Abstract
The autologous ALDH bright (ALDHbr) cell therapy for ischemic injury is clinically safe and effective, while the underlying mechanism remains elusive. Here, we demonstrated that the glycolysis dominant metabolism of ALDHbr cells is permissive to restore blood flow in an ischemic hind limb model compared with bone marrow mononuclear cells (BMNCs). PCR array analysis showed overtly elevated Aldh2 expression of ALDHbr cells following hypoxic challenge. Notably, ALDHbr cells therapy induced blood flow recovery in this model was reduced in case of ALDH2 deficiency. Moreover, significantly reduced glycolysis flux and increased reactive oxygen species (ROS) levels were detected in ALDHbr cell from Aldh2-/- mice. Compromised effect on blood flow recovery was also noticed post transplanting the human ALDHbr cell from ALDH2 deficient patients (GA or AA genotypes) in this ischemic hindlimb mice model. Taken together, our findings illustrate the indispensable role of ALDH2 in maintaining glycolysis dominant metabolism of ALDHbr cell and advocate that patient's Aldh2 genotype is a prerequisite for the efficacy of ALDHbr cell therapy for peripheral ischemia.
Keywords: ALDH2, ALDHbr cells, Glycolysis, Ischemia injury
Graphical abstract

1. Introduction
ALDHbr cells are the stem and progenitor cells that express high level of aldehyde dehydrogenase (ALDH). Till now, 19 ALDHs have been identified [1]. Among them, the cytosolic ALDH1A sub-family, which comprises the primary ALDHs (ALDH1A1/-1A2/-1A3), is known as the major component of ALDHbr cells [2]. The safety and efficiency of autologous ALDHbr cells therapy have been proved by randomized, double-blind pilot study in patients with chronic myocardial ischemia [3] and critical limb ischemia [4], [5]. Robust hematopoietic repopulating function [6] and angiogenesis capacity [7] were also revealed in the immunodeficient mice post administration of ALDHbr cells. However, the underlying factors responsible for the significant benefits of ALDHbr cell therapy remain largely elusive.
Survival and the subsequent tissue renewal capacity of stem cells in the hypoxia niche are dependent of the glycolysis metabolism, the major source of energy supply in the hypoxic circumferences [8], [9], [10]. The status of glycolysis metabolism might thus be a major determinant of the fortune and function of various stem cells. In the present study, we tested the hypothesis that the potent therapeutic efficacy of ALDHbr cell therapy might be related to their individual metabolic pathways in favor of efficient glycolysis.
Our results indicated that the major energy supply of ALDHbr cells is from glycolysis, but not from oxidative phosphorylation (OXPHOS). To further explore the mechanism underlying the efficient therapy of ALDHbr cells for ischemic organs, PCR array was used to detect the differential expressed mRNA related to angiogenesis in hypoxic ALDHbr cells and evidenced significantly upregulated ALDH2 expression. Further studies showed that ALDH2 deficiency reduced the therapeutic effect of ALDHbr cells in the mice model of ischemic hind limb due to decreased anaerobic glycolysis and increased mitochondrial ROS generation. Besides, transplantion of human ALDHbr cells from GG genotype resulted in more significant increased blood flow recovery compared with human ALDHbr cells from GA/AA genotype in the mice model of ischemic hind limb. Above observations highlight the importance of individualized ALDHbr cells therapy for patients with various Aldh2 genotypes.
2. Results
2.1. Therapeutic potency of ALDHbr cells for ischemic hind limb in mice
Mouse ALDHbr cells were purified from bone marrow mononuclear cells by FACS after reacting with Aldefluor substrate, ALDH activity was high and side scatter was low in ALDHbr cells (Fig. 1A). To determine the therapeutic effect of ALDHbr cells, the ischemic injury was induced in left hind limb by unilateral femoral artery ligation in mice. Blood flow recovery of ischemic hind limbs of mice was imaged at different time points after transplantation of PBS, WT ALDHbr cells, and WT BMNCs (Fig. 1B). As shown in Fig. 1C, perfusion rate (PR) of ischemic and non-ischemic hind limb was up to 20.72% at the third day in ALDHbr cells transplanted group, which was about 2-fold higher than that in the control group (p < 0.05). The average PR of ALDHbr cells group was also significantly higher at the seventh day, which was up to 57.12%, while the average PR in other two groups was 28.06% (control, p < 0.01) and 31.26% (BMNCs, p < 0.05) respectively. These findings demonstrated the therapeutic efficiency of ALDHbr cells for ischemic injury.
Fig. 1.
Ischemic reparation capacity of ALDHbrcells in the ischemic hind limb model. A ALDHbr cells was isolated from BMNCs after incubation with Aldefluor reagent. B Laser Doppler imaging of ischemic hind limb and non-ischemic hind limb at day 0, 3, 7, 14 post BMNCs and ALDHbr cells transplantion. Blue indicates ischemic area, red indicates non-ischemic area. C The perfusion ratio (ischemic/non-ischemic hind limb) among the groups at different time points post BMNCs and ALDHbr cells transplantation (*P < 0.05 vs relative group of control, # P < 0.05 relative group of BMNCs, **P < 0.01 vs relative group of control). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Specific metabolic characteristics of ALDHbr cells
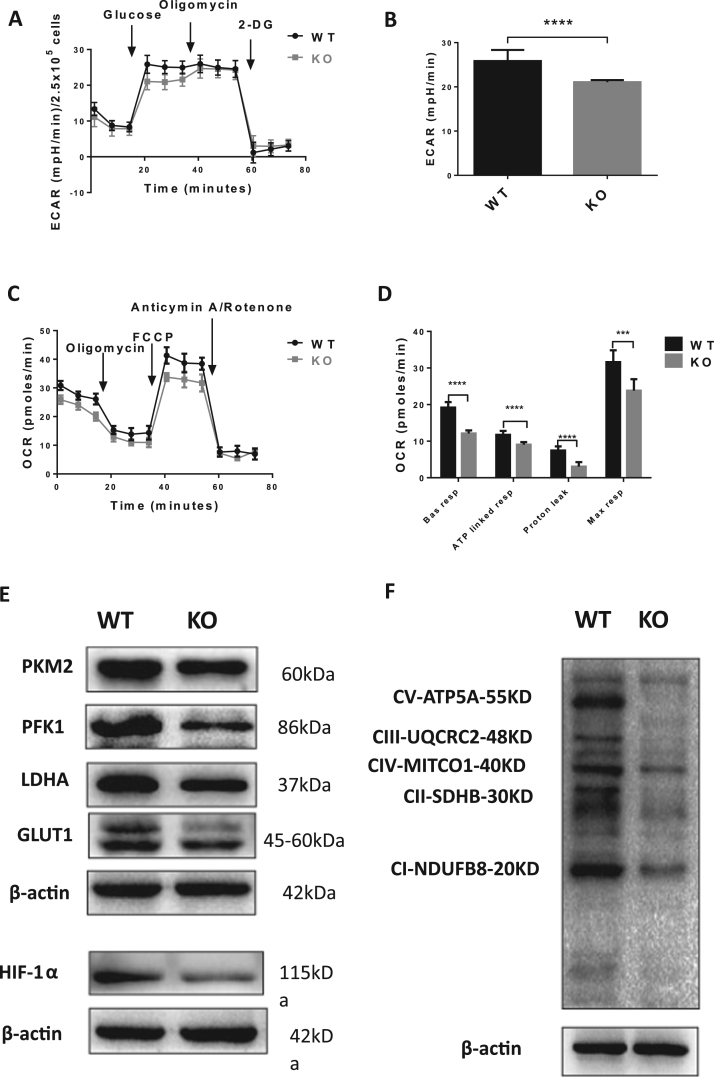
Metabolic characteristics may influence function and fate of transplanted stem cells. Therefore, the metabolic indexes, including ECAR and oxygen consumption rate (OCR), were measured by the XFe96 extracellular flux analyzer in WT ALDHbr cells and WT BMNCs. Analysis of extracellular proton flux demonstrated significantly elevated ECAR in ALDHbr cells (Fig. 2A and B), indicating enhanced glycolysis capacity and glycolysis reserve of ALDHbr cells compared to BMNCs, while the basal respiration, ATP turnover, ATP leak and maximal respiratory capacity of ALDHbr cells were all markedly decreased (Fig. 2C and D), suggesting that the total electron transport capacity was limited in ALDHbr cells. Accordingly, the expression of LDHA, which is responsible for catalyzing the glucose fermentation to lactate, and two rate-limiting enzymes of glycolysis PFK1 and PKM2, were all upregulated in ALDHbr cells. Finally, expression of GLUT1, a glucose transporter for glycolysis, was also upregulated in ALDHbr cells (Fig. 2E).
Fig. 2.
ALDHbrcells heavily rely on anaerobic glycolysis for energy supply. A, B Basal glycolysis, glycolysis capacity and glycolysis reserves were calculated as described in Methods (*P < 0.05, **P < 0.01). C OCR measurement at baseline and after addition of Oligomycin, FCCP, and Anticymin A/Rotenone. D OXPHOS indexes were calculated (**P < 0.01, ****P < 0.0001). E Western blot analysis of representative glycolysis related enzymes (PKM2, PFK1, LDHA), key glucose transporter (GLUT1) in mononuclear cells and KO ALDHbr cells.
2.3. PCR array analysis of differentially expressed genes in hypoxic ALDHbr cells
To analyze the key regulators responsible for ALDHbr cell therapy efficacy under ischemia, PCR array was used to detect the mRNA expression changes of 95 angiogenesis related genes, including cytokines, adhesion, growth factors and receptors, in ALDHbr cells under hypoxia. Data suggested that 44 mRNA expressions were downregulated and 10 were upregulated under hypoxia. One of the most significantly upregulated gene expression was Aldh2 (p < 0.05; Fig. 3A). We thus speculated that Aldh2 might play a key role in the therapeutic process of ALDHbr cells on ischemia injury. Based on above finding, we used ALDH2 deleted mice (Fig. 3B) for further experiments, ALDHbr cells from ALDH2 knockout (KO) or WT mice were isolated by Aldefluor reaction method (Fig. 3C). As shown in Fig. 3D, the number of ALDHbr cells was significantly lower in ALDH2 KO BMNCs than that from WT (11.96 ± 0.75 vs 30.97 ± 1.73, n = 19, p < 0.01).
Fig. 3.
Significant change ofAldh2expression in ALDHbrcells under hypoxia. A Expression of angiogenesis related genes in ALDHbr cells: normoxia relative to hypoxia exception for genes fold change between ± 0.5 (*P < 0.05, n = 3). BAldh2-/- mice was created by homozygous mutant, change of DNA and protein level. C WT ALDHbr cells and KO ALDHbr cells were separated by low side scatter and high ALDH activity. FACS of ALDHbr cells was circled by elliptical ring. D Compared with ALDHbr cells (30.97%, n = 19) in WT BMNCs, ALDH2 deficiency markedly decreased the number of ALDHbr cells (11.96%, n = 19) in KO BMNCs (****P < 0.0001).
2.4. Impact of ALDH2 deficiency on ALDHbr cells therapy for ischemic injury
Our previous study showed that ALDH2 was a key microenvironment homeostasis mediator, which was an essential prerequisite for effective bone marrow MSC therapy [11]. To observe whether the ALDHbr cells therapy for ischemic injury could be affected by ALDH2 genotype alone, we evaluated the blood flow recovery of ischemic hind limbs of mice post ALDH2 KO ALDHbr cells and WT ALDHbr cells transplantation at various time points (Fig. 4A and B). The PR value of ischemic hind limbs at the third day was 2-fold lower in ALDH2 KO ALDHbr cells treated group than in WT ALDHbr cells treated group: PBS (13.81%), WT ALDHbr cells (21.86%) and KO ALDHbr cells (10.25%) (p < 0.01). At seventh day, PR values of various groups were as follows: WT ALDHbr cells (57.22%), ALDH2 KO ALDHbr cells (33.5%) and PBS (31.12%). In addition, ALDH2 KO ALDHbr cells treated hind limb exhibited more severe necrosis than that treated by WT ALDHbr cells (Fig. 4C and D). Similar as PR, same trend was documented on CD31 stained capillary numbers, a representative marker of angiogenesis (p < 0.05; Fig. 4E and F). Therefore, the ALDHbr cells from ALDH2 deficiency mice had reduced therapeutic effect on ischemic injury in this hind limb ischemia model.
Fig. 4.
ALDH2 deficiency compromised the therapeutic effects of ALDHbrcells therapy for hind limb ischemia. A Representative Laser Dopplor imaging of mouse hindquarters in control, WT ALDHbr cells and KO ALDHbr cells injected groups at day 0, 3, 7, 14, the red represents non-ischemic hind limb and blue ischemic hind limb. B PR of ischemic hind limb and non-ischemic hind limb in PBS, WT ALDHbr cells and KO ALDHbr cells transplanted mice at day 1, 3 (**p < 0.01 vs relative group of KO ALDHbr cells n = 12–14), 7 (**p < 0.01 vs relative group of KO ALDHbr cells, ##p < 0.01 vs relative group of control, n = 14). C Morphological images of PBS, WT ALDHbr cells and KO ALDHbr cells administrated ischemic hind limb. Non-isc represents right hind limb, isc represents left hind limb. D Necrosis assessment of ischemic hind limb, the following three grades were used: no necrosis; toe necrosis, necrosis limited to the toes; foot necrosis, necrosis extending to the dorsum pedis. Compared with WT ALDHbr cells, KO ALDHbr cells transplanted ischemic hind limb showed more severely necrosis degree. E, F Immunofluorescence of 4′,6-diamidino-2-phenylindole (DAPI; blue) and CD31 (red) in ischemic tissue after PBS, WT ALDHbr cells and KO ALDHbr cells delivery for 7days (*P < 0.05, n = 3–5). Bar, 10 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Fate of WT ALDHbr and ALDH2 KO ALDHbr cells in vivo and in vitro
To determine the impact of ALDH2 on the fate of ALDHbr cells, retention of ALDHbr cells was monitored by green fluorescent protein GFP (Fig. 5A). Compared with WT ALDHbr cells treated ischemic hind limbs, the in vivo fluorescence imaging was decreased in ALDH2 KO ALDHbr cells on day 3 (Fig. 5B), suggesting that ALDH2 deficiency decreased ALDHbr cells retention in ischemic tissue. However, GFP fluorescence decayed in both WT ALDHbr cells and ALDH2 KO ALDHbr cells treated hind limb at day 7 (Fig. 5C). To further demonstrate the influence of hypoxia in ALDHbr cells, Annexin V assay of WT and ALDH2 KO ALDHbr cells after hypoxia treatment were performed. The results showed that apoptosis was markedly increased in hypoxic ALDH2 KO ALDHbr cells compared to hypoxic WT ALDHbr cells (p < 0.0001; Fig. 5D and E). Therefore, survival of ALDH2 KO ALDHbr cells was significantly reduced in the hypoxic environment compared to WT ALDHbr cells.
Fig. 5.
Retention effect of ALDHbrcells in the ischemic limb. A The whole body fluorescence imaging at day 3 and 7 after transplantation of PBS, GFP labelled WT ALDHbr cells and ALDH2 KO ALDHbr cells. B, C Fluorescence intensity of ALDHbr cells transplanted mice was significantly increased in comparison with control at days 3 (*P < 0.05, n = 5). Fluorescence intensity decayed in both WT ALDHbr cells and ALDH2 KO ALDHbr cells treated mice at the seventh day. D, E WT ALDHbr cells and KO ALDHbr cells were incubated in serum-free medium under hypoxia (1% O2) condition for 3 h, Annexin V apoptosis assay in WT ALDHbr cells and KO ALDHbr cells were measured. (****P < 0.0001).
2.6. Metabolic changes of ALDHbr cells related to ALDH2 deletion
The metabolic status was compared between ALDH2 KO ALDHbr cells and WT ALDHbr cells using XFe96 extracellular flux analysis. Compared with WT ALDHbr cells, glycolysis capacity was significantly reduced in ALDH2 KO ALDHbr cells, which exhibited decreased ECAR (p < 0.0001; Fig. 6A and B). We hypothesized that the impaired glycolysis capacity in ALDH2 KO ALDHbr cells might result in enhanced mitochondrion respiration to keep the metabolic balance of ALDH2 KO ALDHbr cells. In fact, the results of OCR measurement demonstrated that the basal respiration, ATP production, proton leak, maximal respiratory capacity were all markedly reduced in ALDH2 KO ALDHbr cells as compared to WT ALDHbr cells (Fig. 6C and D).
Fig. 6.
The metabolic characteristics was changed by ALDH2 deficiency in ALDHbrcells. A ECAR was detected in WT ALDHbr cells and KO ALDHbr cells by Seahorse XFe96 analyzer following the method mentioned above. B Compared with WT ALDHbr cells, the glycolysis capacity decreased significantly in KO ALDHbr cells (****P < 0.0001). C OCR detection in WT ALDHbr cells and KO ALDHbr cells by Seahorse XF96 analyzer. D Calculation of the basal respiration, ATP linked respiration, proton leak, maximal respiratory capacity of OCR showed a lower oxidative phosphorylation state in ALDH2 KO ALDHbr cells (**P < 0.001, ****P < 0.001). E Western blot analysis of representative glycolysis related enzymes (PKM2, PFK1, LDHA), key glucose transporter (GLUT1) and Expression of HIF-1α in WT ALDHbr cells and KO ALDHbr cells. F Western blot analysis of OXPHOS complexes in ALDHbr cells, including complex I, II, III, cytochrome c oxidase and Mitochondrial membrane ATP synthase. Expression 5 OXPHOS complexes were downregulated in ALDH2 KO ALDHbr cells.
To confirm the change of glycolysis in different ALDHbr cells, the glycolysis associated enzymes, including LDHA, PKF1, PKM2 and GLUT1, were measured in WT ALDHbr and ALDH2 KO ALDHbr cells. As shown in Fig. 6E, the expression of these enzymes was decreased in ALDH2 KO ALDHbr cells, suggesting decreased glucose absorption and glycolysis capacity in ALDH2 KO ALDHbr cells. Given that cell glycolysis capacity is regulated by HIF-1α dependent transcription activation of genes encoding for GLUT1 and glycolytic enzymes in hypoxia niche [12], the expression of HIF-1α was measured. Consistent with the decreased glycolysis flux in ALDH2 KO ALDHbr cells, HIF-1α expression was significantly downregulated in ALDH2 KO ALDHbr cells.
To further investigate the role of mitochondrial respiration in ALDH2 KO ALDHbr cells, Western Blotting analysis was performed to detect the relative levels of 5 OXPHOS complexes in mitochondrial, including complex I, II, III, cytochrome c oxidase and mitochondrial membrane ATP synthase. We found that the expression of OXPHOS complexes in ALDH2 KO ALDHbr cells was significantly downregulated (Fig. 6F), indicating decreased electron transport efficiency.
2.7. ALDH2 deficiency is associated with increased mitochondrial ROS levels in ALDHbr cells
4-HNE was the major substrate of ALDH2 and the biomarkers for oxidative stress, we analyzed the expression of 4-HNE adducts using Western blot. Compared with WT ALDHbr cells, 4-HNE modified protein expressions were dramatically increased in ALDH2 KO ALDHbr cells (Fig. 7A). Moreover, measurement of intracellular ROS levels in WT ALDHbr cells and ALDH2 KO ALDHbr cells showed that ALDH2 deficiency notably increased ROS fluorescent density of ALDHbr cells (p < 0.01; Fig. 7B and C). Cellular ROS is generated from various sources including mitochondria, cytochrome p450, and NADPH oxidase. Previous research of our group indicated the remarkably increased ROS of Aldh2-/- mice majorly derived from mitochondria [13]. Therefore, mitochondrial ROS levels of WT ALDHbr cells and ALDH2 KO ALDHbr cells were measured, the result showed that mitochondrial ROS fluorescent density significantly increased in ALDH2 deleted ALDHbr cells (p < 0.001; Fig. 7D and E).
Fig. 7.
ALDH2 deficiency increased ROS levels of ALDHbrcells. A Western bolt analysis of 4-HNE adducted proteins in WT ALDHbr cells and ALDH2 KO ALDHbr cells. B,C Intracellular ROS levels in ALDH2 KO ALDHbr cells were increased significantly (**P < 0.001). D,E Total fluorescence density of ROS in mitochondria of ALDH2 KO ALDHbr cells markedly increased (***P < 0.001).
2.8. Therapy of human ALDHbr cells with different Aldh2 genotypes in the ischemic hind limb mice model
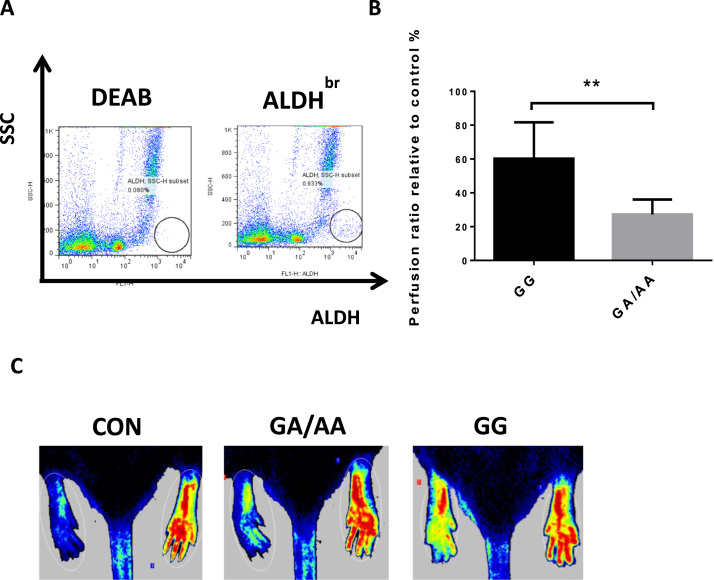
We further separated human ALDHbr cells from BMNCs using a similar method employed in mice. Different with mouse ALDHbr cells, human ALDHbr cells were sorted only about 1% (Fig. 8A). To identify the effect of Aldh2 mutation on ischemic therapy efficacy of human ALDHbr cells, we transplanted human ALDHbr cells with different Aldh2 genotypes into ischemic hind limbs of SOD/SCID mice. One week later, the hindquarters blood flow was scanned by Laser Doppler. Compared with control, PR values of GG and GA/AA ALDHbr cells treated groups were both increased, while the PR increase was more significant in GG ALDHbr cells treated groups than in GA/AA ALDHbr cells treated groups (60.17% vs 27.23%, P < 0.001 Fig. 8B and C). Our results indicated that ALDH2 deficiency reduced the therapeutic capacity of human ALDHbr cells in this ischemic hind limb mice model.
Fig. 8.
HumanAldh2genotype determines the therapeutic potential of ALDHbrcells for ischemic injury. A Purification of human ALDHbr cells from MNCs with low side scatter and high ALDH activity. B PR of control and ALDHbr cells transplanted NOD/SCID mice (**p < 0.01 n ≥ 4). C Laser Dopplor imaging of hindquarters treated with GG-, GA/AA- ALDHbr cells for one week (red: non-ischemic hind limb, blue: ischemic hind limb). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
In this study, we demonstrated that ALDHbr cells restored the blood of ischemic hind limb with higher efficiency compared to BMNCs, and ALDHbr cells utilized anaerobic glycolysis instead of OXPHOS to meet their energy demands. Furthermore, we illuminated that ALDH2 is a key mediator of ALDHbr cells under hypoxic circumference. ALDH2 deficiency also attenuated the therapeutic effect of both mouse and human ALDHbr cells in ischemic hind limb mice model, mostly due to decreased glycolysis and abnormal mitochondrial respiration, as well as increased mitochondrial ROS.
Recent studies have highlighted that hypoxia contributes to the maintenance of an undifferentiated status as well as influences cell proliferation and cell fate [14]. Therefore, hematopoietic stem cells and progenitors likely depend on anaerobic glycolysis, rather than OXPHOS to support ATP production, this unique low mitochondrial activity/glycolysis-dependent subpopulation is also linked with enhanced colony forming capacity and long-term repopulation [15]. In addition, increasing evidence suggests that metabolic transition from glycolysis to oxidative phosphorylation plays an important role in the process of stem cell differentiation [16], [17], [18], [19], [20]. Taken together, glycolysis is a crucial regulator of stemness maintaining. In this study, the heterogeneous population of bone marrow ALDHbr cells also depends on glycolysis-dominated metabolism as those hematopoietic stem cells and progenitors. This metabolic mode may thus underlie ALDHbr cells’ high proliferation capacity and adapting ability in ischemic tissue. Indeed, we showed that the stemness of ALDHbr cells was enhanced compared to that of BMNCs. These observations support the robust therapeutic effect of ALDHbr cells on the ischemic tissue.
Mitochondrial ALDH2 is initially reported as a key enzyme catalyzing acetaldehyde formed from ethanol metabolism [21], [22]. Studies of recent years revealed a vital cardiac protective role of ALDH2 activity. Chen et al. proved that pharmacologic enhancement of ALDH2 activity by Alda-1 reduced ischemic damage to the heart [23]. The ethanol-induced protection from cardiac ischemia was dependent on the activity of ALDH2 mediated by varepsilon PKC [24], while direct activation of ALDH2 conferred similar cardioprotection effect like ethanol [25]. ALDH2 also induced cardioprotective effect against ischemia/reperfusion (I/R) injury via detoxification of toxic aldehyde and regulation of autophagy [26]. Besides, ALDH2 activation contributed to ischemic preconditioning induced cardioprotection by preventing renin release from cardiac mast cells [27]. In addition, ALDH2 protected heart against acute ethanol toxicity by inhibiting protein phosphatase and forkhead transcription factor [28]. The selective activation of ALDH2 sufficiently improved the heart failure outcome [29]. Furthermore, ALDH2 was necessary for aldehyde detoxification in hematopoietic stem and progenitor cells (HSPCs) [30]. Our study evidenced upregulated genes expression especially Aldh2 in hypoxic ALDHbr cells. In case of Aldh2 deficiency, the therapeutic effect of ALDHbr cells on ischemic hind limbs was reduced, which was linked with impaired glycolysis capacity and OXPHOS of ALDHbr cells. The former might diminish the ischemic adaption and proliferation of ALDH2 KO ALDHbr cells, the latter was associated with increased level of ROS. The above mentioned disturbed metabolism might thus responsible for the compromised therapeutic effect on ischemic injury of ALDH2 KO ALDHbr cells.
It is known that expression of key glycolytic genes, including glucose transporters GLUT-1 and GLUT-3, lactate dehydrogenase (LDH), phosphoglycerate kinase (PGK-1), glucose-6-phosphate isomerase (GPI) and PFK-1, could be upregulated by HIF-1α [31], [32]. Our data showed that ALDH2 deficiency decreased the expression of HIF-1α in ALDHbr cells, and downregulated the expression of glycolysis associated proteins GLUT1, PKM2, PFK-1 and LDHA. Therefore, combined with the increased Aldh2 expression in ALDHbr cells under hypoxia condition and the attenuated ECAR induced by ALDH2 deficiency, our data collectively indicated that normal glycolysis in ALDHbr cells may partly be regulated by ALDH2 via regulating HIF-1α expression. Since HIF-1α is essential for cellular and systemic responses to low oxygen availability [33], reduced HIF-1α level, therefore, could be responsible for the reduced survival of ALDH2 KO ALDHbr cells in hypoxic condition.
As a substrate of ALDH2, 4-HNE may suppress ALDH2 activity [34]. ALDH2 inhibition, on the other hand, could increase levels of 4-HNE and superoxide in diabetic cardiomyopathy [35] and alcoholic cardiomyopathy [36]. We also found increased 4-HNE in the ALDH2 deficient ALDHbr cells. The majority of 4-HNE adducted proteins in mitochondria are the members of electron transport chain [37], [38], which could directly affect electron transport efficiency. We evidenced downregulated OXPHOS complexes in ALDH2 KO ALDHbr cells. Our results suggest that ALDH2 deficiency might damage the electron transport efficiency by the combined effect of increased 4-HNE and decreased OXPHOS complexes expression. Like 4-HNE, increased ROS could inhibit ALDH2 activity [39], [40]. Previous studies showed that increased oxidative stress was discernable in both ALDH2 deficient cells [41] and mice [42]. Previous report demonstrated that excessive ROS might damage the stem cell function and reduce the size of stem cell pool. And acute increase in intracellular concentrations of ROS caused inhibition of glycolytic enzyme PKM2 in human cancer cells [43]. Our results indicated that ALDH2 deficiency significantly enhanced ROS generation and downregulated glycolytic enzyme PKM2 in ALDHbr cells. Taken together, we considered that the compromised therapeutic potency of ALDHbr cells with ALDH2 deficiency might be caused by increased ROS levels, which might have resulted in disadvantaged metabolism, low retention and re-adaption capacity under hypoxic condition.
It was reported that mitochondrial ALDH, is a nitrate reductase that performed the biotransformation of nitroglycerin (GNT) and relax the vascular smooth muscle [44], [45]. Mitochondrial ROS overproduction could inhibit ALDH2 activity, finally mediated the process of nitrate tolerance [46], [47]. Conversely, reactivation of ALDH2 by lipoic acid improved the nitrate tolerance [48], [49]. In recent years, studies have revealed that mitochondrial ROS was the central regulator in nitrate tolerance. Inhibition of mitochondria-targeted antioxidants improved the tolerance of GNT [50]. In response to chronic pro-oxidant stimuli, the mitochondrial ROS formation significantly increased in Aldh2-/- mice [51]. Our previous study has demonstrated that the mitochondria were the major source of ROS formation in Aldh2-/- mice [13]. The present study also showed that, ALDH2 deficiency could induce the remarkably increase of mitochondrial ROS formation in ALDHbr cells, which might be one determinant for the reduced anti-ischemic capacity after transplantation.
Based on our research, ALDH2 deficiency decreased the therapeutic capacity of ALDHbr cells, it remains unknown if the small molecule activator of ALDH2, Alda-1 [52], could reverse the effect. Alda-1 was reported to reduce the ischemic heart damage in rodent models [23] and ameliorate vascular remodeling in pulmonary arterial hypertension [53]. Furthermore, selective activation of mitochondrial ALDH2 reduced the aldehydic load and improved mitochondrial function in the failing heart [54]. The application of Alda-1 might pave the way of improving the function of ALDHbr cells in case of ALDH2 deficiency. However, the improved NAD binding for Alda-1 stimulation was needed to establish ALDH2 activity, the functions of ALDH2 exhibited reductase, dehydrogenase and esterase activities, not all of the activities could be activated by Alda-1 [55]. Despite the presence of Alda-1, the catalytic process upon activation took place under saturated conditions [52], the rate of catalysis of ALDH2 was decreased at the low concentrations of aldehyde, the beneficial effects of Alda-1 were only observed under high oxidative stress conditions [56]. In summary, although the beneficial effects of Alda-1 have been elaborated in various disease models, the function of ALDH2 could not be completely restored. Several factors should be taken into consideration when using Alda-1 as an exogenous stimulator of ALDH-2, including the working concentration, half-life period, continuity of stimulation, toxic and side effect.
As a therapeutic candidate for ischemic injury, ALDHbr cells have been tested in clinical trials of ischemia repair with satisfactory results [3], [4], [5], [57]. Our study supply evidences explaining the potential working mechanism by exploring the metabolic characteristic of ALDHbr cells in case of hypoxia and ALDH2 deficiency. More importantly, we found that ALDH2 deficiency compromised ALDHbr cells’ repair capacity for ischemia due to reduced glycolysis capacity and increased ALDHbr cells apoptosis, which might be mediated by downregulated HIF-1α expression, as well as enhanced 4-HNE and ROS levels. In conclusion, our results show that the efficacy of ALDHbr cell therapy depends on ALDH2 activity, individuals with loss of function of ALDH2 are therefore not suitable for ALDHbr cells therapy for ischemic disease.
4. Limitation
Limitation existed in our present study. It is also essential to determine the source of ROS production by using various inhibitors for mitochondrial ROS or NADPH oxidase, these results are yet unavailable now, future studies are warranted to explore this issue.
5. Materials and methods
5.1. Animals and ALDHbr cells preparation
Eight weeks old C57BL/6 male mice (weighing 20–22g) and matched ALDH2 knockout (KO) male mice were used in our study. Among them, C57BL/6 mice were obtained from Shanghai Jiesijie Laboratory Animal Centre (Shanghai, China) and ALDH2 KO mice were generated as described previously [58]. All animals experiment was performed according to institutional guidelines. Bone marrow cells were isolated from 8-weeks-old C57BL/6 wild type and ALDH2 KO male mice respectively. Unpurified mononuclear cells (MNCs) were separated by Ficoll-hypaque gradient centrifugation, the representative buffy coat was collected and washed by stain buffer for eliminating the Ficoll-hypaqyue solution. Finally, cells were stained with Aldefluor reagent and ALDH inhibitor DEAB (Stem Cell Technologies), then ALDHbr cells with high ALDH activity and low side scatter were gated and collected by fluorescence-activated cell sorting FACS Calibur (BD biosciences).
5.2. Murine hind limb ischemia surgery and laser Doppler perfusion imaging
Eight-week-old C57BL/6 mice were anesthetized by intraperitoneally injection of pentobarbital sodium (40 mg/kg body weight), then hind limb ischemia was performed by left femoral artery ligation and excision, Then PBS, 4×106 mononuclear cells, isolated 106 WT ALDHbr cells and 106 ALDH2 KO ALDHbr cells were transplanted within 24 h at the two sides of ligation site. Blood recovery was detected by laser Doppler perfusion imaging (PeriScan PIM 3 system, Perimed, Sweden) at day 0, 3, 7 and 14. In detail, mouse hindquarters were imaged after placing the anesthetized mouse on 37 ℃ heating plate for 5 min, perfusion ratio of ischemic limbs vs non-ischemic limbs were quantified by averaging relative units of flux from the knee to the toe using PIMsoft Software (Perimed med, Sweden) [7].
5.3. Bioenergetics assay
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of WT mononuclear cells, WT ALDHbr cells and ALDH2 KO ALDHbr cells were analyzed in an XFe96 extracellular flux analyzer (Seahorese Bioscience) as described previously [59]. Before the experiment, Seahorse 96-well plate was attached by poly-D-lysine (PDL) (Sigma) for at least 2 h, then isolated cells were plated on PDL attached plates at a density of 5×105 cells/well, then the OCR and ECAR were measured to analyze oxygen consumption rate and glycolytic capacity of cells. Then metabolic profiles of OCR were detected by adding oligomycin A (1 μM), 1 μM FCCP, antimycin A (1 μM) and rotenone (1 μM). ECAR was determined by adding glucose (10 mM), oligomycin A (1 μM) and 2-deoxy-D-glucose (2-DG; 1 M). The following Oxidative Phosphorylation (OXPHOS) and glycolytic indexes were calculated: Basal respiration (OCRpre-Olig-OCRpost-AntA), ATP linked respiration (OCRpre-Olig-OCRpost-Olig), Maximal respiration (OCRpost-FCCP-OCRpost-AntA), Proton leak (OCRpost-Olig-OCRpost-AntA), Basic glycolysis (ECARpre-Olig), Glycolytic capacity (ECARpost-Olig), and Glycolytic capacity (ECARpost-Olig), Glycolytic reserve (ECARpost-Olig-ECARpre-Olig) [20].
5.4. PCR array analysis
After sorting, ALDHbr cells were incubated under hypoxia for 6 h. Then cells were collected by centrifugation and were lysed in TRIZOL regent (Invitrogen). RNA was separated in phases and precipitated, showing a gel-like pellet on the side and bottom of the tube, then the supernatant was removed and RNA pellet was washed with 75% ethanol. At the end of the procedure, RNA was air-dried and dissolved, with an A260/280 ratio < 1.6. The RNA yielded and quality was assessed by UV absorbance (NanoDrop® ND-1000) and denatured agarose gel electrophoresis, then the cDNA was synthetized and the real-time PCR was performed by Real-Time PCR Detection. In the end, the data was analyzed by ΔΔCt method.
5.5. Immunofluorescence and morphological necrosis examination
The quadriceps femoris muscle of ischemic limb were harvested after administration of cells for 7days, the samples were gradient dehydrated with sucrose and embedded with OCT, then frozen in liquid nitrogen for 5 min and transferred into −80 ℃. Each tissue was sectioned at 8 µm. Sections were incubated with rat anti-mouse CD31 (BD Biosciences), Alexa Fluor 549 donkey anti-rat secondary antibodies (Thermo Fisher Scientific) was used for fluorescence imaging. After counterstaining with DAPI, the sections were photographed with fluorescence confocal microscope (Nikon TE2000, Tokyo, Japan). Necrosis degree of ischemic limb was imaged by ordinary camera at days 0, 3, 7, 14. The degree of necrosis was visually evaluated by 3 grades [11]: no necrosis; toe necrosis, necrosis limited to the toes; foot necrosis, necrosis extending to the dorsum pedis.
5.6. In vivo immunofluorescence and apoptosis assay
WT ALDHbr cells and KO ALDHbr cells were labelled with green fluorescent protein (GFP) by transfection with lentivirus, then transplanted into the ischemic area of mouse hindlimb. The whole body immunofluorescent imaging was performed by fluorescence detection system (IVIS Lumin XRMS) at day 3 and 7 after transplantation. Cells apoptosis was analyzed by FITC-Annexin V detection kit (BD Bioscience). Isolated ALDHbr cells and ALDH2 KO ALDHbr cells were treated by hypoxia for 3 h, then harvested cell were washed with cold PBS for two times and suspended in binding buffer, after incubating with FITC-Annexin V and PI, cell apoptosis was detected by FACSCalibur (BD biosciences).
5.7. Western blotting
Proteins extracted from WT mononuclear cells, ALDHbr cells and ALDH2 KO ALDHbr cells were fractionated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), then transferred to the polyvinylidene difluoride (PVDF) (Millipore). After blocking with 5% Bovine Serum Albumin (BSA), the bolted membrane was incubated with rabbit anti-mouse polyclonal antibody Hif-1α(Novus Biologicals, 1:500), rabbit anti-mouse polyclonalantibody LDHA (Cell Signaling Technology, 1:1000), rabbit anti-mouse monoclonal antibody GLUT1 (Cell Signaling Technology, 1:1000), rabbit anti-mouse monoclonal antibody PKM2 (Cell Signaling Technology, 1:1000),rabbit anti-mouse monoclonal antibody PFK1 (Abcam, 1:2000) and β-actin (Kangcheng, 1:5000). Rabbit anti-mouse polyclonal antibody 4-ydroxynonenal (Abcam, 1:3000) and Total OXPHOS rodent antibody(Abcam, 1:250) were incubated on a whole PVDF membrane. Then enhanced chemiluminescence detection was used for imaging.
5.8. Oxidative stress analysis
Fluorometric intracellular ROS kit (Sigma aldrich) were used to detect intracellular ROS (especially superoxide and hydroxyl radicals) in live WT ALDHbr cells and ALDH2 KO ALDHbr cells. WT ALDHbr cells and ALDH2 KO ALDHbr cells were incubated in the working solution (1:1000dilution) for 30 min after FACS sorting. Then cells were washed for three times with PBS. Red fluorescence labelled cells (λex = 520/λem = 605 nm) was analyzed by flow cytometry (BD biosciences). Fluorescence density was calculated as percent of gated cell multiplied by mean of fluorescence density.
MitoSOX red mitochondrial superoxide indicator (Invitrogen) was used to detect mitochondrial superoxide in live WT ALDHbr cells and ALDH2 KO ALDHbr cells. WT ALDHbr cells and ALDH2 KO ALDHbr cells were incubated in the working solution (1:1000 dilution) for 10 min at 37 ℃ after FACS sorting. Then cells were washed for three times with warm PBS. Red fluorescence labelled cells (λex = 510/λem = 580 nm) was analyzed by flow cytometry (BD biosciences).
5.9. Human ALDHbr cells preparation and transplantation
Human breastbone marrow was collected with informed consent from patients undergoing coronary artery disease (CAD). Then mononuclear cells (MNCs) was obtained after gradient centrifugation with human Ficoll-hypaqyue solution (Sigma) and incubated with Aldefluor kit (Stem Cell Technologies). ALDHbr cells was separated by FACS Calibur mentioned above. We ligated the femoral artery of right-hind limb in NOD/SCID mice and immediately transplanted the sorted ALDHbr cells (3–5×104) into ischemic areas. The blood flow of hindquarters were detected one week later. One milliliter peripheral blood was collected from the included patients for DNA extraction, and the genotypes were identified by hybridization instrument (BR-526-24).
6. Statistical analyses
All above quantification analysis of at least 3 independent experiments were performed by GraphPad Prism 6.01. Statistical results was shown as the means ± standard error of mean (SEM). Student's independent t-test was used for analysis of two groups, and one-way ANOVA with the Bonferroni correction for multiple group analysis. The value of P < 0.05 was considered statistically significant.
Disclosures
None.
Acknowledgement
We thank the helpful assistance from Shuhui Sun for cell sorting. This work was supported by grants from National Natural Science Foundation of China (81570224) and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (81521001).
References
- 1.Marchitti S.A., Brocker C., Stagos D., Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storms R.W., Trujillo A.P., Springer J.B. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc. Natl. Acad. Sci. USA. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perin E.C., Silva G.V., Zheng Y. Randomized, double-blind pilot study of transendocardial injection of autologous aldehyde dehydrogenase–bright stem cells in patients with ischemic heart failure. Am. Heart J. 2012;163:415–421. doi: 10.1016/j.ahj.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Perin E.C., Silva G., Gahremanpour A. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheter Cardiovasc. Internv. 2011;78:1060–1067. doi: 10.1002/ccd.23066. [DOI] [PubMed] [Google Scholar]
- 5.Perin E.C., Murphy M.P., March K.L. Evaluation of cell therapy on exercise performance and limb perfusion in peripheral artery disease: the CCTRN patients with intermittent claudication injected with ALDH bright cells (PACE) trial. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.116.025707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess D.A., Meyerrose T.E., Wirthlin L. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 7.Capoccia B.J., Robson D.L., Levac K.D. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suda T., Takubo K., Semenza G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Shyh-Chang N., Daley G.Q., Cantley L.C. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi D.J., Jamieson C.H., Weissman I.L. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H., Sun A., Zhu H. Aldehyde dehydrogenase-2 is a host factor required for effective bone marrow mesenchymal stem cell therapy. Arterioscler. Thromb. Vasc. Biol. 2014;34:894–901. doi: 10.1161/ATVBAHA.114.303241. [DOI] [PubMed] [Google Scholar]
- 12.Iyer N.V., Kotch L.E., Agani F. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Zhang F., Zhao G. Mitochondrial PKC-epsilon deficiency promotes I/R-mediated myocardial injury via GSK3beta-dependent mitochondrial permeability transition pore opening. J. Cell. Mol. Med. 2017 doi: 10.1111/jcmm.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohyeldin A., Garzon-Muvdi T., Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Simsek T., Kocabas F., Zheng J. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folmes C.D., Dzeja P.P., Nelson T.J., Terzic A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. 2012;11:596–606. doi: 10.1016/j.stem.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varum S., Rodrigues A.S., Moura M.B. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folmes C.D., Dzeja P.P., Nelson T.J., Terzic A. Mitochondria in control of cell fate. Circ. Res. 2012;110:526–529. doi: 10.1161/RES.0b013e31824ae5c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folmes C.D., Nelson T.J., Dzeja P.P., Terzic A. Energy metabolism plasticity enables stemness programs. Ann. N.Y. Acad. Sci. 2012;1254:82–89. doi: 10.1111/j.1749-6632.2012.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shum L.C., White N.S., Mills B.N., de Mesy B.K., Eliseev R.A. Energy metabolism in mesenchymal stem cells during osteogenic differentiation. Stem Cells Dev. 2016;25:114–122. doi: 10.1089/scd.2015.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomasson H.R., Crabb D.W., Edenberg H.J., Li T.K. Alcohol and aldehyde dehydrogenase polymorphisms and alcoholism. Behav. Genet. 1993;23:131–136. doi: 10.1007/BF01067417. [DOI] [PubMed] [Google Scholar]
- 22.Iwahashi K., Suwaki H. Ethanol metabolism, toxicity and genetic polymorphism. Addict. Biol. 1998;3:249–259. doi: 10.1080/13556219872065. [DOI] [PubMed] [Google Scholar]
- 23.Chen C.H., Budas G.R., Churchill E.N. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churchill E.N., Disatnik M.H., Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J. Mol. Cell. Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budas G.R., Disatnik M.H., Chen C.H., Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCvarepsilon) knockout mice. J. Mol. Cell. Cardiol. 2010;48:757–764. doi: 10.1016/j.yjmcc.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H., Guo R., Yu L., Zhang Y., Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur. Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koda K., Salazar-Rodriguez M., Corti F. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation. 2010;122:771–781. doi: 10.1161/CIRCULATIONAHA.110.952481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H., Li J., Gao F., Ren J. Aldehyde dehydrogenase 2 ameliorates acute cardiac toxicity of ethanol: role of protein phosphatase and forkhead transcription factor. J. Am. Coll. Cardiol. 2009;54:2187–2196. doi: 10.1016/j.jacc.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 29.Gomes K.M., Campos J.C., Bechara L.R. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc. Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garaycoechea J.I., Crossan G.P., Langevin F. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 31.Hu C.J., Iyer S., Sataur A. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol. Cell. Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong L., D'Urso A., Toiber D. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semenza G.L. Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 34.Oelze M., Knorr M., Schell R. Regulation of human mitochondrial aldehyde dehydrogenase (ALDH-2) activity by electrophiles in vitro. J. Biol. Chem. 2011;286:8893–8900. doi: 10.1074/jbc.M110.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J., Wang H., Hao P. Inhibition of aldehyde dehydrogenase 2 by oxidative stress is associated with cardiac dysfunction in diabetic rats. Mol. Med. 2011;17:172–179. doi: 10.2119/molmed.2010.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandt M., Garlapati V., Oelze M. NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. Sci. Rep. 2016;6:32554. doi: 10.1038/srep32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poli G., Schaur R.J., Siems W.G., Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Miriyala S., Miao L. Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment. Free Radic. Biol. Med. 2014;72:55–65. doi: 10.1016/j.freeradbiomed.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daiber A., Oelze M., Coldewey M. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol. Pharmacol. 2004;66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- 40.Wenzel P., Hink U., Oelze M. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J. Biol. Chem. 2007;282:792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 41.Szocs K., Lassegue B., Wenzel P. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J. Mol. Cell. Cardiol. 2007;42:1111–1118. doi: 10.1016/j.yjmcc.2007.03.904. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel P., Muller J., Zurmeyer S. ALDH-2 deficiency increases cardiovascular oxidative stress–evidence for indirect antioxidative properties. Biochem. Biophys. Res. Commun. 2008;367:137–143. doi: 10.1016/j.bbrc.2007.12.089. [DOI] [PubMed] [Google Scholar]
- 43.Anastasiou D., Poulogiannis G., Asara J.M. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Z., Zhang J., Stamler J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z., Foster M.W., Zhang J. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sydow K., Daiber A., Oelze M. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J. Clin. Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munzel T., Daiber A., Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 48.Wenzel P., Hink U., Oelze M. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J. Biol. Chem. 2007;282:792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 49.Dudek M., Bednarski M., Bilska A. The role of lipoic acid in prevention of nitroglycerin tolerance. Eur. J. Pharmacol. 2008;591:203–210. doi: 10.1016/j.ejphar.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 50.Esplugues J.V., Rocha M., Nunez C. Complex i dysfunction and tolerance to nitroglycerin: an approach based on mitochondrial-targeted antioxidants. Circ. Res. 2006;99:1067–1075. doi: 10.1161/01.RES.0000250430.62775.99. [DOI] [PubMed] [Google Scholar]
- 51.Wenzel P., Müller J., Zurmeyer S. ALDH-2 deficiency increases cardiovascular oxidative stress – evidence for indirect antioxidative properties. Biochem. Biophys. Res. Co. 2008;367:137–143. doi: 10.1016/j.bbrc.2007.12.089. [DOI] [PubMed] [Google Scholar]
- 52.Perez-Miller S., Younus H., Vanam R. Alda-1 is an agonist and chemical chaperone for the common human aldehyde dehydrogenase 2 variant. Nat. Struct. Mol. Biol. 2010;17:159–164. doi: 10.1038/nsmb.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu T., Liu S., Ma T. Aldehyde dehydrogenase 2 protects against oxidative stress associated with pulmonary arterial hypertension. Redox Biol. 2017;11:286–296. doi: 10.1016/j.redox.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes K.M., Campos J.C., Bechara L.R. Aldehyde dehydrogenase 2 activation in heart failure restores mitochondrial function and improves ventricular function and remodelling. Cardiovasc. Res. 2014;103:498–508. doi: 10.1093/cvr/cvu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beretta M., Gorren A.C., Wenzl M.V. Characterization of the East Asian variant of aldehyde dehydrogenase-2: bioactivation of nitroglycerin and effects of Alda-1. J. Biol. Chem. 2010;285:943–952. doi: 10.1074/jbc.M109.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belmont-Diaz J.A., Yoval-Sanchez B., Calleja-Castaneda L.F., Pardo V.J., Rodriguez-Zavala J.S. Alda-1 modulates the kinetic properties of mitochondrial aldehyde dehydrogenase (ALDH2) Febs J. 2016;283:3637–3650. doi: 10.1111/febs.13833. [DOI] [PubMed] [Google Scholar]
- 57.Perin E.C., Murphy M., Cooke J.P. Rationale and design for PACE: patients with intermittent claudication injected with ALDH bright cells. Am. Heart J. 2014;168:667–673. doi: 10.1016/j.ahj.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitagawa K., Kawamoto T., Kunugita N. Aldehyde dehydrogenase (ALDH) 2 associates with oxidation of methoxyacetaldehyde; In vitro analysis with liver subcellular fraction derived from human and Aldh2 gene targeting mouse. Febs Lett. 2000;476:306–311. doi: 10.1016/s0014-5793(00)01710-5. [DOI] [PubMed] [Google Scholar]
- 59.Wu M., Neilson A., Swift A.L. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]