Abstract
Objective
Increasing evidence implicates advanced paternal age at offspring birth in neuropsychiatric disorders. Advanced maternal age has also been associated with schizophrenia and other neurodevelopmental disorders, whereas younger maternal age has been linked with behavioral disorders. Few studies have considered the specificity of the associations with respect to comorbidity. In addition, most prior studies have been conducted in clinical samples or registries that may reflect more severe forms of psychopathology. The aim of this research is to examine the independent and joint associations of maternal and paternal age with specific subtypes of psychopathology in offspring in a pediatric sample of adolescents with emergent psychiatric syndromes.
Method
8,725 youths (age 8–21) from the Philadelphia Neurodevelopmental Cohort were included in the analyses. Logistic regression models with parental age predicting offspring psychopathology were adjusted for sociodemographic factors and comorbid disorders.
Results
We found that younger parental ages were generally associated with increased rates of offspring psychopathology. After controlling for sociodemographic characteristics and comorbidity, both younger maternal and paternal ages were associated with behavior syndromes and psychosis in youth, whereas advanced paternal age was associated with pervasive developmental disorders/autism spectrum disorder (PDD/ASD).
Conclusion
These findings suggest that both younger and older parental age at birth are associated with specific forms of psychopathology in offspring. The persistence of the influence of parental age after control for demographic factors and an index of social environment suggests that additional explanations for these findings should be examined in future studies.
Keywords: epidemiology, psychopathology, maternal age, paternal age, parental age
INTRODUCTION
Increasing evidence implicates parental age at birth in the etiology of neuropsychiatric disorders. Early studies described older parental age among psychiatric inpatients,1 particularly schizophrenia.2 These findings have been extended to other neurodevelopmental disorders (NDDs),3 including autism spectrum disorder (ASD).4 Advanced paternal age has also been implicated in the etiology of bipolar disorder,5 suicide attempts, substance use problems, low educational attainment,6 cognitive deficits,7 and attention-deficit/hyperactivity disorder (ADHD).8 Few studies of advanced paternal age considered the potential joint influence of advanced maternal age on offspring psychopathology, which has also been associated with NDDs, specifically ASD,9 schizophrenia,10 and bipolar disorder.11 For example, Croen et al. reported that both paternal and maternal ages were associated with offspring ASD.12
Younger parental age has also been associated with increased rates of schizophrenia and other disorders. A meta-analysis by Miller et al.13 showed that younger paternal age is associated with schizophrenia risk, but only among male offspring. Younger maternal age at birth is associated with behavioral disorders, including ADHD14 and substance use.15 For example, Chang et al.16 reported a 78% increased risk of ADHD in children of mothers under age twenty.
In addition to differences in risk of psychopathology among both younger and older parents, several studies have found nonlinear associations between maternal or paternal age and psychopathology. Typically these are U-shaped associations that indicate increased risk imparted by both younger and older parents as illustrated by studies of paternal age in bipolar disorder,17 schizophrenia,15 and major depressive disorder.18 Furthermore, in one study,19 both older paternal age and younger maternal age predicted greater positive psychotic symptoms, but not negative or disorganized features of schizophrenia. These nonlinear relationships may reflect different explanations for the impact of maternal versus paternal age, and younger versus older parental age on specific domains of psychopathology in offspring.
There are different proposed biological mechanisms underlying the advanced maternal and paternal age effects. Notably, de novo mutations have been proposed as the causal agent in the association between advanced paternal age and schizophrenia due to the increased rate of de novo mutations with increasing paternal age.20,21 However, it has been reported that age-related mutations may explain only 10–20% of the increased risk of mental illness in children born to older fathers, and that genetic risk factors shared by older fathers and their offspring are a credible alternative explanation for the advanced paternal age effect.22 The potential genetic mechanisms underlying advanced maternal age effects have not been elucidated and are likely a complex combination of genetic and environmental factors, but recent evidence suggests that de novo mutations are also associated with advancing maternal age.23 Alternatively, rather than genetic origins, the association between child psychopathology and younger parental ages is assumed to result from psychosocial factors, including poorer health behaviors during pregnancy and after birth, the potential suboptimal child-rearing environment secondary to the economic and educational disadvantage that parents suffer as a result of early parenthood (especially mothers24). In addition, mothers who give birth in their teens are 2.5 times more likely to have a behavioral disorder themselves,25 which could suggest genetic susceptibility that could be transmitted to their children, or be the result of having children at a young age.
There are several methodological challenges in studying the association between parental ages at birth and offspring psychopathology. First, the strong correlation between maternal and paternal ages leads to co-linearity in regression models that include age as a continuous variable. In order to address this problem, parental age has often been analyzed categorically, but different age binning strategies and different reference age groups in statistical analyses make it difficult to aggregate findings. Second, identifying the specificity of links between parental age and different classes of psychopathology requires large and comprehensively evaluated samples. Third, identifying potential explanations for associations between parental age and offspring psychopathology requires information on the full range of psychopathology that is often unavailable in large data sets derived from most registry or clinical samples, and also tends to reflect the most severe cases.
Aims
The overall goal of this investigation is to examine the joint and independent associations between maternal and paternal age with offspring psychopathology in a community-based sample of youths from the greater Philadelphia area with emergent psychiatric symptoms and syndromes. The specific aims are to: 1) investigate the association and direction of the individual and joint influence of maternal and paternal age on offspring psychopathology, and if an association is found to evaluate whether risk of particular disorders in offspring increases or decreases with parental age; and 2) examine the specificity of parental age at birth of offspring with respect to anxiety, behavior, mood, pervasive developmental disorder (PDD)/ASD, and psychotic symptoms in youth. Clarifying these associations may provide insight into the biological or psychosocial mechanisms that lead to child or adolescent psychopathology, and may provide inroads into prevention.
METHOD
Participants
The Philadelphia Neurodevelopmental Cohort (PNC) procedures have been described elsewhere.26 Briefly, the sample included youths (age 8–21 years) recruited through a National Institute of Mental Health (NIMH)-funded Grand Opportunity study characterizing clinical and neurobehavioral features in a genotyped, prospectively accrued community cohort. All participants were previously consented for genomic studies when they presented for pediatric services within the Children’s Hospital of Philadelphia (CHOP) healthcare network. Participants provided a blood sample for genetic studies and authorized access to electronic medical records. The institutional review boards at the University of Pennsylvania and CHOP approved the protocol.
Demographic Assessment
Race was self-identified as European American and other races, including African American and other non-European American.26 Characterization of the social environment is described in detail in Moore et al.27 Briefly, we linked participant address information to 2010 census and crime data, and then completed exploratory factor analysis to derive social and criminal dimensions of participants’ environments. These were used to calculate environment-level scores, and were merged with individual-level variables. The socioeconomic status (SES)-related variables included percent in poverty, percent married, median family income, and percent with at least a high school education. Here we use these factor scores as a proxy for SES.
Parental Age Assessment
Maternal and paternal ages were calculated from the parental and child’s dates of birth. Extremely low parental age values were eliminated (<12 years). Where only the year of birth of the parent was recorded, July 1 was selected for the date of birth so that the maximum error would be 6 months. Maternal and paternal ages were evaluated both continuously and categorically. Maternal age was divided into six 5-year groups (<20, 20–24, 25–29, 30–34, 35–39, ≥40), and paternal age was divided into seven 5-year groups (<20, 20–24, 25–29, 30–34, 35–39, 40–44, ≥45) (as in 15).
Clinical Assessments
Lifetime psychopathology was assessed with a comprehensive computerized adaptation of the NIMH Genetic Epidemiology Research Branch Kiddie-Schedule for Affective Disorders and Schizophrenia Family Study Interview28 for structured evaluation of psychopathology domains (GOASSESS) that was constructed specifically for the PNC.26 Adult (≥18 years) and middle (11–17 years) probands reported on themselves. Collateral informants (most often the mother) reported on probands aged 8–17 years. Disorders were rated dimensionally (0=did not endorse screening question(s); 1=endorsed one or more screening questions; 2=subthreshold: generally falling short of minimal DSM-IV criteria for disorder or set of disorders; 3=threshold: meeting assessed symptom criteria for DSM-IV criteria for disorder or set of disorders, but with minimal or no associated distress/impairment; 4=significant: meeting assessed symptom criteria for DSM-IV as well as endorsing distress or impairment rating ≥5 on a 0–10 scale, typically meeting DSM-IV disorder criteria), and dichotomized at 4 versus all others for analysis. Disorder classes evaluated by GOASSESS included: anxiety (ANX: generalized anxiety disorder, separation anxiety, specific phobia, social phobia, panic disorder, agoraphobia); behavioral disorders (BEH: ADHD, conduct disorder); mood disorders (MOOD: depression, mania); and psychosis (PSY: psychotic symptoms including hallucinations, delusions). PDD/ASD was assessed in the medical history section (administered to collateral informants of youth age 8–17, and probands age 18–21) by the probe: “Does/did your child have this problem… autism or pervasive developmental disorder?” with a forced-choice yes or no answer. Medical charts, when available, were reviewed for those participants in order to validate collateral/self-reported diagnoses.
Statistical Analyses
Statistical analyses were completed in SAS version 9.329 and R version 3.2.3.30 Because there are related individuals in the PNC sample, one child was randomly selected per family via SAS proc SURVEYSELECT, with the family identifier as the strata, yielding an analytic sample size of 8,725 participants. Both crude and adjusted logistic regression models in R with parental age predicting offspring psychopathology were then completed. Four models were run: 1) crude analyses; 2) adjustment for sociodemographic variables (age, sex, race, SES); 3) adjustment for all other psychiatric syndromes endorsed by the participant (as a test of disorder specificity); and 4) adjustment for both sociodemographic variables and comorbid disorders. First, mean parental age was examined in order to establish if there was an overall parental age effect; then mothers and fathers were examined separately to investigate if there was specificity of the association. Odds ratios (OR) less than 1 indicate an association with younger age, while ORs greater than 1 indicate an association with older age. Models adjusted for the opposite parent’s 5-year age category (with the 25–29-year-old group as the reference group) are presented in Supplement 1, available online.
Due to substantial missing data for paternal age, multiple imputation analysis using fully conditional specification was completed in R (package mice: Multiple Imputation by Chained Equations).31 Five imputations were run, with sex, age, binary race, SES, maternal age (years), maternal age category, paternal age (years), paternal age category, mood, anxiety, behavior, psychosis, and PDD/ASD variables all included in the imputation model. The resultant datasets were analyzed as described above, and their results pooled to create the final parameter estimates and statistical significance. Pooled imputation results are presented below. The imputation plots, original data results, and differences between the participants who were and were not missing parental age information are included in Supplement 1, available online.
RESULTS
Sample Description
Of the 50,540 participants genotyped, 36% met initial inclusion criteria. Among ineligible youths, approximately 31% had serious medical conditions precluding participation in the study (severe developmental delay, significant hearing loss, limited mobility), 44% were outside the study age range or deceased, and 36% declined to be re-contacted for future studies.26 The remaining sample of 18,344 ambulatory youths in stable health, proficient in English, physically and cognitively capable of participating in an interview and performing computerized neurocognitive testing, were stratified for age, sex, and ethnicity, and randomly selected for additional screening. Youths with disorders that impaired motility or cognition (e.g. significant paresis or palsy, intellectual disability) were excluded. Participants were not recruited from psychiatric clinics; therefore, the sample is not enriched for mental health help-seeking individuals. A total of 9,498 participants enrolled in the study, and provided informed consent/assent after receiving a complete description of the study. The sample is 51.7% female, and 55.7% of participants self-identified as European American, and 32.9% as other races, including African American and other non-European American (11.4%: Asian, Native American, Hawaiian Pacific Islander, Multi-Racial). The mean age of the entire sample is 14.2 years. Further recruitment details and a flow chart of sample inclusion and exclusion are provided in Calkins et al.,26 and more information is included in Supplement 1, available online.
As shown in Table 1, after limiting to one child per family and imputing missing parental age information, the final subsample of the PNC (n=8,725) was 51.83% female and 56.72% European American, with a mean age of 14.21 years (SD=3.67). Rates of psychiatric symptomatology varied substantially: 46.34% of the sample reported significant anxiety symptoms; 27.29% of the sample had significant anxiety symptoms when specific phobias are excluded; 21.07% significant behavior symptoms; 12.42% significant mood symptoms; 3.05% PDD/ASD; 3.89% significant positive symptoms of psychosis, and substantial comorbidity was evident (see Supplement 1, available online, for more detail). These rates differed slightly by age, race, and sex. Of those participants with PDD/ASD with available medical records, two thirds had verified or probable PDD/ASD diagnoses.
Table 1.
Philadelphia Neurodevelopmental Cohort: Demographic Characteristics
| Total sample | Proband Age Group (y) | Race | Sex | Missing n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 8–10 | 11–17 | 18–21 | White | Other | Female | Male | |||
| n (%) | 8725 | 3484 (39.93) |
4271 (48.95) |
970 (11.12) |
4949 (56.72) |
3776 (43.28) |
4522 (51.83) |
4203 (48.17) |
|
| Age (years) | |||||||||
| Mean (SD) | 14.21 (3.67) |
10.38 (1.45) |
16.02 (1.72) |
19.96 (0.78) |
14.21 (3.64) |
14.21 (3.71) |
14.55 (3.66) |
13.85 (13.85) |
|
| Sex | |||||||||
| n (%) Female | 4522 (51.83) |
1631 (46.81) |
2319 (54.30) |
572 (58.97) |
2455 (54.29) |
2067 (54.74) |
|||
| Race | |||||||||
| % European American | 56.72 | 56.72 | 57.25 | 54.43 | 54.29 | 59.34 | |||
| % Other | 43.28 | 43.28 | 42.75 | 45.57 | 45.71 | 54.29 | |||
| Parental Age (years) | |||||||||
| Maternal n | 7473 | 3074 | 3655 | 744 | 4395 | 3078 | 3892 | 3581 | 1252 (14.35%) |
| Mean Maternal (SD) | 29.06 (6.12) |
29.21 (6.21) |
29.07 (6.13) |
28.36 (5.67) |
30.96 (5.13) |
26.34 (6.39) |
28.91 (6.14) |
29.22 (6.09) |
|
| Paternal n | 6206 | 2651 | 2967 | 588 | 4108 | 2098 | 3171 | 3035 | 2519 (28.87%) |
| Mean Paternal (SD) | 32.08 (6.49) |
32.27 (6.65) |
32.07 (6.41) |
31.27 (6.05) |
33.19 (5.59) |
29.91 (7.49) |
31.91 (6.51) |
32.26 (6.45) |
|
| Social Environment | |||||||||
| Mean (SD) | 0.03 (0.99) |
0.04 (0.98) |
0.04 (0.98) |
−0.09 (1.04) |
0.62 (0.49) |
−0.75 (0.94) |
−0.01 (1.00) |
0.07 (0.97) |
|
| Psychiatric Syndromes | |||||||||
| Anxiety n (%) | 4043 (46.34) |
1598 (45.87) |
1598 (48.02) |
1598 (40.62) |
2148 (43.40) |
1895 (50.19) |
1727 (51.22) |
1727 (41.09) |
|
| Anxietya n (%) | 2381 (27.29) |
783 (22.47) |
1339 (31.35) |
259 (26.70) |
1202 (24.29) |
1179 (31.22) |
1379 (30.50) |
1002 (23.84) |
|
| Behavior n (%) | 1838 (21.07) |
860 (24.68) |
902 (21.12) |
76 (7.84) |
894 (18.06) |
944 (25.00) |
748 (16.54) |
1090 (25.93) |
|
| Mood n (%) | 1084 (12.42) |
180 (5.17) |
722 (16.90) |
182 (18.76) |
611 (12.35) |
473 (12.53) |
700 (15.48) |
384 (9.14) |
|
| PDD/ASD n (%) | 265 (3.05) |
148 (4.26) |
109 (2.57) |
8 (0.82) |
200 (4.05) |
65 (1.73) |
54 (1.20) |
211 (5.04) |
36 (0.41%) |
| Psychosis n (%) | 339 (3.89) |
107 (3.07) |
211 (4.94) |
21 (2.16) |
122 (2.47) |
217 (5.75) |
156 (4.05) |
156 (3.71) |
|
Note: ASD = autism spectrum disorder; PDD = pervasive developmental disorder.
Without specific phobias.
Parental Age
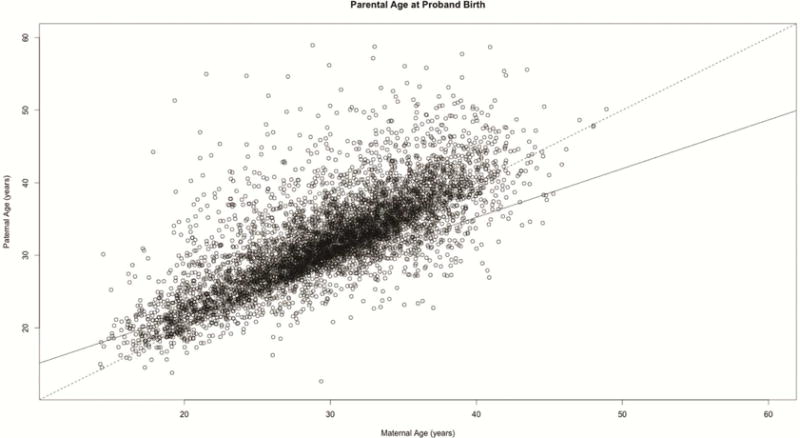
7,473 participants had maternal age data (85.65%, mean=29.06 years, SD=6.12) and 6,206 had paternal age data (71.13%, mean=32.08 years, SD=6.49). The distribution of maternal by paternal age is shown in Figure 1; parental ages were highly correlated (r=0.76). Histograms of maternal and paternal age are shown in Supplement 1, available online. Participants who are missing data are likely to be older and not European American. There were no sex differences between those with and without missing data (see Supplement 1, available online). Results for the pooled imputation logistic regression models of mean parental age predicting psychiatric syndromes are shown in Table 2. In the unadjusted models, younger parental ages at offspring birth were generally associated with increased rates of offspring psychopathology, with the exception of PDD/ASD, which was associated with older parental age. After adjustment for sociodemographic factors, the majority of these associations remained, except for mood disorders and PDD/ASD (anxiety: OR=0.991, p=.049; behavior: OR=0.976, p≤.001; mood: OR=0.990, p=.157; PDD/ASD: 1.018, p=.189; psychosis: OR=0.962, p=.004). After adjustment for comorbid disorders in offspring, there was also an association between older parental age and PDD/ASD (OR=1.065, p≤.001). Combining adjustment to include sociodemographic factors and comorbid psychopathology resulted in a loss of association between parental age and anxiety, but all other associations remained. Thus, after adjusting for sociodemographic correlates and comorbidity, for each year increase in mean parental age, the log odds of having a child with PDD/ASD increases by 3.4%, and the log odds of having a child with a behavior disorder or psychosis decreases by 2.3% and 3.5%, respectively.
Figure 1.
Maternal age by paternal age – identity line (black dashed) and regression line (black) are shown.
Table 2.
Pooled Imputation Results: Parental Age Predicting Psychiatric Syndromes
| Mean Parental Age | Adjusted for Sociodemographics | Adjusted for Comorbidity | Adjusted for Sociodemographics and Comorbidity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% Wald CI | p | OR | 95% Wald CI | p | OR | 95% Wald CI | p | ||||
| Anxiety | 0.991 | 0.982 | 1.000 | .049* | 0.989 | 0.980 | 0.997 | .007* | 0.995 | 0.986 | 1.004 | .307 |
| Anxietya | 0.986 | 0.975 | 0.996 | .008* | 0.980 | 0.970 | 0.990 | <.001* | 0.990 | 0.980 | 1.001 | .062 |
| Behavior | 0.976 | 0.965 | 0.988 | <.001* | 0.966 | 0.956 | 0.976 | <.001* | 0.977 | 0.966 | 0.988 | <.001* |
| Mood | 0.989 | 0.975 | 1.004 | .157 | 0.997 | 0.983 | 1.011 | .684 | 0.997 | 0.982 | 1.012 | .672 |
| PDD/ASD | 1.018 | 0.991 | 1.046 | .189 | 1.065 | 1.039 | 1.091 | <.001* | 1.034 | 1.006 | 1.062 | .018* |
| Psychosis | 0.962 | 0.938 | 0.987 | .004* | 0.946 | 0.925 | 0.969 | <.001* | 0.965 | 0.942 | 0.989 | .004* |
| Maternal Age | Adjusted for Sociodemographics | Adjusted for Comorbidity | Adjusted for Sociodemographics and Comorbidity | |||||||||
| OR | 95% Wald CI | p | OR | 95% Wald CI | p | OR | 95% Wald CI | p | ||||
| Anxiety | 0.992 | 0.983 | 1.000 | .060 | 0.991 | 0.983 | 0.998 | .020* | 0.997 | 0.989 | 1.006 | .542 |
| Anxietya | 0.985 | 0.975 | 0.995 | .003* | 0.981 | 0.972 | 0.990 | <.001* | 0.991 | 0.981 | 1.000 | .062* |
| Behavior | 0.974 | 0.965 | 0.983 | <.001* | 0.965 | 0.956 | 0.974 | <.001* | 0.975 | 0.966 | 0.985 | <.001* |
| Mood | 0.982 | 0.970 | 0.995 | .008* | 0.992 | 0.980 | 1.004 | .193 | 0.989 | 0.976 | 1.003 | .117 |
| PDD/ASD | 1.010 | 0.983 | 1.036 | .474 | 1.056 | 1.032 | 1.080 | <.001* | 1.025 | 0.998 | 1.052 | .070 |
| Psychosis | 0.965 | 0.945 | 0.986 | .002* | 0.952 | 0.933 | 0.971 | <.001* | 0.971 | 0.950 | 0.991 | .006* |
| Paternal Age | Adjusted for Sociodemographics | Adjusted for Comorbidity | Adjusted for Sociodemographics and Comorbidity | |||||||||
| OR | 95% Wald CI | p | OR | 95% Wald CI | p | OR | 95% Wald CI | p | ||||
| Anxiety | 0.994 | 0.986 | 1.001 | .112 | 0.991 | 0.983 | 0.999 | .022* | 0.995 | 0.987 | 1.004 | .269 |
| Anxietya | 0.992 | 0.984 | 1.001 | .071 | 0.987 | 0.978 | 0.995 | .002* | 0.994 | 0.985 | 1.003 | .162 |
| Behavior | 0.988 | 0.978 | 0.998 | .025* | 0.980 | 0.971 | 0.989 | <.001* | 0.988 | 0.978 | 0.997 | .014* |
| Mood | 1.000 | 0.986 | 1.014 | .999 | 1.003 | 0.990 | 1.017 | .648 | 1.005 | 0.991 | 1.019 | .480 |
| PDD/ASD | 1.019 | 0.997 | 1.042 | .095 | 1.046 | 1.025 | 1.067 | <.001* | 1.028 | 1.005 | 1.051 | .015* |
| Psychosis | 0.976 | 0.952 | 0.999 | .043* | 0.962 | 0.940 | 0.985 | .002* | 0.975 | 0.954 | 0.997 | .030* |
Note: Sociodemographic factors include the proband’s age, sex, binary race, and social environment index, with white males as the reference group. Imputed estimates are the pooled results from five imputations. ASD = autism spectrum disorder; PDD = pervasive developmental disorder.
Without specific phobias.
Indicates statistical significance at the α=0.05 level
Maternal Age
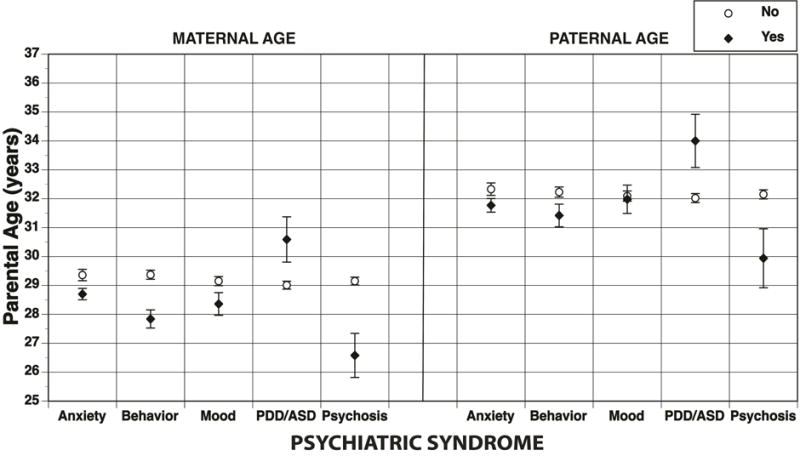
The mean and 95% CI of psychiatric syndromes by maternal age are shown in Figure 2. The results for the pooled imputation logistic regression models of maternal age predicting psychiatric syndromes are shown in Table 2. As in the parental age analyses, in the unadjusted models, younger maternal ages at offspring birth were generally associated with increased rates of offspring psychopathology, with the exception of PDD/ASD, which was associated with older maternal age. The results were broadly consistent in the adjusted models with the parental age results, with the exception that when controlling for sociodemographic factors, there was a significant effect of younger maternal age on mood disorders (OR=0.982; p=.008), but not on anxiety (OR=0.992; p=.060). The effects of younger maternal age persisted for all disorders except mood when adjusting for comorbidity, with an additional association between advanced maternal age and PDD/ASD (OR=1.056; p≤.001). Adjusting for both sociodemographics and comorbidity yielded results that mimicked those shown when adjusting for sociodemographics alone, with the exception of the loss of the mood association.
Figure 2.
Psychiatric syndromes by parental age (years) – mean and 95% CI are shown. Note: ASD = autism spectrum disorder; PDD = pervasive developmental disorder.
Paternal Age
The mean and 95% CI of psychiatric syndromes by paternal age are shown in Figure 2. The results for the pooled imputation logistic regression models of paternal age predicting psychiatric syndromes are shown in Table 2. In the crude models anxiety (OR=0.988; p=.001), behavior (OR=0.981; p≤.001), and psychosis (OR=0.958; p=.001) were associated with younger paternal age, while PDD/ASD was associated with older paternal age (OR=1.035; p≤.001); there was no association between paternal age and mood disorders (OR=0.995; p=.446). Adjusting for sociodemographics reduced the significance of the majority of these associations; only the associations between younger paternal age and behavior (OR=0.988, p=.025) and psychosis (OR=0.976, p=.043) persisted. Adjustment for comorbidity alone yielded quite different results: younger paternal age was associated with anxiety, behavior, and psychosis, and older paternal age was associated with PDD/ASD. Combining adjustment for sociodemographics and comorbidity showed similar results to that of controlling for sociodemographics alone, but with the addition of the association between advanced paternal age and PDD/ASD (OR=1.028; p=.015). Notably, these results do not change when fathers older than 60 years were removed from the analyses (data not shown).
Effects of Covariates
The effects of sociodemographic correlates in this sample were consistent with those of nationally representative community surveys.32,33 These effects were generally consistent across all of the models described above. In the fully adjusted model of mean parental age, we found the typical sex effects in psychopathology, with males having higher odds of behavior problems and PDD/ASD than females; younger youth had greater odds of anxiety with phobias, behavior disorders, and PDD/ASD, and older youth had higher odds of anxiety disorders without phobias, mood disorders, and psychosis. White youth had greater odds of mood disorders and PDD/ASD, whereas non-white youth had greater odds of anxiety, behavior disorders, and psychosis. Finally, those at greater social disadvantage had higher odds of anxiety, behavior disorders, and psychosis, whereas youth with PDD/ASD tended to have less social disadvantage.
Imputation Versus Original Data Results
Overall, the results of the pooled imputation analyses mimic those of the original data (Supplement 1, available online). In general, the imputed estimates were more significant than the original data, probably due to the increased sample size of the imputed data set. Differences were most notable in PDD/ASD, the rarest condition in the sample.
DISCUSSION
We found that there were associations between both younger and older parental age and different maternal and paternal influences on psychopathology in youth. Specifically, in the fully adjusted models, younger maternal and paternal ages were associated with behavior disorders and psychosis, whereas older paternal age was associated with PDD/ASD. To our knowledge, this is the largest study in the US to examine parental age and multiple domains of offspring psychopathology simultaneously in a pediatric sample not selected for specific disorders with direct clinical assessments. These data extend previous parental age research of adult registry and clinical samples to a community-based sample of children and adolescents. These findings on the complex influence of parental age at offspring birth with specific offspring disorders, already evident in early stages of development, may provide insight into their etiology.
The associations between younger parental ages at offspring birth and behavior disorders corroborate previous research,15,16 while the finding of younger parental age association with psychotic symptoms has been reported less frequently. This latter finding was contrary to our expectation that older parental age at birth, particularly paternal age, would be associated with a greater risk of psychotic symptoms in youth. This expectation was based on robust literature documenting associations between older parental age and psychosis, though younger parental age has also been associated with schizophrenia among male offspring.13 Our intentionally broad psychosis spectrum category includes subthreshold symptoms (i.e., odd or unusual thoughts, auditory perceptions, reality confusion, audible thoughts, grandiosity, mind tricks, superstitions, persecutory or suspicious thoughts, predicting the future, mind reading, thought control),34 that are not equivalent to a diagnosis of psychosis or schizophrenia, the outcome of interest in most prior work. That we did find an association between NDDs with advanced paternal age may reflect a broader influence of parental age on neurodevelopment.
The specific association of younger maternal age with behavior and mood disorders confirms previous studies regarding younger maternal age with ADHD and conduct disorders.35 The association between younger maternal age and mood disorders has not been widely reported, though McGrath et al.15 found a small effect of younger maternal age on offspring mood disorders in a registry study, where only the most severe cases would have been included. The finding that the maternal age effect persisted after adjustment for our index of social disadvantage suggests that other factors may influence the increased risk of these conditions among offspring. Neurodevelopmental processes in cognition and behavior may be mediated by correlates of young parenthood that may influence both the child’s biologic (prenatal environment, nutrition, etc.) and environmental (environmental enrichment, parenting structure, etc.) circumstances. The dissipation of the association after controlling for comorbidity indicates the lack of specificity of the association between maternal age and mood disorder symptoms. Other studies25 have reported an increased risk of behavior disorders, anxiety, and posttraumatic stress disorder in low-income mothers who gave birth in their teens, and it is worth considering how this could impact their offspring.
The association between advanced paternal age and PDD/ASD has been shown previously,4 but we did not confirm the association between advanced maternal age and PDD/ASD.9 The specific link between advanced paternal age and PDD/ASD suggests that the parental age effect may apply to NDDs in general rather than solely schizophrenia as indicated by the classic studies of this issue. Furthermore, it has been proposed that the advanced paternal age effect in schizophrenia is limited to those people with a maternal history of psychosis, which we were not able to test in this sample.36 The PDD/ASD association observed in our study may therefore reflect the spectrum of ASD, other NDDs, and the subsequent development of schizophrenia under the hypothesis that there are similar genetic and environmental (i.e. migration, etc.) risks underlying NDDs.37,38 This is a plausible explanation because the youth in our study did not have severe ASD, which was a study exclusion, so these disorders in the PNC represent high functioning individuals with ASD.
There are different proposed biological mechanisms underlying the advanced maternal and paternal age effects. Malaspina et al.20 proposed that de novo mutations may be the causal agent in the association between advanced paternal age and schizophrenia due to the increased rate of de novo mutations with increasing paternal age.21 This is biologically plausible given that de novo mutations have also been associated with schizophrenia.39 Recent work from Gratten et al,22 however, showed that age-related mutations may explain only 10–20% of the increased risk of mental illness in children born to older fathers. They posit that genetic risk factors shared by older fathers and their offspring are a credible alternative explanation for the advanced paternal age effect. That is, psychiatric or personality characteristics that led to older age at fatherhood may be transmitted to their offspring, e.g. fathers of children with ASD exhibiting a broader autism phenotype,40 or schizoid or schizotypal features in the fathers of persons with schizophrenia.41
Potential genetic mechanisms underlying advanced maternal age effects have not been elucidated and are likely a complex combination of genetic and environmental factors. Non-disjunction errors (i.e. autosomal trisomies), such as the well-documented maternal age association with Down syndrome,42 have not been independently associated with psychosis or schizophrenia; however, detection of schizophrenia or other NDDs may not be evident among people with major trisomies. Recent evidence also shows that de novo mutations presumed to explain the paternal age effect are also associated with advancing maternal age.23
Rather than genetic origins, the association between child psychopathology and younger parental ages is assumed to result from psychosocial factors, including poorer health behaviors during pregnancy and after birth, the potential suboptimal child-rearing environment secondary to the economic and educational disadvantage that parents suffer as a result of early parenthood (especially mothers24). One cannot rule out that the traits that led individuals to younger parenthood (e.g. impulse control problems, risk-taking behaviors) were then transmitted to their offspring. Though speculative, and assuming directional causality that cannot be established from this study, this work serves to reinforce current health practice encouraging that delaying pregnancy past adolescence may have major benefits to offspring. Teen pregnancy has generally been on the decline in the US, so the continuation of current strategies seems worthwhile.43 With respect to reducing older parentage, some have suggested sperm banking to prevent the increased rate of paternal de novo mutation,44 but long-term studies on sperm quality after storage have not been completed, so this may be a premature suggestion.45 Furthermore, the slightly increased rates of NDDs after the use of assisted reproduction should be considered,46 noting that studies have presented both positive and negative associations.47,48 NDDs may also result from the risks associated with multiparous birth, rather than of assisted reproduction itself. Furthermore, older parental age effects could be attributable to comorbid medical disorders, e.g. cardiovascular disease, that are more likely in older fathers.
This study has several limitations. The findings may not persist into adulthood, as this young age cohort has not passed through the full risk period for most disorders. It is also possible that there is an impact of informant bias on our diagnoses; even though we did conduct diagnostic screening for major classes of psychopathology, we did not conduct comprehensive diagnostic interviews, particularly for ASD because of a lack of feasibility; however, we were able to validate a substantial proportion of the self-reported diagnoses of PDD/ASD by medical chart review. Previous studies have demonstrated the reliability of parental reports with clinical diagnoses of ASD.49 Moreover, the study inclusion criteria limited our participants with PDD/ASD to the higher-functioning subset, so it is likely that our findings would be strengthened had we completed a more rigorous ASD assessment. Since we do not have systematic information about pre-/perinatal complications, gestational age, and early development, we were unable to examine hypotheses related to this, which may modify the associations presented here. In addition, information about parental characteristics was somewhat limited, but our adjustment for social disadvantage (e.g., poverty, crowding, crime) in the models, even though not an individual-level variable, can inform potential social environmental factors on these conditions in youth.27
Strengths of this study include the large community-based sample recruited from pediatrics rather than clinical psychiatric settings, and the broad range of psychiatric symptomatology assessed that enhances the generalizability of the findings. Although this was not a representative sample of the general population, the prevalence and demographic correlates of the diagnoses are comparable to that of nationally representative samples of youth.33,50 Most research on the parental age effect that has been conducted in older, clinical samples and previous research18 has found different results in clinical and population-based samples. The young age of the participants is an additional strength. Though the majority of study participants have not yet passed through the period of greatest risk for onset of psychiatric disorders, we still found strong associations between parental age and psychosis spectrum symptoms, behavioral disorders, and NDDs. Examining whether parental age influences in disorders across the full age spectrum in youth, including mood and psychotic disorders that emerge in late adolescence and early adulthood, and anxiety disorders that begin earlier51 may facilitate inquiry into potential explanations for these associations. Studies of youth may also inform developmental etiology, as postulated by Jones,52 who suggested that the transformation of the adolescent brain and the balance of connectivity between brain areas might actually indicate that normal development leads to the risk of psychopathology, rather than specifically atypical development. These early manifestations may provide insight into the mechanisms underlying parental age and psychopathology. Finally, by analyzing parental ages as continuous variables, we did not impose arbitrary cut-points for younger or advanced ages.
Future studies in community-based samples are necessary to replicate these findings. Further investigation into the domains underlying the broad categories of psychopathology investigated in this study may enhance our understanding of early manifestations and mechanisms for these findings. Additionally, examining potential correlates that were not included here, including parental disorder, education, or marital status may provide valuable information into the nature of the associations that we have found. Examination of birth order,53 interval,54 or difference in parental ages55 may also be informative to elucidate mechanisms underlying the parental age effect. Finally, assessment of genetic markers that may underlie the parental age phenomena, specifically de novo mutations, may be a beneficial line of inquiry.
This work confirms previous research on the associations between parental age and psychopathology in offspring, and extends this work to a large, pediatric sample assessed for the full range of psychiatric syndromes. We found specific associations between younger parental age and behavior disorders, and psychosis, and advanced paternal age on PDD/ASD in offspring. The persistence of the influence of parental age after control for demographic factors and an index of social environment suggests that additional explanations for these findings should be examined in future studies. The small effect sizes also indicate that parental age is only one of a complex network of vulnerability factors for psychiatric disorders.56
Supplementary Material
Acknowledgments
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was supported by RC2 grants from the National Institute of Mental Health (NIMH): MH089983 and MH089924 (R.E. Gur and H. Hakonarson). The Philadelphia Neurodevelopmental Cohort sample provides a public domain resource for investigation and characterization of gene networks underlying neuronal vulnerability leading to mental disorders. Data generated are available through the NIMH Database of Genotypes and Phenotypes (dbGaP, http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v1.p1, dbGaP Study Accession: phs000607.v1.p1).
Dr. Bilker served as the statistical expert for this research.
The authors are grateful to the participants of the PNC and their families, and all of the members of the recruitment, assessment, and data teams whose contributions made this work possible. Kosha Ruparel, MSE, MS, of the University of Pennsylvania, and Lihong Cui, MS, of NIMH, provided valuable assistance with this work and their input is appreciated. Preliminary discussions with Eleisa Heron, PhD, of Trinity College Dublin, were also fundamental in completing this research.
Dr. Merikangas has received support from a NIMH T32 grant to R.E. Gur (MH019112) and has received travel funding from the Society of Biological Psychiatry (Domestic Travel Fellowship Award 2016). Dr. Bilker has served as a consultant to Janssen Pharmaceutical about unrelated medications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article is discussed in an editorial by Dr. Daniel Dickstein on page xx.
Supplemental material cited in this article is available online.
Disclosure: Drs. Calkins, Moore, R.C. Gur, and R.E. Gur report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Alison K. Merikangas, Neuropsychiatry Section and the Lifespan Brain Institute, Children’s Hospital of Philadelphia and Perelman School of Medicine, Philadelphia.
Dr. Monica E. Calkins, Neuropsychiatry Section and the Lifespan Brain Institute, Children’s Hospital of Philadelphia and Perelman School of Medicine, Philadelphia.
Dr. Warren B. Bilker, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
Dr. Tyler M. Moore, Neuropsychiatry Section and the Lifespan Brain Institute, Children’s Hospital of Philadelphia and Perelman School of Medicine, Philadelphia.
Dr. Ruben C. Gur, Neuropsychiatry Section and the Lifespan Brain Institute, Children’s Hospital of Philadelphia and Perelman School of Medicine, Philadelphia.
Dr. Raquel E. Gur, Neuropsychiatry Section and the Lifespan Brain Institute, Children’s Hospital of Philadelphia and Perelman School of Medicine, Philadelphia.
References
- 1.Hare EH, Moran PA. Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. Br J Psychiatry. 1979;134:169–177. doi: 10.1192/bjp.134.2.169. [DOI] [PubMed] [Google Scholar]
- 2.Johanson E. A study of schizophrenia in the male: a psychiatric and social study based on 138 cases with follow up. Acta Psychiatr Neurol Scand Suppl. 1958;125:1–132. [PubMed] [Google Scholar]
- 3.Goriely A, McGrath JJ, Hultman CM, Wilkie AO, Malaspina D. “Selfish spermatogonial selection”: a novel mechanism for the association between advanced paternal age and neurodevelopmental disorders. Am J Psychiatry. 2013;170:599–608. doi: 10.1176/appi.ajp.2013.12101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichenberg A, Gross R, Weiser M, et al. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63:1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 5.Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Langstrom N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65:1034–1040. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 6.D’Onofrio BM, Rickert ME, Frans E, et al. Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry. 2014;71:432–438. doi: 10.1001/jamapsychiatry.2013.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha S, Barnett AG, Foldi C, et al. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009;6:e40. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saha S, Barnett AG, Buka SL, McGrath JJ. Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophr Res. 2009;115:130–135. doi: 10.1016/j.schres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Durkin MS, Maenner MJ, Newschaffer CJ, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168:1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Castroman J, Gomez DD, Belloso JJ, et al. Differences in maternal and paternal age between schizophrenia and other psychiatric disorders. Schizophr Res. 2010;116:184–190. doi: 10.1016/j.schres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Menezes PR, Lewis G, Rasmussen F, et al. Paternal and maternal ages at conception and risk of bipolar affective disorder in their offspring. Psychol Med. 2010;40:477–485. doi: 10.1017/S003329170999064X. [DOI] [PubMed] [Google Scholar]
- 12.Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med. 2007;161:334–340. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- 13.Miller B, Messias E, Miettunen J, et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull. 2011;37:1039–1047. doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chudal R, Joelsson P, Gyllenberg D, et al. Parental age and the risk of attention-deficit/hyperactivity disorder: a nationwide, population-based cohort study. J Am Acad Child Adolesc Psychiatry. 2015;54:487 e481. doi: 10.1016/j.jaac.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 15.McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry. 2014;71:301–309. doi: 10.1001/jamapsychiatry.2013.4081. [DOI] [PubMed] [Google Scholar]
- 16.Chang Z, Lichtenstein P, D’Onofrio BM, et al. Maternal age at childbirth and risk for ADHD in offspring: a population-based cohort study. Int J Epidemiol. 2014;43:1815–1824. doi: 10.1093/ije/dyu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chudal R, Gissler M, Sucksdorff D, et al. Parental age and the risk of bipolar disorders. Bipolar Disord. 2014;16:624–632. doi: 10.1111/bdi.12182. [DOI] [PubMed] [Google Scholar]
- 18.Buizer-Voskamp JE, Laan W, Staal WG, et al. Paternal age and psychiatric disorders: findings from a Dutch population registry. Schizophr Res. 2011;129:128–132. doi: 10.1016/j.schres.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grattan RE, Morton SE, Warhurst ES, et al. Paternal and maternal ages have contrasting associations with self-reported schizophrenia liability. Schizophr Res. 2015;169:308–312. doi: 10.1016/j.schres.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27:379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crow JF. The high spontaneous mutation rate: is it a health risk? Proc Natl Acad Sci U S A. 1997;94:8380–8386. doi: 10.1073/pnas.94.16.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gratten J, Wray NR, Peyrot WJ, McGrath JJ, Visscher PM, Goddard ME. Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nat Genet. 2016;48:718–24. doi: 10.1038/ng.3577. [DOI] [PubMed] [Google Scholar]
- 23.Goldmann JM, Wong WS, Pinelli M, et al. Parent-of-origin-specific signatures of de novo mutations. Nat Genet. 2016;48:935–939. doi: 10.1038/ng.3597. [DOI] [PubMed] [Google Scholar]
- 24.Fergusson DM, Woodward LJ. Maternal age and educational and psychosocial outcomes in early adulthood. J Child Psychol Psychiatry. 1999;40(3):479–489. [PubMed] [Google Scholar]
- 25.Tabet M, Flick LH, Cook CA, Xian H, Chang JJ. Age at First Birth and Psychiatric Disorders in Low-Income Pregnant Women. J Womens Health (Larchmt) 2016;25(8):810–817. doi: 10.1089/jwh.2015.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calkins ME, Merikangas KR, Moore TM, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56:1356–1369. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore TM, Martin IK, Gur OM, et al. Characterizing social environment’s association with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol Med. 2016;46:599–610. doi: 10.1017/S0033291715002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merikangas K, Avenevoli S, Costello J, Koretz D, Kessler RC. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 2009;48:367–369. doi: 10.1097/CHI.0b013e31819996f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS v9.3 [computer program] Cary, NC: pp. 2002–2012. [Google Scholar]
- 30.R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 31.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- 32.Merikangas KR, He JP, Brody D, Fisher PW, Bourdon K, Koretz DS. Prevalence and treatment of mental disorders among US children in the 2001–2004 NHANES. Pediatrics. 2010;125(1):75–81. doi: 10.1542/peds.2008-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hvolgaard Mikkelsen S, Olsen J, Bech BH, Obel C. Parental age and attention-deficit/hyperactivity disorder (ADHD) Int J Epidemiol. 2016 May 11; doi: 10.1093/ije/dyw073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Miller B, Suvisaari J, Miettunen J, et al. Advanced paternal age and parental history of schizophrenia. Schizophr Res. 2011;133:125–132. doi: 10.1016/j.schres.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crespi B, Stead P, Elliot M. Evolution in health and medicine Sackler colloquium: Comparative genomics of autism and schizophrenia. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1736–1741. doi: 10.1073/pnas.0906080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamlyn J, Duhig M, McGrath J, Scott J. Modifiable risk factors for schizophrenia and autism–shared risk factors impacting on brain development. Neurobiol Dis. 2013;53:3–9. doi: 10.1016/j.nbd.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Rees E, Kirov G, O’Donovan MC, Owen MJ. De novo mutation in schizophrenia. Schizophr Bull. 2012;38:377–381. doi: 10.1093/schbul/sbs047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: a comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zammit S, Allebeck P, Dalman C, et al. Paternal age and risk for schizophrenia. Br J Psychiatry. 2003;183:405–408. doi: 10.1192/bjp.183.5.405. [DOI] [PubMed] [Google Scholar]
- 42.Allen EG, Freeman SB, Druschel C, et al. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Hum Genet. 2009;125(1):41–52. doi: 10.1007/s00439-008-0603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin JA, Hamilton BE, Osterman MJ. Births in the United States, 2014. NCHS Data Brief. 2015;(216):1–8. [PubMed] [Google Scholar]
- 44.Hudson WC. Sperm Banking as a Strategy to Reduce Harms Associated with Advancing Paternal Age. Food Drug Law J. 2015;70(4):573–591, ii. [PubMed] [Google Scholar]
- 45.Carey B. Father’s Age Is Linked to Risk of Autism and Schizophrenia. The New York Times. 2012 08/22/2012. Health. [Google Scholar]
- 46.Sandin S, Nygren KG, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA. 2013;310(1):75–84. doi: 10.1001/jama.2013.7222. [DOI] [PubMed] [Google Scholar]
- 47.Fountain C, Zhang Y, Kissin DM, et al. Association between assisted reproductive technology conception and autism in California, 1997–2007. Am J Public Health. 2015;105(5):963–971. doi: 10.2105/AJPH.2014.302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kissin DM, Zhang Y, Boulet SL, et al. Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART-conceived children. Hum Reprod. 2015;30:454–465. doi: 10.1093/humrep/deu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daniels AM, Rosenberg RE, Anderson C, Law JK, Marvin AR, Law PA. Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. J Autism Dev Disord. 2012;42:257–265. doi: 10.1007/s10803-011-1236-7. [DOI] [PubMed] [Google Scholar]
- 50.Zammit S, Kounali D, Cannon M, et al. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am J Psychiatry. 2013;170:742–750. doi: 10.1176/appi.ajp.2013.12060768. [DOI] [PubMed] [Google Scholar]
- 51.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 52.Jones PB. Adult mental health disorders and their age at onset. Br J Psychiatry Suppl. 2013;54:s5–10. doi: 10.1192/bjp.bp.112.119164. [DOI] [PubMed] [Google Scholar]
- 53.Kemppainen L, Veijola J, Jokelainen J, et al. Birth order and risk for schizophrenia: a 31-year follow-up of the Northern Finland 1966 Birth Cohort. Acta Psychiatr Scand. 2001;104:148–152. doi: 10.1034/j.1600-0447.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 54.Risch N, Hoffmann TJ, Anderson M, Croen LA, Grether JK, Windham GC. Familial recurrence of autism spectrum disorder: evaluating genetic and environmental contributions. Am J Psychiatry. 2014;171(11):1206–1213. doi: 10.1176/appi.ajp.2014.13101359. [DOI] [PubMed] [Google Scholar]
- 55.Sandin S, Schendel D, Magnusson P, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry. 2016;21:693–700. doi: 10.1038/mp.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janecka M, Mill J, Basson MA, et al. Advanced paternal age effects in neurodevelopmental disorders-review of potential underlying mechanisms. Transl Psychiatry. 2017;7:e1019. doi: 10.1038/tp.2016.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


