Abstract
BimC kinesins are required for mitotic spindle assembly in a variety of organisms. These proteins are localized to centrosomes, spindle microtubules, and the spindle midzone. We have previously shown that the Caenorhabditis elegans Aurora B kinase AIR-2 is required for the localization of the ZEN-4 kinesin protein to midzone microtubules. To determine whether the association of BimC kinesins with spindle microtubules is also dependent on AIR-2, we examined the expression pattern of BMK-1, a C. elegans BimC kinesin, in wild-type and AIR-2–deficient embryos. BMK-1 is highly expressed in the hermaphrodite gonad and is localized to meiotic spindle microtubules in the newly fertilized embryo. In mitotic embryos, BMK-1 is associated with spindle microtubules from prophase through anaphase and is concentrated at the spindle midzone during anaphase and telophase. In the absence of AIR-2, BMK-1 localization to meiotic and mitotic spindles is greatly reduced. This is not a consequence of loss of ZEN-4 localization because BMK-1 is appropriately localized in ZEN-4–deficient embryos. Furthermore, AIR-2 and BMK-1 directly interact with one another and the C-terminal tail domain of BMK-1 is specifically phosphorylated by AIR-2 in vitro. Together with our previous data, these results suggest that at least one function of the Aurora B kinases is to recruit spindle-associated motor proteins to their sites of action.
INTRODUCTION
Molecular motor proteins are intimately involved in the microtubule dynamics that are required for accurate chromosome segregation and progress through the cell cycle (Walczak and Mitchison, 1996). Kinesins have been shown to be required for many aspects of mitosis, including centrosome separation, mitotic spindle assembly, chromosome alignment and segregation, and spindle elongation, as well as for cytokinesis (Walczak and Mitchison, 1996; Adams et al., 1998; Powers et al., 1998; Raich et al., 1998). BimC kinesins make up a highly conserved and well-studied kinesin subfamily that includes Aspergillus BimC, Saccharomyces cerevisiae CIN8, Schizosaccharamyces pombe CUT7, Drosophila KLP61F, Xenopus Eg5 (XlEg5), and Human Eg5 (HsEg5) (Enos and Morris, 1990; Hagan and Yanagida, 1990; Le Guellec et al., 1991; Hoyt et al., 1992; Heck et al., 1993; Blangy et al., 1995). These proteins share very similar N-terminal motor (head) domains and also contain a conserved region in the C-terminal tail domain, the BimC box. Genetic, immunodepletion, and antibody microinjection experiments have revealed very similar loss-of-function phenotypes for each BimC family member. All of these proteins are necessary for bipolar mitotic spindle formation and seem to be required for spindle pole body/centrosome separation or the maintenance of pole separation (Enos and Morris, 1990; Hagan and Yanagida, 1990; Hoyt et al., 1992; Sawin et al., 1992; Heck et al., 1993; Blangy et al., 1995). Immunofluorescence experiments have shown that the subcellular localization of the various family members is also highly conserved. BimC kinesins are associated with centrosomes or spindle pole bodies as well as mitotic spindle microtubules and are highly concentrated at the spindle midzone during anaphase (Hagan and Yanagida, 1992; Hoyt et al., 1992; Sawin et al., 1992; Houliston et al., 1994; Blangy et al., 1995; Sharp et al., 1999). Additional studies have shown that phosphorylation of XlEg5, HsEg5, and KLP61F at a consensus CDC2 phosphorylation site in the C-terminal BimC box is required for the localization of each of these proteins to the mitotic spindle (Blangy et al., 1995; Sawin and Mitchison, 1995; Sharp et al., 1999).
BimC kinesins at the spindle midzone are thought to cross-link and bundle antiparallel polar microtubules and assist in the stabilization of the bipolar spindle (Sharp et al., 1999). Other kinesin-related molecules also have been shown to specifically associate with midzone microtubules at anaphase. Mammalian MKLP-1 is localized to the spindle midzone and is thought to be involved in spindle elongation at anaphase B (Nislow et al., 1992). The MKLP-1 homologues Drosophila Pavarotti and C. elegans ZEN-4 are also located at the spindle midzone, but they are not essential for spindle elongation (Adams et al., 1998; Powers et al., 1998; Raich etal., 1998). Instead, loss-of-function experiments have revealed that these proteins are required for cytokinesis (Adams et al., 1998; Powers et al., 1998; Raich et al., 1998). Each of these motor proteins is necessary for the proper organization of the spindle midzone and for the stabilization of the cleavage furrow in their respective organisms (Adams et al., 1998; Powers et al., 1998; Raich et al., 1998). C. elegans ZEN-4 is also required for polar body extrusion during the meiotic divisions of the oocyte nucleus (Severson et al., 2000). Given their strong association with the spindle midzone, it has been suggested that BimC kinesins also may participate in cytokinesis (Giet et al., 1999). In support of this notion, expression of a dominant-negative form of the sea urchin BimC kinesin Boursin results in severe cytokinesis defects in P. lividus embryos (Touitou et al., 2001). Late mitotic functions for other BimC family members may be masked due to the earlier requirement for these proteins in mitotic spindle assembly (Whitehead and Rattner, 1998).
Previous work in our laboratory has shown that the C. elegans Aurora B kinase AIR-2 is required for the localization of ZEN-4 to the spindle midzone (Schumacher et al., 1998b). The Aurora kinases comprise two highly conserved subfamilies, now commonly referred to as the Aurora A and Aurora B kinases. Aurora A kinases are associated with mitotic centrosomes and are required for mitotic spindle formation and chromosome segregation (Bischoff and Plowman, 1999). Aurora B kinases display a “chromosomal passenger” localization pattern during meiosis and mitosis; they associate with centromeres at metaphase and translocate to the central spindle at anaphase (Schumacher et al., 1998b; Adams et al., 2001; Giet and Glover, 2001; Petersen et al., 2001; Terada, 2001). Aurora B kinases are required for chromosome segregation and polar body extrusion during meiosis and for chromosome separation and cytokinesis during mitosis (Schumacher et al., 1998b; Adams et al., 2001; Giet and Glover, 2001; Petersen et al., 2001; Terada, 2001; Rogers et al., 2002). Genetic and immunofluoresence analyses of polar body extrusion and cytokinesis in C. elegans have shown that the Aurora B kinase AIR-2 acts upstream of the MKLP-1–related kinesin ZEN-4 in a linear pathway (Severson et al., 2000). Supporting biochemical studies suggest that these proteins physically associate with one another (Severson et al., 2000).
In addition to ZEN-4, BimC kinesins are also likely candidates for proteins that may act in concert with Aurora kinases. Genetic analysis of the budding yeast Aurora kinase IPL1 has shown that mutations in IPL1 are synthetic lethal with mutations in the yeast BimC family member CIN8 (Geiser et al., 1997; Kim et al., 1999). In addition, biochemical studies have shown that the Xenopus Aurora A kinase Eg2 interacts with and can phosphorylate the XlEg5 BimC kinesin in vitro (Giet et al., 1999). However, the functional consequence of this phosphorylation is unknown. The precedence for interactions between Aurora kinases and BimC kinesins and our previous results regarding the dependence of ZEN-4 localization on AIR-2 expression led us to examine whether there is a functional relationship between the Aurora B kinase AIR-2 and the BimC kinesin BMK-1 in C. elegans.
MATERIALS AND METHODS
C. elegans Strains
C. elegans strains were derived from the wild-type Bristol strain N2 and cultured by standard techniques (Brenner, 1974). bmk-1(ok391) was obtained from the Oklahoma Medical Research Foundation C. elegans Gene Knockout Consortium (http://elegans.bcgsc.bc.ca/knockout.shtml). air-2(or207ts) was obtained from B. Bowerman (University of Oregon, Eugene, OR). zen-4(or153ts) was obtained from the C. elegans Genetics Center (University of Minnesota, St. Paul, MN)
Antibodies
The DNA sequence of the bmk-1 cDNA yk134g8 (obtained from Yuji Kohara (National Institute of Genetics, Mishima, Japan) was determined by automated DNA sequencing (M.D. Anderson DNA Sequence and Analysis Facility, University of Texas, Houston, TX). The DNA sequence of yk134g8 exactly matched the F23B12.8 sequence and gene structure predicted by the C. elegans Sequencing Consortium (http://www.wormbase.org). A peptide corresponding to the C-terminal 12 amino acids of the predicted BMK-1 protein (Figure 1A, box) was coupled to KLH by standard methods and injected into rabbits. Rabbits were boosted for a period of 6 mo, and final bleeds were collected. For affinity purification, the BMK-1 antigenic peptide was coupled to bovine serum albumin and immobilized using an AminoLink kit as described by the manufacturer (Pierce Chemical, Rockford, IL). Using this immobilized BMK-1 peptide, BMK-1–specific antibodies were purified as described previously (Schumacher et al., 1998b).
Figure 1.
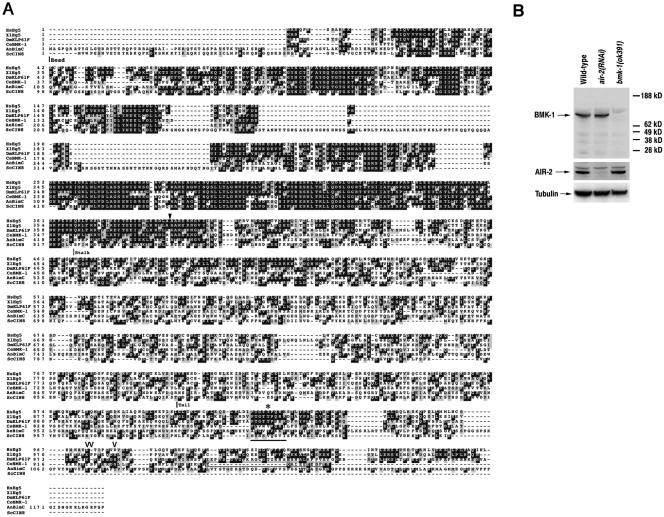
C. elegans BMK-1 is a member of the BimC family of kinesins. (A) An alignment of the predicted protein sequences for C. elegans BMK-1 (CeBMK-1), human Eg5 (HsEg5), Xenopus Eg5 (XlEg5), Drosophila KLP61F (DmKLP61F), A. nidulans BimC (AnBimC), and S. cerevisiae Cin8p (ScCin8) is shown. Identical residues are shaded in black, similar residues are shaded in gray. The head, stalk, and tail domains are indicated. The conserved BimC box is underlined, and an asterisk indicates the threonine residue that is phosphorylated in XlEg5, HsEg5, and DmKLP61F (Blangy et al., 1995; Sawin and Mitchison, 1995; Sharp et al., 1999). An arrowhead indicates the amino acid where the ok391 mutant BMK-1 protein is predicted to be out-of-frame. The C-terminal peptide used as an antigen for generating the BMK-1 antibody is boxed. The residues phosphorylated by AIR-2 are indicated by open arrowheads. C. elegans BMK-1 sequence data are available from GenBank/EMBL/DDBJ under accession no. AF295093.1. (B) Western blot of wild-type, air-2(RNAi), and bmk-1(ok391) embryo lysates probed with an affinity-purified C-terminal specific BMK-1 antibody. The blot was stripped and reprobed with AIR-2 and α-tubulin antibodies as controls.
AIR-2 and ZEN-4 antibodies have been described previously (Raich et al., 1998; Schumacher et al., 1998b). ZEN-4 antibodies were a gift from J. Hardin (University of Wisconsin, Madison, WI). For glutathione S-transferase (GST) and ICP-1 antibodies, recombinant GST and GST-ICP-1 fusion proteins (Bishop and Schumacher, 2002) were injected into rabbits and the immune sera affinity purified by Western blotting against the recombinant antigen as described previously (Sambrook et al., 1989). An α-tubulin monoclonal antibody (T9026) was purchased from Sigma-Aldrich (St. Louis, MO).
RNA-mediated Interference
The bacteria feeding method of RNA interference (RNAi) (Timmons and Fire, 1998) was used to reduce AIR-1, AIR-2, BMK-1, ICP-1, and ZEN-4 protein expression in C. elegans. cDNA clones for air-1 (Schumacher et al., 1998a), air-2 (Schumacher et al., 1998b), bmk-1 (yk134g8), and icp-1 (yk329a11) (yk clones were obtained from Y. Kohara, National Institute of Genetics, Mishima, Japan) were subcloned into the RNAi feeding vector L440 and transformed into HT115 bacteria (Timmons and Fire, 1998). L440/zen-4 was purchased as a component of the C. elegans Chromosome IV RNAi library (J. Ahringer and the Medical Research Council Geneservice, Cambridge, United Kingdom). Transformants were grown in LB + 50 μg/μl ampicillin overnight at 37°C and then spread on NG plates containing 50 μg/μl ampicillin and 0.3 mM isopropyl β-d-thiogalactoside (IPTG). The following day, the plates were seeded with L4 hermaphrodites. Treated animals and their progeny were assayed for RNAi effects after incubation at 15, 20, or 25°C for 20–24 h. HT115 bacteria transformed with the L440 vector without an insert was used as an RNAi control.
Yeast Two-Hybrid Analysis
The full-length air-2 (Schumacher et al., 1998b) and bmk-1 (yk134g8) cDNAs were polymerase chain reaction (PCR) amplified using primers with restriction sites for cloning each cDNA in frame in the appropriate two-hybrid vector (pas2–1 and pACTII; BD Biosciences Clonetech, Palo Alto, CA). The air-2 cDNA was fused in frame with the DNA-binding domain of GAL-4 (pasAIR-2), and the full-length bmk-1 cDNA was fused in frame with the GAL-4 activation domain (pACT-BMK-1). The DNA sequence of each plasmid was confirmed by DNA sequencing. Plasmids were cotransformed into the yeast strain PJ694A (James et al., 1996). Cotransformants were grown on -leu-trp plates and screened for reporter gene expression (histidine) by streaking on -leu-trp-his plates. Control pACT clones included: pACT-AIR-1, pACT-AIR-2, and the pACT vector alone. Control pas clones included: pasBMK-1, pasAIR-1, and the pAS vector alone.
Immunocytochemistry
Immunocytochemistry experiments were performed with gravid wild-type, bmk-1(ok391), bmk-1(RNAi), air-1(RNAi), air-2(RNAi), zen-4(RNAi), icp-1(RNAi), bmk-1(ok391);zen-4(RNAi), and bmk-1(ok391);air-2(RNAi) hermaphrodites reared at 20°C and wild-type, bmk-1(RNAi), air-2(or207ts), zen-4(or153ts), air-2(or207ts);bmk-1(RNAi), and zen-4(or153ts);bmk-1(RNAi) animals reared at 15 and 25°C. Adult animals were placed in 15 μl of phosphate-buffered saline (PBS) on a poly-lysine–coated glass slide. A coverslip was placed over the intact animals and gently tapped to release embryos. The slides were placed on an aluminum sheet on dry ice for 25 min. Coverslips were removed and slides were immediately placed in -20°C methanol for 5 min. Slides were then transferred to 10% formaldehyde for 30 min and washed as described previously (Seydoux and Dunn, 1997). The α-tubulin antibody (Sigma-Aldrich) was used at a dilution of 1:2000 and affinity-purified BMK-1, AIR-2, and ZEN-4 antibodies were used at a dilution of 1:200. Fluorescent secondary antibodies conjugated to fluorescein isothiocyanate and Texas Red (Vector Laboratories, Burlingame, CA) were used at dilutions of 1:500.
Image Analysis
Immunofluorescent images were obtained using MetaMorph software to obtain z-series sections (0.2 μm/slice) by using a 60×/1.45 objective attached to a Nikon 2000U inverted microscope equipped with a motorized x-, y-, and z-stage and a Photometric Quantix 1401E camera. Images were deconvolved for 60 iterations by using Autodeblur software (AutoQuant Imaging, Watervliet, NY). For each individual embryo, the exposure time and any postmicroscopy processing (Autodeblur and Adobe Photoshop) for BMK-1, AIR-2, or ZEN-4 antibody staining was identical to that of α-tubulin staining.
Recombinant Protein Production
HIS-BMK-1 and HIS-AIR-2 were created by PCR amplification of full-length bmk-1 and air-2 cDNAs with primers incorporating restriction sites for cloning the cDNAs in-frame with the HIS tag of the pRSETC vector (Invitrogen, Carlsbad, CA). Construction of GST-AIR-2, GST-AIR-2ts, GST-AIR-2dead, GST-ICP-1, and GST-AIR-1 has been described previously (Bishop and Schumacher, 2002; Rogers et al., 2002). GST-AIR-2KD (kinase domain) and full-length GST-BMK-1 were created by PCR amplification of AIR-2 and BMK-1 cDNA with primers incorporating restriction sites for cloning in frame with GST moiety of the pGEX6P vector (Amersham Biosciences, Piscataway, NJ). GST-AIR-2KD was created by PCR amplification of the full-length AIR-2 cDNA with the following primers: JMS 498: 5′-GCTAGAATTCGAAAACCTGTATTTTCAGGGCGGAAAATTTACTATTAACG-3′ and JMS 499: 5′-GCTACTCGAGTCAGTGATTCCGAAG-3′, and corresponds to amino acids 23–306. GST-BMK-1C was created by PCR amplification of the full-length BMK-1 cDNA with the following primers: JDB 37: 5′-CGTAGGATCCGTCATCGACAACATGACTGCC-3′ and JDB 38: 5′-CGTAGCGGCCGCTTAGTTTTCGAAATC-3′ and corresponds to amino acids 595–958. Point mutations in GST-BMK-1 and GST-BMK-1C were introduced by subjecting the construct to PCR-based site-directed mutagenesis. All constructs were verified by DNA sequencing.
Recombinant HIS and GST fusion proteins were expressed in Escherichia coli strain BL21 (DE3) pLys S by overnight induction with 1 mM IPTG. Fifteen milliliters of bacteria was pelleted, resuspended in 700 μl of lysis buffer (PBS + 40 mM HEPES + 0.2% Triton-X-100, pH 7.6, and briefly sonicated on ice. The sonicates were clarified by centrifugation, the supernatants added to 10 μl of nickel or glutathione beads (Invitrogen and Amersham Biosciences), and incubated on ice for 2 h. Beads were thoroughly washed in PBS + 40 mM HEPES, pH 7.6, and the bound proteins were eluted by addition of 30 μl of elution buffer (as recommended by the manufacturers). Protein concentrations were determined by Bradford assay (Bio-Rad, Hercules, CA).
Western Analysis and Immunoprecipitation
C. elegans embryos were harvested from gravid adult wild-type, air-2(RNAi), or bmk-1(ok391) hermaphrodites as described previously (Schumacher et al., 1998b). Washed embryos were resuspended in lysis buffer (1× PBS, 20 mM HEPES, pH 7.6, 1% NP-40, 50 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM dithiothreitol [DTT], 1 mM EDTA) supplemented with complete protease inhibitors (Roche Diagnostics, Indianapolis, IN) and briefly sonicated on ice. Lysates were centrifuged at 13,000 rpm for 10 min at 4°C to pellet insoluble material, and the protein concentration of the supernatants was determined by Bradford assay (Bio-Rad). Lysates were quick-frozen in a dry ice/ethanol bath and stored at –80°C.
For Western analysis, 100 μg of embryo extract was separated by SDS-PAGE and transferred to nitrocellulose. Blots were probed with the affinity-purified BMK-1 antibody. After overnight incubation in primary antibody, the blots were washed extensively in 137 mM NaCl, 20 mM Tris, pH 8.0, 0.2% Tween 20, 0.2% Triton X-100 (TBST+T) and probed with horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ). Proteins were visualized using a chemiluminescence reagent kit (Amersham Biosciences). Membranes were stripped and reprobed with AIR-2 (Schumacher et al., 1998b) and α-tubulin (Sigma, St. Louis, MO) antibodies as protein loading controls.
For in vitro binding assays, 500 nM GST-BMK-1 + 500 nM GST-AIR-2, GST-AIR-2dead, GST-AIR-2KD, or GST-AIR-1 were mixed and rocked on ice for 2 to 4 h. Five microliters of 70% protein A-Sepharose beads (Amersham Biosciences) in PBS and 1 μl of affinity-purified α-BMK-1 or 1 μl of lysis buffer was added to each binding reaction and rocked for another 1 to 3 h. The beads were washed three times in 500 μl TBST+T. After the last wash, the beads were resuspended in 20 μl of 1× SDS gel loading buffer, heated to 95°C for 3 min, and the proteins separated by SDS-PAGE. Protein gels were electroblotted to nitrocellulose and subjected to Western blot analysis with GST- and BMK-1–specific antibodies.
For coimmunoprecipitation from C. elegans protein extracts, 1 μl of affinity-purified α-BMK-1, 1 μl of affinity-purified α-AIR-2 (Schumacher et al., 1998b), or 1 μl of lysis buffer was added to 400–500 μg of clarified embryo extract. The aliquots were rocked on ice for 2 to 4 h, and 5 μl of 70% protein A-Sepharose beads (Amersham Biosciences) in PBS was added to each aliquot and rocked for another 1 to 3 h. The beads were washed and the proteins separated by SDS-PAGE as described above. Protein gels were electroblotted to nitrocellulose and subjected to Western blot analysis with BMK-1– and AIR-2–specific antibodies. Bacterially produced HIS-BMK-1 and HIS-AIR-2 were used as size controls.
Kinase Assays
Kinase reactions were performed at room temperature for 10 min in kinase buffer (20 mM HEPES, pH 7.6, 5 mM EGTA, 1 mM DTT, 25 mM β-glycerophosphate, 7.5 mM magnesium chloride, 10 nM ATP, 30 μCi [3 Ci/μmol] of [32P]γ-ATP) with ∼100 ng of GST-AIR-2 or GST-AIR-2ts, with and without 100 ng of GST-ICP-1, and 100 ng of GST-BMK-1, GST-BMK-1C, or GST-BMK-1 and GST-BMK-1C point mutants in a total volume of 15 μl. Kinase reactions were terminated by the addition of 7 μl of 3× SDS-PAGE loading buffer, separated by SDS-PAGE, and blotted to nitrocellulose. Radioactive phosphate incorporation was visualized by phosphorimaging. Protein loading was determined by staining membranes with Ponceau-S (Sigma-Aldrich) or by Western blot analysis with GST-, BMK-1–, ICP-1–, or AIR-2-specific antibodies.
RESULTS
BMK-1, a C. elegans BimC Kinesin, Is Associated with Meiotic and Mitotic Spindles
The synthetic lethal interaction between the budding yeast IPL1 and CIN8 genes suggested that members of the Aurora family of kinases might play a regulatory role in the function of BimC kinesins in other organisms (Geiser et al., 1997; Kim et al., 1999). To determine whether this was the case in C. elegans, we searched the C. elegans sequence database for a BimC family member. BLAST searches revealed a single high-scoring homolog encoded by a gene on chromosome V, F23B12.8 (Figure 1A). An expressed sequence tag (EST) clone (yk134g8) corresponding to the predicted gene was obtained from Yuji Kohara (National Institute of Genetics, Mishima, Japan), and DNA sequencing revealed that it is a full-length cDNA that exactly matched the sequence and exon structure predicted by the C. elegans Sequencing Consortium. We have named this protein BMK-1 (BimC Kinesin-1). Although BMK-1 is more similar to BimC kinesins than other kinesins, it diverges significantly from other family members in the signature BimC box (PTGXTPXK/RR) found in the C terminus of many of these proteins (Figure 1A, underline) (Heck, 1999).
To determine the expression pattern of the BMK-1 protein, a rabbit polyclonal antibody was raised against a C-terminal specific peptide (Figure 1A, box). The antiserum was affinity-purified and used to probe a Western blot of protein lysates from wild-type, air-2(RNAi), and bmk-1(ok391) mutant hermaphrodites (Figure 1B). The antibody recognized a single band of the appropriate size (108 kDa) that was not found in bmk-1(ok391) lysates. As described below, BMK-1 localization is disrupted in the absence of the AIR-2 kinase. This disruption is not due to a change in BMK-1 protein levels because there is no change in BMK-1 protein expression in air-2(RNAi) animals (Figure 1B).
The bmk-1(ok391) mutant was isolated by the Oklahoma Medical Research Foundation C. elegans Gene Knockout Consortium and deletes 616 nucleotides from exons 4 and 5, resulting in a protein product that is predicted to be out of frame at amino acid 376 (Figure 1A, arrowhead). The mutant protein truncates at a stop codon three amino acids down-stream of amino acid 376 and should therefore not be recognized by the BMK-1 C-terminal antibody (Figure 1B). Surprisingly, bmk-1(ok391) homozygous mutant and bmk-1(RNAi) animals are viable and have grossly normally meiotic and mitotic spindles that are not characteristic of the monopolar spindles found when BimC expression or function is compromised in other species (Figure 2 and Supplementary Figures 1–3).
Figure 2.
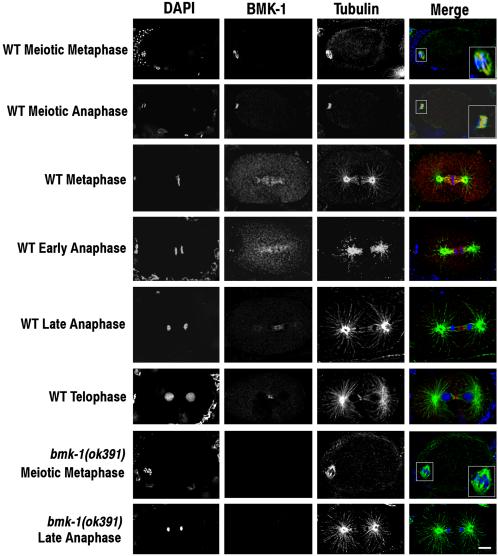
BMK-1 is localized to meiotic and mitotic spindle microtubules. Embryos dissected from wild-type and bmk-1(ok391) adult hermaphrodites were fixed and stained with DAPI, and BMK-1 and α-tubulin antibodies. In the merged image, DAPI is blue, BMK-1 is red, and tubulin is green. One-cell embryos at various stages of the meiotic and mitotic divisions are shown. BMK-1 is localized to spindle microtubules at meiotic and mitotic metaphase and to the meiotic and mitotic central spindle at anaphase. Close-ups of meiotic spindles are shown in the boxed inserts. BMK-1 immunostaining is absent in bmk-1(ok391) embryos. Bar, 5 μm.
The affinity-purified BMK-1 antibody was used to localize BMK-1 in the C. elegans germline and embryos. These experiments revealed that BMK-1 is expressed at high levels in the hermaphrodite germline and is ubiquitously associated with nuclei at every stage of oogenesis (our unpublished data). After fertilization, BMK-1 is localized to the meiotic spindle and with the meiotic midbody (Figure 2). Like AIR-2, it also persists on extruded polar bodies (our unpublished data; Schumacher et al., 1998b). In embryos, BMK-1 localization is dynamic with respect to the cell cycle. In prophase cells, BMK-1 protein begins to be associated with mitotic spindle microtubules (our unpublished data). At metaphase, BMK-1 is strongly concentrated on the inner face of the centrosomes and mitotic spindle kinetochore microtubules, but it is not associated with astral microtubules (Figure 2). In early anaphase, the protein remains associated with kinetochore microtubules and begins to populate the central spindle (Figure 2). By late anaphase, BMK-1 is no longer localized to the poleward side of the separating chromosomes, but it is readily detectable at the central spindle (Figure 2). At telophase, all BMK-1 staining is localized to the spindle midbody (Figure 2). As expected, immunostaining is absent in bmk-1(ok391) and bmk-1(RNAi) embryos at all stages of the cell cycle and development (Figure 2 and Supplementary Figures 2 and 3; our unpublished data).
BMK-1 Localization Is Dependent on AIR-2 and ICP-1
Our previous studies have shown that the C. elegans AIR-2 kinase is required for the organization of the spindle midzone and for the localization of the ZEN-4 kinesin to midzone microtubules (Schumacher et al., 1998b; Severson et al., 2000). To test whether aspects of BMK-1 localization also might be AIR-2 dependent, we examined the localization of BMK-1 in the progeny of air-2(RNAi) and air-2(or207ts) hermaphrodites. Immunostaining of air-2(RNAi) and air-2(or207ts) embryos with the BMK-1 antibody revealed that BMK-1 staining of meiotic and mitotic spindle microtubules was dramatically reduced (Figure 3 and Supplementary Table 1; our unpublished data). The localization of the AIR-2 kinase to meiotic and mitotic chromosomes and spindle microtubules has been shown to be dependent on the presence of the ICP-1 and BIR-1 proteins, the respective C. elegans homologues of vertebrate INCENP and Survivin (Kaitna et al., 2000; Speliotes et al., 2000). In addition, ICP-1 has been shown to increase the kinase activity of AIR-2 in vitro (Bishop and Schumacher, 2002; Romano et al., 2003). Because the localization of BMK-1 is dependent on AIR-2, and AIR-2 localization and full kinase activity is likely to require ICP-1, we asked whether BMK-1 is localized in the progeny of icp-1(RNAi)-treated hermaphrodites. The meiotic and mitotic spindles of icp-1(RNAi) and air-2(RNAi) embryos were indistinguishable, both lacking BMK-1 immunostaining (Figure 3 and Supplementary Table 1).
Figure 3.
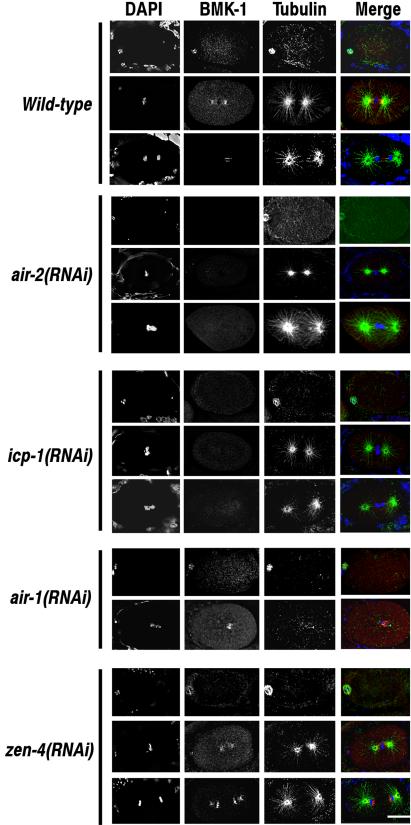
The association of BMK-1 with meiotic and mitotic spindles is greatly reduced in air-2(RNAi) and icp-1(RNAi) embryos. Embryos dissected from wild-type, air-2(RNAi), icp-1(RNAi), zen-4(RNAi), and air-1(RNAi) hermaphrodites were fixed and stained with DAPI, and BMK-1 and α-tubulin antibodies. Meiotic metaphase and metaphase and anaphase of the first mitotic division are shown for each experimental condition with the exception of air-1(RNAi) where only meiotic metaphase and mitotic anaphase are shown. BMK-1 is associated with meiotic and mitotic spindle microtubules in wild-type, zen-4(RNAi), and air-1(RNAi) embryos. BMK-1 immunostaining at all stages of the cell cycle is greatly reduced in air-2(RNAi) and icp-1(RNAi) embryos. Bar, 10 μm.
In wild-type embryos, the ZEN-4 kinesin is localized to the midzone microtubules of meiotic and mitotic spindles during anaphase and telophase (Supplementary Figure 1; Powers et al., 1998; Raich et al., 1998). This localization is dependent on AIR-2 and ICP-1 expression, but it is independent of BMK-1 (Supplementary Figure 1; Schumacher et al., 1998; Severson et al., 2000). To determine whether the lack of BMK-1 localization to meiotic and mitotic spindles in air-2(RNAi), air-2(or207ts), and icp-1(RNAi) embryos might be due to loss of ZEN-4 localization, we examined the localization of BMK-1 in the progeny of zen-4(RNAi)-treated hermaphrodites. BMK-1 remained localized to meiotic spindle microtubules in ZEN-4-depleted embryos and to mitotic spindle microtubules in zen-4(RNAi) embryos at metaphase. However, the association of BMK-1 to mitotic spindle microtubules at anaphase was disrupted. Instead of spreading across the central spindle, BMK-1 immunostaining remained tightly associated with the interior faces of the separating chromosomes (Figure 3). This was not surprising because assembly of the central spindle microtubule bundles is disrupted in the absence of ZEN-4 (Powers et al., 1998). Based on these results, we conclude that the change in BMK-1 behavior noted in air-2(RNAi), air-2(or207ts), and icp-1(RNAi) meiotic and mitotic spindles is not due to loss of ZEN-4 localization.
The Xenopus Aurora A kinase Eg2 has previously been shown to phosphorylate the Xenopus BimC kinesin Eg5 (Giet et al., 1999). Given this interaction, we asked whether BMK-1 localization is also dependent on expression of the C. elegans Aurora A kinase AIR-1. Depletion of AIR-1 by RNAi results in mitotic spindle and chromosome segregation defects that ultimately culminate in lethal embryonic aneuploidy (Schumacher et al., 1998a). However, unlike air-2(RNAi) embryos, the meiotic divisions of the oocyte nucleus are not affected (Schumacher et al., 1998b; Hannak et al., 2001). Immunostaining of air-1(RNAi) embryos with the BMK-1 antibody revealed that BMK-1 was still localized to meiotic and mitotic spindle microtubules in the absence of the AIR-1 kinase (Supplementary Table 1 and Figure 3).
AIR-2 Localization Is Not Dependent on BMK-1 Expression
Because BMK-1 localization is dependent on AIR-2 expression, we asked whether the normal AIR-2 localization pattern during meiosis and mitosis is disrupted in the absence of BMK-1. Staining of wild-type and bmk-1(ok391) embryos revealed that AIR-2 is properly localized to metaphase chromosomes and central spindle microtubules during the meiotic and mitotic divisions in the presence and absence of functional BMK-1 (Figure 4). Furthermore, AIR-2 is properly localized during meiosis and remains associated with metaphase chromosomes in zen-4(RNAi) embryos (Figure 4). However, at anaphase, AIR-2 is no longer localized to the central spindle microtubules in zen-4(RNAi) embryos (Figure 4), but remains associated with the inner faces of the separating sister chromatids, in a pattern identical to BMK-1 localization in zen-4(RNAi) anaphase embryos (Figure 3). As reported previously, AIR-2 is no longer localized to meiotic or mitotic chromosomes or microtubules in the absence of ICP-1 expression (Figure 4) (Kaitna et al., 2000). Together, these data reveal that AIR-2 localization is not dependent on BMK-1.
Figure 4.
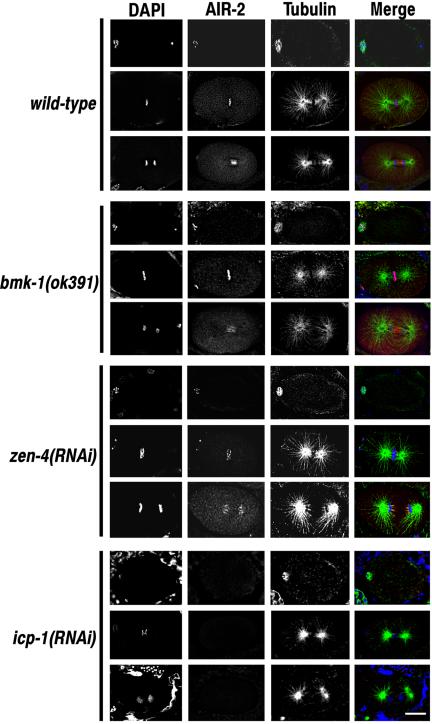
The association of AIR-2 with chromosomes and central spindle microtubules is not dependent on BMK-1 or ZEN-4 expression. Wild-type, bmk-1(ok391), zen-4(RNAi), and icp-1(RNAi) embryos were fixed and stained with DAPI, and AIR-2 and α-tubulin antibodies. Meiotic metaphase and metaphase and anaphase of the first mitotic division are shown for each experimental condition. AIR-2 is localized to meiotic and mitotic chromosomes at metaphase in wild-type, bmk-1(ok391), and zen-4(RNAi) embryos and with midzone microtubules at anaphase in wild-type, and bmk-1(ok391) embryos. AIR-2 immunostaining of metaphase chromosomes and anaphase spindle microtubules is greatly reduced in icp-1(RNAi) embryos. Bar, 10 μm.
Loss of BMK-1 Expression Does Not Enhance or Suppress Meiotic or Mitotic Defects in AIR-2- or ZEN-4–deficient Embryos
To determine whether loss of BMK-1 activity might affect the phenotype of air-2(RNAi) or zen-4(RNAi) embryos, air-2(RNAi) or zen-4(RNAi) was performed with wild-type and bmk-1(ok391) animals. Embryos dissected from treated animals were fixed and stained with 4,6-diamidino-2-phenylindole (DAPI), α-tubulin antibodies, and antibodies specific for AIR-2 or ZEN-4. Loss of BMK-1 expression concomitant with depletion of AIR-2 or ZEN-4 activity had no discernible affect on the expected phenotypes of air-2(RNAi) or zen-4(RNAi) embryos (Figures 5 and 6). To confirm these results, wild-type, air-2(or207ts), and zen-4(or153ts) animals were treated with a control RNAi or bmk-1(RNAi) at permissive (15°C) and restrictive temperatures (25°C). At 15°C, air-2(or207ts) and zen-4(or153ts) embryos are viable, and the majority have no discernible defects in spindle formation, chromosome segregation, or cytokinesis (Severson et al., 2000). At 25°C, air-2(or207ts) and zen-4(or153ts) embryos are inviable and display defects similar to air-2(RNAi) and zen-4(RNAi) embryos (Severson et al., 2000). Embryos dissected from wild-type, air-2(or207ts), and zen-4(or153ts) animals reared and treated with bmk-1(RNAi) at 15 and 25°C were fixed and stained with DAPI, and BMK-1 and α-tubulin antibodies. Like the bmk-1(ok391) deletion strain, bmk-1(RNAi)–treated animals gave rise to viable progeny with no obvious defects in the structure or function of meiotic or mitotic spindles. bmk-1(RNAi) also had no discernible affect on the expected phenotypes of air-2(or207ts) or zen-4(or153ts) embryos at 15 or 25°C (Supplementary Figures 2 and 3; our unpublished data). Together, these results are entirely consistent with our observations that AIR-2 is required for the independent localization of BMK-1 and ZEN-4. Importantly, monopolar spindles were not observed, suggesting that redundancy with AIR-2 or ZEN-4 is not masking a role for BMK-1 in bipolar spindle formation in bmk-1(ok391) or bmk-1(RNAi) embryos.
Figure 5.
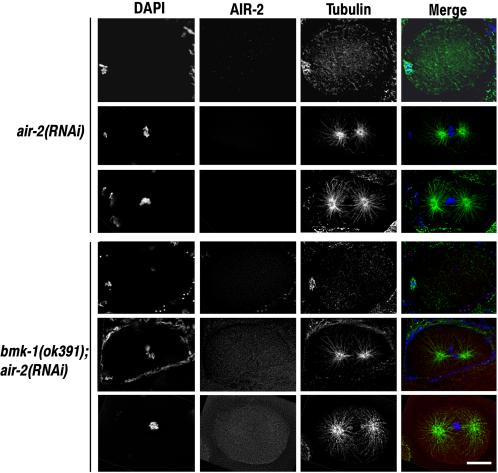
The air-2(RNAi) phenotype is not affected by loss of BMK-1 expression. air-2(RNAi) and bmk-1(ok391);air-2(RNAi) embryos were fixed and stained with DAPI and antibodies to AIR-2 and α-tubulin. One-cell embryos at meiotic metaphase and mitotic metaphase and anaphase are shown for each experimental condition. There were no discernible differences between air-2(RNAi) and bmk-1(ok391);air-2(RNAi) embryos at any stage of the cell cycle or development. Bar, 10 μm.
Figure 6.
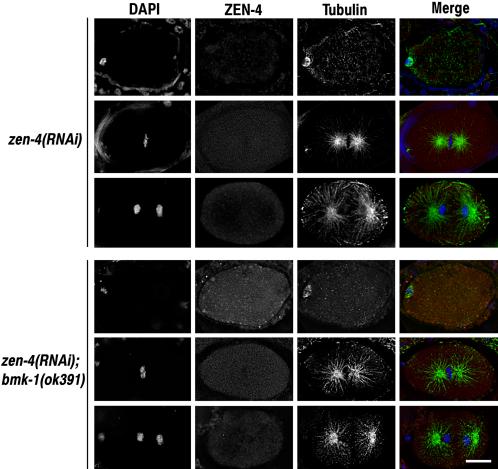
The zen-4(RNAi) phenotype is not affected by loss of BMK-1 expression. zen-4(RNAi) and bmk-1(ok391);zen-4(RNAi) embryos were fixed and stained with DAPI and antibodies to ZEN-4 and α-tubulin. One-cell embryos at meiotic metaphase and mitotic metaphase and anaphase are shown for each experimental condition. There were no discernible differences between zen-4(RNAi) and bmk-1(ok391);zen-4(RNAi) embryos at any stage of the cell cycle or development. Bar, 10 μm.
The BMK-1 Kinesin and the AIR-2 Kinase Physically Interact
Given the striking dependence of BMK-1 localization on the expression of the AIR-2 kinase, we tested whether the requirement for AIR-2 was due to a physical interaction between the two proteins. Initially, we examined whether AIR-2 and BMK-1 could interact in the yeast two-hybrid assay (Fields and Sternglanz, 1994). Coexpression of full-length AIR-2 fused with GAL-4 DNA binding domain (pasAIR-2) and full-length BMK-1 fused with the GAL-4 activation domain (pACT-BMK-1) in a yeast two-hybrid reporter strain (PJ69-4A) (James et al., 1996) resulted in reporter gene expression that allowed for growth in the absence of histidine (our unpublished data). To assess the specificity of the AIR-2:BMK-1 interaction, pasAIR-2 was cotransformed with the GAL-4 activation domain alone (pACT), pACT-AIR-1 (Schumacher et al., 1998a), and pACT-AIR-2, whereas pACT-BMK-1 was coexpressed with the GAL-4 DNA-binding domain alone (pas), pasAIR-1, and pasBMK-1. None of these combinations resulted in growth on media lacking histidine (our unpublished data). Thus, the interaction between AIR-2 and BMK-1 in this assay seemed to be specific.
The interaction between AIR-2 and BMK-1 detected in the yeast two-hybrid assay prompted us to test whether AIR-2 and BMK-1 directly interact in vitro. Recombinant GST-BMK-1, full-length GST-AIR-2, kinase dead GST-AIR-2(GST-AIR-2dead), a GST fusion with the kinase domain of AIR-2 (GST-AIR-2KD), and full-length GST-AIR-1 were expressed and purified from E. coli (Figure 7A). Equal molar amounts of GST-BMK-1 and the GST-AIR-2 or GST-AIR-1 fusion proteins were mixed together and subjected to immunoprecipitation with BMK-1 antibodies. GST-BMK-1 and GST-AIR-2 were found in the BMK-1 immune complexes (Figure 7B). However, GST-AIR-2dead, GST-AIR-2KD, and GST-AIR-1 did not bind to GST-BMK-1 (Figure 7B). These results confirm that BMK-1 and AIR-2 directly interact and that the full-length, kinase active AIR-2 protein is the preferred binding partner. Importantly, although GST-AIR-1 expressed and purified from E. coli is an active kinase (our unpublished data; Bishop and Schumacher, 2002), GST-AIR-1 does not bind to GST-BMK-1 in this assay, suggesting that unlike Xenopus Aurora A (Giet et al., 1999), the C. elegans Aurora A kinase does not directly interact with a BimC kinesin. Similar results were obtained when the AIR-2 antibody was used to immunoprecipitate the GST-AIR-2/GST-BMK-1 proteins (our unpublished data).
Figure 7.
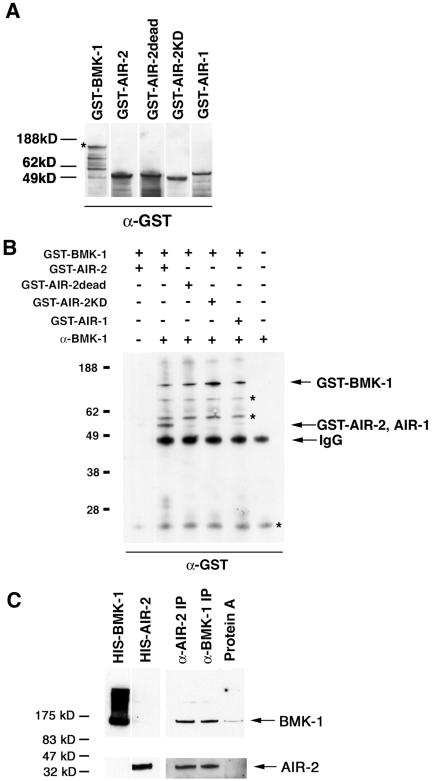
AIR-2 physically interacts with BMK-1. (A) GST-BMK-1, GST-AIR-2, GST-AIR-2dead, GST-AIR-2KD, and GST-AIR-1 were expressed and purified from E. coli. Equal molar amounts were separated by SDS-PAGE, transferred to nitrocellulose, and probed with a GST specific antibody. An asterisk denotes full-length GST-BMK-1. (B) GST-BMK-1 was mixed with equal molar amounts of GST-AIR-2, GST-AIR-2dead, GST-AIR-2KD, or GST-AIR-1 and immunoprecipitated with the BMK-1 antibody. A Western blot of the immune complexes was probed with a GST-specific antibody. GST-BMK-1 and GST-AIR-2 were detected in BMK-1 immunoprecipitates, whereas GST-AIR-2dead, GST-AIR-2KD, and GST-AIR-1 were not. GST-BMK-1 and GST-AIR-2 did not bind to protein A-Sepharose in the absence of the BMK-1 antibody (lane 1). Asterisks denote background bands. (C) A Western blot of BMK-1 and AIR-2 immunoprecipitates from wild-type C. elegans embryo extracts was cut in half and probed with BMK-1 (top) and AIR-2 (bottom) antibodies. Both proteins are detected in immunoprecipitates with either the AIR-2 or BMK-1 antibody, but they were not retained at significant levels with protein A-Sepharose in the absence of antibody. His-tagged BMK-1 and AIR-2 were included as size standards.
To determine whether AIR-2 and BMK-1 physically interact in vivo, we asked whether the endogenous proteins could be coimmunoprecipitated directly from C. elegans embryo extracts. Embryos were isolated from gravid adult hermaphrodites and lysed in coimmunoprecipitation buffer as described in Materials and Methods. When either AIR-2 or BMK-1 was immunoprecipitated with an AIR-2– or BMK-1–specific antibody, the other protein was also detectable in the immune complexes (Figure 7C). Neither protein was retained in significant amounts on protein A-Sepharose beads in the absence of antibody (Figure 7C). This result confirmed our two-hybrid and in vitro data and suggests that AIR-2 and BMK-1 associate with one another in vivo.
BMK-1 Is Phosphorylated by the AIR-2 Kinase In Vitro
Because the localization of BMK-1 is dependent on AIR-2 expression and the two proteins are likely to interact with one another in vivo, we tested whether BMK-1 can be directly phosphorylated by the AIR-2 kinase in vitro. We have previously shown that a GST-AIR-2 fusion protein is an active kinase when purified from E. coli (Bishop and Schumacher, 2002). GST-AIR-2 could phosphorylate full-length GST-BMK-1 (Figure 8A). The kinase activity was specific to GST-AIR-2 and not caused by a contaminating bacterial activity as preparations of a kinase-inactive GST-AIR-2 mutant corresponding to the in vivo mutation or207ts (GST-AIR-2ts) (Bishop and Schumacher, 2002) did not phosphorylate GST-BMK-1 (Figure 8A). We have previously shown that AIR-2 kinase activity is enhanced by the addition of ICP-1 (Bishop and Schumacher, 2002). When GST-ICP-1 was added to the AIR-2/BMK-1 kinase assay, GST-ICP-1 was phosphorylated, and GST-AIR-2 autophosphorylation and GST-BMK-1 phosphorylation were increased (Figure 8A).
Figure 8.
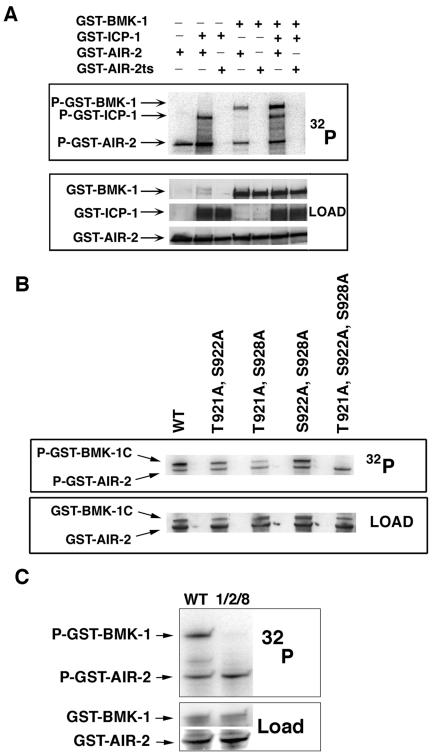
AIR-2 specifically phosphorylates three residues in the BMK-1 tail domain. (A) GST-AIR-2 in the presence or absence of GST-ICP-1 was mixed with GST-BMK-1 in kinase assay conditions in the presence of [γ-32P]ATP. 32P incorporation was measured by phosphorimaging and protein loading was determined by antibody staining. GST-BMK-1 was phosphorylated by GST-AIR-2 in the presence and absence of GST-ICP-1, but it was increased with GST-ICP-1 addition. GST-BMK-1 and GST-ICP-1 were not phosphorylated by GST-AIR-2ts. P-GST-AIR-2 indicates AIR-2 autophosphorylation. (B) GST-BMK-1C and GST-BMK-1C point mutants were incubated with GST-AIR-2 in kinase assay conditions in the presence of [γ-32P]ATP. 32P incorporation was measured by phosphorimaging and protein loading was determined by staining with Ponceau S. Mutation of T921, S922, and S928 to alanine eliminated GST-AIR-2–mediated phosphorylation of GST-BMK-1C. P-GST-AIR-2 indicates AIR-2 autophosphorylation. (C) Full-length wild-type GST-BMK-1 (WT) and GST-BMK-1 mutated to alanine at T921, S922, and S928 (1, 2, and 8) were incubated with GST-AIR-2 in kinase assay conditions in the presence of [γ-32P]ATP. 32P incorporation was measured by phosphorimaging and protein loading determined by Ponceau S (BMK-1) and antibody staining (AIR-2). Mutation of T921, S922, and S928 to alanine eliminated GST-AIR-2–mediated phosphorylation of full-length GST-BMK-1. P-GST-AIR-2 indicates AIR-2 autophosphorylation.
To determine the site(s) of AIR-2 phosphorylation within the BMK-1 protein, GST fusion proteins corresponding to the N-terminal, middle, and C-terminal thirds of BMK-1 were expressed and purified from E. coli and subjected to GST-AIR-2 kinase assays (our unpublished data). These experiments revealed that AIR-2 phosphorylates BMK-1 at a site or sites within the C terminus of the protein. The study of numerous Aurora substrates has revealed a loose consensus site for Aurora kinases (Bishop and Schumacher, 2002; Cheeseman et al., 2002). The C terminus of BMK-1 harbors adjacent threonine and serine residues at amino acids 921 and 922 that fit this consensus. To determine whether these sites are phosphorylated by the AIR-2 kinase, site-directed mutagenesis was used to mutate these sites to alanine within the context of the GST-BMK-1 C-terminal fusion protein (GST-BMK-1C). Compared with the wild-type GST-BMK-1C protein, phosphorylation of GST-BMK-1C(T921A, S922A) was reduced (Figure 8B). However, significant phosphorylation of this protein was still detectable, indicating the presence of additional phosphorylation sites. Mutation of serine and threonine residues in addition to T921A and S922A revealed that serine 928 also contributed to BMK-1 phosphorylation (Figure 8B; our unpublished data). Mutation of all three residues to alanine eliminated phosphorylation of GST-BMK-1C (Figure 8B) and of full-length GST-BMK-1 (Figure 8C). Together, these results revealed that BMK-1 directly interacts with the AIR-2 kinase and can be phosphorylated by AIR-2 at three specific serine/threonine residues in vitro.
DISCUSSION
Here, we report that the localization of the C. elegans BimC kinesin BMK-1 to meiotic and mitotic spindle microtubules is dependent on the appropriate expression and localization of the wild-type C. elegans Aurora B kinase AIR-2. Furthermore, AIR-2 and BMK-1 physically interact and AIR-2 can phosphorylate BMK-1 at three specific residues within the BMK-1 C-terminal tail domain.
The C. elegans BimC kinesin BMK-1 Is Associated with Meiotic and Mitotic Spindles
We have identified a new member of the BimC family of kinesins in C. elegans. Like BimC kinesins in other organisms, BMK-1 is also localized to meiotic and mitotic spindle microtubules and the spindle midzone (Hagan and Yanagida, 1992; Hoyt et al., 1992; Sawin et al., 1992; Houliston et al., 1994; Blangy et al., 1995; Sharp et al., 1999). The localization of some BimC kinesin proteins has been shown to be dependent on phosphorylation of a conserved threonine in the signature BimC box found in the C terminus of most BimC kinesins (PTGXTPXK/RR) (Blangy et al., 1995; Sawin and Mitchison, 1995; Sharp et al., 1999). The cyclin-dependent kinase complex CDK1/cyclin B can phosphorylate this site. Interestingly, C. elegans BMK-1 has a divergent BimC box (PSVNVPQRI) that does not have the conserved threonine that is phosphorylated in other systems (Figure 1A, asterisk). This suggests that the regulation of BMK-1 may differ from other BimC kinesins and distinct phosphorylation sites and/or protein kinases may be involved in the localization of BMK-1 in C. elegans. As we have shown here, a strong candidate for such a kinase is the C. elegans Aurora B kinase AIR-2.
The AIR-2 Kinase Is Required for the Subcellular Localization of BMK-1
The localization of BMK-1 to meiotic and mitotic spindles is dependent on expression of the AIR-2 kinase. Furthermore, AIR-2 must be appropriately localized for BMK-1 to associate with these subcellular structures because BMK-1 localization is also dependent on the expression of ICP-1, a protein that is required for the “chromosomal passenger protein” behavior of AIR-2 (Kaitna et al., 2000; Adams et al., 2001). The localization of BMK-1 is also likely to be dependent on AIR-2 kinase activity, because BMK-1 is not appropriately localized in air-2(or207ts) embryos. The air-2(or207ts) mutant protein harbors a missense mutation at a conserved residue that affects AIR-2 kinase activity in vitro and prohibits the movement of AIR-2 from chromosomes to the spindle midzone in vivo (Severson et al., 2000; Bishop and Schumacher, 2002). These results along with the in vitro kinase assay data presented here suggest that direct phosphorylation of BMK-1 by the AIR-2 kinase may be essential for BMK-1 localization. Unfortunately, attempts to directly prove this hypothesis by expressing wild-type and phosphosite mutant GFP-BMK-1 in transgenic C. elegans have been unsuccessful, perhaps due to BMK-1 overexpression-mediated lethality. We are currently performing in vitro microtubule binding assays and generating phospho-specific antibodies to determine whether AIR-2 phosphorylated BMK-1 is preferentially localized to meiotic and mitotic spindle microtubules.
Aurora Kinases and BimC Kinesins
Previous studies have also shown interactions between Aurora kinases and BimC kinesin proteins. Mutations in the budding yeast Aurora kinase IPL1 are synthetic lethal with mutations in the BimC kinesin CIN8 (Geiser et al., 1997; Kim et al., 1999) and the Xenopus Aurora A kinase Eg2 has been shown to phosphorylate the BimC kinesin Eg5 in vitro (Giet et al., 1999). It has been proposed that the collapse of mitotic spindles in Eg2 antibody injection experiments is due to inhibition of Eg5 microtubule-cross-linking activity (Giet and Prigent, 2000). However, the functional role of Eg2 mediated phosphorylation in regulating Eg5 activity or localization remains unknown. Our findings suggest that C. elegans Aurora A does not interact with or phosphorylate BMK-1. It remains to be determined whether BimC kinesins in different species are differentially regulated by multiple Aurora family members.
AIR-2, BMK-1, and the MKLP-1 Kinesin ZEN-4
This study has shown that AIR-2 is required to localize BMK-1. Previous studies have shown that AIR-2 genetically and physically interacts with the ZEN-4 kinesin and is required for the association of ZEN-4 with the spindle midzone (Schumacher et al., 1998b; Severson et al., 2000). Furthermore, AIR-2 can directly phosphorylate ZEN-4 in vitro (Bishop and Schumacher, unpublished observations). A formal possibility for loss of BMK-1 and ZEN-4 localization in AIR-2–depleted backgrounds is that BMK-1 localization is dependent on ZEN-4 localization and/or ZEN-4 mislocalization is due to the failure to properly localize BMK-1 in the absence of AIR-2. However, although both motor proteins require AIR-2 activity for association with meiotic and mitotic spindles, the localization of ZEN-4 and BMK-1 is independent of one another. Furthermore, concomitant loss of ZEN-4 and BMK-1 expression results in a phenotype that is indistinguishable from ZEN-4 loss-of-function, suggesting that BMK-1 and ZEN-4 do not act redundantly.
Kinesin Proteins and AIR-2–dependent Cellular Functions
The ZEN-4 kinesin is required for cytokinesis in C. elegans, and loss-of-function experiments in other organisms have shown that BimC kinesins are required for centrosome separation and/or the maintenance of bipolar spindles (Enos and Morris, 1990; Le Guellec et al., 1991; Hagan and Yanagida, 1992; Hoyt et al., 1992; Heck et al., 1993; Blangy et al., 1995; Powers et al., 1998; Raich et al., 1998). A role for BimC kinesins in cytokinesis has recently been revealed by expression of a dominant-negative version of the Boursin kinesin during sea urchin embryogenesis (Touitou et al., 2001). However, in C. elegans, the sole BimC kinesin, BMK-1, is not essential. The bmk-1(ok391) deletion mutant was isolated in a PCR-based screen by the Oklahoma Medical Research Foundation C. elegans Gene Knockout Consortium. This mutation truncates the BMK-1 protein at amino acid 376, removing part of the head domain, as well as the entire stalk and tail domains. bmk-1(ok391) homozygotes are viable and fertile; however, they do show a marked decrease in fecundity, likely due to defects during gametogenesis (Schumacher, unpublished observations). Surprisingly, the bmk-1(ok391) embryos that are produced are viable and have no gross defects in bipolar mitotic spindle formation or cytokinesis. The absence of monopolar spindles in BMK-1 mutant animals could be due to genetic redundancy with other motor proteins (Hoyt et al., 1992). Here, we have eliminated ZEN-4 as a potential redundant partner. The C. elegans genome encodes 20 kinesin-like proteins, 11 of which have may have roles in chromosome segregation and/or spindle assembly (Siddiqui, 2002). Experiments addressing whether any of these motor proteins are acting redundantly with BMK-1 are planned.
One interesting facet of our results is the requirement for AIR-2 for all aspects of BMK-1 localization. The lack of BMK-1 immunostaining at the midzone is not surprising given that the midzone is disrupted in the absence of AIR-2 (Schumacher et al., 1998b; Severson et al., 2000). However, the association of BMK-1 with meiotic and mitotic spindle microtubules is also AIR-2 dependent. The results presented here suggest that, beginning in prophase, chromosomal AIR-2 begins to recruit BMK-1 to the meiotic and mitotic spindle. The movement of AIR-2 from the chromosomes to the midzone microtubules may direct the relocalization of BMK-1 from kinetochore microtubules to the polar midzone microtubules and seems to be necessary for the midzone localization of the ZEN-4 kinesin (Schumacher et al., 1998b; Severson et al., 2000). How the AIR-2 kinase orchestrates the intracellular movement of these two kinesin proteins and the respective roles of BMK-1 and ZEN-4 motor activities in AIR-2-dependent processes are important questions for the future.
Supplementary Material
Acknowledgments
We thank Y. Kohara for the yk134b8 EST clone, S. Dent for advice on the two-hybrid analysis, T. Copeland for peptide synthesis, T. Heallen for technical assistance, R. Haynes for media preparation, and members of the Schumacher laboratory for critical reading of this manuscript. This work was supported by a Leukemia Society of America fellow award (to J.M.S) and the National Institutes of Health (R01 GM62181-01) (to J.M.S). J.D.B. was supported by the Training Program in Molecular Genetics of Cancer, National Institutes of Health, CA09299-22. The M.D. Anderson DNA Sequencing and Analysis Facility is supported by National Cancer Institute grant CA-16672.
Article published online ahead of print in MBC in Press on November 17, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0682).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, R. R., Carmena, M., and Earnshaw, W. C. (2001). Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11, 49-54. [DOI] [PubMed] [Google Scholar]
- Adams, R. R., Tavares, A. A., Salzberg, A., Bellen, H. J., and Glover, D. M. (1998). pavarotti encodes a kinesin-like protein required to organize the central spindle and contractile ring for cytokinesis. Genes Dev. 12, 1483-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, J. R., and Plowman, G. D. (1999). The Aurora/Ipl1p kinase family: regulators of chromosome segregation and cytokinesis. Trends Cell Biol. 9, 454-459. [DOI] [PubMed] [Google Scholar]
- Bishop, J. D., and Schumacher, J. M. (2002). Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B kinase stimulates Aurora B kinase activity. J. Biol. Chem. 277, 27577-27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy, A., Lane, H. A., d'Herin, P., Harper, M., Kress, M., and Nigg, E. A. (1995). Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159-1169. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman, I. M., Anderson, S., Jwa, M., Green, E. M., Kang, J., Yates, J. R., 3rd, Chan, C. S., Drubin, D. G., and Barnes, G. (2002). Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163-172. [DOI] [PubMed] [Google Scholar]
- Enos, A. P., and Morris, N. R. (1990). Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell 60, 1019-1027. [DOI] [PubMed] [Google Scholar]
- Fields, S., and Sternglanz, R. (1994). The two-hybrid system: an assay for protein-protein interactions. Trends Genet 10, 286-292. [DOI] [PubMed] [Google Scholar]
- Geiser, J. R., Schott, E. J., Kingsbury, T. J., Cole, N. B., Totis, L. J., Bhattacharyya, G., He, L., and Hoyt, M. A. (1997). Saccharomyces cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol. Biol. Cell 8, 1035-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and Glover, D. M. (2001). Drosophila Aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 152, 669-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet, R., and Prigent, C. (2000). The Xenopus laevis Aurora/Ip11p-related kinase pEg2 participates in the stability of the bipolar mitotic spindle. Exp. Cell Res. 258, 145-151. [DOI] [PubMed] [Google Scholar]
- Giet, R., Uzbekov, R., Cubizolles, F., Le Guellec, K., and Prigent, C. (1999). The Xenopus laevis Aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J. Biol. Chem. 274, 15005-15013. [DOI] [PubMed] [Google Scholar]
- Hagan, I., and Yanagida, M. (1990). Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature 347, 563-566. [DOI] [PubMed] [Google Scholar]
- Hagan, I., and Yanagida, M. (1992). Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature 356, 74-76. [DOI] [PubMed] [Google Scholar]
- Hannak, E., Kirkham, M., Hyman, A. A., and Oegema, K. (2001). Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155, 1109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck, M. M. (1999). Dr. Dolittle and the making of the mitotic spindle. Bioessays 21, 985-990. [DOI] [PubMed] [Google Scholar]
- Heck, M. M., Pereira, A., Pesavento, P., Yannoni, Y., Spradling, A.C., and Goldstein, L. S. (1993). The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J. Cell Biol. 123, 665-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston, E., Le Guellec, R., Kress, M., Philippe, M., and Le Guellec, K. (1994). The kinesin-related protein Eg5 associates with both interphase and spindle microtubules during Xenopus early development. Dev. Biol. 164, 147-159. [DOI] [PubMed] [Google Scholar]
- Hoyt, M. A., He, L., Loo, K. K., and Saunders, W. S. (1992). Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J. Cell Biol. 118, 109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, P., Halladay, J., and Craig, E. A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna, S., Mendoza, M., Jantsch-Plunger, V., and Glotzer, M. (2000). INCENP and an Aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 10, 1172-1181. [DOI] [PubMed] [Google Scholar]
- Kim, J. H., Kang, J. S., and Chan, C. S. (1999). Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 145, 1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guellec, R., Paris, J., Couturier, A., Roghi, C., and Philippe, M. (1991). Cloning by differential screening of a Xenopus cDNA that encodes a kinesin-related protein. Mol. Cell. Biol. 11, 3395-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nislow, C., Lombillo, V. A., Kuriyama, R., and McIntosh, J. R. (1992). A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature 359, 543-547. [DOI] [PubMed] [Google Scholar]
- Petersen, J., Paris, J., Willer, M., Philippe, M., and Hagan, I. M. (2001). The S. pombe Aurora-related kinase Ark1 associates with mitotic structures in a stage dependent manner and is required for chromosome segregation. J. Cell Sci. 114, 4371-4384. [DOI] [PubMed] [Google Scholar]
- Powers, J., Bossinger, O., Rose, D., Strome, S., and Saxton, W. (1998). A nematode kinesin required for cleavage furrow advancement. Curr. Biol. 8, 1133-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich, W. B., Moran, A. N., Rothman, J. H., and Hardin, J. (1998). Cytokinesis and midzone microtubule organization in Caenorhabditis elegans require the kinesin-like protein ZEN-4. Mol. Biol. Cell 9, 2037-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, E., Bishop, J. D., Waddle, J. A., Schumacher, J. M., and Lin, R. (2002). The Aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J. Cell Biol. 157, 219-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano, A., Guse, A., Krascenicova, I., Schnabel, H., Schnabel, R., and Glotzer, M. (2003). CSC-1, a subunit of the Aurora B kinase complex that binds to the survivin-like protein BIR-1 and the INCENP-like protein ICP-1. J. Cell Biol. 161, 229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Sawin, K. E., LeGuellec, K., Philippe, M., and Mitchison, T. J. (1992). Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359, 540-543. [DOI] [PubMed] [Google Scholar]
- Sawin, K. E., and Mitchison, T. J. (1995). Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc. Natl. Acad. Sci. USA 92, 4289-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, J. M., Ashcroft, N., Donovan, P. J., and Golden, A. (1998a). A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 125, 4391-4402. [DOI] [PubMed] [Google Scholar]
- Schumacher, J. M., Golden, A., and Donovan, P. J. (1998b). AIR-2, An Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 143, 1635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson, A. F., Hamill, D. R., Carter, J. C., Schumacher, J., and Bowerman, B. (2000). The Aurora-related kinase AIR-2 recruits ZEN-4/CeMKLP1 to the mitotic spindle at metaphase and is required for cytokinesis. Curr. Biol. 10, 1162-1171. [DOI] [PubMed] [Google Scholar]
- Seydoux, G., and Dunn, M. A. (1997). Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 124, 2191-2201. [DOI] [PubMed] [Google Scholar]
- Sharp, D. J., McDonald, K. L., Brown, H. M., Matthies, H. J., Walczak, C., Vale, R. D., Mitchison, T. J., and Scholey, J. M. (1999). The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J. Cell Biol. 144, 125-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, S. S. (2002). Metazoan motor models: kinesin superfamily in C. elegans. Traffic 3, 20-28. [DOI] [PubMed] [Google Scholar]
- Speliotes, E. K., Uren, A., Vaux, D., and Horvitz, H. R. (2000). The survivin-like C. elegans BIR-1 protein acts with the Aurora-like kinase AIR-2 to affect chromosomes and the spindle midzone. Mol. Cell 6, 211-223. [DOI] [PubMed] [Google Scholar]
- Terada, Y. (2001). Role of chromosomal passenger complex in chromosome segregation and cytokinesis. Cell Struct. Funct. 26, 653-657. [DOI] [PubMed] [Google Scholar]
- Timmons, L., and Fire, A. (1998). Specific interference by ingested dsRNA. Nature 395, 854. [DOI] [PubMed] [Google Scholar]
- Touitou, I., Lhomond, G., and Pruliere, G. (2001). Boursin, a sea urchin bimC kinesin protein, plays a role in anaphase and cytokinesis. J. Cell Sci. 114, 481-491. [DOI] [PubMed] [Google Scholar]
- Walczak, C. E., and Mitchison, T. J. (1996). Kinesin-related proteins at mitotic spindle poles: function and regulation. Cell 85, 943-946. [DOI] [PubMed] [Google Scholar]
- Whitehead, C. M., and Rattner, J. B. (1998). Expanding the role of HsEg5 within the mitotic and post-mitotic phases of the cell cycle. J. Cell Sci. 111, 2551-2561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


