously determined LD25 doses. To validate the results obtained with the SRB assays (Figure 6, depicted in red bars), additional CellTiter-Blue (CTB) viability assays were performed (Figure 6, depicted in blue bars).
Figure 6.
Combinatorial Chemovirotherapeutic Treatment of Four Different Pancreatic Tumor Cell Lines with GLV-1h68 and nab-Paclitaxel Plus Gemcitabine Dual Chemotherapy
(A) Triple therapy setting. Chemotherapy was started at 1 hpi by adding medium containing nab-paclitaxel. Half an hour later (1.5 hpi), gemcitabine was added. (B) The remaining tumor cell masses and cell viability after 72-hr treatment with the triple therapy at increasing doses of GLV-1h68 were analyzed by sulforhodamine B assays and by CellTiter-Blue assays, respectively (n ≥ 3, mean and SD are shown). Dotted lines indicate a tumor cell mass of 75% being remnant at 72 hpi. CTB, CellTiter-Blue; Gem, gemcitabine; hpi, hour(s) post-infection; MOCK, untreated control; MOI, multiplicity of infection; nab-PTX, nab-paclitaxel; SRB, sulforhodamine B; VV, vaccinia virus GLV-1h68.
As a result, both assays showed that the triple-therapy approach resulted in an enhanced cytotoxic effect in BxPc-3 and MIA PaCa-2 tumor cells, which could be further enhanced by increasing the viral dose of GLV-1h68 from MOI 0.01 to 0.05 and from MOI 0.5 to 1, respectively (Figure 6). In contrast, no additional effects of the chemovirotherapeutic combination were observed in AsPc-1 and Panc-1 cells. Interestingly, monovirotherapy with GLV-1h68 even led to an increase in tumor cell metabolism in AsPc-1 cells, which was restrained in combination with the dual chemotherapy.
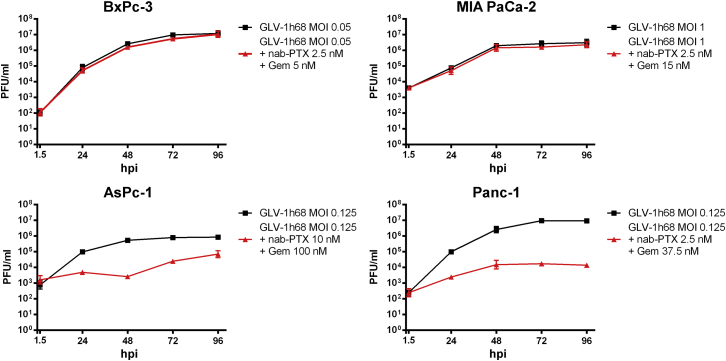
Effect of Dual Chemotherapy with nab-Paclitaxel Plus Gemcitabine on Viral Replication of GLV-1h68
Both oncolysis and potential long-term consequences of oncolytic virotherapy are believed to depend on a profound viral replication, which might be influenced by the chemotherapeutic agents being applied in combination in the case of chemovirotherapy. To assess whether the previously obtained results of the triple chemovirotherapy were linked to an altered viral replication of our study virus GLV-1h68, virus growth curves were generated by measuring viral titers at different time points after initiation of treatment (Figure 7). In fact, after the dual chemotherapy with nab-paclitaxel plus gemcitabine was added, a reduction in viral replication was seen in AsPc-1 and Panc-1 cells, whereas replication of GLV-1h68 in the previously better responding BxPc-3 and MIA PaCa-2 cells remained as potent as in the monovirotherapeutic treatment. Thus, therapeutic benefits of the combination of dual chemotherapy with nab-paclitaxel plus gemcitabine with GLV-1h68 was linked to an unaltered replication of the viral agent in BxPc-3 or MIA PaCa-2 cells.
Figure 7.
Effect of the nab-Paclitaxel Plus Gemcitabine Dual Chemotherapy on Replication of Study Virus GLV-1h68 in Four Different Pancreatic Tumor Cell Lines
Tumor cells were treated as described previously with the highest MOI used in either cell line. At five given time points (1.5, 24, 48, 72, and 96 hpi), tumor cells were harvested, followed by virus titer determination (n = 3, mean and SD are shown). Gem, gemcitabine; hpi, hour(s) post-infection; MOI, multiplicity of infection; nab-PTX, nab-paclitaxel; PFU, plaque-forming unit.
Discussion
To date, only a few chemovirotherapeutic trials encompassing higher numbers of patients have been completed. Fortunately, a wide range of promising chemovirotherapeutic protocols are undergoing clinical investigation and their results may possibly help to evaluate the future of multimodal virotherapeutic approaches.17
Rationale for Chemovirotherapy
One rationale for chemovirotherapy lies within cancer biology itself. Targeting tumor cells with distinct mechanisms at different sites of the tumor cell metabolism promises to be a more effective and more rapid type of antitumor treatment by helping to prevent any evolutionary selection of tumor cell subpopulations with primary and secondary acquired resistances. In addition, highly immunogenic “foreign” agents, such as oncolytic viruses, can be instrumental in arousing the dormant pathways of antitumor immunity, thereby utilizing the body’s own capacity for tumor cell clearance, especially in long-term antitumor treatment. Pancreatic cancer, with its highly instable genome and strongly immunosuppressive microenvironment, combines both of these key mechanisms for rapid tumor progression and the acquisition of high-grade resistance to standard chemotherapy protocols. Therefore, it seems logical that any form of antitumor treatment aiming for more efficient tumor cell killing has to strike hard and, more importantly, operate in a multimodal manner. The combination of clinically approved chemotherapeutic agents, acting by curbing cell growth in general, and oncolytic viruses that selectively target tumor cells holds great promise for clinical evaluation by potentially arousing a profound antitumor immune response. Of note, a recent randomized phase II trial in upfront treatment of metastatic pancreatic adenocarcinoma (MPA) showed that a chemovirotherapeutic regimen employing Reolysin with carboplatin and paclitaxel was safe but did not improve progression-free survival, regardless of KRAS mutational status.18 Therefore, it is of major interest to develop and test alternative chemovirotherapeutic regimens such as GLV-1h68 plus nab-paclitaxel plus gemcitabine.
Influence of Chemotherapy on Viral Replication
The main focus of chemovirotherapeutic regimens lies in harnessing the oncolytic and, more importantly, immunotherapeutic potential of the applied viral agents. Since both parameters are presumed to depend on a strong viral replication, it has to be ascertained that the application of chemotherapeutic agents does not interfere with viral replication and spread of infectious progeny virus particles in a negative manner. Such interactions depend not only on the chosen agents but also on their dosages and the order of administration of the respective therapeutic compounds, basic determinants that have to be considered for the design of any successful combination therapy.
Thus far, therapeutic benefit after chemovirotherapy both in vitro and in vivo has been shown to be linked to an unchanged or even enhanced viral replication in most cases. In some cases, chemotherapy-induced DNA damage resulted in a cellular overexpression of GADD34 or ribonucleotide reductase (RR), which led to an increased replication of herpes virus-based agents if homologous viral gene products had previously been deleted.19, 20, 21, 22 Similarly, chemotherapy was shown to increase the levels of E1A, an early expressed adenoviral gene product that not only regulates a multitude of both cellular and viral genes to initiate the adenoviral replication cycle but is also known for its chemosensitizing effects.23, 24 The mitotic inhibitor paclitaxel was found to increase adenoviral assembly and subsequent release from the host cell while leaving DNA synthesis unaffected.25 Furthermore, our group showed that chemotherapy-induced senescence promoted replication of a measles vaccine virotherapeutic virus and led to increased tumor cell killing.26 However, in most cases, it remains unclear whether an enhanced viral replication constitutes the main determinant for therapeutic efficacy.
In contrast, it was previously shown that chemotherapeutics such as 5-fluorouracil (5-FU) or irinotecan induce an unfavorable environment for viral replication.27 SN-38, an active metabolite of clinically used irinotecan, was found to inhibit replication of the HSV-1 vector G47Δ, which decreased the therapeutic benefit otherwise seen in combination with etoposide where viral replication was not influenced.28 Interestingly, therapeutic benefit could be observed despite reduced viral titers. Enhanced levels of apoptosis in response to chemovirotherapeutic treatment were found to result in enhanced therapeutic efficacy and therefore outweighed detrimental decreases of the effective viral dose.29, 30 Prodrug converting strategies led to a powerful bystander effect, although viral replication was inhibited by the converted cytotoxic compound 5-FU.31, 32
Vaccinia Virus in the Context of Chemovirotherapy
Due to their extensive use in the eradication of smallpox, vaccinia virus strains are known to induce a potent immunological response in the human host.33 Among other reasons their capacity (1) to infect almost any cell type, (2) to transport and express large amounts of foreign DNA, as well as (3) their cytoplasmic replication cycle being independent from the cell’s own nuclear gene transcription make them favorable agents for virotherapy.
GLV-1h68, a Lister strain derivative recombinant vaccinia virus, has been part of many preclinical and clinical investigations. By inserting three expression cassettes (β-galactosidase, β-glucuronidase, and Ruc-GFP), GLV-1h68 unifies both diagnostic and therapeutic properties of cancer treatment and therefore belongs to a group of agents usually referred to as “theranostics.”34 GLV-1h68 was shown to not only exhibit proinflammatory35, 36, 37, 38 and antivascular39 properties but to also be able to colonize lymph node metastases40, 41 and infect cells with stem-cell-like features.42 However, the most crucial predictive marker for potent antitumor efficacy seems to be a strong viral replication in tumor cells.13, 35, 38, 39, 43, 44, 45, 46
Here, we investigated the cytotoxic effect of the chemovirotherapeutic combination of oncolytic vaccinia virus GLV-1h68 with nab-paclitaxel and/or gemcitabine on four well-characterized cell lines of pancreatic adenocarcinoma origin. As a result, chemovirotherapeutic protocols combining GLV-1h68 with each of both chemotherapeutic agents separately were seen to result in either no or only marginally enhanced rates of tumor cell killing, independent of the time lag between the initiation of viro- and chemotherapy. We therefore concluded that GLV-1h68 could be combined with chemotherapeutic agents (e.g., nab-paclitaxel and gemcitabine) in a more flexible time pattern, which helps to avoid the necessity of strict time intervals between the application of the chemotherapeutic and virotherapeutic compounds.
Based on the fact that virotherapeutic agents used in chemovirotherapy regimens are considered to serve as an add-on module to established chemotherapy protocols, a triple-therapy protocol combining GLV-1h68 with clinically approved dual chemotherapy with nab-paclitaxel plus gemcitabine was devised. We found that the triple chemovirotherapy resulted in a considerable increase in tumor cell killing in two pancreatic carcinoma cell lines (BxPc-3 and MIA PaCa-2), whereas the response in AsPc-1 and Panc-1 tumor cells after triple therapy resembled the response after single viro- or dual chemotherapy alone.
Furthermore, our data clearly demonstrate that the therapeutic benefit of triple therapy with GLV-1h68 plus nab-paclitaxel plus gemcitabine in treating different pancreatic cancer cell lines depends on an unaltered viral replication in vitro. These findings are of special interest, since, in view of future clinical applications, GLV-1h68 provides the possibility to non-invasively monitor viral replication as a surrogate marker for an (immuno)therapeutic effect in animal models or human patients. Any approach trying to prove its therapeutic benefit besides investigating its cytotoxic effect would have to focus especially on viral replication and its consequence on antitumor immunity.
Interactions between Chemo- and Virotherapeutics Depend on Their Sequence of Administration
Although identifying sequence-dependent interactions between the applied agents is highly complex, a few basic considerations can be pointed out. Generally, three distinct administration sequences can be differentiated: pretreatment with either (1) the chemo- (C) or (2) the virotherapeutic (V) agent (C→V / V→C) or (3) their concurrent administration (C⇔V). However, chemovirotherapeutic strategies are still experimental, rather than based on a profound understanding of the underlying mechanisms, and sequence-dependent interactions are not easy to predict. Of special interest are synergistic interactions independently from the treatment order but mediated via different antitumoral mechanisms. Huang et al.47 postulated that pretreatment with paclitaxel (C→V) induced a cell cycle arrest of colorectal cancer cells in the G2/M phase, which rendered them more susceptible to vaccinia virus infection. Pretreatment with the virotherapeutic (V→C), on the other hand, was shown to sensitize for an adjacent chemotherapy by the release of cellular danger signals.
Hypothetic considerations of one therapeutic approach sensitizing for the other hint at superior treatment outcomes after sequential administration of the agents (C→V / V→C) and, to a lesser extent, in the concurrent setting (C⇔V) as well. Any therapeutic effect based on an augmented viral replication would therefore suggest a benefit of administering chemotherapeutics first.20, 48 However, similar increases in adenoviral replication have also been found to be independent of the treatment order (C→V/ V→C).49
Then again, the requirement of a strong viral replication (or rather a high number of viral gene products) possibly sensitizing for adjacent chemotherapy suggests a therapeutic benefit if tumor cells are pretreated with the virotherapeutic agent (V→C).50 Nevertheless, some of the synergistic interactions associated with a potent viral replication have been found to be sequence independent.51, 52 In contrast, although gemcitabine negatively influenced the viral life cycle of parvovirus H-1PV in the concurrent setting (C→V), it was found to prolong survival of tumor-bearing rats when its administration took place much earlier (i.e., 2 weeks).53 Such combination protocols demonstrate the possibility to employ more flexible time patterns between chemo- and virotherapy in case of detrimental interactions in the concurrent setting. Additionally, unraveling treatment protocols by applying the agents separately, which to date is frequently used in multimodal chemotherapy protocols, possesses the benefit of reduced toxicity.
Unfortunately, the experimental settings of investigations addressing sequence-dependent effects are not always conclusive. In one case, both concurrent and delayed administration of cisplatin (C⇔V / V→C) resulted in a similar therapeutic benefit; however, the increase in cisplatin-induced apoptosis was only investigated in the former and therefore might not contribute to the therapeutic success in the sequential administration setting.54 Similarly, although pretreatment with either agent (C→V / V→C) often results in superior treatment outcomes, the effect of the chemotherapeutic agents on viral replication is frequently measured only in the concurrent setting. Depending on the respective treatment order, chemotherapeutics are known to also affect other parameters determining the cellular environment for viral replication, such as the induction of cell cycle arrest55 or changes in gene expression.19, 20, 21, 22 Therefore, although chemotherapeutics may directly interfere with the viral life cycle (as measured in the concurrent setting), it would be shortsighted to extrapolate such results for the sequential setting.
In our setting, the chronological order of the viro- and chemotherapeutic agents (GLV-1h68 plus nab-paclitaxel or gemcitabine) did not influence the therapeutic effect in either of the four tumor cell lines (C→V; data not shown). As a result, we concluded that chemo- and virotherapy could be administered in a more flexible time pattern and we devised a triple chemovirotherapy protocol in which nab-paclitaxel and gemcitabine were added directly after the initial virus infection (at 1 plus 1.5 hpi). Moreover, analyses of the antitumoral effect as well as of the influence of the dual chemotherapy on viral replication of GLV-1h68 were performed under similar conditions. Therapeutic success of the triple chemovirotherapy in the concurrent setting was therefore clarified to depend on an effective chemotherapy in addition to an unaltered viral replication.
Dose-Dependent Effects Determine the Therapeutic Outcome, Especially If Chemotherapy Influences Viral Replication
The positive/negative influence of one agent on the other likely depends on the concentrations used in the respective settings. Possible synergistic interactions may intensify therapeutic success and allow for dose reductions of the applied agents to a less toxic degree.56, 57, 58 High-dose combination therapy was actually unable to further increase the levels of tumor cell death already being induced by low-dose chemovirotherapy.59, 60 Furthermore, virus-mediated chemosensitization was shown to be powerful enough to render chemotherapy-resistant tumor cells sensitive for low-dose chemotherapy.61, 62
Since some chemotherapeutic agents are known to directly interfere with the viral life cycle, dose-dependent relations in this regard have been in the focus of diverse investigations. Although application of high-dose mitomycin C was found to severely reduce replication of an oncolytic herpes simplex virus, viral titers were found to be unchanged when administered in lower and thereby “beneficial” doses.63 Furthermore, low-dose chemotherapy was found to increase viral titers to a greater extent than its high-dose application.20, 49
The applied dose of an agent in combination therapy is usually determined by its cytotoxic effect in monotherapy. However, to demonstrate therapeutic benefit of the combination, suitable doses of single agents have to be chosen carefully. If doses are too high, single agents will be too “successful” in killing tumor cells on their own and the readout of potential combinatorial therapeutic benefits could be threatened. Moreover, even if the chemotherapy does not directly interfere with the viral life cycle, high concentrations would be immediately cytotoxic and therefore prevent effective viral replication by killing tumor cells, which function as hosts for replicating virotherapeutics.64 Accordingly, higher gemcitabine doses in gemcitabine-insensitive pancreatic cancer cell lines were assumed to cause a greater inhibition of viral replication and in accordance to also prevent therapeutic benefit.65
In line with these considerations, we designed our chemovirotherapeutic protocols by carefully adjusting concentrations of the respective compounds, ensuring remaining tumor cell masses of ≈75% after 72 hr of chemo- or virotherapeutic treatment in monotherapy (designated as a so-called LD25). By doing so, we set out to prevent excessive tumor cell killing possibly disguising additional effects of the chemovirotherapeutic combination. Nonetheless, reductions in the viral titers in tumor cell lines that had been non-responsive to the triple chemovirotherapy still could be the result of overdosing chemotherapy. On closer inspection, AsPc-1 and Panc-1 tumor cells indeed received higher concentrations of nab-paclitaxel and/or gemcitabine than the triple-chemovirotherapy-responsive tumor cell lines BxPc-3 and MIA PaCa-2. Therefore, chemotherapeutic doses still might have been adjusted in a too-high range and could potentially have negatively influenced viral replication as a result. Since we strongly believe that an unaltered replication of GLV-1h68 constitutes an important key to chemovirotherapeutic success, we call for further investigations on this matter.
Triple Chemovirotherapy Regimen with GLV-1h68 Plus nab-paclitaxel Plus Gemcitabine in Animal Models
In the further development of chemovirotherapy regimens, it would be of great interest to come to a preclinical evaluation of potential immunotherapeutic effects of the combination GLV-1h68 plus nab-PTX plus gemcitabine. However, when employing human pancreatic ductal adenocarcinoma (hPDA) cell lines as investigated in this work (AsPc-1, BxPc-3, MIA-PaCa-2, and Panc-1), such experiments could only be performed in xenograft animal models (e.g., in nude or severe combined immunodeficiency [SCID] mice). Unfortunately, these immunodeficient mice are lacking important features of adaptive immunity. As an alternative, usage of humanized mice with a partially or nearly fully reconstituted immune system could provide insights on (1) how this triple therapeutic regimen would affect antitumor immunity, (2) how immune checkpoint inhibitors could be placed on top, and (3) how means aiming at a depletion of the immunosuppressive phenotypes of human pancreatic cancer could be made successful; however, proper answers to these highly interesting questions can only be provided by future clinical trials. Of further interest are investigations on how the triple chemovirotherapy regimen with GLV-1h68 plus nab-PTX plus gemcitabine would affect the dense stroma being associated with hPDA. Again, xenograft mouse models are not suitable for such investigations due to the fact that hPDA cells cannot be mixed with human pancreatic stromal cells for a remodeling of the specific histological features of hPDA. As an alternative, organotypic culture models have emerged as tractable systems to recapitulate the complex three-dimensional organization of hPDA66 and could be implemented for such analyses in the future. Such hPDA organoids would also be highly instrumental for further investigations on the mechanistic effects of the triple-chemovirotherapy regimen with GLV-1h68 plus nab-PTX plus gemcitabine.
Conclusions
Identifying agent-, sequence-, and dose-dependent interactions may not be as easy as confirming a supposedly reasonable hypothesis. Differences between the in vitro and in vivo settings as well as in different tumor cell lines further aggravate this complex matter.24, 29, 67 However, since the immunotherapeutic effects of oncolytic viruses are assumed to be the key players mediating durable disease regressions, patients will respond differently to distinct oncolytic agents and more than one combination protocol might be required. In chemovirotherapy, immunogenic oncolytic viruses are primarily thought to operate as add-ons to already existing chemotherapy protocols. Many chemovirotherapeutic protocols thus far have demonstrated their safety in patients,17 and it may be of utmost importance to identify non-responders at an early stage of therapy to change the virotherapeutic component if necessary. Non-invasive monitoring of viral replication and therapeutic efficacy as surrogate markers for (immuno)therapeutic success is therefore of special interest. In this regard, viral agents such as the prototypic theranostic vector GLV-1h68 are favorable in providing the required insights and are therefore promising candidates for personalized cancer therapy in the near future.
Materials and Methods
Cell Culture
The pancreatic adenocarcinoma cell lines AsPc-1 and Panc-1 were purchased from Sigma-Aldrich. BxPc-3 and Panc-1 pancreatic cancer cells were purchased from the American Type Culture Collection (ATCC). Tumor cells were seeded for subsequent experiments in 24-well plates at a density of 4 × 104/well (BxPc-3, MIA PaCa-2, and Panc-1) or 5 × 104/well (AsPc-1). African green monkey kidney cells (CV-1) were provided by Genelux. All cell lines were cultivated in DMEM (Biochrom) supplemented with 10% fetal calf serum (FCS; PAA Laboratories) (growth medium). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Monovirotherapeutic Treatment with GLV-1h68
Following seeding, BxPc-3, MIA PaCa-2, Panc-1, and AsPc-1 cells were infected the next day with GLV-1h68 at different MOIs. Suspensions of GLV-1h68 were prepared by thawing and sonicating frozen virus solutions for 30 s at 4°C, followed by dilution in DMEM supplemented with 2% FCS (infection medium). During the following hour, plates were swayed every 15 min. At 1 hpi, the infection medium was replaced with cell growth medium. At 72 hpi, remaining tumor cell masses were measured by the SRB viability assay.
Monochemotherapeutic Treatment with nab-Paclitaxel or Gemcitabine
Following seeding, the culture medium of BxPc-3, MIA PaCa-2, Panc-1, and AsPc-1 cells was replaced the next day with medium containing different concentrations of the respective chemotherapeutic agent. At 72 hr post-treatment (hpt), the remaining tumor cell masses were measured by the SRB assay.
Dual Chemovirotherapy with GLV-1h68 and Either nab-Paclitaxel or Gemcitabine
Following seeding, BxPc-3, MIA PaCa-2, Panc-1, and AsPc-1 cells were infected the next day with GLV-1h68 as described above. At either 1 hpi or 24 hpi, the culture medium was replaced with medium containing the LD25 dose of the respective chemotherapeutic agent (either nab-paclitaxel or gemcitabine). At 72 hpi, the remaining tumor cell masses were measured by the SRB viability assay.
Triple Chemovirotherapy with GLV-1h68 and nab-Paclitaxel Plus Gemcitabine
Following seeding, BxPc-3, MIA PaCa-2, Panc-1, and AsPc-1 cells were infected the next day with GLV-1h68 as described above. At 1 hpi, medium was replaced with nab-paclitaxel-containing growth medium. At 1.5 hpi, gemcitabine-containing growth medium was added. Concentrations of both agents were calculated to result in the proper (tumor cell line-adjusted) LD25 doses in the entire medium. At 72 hpi, the remaining tumor cell masses were measured by the SRB assay and the CellTiter-Blue assay, respectively.
SRB Assay
Tumor cells were seeded in 24-well plates. The next day, the respective chemo- and/or virotherapeutic treatment was performed as described above. At 72 hpt or 72 hpi, tumor cells were washed with cold PBS and fixed by administering cold 10% trichloroacetic acid (TCA) solution. After 30 min of incubation at 4°C, TCA was removed and cells were washed with tap water and dried in a drying chamber at 40°C. Cellular proteins were stained by adding 0.4% (w/v) SRB (Sigma-Aldrich) dissolved in 1% acetic acid for 10 min at room temperature (RT). Plates were then washed with 1% acetic acid and dried another time. Protein-bound dye was solubilized in 10 mM Tris base (pH 10.5), after which optical density was measured at a wavelength of 550 nm using the Tecan GENios Plus multifunction fluorescence microtiter plate reader (Tecan Deutschland).
CellTiter-Blue Assay
Tumor cells were seeded in 24-well plates. The next day, the triple therapy was performed as described above. At 72 hpi, 100 μL medium of each well was replaced with CellTiter-Blue reagent (Promega). Cells were further incubated for 1 up to 4 hr, depending on the particular metabolic rate of each cell line. End-point fluorescence was then quantified at a wavelength of 595 nm with the Tecan GENios Plus multifunction microtiter plate reader (excitation wavelength of 550 nm).
Virus Titration of GLV-1h68
Tumor cells were seeded in 24-well plates. The next day, the triple therapy was performed as described above. At different time points (1.5 hpi, 24 hpi, 48 hpi, 72 hpi, and 96 hpi), cells were harvested by scraping them into their medium. A subsequent freeze/thaw cycle led to cell lysis and the release of cell-bound viral particles. CV-1 cells were seeded in 24-well-plates and were infected on the following day with 1:10 serial dilutions (10−1 to 10−6) of the collected virus samples. After primary infection, plates were swayed every 20 min. At 1 hpi, each well received overlay medium containing 1.5% carboxymethylcellulose (CMC) (Sigma-Aldrich) additionally supplemented with Gibco Antibiotic-Antimycotic (Life Technologies) and 5% FCS. Cells were further incubated for 2 days. Staining of the virus plaques was performed by adding crystal violet for 4 hr at RT. Subsequently, cells were washed with tap water. Stained virus plaques were counted and the corresponding virus titers (plaque forming units [PFU] per milliliter) were calculated.
Statistical Analysis
All results are expressed as means ± SD and were calculated using GraphPad Prism software (version 6; GraphPad Software).
Author Contributions
Conceptualization, E.B., S.B., J.B., M.S., and U.M.L.; Methodology, E.B., S.B., J.B., M.S., and U.M.L.; Investigation, E.B., C.G., and I.S.; Writing – Original Draft, E.B., S.B., and U.M.L.; Writing – Review & Editing, E.B., S.B., and U.M.L.; Funding Acquisition, E.B., S.B., and U.M.L.; Resources, S.B., J.B., and U.M.L.; Supervision, S.B., J.B., M.S., and U.M.L.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
Oncolytic vaccinia virus GLV-1h68 was kindly provided by Genelux. E.B. was funded by a German Cancer Aid (DKH) Mildred Scheel Scholarship for postgraduates (reference no. 111045). We further acknowledge support from the German Research Foundation (DFG) and the German Cancer Consortium (DKTK).
Footnotes
Supplemental Information includes one figure and can be found with this article online at http://dx.doi.org/10.1016/j.omto.2017.04.001.
Supplemental Information
References
- 1.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo M. New insights into pancreatic cancer biology. Ann. Oncol. 2012;23(Suppl 10):135–138. doi: 10.1093/annonc/mds313. [DOI] [PubMed] [Google Scholar]
- 4.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melcher A., Parato K., Rooney C.M., Bell J.C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffin R.S. From virotherapy to oncolytic immunotherapy: where are we now? Curr. Opin. Virol. 2015;13:93–100. doi: 10.1016/j.coviro.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Kelly E., Russell S.J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 8.Russell S.J., Federspiel M.J., Peng K.W., Tong C., Dingli D., Morice W.G., Lowe V., O’Connor M.K., Kyle R.A., Leung N. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014;89:926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amgen. (2015). FDA approves IMLYGIC™ (talimogene laherparepvec) as first oncolytic viral therapy in the US. https://www.amgen.com/media/news-releases/2015/10/fda-approves-imlygic-talimogene-laherparepvec-as-first-oncolytic-viral-therapy-in-the-us/.
- 10.Amgen. (2015). European Commission approves Amgen’s IMLYGIC™ (talimogene laherparepvec) as first oncolytic immunotherapy in Europe. http://www.amgen.com/media/news-releases/2015/12/european-commission-approves-amgens-imlygic-talimogene-laherparepvec-as-first-oncolytic-immunotherapy-in-europe/.
- 11.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oncolytics Biotech. (2014). Oncolytics Biotech Inc. announces additional data from REO 018 randomized study of REOLYSIN in head and neck cancers. http://www.oncolyticsbiotech.com/news/oncolytics-biotech-inc-announces-additional-data-from-reo-018-randomized-study-of-reolysin-in-head-and-neck-cancers/.
- 13.Zhang Q., Yu Y.A., Wang E., Chen N., Danner R.L., Munson P.J., Marincola F.M., Szalay A.A. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q., Liang C., Yu Y.A., Chen N., Dandekar T., Szalay A.A. The highly attenuated oncolytic recombinant vaccinia virus GLV-1h68: comparative genomic features and the contribution of F14.5L inactivation. Mol. Genet. Genomics. 2009;282:417–435. doi: 10.1007/s00438-009-0475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Y.A., Shabahang S., Timiryasova T.M., Zhang Q., Beltz R., Gentschev I., Goebel W., Szalay A.A. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat. Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 16.Lauer U.M., Zimmermann M., Sturm J., Koppenhoefer U., Bitzer M., Malek N.P., Glatzle J., Koenigsrainer A., Moehle R., Fend F. Phase I/II clinical trial of a genetically modified oncolytic vaccinia virus GL-ONC1 in patients with unresectable, chemotherapy-resistant peritoneal carcinomatosis. J. Clin. Oncol. 2013;31(Suppl):3098. [Google Scholar]
- 17.Binz E., Lauer U.M. Chemovirotherapy: combining chemotherapeutic treatment with oncolytic virotherapy. Oncolytic Virother. 2015;4:39–48. doi: 10.2147/OV.S54780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noonan A.M., Farren M.R., Geyer S.M., Huang Y., Tahiri S., Ahn D., Mikhail S., Ciombor K.K., Pant S., Aparo S. Randomized phase 2 trial of the oncolytic virus Pelareorep (Reolysin) in upfront treatment of metastatic pancreatic adenocarcinoma. Mol. Ther. 2016;24:1150–1158. doi: 10.1038/mt.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghi M., Rabkin S., Martuza R.L. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J. Natl. Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 20.Adusumilli P.S., Chan M.K., Chun Y.S., Hezel M., Chou T.C., Rusch V.W., Fong Y. Cisplatin-induced GADD34 upregulation potentiates oncolytic viral therapy in the treatment of malignant pleural mesothelioma. Cancer Biol. Ther. 2006;5:48–53. doi: 10.4161/cbt.5.1.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrowsky H., Roberts G.D., Kooby D.A., Burt B.M., Bennett J.J., Delman K.A., Stanziale S.F., Delohery T.M., Tong W.P., Federoff H.J., Fong Y. Functional interaction between fluorodeoxyuridine-induced cellular alterations and replication of a ribonucleotide reductase-negative herpes simplex virus. J. Virol. 2001;75:7050–7058. doi: 10.1128/JVI.75.15.7050-7058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberg D.P., Adusumilli P.S., Hendershott K.J., Yu Z., Mullerad M., Chan M.K., Chou T.C., Fong Y. 5-fluorouracil and gemcitabine potentiate the efficacy of oncolytic herpes viral gene therapy in the treatment of pancreatic cancer. J. Gastrointest. Surg. 2005;9:1068–1077. doi: 10.1016/j.gassur.2005.06.024. discussion 1077–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng P.H., Lian S., Zhao R., Rao X.M., McMasters K.M., Zhou H.S. Combination of autophagy inducer rapamycin and oncolytic adenovirus improves antitumor effect in cancer cells. Virol. J. 2013;10:293. doi: 10.1186/1743-422X-10-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheong S.C., Wang Y., Meng J.H., Hill R., Sweeney K., Kirn D., Lemoine N.R., Halldén G. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AbouEl Hassan M.A., Braam S.R., Kruyt F.A. Paclitaxel and vincristine potentiate adenoviral oncolysis that is associated with cell cycle and apoptosis modulation, whereas they differentially affect the viral life cycle in non-small-cell lung cancer cells. Cancer Gene Ther. 2006;13:1105–1114. doi: 10.1038/sj.cgt.7700984. [DOI] [PubMed] [Google Scholar]
- 26.Weiland T., Lampe J., Essmann F., Venturelli S., Berger A., Bossow S., Schulze-Osthoff K., Lauer U.M., Bitzer M. Enhanced killing of therapy-induced senescent tumor cells by oncolytic measles vaccine viruses. Int. J. Cancer. 2014;134:235–243. doi: 10.1002/ijc.28350. [DOI] [PubMed] [Google Scholar]
- 27.Kulu Y., Kawasaki H., Donahue J.M., Kasuya H., Cusack J.C., Choi E.W., Kuruppu D.K., Fuchs B.C., Tanabe K.K. Concurrent chemotherapy inhibits herpes simplex virus-1 replication and oncolysis. Cancer Gene Ther. 2013;20:133–140. doi: 10.1038/cgt.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheema T.A., Kanai R., Kim G.W., Wakimoto H., Passer B., Rabkin S.D., Martuza R.L. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin. Cancer Res. 2011;17:7383–7393. doi: 10.1158/1078-0432.CCR-11-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottolino-Perry K., Acuna S.A., Angarita F.A., Sellers C., Zerhouni S., Tang N., McCart J.A. Oncolytic vaccinia virus synergizes with irinotecan in colorectal cancer. Mol. Oncol. 2015;9:1539–1552. doi: 10.1016/j.molonc.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong S., Yu D., Wang Y., Qiu S., Wu S., Liu X.Y. An armed oncolytic adenovirus ZD55-IL-24 combined with ADM or DDP demonstrated enhanced antitumor effect in lung cancer. Acta Oncol. 2010;49:91–99. doi: 10.3109/02841860903246557. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S., Kuroda T., Fuchs B.C., He X., Supko J.G., Schmitt A., McGinn C.M., Lanuti M., Tanabe K.K. Oncolytic herpes simplex virus expressing yeast cytosine deaminase: relationship between viral replication, transgene expression, prodrug bioactivation. Cancer Gene Ther. 2012;19:160–170. doi: 10.1038/cgt.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foloppe J., Kintz J., Futin N., Findeli A., Cordier P., Schlesinger Y., Hoffmann C., Tosch C., Balloul J.M., Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 33.Thorne S.H. Immunotherapeutic potential of oncolytic vaccinia virus. Immunol. Res. 2011;50:286–293. doi: 10.1007/s12026-011-8211-4. [DOI] [PubMed] [Google Scholar]
- 34.Rojas J.J., Thorne S.H. Theranostic potential of oncolytic vaccinia virus. Theranostics. 2012;2:363–373. doi: 10.7150/thno.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrig K., Kilinc M.O., Chen N.G., Stritzker J., Buckel L., Zhang Q., Szalay A.A. Growth inhibition of different human colorectal cancer xenografts after a single intravenous injection of oncolytic vaccinia virus GLV-1h68. J. Transl. Med. 2013;11:79. doi: 10.1186/1479-5876-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentschev I., Donat U., Hofmann E., Weibel S., Adelfinger M., Raab V., Heisig M., Chen N., Yu Y.A., Stritzker J., Szalay A.A. Regression of human prostate tumors and metastases in nude mice following treatment with the recombinant oncolytic vaccinia virus GLV-1h68. J. Biomed. Biotechnol. 2010;2010:489759. doi: 10.1155/2010/489759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gentschev I., Ehrig K., Donat U., Hess M., Rudolph S., Chen N., Yu Y.A., Zhang Q., Bullerdiek J., Nolte I. Significant growth inhibition of canine mammary carcinoma xenografts following treatment with oncolytic vaccinia virus GLV-1h68. J. Oncol. 2010;2010:736907. doi: 10.1155/2010/736907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worschech A., Chen N., Yu Y.A., Zhang Q., Pos Z., Weibel S., Raab V., Sabatino M., Monaco A., Liu H. Systemic treatment of xenografts with vaccinia virus GLV-1h68 reveals the immunologic facet of oncolytic therapy. BMC Genomics. 2009;10:301. doi: 10.1186/1471-2164-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gentschev I., Müller M., Adelfinger M., Weibel S., Grummt F., Zimmermann M., Bitzer M., Heisig M., Zhang Q., Yu Y.A. Efficient colonization and therapy of human hepatocellular carcinoma (HCC) using the oncolytic vaccinia virus strain GLV-1h68. PLoS ONE. 2011;6:e22069. doi: 10.1371/journal.pone.0022069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donat U., Weibel S., Hess M., Stritzker J., Härtl B., Sturm J.B., Chen N.G., Gentschev I., Szalay A.A. Preferential colonization of metastases by oncolytic vaccinia virus strain GLV-1h68 in a human PC-3 prostate cancer model in nude mice. PLoS ONE. 2012;7:e45942. doi: 10.1371/journal.pone.0045942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly K.J., Brader P., Woo Y., Li S., Chen N., Yu Y.A., Szalay A.A., Fong Y. Real-time intraoperative detection of melanoma lymph node metastases using recombinant vaccinia virus GLV-1h68 in an immunocompetent animal model. Int. J. Cancer. 2009;124:911–918. doi: 10.1002/ijc.24037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Chen N.G., Minev B.R., Szalay A.A. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J. Transl. Med. 2012;10:167. doi: 10.1186/1479-5876-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Advani S.J., Buckel L., Chen N.G., Scanderbeg D.J., Geissinger U., Zhang Q., Yu Y.A., Aguilar R.J., Mundt A.J., Szalay A.A. Preferential replication of systemically delivered oncolytic vaccinia virus in focally irradiated glioma xenografts. Clin. Cancer Res. 2012;18:2579–2590. doi: 10.1158/1078-0432.CCR-11-2394. [DOI] [PubMed] [Google Scholar]
- 44.Chen N.G., Yu Y.A., Zhang Q., Szalay A.A. Replication efficiency of oncolytic vaccinia virus in cell cultures prognosticates the virulence and antitumor efficacy in mice. J. Transl. Med. 2011;9:164. doi: 10.1186/1479-5876-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin S.F., Yu Z., Riedl C., Woo Y., Zhang Q., Yu Y.A., Timiryasova T., Chen N., Shah J.P., Szalay A.A. Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery. 2007;142:976–983. doi: 10.1016/j.surg.2007.09.017. discussion 976–983. [DOI] [PubMed] [Google Scholar]
- 46.Weibel S., Raab V., Yu Y.A., Worschech A., Wang E., Marincola F.M., Szalay A.A. Viral-mediated oncolysis is the most critical factor in the late-phase of the tumor regression process upon vaccinia virus infection. BMC Cancer. 2011;11:68. doi: 10.1186/1471-2407-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang B., Sikorski R., Kirn D.H., Thorne S.H. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2011;18:164–172. doi: 10.1038/gt.2010.121. [DOI] [PubMed] [Google Scholar]
- 48.Li H., Zeng Z., Fu X., Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67:7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- 49.Quirin C., Mainka A., Hesse A., Nettelbeck D.M. Combining adenoviral oncolysis with temozolomide improves cell killing of melanoma cells. Int. J. Cancer. 2007;121:2801–2807. doi: 10.1002/ijc.23052. [DOI] [PubMed] [Google Scholar]
- 50.Heise C., Lemmon M., Kirn D. Efficacy with a replication-selective adenovirus plus cisplatin-based chemotherapy: dependence on sequencing but not p53 functional status or route of administration. Clin. Cancer Res. 2000;6:4908–4914. [PubMed] [Google Scholar]
- 51.Yu D.C., Chen Y., Dilley J., Li Y., Embry M., Zhang H., Nguyen N., Amin P., Oh J., Henderson D.R. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–525. [PubMed] [Google Scholar]
- 52.Fujiwara T., Kagawa S., Kishimoto H., Endo Y., Hioki M., Ikeda Y., Sakai R., Urata Y., Tanaka N., Fujiwara T. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int. J. Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- 53.Angelova A.L., Aprahamian M., Grekova S.P., Hajri A., Leuchs B., Giese N.A., Dinsart C., Herrmann A., Balboni G., Rommelaere J. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin. Cancer Res. 2009;15:511–519. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 54.Moehler M., Sieben M., Roth S., Springsguth F., Leuchs B., Zeidler M., Dinsart C., Rommelaere J., Galle P.R. Activation of the human immune system by chemotherapeutic or targeted agents combined with the oncolytic parvovirus H-1. BMC Cancer. 2011;11:464. doi: 10.1186/1471-2407-11-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma G., Kawamura K., Li Q., Okamoto S., Suzuki N., Kobayashi H., Liang M., Tada Y., Tatsumi K., Hiroshima K. Combinatory cytotoxic effects produced by E1B-55kDa-deleted adenoviruses and chemotherapeutic agents are dependent on the agents in esophageal carcinoma. Cancer Gene Ther. 2010;17:803–813. doi: 10.1038/cgt.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma B., Wang Y., Zhou X., Huang P., Zhang R., Liu T., Cui C., Liu X., Wang Y. Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin. J. Cancer Res. Clin. Oncol. 2015;141:419–429. doi: 10.1007/s00432-014-1835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng W.G., Li J.J., Hu P., Lei L., Wang J.N., Liu R.B. An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer. Oncol. Rep. 2013;29:2355–2361. doi: 10.3892/or.2013.2359. [DOI] [PubMed] [Google Scholar]
- 58.Shen W., Tu J.K., Wang X.H., Fu Z.X. Oncolytic adenovirus mediated Survivin RNA interference and 5-fluorouracil synergistically suppress the lymphatic metastasis of colorectal cancer. Oncol. Rep. 2010;24:1285–1290. doi: 10.3892/or_00000984. [DOI] [PubMed] [Google Scholar]
- 59.Rein D.T., Volkmer A., Bauerschmitz G., Beyer I.M., Janni W., Fleisch M.C., Welter A.K., Bauerschlag D., Schöndorf T., Breidenbach M. Combination of a MDR1-targeted replicative adenovirus and chemotherapy for the therapy of pretreated ovarian cancer. J. Cancer Res. Clin. Oncol. 2012;138:603–610. doi: 10.1007/s00432-011-1135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oberg D., Yanover E., Adam V., Sweeney K., Costas C., Lemoine N.R., Halldén G. Improved potency and selectivity of an oncolytic E1ACR2 and E1B19K deleted adenoviral mutant in prostate and pancreatic cancers. Clin. Cancer Res. 2010;16:541–553. doi: 10.1158/1078-0432.CCR-09-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takakura M., Nakamura M., Kyo S., Hashimoto M., Mori N., Ikoma T., Mizumoto Y., Fujiwara T., Urata Y., Inoue M. Intraperitoneal administration of telomerase-specific oncolytic adenovirus sensitizes ovarian cancer cells to cisplatin and affects survival in a xenograft model with peritoneal dissemination. Cancer Gene Ther. 2010;17:11–19. doi: 10.1038/cgt.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G., Zhou J., Gao Q., Huang X., Li K., Zhuang L., Huang M., Xu G., Wang S., Lu Y., Ma D. Oncolytic adenovirus-mediated transfer of the antisense chk2 selectively inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. 2006;13:930–939. doi: 10.1038/sj.cgt.7700967. [DOI] [PubMed] [Google Scholar]
- 63.Toyoizumi T., Mick R., Abbas A.E., Kang E.H., Kaiser L.R., Molnar-Kimber K.L. Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-1716) in human non-small cell lung cancer. Hum. Gene Ther. 1999;10:3013–3029. doi: 10.1089/10430349950016410. [DOI] [PubMed] [Google Scholar]
- 64.Leitner S., Sweeney K., Oberg D., Davies D., Miranda E., Lemoine N.R., Halldén G. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin. Cancer Res. 2009;15:1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cherubini G., Kallin C., Mozetic A., Hammaren-Busch K., Müller H., Lemoine N.R., Halldén G. The oncolytic adenovirus AdΔΔ enhances selective cancer cell killing in combination with DNA-damaging drugs in pancreatic cancer models. Gene Ther. 2011;18:1157–1165. doi: 10.1038/gt.2011.141. [DOI] [PubMed] [Google Scholar]
- 66.Baker L.A., Tiriac H., Clevers H., Tuveson D.A. Modeling pancreatic cancer with organoids. Trends Cancer. 2016;2:176–190. doi: 10.1016/j.trecan.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nelson A.R., Davydova J., Curiel D.T., Yamamoto M. Combination of conditionally replicative adenovirus and standard chemotherapies shows synergistic antitumor effect in pancreatic cancer. Cancer Sci. 2009;100:2181–2187. doi: 10.1111/j.1349-7006.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.