Abstract
The Mre11-Rad50-Xrs2 (MRX) protein complex plays pivotal roles in meiotic recombination, repair of damaged DNA, telomere elongation, and cell cycle checkpoint control. Xrs2p is known to be essential for all the functions of the complex, but its role in the complex has not been clearly elucidated. A 32-amino acid region near the C terminus of Xrs2p was identified as an Mre11p-binding site. No more function of Xrs2p than translocation of Mre11p from the cytoplasm to the nucleus is necessary for response to DNA damage. However, domains in Xrs2p located both 49 amino acids upstream and 104 amino acids downstream of the Mre11p binding site are required for meiotic recombination and telomere elongation, respectively, in addition to the 32-amino acid region. These findings demonstrate that Xrs2p acts as a specificity factor that allows the MRX complex to function in meiotic recombination and in telomere elongation.
INTRODUCTION
The MRX complex in Saccharomyces cerevisiae acts to maintain genome stability by functioning in both DNA repair and genetic recombination pathways (for reviews, see Haber, 1998; D'Amours and Jackson, 2002). It is composed of Mre11p, Rad50p, and Xrs2p subunits (Johzuka and Ogawa, 1995; Usui et al., 1998) and is necessary for phosphorylation and activation of both Rad53p and Chk1p checkpoint kinases on a Tel1p-dependent checkpoint pathway (Grenon et al., 2001; Usui et al., 2001). One the other hand, Mre11p and Xrs2p are phosphorylated in a Tel1p-dependent manner in response to DNA damage, indicating that the MRX complex functions as a sensor and a signal transducer for DNA double-strand breaks (DSBs). The complex also is involved in telomere elongation through a Tel1 pathway (Ritche and Petes 2000) and is suggested to recruit telomerase to telomeres (Tsukamoto et al., 2001). In addition, it plays at least two roles in meiotic recombination. One is introduction of meiosis-specific DSBs with an aid of a meiosis-specific DNA endonuclease, Spo11p. The other is successive removal of Spo11p-DNA complexes and resection of the broken DNA ends (for reviews, see Haber, 1998; D'Amours and Jackson, 2002). There is separation of function alleles of the rad50 and mre11 mutants that are defective in the processing of meiotic DSBs; however, xrs2 mutants that show similar phenotype to these mutants are not isolated. Null strains defective in any one of these subunits display the same phenotype, which includes defects in meiotic homologous recombination, nonhomologous end-joining, DNA damage repair, S-phase checkpoint control, and telomere elongation.
The Mre11p and Rad50p components of the complex have been characterized extensively. Mre11p displays several biochemical properties, including 3′-to-5′ single-strand (ssDNA) and double-strand DNA (dsDNA) exonuclease activity, ssDNA endonuclease activity, a limited DNA unwinding activity, and binding to both ssDNA and dsDNA (Furuse et al., 1998; Usui et al., 1998). Rad50p exhibits ATP-dependent binding to dsDNA, and the binding involves dimer formation of the protein (Raymond and Kleckner, 1993). The dimerization is mediated through globular ATP binding domains at the N and C terminals, and the heptad repeat regions form intramolecular coiled-coil structure (Anderson et al., 2001). Mre11p binds as a dimer to a region in Rad50p located between the two catalytic domains.
Xrs2p consists of 854 amino acids and binds to Mre11p, but not to Rad50p (Usui et al., 1998). Xrs2p was recently shown to display DNA binding activity that is specific for DNA structure and enhances the Mre11p nuclease activity (Trujillo et al., 2003). Similar enhancements of hMre11p activities have been reported in a study of the human homologue of the XRS2 gene NBS1. Nbs1p binds at the C-terminal region to hMre11p and enhances the activities of hMre11p, as measured in in vitro DNA binding, ssDNA endonuclease, and DNA unwinding assays (Carney et al., 1998; Paull and Gellert, 1999; Desai-Mehta et al., 2001, Tauchi et al., 2001). Interestingly, Nbs1p is located at the telomere region only in S phase when telomeres are elongated in HeLa cells, whereas hMre11p and hRad50p are associated with telomeres throughout the cell cycle (Zhu et al., 2000), suggesting that Nbs1p acts a factor that allows hMre11p and hRad50p to function in telomere elongation. This result prompted us to speculate that Nbs1p and Xrs2p are regulators that permit the Mre11p and Rad50p complexes to function specifically when their respective activities are required. To test this possibility, we examined various mutants with specific functionalities, and found that Xrs2p contains distinct domains involved in the respective functions. Xrs2p binds to Mre11p with a 32-amino acid region and translocates Mre11p to the nucleus. In addition, Xrs2p plays essential roles in telomere elongation and meiotic recombination with amino acids 630-765 and 581-661 of Xrs2p, respectively.
MATERIALS AND METHODS
Media
Media were prepared as described previously (Burke et al., 2000), with the exception of YPG (1% Bacto Yeast Extract, 2% Bacto Peptone, 2% glycerol), presporulation (1% Bacto Yeast Extract, 2% Bacto Peptone, and 1% potassium acetate), and sporulation media (0.3% potassium acetate and 0.02% raffinose).
Plasmids
To create pTY1269, a 3.2-kb XRS2 gene fragment amplified by polymerase chain reaction (PCR) was cloned into pRS318 (ARSH4 CEN6 LEU2 CYH2; Sikorski and Boeke, 1991). pYT1501 was constructed by inserting a 3.1-kb HDF1 gene fragment amplified by PCR into pRK900 (MEC1 ARS1 CEN4 URA3; Kato and Ogawa, 1994). To construct integration vectors pYT1608, pYT1610, and pCM76a, a 0.5-kb fragment containing a part of the MET17 gene was amplified by PCR and then cloned into pBluescript II KS+ (Stratagene, La Jolla, CA), pRS404 (TRP1; Sikorski and Hieter, 1989), and YIplac204 (TRP1; Gietz and Sugino, 1988), respectively. xrs2 alleles bearing truncation or point mutations were subcloned into these vectors.
Strains
Methyl methanesulfonate (MMS) and hydroxyurea (HU) sensitivity assays and telomere length assays were performed in a SYT358a background (MATa xrs2Δ::URA3 ade2 ade3 trp1Δ leu2-3,112 ura3Δ lys2-801 can1). SYT301a (MATα xrs2Δ::TRP1 mec1Δ::leu2::hisG sml1Δ::leu2::hisG hdf1Δ::leu2::hisG ade2 ade3 trp1Δ leu2-3,112 ura3Δ lys2-801 met17Δ::leu2::hisG can1 cyh2) harboring the plasmid pYT1501 (ARS1 CEN4 URA3 MEC1 HDF1) was used in the synthetic senescence assay. SYT9a (MATα ade2 ade3 trp1Δ leu2-3,112 ura3Δ lys2-801 met17Δ::leu2::hisG can1 cyh2; Tsukamoto et al., 2001) was used to construct truncated xrs2 alleles containing a Myc epitope tag. SYT358a, SYT301a, and SYT9a are derivatives of VPS105 (MATα ade2 ade3 trp1Δ leu2-3,112 ura3Δ lys2-801 can1; Schulz and Zakian, 1994) or VPS106 (as for VPS105, except with MATa).
To study the phenotype conferred by the xrs2 truncation alleles, an xrs2 deletion construct in which the XRS2 open reading frame (ORF) is replaced with either the TRP1 or URA3 marker was introduced by PCR-mediated one-step gene disruption. The mre11 or rad50 deletion alleles were introduced using similar methods. The DNA sequences of the primers used for gene disruption are described in the Supplemental Data. xrs2 mutant alleles were targeted to the MET17 locus by using integration plasmids pYT1608 (met17ΔNΔC), pYT1610 (met17ΔNΔC TRP1), or pCM76a (met17ΔNΔC TRP1). Disruption of the MEC1, SML1, and HDF1 genes was performed as described previously (Tsukamoto et al., 2001). Meiotic studies were performed in CMY9 (MATα xrs2Δ::URA3 ho::LYS2 trp1 ura3 lys2 his4X::LEU2 arg4-nsp) and CMY10 (MATa xrs2Δ::URA3 ho::LYS2 trp1 ura3 lys2 his4B::LEU2 arg4-bgl) derivatives. CMY9 and CMY10 are SK1-derived strains.
PJ69-4A (MATa trp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ LYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ; James et al., 1996) was used in the two-hybrid assay.
Screening of xrs2 Point Mutants with a Defect in Telomere Elongation
XRS2 was randomly mutagenized by error prone PCR in the presence of 0.2-0.4 mM MnCl2, by using TaqDNA polymerase (Promega, Madison, WI), the primers T3 (5′-AATTAACCCTCACTAAAGGG-3′) and T7 (5′-GTAATACGACTCACTATAGGGC-3′), and the pYT1269 DNA template. PCR products were digested with BamHI and cloned into the BamHI site of pRS318. The resulting mutant pools were introduced into SYT301 harboring the plasmid pYT1501. The transformants were streaked onto plates containing 5-fluoroorotic acid (5-FOA) to screen xrs2 mutants that are inviable after loss of pYT1501. xrs2 mutants displaying an accelerated senescence phenotype in a mec1 sml1 hdf1 mutant background were isolated by plasmid shuffling method. Then, xrs2 mutant plasmids were recovered by transforming whole yeast DNA derived from an xrs2 mutant into the Escherichia coli strain XL1-Blue (Stratagene, La Jolla, CA). The location of the xrs2 mutation was determined by sequencing both strands of the XRS2 gene.
Construction of xrs2 Mutants with Truncations
A series of xrs2 mutants with C-terminal truncations was constructed by replacing the specified C-terminal region of the XRS2 gene on pYT1269 in SYT9a with a 9Myc-klTRP1 unit (Knop et al., 1999), by using PCR-mediated one-step gene replacement. Similarly, a series of N-terminal truncated xrs2 mutants were constructed by replacing the specified N-terminal region with a 3Myc-URA3-3Myc fragment (Schneider et al., 1995). Pop out events, which eliminated one 3Myc fragment and the URA3 gene, were selected on 5-FOA media to obtain 3Myc-tagged xrs2-ΔN mutants. For construction of the xrs2-ΔM mutant, the amino acid residues 630-661 of xrs2-ΔC0 on pRS318 was replaced with a 3HA-URA3-3HA fragment (Schneider et al., 1995). The DNA sequences of the primers used for the truncations are described in the Supplemental Data. These sets of mutations were subcloned into the integration vectors and introduced into xrs2 null strains.
Analysis of Telomere Length by Southern Blotting
For each mutant, genomic DNA was prepared after ∼100 cell divisions after strain construction. The DNA from each strain was digested with XhoI, electrophoresed in a 0.9% TAE agarose gel, transferred to a nylon membrane (Roche Diagnostics, Indianapolis, IN), and hybridized with a 247-base pair probe bearing Y′ element DNA. The probe was labeled with digoxigenin, by using a PCR DIG Probe Synthesis kit (Roche Diagnostics) and primer pairs 5′-CAGTTTAGCAGGCATCATCG-3′ and 5′-CGAGAACTTCAACGTTTGCC-3′. The Southern blot signals were quantified with a DIG Luminescent Detection kit (Roche Diagnostics) and a LAS1000 chemiluminescence image analyzer (Fuji Film, Tokyo, Japan). The digoxigenin-labeled molecular weight markers (Roche Diagnostics) were λDNA digested with EcoRI and HindIII.
Synchronous Entry of Cells into Meiosis
Cells from a frozen stock were grown on a YPG plate for 12 h to remove ρ- cells. The cells were then streaked on a YPAD plate and incubated for 2 d. A single colony was incubated in YPAD liquid medium and grown for 24 h. The resulting culture was added to presporulation medium at a 1/100 dilution and incubated with vigorous aeration for 12 h. Cells were then harvested, washed twice with water, and transferred to sporulation medium to induce meiosis. All experiments were performed at 30°C.
Immunostaining
Immunostaining was carried out as described previously (Burke et al., 2000). For staining of Myc-tagged Xrs2p or Mre11p, cells prepared on slides were first treated with α-Myc (9E10) mouse monoclonal antibody (mAb) (Sigma-Aldrich, St. Louis, MO) and then stained with Alexa-Fluor-488-conjugated α-mouse IgG (Molecular Probes, Eugene, OR). The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Immunofluorescence microscopy and image processing were carried out as described previously (Terasawa et al., 1995).
Two-Hybrid Analysis
DNA fragments encoding the Mre11p-binding domain for Xrs2p (MBX) of Xrs2p, full-length Xrs2p and full-length Mre11p were cloned into both pG-BDU-c2 (2 μm, URA3 and GAL4 DNA binding domain fusion construct) and pGAD424 (2 μm, LEU2 and GAL4 activation domain fusion construct). The resulting plasmids were introduced into the PJ69-4A strain to examine two-hybrid interactions through HIS3 expression after a 2-d incubation on SC plates (-His -Ura -Leu).
Immunoprecipitation
α-Mre11 rabbit serum was prepared by Tanpaku Seisei Kogyo (Gunma, Japan). The IgG fraction of α-Mre11 rabbit serum was affinity purified using a HiTrap affinity column (Amersham Biosciences, Piscataway, NJ). For each immunoprecipitation experiment, ∼5 × 108 cells were suspended in 300 μl of lysis buffer (20 mM HEPES, pH 7.5, 300 mM NaCl, 5 mM EDTA, 0.01% NP-40, 5 mM dithiothreitol, 0.04% 1-octanol, 10% glycerol, 0.8 mM phenylmethyl-sulfonyl fluoride, and Roche protease inhibitor cocktail [Complete, EDTA free]) and lysed by glass bead disruption by using a mini Bead-Beater (Biospec, Bartlesville, OK). After centrifugation at 20,000 × g for 10 min, α-Myc serum (9E10) agarose conjugate (Santa Cruz Biotechnology, Santa Cruz, CA) or affinity-purified α-Mre11 rabbit serum cross-linked to protein A-Sepharose 4FF (Amersham Biosciences) by dimethyl pimelimidate was mixed with the supernatant at 4°C for 45 min. The immunoprecipitates collected by centrifugation at 2000 × g for 15 s were washed with 600 μl of the lysis buffer four times, fractionated using 7 or 8.5% SDS-PAGE, and analyzed by Western blotting by using ECL Plus Western blotting detection reagents (Amersham Biosciences). For Western blotting, α-Mre11 guinea pig serum (Usui et al., 1998) and α-Myc (9E10) mAb (Sigma-Aldrich) were used at a 1/1500 and 1/1000 dilutions, respectively. Horseradish peroxidase-conjugated antiguinea pig (Chemicon International, Temecula, CA) and anti-mouse (Promega) IgGs were used, as instructed by the manufacturer.
RESULTS
Identification of a Minimum Region of Xrs2p Necessary for Telomere Elongation
To determine the functional regions associated with the respective functions of Xrs2p, we first constructed a system to screen xrs2 mutants that are defective in telomere elongation. mec1 strains lacking any one of the MRX components display an ever shorter telomeres (est) phenotype, which is referred to as senescence (Ritchie and Petes, 2000). This phenotype is accelerated in an hdf1 mutant background (Tsukamoto et al., 2001) and therefore xrs2 mutants with a telomere elongation defect can be isolated in a mec1 hdf1 background. The mec1 hdf1 strain we constructed also contains sml1 to suppress the lethality of the mec1 null mutation. From the 2160 clones with an xrs2 mutant gene that was mutagenized randomly by error prone PCR in the presence of MnCl2, 33 mutants were isolated by screening for the senescence phenotype. The DNA sequences of mutation sites were then analyzed, and 31 were found to be frameshift mutations or nonsense mutations that caused deletion of the C-terminal region, suggesting that the region necessary for telomere elongation is located near the C terminus. Moreover, 15 mutants showed resistance to DNA damaging agents, indicating that the region of Xrs2p necessary for telomere elongation partially overlaps with, but is separable from, that the region required for response to DNA damage (our unpublished data).
To better understand the Xrs2p domain associated with telomere elongation, we constructed a series of xrs2 mutants with various lengths of C-terminal or N-terminal truncations (Figure 1A). The truncation points were determined by the location of the xrs2 frameshift mutations and nonsense mutations described above, as well as by changes in secondary structure predicted by Chou-Fasman analysis (Chou and Fasman, 1978). The wild-type and truncated mutant proteins were tagged with 9Myc at their C termini or with 3Myc at their N termini. The xrs2-ΔC0 strain, expressing wild-type Xrs2p with 9Myc, displayed telomere lengths that were indistinguishable from wild type (Figure 2A). The length of telomeres in the xrs2-ΔC1 mutant strain, which encodes an Xrs2p lacking 89 C-terminal residues, was slightly shorter (∼50 base pairs). In contrast, the xrs2-ΔC2 to -ΔC12 mutant strains displayed telomere elongation defects similar to that observed in the null strain (Figure 2A; unpublished data for xrs2-ΔC5 to -ΔC12). Consistent with this, xrs2-ΔC2 to -ΔC12, but not the xrs2-ΔC0 and -ΔC1 mutants, displayed a senescent phenotype in the mec1 hdf1 strain background (Figure 2B; unpublished data for xrs2-ΔC5 to -ΔC12). The XRS2 construct containing a Myc epitope tag at the N terminus caused a moderate telomere length defect (∼100 base pairs shorter than wild type; xrs2-ΔN0, Figure 2A). The lengths of telomeres in the xrs2-ΔN1 to -ΔN20 mutant strains were similarly shorter than in the wild type, but longer than in the null strain. This observation, in conjunction with the fact that these mutants did not show senescence in the mec1 hdf1 strain background, suggests that the xrs2-ΔN1 to -ΔN20 mutants are functional for telomere elongation (Figure 2B). In contrast, telomere length in the xrs2-ΔN21 mutant strain was similar to that in the null strain, and the xrs2-ΔN21 mutant allele conferred a senescence phenotype in the mec1 hdf1 strain background. Hence, the results obtained from the analysis of the N- and C-terminal truncation alleles identify amino acid residues 630-765 as the region that is important for the telomere elongation function of Xrs2p.
Figure 1.
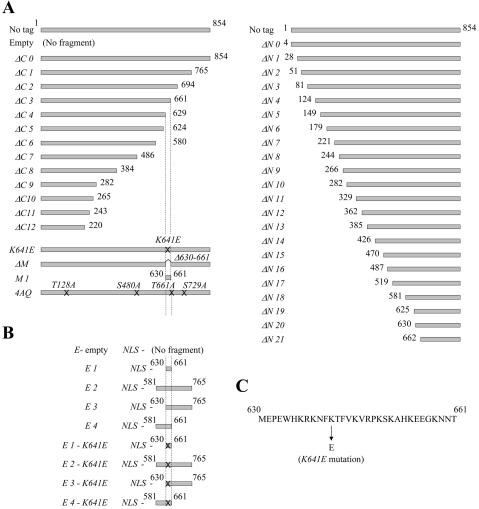
Diagram of the wild-type and the constructed xrs2 mutants. (A) xrs2 mutations analyzed in this study. No tag indicates the wild-type XRS2 gene without an epitope tag sequence. The xrs2-ΔC0 allele is abbreviated to ΔC0; similar abbreviations are used for both the C- and N-terminal xrs2 mutations. Crosses on the gray bars indicate xrs2 alleles containing point mutations created by PCR mutagenesis. All of the xrs2 alleles containing N-terminal truncations encode the first three amino acid residues of Xrs2p, followed by the 3Myc tag at the deletion point. (B) Constructs of Xrs2-E1 to -E4 proteins and their derivatives. All bear a nuclear localization signal (NLS) and the 9Myc tag at their N and C termini, respectively. All of the constructs shown were integrated into the MET17 locus of xrs2 null strains. They also were cloned into the ARS CEN plasmid pRS318 for analysis in the synthetic senescence assay. (C) Amino acid sequence of the MBX.
Figure 2.
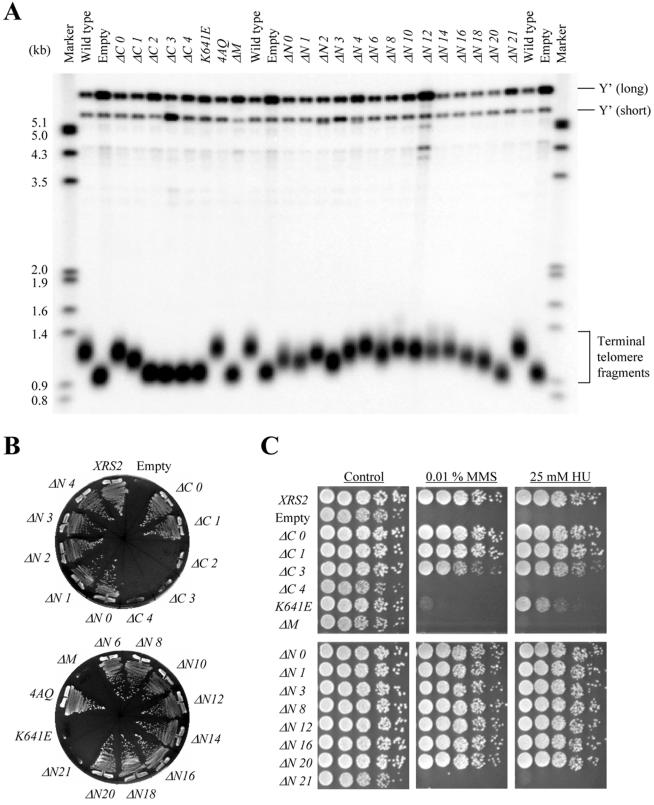
Characterization of the xrs2 mutants. (A) SYT358a (MATa xrs2Δ::URA3 ade2 ade3 trp1Δ leu2-3,112 ura3Δ lys2-801 can1) containing integrations of the indicated xrs2 alleles were examined for telomere length. (B) Complementation of the synthetic senescence phenotype exhibited in xrs2 mec1 sml1 hdf1 mutants. The indicated xrs2 alleles present on CEN plasmids (ARSH4 CEN6 LEU2 CYH2) were introduced into SYT301 harboring the plasmid pYT1501. The cells that are viable after loss of pYT1501 can grow on SC (+5-FOA -Leu) plates. (C) Sensitivity to MMS and HU in the xrs2 mutants with indicated alleles. Fivefold serial dilutions of the indicated xrs2 mutants were spotted onto YPAD plates containing 0.01% MMS or 25 mM HU. The xrs2 mutants examined in this assay are the SYT358a derivatives in which the truncated xrs2 genes were integrated into the genome.
A 32-Amino Acid Region of Xrs2p Is Necessary for Response to DNA Damage
The domain of Xrs2p necessary for response to DNA damage is separable from that for telomere elongation, as shown in the previous section. To identify the regions of Xrs2p necessary for response to DNA damage, the xrs2 truncation mutants were analyzed for sensitivity to the alkylating agent MMS and the ribonucleotide reductase inhibitor HU. HU reversibly disrupts the progression of replication forks from early-firing origins and also inhibits the firing of late origins (Santocanale and Diffley, 1998). Epitope tagging at either the N or C termini of Xrs2p did not affect function, as measured by complementation of xrs2 null strains by the tagged XRS2 constructs in the MMS and HU sensitivity assays (Figure 2C). As shown in Figure 2C, the xrs2-ΔC0 to -ΔC3 and xrs2-ΔN0 to -ΔN20 mutant strains showed wild-type resistance to both HU and MMS. N- and C-terminal mutants containing truncations larger than xrs2-ΔN21 and xrs2-ΔC4, respectively, displayed sensitivities that were similar to the xrs2 null strain (Figure 2C; unpublished data for xrs2-ΔC5 to -ΔC12). These results predict that only 32 amino acids located at positions 630-661 and included in the region required for telomere elongation are sufficient for the Xrs2p DNA damage response.
Regions in Xrs2p Necessary for Spore Formation, Spore Viability, and Meiotic Recombination
The series of xrs2 truncation mutations analyzed above were introduced into an SK1 xrs2/xrs2 null mutant strain to identify regions in Xrs2p necessary for meiotic events. The resulting strains were induced to enter meiosis and then examined for spore formation and viability. Meiotic functions were impaired in the xrs2-ΔC4 mutant strain, but not in the xrs2-ΔC0 to -ΔC3 mutant strains (Table 1). Therefore, a region necessary for both spore formation and meiotic viability is located between the N terminus and the 32-amino acid region. To identify this region, the N-terminal xrs2 truncation mutants were examined. Mutants xrs2-ΔN1, -ΔN2, -ΔN3, -ΔN20, and -ΔN21 displayed severe meiotic defects. These defects were not caused by a lack of expression of the mutant genes, because a normal level of protein expression was observed in meiotic extracts obtained from each mutant strain (our unpublished data). Furthermore, the meiotic defects in the xrs2-ΔN1 to -ΔN3 mutant strains seemed not to be caused by the loss of a specific region of Xrs2p, because some mutant strains with deletions longer than xrs2-ΔN1 to -ΔN3 were proficient in meiotic function. In support of this idea, we found that xrs2-ΔN12 to -ΔN16 mutant strains were partially defective in meiotic function, but that the xrs2- ΔN17 and -ΔN18 mutant strains, which have extensive deletions in this region, were functional.
Table 1.
Meiotic division and meiotic recombination in the xrs2 mutants
| Fold increase of recombinant fraction
|
||||
|---|---|---|---|---|
| Mutants | Spore formation | Spore viability | (24 h/0 h in SPM) | |
| (%) | (%) | Arg+ | His+ | |
| No tag | 86 | 97 | 600 | 340 |
| Empty | 36 | 0 | 1.8 | 1.6 |
| ΔC 0 | 88 | 99 | 1600 | 330 |
| ΔC 1 | 87 | 100 | 620 | 260 |
| ΔC 2 | 87 | 92 | 1300 | 270 |
| ΔC 3 | 85 | 86 | 840 | 190 |
| ΔC 4 | 47 | 0 | 2.2 | 1.1 |
| ΔN 0 | 73 | 76 | 290 | 51 |
| ΔN 1 | 18 | 3 | 30 | 22 |
| ΔN 2 | 31 | 0 | 24 | 5.8 |
| ΔN 3 | 60 | 21 | 140 | 25.1 |
| ΔN 4 | 87 | 86 | 930 | 130 |
| ΔN 8 | 84 | 88 | 1900 | 290 |
| ΔN 11 | 80 | 96 | 470 | 150 |
| ΔN 12 | 66 | 18 | 140 | 51 |
| ΔN 13 | 82 | 8 | 110 | 53 |
| ΔN 14 | 78 | 38 | 110 | 77 |
| ΔN 15 | 74 | 54 | 160 | 57 |
| ΔN 16 | 87 | 40 | 550 | 110 |
| ΔN 17 | 82 | 78 | 100 | 80 |
| ΔN 18 | 77 | 96 | 650 | 180 |
| ΔN 20 | 69 | 0 | 9.4 | 2.4 |
| ΔN 21 | 35 | 0 | 1.3 | 1.1 |
| K641E | 46 | 0 | 1.7 | 1.7 |
| 4AQ | 81 | 100 | 1300 | 240 |
| ΔM | 45 | 0 | 2.2 | 1.3 |
| E-empty | 46 | 0 | 2.9 | 1.3 |
| E 1 | 56 | 3 | 5.0 | 6.9 |
| E 4 | 67 | 39 | 76 | 19 |
| E 1-K641E | 53 | 0 | 3.6 | 0.8 |
| E 4-K641E | 31 | 0 | 4.6 | 2.3 |
Spore formation indicates percentage of cells containing more than one spore in all visible cells after 24 h. Spore viability was measured by dissection of asci by micromanipulation, and the proportion of spores germinating to give visible colonies was assessed. Eighteen asci were dissected for each strain.
Because defects in spore formation and viability are often associated with a deficiency in meiotic recombination, we examined recombination functions by using the return-to-growth protocol (Sherman and Roman, 1963). xrs2 mutant strains bearing the arg4-nsp/arg4-bgl and his4X::LEU2/his4B heteroalleles (Cao et al., 1990) were constructed, and their recombination functions were examined by measuring the frequency of Arg+ and His+ recombinants. In the wild-type strain, the fractions of recombinants increased with incubation time and reached a maximal level of ∼1000-fold at 6 h postmeiotic induction, relative to uninduced cultures (Figure 3). The recombination frequency in the xrs2 null strain was ∼10 times higher than in wild type, consistent with null mutations in the MRX complex conferring a hyperrecombination phenotype in vegetative growth (Figure 3A; Alani et al., 1990; Malone et al., 1990; Ivanov et al., 1992; Ajimura et al., 1993). As predicted by the spore formation and viability assays, meiotic recombination was poorly induced in xrs2- ΔN1, -ΔN2, -ΔN3, -ΔN20, and -ΔN21 strains (Figure 3, B and C). A less severe defect in meiotic recombination was observed in xrs2-ΔN12 to -ΔN16 mutant strains, which showed moderate defects in spore viability. In meiotic recombination, the MRX complex is involved in at least two steps of the processes. One is the recognition step of the hot-spots, where introduction of DSBs are followed by Spo11p, and the other is the resection step at the broken ends for formation of 3′ overhang (Usui et al., 1998; for reviews, see Haber 1998; D'Amours and Jackson 2002). The formation of meiotic DSBs was not detected in the xrs2-ΔN1 to -ΔN3, -ΔN13, and -ΔN20 similar to null strain (our unpublished data).
Figure 3.
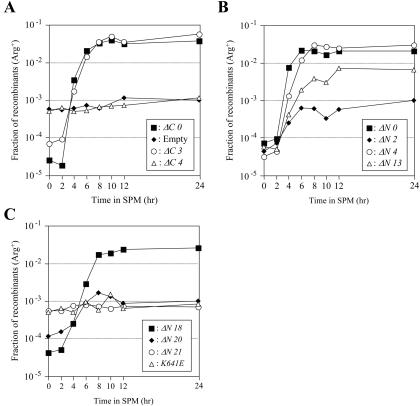
Commitment to meiotic recombination in the constructed xrs2 mutants; Return to growth experiment. Meiosis was induced in diploid strains containing the indicated xrs2 alleles. At various times after induction, cells were returned to vegetative growth media, and the fraction of Arg+ and His+ prototrophs was measured.
The above-mentioned analysis suggests that two regions, comprising amino acids 581-661 and 5-123, respectively, are important for meiotic function in Xrs2p, in particular for formation of meiotic DSBs. A third region, amino acids 330-518, seems to play an important but less essential role.
Complementation of Defects in the xrs2 Null Strain by the Selected Domains
We examined whether the minimum regions of Xrs2p identified above complement the defects of the null strain. The 32 amino acids located at positions 630-661 are suggested to be sufficient for the Xrs2p DNA damage response, based on the analysis of truncation mutants (Figure 2C). To examine whether this 32-amino acid region can complement the MMS and HU sensitivity exhibited by xrs2 null strains, we constructed an xrs2-M1 mutant that expresses amino acids 630-661 of Xrs2p fused to the Myc tag. The xrs2-M1 allele, which expressed Xrs2-M1 protein at wild-type levels, but did not complement the HU and MMS sensitivity of xrs2 null strains (Figure 4, A and B). Because the failure in complementation may be caused by absence of a nuclear localization signal in the 32-amino acid region, we examined the cellular localization of Xrs2-M1 protein, which was shown to remain in the cytoplasm (Figure 4C). We then made a fusion construct in which amino acids 630-661 of Xrs2p were fused to the Gal4p activation domain (Gal4AD), which contains a nuclear localization signal (xrs2-E1; Figure 1B). The resulting Xrs2-E1 peptide was able to localize to the nucleus (Figure 4C), and the xrs2-E1 construct, but not a control construct (xrs2-E-empty) containing only the nuclear localization signal, complemented the MMS and HU sensitivity of the xrs2 null, as shown in Figure 5A. Collectively, these experiments suggest that amino acids 630-661 of Xrs2p, when localized to the nucleus, can reproduce the Xrs2p DNA damage response.
Figure 4.
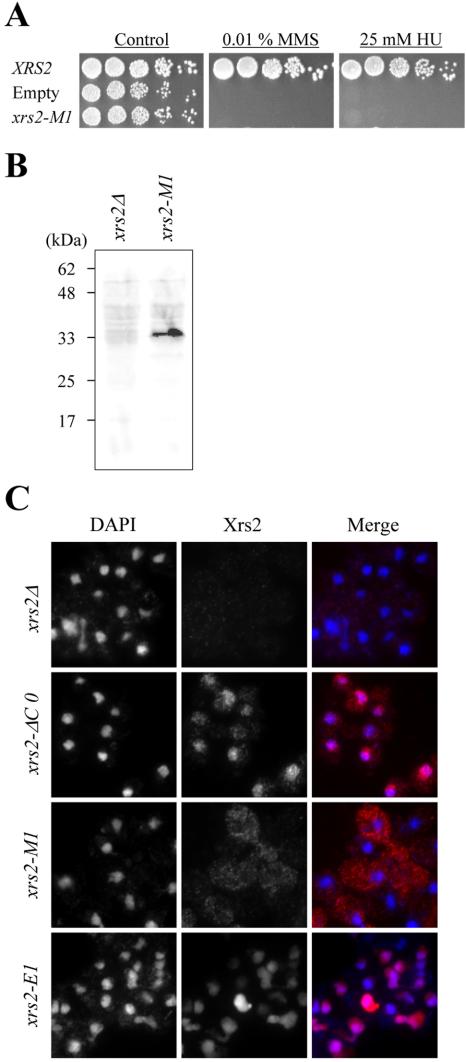
Cellular localization of the 32-amino acid peptide of Xrs2p. (A) Sensitivity to MMS and HU in the xrs2-M1 mutants with truncations. Fivefold serial dilutions of the indicated xrs2 mutants were spotted onto YPAD plates containing 0.01% MMS or 25 mM HU. The xrs2-M1 is SYT358a derivative in which the truncated xrs2 mutant gene was integrated into the genome. (B) Expression of the mutant Xrs2-M1 peptide was detected in a Western blot by immunostaining with α-Myc antibody. (C) Cellular localization of mutant Xrs2p. xrs2 mutant cells in vegetative growth were immunostained with α-Myc antibody to examine the localization of the mutant Xrs2 protein. The middle panels are images of the localization of Xrs2p, obtained by staining with Alexa-Fluor-488-conjugated α-mouse IgG (specific for α-Myc antibody). The left panels are images of the localization of chromosomes, obtained by DAPI staining. The right panels show the merged images of the left (blue) and middle (red) images.
Figure 5.
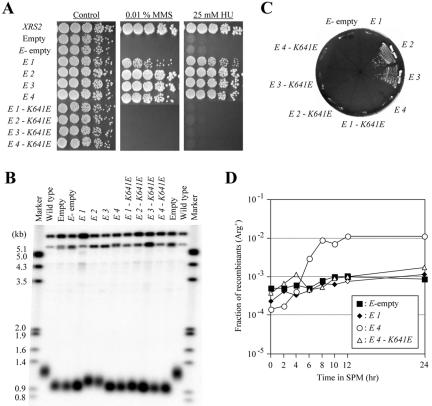
Characteristics of the xrs2-E1 to -E4 mutants and their derivatives. (A) Sensitivity to MMS and HU in the strain with xrs2-E1 to -E4 mutations and their derivatives. Fivefold serial dilutions of the indicated xrs2 alleles were spotted onto YPAD plates containing 0.01% MMS or 25 mM HU. The xrs2 mutants examined in this assay are SYT358a derivatives in which mutant xrs2 genes were integrated into the genome. (B) The mutants were examined for telomere length. Southern blot analysis was performed as described in Figure 2. (C) Complementation of the synthetic senescence phenotype exhibited in xrs2 mec1 hdf1 mutants with xrs2-E1 to -E4 mutations and their derivatives. The synthetic senescence assay was performed as described in Figure 2. (D) Meiosis was induced in diploid strains with the xrs2-E1, -E4, and -E4-K641E mutations. At various times after induction, cells were returned to vegetative growth media, and the fraction of Arg+ and His+ prototrophs was measured.
Next, to test whether a peptide spanning amino acids 630-765 is sufficient to complement the telomere elongation defects of xrs2 mutants, we made a fusion construct containing Gal4AD and amino acids 630-765 of Xrs2p (xrs2-E3). xrs2-E3 strains displayed a longer telomere length than null strains, and furthermore, the xrs2-E3 allele suppressed the senescence of the xrs2 mec1 hdf1 mutant (Figure 5, B and C).
Amino acids 581-661 of Xrs2p, the predicted minimum region for meiotic function, were then tested to determine whether this region alone is sufficient for meiotic function. The xrs2-E4 construct (Gal4AD with amino acids 581-661 of Xrs2p) was examined to determine whether it was able to complement the meiotic defects observed in the xrs2 null strain. As shown in Table 1 and Figure 5D, xrs2-E4 significantly restored the meiotic function of xrs2 null strains.
Hence, the above-mentioned results indicate that peptides comprising the amino acid regions 630-765, 630-661, and 581-661 of Xrs2p are functional for telomere elongation, response to DNA damage, and meiotic function, respectively, provided that they are localized to the nucleus.
The 32-Amino Acid Peptide of Xrs2p Binds to Mre11p
The 32 amino acids located at positions 630-661 of Xrs2p are required commonly for response to DNA damage, telomere elongation, and meiotic functions. A K641E point mutation in this region, which was identified in initial screening, causes defects in all three functions (Figures 2 and 3C), and the introduction of the corresponding point mutation into the xrs2-E3, -E1, and -E4 alleles resulted in mutants that failed to complement defects in telomere elongation, response to DNA damage, and meiotic events, respectively (Figure 5). This suggested that K641 is essential for each function of Xrs2p. Strains bearing full-length Xrs2p but lacking amino acid residues 630-661 displayed defects similar to the null strain (xrs2-ΔM; Figures 2 and 3C), and collectively these experiments suggest that amino acid residues 630-661 of Xrs2p are indispensable for Xrs2p function.
Xrs2p binds to Mre11p but not to Rad50p (Usui et al., 1998). Hence, we tested whether the Xrs2-E1 peptide, containing amino acids 630-661 of Xrs2p and a nuclear localization signal, can bind to Mre11p, by using coimmunoprecipitation (see Materials and Methods). Extracts obtained from log-phase cell lysates of the xrs2-E1 strain were treated with agarose-conjugated α-Myc antibody to precipitate the Myctagged Xrs2-E1 peptide. As shown in Figure 6A, Mre11 protein was detected in coimmunoprecipitates prepared from xrs2-E1 cell extracts, but the protein was not detected in immunoprecipitates from xrs2-E1-K641E extracts. Conversely, Xrs2-E1 peptide, but not Xrs2-E1-K641E, was detected in immunoprecipitates by using α-Mre11 rabbit antibody. An interaction between the 32-amino acid region of Xrs2p and Mre11p also was observed in a two-hybrid analysis (Figure 6B; see Materials and Methods), and introduction of the K641E mutation disrupted the two-hybrid interaction. Collectively, these data indicate that amino acids 630-661 of Xrs2p can bind to Mre11p, and consequently we have named this region MBX. The results also suggest that binding of Mre11p to the MBX site of Xrs2p is necessary for the MRX complex to function in telomere elongation, DNA damage response, and meiotic events.
Figure 6.
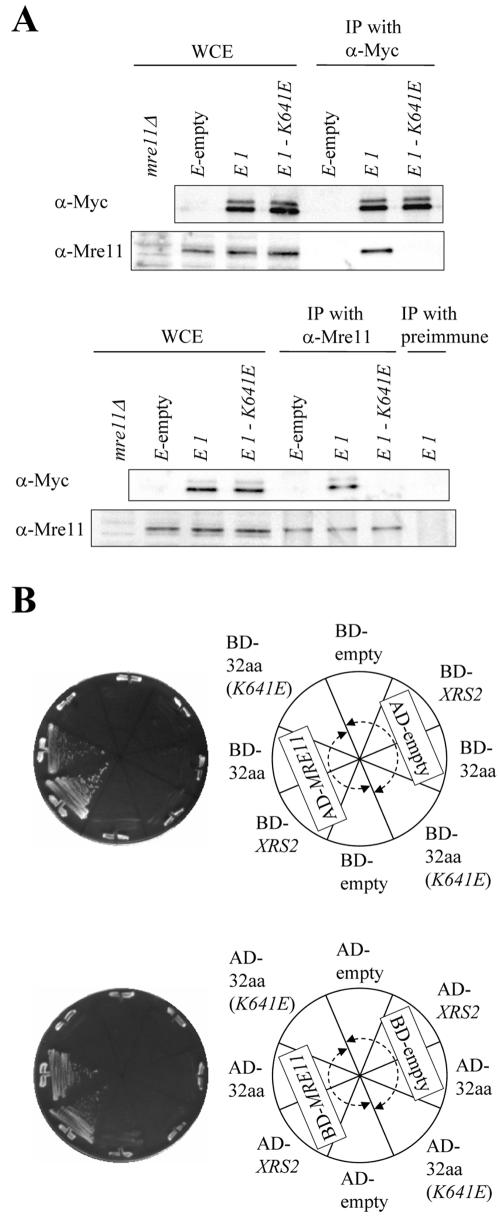
The 32-amino acid peptide of Xrs2p is responsible for binding to Mre11p. (A) Western blotting analyses of immunoprecipitates from cell extracts of mutant strains. The whole cell extracts (WCE) and the immunoprecipitates (IP) with α-Myc antibody were electrophoresed using 7 or 8.5% SDS-PAGE for detection of Mre11 or Xrs2-E1 proteins, respectively (top). The whole cell extracts, the α-Mre11 precipitates, and the preimmune serum precipitates were fractioned by SDS-PAGE for detection of Mre11 or Xrs2-E1 proteins (bottom). The blots were immunostained with α-Myc or α-Mre11 antibodies. (B) Two-hybrid interaction between the 32-amino acid region of Xrs2p and Mre11p. The yeast strain PJ69-4A containing the GAL4-DNA-binding-fusion plasmids and the GAL4-activation-domain-fusion plasmids was streaked onto SC (-His -Ura -Leu) plates. The physical interaction was examined after a 2-d incubation on SC plates at 30°C. BD-, fusion with the GAL4 DNA binding domain; AD, fusion with the GAL4 activation domain; empty, no insertion; XRS2: full-length XRS2 gene; MRE11, full-length MRE11 gene; 32aa, 32-amino acid region of Xrs2p; 32aa (K641E), 32-amino acid region of Xrs2p with the K641E mutation.
xrs2 DNA Repair Defects, but Not Defects in Telomere Elongation and Meiotic Recombination, Can Be Suppressed by Fusing a Nuclear Localization Signal to Mre11p
Mutation of the NBS1 gene causes mislocalization of hMre11p to the cytoplasm (Carney et al., 1998; Maser et al., 2001; Tauchi et al., 2001). Similarly, the nuclear localization of Mre11p is dependent on Xrs2p in yeast (Figure 7A), indicating that one of the roles of Xrs2p is to translocate Mre11p from the cytoplasm to the nucleus. To test this, we made a construct in which Gal4AD bearing a nuclear localization signal was fused to Mre11p (NLS-MRE11). xrs2 null strains including this construct displayed resistance to DNA damage at levels nearly similar to wild type (Figure 7B), indicating that Xrs2p is not required for the DNA damage response if Mre11p is present in the nucleus. In contrast, the DNA damage sensitivity exhibited by rad50 null strains was not suppressed by the NLS-MRE11, indicating that Rad50p is required for the DNA damage response, even if Mre11p is present in the nucleus. Finally, we tested whether the NLS-MRE11 was able to suppress the telomere elongation and meiosis defects observed in xrs2 null strains. As shown in Figure 7, C-F, the fusion construct did not suppress these defects, indicating that Xrs2p has other function(s) that are required for these processes. xrs2-E3 constructs lacking Gal4AD, with the MBX domain deleted, or containing mutations that disrupt Mre11p binding, conferred a null-like phenotype in the telomere elongation assay, even when NLS-Mre11p was coexpressed (our unpublished data). Collectively, these data suggest that binding of Xrs2p to Mre11p is necessary for telomere elongation after the two proteins are translocated to the nucleus.
Figure 7.
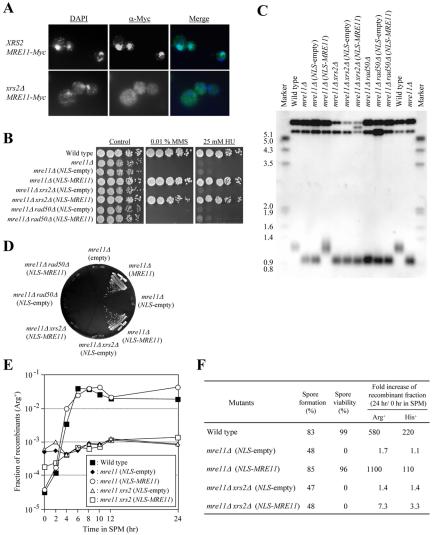
The MRE11 gene with a heterologous nuclear localization signal suppresses xrs2 DNA damage sensitivity but not meiotic or telomere elongation defects. (A) Mitotic cells of the xrs2 mutant were immunostained with α-Myc antibody to examine localization of the Mre11 protein tagged with 9Myc at the C terminus. The middle panels are images of the localization of Mre11p, obtained by staining with Alexa-Fluor-488-conjugated α-mouse IgG (specific for α-Myc antibody). Left, images of the localization of chromosomes, obtained by DAPI staining. Right, merged images of the left (blue) and middle (green) images. (B) Sensitivity to MMS and HU in the mre11, mre11 xrs2, and mre11 rad50 null strains with the NLS-MRE11 fusion constructs. The fusion bears a nuclear localization signal (NLS) of the Gal4 activation domain at the N-terminal of Mre11p. Fivefold serial dilutions of yeast cells were spotted onto YPAD plates containing 0.01% MMS or 25 mM HU. (C) Lengths of telomeres in the mre11, mre11 xrs2, and mre11 rad50 null strains with the NLS-MRE11 fusion constructs. Southern blot analysis was performed as described in Figure 2. (D) Complementation of the synthetic senescence phenotype exhibited by mec1 sml1 hdf1 mutants with the mre11, mre11 xrs2, and mre11 rad50 mutations by the NLS-MRE11 fusion constructs. The NLS-MRE11 plasmid was introduced into SYT301a harboring the plasmid pYT1501. The senescence assay was performed as described in Figure 2. (E and F) Meiosis was induced in the indicated diploid strains. At various times after induction, cells were returned to vegetative growth media, and the fraction of Arg+ and His+ prototrophs was measured. Fractions of spore formation, spore viability, and recombinants were measured as described in Table 1.
DISCUSSION
In this study, we have identified a 32-amino acid region of Xrs2p that is required for binding to Mre11p, and we refer to this as the MBX region. This binding is necessary for translocation of Mre11p from the cytoplasm to the nucleus. We showed that Xrs2p is not required in repair of DNA damage when Mre11p is localized in the nucleus. However, two other defined domains, which are upstream and downstream of the MBX region, respectively, are absolutely required for meiotic recombination and telomere elongation, respectively, in addition to the Mre11p binding domain (Figure 8).
Figure 8.
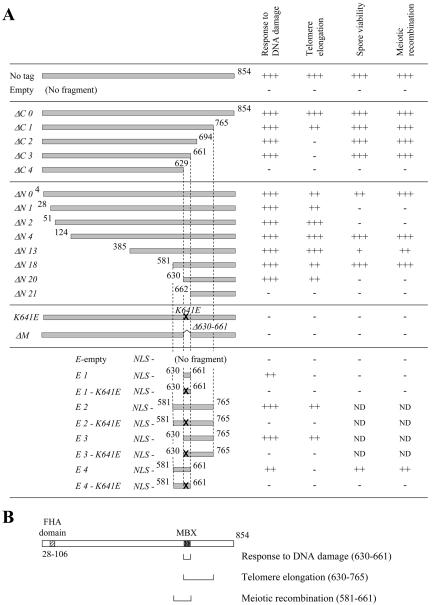
Summary of the domain analyses of Xrs2p. (A) Summary of the effects of the various xrs2 mutations on DNA damage response, telomere elongation, spore viability, and meiotic recombination. The symbols indicate the level of function: +++, comparable to wild-type; ++, slight defect; +, moderate defect; -, null. (B) Minimum regions of Xrs2p required for DNA damage response, telomere elongation, and meiotic recombination. The MBX region and the FHA-like domain of Xrs2p are indicated.
Roles of Xrs2p Domains Adjacent to the MBX Region
The MBX region and a downstream region (amino acids 662-765) of Xrs2p were identified as essential for telomere elongation function. Recently, Tel1p, an ATM homologue that phosphorylates Xrs2p in response to DNA double-strand breaks, was shown to bind to Xrs2p (D'Amours and Jackson, 2001; Usui et al., 2001; Nakada et al., 2003). This binding is eliminated in an Xrs2p mutant protein that lacks the C-terminal 162 amino acid residues (Nakada et al., 2003). This truncated version of Xrs2p is only two amino acids shorter than Xrs2-ΔC2, which is defective in telomere elongation (Figures 1 and 2), and therefore these data suggest that the telomere elongation defect observed in xrs2-ΔC2 to -ΔC12 mutants is caused by a defect in binding to Tel1p.
The MBX region and a upstream region (amino acids 581-629) are both required for meiotic recombination and may regulate the activity of Mre11p. In support of this idea, we showed that meiotic processing of DSBs by MRX requires a stable MRX complex (Usui et al., 1998). In addition, Xrs2p binds to the N-terminal half of Mre11p, in which its nuclease domains are located (our unpublished data). These data imply that not only the domain for meiotic recombination but also that for telomere elongation may induce conformational changes of Mre11p or act to regulate Mre11p activity. An analysis of whether the Xrs2p domains modify Mre11p properties such as nuclease activity and DNA binding activity, and screening for transacting factors to these regions, would provide valuable information regarding their function.
We showed that amino acids 581-661 and 630-765 of Xrs2p complement defects in meiotic functions and telomere elongation, respectively, when they are fused to a nuclear localization signal (Figure 5). However, other regions located in the N terminus of Xrs2p also have some effect on meiosis and telomere elongation (Figures 4A and 5 and Table 1). In particular, the xrs2-ΔN1, -ΔN2, and -ΔN13 mutants are severely defective in meiosis, whereas the xrs2- ΔN4, -ΔN8, -ΔN11, and -ΔN18 mutants display meiotic function, even though they contain deletions in XRS2 that are more extensive than in other truncation mutants that are strongly defective in meiosis (Figures 2 and 3 and Table 1). These differences are unlikely to be due to protein expression, because on the whole the expression of the mutant proteins is comparable with wild type in vegetative growth and during meiosis (our unpublished data). We favor the idea that certain truncations at the N-terminal region of Xrs2p might induce conformational changes that impair the meiotic functions of the Mre11 complex, whereas more extensive truncations in Xrs2p remove such impairment.
A Comparison of Xrs2p with Its Human Homologue Nbs1p
Xrs2p and Nbs1p share several features, in addition to the enhancement of the in vitro DNA binding and DNA nuclease activities of Mre11p (Paull and Gellert, 1999; Trujillo et al., 2003). For both proteins, their binding to Mre11p seems essential for MRX or Mre11p-Rad50p-Nbs1p complex function (Figures 2, 3, 5, and 6; Desai-Mehta et al., 2001, Tauchi et al., 2001). The Mre11p binding site in both Xrs2p and Nbs1p is located near the C terminus, and the amino acid sequence of the binding site is conserved between Xrs2p and Nbs1p and also in other species (Figure 2; Tauchi et al., 2001). The K641 residue of Xrs2p, mutation of which causes an almost null phenotype, is highly conserved across species, and this strongly suggests that Xrs2p and its homologues bind to a conserved structure in Mre11p.
Nbs1p is suggested to affect nuclear location of hMre11p because hMre11p is mislocalized to the cytoplasm in nbs1 mutants with the 657del5 allele, which is found in ∼90% of Nijmegen breakage syndrome patients (Carney et al., 1998; Tauchi et al., 2001). In this study, Mre11p was shown not to be localized to the nucleus in the xrs2 null strain (Figure 7A). Furthermore, Mre11p fused to a nuclear localization signal completely suppresses the MMS sensitivity of the xrs2 null strain. Hence, these results show that Xrs2p has a role in translocation of Mre11p from the cytoplasm to nucleus. Three nuclear localization signal motifs have been located near the hMre11 binding site of Nbs1p, one of which is located at the C-terminal side of the site (Tauchi et al., 2002). Xrs2p is also suggested to have such signals that located at both sides of the MBX, in regions amino acids 320-629 and 766-854 (Tsukamoto, Ogawa, and Ogawa; unpublished data). Alternatively, Xrs2p might have interaction sites with another protein, which possesses a nuclear localization signal, at both sides of the MBX.
An FHA motif, corresponding to a phosphopeptide recognition motif found in many proteins that act in nuclear signaling, is located at the N-terminal regions of both Nbs1p (amino acids 24-100) and Xrs2p (amino acids 28-106). The 70-kDa Nbs1 mutant protein, which the nbs1-657del5 mutant cells express, lacks the N-terminal region, including the FHA domain, but still possesses the hMre11 binding domain and a nuclear localization signal (Maser et al., 2001). The absence of this motif in Nbs1p causes sensitivity to DNA damage, radioresistant DNA synthesis, and less foci formation of the hMre11 complex in response to DNA damage (Petrini, 2000; Cerosaletti and Concannon 2003). On the other hand, analogous truncation mutations on the XRS2 gene do not cause deficiency in terms of sensitivity to DNA damage (Figure 2C). Expression of the 70-kDa Nbs1 mutant protein in nbs1-657del5 mutant cells is reduced (Maser et al., 2001), and the x-ray sensitivity of the nbs1 mutant is greatly compensated for by overexpression of Nbs1 mutant proteins lacking the N-terminal regions, although properties of radioresistant DNA synthesis and reduced foci formation of the hMre11 complex were not recovered (Desai-Mehta et al., 2001; Tauchi et al., 2001). Therefore, a reduced amount of the N-terminal truncated Nbs1p may cause different effects of similar mutations in human and yeast cells, in terms of response to DNA damage. To validate this possibility, an examination of radioresistant DNA synthesis and foci formation of the Mre11 complex in response to DNA damage in yeast is required.
Xrs2p Is Unique in Eukaryotes
The Mre11p and Rad50p components of MRX are conserved from bacteria to vertebrates. The bacterial SbcD proteins of E. coli, Bacillus subtilis, and Rhodobacter capsulatus, and gp47 of bacteteriophage T4 are homologues of Mre11p, and the SbcC proteins of these bacteria and gp46 of the bacteriophage are homologues of Rad50p (Sharples and Leach, 1995). Similarly to yeast Mre11p and Rad50p, E. coli SbcD and SbcC proteins form a complex and have 3′-to-5′ exonuclease and ssDNA endonuclease activities, which are important for DNA repair and recombination functions mediated by the complex (Connelly and Leach, 1996). However, homologues of Xrs2p are not found in bacteria. We hypothesize that bacteria do not require such homologues because they have no need to translocate Mre11 homologues into a nuclear compartment. Also, the functions of Xrs2p in telomere elongation and meiotic processes are unlikely to be required in organisms that contain circular genomes and do not have a meiotic recombination pathway analogous to that observed in eukaryotes.
In conclusion, our results suggest that Xrs2p has important roles in regulation of the extensive activities of the Mre11 complex, as well as for a role in the translocation of Mre11p into the nucleus. We anticipate that an understanding of the yeast MRX complex will aid in studies on the functions of the human MRN complex in telomere elongation and meiotic recombination, which are difficult to analyze in humans. Our results also should contribute to the understanding of genetic disorders associated with defects in this complex, such as Nijmegen breakage syndrome and ataxia-telangiectasia-like disease (Carney et al., 1998; Matsuura et al., 1998; Varon et al., 1998; Stewart et al., 1999).
Supplementary Material
Acknowledgments
We thank Dr. Eric Alani for helpful discussion and critical reading of manuscript, and Dr. M. Shinohara for communication of unpublished data. We also thank Drs. T. Usui and H. Oshiumi for providing α-Mre11 guinea pig serum and strains, respectively. This work was supported by a grant-in aid for Young Scientists to Y. T. (14780530) and Specially Promoted Research to T. O. (11101003) from the Ministry of Education, Science, Sports, and Culture of Japan, and by Core Research for Evolutional Science and Technology of Japan Science and Technology to H. O.
Article published online ahead of print in MBC in Press on November 17, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-09-0782).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Ajimura, M., Leem, S. H., and Ogawa, H. (1993). Identification of new genes required for meiotic recombination in Saccharomyces cerevisiae. Genetics 133, 51-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alani, E., Padmore, R., and Kleckner, N. (1990). Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell 61, 419-436. [DOI] [PubMed] [Google Scholar]
- Anderson, D. E., Trujillo, K. M., Sung, P., and Erickson, H. P. (2001). Structure of the Rad50·Mre11 DNA repair complex from Saccharomyces cerevisiae by electron microscopy. J. Biol. Chem. 276, 37027-37033. [DOI] [PubMed] [Google Scholar]
- Burke, D., Dawson, D., and Stearns, T. (2000). Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
- Cao, L., Alani, E., and Kleckner, N. (1990). A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61, 1089-1101. [DOI] [PubMed] [Google Scholar]
- Carney, J. P., Maser, R. S., Olivares, H., Davis, E. M., Le Beau, M., Yates, J. R., 3rd, Hays, L., Morgan, W. F., and Petrini, J. H. (1998). The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93, 477-486. [DOI] [PubMed] [Google Scholar]
- Cerosaletti, K. M., and Concannon, P. (2003). Nibrin forkhead-associated domain and breast cancer C-terminal domain are both required for nuclear focus formation and phosphorylation. J. Biol. Chem. 278, 21944-21951. [DOI] [PubMed] [Google Scholar]
- Chou, P. Y., and Fasman, G. D. (1978). Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 47, 45-148. [DOI] [PubMed] [Google Scholar]
- Connelly, J. C., and Leach, D. R. (1996). The sbcC and sbcD genes of Escherichia coli encode a nuclease involved in palindrome inviability and genetic recombination. Genes Cells 1, 285-291. [DOI] [PubMed] [Google Scholar]
- D'Amours, D., and Jackson, S. P. (2001). The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes Dev. 15, 2238-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amours, D., and Jackson, S. P. (2002). The Mre11 complex: at the crossroads of DNA repair and checkpoint signalling. Nat. Rev. Mol. Cell. Biol. 3, 317-327. [DOI] [PubMed] [Google Scholar]
- Desai-Mehta, A., Cerosaletti, K. M., and Concannon, P. (2001). Distinct functional domains of nibrin mediate Mre11 binding, focus formation, and nuclear localization. Mol. Cell. Biol. 21, 2184-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, M., Nagase, Y., Tsubouchi, H., Murakami-Murofushi, K., Shibata, T., and Ohta, K. (1998). Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 17, 6412-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R. D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527-534. [DOI] [PubMed] [Google Scholar]
- Grenon, M., Gilbert, C., and Lowndes, N. F. (2001). Checkpoint activation in response to double-strand breaks requires the Mre11/Rad50/Xrs2 complex. Nat. Cell Biol. 3, 844-847. [DOI] [PubMed] [Google Scholar]
- Haber, J. E. (1998). The many interfaces of Mre11. Cell 95, 583-586. [DOI] [PubMed] [Google Scholar]
- Ivanov, E. L., Korolev, V. G., and Fabre, F. (1992). XRS2, a DNA repair gene of Saccharomyces cerevisiae, is needed for meiotic recombination. Genetics 132, 651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., Halladay, J., and Craig, E. A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johzuka, K., and Ogawa, H. (1995). Interaction of Mre11 and Rad 50: two proteins required for DNA repair and meiosis-specific double-strand break formation in Saccharomyces cerevisiae. Genetics 139, 1521-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, R., and Ogawa, H. (1994). An essential gene, ESR1, is required for mitotic cell growth, DNA repair and meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 22, 3104-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop, M., Siegers, K., Pereira, G., Zachariae, W., Winsor, B., Nasmyth, K., and Schiebel, E. (1999). Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15, 963-972. [DOI] [PubMed] [Google Scholar]
- Malone, R. E., Ward, T., Lin, S., and Waring, J. (1990). The RAD50 gene, a member of the double strand break repair epistasis group, is not required for spontaneous mitotic recombination in yeast. Curr. Genet. 18, 111-116. [DOI] [PubMed] [Google Scholar]
- Maser, R. S., Zinkel, R., and Petrini, J. H. (2001). An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele. Nat. Genet. 27, 417-421. [DOI] [PubMed] [Google Scholar]
- Matsuura, S., et al. (1998). Positional cloning of the gene for Nijmegen breakage syndrome. Nat. Genet. 19, 179-181. [DOI] [PubMed] [Google Scholar]
- Nakada, D., Matsumoto, K., and Sugimoto, K. (2003). ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 17, 1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull, T. T., and Gellert, M. (1999). Nbs1 potentiates ATP-driven DNA unwinding and endonuclease cleavage by the Mre11/Rad50 complex. Genes Dev. 13, 1276-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini, J. H. (2000). The Mre11 complex and ATM: collaborating to navigate S phase. Curr. Opin. Cell Biol. 12, 293-296. [DOI] [PubMed] [Google Scholar]
- Raymond, W. E., and Kleckner, N. (1993). RAD50 protein of S. cerevisiae exhibits ATP-dependent DNA binding. Nucleic Acids Res. 21, 3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, K. B., and Petes, T. D. (2000). The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics 155, 475-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santocanale, C., and Diffley, J. F. (1998). A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature 395, 615-618. [DOI] [PubMed] [Google Scholar]
- Schneider, B. L., Seufert, W., Steiner, B., Yang, Q. H., and Futcher, A. B. (1995). Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11, 1265-1274. [DOI] [PubMed] [Google Scholar]
- Schulz, V. P., and Zakian, V. A. (1994). The Saccharomyces PIF1 DNA helicase inhibits telomere elongation and de novo telomere formation. Cell 76, 145-155. [DOI] [PubMed] [Google Scholar]
- Sharples, G. J., and Leach, D. R. (1995). Structural and functional similarities between the SbcCD proteins of Escherichia coli and the RAD50 and MRE11 (RAD32) recombination and repair proteins of yeast. Mol. Microbiol. 17, 1215-1217. [DOI] [PubMed] [Google Scholar]
- Sherman, F., and Roman, H. (1963). Evidence for two types of allelic recombination in yeast. Genetics 48, 255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and Boeke, J. D. (1991). In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 194, 302-318. [DOI] [PubMed] [Google Scholar]
- Stewart, G. S., Maser, R. S., Stankovic, T., Bressan, D. A., Kaplan, M. I., Jaspers, N. G., Raams, A., Byrd, P. J., Petrini, J. H., and Taylor, A. M. (1999). The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99, 577-587. [DOI] [PubMed] [Google Scholar]
- Tauchi, H., Kobayashi, J., Morishima, K., Matsuura, S., Nakamura, A., Shiraishi, T., Ito, E., Masnada, D., Delia, D., and Komatsu, K. (2001). The forkhead-associated domain of NBS1 is essential for nuclear foci formation after irradiation but not essential for hRAD50-hMRE11-NBS1 complex DNA repair activity. J. Biol. Chem. 276, 12-15. [DOI] [PubMed] [Google Scholar]
- Tauchi, H., Matsuura, S., Kobayashi, J., Sakamoto, S., and Komatsu, K. (2002). Nijmegen breakage syndrome gene, NBS1, and molecular links to factors for genome stability. Oncogene 21, 8967-8980. [DOI] [PubMed] [Google Scholar]
- Terasawa, M., Shinohara, A., Hotta, Y., Ogawa, H., and Ogawa, T. (1995). Localization of RecA-like recombination proteins on chromosomes of the lily at various meiotic stages. Genes Dev. 9, 925-934. [DOI] [PubMed] [Google Scholar]
- Trujillo, K. M., Roh, D. H., Chen, L., Van Komen, S., Tomkinson, A., and Sung, P. (2003). Yeast xrs2 binds DNA and helps target rad50 and mre11 to DNA ends. J. Biol. Chem. 278, 48957-48964. [DOI] [PubMed] [Google Scholar]
- Tsukamoto, Y., Taggart, A. K., and Zakian, V. A. (2001). The role of the Mre11-Rad50-Xrs2 complex in telomerase-mediated lengthening of Saccharomyces cerevisiae telomeres. Curr. Biol. 11, 1328-1335. [DOI] [PubMed] [Google Scholar]
- Usui, T., Ogawa, H., and Petrini, J. H. (2001). A DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7, 1255-1266. [DOI] [PubMed] [Google Scholar]
- Usui, T., Ohta, T., Oshiumi, H., Tomizawa, J., Ogawa, H., and Ogawa, T. (1998). Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell 95, 705-716. [DOI] [PubMed] [Google Scholar]
- Varon, R., et al. (1998). Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 93, 467-476. [DOI] [PubMed] [Google Scholar]
- Zhu, X. D., Kuster, B., Mann, M., Petrini, J. H., and de Lange, T. (2000). Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat. Genet. 25, 347-352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


