Abstract
The heterodimeric splicing factor U2AF plays an important role in 3′ splice site selection, but the division of labor between the two subunits in vivo remains unclear. In vitro assays led to the proposal that the human large subunit recognizes 3′ splice sites with extensive polypyrimidine tracts independently of the small subunit. We report in vivo analysis demonstrating that all five domains of spU2AFLG are essential for viability; a partial deletion of the linker region, which forms the small subunit interface, produces a severe growth defect and an aberrant morphology. A small subunit zinc-binding domain mutant confers a similar phenotype, suggesting that the heterodimer functions as a unit during splicing in Schizosaccharomyces pombe. As this is not predicted by the model for metazoan 3′ splice site recognition, we sought introns for which the spU2AFLG and spU2AFSM make distinct contributions by analyzing diverse splicing events in strains harboring mutations in each partner. Requirements for the two subunits are generally parallel and, moreover, do not correlate with the length or strength of the 3′ pyrimidine tract. These and other studies performed in fission yeast support a model for 3′ splice site recognition in which the two subunits of U2AF functionally collaborate in vivo.
INTRODUCTION
The removal of noncoding introns and the joining of coding exons via splicing, an essential step in the eukaryotic pre-mRNA processing pathway, requires accurate splice site selection by the spliceosome. Initial recognition of the 5′ exon/intron boundary is achieved via base pairing with the U1 snRNA component of the U1 snRNP (Zhuang and Weiner, 1986), whereas U2AF (U2 snRNP auxiliary factor) is the first factor bound to the 3′ splice site (Ruskin et al., 1988). Biochemical complementation assays demonstrated that U2AF is required for the subsequent ATP-dependent association of U2 snRNP with pre-mRNA branchpoints (Ruskin et al., 1988; Valcarcel et al., 1996). Purified U2AF is a heterodimer composed of large and small subunits in humans (Zamore and Green, 1989), Drosophila melanogaster (Kanaar et al., 1993; Rudner et al., 1996), Caenorhabditis elegans (Zorio et al., 1997; Zorio and Blumenthal, 1999) and Schizosaccharomyces pombe (Potashkin et al., 1993; Wentz-Hunter and Potashkin, 1996). Functional conservation of this splicing factor is evidenced by 1) the restoration of splicing activity to U2AF-depleted HeLa nuclear splicing extracts via addition of Drosophila U2AF large subunit (dmU2AFLG; Zamore and Green, 1991) and 2) the ability of human U2AF35 (hsU2AFSM) to restore growth to an S. pombe strain lacking the small subunit (Webb and Wise, 2004).
The structural domains of U2AF are also conserved except in Saccharomyces cerevisiae, where the large subunit is highly divergent and the small subunit is absent entirely (Abovich et al., 1994). The small subunit of U2AF consists of two zinc-binding domains (ZBDs) surrounding a central pseudo-RNA recognition motif (ψRRM; Rudner et al., 1998b), also known as a PUMP (PUF60/U2AF/MUD2 protein-protein interaction) domain (Page-McCaw et al., 1999) or a UHM (U2AF homology motif; Kielkopf et al., 2004), which are highly conserved between S. pombe and humans, followed by a C-terminal domain that consists of RS or RS/glycine repeats in metazoan orthologues, which are not present in the fission yeast protein. We recently demonstrated that the three conserved domains including both ZBDs and the ψRRM of S. pombe U2AF small subunit (spU2AFSM) contribute to RNA binding and are essential for function in vivo, whereas the more divergent C-terminal domain is dispensable (Webb and Wise, 2004). A comparable domain ablation analysis in vivo has not been carried out for the U2AF large subunit, which consists of an N-terminal RS domain, a linker region, two classical RRMs (RNA recognition motifs) and a ψRRM (Zamore et al., 1992). Such an analysis would complement extensive biochemical data demonstrating that this subunit is the major contributor to RNA binding (Rudner et al., 1998a; Wu et al., 1999; Kielkopf et al., 2001) and interacts with multiple protein partners implicated in splicing including p54 (Zhang and Wu, 1996), UAP56 (Fleckner et al., 1997), Sip1 (Zhang and Wu, 1998), SF1/BBP (Berglund et al., 1998), and SAP155 (Gozani et al., 1998), as well as nonsplicing factors such as WT1 (Davies et al., 1998) and poly(A) polymerase (Vagner et al., 2000).
RNA-binding assays (Zamore et al., 1992; Rudner et al., 1998a) and in vitro selection studies (Singh et al., 1995; Wu et al., 1999; Banerjee et al., 2004) demonstrated that the large subunit of U2AF interacts with the 3′ polypyrimidine tract, whereas the small subunit functions in recognition of the 3′ AG dinucleotide (Merendino et al., 1999; Wu et al., 1999; Zorio and Blumenthal, 1999b). The bipartite nature of the RNA target sequences for the two subunits, in combination with biochemical complementation data demonstrating that addition of the human large subunit (hsU2AFLG) alone to U2AF-depleted HeLa nuclear extracts can rescue splicing of substrates that contain long polypyrimidine tracts (Wu et al., 1999; Guth et al., 2001), led to a widely accepted model for 3′ splice site recognition by U2AF. The central tenet of this model is that the binding energy contributed by the small subunit/AG interaction is essential only for introns with less extensive polypyrimidine tracts (reviewed in Moore, 2000), consistent with earlier splicing assays of mutant human pre-mRNAs in vitro, which indicated that the requirement for a 3′ AG to proceed through the first step of splicing (AG-dependence) could be eliminated by expanding the polypyrimidine tract (Reed, 1989). In S. pombe, mutating the terminal AG dinucleotide prevented the first transesterification reaction for all three introns examined (Romfo and Wise, 1997), as for a subset of mammalian pre-mRNAs (Reed, 1989; Wu et al., 1999). However, doubling the length of the polypyrimidine tract in two different fission yeast introns did not render the AG dinucleotide dispensable in vivo (Romfo and Wise, 1997), providing the first hint that both subunits of U2AF may be important for initial recognition of a broad spectrum of introns in this organism.
In this report, we demonstrate that S. pombe U2AF large subunit (spU2AFLG) mutants lacking the linker region or any of the C-terminal RRMs or ψRRM are recessive lethal, whereas deleting the N-terminal RS domain confers dominant lethality. A partial deletion of the spU2AFLG linker, which interacts with the small subunit in S. pombe (Wentz-Hunter and Potashkin, 1996) as well as humans (Zhang et al., 1992; Kielkopf et al., 2001) and Drosophila (Rudner et al., 1998c), produces an unusual branched morphology that is also observed in a triple substitution within the second zinc-binding domain of spU2AFSM. As the aberrant morphology is likely to reflect a common underlying splicing defect, this result, in conjunction with our previous data (Romfo and Wise, 1997), prompted us to ask whether splicing of different introns in vivo requires parallel contributions from the two subunits of spU2AF. Analysis of 22 diverse pre-mRNAs after separate mutational inactivation of each subunit reveals that those introns that are most highly dependent on spU2AFLG also exhibit a marked dependence on spU2AFSM. Moreover, counter to what would be predicted from the model for metazoan U2AF function (Moore, 2000), the length and sequence of the polypyrimidine tract do not correlate with the requirement for either subunit. In addition to explaining our previous finding that expanding the polypyrimidine tract did not negate the requirement for the AG dinucleotide, these results are consistent with the recent demonstration that, in S. pombe, most of the U2AF heterodimer is maintained in a tight complex with SF1/BBP, apparently optimized to recognize the branchpoint/3′ splice site region as a functional unit (Huang et al., 2002).
MATERIALS AND METHODS
Plasmid Construction and Mutagenesis
All oligonucleotides used in this study are listed in Table 1. spU2AFLG mutants were generated using either site-directed mutagenesis of single-stranded DNA (ΔL3-P102; ΔD2-P140; ΔP139-P189; F247D, I249D; F353, F355D; F476D, Y479D) using commercially available reagents (Amersham, Arlington Heights, IL) with oligonucleotides 1-6 (Table 1) or by overlap extension PCR (ΔP124-P189; W135F; W135A) using Platinum pfx DNA polymerase (Invitrogen, Carlsbad, CA) with the flanking primers prp-Nde and prp-Bam (Romfo et al., 1999) in combination with appropriate internal primers (9-14) listed in Table 1. Mutants generated by overlap extension PCR were subcloned into the S. pombe plasmid prp2+/PIRT3 (Romfo et al., 1999) as NruI-NheI or BglII fragments. The presence of each mutation was confirmed by DNA sequencing (Cleveland Genomics, Cleveland, OH). Two-hybrid alleles were constructed by amplification of spU2AFLG mutant templates with the primers prp-Nde and prp-Bam (Romfo et al., 1999) and oligonucleotides 7 and 8. The resulting fragments were inserted into pAS2-1 (BD Sciences Clontech, Palo Alto, CA) and sequenced. To express a fragment derived from the β-globin 3′ intron/exon boundary (Wu et al., 1999) in the modified three-hybrid system (Webb and Wise, 2004), the 5′ phosphorylated oligonucleotides 15 and 16 were annealed and inserted into the XmaI and ClaI sites of p4130 (kindly provided by John Woolford, Carnegie Mellon University; described in Fewell and Woolford, 1999) to generate the plasmid β-globin/pIII MS2-1 B,H,C.
Table 1.
Oligonucleotides and primers used in these studies
| Oligonucleotide or primer and function | Designation | Sequence |
|---|---|---|
| Oligonucleotides for uaf1+ phosphorothioate-based site-directed mutagenesis | ||
| 1 | D7RS mut | 5′ gcttctttctcggctgggaggcaaatccatgttagtgaat gaatatg 3′ |
| 2 | D11RS mut | 5′ cagcagtaaccaattcataaccaggatccatgttagt gaatgaatatg 3′ |
| 3 | DHinge | 5′ ctagcacctggttgtaagggtggtttaatgtcccataaag a 3′ |
| 4 | F247D/I249D | 5′ cgacctcaagatcagcatcgttttcttctttacag 3′ |
| 5 | F353D/F355D | 5′ aggatttttaaattcgcaatcacaatcacccttcgaagat c 3′ |
| 6 | F476D/Y479D | 5′ gatatcggagtctcgtacatcaacctttccagttc 3′ |
| Oligonucleotides for making two-hybrid constructs | ||
| 7 | D7RS-Nde | 5′ tagaagtccatatggatttgcctcccagccgagaa 3′ |
| 8 | slrDRS Nde | 5′ ggacattaaaccatatggatcctggttatgaattg 3′ |
| Oligonucleotides for uaf1+ overlap extension PCR mutagenesis | ||
| 9 | PrpF476AY479Apcrmut1 | 5′ cgggattaggaactggaaaggttgctgtacgagcttccgatatcagatctgcagaggtc 3′ |
| 10 | PrpF476AY479Apcrmut2 | 5′ aacctttccagttcctaatcccg 3′ |
| 11 | PrpΔP124-P188pcrmut3 | 5′ ggaactcgaacaattgagagatgtaacacccttacaac caggtgctagcagac 3′ |
| 12 | PrpΔP124-P188pcrmut4 | 5′ tgttacatctctcaattgttcgagttcc 3′ |
| 13 | Prp2.W135F.1 | 5′ cagtggaaaaggaagcgctctttatttgacattaaacca cctggttatg 3′ |
| 14 | Prp2.W135-.2 | 5′ taaagagcgcttccttttccactg 3′ |
| Oligonucleotides for making RNA three-hybrid construct | ||
| 15 | β-globin.XmaI.s | 5′ ccgggtctcttcctttgtcagggaaatgggaac 3′ |
| 16 | β-globin.ClaI.as | 5′ cggttcccatttccctgacaaaggaagagac 3′ |
| Primers for RT-PCR splicing assays | ||
| 17 | SPCC16A11.08-I2.exon.2 | 5′ gatgcgtcggcagtatcccg 3′ |
| 18 | SPCC16A11.08-I2.exon.3 | 5′ gaagagcgcaaatgagcgaatgg 3′ |
| 19 | SPCC830.12-I1.exon1 | 5′ gaggaccagggtcctctgctg 3′ |
| 20 | SPCC830.12-I1.exon2 | 5′ cacagcgaatctttatagtcactg 3′ |
| 21 | SPAC17A5.16-I3.exon3 | 5′ ccgttggatgatgaagtttggg 3′ |
| 22 | SPAC17A5.16-I3.exon4 | 5′ gtaggcgaatgcagtttaacgc 3′ |
| 23 | pyp3-I1.exon1 | 5′ ctcgtgttcgtttagatccaatg 3′ |
| 24 | pyp3-I1.exon2 | 5′ ggcgttccctcaatttggtaagc 3′ |
| 25 | erf1-I2.exon2 | 5′ ggagatcagctgaaggcttctac 3′ |
| 26 | erf1-I2.exon3 | 5′ tacaagcagcatctacacggtcc 3′ |
| 27 | mcs2-I2.exon2 | 5′ tttatctaattaactctgtcatggag 3′ |
| 28 | mcs2-I2.exon3 | 5′ aggacgaaatggaagccagacg 3′ |
| 29 | cdc16-I2.exon2 | 5′ ttattggataaatgccttgaagtcc 3′ |
| 30 | cdc16-I2.exon3 | 5′ tcggaaaataaacatttgtgcaatc 3′ |
| 31 | ypt5-I1.exon1 | 5′ cttgttgttacactcaaaataaatatggc 3′ |
| 32 | ypt5-I1.exon2 | 5′ catcaaattgatctttaacgaatcgaag 3′ |
| 33 | cdc17-I2.exon2 | 5′ actagcaaacggttggaaatcattg 3′ |
| 34 | cdc17-I2.exon3 | 5′ agctacaagaccaagatcaccaac 3′ |
| 35 | SPAC22F8.13-I2.exon2 | 5′ gatcttcctaaaggatcaaggac 3′ |
| 36 | SPAC22F8.13-I2.exon3 | 5′ cagttcctaaataaacaaatgcagaac 3′ |
| 37 | erf1-I4.exon4 | 5′ cttgccaatatttgtctcattacag 3′ |
| 38 | erf1-I4.exon5 | 5′ ggtccttcaaaatttcattaaggg 3′ |
| 39 | cdc2-I2.exon2 | 5′ gggaattccatatgttgtcagggcgtattgtg 3′ |
| 40 | cdc2-I2.exon3 | 5′ gaaggatccggtaaacttttgaacaagtctc 3′ |
| 41 | cdc16-I1.exon1 | 5′ gtgtgtctcaatcgtctctttcac 3′ |
| 42 | cdc16-I1.exon2 | 5′ atacgtgtacaaacgatggtcaag 3′ |
| 43 | pim1-I1.exon1 | 5′ gaaggtgctttatctcatctacg 3′ |
| 44 | pim1-I1.exon2 | 5′ gtagtcaaggcaataatatggtcc 3′ |
| 45 | rad9-I3.exon3 | 5′ gatggcagcacagttcaacacc 3′ |
| 46 | rad9-I3.exon4 | 5′ aagaacaatgattgaccagcatcg 3′ |
| 47 | pck1-I1.exon1 | 5′ gcaagctttcaaactagtaccagg 3′ |
| 48 | pck1-I1.exon2 | 5′ tcgtatatgaccatcaggacaaag 3′ |
| 49 | rad26-I2.exon2 | 5′ aatcccaagaatcactcatgcaac 3′ |
| 50 | rad26-I2.exon3 | 5′ agtccgatgcatactattaaggg 3′ |
| 51 | top1-I2.exon2 | 5′ gaaggaggcaaagacgcggttc 3′ |
| 52 | top1-I2.exon3 | 5′ ccaccatttcccattactttagc 3′ |
| 53 | cdc16-I3.exon3 | 5′ ttaccatatgggattttttgtttgc 3′ |
| 54 | cdc16-I3.exon4 | 5′ cagaacaccagcttcagaatcc 3′ |
| 55 | chk1-I3.exon3 | 5′ caccttgggatgaagcaattagc 3′ |
| 56 | chk1-I3.exon4 | 5′ tagttcgaaatggtgtacttgagg 3′ |
| 57 | erf1-I3.exon3 | 5′ atgggatcctaccataccttgg 3′ |
| 58 | erf1-I3.exon4 | 5′ gtcccctctccgtttccttgg 3′ |
| 59 | swi4-I1.exon1 | 5′ cgatctcgaaaagggtctgtcc 3′ |
| 60 | swi4-I1.exon2 | 5′ accttgttgttattttcagcttctttttg 3′ |
Two-hybrid Analysis
To analyze heterodimer formation, mutant alleles of uaf1+ (spU2AFLG) were expressed in pAS2-1, as noted above, and the interacting partner, wild-type uaf2+ (spU2AFSM), was expressed in pACT2 (James et al., 1996). Two-hybrid analysis was performed as previously described (Webb and Wise, 2004). Briefly, the fusion protein constructs were cotransformed into the S. cerevisiae strain PJ69-4A (MATa trp1-901 leu2-3112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ; 20) as described (James et al., 1996) except that dimethyl sulfoxide was added to the final incubation step.
Three-hybrid Analysis
Three-hybrid assays to measure RNA-protein interactions were conducted as described previously (Webb and Wise, 2004). Briefly, one plasmid carrying either the large subunit gene or the large and small subunit genes and another expressing a hybrid RNA containing a human β-globin 3′ splice site were cotransformed into the L40 S. cerevisiae strain (MATa ura3-52 leu2-3112 his3Δ200 trp1Δ1 ade2 LYS2::(lexAop)-HIS3 ura3::(lexAop)-lacZ; 44), kindly provided by Dr. J. Woolford. Duplicate transformants were grown to midlog phase and replica plated onto media containing varying amounts of 3-aminotriazole (3-AT). Plate assays were reproducible, with visually indistinguishable results.
Phenotypic Analysis in S. pombe
The ability of large subunit mutants to support growth was determined using the complementation assay previously described in the strain SpCr1, which is heterozygous for deletion of the gene encoding spU2AFLG (Romfo et al., 1999). Dominant negative effects of large subunit mutants were performed as described earlier (Romfo et al., 1999).
To examine the morphology of spU2AF subunit mutants, microscopy was performed with exponentially growing cells using a Zeiss (Jena, Germany) Axioplan-2 microscope equipped with Nomarski differential interference (DIC) optics, a plan-NEOFLUAR (100×, numerical aperture 1.3) oil immersion objective and a Hamamatzu (Tokyo, Japan) C4742-95 progressive scan cooled charge-coupled device camera. Images were captured with QED acquisition software.
RNA Preparation and Analysis
To assay for splicing defects, the temperature-sensitive (ts) mutants spU2AFSM [Y108A, F111A] and spU2AFLG C387Y and corresponding isogenic wild-type strains were grown in selective media to midlog phase at the permissive temperature (30°C) followed by inoculation of equal aliquots into media prewarmed to 30 or 37°C. After 2 h (2, 4, and 6 h for ypt5-I1) at the nonpermissive temperature, cells were harvested and total RNA extracted as previously described (Alvarez et al., 1996). Semiquantitative RT-PCR assays were performed and the results analyzed as in published work (Webb and Wise, 2004) using the primers (17-60) listed in Table 1.
RESULTS
The Dominant Lethality Conferred by U2AFLG RS Domain Deletions Requires Binding to U2AFSM
Two recombinant human U2AFLG mutant proteins carrying deletions in the N-terminal domain were previously analyzed in nuclear splicing extracts from HeLa cells (Valcarcel et al., 1996). In hsU2AFLG Δ1-63, removal of all but one RS/SR dipeptides dramatically reduced the protein's ability to restore splicing activity to an extract depleted of hsU2AF, whereas the more extreme mutant, hsU2AFLG Δ1-94, which eliminates the entire RS domain and approximately half of the linker region, displayed no detectable activity in vitro. To assess the role of the N-terminal RS domain in vivo, we analyzed two similar alleles (ΔL3-P102 and ΔD2-P140; Figure 1) of S. pombe U2AFLG, which contains the same number of RS/SR dipeptide repeats (10) in the intact N-terminal domain as hsU2AFLG. To avoid structural perturbations, we constructed deletions that extended between two prolines.
Figure 1.
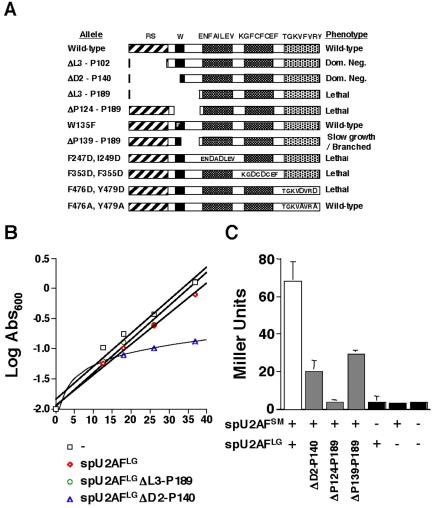
Domain structure of spU2AFLG and mutant phenotypes. (A) The relative lengths and positions of the conserved domains of spU2AFLG are depicted as rectangles with amino acids or motifs targeted by mutations written above the wild-type schematic. Also included in the figure is the previously described ΔRS/Hinge (ΔL3-P189) mutant (Romfo et al., 1999). RS domain, diagonal stripes; linker region, black with the phenylalanine replacement of the key tryptophan (Kielkopf et al., 2001) shown in white; RRM1 and RRM2, cross-hatched with the RNP-1 motif shown on a white background with mutated amino acids in large type; ψRRM, stippled with the RNP1-like motif shown on a white background with mutated amino acids in large type. Phenotypes were assessed as described previously (Romfo et al., 1999; Webb and Wise, 2004). (B) Dominant negative effect on growth of overexpressing the ΔD2-P140 allele. A wild-type fission yeast strain (DS2) was transformed with the indicated plasmids in the presence of thiamine to repress the nmt1 promoter (Romfo et al., 1999). Growth was monitored by measuring absorbance600 after a shift to medium lacking thiamine at time 0. In addition to the vector only and wild-type controls, we also analyzed growth of a strain harboring a plasmid carrying the ΔRS/Hinge allele, which we previously showed confers a recessive lethal phenotype (Romfo et al., 1999). (C) Two-hybrid analysis of heterodimer formation in mutants predicted to disrupt the large and small subunit interface. The figure shows the results of β-galactosidase assays, with the data expressed in Miller Units. Error bars, SD for n = 3. Wild-type, white bars; mutants, gray bars; negative controls, black bars.
Our initial phenotypic analysis suggested that an allele carrying a complete deletion of the N-terminal domain of spU2AFLG (ΔD2-P140; Figure 1A) complements the otherwise lethal uaf1::ura4 gene disruption described earlier (Romfo et al., 1999). However, upon recovery and sequencing of plasmid from the haploid derivatives, we discovered that this allele had in fact been repaired by gene conversion because of dominant negative selective pressure, similar to what we previously found for U1 snRNA mutants (Alvarez et al., 1996). Gene conversion was confirmed by Western blot analysis (unpublished data), which showed that the sole protein expressed in these cells is full-length. The dominant effect of the spU2AFLG ΔD2-P140 allele was unequivocally demonstrated by overexpressing it from the derepressible nmt1 (no message on thiamine) promoter in an otherwise wild-type S. pombe strain. Under derepressing conditions, the presence of the deletion allele nearly abolishes growth as judged by failure to form colonies after streaking (unpublished data) and by growth analysis (Figure 1B). In contrast to cells harboring the vector only or a wild-type control, the strain overexpressing the DRS (ΔD2-P140) allele virtually ceases growth ∼12 h after the shift to medium lacking thiamine. Analogous experiments indicate that the less extreme RS domain deletion ΔL3-P102, which retains three RS/SR dipeptides as well as the entire linker region, also dominantly interferes with growth in fission yeast.
Our previous work indicated that a more extensive spU2AFLG deletion (ΔRS/Hinge = ΔL3-P189) lacking the linker region, which has been implicated in small subunit interactions (Zhang et al., 1992; Wentz-Hunter and Potashkin, 1996; Rudner et al., 1998c; Kielkopf et al., 2001), as well as the RS domain, produced recessive lethality (Romfo et al., 1999); these findings are recapitulated in Figure 1, A and B. In light of the contrast with the dominant lethality conferred by the ΔD2-P140 allele, we hypothesized that spU2AFSM plays a role in the dominant negative effects of spU2AFLG deletion mutants. Consistent with this idea, the spU2AFLG ΔP124-P189 mutant, which removes the linker region alone, is recessive lethal (Figure 1A).
The results of two-hybrid assays further support a role for the small subunit in dominant lethality, as the spU2AFLG ΔP124-P189 mutant interacts with spU2AFSM at background levels, whereas the spU2AFLG ΔD2-P140 mutant, in which only the N-terminal portion of the linker is deleted, shows significant small subunit binding (Figure 1C). Notably, the ΔD2-P140 mutant eliminates the highly conserved tryptophan at position 135, which was proposed based on structural data for a minimal human heterodimer to make a key contact with U2AFSM in a manner dependent on the unique properties of this amino acid (Kielkopf et al., 2001). Consistent with our two-hybrid data, however, we find that an allele carrying a phenylalanine substitution at amino acid 135 of spU2AFLG supports growth of fission yeast (Figure 1A). Although this is a conservative substitution that is also found in both the large and small subunits of plant U2AF orthologues, modeling studies predict that reciprocal changes in the binding pockets coevolved in plants to compensate for the lack of an additional five-membered ring on the phenylalanine side-chains that is present in tryptophan (Kielkopf et al., 2001). In contrast to plants, both subunits of fission yeast U2AF contain tryptophans at the relevant positions and are thus likely to form a tongue-in-groove heterodimeric interface similar to that proposed for the minimal human heterodimer (Kielkopf et al., 2001). This view is bolstered by our finding that human U2AFSM can support S. pombe viability in the absence of spU2AFSM (Webb and Wise, 2004). Thus, the phenylalanine substitution is most likely tolerated in spU2AFLG because this amino acid can still be accommodated in the larger binding pocket that normally envelops a tryptophan. Taken together, our data support the idea that both the N-terminal portion of the linker emphasized by in vitro studies and the C-terminal region make substantial contributions to spU2AF function in vivo.
In analyzing the pattern of dominant lethality conferred by large subunit mutations, we also noted that this phenotype arises only when the RS domain is absent. In particular, the spU2AFLG ΔP139-P189 mutant, which removes the C-terminal portion of the linker (Figure 1A), is recessive. The phenotypic consequences of the ΔP139-P189 mutant in S. pombe include extremely slow growth and an aberrant morphology (described below), and the encoded protein displays an intermediate level of binding to the small subunit in the two-hybrid system (Figure 1C). In keeping with our data, it was possible to identify alleles carrying multiple point mutations within the linker region of the Drosophila U2AF large subunit that compromise, but do not abolish, heterodimer formation in vitro and affect viability of the intact organism (Rudner et al., 1998c). Inactivating substitutions within RRM1, RRM2, and the ψRRM are recessive lethal.
To determine whether the two canonical RRMs of spU2AFLG are critical for viability, we replaced two large hydrophobic residues within RNP1 of RRM1 or RRM2, which were suggested by modeling to interact with RNA via intercalation (Ito et al., 1999), with aspartates, which are predicted to repel a negatively charged RNA. The [F247D, I249D] double substitution in RRM1 and the [F353D, F355D] RRM2 mutant both produce recessive lethality (Figure 1A), consistent with the complete abolition of RNA-binding activity in vitro conferred by analogous substitutions in the mammalian SR protein ASF/SF2 (Caceres and Krainer, 1993). The lethal effect of mutating either RRM1 or RRM2 indicates that each is independently essential for viability in S. pombe, consistent with the finding that both canonical RRMs of hsU2AFLG have RNA-binding activity when tested individually in vitro (Ito et al., 1999).
Recent evidence indicates that deleting the entire ψRRM of spU2AFLG is lethal in vivo even though the hsU2AFLG ψRRM does not contribute to RNA binding in vitro (Banerjee et al., 2004). At first pass, our finding that a double aspartate substitution within the RNP1-like sequence, [F476D, Y479D], is lethal (Figure 1A) might seem at odds with the lack of a role in RNA binding, as charged substitutions of large hydrophobic amino acids within RNP1 of canonical RRMs disrupt contacts with RNA (e.g., Caceres and Krainer, 1993). Furthermore, the analogous substitution in the spU2AFSM ψRRM was also lethal, and our data indicate that this domain does contribute to RNA binding (Webb and Wise, 2004). The discrepancy can be reconciled by structural data implicating the hsU2AFLG amino acid analogous to F476 in packing helix C against the β-sheet that contains the RNP1-like sequence (Selenko et al., 2003), which would block the potential RNA binding surface, whereas the small subunit of U2AF does not have a recognizable helix C (Kielkopf et al., 2001). The inability of the [F476D, Y479D] substitution to support growth also echoes the effect of the corresponding mutation in the ψRRM of the S. cerevisiae large subunit orthologue MUD2. Specifically, MUD2 is essential only when the U1A gene is also deleted, and the synthetic lethality cannot be rescued by an allele carrying a double aspartate substitution in the ψRRM (Abovich et al., 1994).
Whereas replacement of the large hydrophobic amino acids in the RNP1-like peptide of the S. pombe U2AFLG ψRRM is lethal, a more conservative double alanine substitution is phenotypically silent (Figure 1A). Again, a structural comparison with its human counterpart may help to explain the data, as Y479 of spU2AFLG is likely to contribute a core side-chain that stabilizes the protein fold against helix B. The Y479D mutation may not be tolerated because the charge perturbs the structure, while a more conservative alanine substitution would allow the fold to be retained. In contrast to the [F476A, Y479A] spU2AFLG mutant, the analogous double alanine replacement in the ψRRM of spU2AFSM leads to temperature-sensitive growth (Webb and Wise, 2004). A likely explanation is that these substitutions disrupt RNA binding.
A Partial Deletion of the U2AFLG Linker Region Confers a Phenotype Similar to a U2AFSM Zinc-binding Domain Mutant
Our mutagenesis strategy, which involved making deletions that extend between prolines, yielded an spU2AFLG allele (ΔP139-P189) that only partially deletes the linker, retaining the twelve N-terminal amino acids of the region defined in an earlier structural study (Kielkopf et al., 2001). As noted above, the spU2AFLG ΔP139-P189 mutant displays a reduced two-hybrid interaction with spU2AFSM (Figure 1C) but does support growth of S. pombe (Figure 1A), albeit poorly compared with an isogenic wild-type strain as judged by the dramatically reduced colony size. An even more notable phenotype of the spU2AFLG ΔP139-P189 mutant is that individual cells harboring this deletion allele as their sole source of U2AF large subunit display an aberrant branched morphology (Figure 2, compare A and B). Strikingly, a triple substitution (C157S, C163S, H167I) within the second zinc-binding domain (ZBDII) of the S. pombe U2AF small subunit identified in a separate study (Webb and Wise, 2004) also exhibits slow growth and a branched morphology (Figure 2, compare C and D). Like the other 35 small subunit alleles analyzed to date (Webb and Wise, 2004), this mutant is recessive as judged by the reversal of its deleterious effects on introduction of a plasmid-borne wild-type allele. The unusual nature, yet remarkable similarity, of the phenotypes conferred by the spU2AFLG ΔP139-P189 and spU2AFSM [C157S, C163S, H167I] alleles suggest that they may have the same underlying molecular basis.
Figure 2.
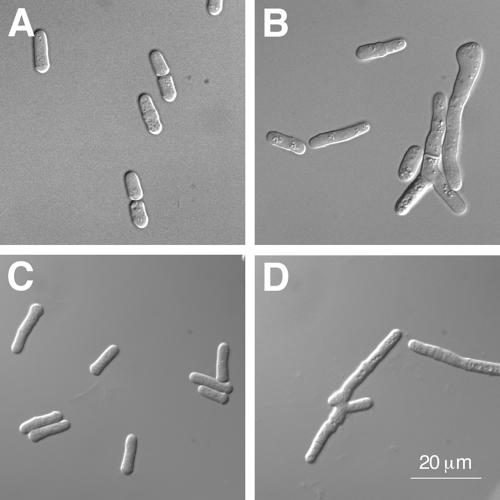
Microscopic analysis of the spU2AFLG ΔP139-P189 and spU2AFSM [C157S, C163S, H167I] mutants. (A) A haploid strain with a plasmid harboring wild-type spU2AFLG covering a disruption of the uaf1+ locus (Romfo et al., 1999). (B) A haploid strain with a plasmid harboring the spU2AFLG ΔP139-P189 mutant covering a disruption of the uaf1+ locus. (C) A haploid strain with a plasmid harboring wild-type spU2AFSM covering a disruption of the uaf2+ locus (Webb and Wise, 2004). (D) A haploid strain with a plasmid harboring the spU2AFSM [C157S, C163S, H167I] mutant covering a disruption of the uaf2+ locus. Magnification, ×1000.
As previously characterized S. pombe mutants that exhibit a similar pseudohyphal appearance have generally been linked to septation or cytokinetic defects such as cell separation (Le Goff et al., 1999), we tested the three introncontaining pre-mRNAs implicated in these processes for splicing defects in the small subunit pZBDII mutant. Although precursor accumulation was observed, it was not dramatic; most definitively, a cDNA encoding imp2, which displayed the most substantial splicing defect, was unable to reverse either the growth defect or the branched morphology (unpublished data). Thus, although we still consider defective splicing of one or more pre-mRNAs that encode gene product(s) required for cytokinesis the most reasonable explanation for the morphology of both the spU2AFSM pZBDII mutant and the spU2AFLG mutant carrying a partial deletion of the linker region, further work will be required to identify the pre-mRNAs whose product(s) have become rate-limiting for growth.
Given that the spU2AFLG mutant carrying a partial deletion of the linker region is defective in heterodimer formation (Figure 1C), we performed two-hybrid analysis on the small subunit ZBDII mutant. As shown in Figure 3A, the [C157S, C163S, H167I] spU2AFSM mutant shows a two-hybrid interaction with spU2AFLG comparable to wild-type, consistent with our previous finding that even lethal amino acid substitutions in either zinc-binding domain did not disrupt large subunit interactions (Webb and Wise, 2004). As noted in the Introduction, U2AF function involves formation of a complex between the heterodimer and the bipartite (polypyrimidine tract/AG dinucleotide) 3′ splice site. Defective heterodimer formation by the spU2AFLG ΔP139-P189 mutant is predicted to destabilize this complex and thus disrupt splicing. However, a similar effect could also result from disrupting the interaction of U2AF with the 3′ splice site. We therefore tested whether the [C157S, C163S, H167I] spU2AFSM mutant shows diminished binding to RNA in a modified three-hybrid assay where the large subunit is coexpressed (Figure 3B). Consistent with our previous finding that S. pombe U2AFSM ZBD mutations decrease the interaction of the heterodimer with small RNAs carrying 3′ splice sites (Webb and Wise, 2004), the [C157S, C163S, H167I] allele does indeed produce a lower signal in the three-hybrid system (Figure 3B). The most parsimonious interpretation of these data in aggregate is that the molecular defect conferred by both the spU2AFLG linker region deletion and the ZBDII spU2AFSM triple substitution is an unstable complex between the U2AF heterodimer and one or more 3′ splice sites.
Figure 3.
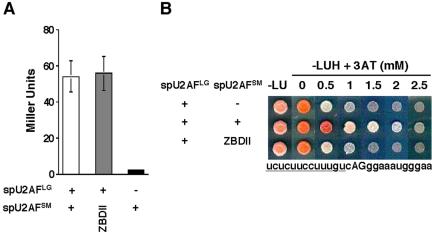
(A) Two-hybrid analysis of heterodimer formation in the [C157S, C163S, H167I] small subunit ZBDII mutant. Two-hybrid assays were performed as in Figure 1C. Wild-type, white bar; mutant, gray bar; negative control, black bar. (B) Plate assays to test the contribution to RNA binding of the [C157S, C163S, H167I] small subunit ZBDII mutant in the modified RNA three-hybrid system (Webb and Wise, 2004). The strength of the RNA-protein interaction is reflected by growth in the presence of increasing amounts of 3-amino-triazole (SenGupta et al., 1996; Eckman et al., 2002). The data shown are for the human β-globin 3′ splice site that is similar to many S. pombe 3′ splice sites; its sequence extending from just downstream of the branchpoint to 10 bases beyond the 3′ AG dinucleotide is shown beneath the plate assays.
The Length and Pyrimidine Content of 3′ Splice Sites Do not Correlate with U2AF Requirements in S. pombe
The correlation between the dominant negative phenotypes of large subunit RS domain deletions and their ability to bind the small subunit (Figure 1) led us to hypothesize that the U2AF heterodimer obligatorily functions as a unit during splicing in S. pombe, a view reinforced by the identification of spU2AFLG and spU2AFSM mutants with similar phenotypic consequences (Figure 2) that are not shared by other fission yeast splicing factor mutants (e.g., Beales et al., 2000; Urushiyama et al., 1996). This proposal is counter to the model for the division of labor between the two subunits in metazoans, which is based on in vitro evidence that the large subunit participates in a subset of splicing events in the absence of a contribution from the small subunit (reviewed in Moore, 2000). Note that it remains possible that mammalian U2AF acts as a unit in vivo, since free large or small subunits are not present in significant amounts inside the cell (Zhang et al., 1992). To test our functional interdependence hypothesis, we decided to perform splicing assays on chromosomally encoded RNAs extracted from two temperature-sensitive fission yeast U2AF mutants isolated in previous studies. The C387Y spU2AFLG allele has been extensively characterized previously and shown to cease growth after approximately half a generation (Potashkin et al., 1993; Romfo et al., 1999). The [Y108A, F111A] spU2AFSM allele was isolated as part of a comprehensive structure-function analysis of the small subunit (Webb and Wise, 2004), but was not analyzed with respect to its effect on growth or splicing in that study.
Shown in Figure 4A is a growth curve for the [Y108A, F111A] spU2AFSM mutant, as well as an isogenic wild-type strain, at both the permissive (30°C) and nonpermissive (37°C) temperatures. The growth rate of the small subunit mutant begins to decrease ∼4 h after shifting to the nonpermissive temperature and plateaus after ∼10 h. To determine whether the growth defect might be due to diminished splicing, we performed RT-PCR analysis to determine the ratio of linear precursor RNA to mature mRNA for the first intron in the ypt5 pre-mRNA (ypt5-I1) at three time points after the temperature shift. Even though the cells are still growing at the wild-type rate, splicing is already impaired after 2 h under nonpermissive conditions, with linear precursor representing 10% of the total ypt5-I1 RNA. A comparatively more dramatic accumulation of precursor (18%) is observed after 4 h, and an even higher level (21%) after 6 h. In parallel cultures grown at the permissive temperature (30°C), the fraction of precursor is very low at the 2 h time point (2%); although increases are observed in the 4- and 6-h samples (7 and 6%, respectively), the accumulation of precursor is quite modest compared with that observed at the nonpermissive temperature.
Figure 4.
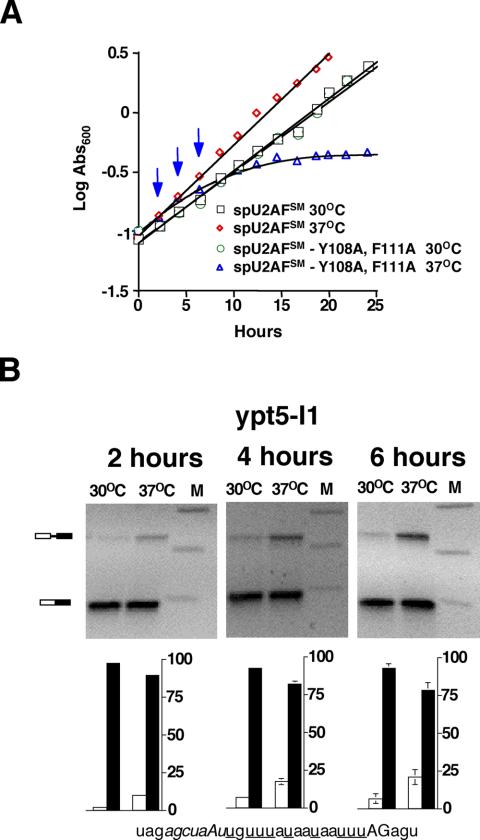
(A) Growth curve for the temperature-sensitive [Y108A, F111A] spU2AFSM mutant. Growth of isogenic strains harboring either a wild-type or mutant allele was monitored by measuring the absorbance600 of cultures propagated at the standard growth temperature (30°C) or at high temperature (37°C). Vertical blue arrows designate time points at which RNA was extracted. (B) RT-PCR assays of splicing in the ts spU2AFSM mutant. (Top) Gel electrophoretic analysis of products from chromosomally expressed ypt5-I1 at three different time points after the shift to nonpermissive temperature and in parallel cultures propagated at the permissive temperature. The positions of linear pre-mRNA and mature mRNA are indicated schematically at the left. Bottom: histogram showing quantitation of RT-PCR data. White bars, percent precursor mRNA; black bars, percent mature mRNA. Error bars, SD for three RT-PCR splicing assays. Shown at the bottom is the sequence of the 3′ end of ypt5-I1 with the branchpoint sequence indicated in italics, the pyrimidine tract underlined, and the terminal AG in uppercase.
The fact that the temperature-sensitive [Y108A, F111A] fission yeast small subunit mutant does indeed display a splicing defect at the nonpermissive temperature placed us in a position to directly test our hypothesis that the two subunits of U2AF are functionally interdependent in S. pombe. Specifically, our model predicts that splicing of any given pre-mRNA will display 1) parallel requirements for the large and small subunits of U2AF and 2) no correlation between the strength of the signals at the 3′ splice site (branchpoint sequence/polypyrimidine tract/yAG) and the requirement for either subunit of spU2AF. Figure 5A shows splicing data for the first pre-mRNA we tested, cdc16-I2, at both the permissive (30°C) and nonpermissive (37°C) temperature for either the spU2AFSM (left panel) or the spU2AFLG (right panel) ts mutant. As an additional control, we assayed RNA from the corresponding wild-type strains at both temperatures. Because extensive data have been published for the effect of the large subunit mutant 2 h after shifting to nonpermissive conditions (Potashkin et al., 1993; Romfo et al., 1999) and to minimize secondary mutational effects after cessation of growth, all assays were conducted at this time point even though the ypt5-I1 data show that the magnitude of the small subunit splicing defect increases after longer periods of time at the nonpermissive temperature (Figure 4B). Interestingly, cdc16-I2 is not very efficiently spliced even at the permissive temperature or in a wild-type strain (Figure 5A). However, inactivation of spU2AFSM by shifting the [Y108A, F111A] mutant to 37°C dramatically increases the level of precursor, from 24 to 60%. spU2AFLG inactivation causes an even more dramatic splicing defect, with <10% mature mRNA remaining after 2 h at the nonpermissive temperature. Splicing of this intron was similarly impaired when we used a different means of reducing the levels of either U2AF subunit, namely metabolic depletion (Webb and Wise, 2004).
Figure 5.
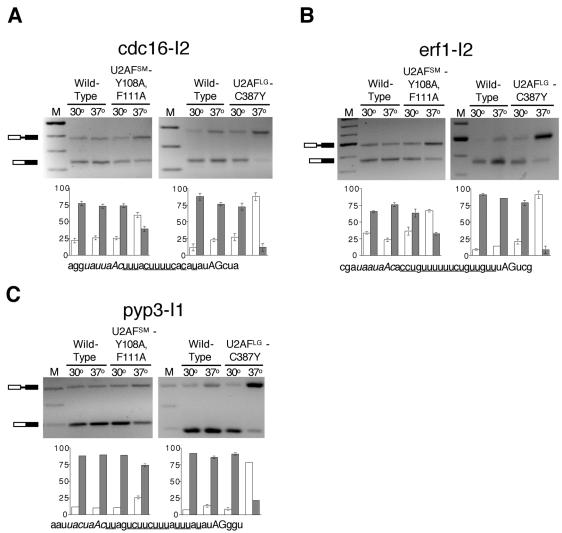
RT-PCR assays of cdc16-I2, erf1-I2, and pyp3-I1 splicing in the ts spU2AFSM and spU2AFLG mutants. (A) Top left: gel electrophoretic analysis of products from cdc16-I2 in the spU2AFSM [Y108A, F111A] mutant. Bottom left: histogram showing quantitation of RT-PCR data with percent precursor mRNA indicated by white bars and percent mature mRNA indicated by black bars. Error bars, SD for three RT-PCR splicing assays. (Right: as in the left panels except that the experiment was performed with the spU2AFLG C387Y mutant. Bottom: the sequence of the 3′ end of cdc16-I2 with the branchpoint sequence indicated in italics, the pyrimidine tract underlined, and the terminal AG in uppercase. (B) As in A except that the splicing data are for erf1-I2. (C) As in A except that the splicing data are for pyp3-I1.
Our finding that cdc16-I2 precursor accumulates in the absence of functional S. pombe U2AF small subunit is notable in light of the model for metazoan U2AF function, which postulates that introns containing strong polypyrimidine tracts do not require the small subunit for splicing (reviewed in Moore, 2000). The polypyrimidine tract of cdc16-I2 consists of 11 pyrimidines with 3 single purine interruptions (Figure 5A, bottom panel), which places it at the “strong” end of S. pombe polypyrimidine tracts (Zhang and Marr, 1994; Wood et al., 2002). To determine whether fission yeast pre-mRNAs with extensive polypyrimidine tracts generally have a strong dependence on both subunits of U2AF, we examined the splicing profile of erf1-I2, which has a branchpoint to 3′ splice site distance and base composition comparable to cdc16-I2 (Figure 5B). Shifting the spU2AFSM mutant to 37°C yields substantially more precursor (67%) than at 30°C (36%), demonstrating that this intron also requires the small subunit for efficient splicing. As for cdc16-I2, inactivation of spU2AFLG results in a higher level of unspliced erf1-I2 (>90%; Figure 5B).
Because erf1 and cdc16 are multi-intronic, we examined the splicing profile of pyp3, a pre-mRNA interrupted by a single central intron with above average 3′ pyrimidine content for S. pombe. pyp3-I1 reproducibly shows modest but readily discernible precursor accumulation in the spU2AFSM mutant at 37°C (25%) and a more dramatic splicing defect in the spU2AFLG mutant (76%; Figure 5C). We conclude that, in S. pombe, the small subunit of U2AF is required for splicing of pre-mRNAs with strong polypyrimidine tracts, supporting our hypothesis that when one subunit of U2AF is required for splicing of a given pre-mRNA in S. pombe, so is the other.
Splicing Assays on a Panel of Introns Reveal Diverse but Generally Parallel Requirements for the Two Subunits of S. pombe U2AF
In an effort to identify introns that show distinct requirements for spU2AFLG and spU2AFSM, we analyzed nineteen additional introns that span the continuum of branchpoint to 3′ splice site distances in this organism. Note that branch-point assignments are based on in silico sequence comparisons, as physical mapping has been impeded by the finding that many splice site mutants that lead to lariat accumulation in mammals and budding yeast block the first step of splicing in fission yeast (Romfo and Wise, 1997; Alvarez and Wise, 2001). Nevertheless, we believe these assignments are likely to be accurate because branchpoint sequences are much more readily recognized in S. pombe than in metazoans, although less so than in S. cerevisiae (Wood et al., 2002). Although the first two positions of the UACUAAC consensus that is virtually invariant in budding yeast are not appreciably conserved in fission yeast, the other five positions show substantial conservation, particularly when purine/purine and pyrimidine/pyrimidine substitutions are taken into account. The frequency breakdown among the 4565 total introns is as follows: ctaac (1943), ctaat (1038), ttaac (714), ctgac (238), ttaat (178), cttac (130), ctaaa (104), ttgac (60), ctgat (60), ataac (50), ttgat (36) and gtaac (14); taken from http://www.sanger.ac.uk/Projects/S_pombe/intron.shtml.
The entire panel of introns examined, shown schematically in Figure 6A, includes the two introns identified by the S. pombe genome project with the longest (SPCC16A11.08-I2) and shortest (swi4-I1) known distances between the designated branchpoint sequence and the terminal 3′ YAG. The other 17 introns were selected to represent a continuum of branchpoint to 3′ splice site distances and variable pyrimidine contents in this interval. Quantitation of the splicing assays is depicted in Figure 6B; for simplicity, the isogenic wild-type controls are unpublished data, but these strains did not display a significant change in precursor accumulation at 37°C compared with 30°C (≤13%). The first notable result is that chk1-I3, which contains just three nucleotides, only one of them a pyrimidine, between the branchpoint sequence and 3′ splice site (Figure 6A, 20), displays a strong requirement for both subunits of S. pombe U2AF (Figure 6B, 20). The 3′ architecture of erf1-I3 is similar to chk1-I3, and this intron displays similar levels of precursor accumulation in the spU2AF mutants (Figure 6B, 21). swi4-I1, which has an even shorter branchpoint to 3′ splice site distance, is affected to a lesser extent by mutational inactivation of either subunit of spU2AF (Figure 6B, 22). Finally, three of the introns with short branchpoint to 3′ splice site distances (Figure 6B, 17-19) exhibit moderate precursor accumulation upon inactivation of spU2AFLG and no discernible splicing defect in the spU2AFSM mutant.
Figure 6.
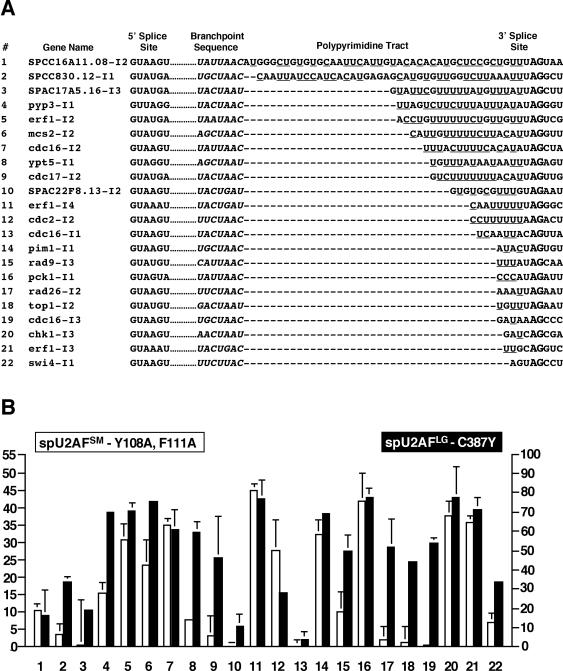
Analysis of splicing in vivo for 22 introns in the ts spU2AFSM and spU2AFLG mutants. (A) Each number is followed by the gene name and sequence of the 5′ splice site, branchpoint, polypyrimidine tract, 3′ splice site, and three nucleotides beyond the AG at the intron/exon boundary. Complete sequences can be found at http://www/Sanger.ac.uk/Projects/S_pombe/. (B) Histograms showing quantitation of RT-PCR assays for the 22 introns depicted in A after heat inactivation of either subunit. Data were collected as in Figure 5 but are expressed as the percent precursor observed at 30°C subtracted from the percent precursor observed at 37°C (Δ precursor accumulation from 30 to 37°C). White bars, data for the spU2AFSM [Y108A, F111A] mutant; black bars, the spU2AFLG C387Y mutant data. Note that the scales are different for each subunit, owing to the more dramatic splicing defects caused by the large subunit ts allele at the 2 h time point (see text for details).
The mean branchpoint to 3′ splice site distance is 9.7 nucleotides in S. pombe (Zhang and Marr, 1994), and introns close to this average also show diverse U2AF requirements for efficient splicing (Figure 6B, 9-13) that do not correlate in any obvious way with 3′ pyrimidine content. More noteworthy is the fact that the intron with the longest branchpoint to 3′ splice site distance in the S. pombe genome does not exhibit dramatic accumulation of precursor after mutational inactivation of either the small or the large subunit of U2AF (Figure 6B, 1). The branchpoint to 3′ splice site interval of SPCC16A11.08-I2 is biased toward pyrimidines (24/42 nucleotides), and a similar ratio (19/40 nucleotides) is found in SPCC830.12-I1 (Figure 6A, 2), which also has an unusually long 3′ interval. Splicing of the latter intron is affected to a modest degree by inactivation of the spU2AF large subunit and less so by inactivation of the small subunit (Figure 6B, 2).
Assaying splicing of different introns in multi-intronic pre-mRNAs after U2AF inactivation demonstrates that the order in which an intron appears is not a key determinant of U2AF dependence in S. pombe. For example, the second and third (middle) introns and the fourth (terminal) intron of erf1 all show a pronounced requirement for both subunits of the S. pombe heterodimer (Figure 6B, 5, 21, and 11). In contrast, introns 1, 2, and 3 of cdc16 exhibit variable spU2AF requirements, with the first intron remaining largely unspliced after inactivation of either spU2AFLG or spU2AFSM (Figure 6B, 13). cdc16-I3 accumulates precursor only after inactivation of the large subunit (Figure 6B, 19), and cdc16-I1 does not accumulate precursor after inactivation of either subunit of spU2AF (Figure 6B, 13). The observation that individual introns within the same pre-mRNA are retained to varying extents after spU2AF inactivation strongly argues against the idea that differences in RNA stability due to either nuclear turnover or cytoplasmic nonsense-mediated decay account for the distinct levels of precursor observed in different pre-mRNAs.
U2AF-dependence Does Not Correlate with Other Obvious Features of S. pombe pre-mRNAs
In an effort to determine the mechanistic basis for the differential U2AF requirements in S. pombe, we proceeded to examine features of the 22 pre-mRNAs in addition to 3′ pyrimidine content, branchpoint to 3′ splice site distance and intron position within multi-intronic precursors. First, because hsU2AFLG binds to and collaborates with SF1/BBP (Berglund et al., 1998; Selenko et al., 2003), we first asked whether the strength of the branchpoint correlates, either directly or inversely, with spU2AF-dependence; no relation was found between the match to the branchpoint consensus (Zhang and Marr, 1994) and spU2AF requirements (see Figure 6). Second, given that introns are bridged by a complex anchored at one end by the U1 snRNP and at the other by U2AF (Abovich et al., 1994; Berglund et al., 1998), we examined the 5′ splice sites of the 22 introns; there was also no correlation between the strength of the 5′ splice site and spU2AF requirements (see Figure 6). Finally, because U2AF can be recruited to weak 3′ splice sites by exonic enhancers (Zuo and Maniatis, 1996) that promote splicing in S. pombe (Webb, Romfo, and Wise, unpublished data), we looked for a correlation with the presence of purine-rich elements downstream; again, none was found (unpublished data). We conclude that neither known cis-acting splicing signals nor other obvious architectural features dictate the requirement for U2AF during splicing in S. pombe. Similarly, the Ares laboratory found that models for features recognized by S. cerevisiae splicing factors did not hold up when a more extensive panel of pre-mRNAs was examined (Clark et al., 2002).
Although we cannot definitively ascertain which features of the pre-mRNA determine spU2AF requirements, the data presented here reinforce the view that the polypyrimidine tract plays a diminished role in S. pombe splicing compared with metazoans. This is consistent with the observation that two different S. pombe introns could not be converted to AG-independence by expansion of the polypyrimidine tract (Romfo and Wise, 1997), in contrast to AG-dependent mammalian substrates (Reed, 1989). Thus, the information content of the polypyrimidine tract alone is not sufficient for 3′ splice site recruitment in S. pombe. Stated otherwise, spU2AFSM recognition of the 3′ splice site cannot be by-passed by improving the target sequence for spU2AFLG. In aggregate, our results can be explained most parsimoniously by a model in which the small and large subunits of U2AF collaborate during initial 3′ splice site recognition in S. pombe. Fifteen of the 22 introns analyzed in this report display parallel spU2AF subunit requirements for efficient splicing. Thirteen of these exhibit readily discernible splicing defects after inactivation of either subunit of the spU2AF heterodimer, and two do not. Most notably, nine of the 10 introns whose splicing is most dramatically affected by spU2AFSM inactivation are among the 10 most strongly affected by spU2AFLG inactivation (Figure 6B, 4-8, 11, 14, 16, 20, and 21). The more dramatic splicing defects observed with the large subunit mutant suggest that it is a more stringent ts allele, consistent with the Rio laboratory's recent finding that a mutation equivalent to spU2AFLG C387Y conferred a temperature-sensitive phenotype in Drosophila, whereas other point mutations that yielded ts phenotypes in fission yeast did not (Blanchette et al., 2004). This interpretation is also consistent with our finding that the small subunit mutant displays more dramatic growth and splicing defects later in the time course (Figure 4), suggesting that it may be impaired in folding or assembly into the heterodimer, whereas the rapid inactivation of the large subunit has been interpreted as evidence of a functional defect (Potashkin et al., 1993; Romfo et al., 1999).
DISCUSSION
In this study, we first demonstrated by ablation analysis that each of the five domains of the S. pombe U2AF large subunit is independently essential for viability. Second, we isolated spU2AF large and small subunit mutants that share a remarkably similar and highly unusual phenotype; in conjunction with the dominant negative effects of large subunit mutants that lack the RS domain but can still bind the small subunit, this observation led us to hypothesize an extraordinary degree of cooperation between the heterodimeric partners in fission yeast. To test this idea, we examined the splicing in vivo of a diverse panel of pre-mRNAs after inactivation of either spU2AFLG or spU2AFSM. In aggregate, the results of this study provide strong evidence from diverse experimental strategies to support the view that the two subunits of S. pombe U2AF function in a highly collaborative manner in vivo.
Novel Contributions from Genetics Shed Light on U2AF Function
Although the present study reports the first comprehensive domain ablation analysis of the U2AF large subunit in vivo, reverse genetic experiments have previously been conducted on the two N-terminal domains in Drosophila. As noted in Results, our finding that a partial deletion of the spU2AFLG linker region implicated in small subunit interactions confers a growth defect echoes the reduced viability observed in flies carrying alleles with multiple point mutations in the linker as their sole source of the large subunit (Rudner et al., 1998c). On the other hand, the results of deleting the U2AFLG RS domain differ between flies and fission yeast. In contrast to the dominant lethality observed in fission yeast, simple deletion of the RS domain of dmU2AFLG was phenotypically silent (Rudner et al., 1998b). However, when this allele was combined with a deletion of the C-terminal RS/G-rich domain of dmU2AFSM, lethality ensued. The authors concluded that the RS domains of the two subunits of U2AF have redundant or overlapping functions in Drosophila. Notably, fission yeast is the only organism in which only one of the U2AF subunits contains a domain with repetitive amino acid sequences (Zorio and Blumenthal, 1999a). Thus, the inability of the S. pombe large subunit to function without its RS domain may simply reflect the absence of a repeat-containing domain at the S. pombe U2AFSM C-terminus. In other words, it is likely that evolution has carried out a successful experiment in fission yeast similar to the domain deletion analysis carried out by Rio and colleagues in the fruit fly.
Mutations designed to abolish the capacity of the two canonical RRMs to bind RNA have not been studied previously in vivo. Notably, both the [F247D, I249D] substitution in RRM1 and the [F353D, F355D] RRM2 mutant produce recessive lethality, in contrast to the dominant effect of deleting the N-terminal RS domain. In light of evidence that the N-terminal domain of human U2AFLG promotes the transition from the E (early) complex containing U1 snRNP, U2AF, and SF1/BBP to the A complex (prespliceosome) by facilitating annealing of U2 snRNA to the branchpoint sequence (Valcarcel et al., 1996), a reasonable interpretation of our data is that the protein lacking an RS domain forms E complex but is unable to continue spliceosome assembly, whereas a mutant unable to bind RNA does not even enter the pathway. The relief of dominance when the linker region is also eliminated (Romfo et al., 1999) suggests further that U2AF heterodimer formation is also necessary to form E complex, consistent with our splicing data supporting parallel requirements for the two subunits in vivo. It is these functional data that distinguish our study from those carried out previously, as splicing was not analyzed in the Drosophila linker domain mutants (Rudner et al., 1998c), and introns with dramatic splicing defects (i.e., that showed more than a few percent precursor accumulation) were not identified in the fly U2AFLG temperature-sensitive mutants (Blanchette et al., 2004) even though one of these is analogous to the allele we analyzed here (C387Y). One possible reason for the discrepancy is that nuclear RNA turnover is much more rapid or efficient in Drosophila than in S. pombe. Most importantly, our study was the first to analyze both subunits of U2AF and was uniquely possible in S. pombe, the only organism in which a conditional small subunit mutant has been identified.
A key question is whether the few introns that showed no or barely detectable splicing defects even in the very tight fission yeast large subunit conditional mutant are truly spU2AF-independent. We have previously reported that different introns display differential splicing defects in the presence of spU2AF large subunit mutations (Romfo et al., 1999). One possible explanation for why some introns show little response to spU2AF mutations even at the nonpermissive temperature is that they have better spU2AF-binding sites capable of attracting the limiting quantities of functional spU2AF. However, given the lack of a correlation between spU2AF dependence and 3′ pyrimidine content, or for that matter any other obvious feature of the intron or downstream exon, we favor the alternative explanation that these data reflect the cooperative nature of spliceosome assembly in vivo.
The idea that U2AF's role in some splicing events in vivo can be masked by redundant interactions with other factors may be related to another apparent discrepancy between our work and results generated in vitro. In particular, structural and biochemical data support the view that a tryptophan corresponding to position 135 of S. pombe U2AFLG is crucial for heterodimer formation (Kielkopf et al., 2001), whereas our data demonstrate that this amino acid can be mutated to a phenylalanine in vivo with no discernible phenotypic consequences. Trp135 is found in the linker region of the large subunit and we have shown previously that mutating the corresponding tryptophan (W128) in the ψRRM of U2AFSM to either phenylalanine or alanine is tolerated in fission yeast (Webb and Wise, 2004). Thus, experiments carried out in vitro with only two components may amplify the importance of particular interactions.
Is U2AF Function in S. pombe Unique?
Recent biochemical data demonstrate that, in S. pombe, the U2AF heterodimer exists in a tight 1:1:1 complex with SF1 (Huang et al., 2002). The authors of this study proposed that S. pombe has optimized recognition of the 3′ splice site via the concerted interaction of the SF1/spU2AFLG/spU2AFSM complex and the tripartite intronic 3′ splicing signals. The parallel splicing defects we observe after separate inactivation of the large or small subunit of spU2AF are consistent with participation of this factor in such a streamlined system of 3′ splice site recognition. A related model for concerted 3′ splice site recognition by the U2AF heterodimer has been proposed for C. elegans, where the intron/exon boundary is preceded by a short, highly conserved polypyrimidine tract followed immediately by the universal AG dinucleotide (U4CAG/R), and the branchpoint sequence has diverged to an unrecognizable state (Blumenthal and Steward, 1997; Zorio and Blumenthal, 1999b).
It remains to be determined whether an SF1/hsU2AFLG/hsU2AFSM complex exists in mammalian cells or if such a complex concurrently recognizes the branchpoint/polypyrimidine tract/AG dinucleotide at the 3′ splice site. In support of this possibility, an interaction between the large subunit of human U2AF and SF1 promotes cooperative binding to an RNA containing a branchpoint and polypyrimidine tract in vitro (Berglund et al., 1998). Concerted recognition is an attractive model to explain how bona fide 3′ splice sites are correctly identified in mammals, where reasonable matches to the branchpoint and 3′ splice site consensus including the polypyrimidine tract occur randomly every 24 and 490 nucleotides, respectively (Burge et al., 1999). Clearly, however, splicing of a subset of metazoan introns does not require the small subunit of U2AF, at least in vitro, and thus the S. pombe model may not apply to such pre-mRNAs. A dichotomy between fission yeast and mammals is supported by the observation that phosphorylation of a serine that is conserved among SF1 orthologues in metazoans, but not in S. pombe (Mazroui et al., 1999), prevents binding to the large subunit of hsU2AF (Wang et al., 1999). If the available hsU2AF and SF1 are not obligatorily bound together in a complex but rather are free to dissociate in a phosphorylation-dependent manner, this could provide an opportunity for these factors to participate in temporal and spatial regulation of splicing that is not possible in S. pombe. Indeed, inhibition of Drosophila msl-2 splicing by Sex-lethal appears to rely on an unusually long branchpoint to 3′ splice site distance to allow the alternative splicing factor to displace U2AF (Merendino et al., 1999). In addition, the presence in metazoans of genes related to the hsU2AF small subunit (Tronchere et al., 1997; Mount and Salz, 2000; Tupler et al., 2001; Pacheo et al., 2004) as well as tissue-specific alternatively spliced isoforms (Pacheo et al., 2004) suggests the possibility of modular hsU2AF function, wherein 3′ splice site recognition depends on the attributes of different heterodimeric partners for hsU2AFLG.
Collectively, the data from a variety of studies in metazoans and both budding and fission yeast suggest that, unlike the evolutionarily conserved process of 5′ splice site recruitment via complementary base-pairing to U1 snRNA, the interplay between cis-acting elements and trans-acting factors during initial recognition of the 3′ splice site has diverged significantly during evolution.
Acknowledgments
We thank Frank Campbell, Jonatha Gott, Michael Harris, Hua Lou, David McPheeters, and Helen Salz for critical comments on the manuscript. We thank Carissa Romano for expert technical assistance, the curators of the Sanger Center S. pombe genome project for providing a list of introns classified according to branchpoint to 3′ splice site distance and pyrimidine content, and Shelley Sazer (Baylor College of Medicine) for prep41X-imp2. This research was supported by a grant to J.A.W. from the National Institutes of Health; C.J.W. was supported in part through a Cell and Molecular Biology Training Grant awarded through the National Institute of General Medical Sciences.
Article published online ahead of print in MBC in Press on November 17, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0768).
Abbreviations used: U2AFSM, U2AF small subunit; U2AFLG, U2AF large subunit; SF1/BBP, splicing factor 1/branchpoint bridging protein; ψRRM, pseudo-RNA recognition motif.
References
- Abovich, N., Liao, X., and Rosbash, M. (1994). The yeast MUD2 protein: an interaction with PRP11 defines a bridge between commitment complex and U2 snRNP addition. Genes Dev. 8, 843-854. [DOI] [PubMed] [Google Scholar]
- Alvarez, C. J., Romfo, C. M., VanHoy, R. W., Porter, G. L., and Wise, J. A. (1996). Mutational analysis of U1 function in Schizosaccharomyces pombe: pre-mRNAs differ in the extent and nature of their requirements for this snRNA in vivo. RNA 2, 404-418. [PMC free article] [PubMed] [Google Scholar]
- Alvarez, C. J., and Wise, J. A. (2001). Activation of a cryptic 6′ splice site by U1 snRNA. RNA 7, 342-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, H., Rahn, A., Gawande, B., Guth, S., Valcarcel, J., and Singh, R. (2004). The conserved RNA recognition motif 3 of U2 snRNA auxiliary factor (U2AF 65) is essential in vivo but dispensable for activity in vitro. RNA 10, 240-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales, M., Flay, N., McKinney, R., Habara, Y., Ohshima, Y., Tani, T., and Potashkin, J. (2000). Mutations in the large subunit of U2AF disrupt pre-mRNA splicing, cell cycle progression and nuclear structure. Yeast 16, 1001-1013. [DOI] [PubMed] [Google Scholar]
- Berglund, J. A., Abovich, N., and Rosbash, M. (1998). The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Genes Dev. 12, 858-867. [DOI] [PubMed] [Google Scholar]
- Blanchette, M., Labourier, E., Green, R. E., Brenner, S. E., and Rio, D. C. (2004). Genome-wide analysis reveals an unexpected function for the Drosophila splicing factor U2AF50 in the nuclear export of intronless mRNAs. Mol. Cell 14, 775-786. [DOI] [PubMed] [Google Scholar]
- Blumenthal, T., and Steward, K. (1997). RNA processing and gene structure. In: C. elegans II, ed. D. Riddle, T. Blumenthal, B. Meyers, and J. Priess, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 117-145. [PubMed]
- Burge, C. B., Tuschl, T., and Sharp, P. A. (1999). Splicing of Precursors to mRNAs by the Spliceosome. In: The RNA World, ed. R. Gesteland, T. Cech, and J. Atkins, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 525-560.
- Caceres, J. F., and Krainer, A. R. (1993). Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J. 12, 4715-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, T. A., Sugnet, C. W., and Ares, M., Jr. (2002). Genomewide analysis of mRNA processing in yeast using splicing-specific microarrays. Science 296, 907-910. [DOI] [PubMed] [Google Scholar]
- Davies, R. C., Calvio, C., Bratt, E., Larsson, S. H., Lamond, A. I., and Hastie, N. D. (1998). WT1 interacts with the splicing factor U2AF65 in an isoform-dependent manner and can be incorporated into spliceosomes. Genes Dev. 12, 3217-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann, C. R., Kraemer, B., Wickens, M., and Kimble, J. (2002). GLD-3, a bicaudal-C homolog that inhibits FBF to control germline sex determination in C. elegans. Dev. Cell 3, 697-710. [DOI] [PubMed] [Google Scholar]
- Fewell, S. W., and Woolford, J. R. (1999). Ribosomal protein S14 of Saccharomyces cerevisiae regulates its expression by binding to RPS14B pre-mRNA and to 18S rRNA. Mol. Cell. Biol. 19, 826-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckner, J., Zhang, M., Valcarcel, J., and Green, M. R. (1997). U2AF65 recruits a novel human DEAD box protein required for the U2 snRNP-branchpoint interaction. Genes Dev. 11, 1864-1872. [DOI] [PubMed] [Google Scholar]
- Gozani, O., Potashkin, J., and Reed, R. (1998). A potential role for U2AF-SAP 155 interactions in recruiting U2 snRNP to the branch site. Mol. Cell. Biol. 18, 4752-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth, S., Tange, T. O., Kellenberger, E., and Valcarcel, J. (2001). Dual function for U2AF35 in AG-dependent pre-mRNA splicing. Mol. Cell. Biol. 21, 7673-7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T., Vilardell, J., and Query, C. (2002). Pre-spliceosomal formation in S. pombe requires a stable complex of SF1-U2AF59-U2AF23. EMBO J. 21, 5516-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., Muto, Y., Green, M. R., and Yokoyama, S. (1999). Solution structures of the first and second RNA-binding domains of human U2 small nuclear ribonucleoprotein particle auxiliary factor (U2AF65). EMBO J. 18, 453-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., Halladay, J., and Craig, E. A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaar, R., Roche, S. E., Beall, E. L., Green, M. R., and Rio, D. C. (1993). The conserved pre-mRNA splicing factor U2AF from Drosophila: requirement for viability. Science 262, 569-573. [DOI] [PubMed] [Google Scholar]
- Kielkopf, C. L., Rodionova, N. A., Green, M. R., and Burley, S. K. (2001). A novel peptide recognition mode revealed by the X-ray structure of a core U2AF35/U2AF65 heterodimer. Cell 106, 595-605. [DOI] [PubMed] [Google Scholar]
- Kielkopf, C. L., Lucke, S., and Green, M. R. (2004). U2AF homology motifs: protein recognition in the RRM world. Genes Dev. 18, 1513-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff, X., Utzig, S., and Simanis, V. (1999). Controlling septation in fission yeast: finding the middle, and timing it right. Curr. Genet. 35, 571-584. [DOI] [PubMed] [Google Scholar]
- Mazroui, R., Puoti, A., and Kramer, A. (1999). Splicing factor SF1 from Drosophila and Caenorhabditis: presence of an N-terminal RS domain and requirement for viability. RNA 5, 1615-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merendino, L., Guth, S., Bilboa, D., Martinez, C., and Valcarcel, J. (1999). Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature 402, 838-841. [DOI] [PubMed] [Google Scholar]
- Moore, M. (2000). Intron recognition comes of AGe. Nature 7, 14-16. [DOI] [PubMed] [Google Scholar]
- Mount, S. M., and Salz, H. K. (2000). Pre-messenger RNA processing factors in the Drosophila genome. J. Cell Biol. 150, F37-F44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheo, T. R., Gomes, A. Q., Barbosa-Morais, N. L., Benes, V., Ansorge, W., Wollerton, M., Smith, C. W., Valcarcel, J., and Carmo-Fonseca, M. (2004). Diversity of vertebrate splicing factor U2AF35: identification of alternatively spliced U2AF1 mRNAs. J. Biol. Chem. 279, 27039-27049. [DOI] [PubMed] [Google Scholar]
- Page-McCaw, P., Amonlirdviman, K., and Sharp, P. (1999). PUF60, a novel U2AF65-related splicing activity. RNA 5, 1548-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashkin, J., Naik, K., and Wentz-Hunter, K. (1993). U2AF homolog required for splicing in vivo. Science 262, 573-575. [DOI] [PubMed] [Google Scholar]
- Reed, R. (1989). The organization of 3′ splice-site sequences in mammalin introns. Genes Dev. 3, 2113-2123. [DOI] [PubMed] [Google Scholar]
- Romfo, C., Lakhe-Reddy, S., and Wise, J. A. (1999). Molecular genetic analysis of U2AF59 in Schizosaccharomyces pombe: differential sensitivity of introns to mutational inactivation. RNA 5, 49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romfo, C. M., and Wise, J. A. (1997). Both the polypyrimidine tract and the 3′ splice site function prior to the first step of splicing in fission yeast. Nucleic Acids Res. 25, 4658-4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, D., Breger, K., Kanaar, R., Adams, M., and Rio, D. (1998a). RNA binding activity of heterodimeric splicing factor U2AF: at least one RS domain is required for high-affinity binding. Mol. Cell. Biol. 18, 4004-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, D. Z., Breger, K. S., and Rio, D. (1998b). Molecular genetic analysis of the heterodimeric splicing factor U2AF: the RS domain on either the large or small Drosophila subunit is dispensable in vivo. Genes Dev. 12, 1010-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, D., Kanaar, R., Breger, K., and Rio, D. (1998c). Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol. Cell. Biol. 18, 1765-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, D., Kanaar, K., Breger, S., and Rio, D. (1996). Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc. Natl. Acad. Sci. USA 93, 10333-10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin, B., Zamore, P. D., and Green, M. R. (1988). A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell 52, 207-219. [DOI] [PubMed] [Google Scholar]
- Selenko, P., Gregorovic, G., Sprangers, R., Stier, G., Rhani, Z., Kramer, A., and Sattler, M. (2003). Structural basis for the molecular recognition between human splicing factors U2AF65 and SF1/mBBP. Mol. Cell 11, 965-976. [DOI] [PubMed] [Google Scholar]
- SenGupta, D. J., Zhang, B., Kraemer, B., Pochart, P. Fields, S., and Wickens, M. (1996). A three-hybrid system to detect RNA-protein interactions in vivo. Proc. Natl. Acad. Sci. USA 93, 8496-8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R., Valcarcel, J., and Green, M. R. (1995). Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science 268, 1173-1176. [DOI] [PubMed] [Google Scholar]
- Tronchere, H., Wang, X., and Fu, X. D. (1997). A protein related to U2AF35 that interacts with U2AF65 and SR proteins in splicing of pre-mRNA. Nature 388, 397-400. [DOI] [PubMed] [Google Scholar]
- Tupler, R., Perini, G., and Green, M.R. (2001). Expressing the human genome. Nature 409, 832-833. [DOI] [PubMed] [Google Scholar]
- Uruyishiyama, S., Tani, T., and Ohshima, Y. (1996). Isolation of novel pre-mRNA splicing mutants of Schizosaccharomyces pombe. Mol. Gen. Genet. 253, 118-127. [DOI] [PubMed] [Google Scholar]
- Vagner, S., Vagner, C., and Mattaj, I. W. (2000). The carboxyl terminus of vertebrate poly(A) polymerase interacts with U2AF65 to couple 3′-end processing and splicing. Genes Dev. 14, 403-413. [PMC free article] [PubMed] [Google Scholar]
- Valcarcel, J., Gaur, R. K., Singh, R., and Green, M. R. (1996). Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA. Science 273, 1706-1709. [DOI] [PubMed] [Google Scholar]
- Wang, X., Bruderer, S., Rafi, Z., Xue, J., Milburn, P. J., Kramer, A., and Robinson, P. J. (1999). Phosphorylation of splicing factor SF1 on Ser20 by cGMP-dependent protein kinase regulates spliceosome assembly. EMBO J. 18, 4549-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, C. J., and Wise, J. A. (2004). The splicing factor U2AF small subunit is functionally conserved between fission yeast and humans. Mol. Cell. Biol. 24, 4229-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentz-Hunter, K., and Potashkin, J. (1996). The small subunit of the splicing factor U2AF is conserved in fission yeast. Nucleic Acids Res. 24, 1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, V. et al. (2002). The genome sequence of Schizosaccharomyces pombe. Nature 415, 871-880. [DOI] [PubMed] [Google Scholar]
- Wu, S., Romfo, C., Nilsen, T., and Green, M. (1999). Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature 402, 832-835. [DOI] [PubMed] [Google Scholar]
- Zamore, P. D., and Green, M. R. (1991). Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 10, 207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore, P. D., and Green, M. R. (1989). Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc. Natl. Acad. Sci. USA 86, 9243-9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore, P., Patton, J., and Green, M. (1992). Cloning and domain structure of the mammalian splicing factor U2AF. Nature 355, 609-614. [DOI] [PubMed] [Google Scholar]
- Zhang, M., Zamore, P. D., Carmo-Fonseca, M., Lamond, A. I., and Green, M. R. (1992). Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc. Natl. Acad. Sci. USA 89, 8769-8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. Q., and Marr, T. G. (1994). Fission yeast gene stucture and recognition. Nucleic Acids Res. 22, 1750-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. J., and Wu, J. Y. (1996). Functional properties of p54, a novel SR protein active in constitutive and alternative splicing. Mol. Cell. Biol. 16, 5400-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. J., and Wu, J. Y. (1998). Sip1, a novel RS domain-containing protein essential for pre-mRNA splicing. Mol. Cell. Biol. 18, 676-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, Y., and Weiner, A. M. (1986). A compensatory base change in U1 snRNA suppresses a 5′ splice site mutation. Cell 46, 827-835. [DOI] [PubMed] [Google Scholar]
- Zorio, D. A., Lea, K., and Blumenthal, T. (1997). Cloning of Caenorhabditis U2AF65: an alternatively spliced RNA containing a novel exon. Mol. Cell. Biol. 17, 946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorio, D.A., and Blumenthal, T. (1999a). U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans. RNA 5, 487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorio, D., and Blumenthal, T. (1999b). Both subunits of U2AF recognize the 3′ splice site in Caenorhabditis elegans. Nature 402, 835-838. [DOI] [PubMed] [Google Scholar]
- Zuo, P., and Maniatis, T. (1996). The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 10, 1356-1368. [DOI] [PubMed] [Google Scholar]


