Abstract
Phosphorylated derivatives of phosphatidylinositol are essential regulators of both endocytic and exocytic trafficking in eukaryotic cells. In Saccharomyces cerevisiae, the phosphatidylinositol 4-kinase, Pik1p generates a distinct pool of PtdIns(4)P that is required for normal Golgi structure and secretory function. Here, we utilize a synthetic genetic array analysis of a conditional pik1 mutant to identify candidate components of the Pik1p/PtdIns(4)P signaling pathway at the Golgi. Our data suggest a mechanistic involvement for Pik1p with a specific subset of Golgi-associated proteins, including the Ypt31p rab-GTPase and the TRAPPII protein complex, to regulate protein trafficking through the secretory pathway. We further demonstrate that TRAPPII specifically functions in a Ypt31p-dependent pathway and identify Gyp2p as the first biologically relevant GTPase activating protein for Ypt31p. We propose that multiple stage-specific signals, which may include Pik1p/PtdIns(4)P, TRAPPII and Gyp2p, impinge upon Ypt31 signaling to regulate Golgi secretory function.
INTRODUCTION
The Golgi apparatus plays an essential role in the intracellular trafficking and sorting of proteins and lipids within the secretory pathway. Although the Golgi complex in mammalian and yeast cells are morphologically different, they are both organized into functionally distinct compartments: the cis-, medial- and trans-Golgi (Franzusoff and Schekman, 1989; Graham and Emr, 1991). Each Golgi compartment has a unique repertoire of resident proteins that are responsible for the sequential posttranslational modification and maturation of cargo proteins, which are then secreted out of the Golgi and transported to their cellular destinations (i.e., to the plasma membrane, endosome, or lysosome/vacuole). Transport to and from the Golgi requires the concerted actions of specific rab-GTPases, SNAREs, phosphoinositides, and hetero-oligomeric protein complexes (Segev, 2001; Zerial and McBride, 2001; Pfeffer, 2003). The coordinated action of these diverse transport components provides specificity in each trafficking step, although the mechanism by which these components cooperate in Golgi trafficking is only partially understood.
Phosphorylated derivatives of phosphatidylinositol are particularly versatile regulators of protein transport and carry out essential modulatory functions within the secretory pathway by interacting with distinct lipid-binding effector molecules (Simonsen et al., 2001). For instance, phosphatidylinositol 3-phosphate (PtdIns(3)P) is required for the anterograde traffic of cargo proteins from the Golgi to the vacuole by interacting with specific proteins that contain either a FYVE domain or a Phox homology (PX) domain, two motifs which specifically bind PtdIns(3)P (Simonsen et al., 2001). Conversely, phosphatidylinositol 4-phosphate (PtdIns(4)P), regulates the traffic of cargo proteins from the Golgi to the plasma membrane (Hama et al., 1999; Walch-Solimena and Novick, 1999; Audhya et al., 2000) by a yet undefined mechanism.
Saccharomyces cerevisiae contains two essential phosphatidylinositol 4-kinases, Stt4p and Pik1p. Functional studies have demonstrated that Pik1p and Stt4p catalyze the production of the majority of PtdIns(4)P in yeast (Audhya et al., 2000) and suggest unique roles for the two pools of PtdIns(4)P synthesized by each PtdIns 4-kinase. In part this is due to the differential subcellular targeting of Stt4p and Pik1p. Stt4p localizes to the cell periphery (Audhya and Emr, 2002) and regulates cell wall integrity and vacuole morphology but has no detected role in secretion (Audhya et al., 2000). In contrast, Pik1p localizes to both the Golgi (Walch-Solimena and Novick, 1999) and the nucleus (Garcia-Bustos et al., 1994; Walch-Solimena and Novick, 1999). Inactivation of Pik1p decreases PtdIns(4)P levels, impairs secretory transport from the Golgi, and disrupts the structural integrity of the Golgi and vacuole (Hama et al., 1999; Walch-Solimena and Novick, 1999; Audhya et al., 2000). In addition, defects in cytokinesis and actin cytoskeletal organization have been observed after loss of Pik1p function (Garcia-Bustos et al., 1994; Walch-Solimena and Novick, 1999). Consequently, the pleiotropic effects caused by the inactivation of Pik1p have obscured the precise function of PtdIns(4)P at the Golgi.
Mammalian cells express at least three Golgi-localized PtdIns 4-kinases, PI4KIIα (Wang et al., 2003), PI4KIIIα (Nakagawa et al., 1996), and PI4KIIIβ (Wong et al., 1997). Subsequent studies have found that PI4KIIIβ is recruited to the Golgi by active, GTP-bound Arf (Godi et al., 1999). Interfering with PI4KIIIβ by the overexpression of a kinase dead, dominant-negative mutant, disrupted the structural integrity of the Golgi (Godi et al., 1999), providing additional evidence for PtdIns(4)P in the regulation of Golgi function. Arf also recruited an unidentified PtdIns(4)P 5-kinase to the Golgi, resulting in the conversion of PtdIns(4)P to PtdIns(4,5)P2, making the respective roles of PtdIns(4)P and PtdIns(4,5)P2 at the Golgi less clear (Godi et al., 1999). Inactivation of temperature-sensitive alleles of MSS4, the sole phosphoinositide kinase responsible for the synthesis of PtdIns(4,5)P2 in yeast, does not affect the morphology or secretory function of the Golgi complex (Audhya and Emr, 2002; Stefan et al., 2002). Collectively, these data suggest that PtdIns(4)P serves multiple functions, as a direct mediator in Golgi function, and as a precursor to PtdIns(4,5)P2.
To gain a better understanding of the essential role of Pik1p in yeast Golgi function, we have utilized a synthetic genetic array (SGA) analysis to identify genes that may participate in a Pik1p-dependent pathway. We report the identification of PIK-specific genetic interactions with genes known to have distinct roles in membrane trafficking and Golgi function. We demonstrate that Pik1p functionally interacts with Ypt31p, a Golgi-localized rab-GTPase, to regulate the trafficking of cargoes that require recycling through the early endosome, including the yeast SNARE, Snc1p, and chitin synthase, Chs3p. Using information obtained from the screen, we present evidence that transport protein particle (TRAPP) complex II, a large protein complex localized to the Golgi (Sacher et al., 2001), interacts genetically with PIK1 and functionally with Ypt31p. Further analysis of the interactions between TRAPPII and Ypt31p led to the identification of Gyp2p as a biologically relevant GTPase activating protein (GAP) for Ypt31p in vivo. Our comprehensive genetic analysis of the pik1 mutation led to the dissection of relevant PtdIns(4)P-dependent pathways and to defining regulatory components that function in Ypt31p rab-GTPase signaling at the Golgi.
MATERIALS AND METHODS
Strains and Media
The genotypes of S. cerevisiae strains used in this study are listed in Table 1. All yeast strains were grown in YPD or SD minimal media containing the necessary amino acid supplements. Transformations into yeast were performed by the lithium acetate method (Ito et al., 1983). All strains were constructed either by tetrad dissection of sporulated diploid strains or by integration of indicated disruption cassettes as previously described (Longtine et al., 1998). Disruptions were confirmed by PCR. pik1-139ts ypt31Δ, pik1-139ts kre11Δ,and pik1-139ts trs33Δ, and pik1–83tsypt31Δ were isolated by tetrad dissection and maintained on YPD containing 1 M sorbitol at 26°C. SEY6210 expressing TAT2-GFP or CHS3-GFP were engineered by transformation of PCR-amplified genomic integration construct using GFP-His3MX6 plasmid as template (Longtine et al., 1998) and verified by PCR analysis generating CJSY298 and VSY184. SEY6210 expressing GFP-SNC1 (VSY167) was engineered by integrating an expression plasmid linearized with StuI at URA3 (Lewis et al., 2000) generating VSY167. pik1-139ts, ypt31Δ and pik1-139ts ypt31Δ double mutant cells expressing TAT2-GFP, CHS3-GFP, and GFP-SNC1 were isolated by tetrad dissection. Expression of TAT2-GFP, CHS3-GFP, and GFP-SNC1 were screened by fluorescence microscopy and Western blotting.
Table 1.
S. cerevisiae strains used in this study
| Strains | Genotype | Reference or source |
|---|---|---|
| SEY6210 | MAT a leu2-3112 ura3-52 his3-Δ200 trp1-Δ901 lys2–801 suc2-Δ9 | Robinson et al. (1988) |
| SEY6210.1 | MAT a leu2-3112 ura3-52 his3-Δ200 trp1-Δ901 lys2–801 suc2-Δ9 | Robinson et al. (1988) |
| SEY6210α/a | MAT α/MAT a homozygous diploid strain from SEY6210/6210.1 cross | Robinson et al. (1988) |
| AAY1139 | SEY6210; pik1Δ::HIS3 carrying pRS415 pik1-139 (LEU2 CEN6 pik1-139) | This study |
| AAY102 | SEY6210; stt4Δ::HIS3 carrying pRS415 stt4-4 (LEU2 CEN6 stt4-4) | Audhya et al. (2000) |
| AAY104 | SEY6210; pik1Δ::HIS3 carrying pRS314 pik1-83 (TRP1 CEN6 pik1-83) | Audhya et al. (2000) |
| AAY202 | SEY6210; mss4Δ::HIS3MX6 carrying pYCplac111 mss4-102 (LEU2 CEN6 mss4-102) | Stefan et al. (2002) |
| AAY1167 | SEY6210; drs2Δ::HIS3 | This study |
| AAY1169 | SEY6210.1; kre11Δ::HIS3 | This study |
| VSY378 | SEY6210; trs33Δ::HIS3 | This study |
| AAY1171 | SEY6210.1; trs33Δ::HIS3 | This study |
| VSY232 | SEY6210.1; ypt31Δ::TRP1 | This study |
| VSY322 | SEY6210; ypt31Δ::HIS3 | This study |
| AAY1182 | AAY1139; kre11Δ::HIS3 | This study |
| AAY1184 | AAY1139; trs33Δ::HIS3 | This study |
| VSY254 | AAY1139; ypt31Δ::TRP1 | This study |
| AAY1191 | AAY102; drs2Δ::HIS3 | This study |
| VSY475 | AAY102; kre11Δ::HIS3 | This study |
| VSY500 | AAY102; trs33Δ::HIS3 | This study |
| VSY312 | AAY102; ypt31Δ::TRP1 | This study |
| VSY479 | AAY104; ypt31Δ::TRP1 | This study |
| VSY342 | AAY202; drs2Δ::HIS3 | This study |
| VSY388 | AAY202; ypt31Δ::TRP1 | This study |
| VSY328 | SEY6210; drs2Δ::HIS3 ypt31Δ::TRP1 | This study |
| VSY409 | SEY6210; drs2Δ::HIS3 kre11Δ::HIS3 | This study |
| VSY167 | SEY6120; GFP-SNC1:URA3 | This study |
| VSY307 | VSY232; GFP-SNC1:URA3 | This study |
| VSY309 | AAY1139; GFP-SNC1:URA3 | This study |
| VSY314 | VSY254: GFP-SNC1:URA3 | This study |
| VSY184 | SEY6210; CHS3-GFP: HIS3MX6 | This study |
| VSY497 | VSY184; ypt31Δ::TRP1 | This study |
| VSY498 | AAY1139; CHS3-GFP:HIS3MX6 | This study |
| VSY499 | VSY254; CHS3-GFP: HIS3MX6 | This study |
| CJSY298 | SEY6210; TAT2-GFP:HIS3MX6 | This study |
| VSY492 | CJS298; ypt31D::TRP1 | This study |
| VSY491 | AAY1139; TAT2-GFP:HIS3MX6 | This study |
| VSY493 | VSY254; TAT2-GFP:HIS3MX6 | This study |
| VSY197 | SEY6210.1; ypt32D::TRP1 | This study |
| VSY468 | SEY6210; ypt32D::TRP1 ypt31-101ts:URA3 | This study |
| VSY484 | VSY468; GFP-SNC1:URA3 | This study |
| AAY1151 | SEY6210; pik1D::HIS3 carrying pRS414 GFP-PIK1 | This study |
| VSY531 | VSY468; pik1D::HIS3 carrying pRS414 GFP-PIK1 | This study |
| VSY407 | SEY6210; ypt31D::HIS3 ypt32 D::TRP1 carrying pRS415 GFP-YPT31 | This study |
| VSY380 | SEY6210α/a; trs130D::HIS3/TRS130 | This study |
| VSY379 | SEY6210α/a; trs120D::HIS3/TRS120 | This study |
| VSY446 | SEY6210; trs130-(33aa truncation)-HAts :HIS3MX6 | This study |
| VSY459 | SEY6210; TRS130-HA:HIS3MX6 | This study |
| VSY470 | SEY6210.1; gyp1D::HIS3 | This study |
| VSY471 | SEY6210.1; gyp2D:HIS3 | This study |
| VSY495 | VSY470; trs130-(33aa truncation)-HAts :HIS3MX6 | This study |
| VSY486 | VSY446; gyp2D::HIS3 | This study |
| VSY488 | VSY468; gyp1D::HIS3 | This study |
| VSY483 | VSY468; gyp2D::HIS3 | This study |
| VSY433 | SEY6210; trs33D::HIS3 kre11D::HIS3 carrying pRS425 ypt31Q72L | This study |
| VSY419 | SEY6210; trs130D::HIS3 carrying pRS425-YPT31 | This study |
| VSY421 | SEY6210; trs130D::HIS3 carrying pRS425-ypt31Q72L | This study |
| VSY428 | SEY6210; trs120D::HIS3 carrying pRS425-YPT31 | This study |
| VSY423 | SEY6210; trs120D::HIS3 carrying pRS425-ypt31Q72L | This study |
Genetic and DNA Manipulations
SGA analysis was performed as described previously (Tong et al., 2001). Standard molecular biology techniques were used for DNA manipulations (Maniatis et al., 1992). Enzymes used for recombinant DNA techniques were purchased from Roche (Nutley, NJ) and New England Biolabs (Beverly, MA). PCR reactions were conducted as directed by manufacturer's instructions using PfuTurbo (Stratagene, La Jolla, CA) or AmpliTaq (Applied Biosystems, Foster City, CA) DNA polymerases for cloning and diagnostic reactions, respectively. Yeast genomic DNA was isolated by the method of Hoffman and Winston (1987). A 1.8-kb fragment containing the open reading frame (ORF) of YPT31 was amplified from genomic DNA prepared from SEY6210 using oligonucleotide primers that incorporated unique BamHI and SmaI restriction enzyme sites at the 5′ and 3′ ends, respectively. The PCR products were digested with BamHI and SmaI and subcloned into BamHI-SmaI–digested pRS416 (Sikorski and Hieter, 1989) creating VSB283. Haploid ypt31Δ and ypt32Δ single deletion mutants of opposite mating types, each expressing low-copy YPT31 were mated and sporulated and tetrads were isolated. ypt31Δ ypt32Δ double mutants carrying YPT31 were confirmed by PCR and demonstrated functionality of the YPT31 clone. ypt31 Q72L (VSB333) and N126I (VSB334) were generated by site-directed mutagenesis of the wild-type plasmid (VSB327) using the Quik-change kit (Stratagene) and confirmed by DNA sequencing. To generate GFP-YPT31, the YPT31 ORF (672 bp) was amplified from pRS416-YPT31 engineered with BglII (5′) and PstI (3′) and subcloned into pGO37 (Burd and Emr, 1998) creating VSB303. Expression of GFP-YPT31p was confirmed by Western blot analysis. GFP-YPT31 was subsequently subcloned into NotI and SalI sites of pRS415 (Sikorski and Hieter, 1989) creating VSB311. ypt31Δ ypt32Δ double mutants carrying low copies of GFP-YPT31 were isolated and confirmed as described above.
To generate a construct for expression of GFP-PIK1, a XhoI-KpnI fragment from pYcp50-PIK1, corresponding to the C-terminus of PIK1, was first ligated into XhoI-KpnI–digested pGO36 (Burd and Emr, 1998), creating pJA519. The N-terminus of PIK1 with an 8-alanine linker and XhoI sites was amplified by PCR and cloned into XhoI-digested pJA519, creating pJA520 (GFP-PIK1). Expression of GFP-PIK1 in either pik1Δ or pik1ts mutant cells rescued lethality. To generate GFP-GYP2, the GYP2 ORF was amplified from genomic DNA prepared from SEY6210 using oligonucleotide primers that incorporated unique EcoRI and ClaI restriction enzyme sites at the 5′ and 3′, respectively. The PCR product was digested with EcoRI and ClaI and ligated into EcoRI-ClaI–digested pGO35 (Burd and Emr, 1998) creating VSB363. In frame GFP fusions were confirmed by Western blot analysis. An NotI-ClaI fragment containing GFP-GYP2 was subcloned into NotI-SmaI sites of pRS415 (Sikorski and Hieter, 1989) creating VSB367. GFP-gyp2 G80E (VSB368) and GFP-gyp2 R295K (VSB369) were generated by site-directed mutagenesis, as described above.
A strain expressing the pik1-139ts mutant plasmid was generated in several steps. First, pRS415-PIK1 was linearized with NheI-NruI. Together with a randomly mutagenized PCR product corresponding to the C-terminal kinase domain of PIK1, these DNA fragments were transformed into pik1Δ::HIS3 cells carrying pRS416-PIK1. Cells were incubated at 26°C and then transferred to plates containing 5-FOA (to eliminate pRS416-PIK1) at both 26 and 37°C. Temperature-sensitive colonies were isolated, and individual plasmids were recovered. Temperature-sensitive alleles were retested, and pik1-139 was chosen based on its ability to support robust growth of pik1Δ::HIS3 cells at 26°C but not at temperatures above 35°C.
The ypt32Δypt31-101ts temperature-sensitive mutant was generated by a multistep process in which YPT31 (i.e., the entire YPT31 ORF including a 200-base pair fragment downstream of the YPT31 stop codon) was randomly mutagenized using AmpliTaq DNA polymerase under error-prone PCR conditions. URA3 was independently amplified under nonerror prone PCR conditions. The mutagenized ypt31 and URA3 PCR fragments were cotransformed, fused to each other, and integrated into the YPT31 locus of ypt32Δ cells by homologous recombination. Ura+ prototrophic transformants were selected and screened for temperature-sensitive growth on YPD at 26 and 38°C. A ypt32Δ strain harboring ypt31-101 (VSY468) displayed strong temperature-sensitive phenotypes was selected for further characterization.
pRC2240 SEC7-DsRED (Calero et al., 2003) was a gift from R. Collins (Cornell University). pHXT1-GFP (Malinska et al., 2003) was obtained from W. Tanner (Universitat Regensberg). pRS315-YPT6 (Bensen et al., 2001) was obtained from G. Payne (UCLA) and the 1.45-kb SacI-BamHI fragment from pRS315-YPT6 was subsequently subcloned into pRS425 (Sikorski and Hieter, 1989) creating VSB372. Plasmids for the expression of SEC4 and sec4 Q72L were gifts from P. Brenwald (UNC, Chapel Hill, NC). GFP-SNC1 integration plasmid was from H. Pelham (MRC, United Kingdom).
Fluorescence and Electron Microscopy
Cells expressing GFP-PIK1 or GFP-YPT31 (or coexpressing SEC7-DsRED) were grown to early log phase at 26°C. After shifting to 38°C for the indicated duration, cells were concentrated and visualized by fluorescence microscopy. Cells expressing GFP-SNC1, CHS3-GFP, TAT2-GFP, or HXT1-GFP were grown to early log phase and visualized at 26°C. Fluorescent images were visualized using a Zeiss Axiovert S1002TV inverted fluorescent microscope (Thornwood, NY), acquired, and processed using a Delta Vision deconvolution system (Applied Precision Instruments, Issaquah, WA) and Adobe Photoshop 6.0 (San Jose, CA). Observations were based on examination of at least 200 cells. Vacuole morphology was examined by visualization of vacuole membranes by labeling with the fluorescent dye, FM4–64 (Molecular Probes, Eugene, OR) as previously described by Vida and Emr (1995). For ultrastructural analysis, 50 OD600 of early log-phase cells incubated at the appropriate temperature were harvested from YPD and fixed in 3% glutaraldehyde, 0.1 M Na cacodylate (pH 7.4), 5 mM CaCl2, 5 mM MgCl2, and 2.5% sucrose for 1 h. Cells were further processed for electron microscopy as described previously (Audhya et al., 2000).
In Vivo Analysis of Phosphoinositides
Analysis of phosphoinositides levels was carried out as previously described (Audhya et al., 2000; Rudge et al., 2004). Briefly, cells were grown in synthetic medium with the appropriate amino acids. Cells from an early log phase culture were harvested, washed, and resuspended in inositol-free synthetic medium (IFM). Cells were shifted to the appropriate temperature (26 or 38°C) for 10 min and labeled with 60 μCi of myo-[2-3H]inositol (Amersham Biosciences, Piscataway, NJ) in IFM for an additional 45 min. Phosphoinositides were extracted by ice-cold precipitation with a final concentration of 4.5% perchloric acid and deacylated by treatment with methylamine. Extracts of deacylated lipids were further processed as described in Rudge et al. (2004). [3H]glycerol-phosphoinositides were separated using an anion-exchange Partisphere SAX column (Whatman, Clifton, NJ) coupled to a GOLD HPLC system (Beckman Coulter, Fullerton, CA) and quantitated by liquid scintillation counting using an on-line radiomatic detector (Packard Instrument Co., Meriden, CT) utilizing Ultima Flo scintillation fluid (Packard).
Metabolic Labeling and Immunoprecipitation
Cell labeling and immunoprecipitations were performed as previously described (Audhya et al., 2000). Log-phase cultures were concentrated to 5 OD600/ml and labeled with 2 μl/OD600 Trans-35S label mix (DuPont New England Nuclear, Boston, MA) for 10 min in YNB, supplemented with 2% glucose, appropriate amino acids, and 100 μg/ml bovine serum albumin. Cells were chased with an excess of unlabeled methionine/cysteine for the indicated times, and proteins were precipitated with 9% trichloroacetic acid (TCA) on ice. For temperature shift experiments, cells were shifted to the appropriate temperature for 10–30 min before labeling. For analysis of media-secreted proteins, cells were separated from media by gentle centrifugation before precipitation. Extracts were immunoprecipitated with antisera against CPY (Cowles et al., 1997) or Gas1p (R. Schekman, UC Berkeley). Immunoprecipitated proteins were resuspended in sample buffer, resolved by SDS-PAGE, and subjected to autofluorography.
Miscellaneous
Western blot analysis of SDS-PAGE–resolved proteins were performed as previously described (Rudge et al., 2004). GFP and HA fusion proteins were detected using mouse monoclonal antibodies against GFP (Santa Cruz Biotechnology, Santa Cruz, CA) and HA (12CA5; Roche). Antibody against G6PDH was purchased from Sigma (St. Louis, MO). Immunoreactive proteins were detected using horseradish peroxidase–conjugated goat anti-mouse or anti-rabbit (Zymed Laboratories, South San Francisco, CA) and SuperSignal chemiluminescent substrate (Pierce Chemical, Rockford, IL).
RESULTS
SGA Analysis Reveals a Network of PIK1 Genetic Interactions
To identify components of the Pik1p-dependent secretory apparatus required during transport out of the Golgi, we conducted a synthetic genetic interaction screen (Tong et al., 2001) using a yeast strain expressing a functionally impaired temperature-sensitive allele of pik1. From such a sensitized genetic screen, we anticipated that we would identify gene products essential for Pik1p-dependent pathways. We used error-prone PCR mutagenesis targeted to the catalytic kinase domain of Pik1p, to engineer the pik1-139ts mutant strain that could grow robustly up to temperatures of 35°C. Under permissive conditions (26°C), pik1-139ts cells have a modest 20% reduction in PtdIns(4)P levels (SEY6210; 0.88 ± 0.06% compared with pik1-139ts; 0.70 ± 0.06% of PtdIns labeled). Accordingly, at 26°C, pik1-139ts cells lack any detectable growth defects associated with the loss of PtdIns(4)P signaling. Previously, it was shown that Pik1p and Stt4p each contribute ∼50% of the total cellular PtdIns(4)P (Audhya et al., 2000). As such, at the nonpermissive temperature (38°C, see Audhya et al., 2000), pik1-139ts cells have 43% reduced levels of PtdIns(4)P (SEY6210; 0.99 ± 0.08% compared with pik1-139ts; 0.56 ± 0.08% of PtdIns labeled) and consequentially accumulate exaggerated Golgi as well as other membranous structures (unpublished data). Most of the remaining PtdIns(4)P observed in pik1-139ts cells grown under nonpermissive conditions is due to the unaltered PtdIns 4-kinase activity of Stt4p (Audhya et al., 2000 and unpublished data). These results are consistent with the proposed role for Pik1p-generated PtdIns(4)P in Golgi structure/function (Hama et al., 1999; Walch-Solimena and Novick, 1999; Audhya et al., 2000).
The pik1-139ts strain was then individually crossed with ∼4700 single deletion strains that represent the complete set of viable haploid deletion mutants in S. cerevisiae. Double mutants exhibiting reduced growth at 26°C relative to the parental strains were scored three independent times. The results of the screen are listed in Supplementary Table 1. More than 30 genes, when deleted in the pik1-139ts background, displayed a synthetic growth defect. The pik1 interactions were highly enriched for genes with roles in membrane trafficking (Supplementary Table 1 and Figure 1A), indicative of an essential function for Pik1p in secretion. Consistent with previous studies suggesting roles for Pik1p/PtdIns(4)P in cytokinesis (Garcia-Bustos et al., 1994), actin rearrangement (Walch-Solimena and Novick, 1999) and vacuole function (Audhya et al., 2000), several genes involved in these processes were isolated. Surprisingly, gene products with known PtdIns(4)P-binding modules, such as a pleckstrin homology (PH) domain (Yu et al., 2004), were not isolated. Further analysis of results obtained from this study and published studies revealed multiple interactions within subsets of genes involved in anterograde and retrograde Golgi traffic (Figure 1A).
Figure 1.
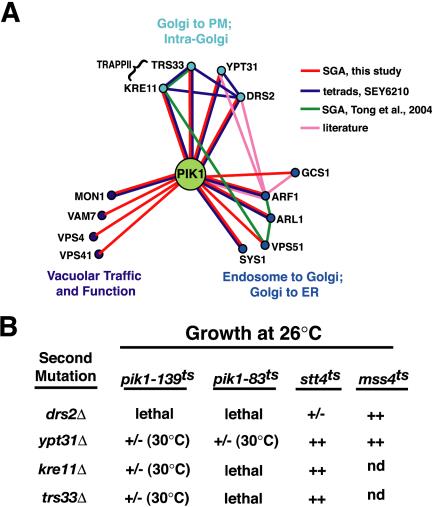
Phosphoinositide kinase specific genetic interactions. (A) Schematic of genes identified by SGA involved in membrane trafficking with known genetic interactions (synthetic sick or lethal). (B) Genetic interactions of four trafficking genes obtained from SGA (drs2Δ, ypt31Δ, kre11Δ, and trs33Δ) were analyzed in pik1-139ts, pik1–83ts, stt4ts, and mss4ts. Tetrads were isolated at 26°C and the growth of the double mutants was assessed at 26°C. +/-; reduced growth, ++; similar to growth of single mutants. Temperature at which lethality is observed is given in parentheses.
Specific Interactions with the Pik1p PtdIns 4-kinase
SGA analysis identified four genes: DRS2, TRS33, KRE11, and YPT31, with previous ascribed roles in Golgi anterograde trafficking to the plasma membrane. Drs2p, a Golgi-associated, integral membrane aminophospholipid translocase, is required for generating clathrin-coated exocytic vesicles (Chen et al., 1999; Gall et al., 2002). TRS33 and KRE11 encode nonessential subunits of the TRAPPII, a large Golgi-associated complex consisting of 10 proteins (Bet3p, Bet5p, Trs20p, Trs23p, Trs31p, Trs33p, Trs85p, Kre11p, Trs120p, and Trs130p; Sacher et al., 2001). Temperature-sensitive mutants of the essential subunits display severe secretion defects, as well as an accumulation of aberrant Golgi structures (Sacher et al., 2001). YPT31 encodes a member of the Ypt/rab family of GTPases that is required for secretory transport, possibly by regulating vesicle formation at the trans-Golgi (Benli et al., 1996; Jedd et al., 1997).
Deletion of DRS2, TRS33, KRE11, or YPT31 in either the pik1-139ts or another temperature-sensitive allele of PIK1, pik1–83ts strain (Hama et al., 1999; Audhya et al., 2000) resulted in either lethality or a severe growth defect (Figure 1B). Although Ypt31p shares 81% sequence identity with Ypt32p, only YPT31 was uncovered in our SGA analysis using the pik1ts mutation. Consistent with this observation, pik1-139ts ypt32Δ double mutant cells did not exhibit a significant synthetic growth defect (lethality at 34°C) in contrast to pik1-139ts ypt31Δ double mutant cells (Figure 1B). Because Ypt31p is more abundant than Ypt32p in the cell (Jedd et al., 1997), these data suggest that Ypt31p may play a more dominant role in cells compared with Ypt32p.
We next compared the specificity of the genetic interactions obtained by SGA analysis by examining the effects of deleting these genes in strains harboring temperature-sensitive Stt4p, the second essential PtdIns 4-kinase in yeast, and Mss4p, the essential yeast PtdIns(4)P 5-kinase. As shown in Figure 1B, no significant genetic interactions with these PtdIns-kinases were identified. The PtdIns-kinase specificity of these genetic interactions suggests roles for Drs2p, Trs33p, Kre11p, and Ypt31p in a common or parallel pathway to Pik1p in Golgi function.
Coordinated Regulation of the Secretory Pathway by Pik1p and Ypt31p
It has become increasingly apparent that cooperative protein-phosphoinositide and protein-protein interactions are required for the localization of specific signaling molecules to their site of action (Levine and Munro, 2002). For instance, localization of early endosome antigen 1 to the endosome requires its interaction with both active, GTP-bound Rab5 (Simonsen et al., 1998), and PtdIns(3)P (Burd and Emr, 1998). In accordance with this model, we hypothesized that active, GTP-bound Ypt31p, and Pik1p-generated PtdIns(4)P may recruit effector molecules to function at the Golgi. We first investigated the phenotypes of pik1-139ts ypt31Δ double mutant cells to determine whether specific membrane trafficking pathways were attenuated in these cells. Growth and organelle morphology of pik1-139ts cells (grown at the permissive temperature) or ypt31Δ cells (Benli et al., 1996; Jedd et al., 1997) were similar to wild-type cells. However, electron microscopy of pik1-139tsypt31Δ double mutant cells grown under the same conditions (26°C) revealed an accumulation of abnormal Golgi structures, including Berkeley bodies, and fragmented vacuoles (unpublished data), indicating a synthetic defect in Golgi function.
We next investigated the localization of proteins that traffic from the Golgi to the plasma membrane. Snc1p is a yeast SNARE that mediates the fusion of exocytic vesicles with the plasma membrane (Protopopov et al., 1993). Snc1p, which localizes to the plasma membrane at sites of bud formation, undergoes rapid endocytosis into early endocytic structures and is recycled to the Golgi where it is incorporated into another round of secretory vesicles (Lewis et al., 2000). Consistent with previous studies (Lewis et al., 2000), wild-type cells expressed GFP-Snc1p at the plasma membrane of newly formed daughter cells, and at the mother-daughter neck (Figure 2A). A few punctate structures were also visible, consistent with the dynamic recycling of this SNARE to and from the Golgi complex. Similar localization patterns of GFP-Snc1p fluorescence were also seen in ypt31Δ and pik1-139ts cells at the permissive temperature (Figure 2A). In pik1-139tsypt31Δ double mutant cells grown at the permissive temperature (26°C), the polarized plasma membrane localization of GFP-Snc1p was no longer observed, and instead GFP-Snc1p resided in punctate structures, some of which resembled “swollen Golgi,” whereas others resembled tubular rings (Figure 2A). These data suggest that Pik1p and Ypt31p are required for GFP-Snc1p recycling to and/or secretion from the Golgi.
Figure 2.
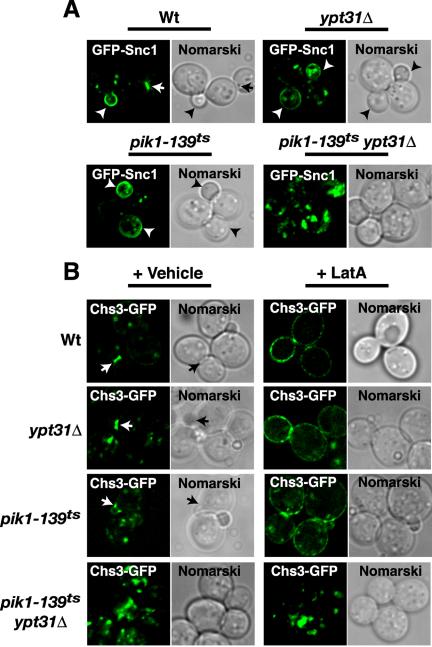
Trafficking of Snc1p and Chs3p requires Pik1p and Ypt31p. (A) GFP-Snc1p or (B) Chs3p-GFP distribution at 26°C in live cells, either wild-type (Wt) or carrying the indicated mutation, ypt31Δ, pik1-139ts, and pik1-139ts ypt31Δ. Arrows indicate bud necks and arrowheads indicate bud tips. (B) Chs3p-GFP transport to the cell surface was monitored by blocking endocytosis with LatA (200 μM, Molecular Probes) or treated with vehicle (dimethyl sulfoxide) as described in Valdivia et al. (2002).
Chitin synthase III (Chs3p), a cell surface enzyme necessary for cell wall assembly, follows a recycling pattern similar to that of Snc1p. Chs3p localizes to the cell surface, to the mother-bud junction, and to intracellular punctate structures, referred to as chitosomes (Valdivia et al., 2002). Because chitosomes resemble early endosomes and Chs3p follows a trafficking pathway similar to Snc1p (Lewis et al., 2000; Valdivia et al., 2002), we next determined whether the transport of Chs3p to the bud neck required Pik1p and/or Ypt31p. Chs3p tagged with GFP at its C-terminus was previously found to be fully functional (Schorr et al., 2001; Valdivia et al., 2002). We examined the localization of Chs3p, chromosomally tagged with GFP (Figure 2B). In 48% of budded wild-type cells, Chs3p-GFP localized to the mother-bud neck. A modest decrease in the targeting of Chs3p-GFP to the bud neck was observed in ypt31Δ cells (39% of budded cells). Chs3p-GFP was localized to the bud neck in only 22% of budded pik1-139ts cells at the permissive temperature (26°C), suggesting that Pik1p/PtdIns(4)P is required for proper Chs3p targeting. Consistent with the defect in GFP-Snc1p localization (Figure 2A), <1% of budded pik1-139ts ypt31Δ double mutant cells (26°C) expressed Chs3p-GFP at the bud neck; Chs3p mostly accumulated intracellularly (Figure 2B). We also monitored the transport of Chs3p-GFP to the plasma membrane by blocking endocytosis with LatA (Figure 2B). Consistent with a defect in Golgi to plasma membrane traffic, Chs3p-GFP remained predominately in intracellular structures in pik1-139ts ypt31Δ double mutant cells treated with LatA (Figure 2B). These results provide evidence for a functional interaction between Pik1p and Ypt31p in Golgi to plasma membrane traffic.
We next examined the localization of other plasma membrane proteins that unlike Snc1p and Chs3p are trafficked to and degraded in the vacuole in response to environmental conditions. Consistent with previous reports (Malinska et al., 2003), the plasma membrane hexose transporter, Hxt1p, localized uniformly at the plasma membrane, with some fluorescence localized to punctate structures and/or the vacuole in wild-type cells (Figure 3A). This pattern of Hxt1p-GFP localization was also observed in ypt31Δ cells, pik1-139ts cells, and pik1-139tsypt31Δ double mutant cells, demonstrating that transport of Hxt1p from the Golgi to the plasma membrane was unaffected (Figure 3A). We also examined the localization of another plasma membrane transporter, the tryptophan permease, Tat2p, chromosomally tagged with GFP to circumvent potential trafficking artifacts due to overexpression. Tat2p-GFP, like Hxt1p-GFP, is expressed uniformly at the plasma membrane with punctate and/or vacuole fluorescence observed in some cells (Umebayashi and Nakano, 2003 and Figure 3A). Under permissive growth conditions, the localization of Tat2p-GFP in ypt31Δ cells, pik1-139ts cells, and pik1-139tsypt31Δ double mutant cells did not change relative to wild-type cells (Figure 3A). Additionally, the processing of several cargo proteins, the soluble vacuolar carboxypeptidase Y (CPY), and a glycophospholipid-anchored surface protein (Gas1p) were unaffected in ypt31Δ cells, pik1-139ts cells, and pik1-139tsypt31Δ double mutant cells relative to wild-type cells (Figure 3B). Collectively, these results provide evidence for a functional interaction between Pik1p and Ypt31p in the specific targeting of proteins that continuously recycle during polarized growth.
Figure 3.
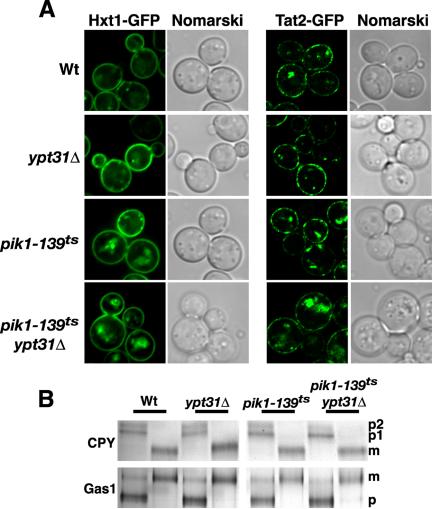
Trafficking competence of pik1-139ts ypt31Δ double mutant cells at permissive temperature. (A) Hxt1p-GFP and Tat2p-GFP distribution at 26°C in Wt, ypt31Δ, pik1-139ts, and pik1-139ts ypt31Δ. (B) Indicated yeast strains (Wt, ypt31Δ, pik1-139ts, and pik1-139ts ypt31Δ) were metabolically labeled with Tran-35S for 10 min, and chased in the presence of excess methionine/cysteine for 30 min at 26°C. CPY and Gas1p were immunoprecipitated from cell lysates, resolved by SDS-PAGE, and visualized by fluorography. p, precursor form; m, mature form.
Pik1p and Ypt31p Function in a Common Set of Trafficking Pathways
If Pik1p and Ypt31p function in similar trafficking pathways, then inactivation of Ypt31p should display similar defects in membrane traffic as the inactivation of Pik1p. To test our hypothesis, we generated a temperature-sensitive mutant of Ypt31p and then compared the phenotypes with those seen during the inactivation of Pik1p, in the same strain background, SEY6210. As previously mentioned, Ypt31p shares a high degree of sequence similarity with the Ypt32p GTPase, and deletion of both genes is lethal, indicating functional redundancy (Benli et al., 1996; Jedd et al., 1997). We therefore generated new temperature-sensitive alleles of YPT31 by first mutating the ORF by random PCR mutagenesis and then integrating, via homologous recombination, the mutated PCR products into the genome of ypt32Δ mutant cells. We isolated two strains that exhibited temperature-sensitive growth. The strain, ypt32Δypt31-101ts that grew most robustly at 26°C was characterized further. ypt32Δypt31-101ts double mutant cells are temperature-sensitive for growth at elevated temperatures (>37°C), which can be fully rescued by the introduction of wild-type YPT31 expressed from a low-copy plasmid (Figure 4A).
Figure 4.
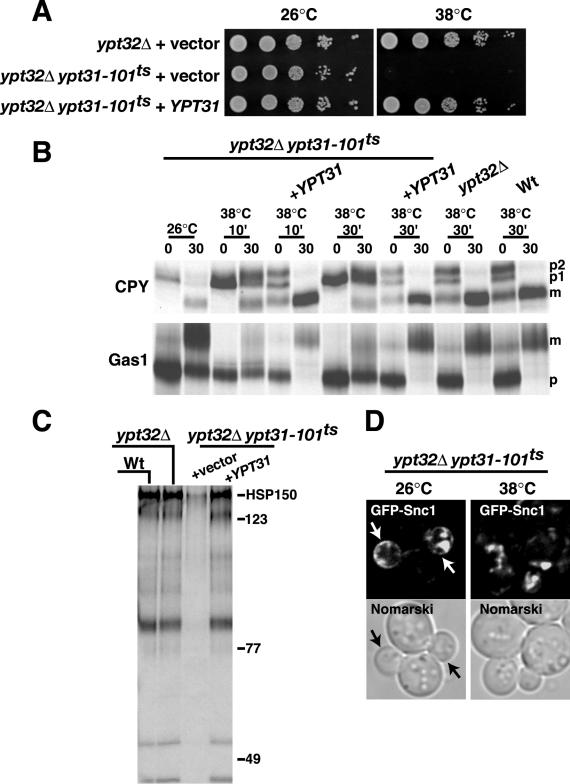
Pik1p and Ypt31p act in similar biological pathways. (A) Temperature-sensitive growth of yeast expressing ypt31-101ts in ypt32Δ background is rescued by expressing low-copy YPT31. (B and C) Wt, ypt32Δ, and ypt32Δypt31-101ts double mutant cells were incubated at the indicated temperature for 10 or 30 min, metabolically labeled with Tran-35S for 10 min, and chased in the presence of excess methionine/cysteine for 30 min. CPY and Gas1p were analyzed as described in the legend to Figure 3. To assay for general secretion competence, cells and media were separated by centrifugation, and proteins secreted into the media during the pulse-chase were visualized by SDS-PAGE and fluorography. (D) ypt32Δypt31-101ts double mutant cells expressing GFP-Snc1p were grown to early/midlog phase, shifted to 26°C or 38°C for 30 min, and observed by fluorescence microscopy.
To further characterize ypt32Δypt31-101ts double mutant cells, we assessed the anterograde secretory transport of CPY and Gas1p. At the permissive temperature, CPY transport to the vacuole was unaffected (Figure 4B). However, after a 10- or 30-min shift to the restrictive temperature (38°C), CPY was predominately in the Golgi-modified form (p2) with minor amounts in the ER-modified (p1) and mature (m) forms. Gas1p acquires a glycophosphoinositol linkage in the ER (p) and is then glycosylated in the Golgi (m) before its transport to the plasma membrane. Although a modest defect is observed at the permissive temperature, shifting of ypt32Δypt31-101ts double mutant cells to the nonpermissive temperature (38°C, for 10 or 30 min) resulted in the accumulation of the ER-modified form (p) of Gas1p (Figure 4B). Expression of YPT31 in the ypt32Δypt31-101ts double mutant cells resulted in full suppression of CPY and Gas1p trafficking defects (Figure 4B). We also examined general secretion competence of ypt32Δypt31-101ts double mutant cells, by taking advantage of the fact that only a few yeast proteins are efficiently secreted into the growth medium (Robinson et al., 1988). As expected, at the nonpermissive temperature, ypt32Δypt31-101ts double mutant cells exhibited a near complete block in protein secretion into the medium, whereas double mutant cells expressing wild-type Ypt31p exhibited a normal pattern of protein secretion (Figure 4C). ypt32Δypt31-101ts double mutant cells at the nonpermissive temperature (38°C for 30 min) also accumulated GFP-Snc1p intracellularly, whereas ypt32Δypt31-101ts double mutant cells at the permissive temperature had only a partial defect in the polarized membrane localization of GFP-Snc1p (Figure 4D). These results reinforce the requirement of a functional Golgi for efficient Snc1p recycling. Collectively, the Golgi transport phenotypes observed upon Ypt31/32p inactivation resemble those observed when Pik1p is inactivated (Audhya et al., 2000), suggesting that these proteins are involved in similar biological functions at the Golgi, consistent with the genetic interactions identified by our SGA analysis.
Ypt31/32 Does Not Regulate PtdIns(4)P Synthesis or Pik1p Localization
Because our genetic and phenotypic analysis suggests that Ypt31p and Pik1p functionally interact in specific biological pathways, it is possible that Ypt31p acts upstream of Pik1p by stimulating the kinase activity of Pik1p and/or by recruiting Pik1p to the Golgi. We next determined the levels of PtdIns(4)P under conditions where Ypt31/32p is transiently inactivated. ypt32Δypt31-101ts double mutant cells were grown at the permissive temperature (26°C) and then shifted to the nonpermissive temperature (38°C) for 10 min before labeling with myo-[3H]inositol for an additional 45 min at 38°C. Subsequent analysis of [3H]inositol–labeled lipids revealed no significant changes in the amounts of PtdIns(4)P at the nonpermissive temperature (Figure 5). This result suggests that the inactivation of Ypt31/32p does not alter production of PtdIns(4)P and therefore does not appear to regulate PtdIns 4-kinase activity in cells. Additionally, overexpression of YPT31 or a GTPase-deficient mutant, ypt31 Q72L, does not restore the temperature-sensitive growth defect of pik1ts cells, nor does it affect PtdIns(4)P levels in wild-type or ypt31Δ cells (unpublished data). A 4.5-fold increase in PtdIns(3,5)P2 was observed (SEY6210; 0.12 ± 0.02%; ypt32Δ; 0.16 ± 0.03%; ypt32Δypt31-101ts; 0.54 ± 0.11% of PtdIns labeled), and concurrently the vacuoles in ypt32Δypt31-101ts double mutant cells are highly fragmented (unpublished data), consistent with the role for PtdIns(3,5)P2 in vacuolar membrane morphology (Rudge et al., 2004). The levels of PtdIns(4,5)P2 are significantly reduced by 2.8-fold (SEY6210; 2.13 ± 0.18%; ypt32Δ; 2.39 ± 0.04%; ypt32Δypt31-101ts; 0.77 ± 0.18% of PtdIns labeled) at the nonpermissive temperature (38°C). The underlying basis of the elevated PtdIns(3,5)P2 levels and the reduced PtdIns(4,5)P2 levels is not clear. One possibility is that the observed accumulation of Golgi membranes and inappropriate sorting of several proteins may indirectly affect the distribution of the kinases and phosphatases involved in maintaining the levels of PtdIns(3,5)P2 and PtdIns(4,5)P2 in ypt32Δypt31-101ts double mutant cells.
Figure 5.
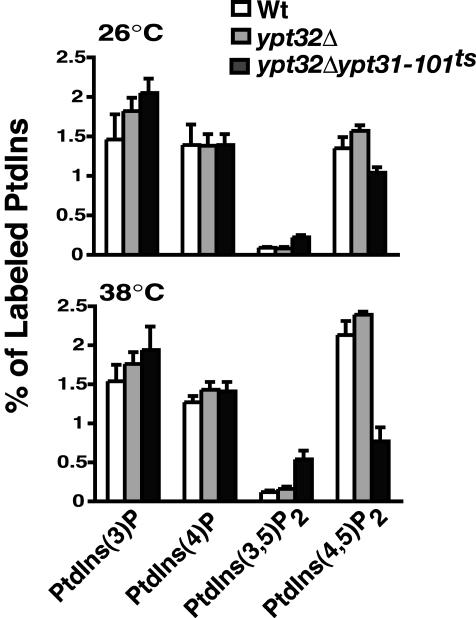
Phosphoinositide levels in Wt, ypt32Δ, and ypt32Δypt31-101ts double mutant cells. Cells were incubated at the temperature indicated for 10 min and labeled with myo-[2-3H]inositol for 45 min, and phosphoinositides were analyzed as previously described in Rudge et al. (2004). The levels of each indicated phosphoinositide are expressed as a percentage of the total 3H-labeled phosphoinositides analyzed by HPLC and represent the average of two independent experiments done in duplicate (n = 4).
We next epitope-tagged Pik1p with GFP to observe the cellular localization of GFP-Pik1p under conditions where Ypt31p is transiently inactivated. Haploid pik1Δ cells expressing GFP-Pik1p from a low-copy plasmid (as the only source of PIK1) were generated, indicating that GFP-Pik1p is functional. In wild-type backgrounds, GFP-Pik1p fluorescence localizes predominantly to concentrated puncta that colocalized with a Golgi marker protein, Sec7p (Figure 6A). Our observed localization of GFP-Pik1p is consistent with the primary role of Pik1p in Golgi structure and function. In ypt32Δypt31-101ts mutant background at both the permissive and nonpermissive temperatures, GFP-Pik1p remained associated with punctate structures (Figure 6A), demonstrating that a functional Ypt31/32p is not required for Pik1p to associate with intracellular membranes. Because overexpression of Sec7p in ypt32Δypt31-101ts double mutant cells has adverse effects on growth (Jones et al., 1999), we were unable to determine whether the fluorescent puncta of GFP-Pik1p and Sec7p-DsRED colocalized. These data suggest that Ypt31p does not function as an upstream regulator of Pik1p kinase activity or localization.
Figure 6.
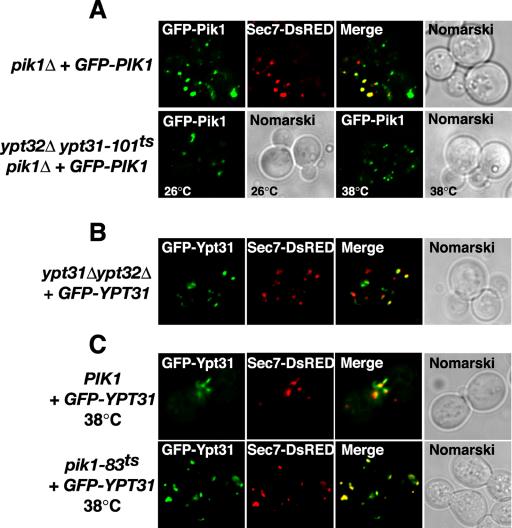
Independent localization of Pik1p and Ypt31p to the trans-Golgi network. (A) pik1Δ cells expressing GFP-Pik1p and Sec7p-DsRed were examined by fluorescence microscopy at 26°C. ypt32Δypt31-101tspik1Δ cells expressing GFP-Pik1p were grown at 26°C or shifted for 1 h at 38°C and examined by fluorescence microscopy. (B) ypt31Δ ypt32Δ cells expressing GFP-Ypt31p and Sec7p-DsRed were grown at 26°C and examined by fluorescence microscopy. (C) Wild-type (PIK1), pik1–83ts, or pik1-139ts (unpublished data) cells expressing GFP-YPT31 from a low copy plasmid and Sec7p-DsRed were grown at 26°C (unpublished data) or shifted 1 h at 38°C and examined by fluorescence microscopy.
Ypt31p Colocalizes with Sec7p at the trans-Golgi
Ypt31/32p has been reported to have a punctate distribution like that of the Golgi (Benli et al., 1996; Jedd et al., 1997), and we confirmed this using a functional GFP-tagged version of Ypt31p (Figure 6B). Furthermore, we extended this observation by demonstrating that GFP-Ypt31p localization was concentrated to Sec7p-containing compartments, indicative of trans-Golgi localization (Figure 6B). Similar to a previous report (Jedd et al., 1997), GFP-Ypt31p fluorescence appeared to have a polarized distribution, being concentrated at the emerging bud or small bud tip in rapidly dividing cells (unpublished data). This localization pattern of Ypt31p is most consistent with the observations that late Golgi can preferentially localize to sites of polarized growth (Rossanese et al., 2001).
We next investigated whether the localization of GFP-Ypt31p to the Golgi is mediated by Pik1p-generated PtdIns(4)P. When GFP-Ypt31p is expressed in yeast with a temperature-sensitive allele of pik1, incubation of cells at a nonpermissive temperature, which causes a substantial reduction in PtdIns(4)P (Audhya et al., 2000 and this study) did not affect the localization of GFP-Ypt31p at Sec7p-enriched puncta compared with that of wild-type PIK1 control cells (Figure 6C). These data indicate that Ypt31p does not require Pik1p kinase activity for normal membrane recruitment. We did note that the mobility of Sec7p/Ypt31p-positive compartments was reduced in pik1–83ts cells when shifted to the nonpermissive temperature, and as a result of the reduced membrane dynamics, we were able to observe significant colocalization of GFP-Ypt31p fluorescence to Sec7p-labeled Golgi (Figure 6B). Taken together, the above results are consistent with a coordinated function of Ypt31p and Pik1p at a common membrane compartment, the trans-Golgi.
TRAPPII Functions Upstream of Ypt31
Previous studies have demonstrated that overexpression of YPT31 can suppress the lethality of trs130, which encodes an essential TRAPPII component (Yamamoto and Jigami, 2002; Zhang et al., 2002), suggesting a functional relationship between TRAPPII and Ypt31p. TRS33 and KRE11 are nonessential subunits of the TRAPPII complex and were identified in our SGA analysis as having a functional interaction with pik1. To provide additional evidence that TRAPPII functions in Ypt31p signaling, we took advantage of our observation that deletion of KRE11 in trs33Δ cells was lethal and tested whether the lethality of kre11Δ trs33Δ double mutants could be suppressed by the overproduction of YPT31. Haploid trs33Δ and kre11Δ single deletion mutants of opposite mating types, each expressing YPT31 were mated and sporulated and tetrads were isolated. As shown in Figure 7A, kre11Δ trs33Δ double mutants were never isolated. We next made a point mutation in YPT31 to mimic the active, GTP-bound state (Walworth et al., 1989) and expressed this mutant, ypt31 Q72L, in both haploid trs33Δ and kre11Δ single deletion mutants. Surprisingly, kre11Δ trs33Δ double mutants were now isolated only when ypt31 Q72L was overexpressed (Figure 7A). Consistent with previous findings (Zhang et al., 2002), overexpression of YPT31 in a trs130Δ (and to a lesser extent trs120Δ) deletion strains rescued the lethality in the SEY6210 background (Figure 7A). However, we found that trs120Δ deletion mutants obtained from trs120Δ heterozygotes grew better when YPT31 was overexpressed in its GTP-locked form. Our data demonstrate that activated Ypt31p suppresses the mutant growth phenotypes exhibited by trs130Δ mutants, trs120Δ mutants, and kre11Δ trs33Δ double mutants.
Figure 7.
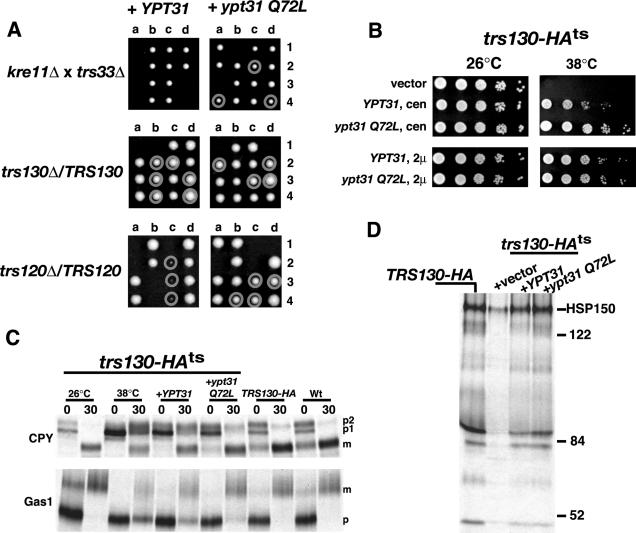
GTP-dependent suppression of TRAPPII lethality and trafficking defects by YPT31. (A) Tetrad analysis of diploid strains were transformed with high-copy wild-type YPT31, or ypt31 Q72L, sporulated, and dissected on YPD plates at 26°C. Haploid cells with deleted gene(s) carrying either YPT31 or ypt31 Q72L are circled (confirmed by PCR and growth on selection media). (B) trs130-HAts (33 aa deleted from C-terminus of TRS130, and tagged with HA) was examined for temperature-sensitive growth when expressing low (cen) or high (2 μ) copies of YPT31 or ypt31 Q72L. (C) Wt, TRS130-HA, or trs130-HAts cells expressing vector, YPT31 or ypt31 Q72L, were incubated at 26 or 38°C for 30 min, metabolically labeled with Tran-35S for 10 min, and chased in the presence of excess methionine/cysteine for 30 min. CPY and GAS1p were analyzed as described in the legend to Figure 3. (D) General secretion competence was analyzed as described in the legend to Figure 4.
To characterize the genetic interaction of YPT31 and TRS130 further, we constructed a temperature-sensitive mutant of TRS130 by deleting the 33 amino acids from the C terminus as described by Sacher et al. (2001). Temperature-sensitive growth of trs130-HAts mutant cells could be suppressed when cells expressed either wild-type or GTP-locked forms of YPT31, similar to the suppression observed in the trs130Δ deletion mutants (Figure 7B). We next examined whether the expression of YPT31 could suppress the trafficking defects associated with inactivation of TRAPPII. In trs130-HAts mutant cells shifted to the restrictive temperature, the majority of CPY was detected as a mixture of p1, p2, and mature forms, indicative of a defect in CPY trafficking out of the Golgi. Similarly, trs130-HAts mutant cells exhibited a defect in the processing of Gas1p at the nonpermissive temperature. Expression of YPT31 from a low-copy plasmid, which partially suppressed the growth phenotype (Figure 7B), resulted in partial suppression of the CPY and Gas1p trafficking defect (Figure 7C). However, expression of ypt31 Q72L from a low-copy plasmid, which suppresses the growth phenotype (Figure 7B), resulted in near complete suppression of the CPY and Gas1p trafficking defect (Figure 7C). Lastly, trs130-HAts mutant cells at the nonpermissive temperature exhibit a strong block in protein secretion, whereas trs130-HAts mutant cells expressing wild-type YPT31 or ypt31 Q72L secreted proteins into the medium, similar to that of wild-type cells (Figure 7D). Moreover, expression of a nucleotide-free mutant of YPT31, ypt31 N126I was lethal when expressed in trs130-HAts mutant cells (unpublished data). Collectively, these results support a role for TRAPPII functioning upstream of Ypt31p, potentially participating in the activation of Ypt31p.
Gyp2p Functions as a GAP for Ypt31p In Vivo
To provide additional evidence that TRAPPII functions upstream of Ypt31p, we reasoned that deletion of a specific rab-GAP would prolong the activated state of Ypt31p and thereby shift endogenous Ypt31p to an activated, GTP-bound state, analogous to the expression of ypt31 Q72L, which mimics the active, GTP-bound state (Figure 7). To date, a GAP for Ypt31p has not been identified. However, a similar approach was successful in identifying Gyp1p, a cis-Golgi localized protein, as the GAP for Ypt1p (Du and Novick, 2001), a rab-GTPase functioning in ER to Golgi transport. Deletion of either GYP1 or a related homologue, GYP2, in wild-type cells did not have any effect on growth or trafficking (Figure 8A and unpublished data). Deletion of the GYP1 in the trs130-HAts mutant cells failed to suppress the growth defect at the nonpermissive temperature (Figure 8A), suggesting that Gyp1p does not exert significant GAP activity toward Ypt31p, which is consistent with in vitro studies (Albert et al., 1999). It has been documented that Gyp2p has relatively low GAP activity toward Ypt31p in vitro (Albert and Gallwitz, 1999). Surprisingly, deletion of GYP2 in trs130-HAts mutant cells restored growth at the nonpermissive temperature of 37°C (Figure 8A) and partially at 38°C (unpublished data). Consistent with the restoration of growth at 37°C, deletion of GYP2 also suppressed the trafficking defects (CPY, Gas1p, and HSP150 secretion) associated with the loss of Trs130p function (unpublished data).
Figure 8.
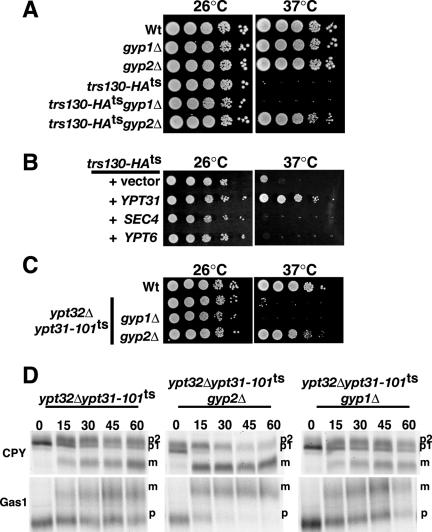
Gyp2p is a GAP for Ypt31p in vivo. (A) gyp2 deletion suppresses the temperature-sensitive growth of trs130-HAts. (B) SEC4 and YPT6 (high-copy) do not suppress the temperature-sensitive growth defect of trs130-HAts. (C) gyp2 deletion suppresses the temperature-sensitive growth of ypt32Δypt31-101ts double mutant cells. (D) gyp2 deletion specifically rescues trafficking defects of ypt32Δypt31-101ts double mutant cells. ypt32Δypt31-101ts, ypt32Δypt31-101ts gyp1Δ, and ypt32Δypt31-101tsgyp2Δ were incubated at 37°C for 30 min, metabolically labeled with Tran-35S for 10 min, and chased in the presence of excess methionine/cysteine for the indicated minutes. CPY and Gas1p were analyzed as described in the legend to Figure 3.
It has been previously demonstrated that Gyp2p has high in vitro GAP activity toward Sec4p (which regulates the traffic and fusion of post-Golgi vesicles to the plasma membrane; Salminen and Novick, 1987) and Ypt6p (which regulates the fusion of late endocytic vesicles to the trans-Golgi; Siniossoglou and Pelham, 2001). Therefore, in order to eliminate the possibility that the suppression observed in gyp2Δ trs130-HAts double mutant cells is due to the activity of Gyp2p toward Sec4p and/or Ypt6p, we determined whether the overexpression of either SEC4 or YPT6 could suppress the temperature-sensitive growth of trs130-HAts mutant cells. However, no suppression of the trs130-HAts mutant cells was observed when the wild-type or GTPase-deficient forms of SEC4 or YPT6 were expressed (Figure 8B and unpublished data). Our results suggest that Gyp2p functions as a physiologically relevant GAP for Ypt31p and that GAP activities measured in vitro may not reveal a true in vivo rab target.
Having demonstrated a role for Gyp2p as a potential GAP for Ypt31p in vivo, we rationalized that deletion of GYP2 might also suppress the growth and trafficking defects of ypt32Δypt31-101ts double mutant cells. As anticipated, deletion of GYP2, but not GYP1, suppressed the growth defect of ypt32Δypt31-101ts double mutant cells fully at 37°C (Figure 8C) and partially at 38°C (unpublished data). More significantly, deletion of GYP2 and not GYP1, suppressed the defects of CPY and Gas1p processing in ypt32Δypt31-101ts double mutant cells grown at 37°C (Figure 8D). This result demonstrates that the deletion of GYP2 can alleviate the trafficking defects associated with loss of Ypt31p-dependent Golgi function.
GYP2, Conserved Member of GRAM-Domain Family of rab-GAPs
Most of the known rab GAPs share a region of homology, referred to as the TBC (Tre2, Bub2, Cdc16) domain (Neuwald, 1997), encoding the core catalytic machinery for GTPase activity (Albert et al., 1999). The S. cerevisiae genome contains 11 genes encoding TBC/rab-GAP domain proteins. Compared with the other rab-GAP domain-containing proteins in yeast, Gyp2p is the only rab-GAP recognized to contain any additional protein motifs. In addition to a TBC/rab-GAP domain, protein motif search using SMART (Schultz et al., 2000) revealed a GRAM (Glucosyltransferases, Rab-like GTPase activators, and Myotubularian) domain and an EfHand within Gyp2p (Figure 9A). Additional database searches revealed that the domain organization of Gyp2p is evolutionarily conserved, with putative orthologues in human (VRP and KIAA0676), Drosophila (CG7324) and Caenorhabditis elegans (Y45F10A.6; Figure 9A). This suggests that Gyp2p may represent a member of a unique set of conserved rab-GAPs.
Figure 9.
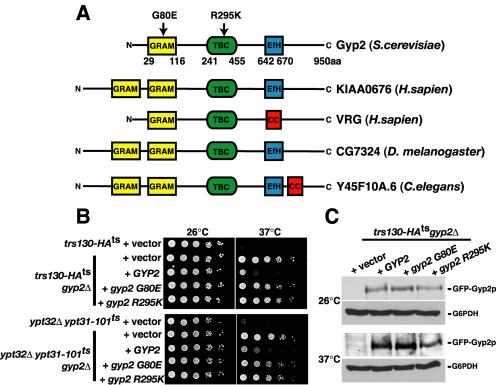
Gyp2p requires its GRAM domain and GAP activity for function in cells. (A) Schematic representation of the conserved domains in Gyp2 family in human, Drosophila, C. elegans, and yeast. EfH, EfHand; cc, coiled-coil. (B) Serial dilution of yeast cells expressing wild-type or mutants of GYP2 from low-copy plasmids at 26 and 37°C. (C) trs130-HAts gyp2Δ cells expressing GFP-GYP2, GFP-gyp2 G80E, or GFP-gyp2 R295K from low-copy plasmids were grown at 26°C and shifted to 37°C for 1 h, and total cellular protein content were TCA-precipitated and resolved by Western blotting. Glucose 6-phosphate dehydrogenase (G6PDH) was examined as a loading control.
To determine the in vivo functionality of the domains in Gyp2p, we developed an assay system based on our observation that deletion of GYP2 restored the growth of the trs130-HAts and ypt32Δypt31-101ts double mutant cells at 37°C (Figure 8). Suppression of the temperature-sensitive growth of the trs130-HAts and ypt32Δypt31-101ts double mutant cells conferred by deleting GYP2 is abrogated by the expression of wild-type GYP2 from a low-copy plasmid (Figure 9B), demonstrating that GYP2 expresses a functional protein and reinforces Gyp2p as a negative regulator of Ypt31p. To confirm that the GAP activity of Gyp2p is required for its in vivo function, we mutated the invariable arginine residue in the catalytic TBC/rabGAP domain (R295) that is predicted to be essential for GAP activity (Albert et al., 1999). Expression of gyp2 R295K from a low copy plasmid in the gyp2Δ trs130-HAts or gyp2Δ ypt32Δypt31-101ts mutant cells, failed to restore Gyp2p GAP function as shown by the ability of these mutants to grow at the elevated temperature (Figure 9B). Because the mutated protein, gyp2 R295K is stably expressed (Figure 9C), these results provide evidence that the GAP activity of Gyp2p is required to regulate Ypt31p function.
The GRAM domain is a protein module of ∼70 amino acids that has been predicted to function as a protein- or lipid-binding motif (Doerks et al., 2000). In the sequences identified so far, an invariable glycine residue in the GRAM domain (G80 in Gyp2p) has been proposed to be essential for function (Doerks et al., 2000). When gyp2 G80E is expressed in gyp2Δ trs130-HAts or gyp2Δ ypt32Δypt31-101ts mutant cells, they were still able to grow at the elevated temperature (Figure 9B), demonstrating that an intact GRAM domain is required for Gyp2p regulation of Ypt31p. Mutation of this residue (G80E), while not affecting the stability of Gyp2p (Figure 9C), impaired the Ypt31p-GTPase activating function of Gyp2p. The mechanism by which the GRAM motif participates in the GAP function of Gyp2p is presently unclear. Our data do not preclude the possibility that the GRAM motif transiently interacts with a protein or lipid to regulate Gyp2p function (see Discussion).
DISCUSSION
A Role for Pik1p in Vesicular Transport
Previous work (Hama et al., 1999; Walch-Solimena and Novick, 1999; Audhya et al., 2000) suggested that Pik1p-generated PtdIns(4)P plays a direct role in the regulation of Golgi traffic and function. To date, the only identified component of a Pik1p-dependent signaling pathway in yeast is Frq1p, a calcium-binding frequenin homologue that directly activates Pik1p catalytic activity in vitro (Hendricks et al., 1999). It is currently unclear whether Frq1p regulates the pool of PtdIns(4)P that is relevant to vesicular trafficking (Hendricks et al., 1999). To identify additional signaling pathway components of Pik1p, particularly those that function together with Pik1p in Golgi-mediated vesicle trafficking, an SGA analysis utilizing a temperature-conditional pik1 mutant was undertaken.
In this study, we report the first genome-wide SGA analysis of pik1. This approach identified genetic interactions unique to pik1, which both confirm an essential role for Pik1p in Golgi-dependent trafficking and suggest a mechanistic involvement for Pik1p with a specific subset of Golgi-associated proteins, including the Ypt31p rab-GTPase, the multicomponent TRAPPII protein complex, and the aminophospholipid translocase, Drs2p. We further show that Pik1p and Ypt31p coordinately regulate traffic at the trans-Golgi, and that Ypt31p functions downstream of multiple TRAPPII components. On the basis of these genetic relationships, we were able to further elucidate the Ypt31p signaling pathway by identifying Gyp2p as a likely GAP for Ypt31p. From our SGA and phenotypic analysis, we infer that a complex spatial and temporal interplay among Pik1p, Ypt31p, TRAPPII and possibly additional trafficking components converge to regulate Golgi secretory function.
Pik1p and Ypt31p Function Synergistically in Regulating Golgi Structure and Secretory Function
EM analysis demonstrated that pik1-139ts (38°C), ypt32Δypt31-101ts (38°C), and pik1-139ts ypt31Δ double mutant cells (26°C) resulted in severe structural abnormalities of the Golgi (our unpublished results). These results suggest that the concerted action of Pik1p and Ypt31p was required to maintain the structural integrity and the unique membrane identity of the Golgi. Consistent with a primary role in Golgi function, we demonstrate that both Pik1p and Ypt31p colocalize to Sec7p-enriched puncta, indicative of trans-Golgi localization. Although the Golgi in pik1-139tsypt31Δ double mutant cells remains remarkably competent in the processing of several cargoes (CPY, Gas1p) and the traffic of several plasma membrane resident proteins (Tat2p, Hxt1p), we found that the collaborative function of Pik1p and Ypt31p was required for the targeting of two proteins, the yeast SNARE Snc1p and the cell wall enzyme Chs3p to sites of polarized growth and membrane fusion (i.e., growing bud or septum). Snc1p and Chs3p are distinct from other transport cargoes in yeast, as they require continual recycling via early endosomes to maintain their proper subcellular localization (Lewis et al., 2000; Valdivia et al., 2002). Hence, the molecular components involved in the efficient recycling of Snc1p and Chs3p are starting to emerge, and our data suggest that Pik1p and Ypt31p activities are particularly important for this trafficking pathway (Figure 10A).
Figure 10.
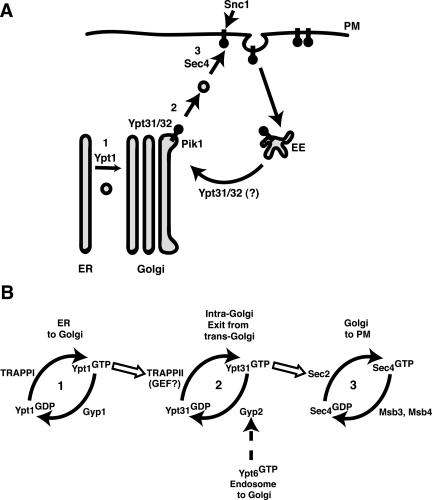
(A) Snc1p trafficking to the plasma membrane (PM) in yeast requires Golgi and early endosome (EE) function. Inactivation of Pik1p or Ypt31p causes Snc1p to accumulate intracellularly. We propose that the primary role for Pik1p/PtdIns(4)P is in maintaining the structural integrity and molecular identity of the trans-Golgi and that unidentified multivalent adaptor proteins may be recruited by Pik1p/PtdIns(4)P and/or activated Ypt31/32p for the coordination of cargo-loading, budding, and/or fission at the trans-Golgi. Alternatively, Ypt31/32p may also participate in retrograde traffic of recycled cargoes by regulating early endosome-to-Golgi transport. (B) The orderly cascade of yeast exocytic rab-GTPases. The cis-Golgi rab-GTPase, Ypt1p and the multicomponent complex, TRAPPII act upstream to the trans-Golgi rab-GTPase, Ypt31p, possibly leading to its activation. Activated, GTP-bound Ypt31p interacts with Sec2p, the GEF for Sec4p (Ortiz et al., 2002), whereas activated-Ypt6p may recruit Gyp2p (Siniossoglou and Pelham, 2001), which inactivates Ypt31p, allowing the rab-GTPase cycle of activation/inactivation at the Golgi to continue.
The observed genetic interaction between pik1 and ypt31 is indicative of an involvement of Pik1p and Ypt31p in a common signaling pathway that facilitates the transport function of the Golgi. The molecular basis of this genetic relationship is currently unclear. The simplest, putative explanation for the mis-localization of secretory cargoes in pik1-139tsypt31Δ double mutant cells is that the simultaneous perturbation of Pik1p and Ypt31p function resulted in severe defects in the generation and/or loading of cargoes into Golgi-derived vesicles (Figure 10A). It is conceivable that Pik1p and Ypt31p cooperatively recruit as yet unrecognized accessory proteins to the Golgi that efficiently package cargo into exocytic vesicles. It should be noted that no PH domain containing proteins (Yu et al., 2004) and in addition no obvious candidate coat proteins that could drive cargo selection and secretory vesicle formation were identified in our SGA analysis. Another possible Pik1p/Ypt31p function stems from observations that some effectors of rab-GTPases interact with the SNARE machinery in ensuring the specificity and efficiency of vesicle fusion. For example, the Vps21p/Ypt51p rab-GTPase effector, Vac1p, associates with the t-SNARE, Pep12p and the Sec1 homolog, Vps45p (Peterson et al., 1999). It is interesting to note that Vac1p also binds PtdIns(3)P, a phosphoinositide mediator of Golgi-to-vacuole transport (Burd and Emr, 1998). These studies suggest that through common effectors, rab-GTPases and phosphoinositides can coordinately function to ensure specificity of vesicular transport reactions
Recently, it was reported that FAPPs (glycolipid transfer proteins) interact with PtdIns(4)P and Arf through their PH domain necessary for their function at the Golgi in mammalian cells (Godi et al., 2004). However, there are no true yeast homologues for the FAPPs, but a related family of proteins, the oxysterol-binding proteins (OSBPs), which also contain PtdIns(4)P-interacting PH domains, require both PtdIns(4)P and Arf1p for their Golgi localization (Levine and Munro, 2002). Genes encoding the yeast OSBPs were not isolated in our SGA analysis and furthermore, deletion of all three yeast homologues did not affect viability nor Golgi morphology (Levine and Munro, 2001). At this time, it is not clear if our SGA analysis uncovered true effectors of Pik1p-generated PtdIns(4)P. It is likely that multiple effectors for PtdIns(4)P exist, possibly including effectors containing as yet unrecognized PtdIns(4)P-binding motifs or that are essential for viability (thereby absent from the array), contributing to the complexity of PtdIns(4)P signaling. These effectors may also be recognized by other Golgi-determinants, such as by Ypt31p or Arf1p that collectively contribute to the maintenance of Golgi structure and function. It is of interest to note that ARF was also identified in our SGA analysis to functionally interact with PIK1 and it also interacts with YPT31, DRS2, and TRS130 (see Figure 1A).
Another interpretation of our results invokes a model whereby Pik1p and Ypt31p physically interact. This hypothesis is supported by a recent report that the mammalian Pik1p homolog, PI4KIIIβ, associates with the GTP-bound form of the mammalian Ypt31p homolog, rab11, implying a role for PI4KIIIβ/Pik1p as an effector of rab11/Ypt31p (de Graaf et al., 2004). However, our data do not support this interpretation because the inactivation of Ypt31p did not alter the localization of Pik1p or influence PtdIns(4)P levels in vivo (Figures 5 and 6). Additionally, in contrast to de Graaf et al. (2004), we did not detect an interaction between Pik1p and Ypt31p using two-hybrid analysis (Liang and Segev, unpublished results). Collectively, our work and the findings of deGraff et al. (2004) favor a close relationship between Pik1p and Ypt31p. Further study will be necessary to mechanistically define the Pik1p/Ypt31p interaction.
TRAPPII in Ypt31 Signaling at the Golgi
Recent studies have suggested that phosphoinositides regulate the activity of small GTP-binding proteins by interacting with specific guanine-nucleotide exchange factors (GEFs) and/or GTPase activating proteins (GAPs); factors that control rab cycling between GTP-(active) and GDP-(inactive) bound forms. For instance, Mss4p-generated PtdIns(4,5)P2 is required for the plasma membrane recruitment of Rom2p, a Rho1-GEF, and the activation of Rho1 (Audhya and Emr, 2002). Based on these models, we cannot overlook the possibility that Pik1-generated PtdIns(4)P regulates the GEF and/or GAP activities specific toward Ypt31/32p. It has been suggested that a TRAPPII complex component(s) functions as a GEF for Ypt31p (Jones et al., 2000). GEF activity toward Ypt31p by an individual component of the TRAPPII complex has yet to be demonstrated (Wang and Ferro-Novick, 2002). Consistent with previous reports (Zhang et al., 2002), we found that overexpression of the GTPase-deficient form of YPT31, but not the activated mutants of other rab-GTPases, can compensate for the loss of TRAPPII function (see Figure 7). This suggests that the TRAPPII components identified in our SGA analysis of pik1 act upstream of Ypt31p. However, the precise biochemical function of TRAPPII (and its individual components) in Ypt31p signaling is unclear.
GYP2 Encodes a Biologically Relevant Ypt31p GAP
Before this study, the GAP or other downstream regulators for Ypt31p had not been identified. It has been suggested that Gyp2p interacts specifically with the GTP-bound form of Ypt6p (Siniossoglou and Pelham, 2001), a rab GTPase required for retrograde transport from the endosome to the Golgi, and that Gyp2p has GTPase activity toward Ypt6p in vitro (Albert et al., 1999). It was thus inferred that Gyp2p functions as a GAP for Ypt6p in vivo. However, overexpression of YPT6 (Bensen et al., 2001) but not the deletion of GYP2 (Lafourcade et al., 2003) suppressed the recycling defects associated with ric1 and rgp1 mutants, components of the heterodimeric GEF for Ypt6p. These results suggest that deletion of GYP2 did not significantly shift Ypt6p to its active, GTP-bound form in vivo. Our observations complement these data because we demonstrate that the deletion of GYP2 can suppress the lethality and trafficking defects associated with temperature-sensitive alleles of YPT31 and TRS130, suggesting that Gyp2p functions as a GAP for Ypt31p in vivo.
Gyp2p is the only known rab-GAP protein in yeast that contains the GRAM domain, a newly recognized protein motif (Doerks et al., 2000). Based on the crystal structure of the GRAM domain-containing phosphoinositide phosphatase, MTMR2, the GRAM domain contains five beta strands that are part of a larger structural motif that resembles a PH domain (Begley et al., 2003). In common with the phosphoinositide-binding activity of the PH domain (Yu et al., 2004), recent reports have suggested that the GRAM domain also binds phosphoinositides (Berger et al., 2003). The GRAM domain of MTMR2 bound PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2, and PtdIns(3,4,5)P3 and was required for MTMR2 correct subcellular targeting (Berger et al., 2003). The phosphoinositide binding potential of the GRAM domain has become controversial, however, because another report did not detect significant binding using similar assay systems (Begley et al., 2003). We find that the GRAM domain of Gyp2p bound mono-phosphoinositides (PtdIns(3)P, PtdIns(4)P, and PtdIns(5)P) using a similar protein-lipid overlay assay; however, mutations in the GRAM domain, which renders Gyp2p dysfunctional (Figure 9), did not significantly alter the cytoplasmic localization of the full-length protein (our unpublished results). Gyp2p, via its GRAM domain, therefore has the potential to couple phosphoinositides to the regulation of Ypt31p rab-GTPase activity.
Novick and colleagues recently proposed a rab-GTPase protein cascade in the regulation of yeast exocytosis, by which individual transport steps are functionally linked with each rab-GTPase and the corresponding regulators of each rab-GTPase in the cascade (Ortiz et al., 2002). As illustrated in Figure 10, genetic and phenotypic analysis previously demonstrated that 1) the cis-Golgi associated Ypt1p rab-GTPase (Segev et al., 1988) acts upstream of 2) the trans-Golgi-associated Ypt31/32p rab-GTPase (Figure 6B), both of which act upstream of 3) Sec4p, a rab-GTPase associated with post-Golgi–derived secretory vesicles (Salminen and Novick, 1987). The orderly regulation of rab-GTPase activity would then ensure the sequential completion of transport steps necessary for proper transport through the Golgi (Figure 10B). Likewise, recycling back to the trans-Golgi must be coupled with exit from the trans-Golgi in order to maintain its structure and function. Consistent with a rab-GTPase cascade of sequential activation events (Ortiz et al., 2002), we propose that multiple, stage-specific signals impinge upon each rab-GTPase to regulate the rab activation/inactivation cycle. In the case of Ypt31p, our data suggest that these signaling interactions are likely to include the activities of the Pik1p PtdIns 4-kinase, the TRAPPII complex and the inactivating function of the Gyp2p GAP.
Supplementary Material
Acknowledgments
We thank R. Collins, G. Payne, P. Brenwald, H. Pelham, and W. Tanner for generously providing plasmids; C. Stefan for yeast strain CJSY298; and R. Schekman for Gas1 antibody. We thank Ingrid Niesman for assisting with the electron microscopic analysis (Immunoelectron Microscopy Core B of Program Project Grant CA58689 headed by M. Farquhar) and Perla Arcaira for technical assistance. We also thank J. Thorner and T. Graham for many helpful suggestions and for communicating unpublished data. We are indebted to S. Rudge and A. Wurmser for useful discussion and critical reading of the manuscript. SD.E. is supported as an Investigator of the Howard Hughes Medical Institute.
Article published online ahead of print in MBC in Press on December 1, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0700).
Abbreviations used: CPY, carboxypeptidase Y; GAP, GTPase-activating protein; GFP, green fluorescent protein; PtdIns(4)P, phosphatidylinositol 4-phosphate; PtdIns(4,5)P2, phosphatidylinositol 4,5 bisphosphate; PH, pleckstrin homology; TRAPP, transport protein particle.
The online version of this article contains supplemental material on MBC Online (http://www.molbiolcell.org).
References
- Albert, S., and Gallwitz, D. (1999). Two new members of a family of Ypt/Rab GTPase activating proteins. J. Biol. Chem. 274, 33186-33189. [DOI] [PubMed] [Google Scholar]
- Albert, S., Will, E., and Gallwitz, D. (1999). Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J. 18, 5216-5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya, A., Foti, M., and Emr, S. D. (2000). Distinct roles for the yeast phosphoinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya, A., and Emr, S. D. (2002). Stt4 PI4-kinase localizes to the plasma membrane and functions I the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593-605. [DOI] [PubMed] [Google Scholar]
- Begley, M. J., Taylor, G. S., Kim, S.-A., Veine, D. M., Dixon, J. E., and Stuckey, J. A. (2003). Crystal structure of a phosphoinsitide phosphatase, MTMR 2, insights into myotubular myopathy and Charcot-Marie Tooth Syndrome. Mol. Cell 12, 1391-1402. [DOI] [PubMed] [Google Scholar]
- Benli, M., Doring, F., Robinson, D. G., Yang, X., and Gallwitz, D. (1996). Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 15, 6460-6475. [PMC free article] [PubMed] [Google Scholar]
- Bensen, E. S., Yeung, B. G., and Payne, G. S. (2001). Ric1p and the Ypt6p GTPase function in a common pathway required for location of trans-Golgi network membrane proteins. Mol. Biol. Cell 12, 13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, P., Schaffitzel, C., Berger, I., Ban, N., and Suter, U. (2003). Membrane association of myotubularian-related protein 2 is mediated by a pleckstrin homology GRAM domain and a coiled-coil dimerization module. Proc. Natl. Acad. Sci. USA 100, 12177-12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, C., and Emr, S. D. (1998). Phosphatidylinositol (3) phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell 2, 157-162. [DOI] [PubMed] [Google Scholar]
- Calero, M., Chen, C. Z., Zhu, W., Winand, N., Havas, K. A., Gilbert, P. M., Burd, C. G., and Collins, R. N. (2003). Dual prenylation is required for Rab protein localization and function. Mio. Biol. Cell 14, 1852-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.-Y., Ingram, M. F., Rosal, P. H., and Graham, T. R. (1999). Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase in yeast late Golgi function. J. Cell Biol. 147, 1223-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles, C. R., Synder, W. B., Burd, C. G., and Emr, S. D. (1997). Novel Golgi tovacuole delivery pathway in yeast. EMBO J. 16, 2769-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, P. et al. (2004). Phosphatidyylinositol 4-kinase β is critical for functional association of rab11 with the Golgi complex. Mol. Biol. Cell 15, 2038-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerks, T., Strauss, M., Brendel, M., and Bork, P. (2000). GRAM, a novel domain in glucosyltransferases, myotubularins, and other putative membrane-associated proteins. Trends Biochem. Sci. 25, 483-485. [DOI] [PubMed] [Google Scholar]
- Du, L., and Novick, P. (2001). Yeast rab-GTPase activating protein Gyp1p localizes to the Golgi apparatus and is a negative regulator of Ypt1p. Mol. Biol. Cell 12, 1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff, A., and Schekman, R. (1989). Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 8, 2695-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall, W. E., Geething, N. C., Hua, Z., Ingram, M. F., Liu, K., Chen, S. I., and Graham, T. R. (2002). Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 12, 1623-1627. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos, J. F., Marini, F., Stevenson, I., Frei, C., and Hall, M. N. (1994). PIK1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J. 15, 2352-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godi, A., DiCampli, A., Konstantakopoulos, A., DiTullio, G., Alessi, D. R., Kular, G. S., Daniele, T., Marra, P., Lucocq, J. M., and DeMatteis, A. (2004). FAPPs control Golgi to cell surface membrane traffic by binding ARF and PtdIns(4)P. Nat. Cell Biol. DOI:10.1038/ncb1119. [DOI] [PubMed]
- Godi, A., Pertile, P., Meyers, R., Marra, P., DiTullio, G., Iurusci, C., Luini, A., Corda, D., and De Matteis, A. (1999). ARF mediates recruitment of PtdIns 4-OH kinase β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1, 280-287. [DOI] [PubMed] [Google Scholar]
- Graham, T. R., and Emr, S. D. (1991). Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 mutant. J. Cell Biol. 114, 207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama, H., Schnieders, E. A., Thorner, J., Takemoto, J. Y., and DeWald, D. B. (1999). Direct involvement of phosphatidiylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 34294-34300. [DOI] [PubMed] [Google Scholar]
- Hendricks, K. B., Wang, B. Q., Schnieders, E. A., and Thorner, J. (1999). Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat. Cell Biol. 4, 234-241. [DOI] [PubMed] [Google Scholar]
- Hoffman, C. S., and Winston, F. (1987). A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene 57, 267-272. [DOI] [PubMed] [Google Scholar]
- Ito, H., Fukuda, Y., Murata, K., and Kimura, A. (1983). Transformations of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd, G., Mulholland, J., and Segev, N. (1997). Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 137, 563-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S., Jedd, G., Kahn, R. A., Franzusoff, A., Bartolini, F., and Segev, N. (1999). Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics 152, 1543-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S., Newman, C., Liu, F., and Segev, N. (2000). The TRAPP complex is a nucleotide exchanger for Ypt1 and Ypt31/32. Mol. Biol. Cell 11, 4403-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade, C., Galan, J.-M., and Peter, M. (2003). Opposite roles of the F-box protein Rcp1p and the GTPase-activating protein Gyp2p during recycling of internalization proteins in yeast. Genetics 164, 469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, T. P., and Munro, S. (2002). Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase dependent and independent components. Curr. Biol. 12, 695-704. [DOI] [PubMed] [Google Scholar]
- Levine, T. P., and Munro, S. (2001). Dual targeting of Osh1p, a yeast homologue of oxysterol-binding protein, to both the Golgi and the nucleus-vacuole junction. Mol. Biol. Cell 12, 1633-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M. J., Nichols, B. J., Prescianotto-Baschong, C., Riezman, H., and Pelham, H.R.B. (2000). Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11, 23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M. S., McKenzie, A., 3rd, Demarini, D. J., Shah, N. G., Walsh, A., Brachat, A., Philippsen, P., and Pringle, J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in S. cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Malinska, K., Malinsky, J., Opekarova, M., and Tanner, W. (2003). Visualization of protein compartmentation within the plasma membrane of living yeast cells. Mol. Biol. Cell 14, 4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis, T., Fritsch, E. F., and Sambrook, J. (1992). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Nakagawa, T., Goto, K., and Kondo, H. (1996). Cloning, expression, and localization of 230-kDa phosphatidylinositol 4-kinase. J. Biol. Chem. 271, 12088-12094. [DOI] [PubMed] [Google Scholar]
- Neuwald, A. F. (1997). A shared domain between a spindle assembly check-point protein and Ypt/Rab-specific GTPase activators. Trends Biochem. Sci. 22, 243-244. [DOI] [PubMed] [Google Scholar]
- Ortiz, D., Medkova, M., Walch-Solimena, C., and Novick, P. (2002). Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157, 1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, M. R., Burd, C. G., and Emr, S. D. (1999). Vac1p coordinates Rab and phosphatidylinositol 3-kinase signaling in Vps45p-dependent vesicle docking/fusion at the endosome. Curr. Biol. 9, 159-162. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. (2003). Membrane domains in the secretory and endocytic pathways. Cell 112, 507-517. [DOI] [PubMed] [Google Scholar]
- Protopopov, V., Govindan, B., Novick, P., and Gerst, J. E. (1993). Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function of the late secretory pathway in S. cerevisiae. Cell 74, 855-861. [DOI] [PubMed] [Google Scholar]
- Robinson, J. S., Klionsky, D. J., Banta, L. M., and Emr, S. D. (1988). Protein sorting in S. cerevisiae: isolation of mutants defects in the delivery and sorting of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossanese, O. W., Reinke, C. A., Bevis, B. J., Hammond, A. T., Sears, I. B., O'Connor, J., and Glick, B. S. (2001). A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J. Cell Biol. 153, 47-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge, S. A., Anderson, D. M., and Emr, S. D. (2004). Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol. Biol. Cell 15, 24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher, M., Barrowman, J., Wang, W., Horecka, J., Zhang, Y., Pypaert, M., and Ferro-Novick, S. (2001). TRAPPI implicated in the specificity of tethering in ER-to-Golgi transport. Mol. Cell 7, 433-442. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and Novick, P. J. (1987). A ras-like protein is required for a post-Golgi event in yeast secretion. Cell 49, 527-538. [DOI] [PubMed] [Google Scholar]
- Schorr, M., Then, A., Tahirovic, S., Hug, N., and Mayinger, P. (2001). The phosphoinositide phosphatase Sac1p controls trafficking of the yeast Chs3p chitin synthase. Curr. Biol. 11, 1421-1426. [DOI] [PubMed] [Google Scholar]
- Schultz, J., Copley, R. R., Doerks, T., Ponting, C. P., and Bork, P. (2000). SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 1, 213-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev, N. (2001). Ypt/Rab GTPases: regulators of protein trafficking. Sci STKE 2001, re11. [DOI] [PubMed]
- Segev, N., Mulholland, J., and Botstein, D. (1988). The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretory machinery. Cell 52, 915-924. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in S. cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J. M., Brech, A., Callaghan, J.,. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494-498. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Wurmser, A. E., Emr, S. D., and Stenmark, H. (2001). The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485-492. [DOI] [PubMed] [Google Scholar]
- Siniossoglou, S., and Pelham, H.R.B. (2001). An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 20, 5991-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan, C. J., Audhya, A., and Emr, S. D. (2002). The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell 13, 542-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, A. et al. (2001). Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364-2367. [DOI] [PubMed] [Google Scholar]
- Tong, A. et al. (2004). Global mapping of the yeast genetic interaction network. Science 6, 808-813. [DOI] [PubMed] [Google Scholar]
- Umebayashi, K., and Nakano, A. (2003). Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J. Cell Biol. 161, 1117-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia, R. H., Baggott, D., Chuang, J. S., and Schekman, R. W. (2002). The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late golgi membrane proteins. Dev. Cell 2, 283-294. [DOI] [PubMed] [Google Scholar]
- Vida, T. A., and Emr, S. D. (1995). A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128, 779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena, C., and Novick, P. (1999). The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat. Cell Biol. 1, 523-525. [DOI] [PubMed] [Google Scholar]
- Walworth, N. C., Goud, B., Kabcenell, A. K., and Novick, P. J. (1989). Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 8, 1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., and Ferro-Novick, S. (2002). A Ypt32p exchange factor is a putative effector of Ypt1p. Mol. Biol. Cell 13, 3336-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. J., Wang, J., Sun, H. S., Martinez, M., Sun, Y. X., Macia, E., Kirchhausen, T., Albanesi, J. P., Roth, M. G., and Yin, H. L. (2003). Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299-310. [DOI] [PubMed] [Google Scholar]
- Wong, K., Meyers, R., and Cantley, L. C. (1997). Subcellar localizations of phosphatidylinositol 4-kinase isoforms. J. Biol. Chem. 272, 13236-13241. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K., and Jigami, Y. (2002). Mutation of TRS130, which encodes a component of the TRAPPII complex, activates transcription of OCH1 in S. cerevisiae. Curr. Genet. 42, 85-93. [DOI] [PubMed] [Google Scholar]
- Yu, J. W., Mendrola, J. M., Audhya, A., Singh, S., Keleti, D., DeWald, D. B., Murray, D., Emr, S. D., and Lemmon, M. A. (2004). Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell 13, 677-688. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
- Zhang, C., Bowzard, B., Greene, M., Anido, A., Stearns, K., and Kahn, R. A. (2002). Genetic interactions link ARF1, YPT31/32, and TRS130. Yeast 19, 1075-1086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


