Abstract
Human alveolar macrophages (AMs) phagocytose Pneumocystis (Pc) organisms predominantly through mannose receptors, although the molecular mechanism mediating this opsonin-independent process is not known. In this study, using AMs from healthy individuals, Pc phagocytosis was associated with focal F-actin polymerization and Cdc42, Rac1, and Rho activation in a time-dependent manner. Phagocytosis was primarily dependent on Cdc42 and RhoB activation (as determined by AM transfection with Cdc42 and RhoB dominant-negative alleles) and mediated predominantly through mannose receptors (as determined by siRNA gene silencing of AM mannose receptors). Pc also promoted PAK-1 phosphorylation, which was also dependent on RhoGTPase activation. HIV infection of AMs (as a model for reduced mannose receptor expression and function) was associated with impaired F-actin polymerization, reduced Cdc42 and Rho activation, and markedly reduced PAK-1 phosphorylation in response to Pc organisms. In healthy AMs, Pc phagocytosis was partially dependent on PAK activation, but dependent on the Rho effector molecule ROCK. These data provide a molecular mechanism for AM mannose receptor-mediated phagocytosis of unopsonized Pc organisms that appears distinct from opsonin-dependent phagocytic receptors. Reduced AM mannose receptor-mediated Cdc42 and Rho activation in the context of HIV infection may represent a mechanism that contributes to the pathogenesis of opportunistic pneumonia.
INTRODUCTION
Opportunistic lung infections such as Pneumocystis pneumonia frequently complicate HIV disease, although the underlying mechanisms of increased susceptibility and disease pathogenesis remain incompletely understood. Alveolar macrophages (AMs) are critical components of the pulmonary innate immune response to infectious challenge and likely represent important effector cells in the host response to Pneumocystis (Limper et al., 1997; Steele et al., 2003). Human AMs express surface innate immune receptors such as mannose receptors (Greenberg and Grinstein, 2002) that can mediate Pneumocystis phagocytosis (Ezekowitz et al., 1991). AMs from asymptomatic HIV-infected individuals at high clinical risk for Pneumocystis pneumonia (peripheral CD4+ T-lymphocyte counts <200 cells/mm3) demonstrate significantly reduced Pneumocystis phagocytosis compared with AMs from healthy individuals (Koziel et al., 1998a). Although the reduced Pneumocystis phagocytosis is in part associated with reduced AM mannose receptor surface expression (Koziel et al., 1998a), the mechanism of reduced phagocytosis has not been completely established.
The family of small Rho guanosine triphosphatases (GTPases) link membrane receptors to the actin cytoskeleton and represent molecular switches that control a variety of signal transduction pathways including phagocytosis (Etienne-Manneville and Hall, 2002). Rho GTPases are a subgroup of the Ras superfamily of 20–30-kDa GTP-binding proteins, and include Cdc42, Rac, and Rho (-A, -B, and -C). Specific Rho GTPase activation may provide the molecular basis for the observed differences in opsonin-dependent receptor-mediated phagocytosis described for immune globulin receptors (FcγR) and complement receptors (C3R). FcγR-mediated phagocytosis of appropriately opsonized particles may be mediated predominantly by Cdc42 and Rac, whereas C3R-mediated phagocytosis of appropriately opsonized particles may be mediated primarily by Rho (Caron and Hall, 1998). However, the role of the actin-based cytoskeleton and the molecular mechanisms that mediate opsonin-independent phagocytosis of Pneumocystis by human AMs have not been investigated. The purpose of this study was to examine the role of RhoGTPases in opsonin-independent receptor-mediated phagocytosis of Pneumocystis organisms by human AMs and to examine the influence of HIV infection on Pneumocystis-mediated AM RhoGTPase expression and activation.
MATERIALS AND METHODS
Study Subjects
Prospectively recruited healthy and asymptomatic HIV seropositive (HIV+) individuals were without evidence for active pulmonary disease and had normal spirometry. Healthy individuals were without known risk factors for HIV infection and confirmed to be HIV seronegative by ELISA (ELISA performed according to the manufacturers instructions; Abbott Diagnostics; N. Chicago, IL). Demographic characteristics for all participants included age, gender, smoking status, HIV risk factor, prior opportunistic infections, and prescribed antiretroviral medications.
Bronchoscopy
Pulmonary immune cells were obtained by bronchoalveolar lavage (BAL) using standard technique as previously described (Koziel et al., 1998a, 1998b). All procedures are performed on consenting adults following protocols approved by the Beth Israel Deaconess Medical Center institutional review board and Committee for Clinical Investigations. The cells were separated from the pooled BAL fluid as previously described (Koziel et al., 1998a) and counted on a hemacytometer with light microscopy.
Human AMs
AMs were isolated by adherence to plastic-bottom tissue culture plates (1–3 × 106 cells/well in six-well plates for Western blot, transfection and immunoprecipitation) or 13-mm round glass coverslips (1–2.5 × 105 cells/slip in 24-well plates for phagocytosis and immunofluorescence staining) as previously described (Koziel et al., 1998a). Isolation of AMs from all healthy and HIV+ persons yielded cells that were ≥98% viable as determined by trypan blue dye exclusion and demonstrated >95% positive nonspecific esterase staining.
In Vitro HIV-1 Infection of AMs from Healthy Individuals
To assess the direct effect of HIV-1 infection on macrophage RhoGTPase activation, AMs from healthy individuals were infected in vitro as previously described (Koziel et al., 1998a; Salahuddin et al., 1986), utilizing a monocytotropic (R5) HIV-1 isolate (HIV Bal). Uninfected macrophages were also maintained as control conditions. Culture media was changed every 3–4 d, and productive HIV-1 infection was verified by the measurement of HIV p24 antigen in the culture supernatants by ELISA (Dupont, Boston, MA). At 2 wk after HIV-1 infection, viability was determined on representative cells by trypan blue dye exclusion, and experiments were performed comparing HIV-1–infected to –uninfected AMs.
AM Innate Immune Receptor Stimulation
To examine the role of AM Rho GTPase signaling pathway during opsonin-independent phagocytosis, experiments used PMA (phorbol myristate acetate; Sigma Chemical, St. Louis, MO), zymosan (Sigma Chemical) and Pneumocystis. PMA is a recognized inducer of Rho GTPase in neutrophils and served as a positive control. Unopsonized zymosan and Pneumocystis are recognized by macrophage innate immune receptors (Ezekowitz et al., 1991; Hoffmann et al., 1999). Because sustainable cultivation of Pneumocystis is not possible, and Pneumocystis derived from human disease (P. jiriveci; Stringer et al., 2002) is rarely available, Pneumocystis organisms were obtained from chronically immunosuppressed male Lewis rats (University of Cincinnati Lab Animal Medicine Facility, Cincinnati, OH) as previously described (Zhang et al., 2004). Isolated Pneumocystis mixed-life cycle preparations yield ∼90% trophozoite and 10% cyst forms, and viability was >85% (Chen and Cushion, 1994). Pneumocystis preparations were relatively free of contaminating rat-derived proteins (Koziel et al., 1998b), and preparations were endotoxin-free (<1.0 endotoxin units/ml) as determined by E-toxate Limulus polyphemus assay (Sigma Chemical).
Immunofluorescence Microscopy
To demonstrate intracellular localization of F-actin, Cdc42, Rac1, Rho, and β-tubulin, isolated AMs (2 × 105 cells/coverslip) were rinsed twice with cytoskeletal buffer, fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in phosphate-buffered saline (PBS) for 10 min at room temperature (RT), and permeabilized with 2% triton in 1% bovine serum albumin (BSA) in PBS, and then incubated with unconjugated primary antibodies for 1 h at 37°C. Primary antibodies included the following: anti-Cdc42, anti-Rac1, anti-RhoB (Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-tubulin and anti-β-actin (Sigma-Aldrich). After washing with PBS, cells were incubated a goat anti-rabbit secondary antibody conjugated either with Cy3 (Sigma Chemical) or a goat antimouse secondary antibody conjugated with fluorescein isothiocyanate (FITC; Sigma Chemical) for 1 h at 37°C. Control conditions included cells stained with primary or secondary antibody only. For select experiments cells are counterstained with the nucleic acid stain DAPI (1:500; Molecular Probes, Eugene, OR; 1:500) for 30 min at RT. For detection of F-actin polymerization, cells were stained with Rhodamine-phalloidin (Molecular Probes). After washing five times with PBS, coverslips were mounted with Mowiol 4–88 (Hoechst Celanese, Somerville, NJ), and images were captured using a fluorescence microscope equipped with a digital camera (Nikon Eclipse E800; Diagnostic Instruments; Sterling Heights, MI).
Western Blot
Western blot was performed as previously described (Zhang et al., 2004) using polyclonal anti-Cdc42, anti-Rac, anti-RhoB and anti-PAK, phospho-PAK, and monoclonal anti-Rho A (Santa Cruz Biotechnology). After blocking with 4% nonfat dry milk, membranes were incubated with appropriate antibodies overnight at 4°C, washed three times with TTBS, incubated with conjugated secondary antibody (1:3000) for 2 h, and washed three times with TTBS, and then the signal was detected by SuperSignal West Pico protein detection system (Pierce, Rockford, IL). Protein concentrations were determined by Bio-Rad protein assay using BSA as standard (Bio-Rad, Hercules, CA). Select experiments used the general Rho GTPase inhibitor, Clostridium difficile toxin B (Sigma) or the specific Rho Kinase inhibitor, (+)-R-trans-4-(aminoethyl)-N-4-pyridyl) cyclohexanecarboxamide (Y-27632; Calbiochem, San Diego, CA; Uehata et al., 1997).
Cdc42, Rac, and Rho Activation Assay
AM cytoplasmic extracts were prepared, and activation of Cdc42, Rac (Benard et al., 1999), and Rho (Ren et al., 1999) were determined by affinity precipitation using GST fusion-protein–based kits according to the manufacturer's protocols (Rac1/Cdc42 assay reagent using GST-PBD, and Rhotekin Rho binding domain using GST-RBD; Upstate Biotechnology, Lake Placid, NY). For Cdc42 and Rac1-GTP, proteins were separated by 12% SDS-PAGE, transferred to nitrocellulose membrane, and blotted for the appropriate specific monoclonal antibodies (Santa Cruz Biotechnology). To identify Rho-GTP, immobilized Rhotekin (Rho binding domain) was used to selectively precipitate Rho-GTP (Upstate Biotechnology), and precipitated GTP-Rho was detected by immunoblot using polyclonal antibody (-A, -B and -C; Upstate Biotechnology). For all assays, signal was detected by SuperSignal West Pico protein detection system (Pierce).
Dominant Negative Cdc42, Rac, and Rho Allele Amplification, Purification, and Transfection
Guanine nucleotide binding-deficient (dominant negative) N-terminal 3× hemaglutinin (HA)-tagged alleles Cdc42 T 17 N, Rac1 T17 N, Rho A T19 N, and Rho B T19 N subcloned in pcDNA 3.1+ vector (Guthrie cDNA Resource Center, Sayre, PA) were used for transfections. The empty vector pcDNA 3.1+ (Invitrogen, Carlsbad, CA) was used as a control. Plasmid amplification was performed using MAX Efficiency DH5α MAX Competent Escherichia coli (Invitrogen) as previously described (Miralem and Avraham, 2003). DNA purification was performed using Geno Pure Plasmid Maxi Kit (Roche, Indianapolis, IN) following the manufacturer's protocol, and cDNA was confirmed by resolution on agarose gel and optical density measurement (260 nm) by spectrophotometry. Transfection of AMs (1 × 106 cells/well of six-well plate) of each dominant negative allele was performed using Effectene Transfection Reagent (Qiagen, Valencia, CA) for 60 h.
Immunoprecipitation
Transfected AM cell cytoplasmic protein was prepared using NE-PER kit (Pierce) according to the manufacturer's protocol. N-terminal 3-× HA–tagged dominant negative Rho GTPase proteins were immunoprecipitated by incubation cytoplasmic protein with monoclonal antibody against 3× HA for overnight at 4°C and then the antibody-protein complexes were incubated with protein A-agrose (Upstate Biotechnology) for 2 h at RT. Each immunoprecipitate was washed three times with PBST. The agrose beads and protein complex were solved by SDS-PAGE as described above, and immunoblot was probed for 3× HA.
Fluorescein Isothiocyanate Labeling of Pneumocystis
To facilitate the identification of Pneumocystis in macrophage phagocytosis studies, organisms were labeled with FITC (Sigma Chemical), as previously described (Ezekowitz et al., 1991). The FITC-labeling process stained both trophic and cyst form surfaces uniformly and did not affect organism viability as determined by flow cytometry (Armstrong et al., 1991).
AM Phagocytosis of Pneumocystis
This technique allowed for the discrimination of FITC-labeled Pneumocystis phagocytosed by the macrophages from those only bound to the surface. AMs adherent to round glass cover slips were incubated with FITC-labeled Pneumocystis at an organism-to-cell ratio of 5:1 for 60 min at 37°C in 5% CO2. After washing with Hanks' balanced salt solution containing magnesium and calcium (Hanks' balanced salt solution+), the AM monolayers were fixed in 4% paraformaldehyde overnight at 4°C, and confocal microscopy was used to determine the phagocytic and binding index as previously described (Koziel et al., 1998a), using a Sarastro 2000 confocal laser scanning microscope (Molecular Dynamics, Sunnyvale, CA) fitted with a 25-mW argon-ion laser. Experimental conditions were performed in duplicate, and the number of FITC-labeled organisms phagocytosed per 200 macrophages was counted on at least two separate slides.
siRNA Gene Silencing of AM Mannose Receptors and PAK1
To determine the specific contribution of AM mannose receptors to Pneumocystis-mediated RhoGTPase signaling and to determine the role of PAK1 in Pneumocystis phagocytosis, siRNA gene silencing was used to produce functional “knock-down” of human AM mannose receptors or PAK1 as previously described (Zhang et al., 2004). Experiments utilized the following oligonucleotide (annealed double-stranded siRNA; Qiagen): Human mannose receptor siRNA (siRNA-MR) sequences: DNA target sequences: AAGTGGTACGCAGATTGCACG begin from 528 base pairs to 549 base pairs; 5′-GUGGUACGCAGAUUGCACG-3′; and 3′-CGUGCAAUCUGCGUACCAC-5′. Human PAK1 siRNA (siRNA-PAK1) sequences: DNA target sequences: 5′-AAGGAGAAGAAAAAGAAGGAC-3′. Sense: 5′-GGAGAAGAAAAAGAAGGACtt-3′. Antisense: 5′-GUCCUUCUUUUUCUUCUCCtc-3′. MR siRNA and PAK1 siRNA were individually transfected into AMs using TransMessenger Transfection reagent (Qiagen).
Detailed procedures were performed following the manufacturer's protocol. Briefly, 2 μg siRNA of each was mixed with 4 μl enhancer R and 100 μl buffer EC-R, incubated at RT for 5 min, and then spun down to collect supernatant. Then 8 μl transMessenger transfection reagent was added to this supernatant. The mixture was vortexed and incubated for 10 min at RT. AMs (3 × 106 cells) were washed with sterile PBS once, and 900 μl medium without serum and antibiotics together with siRNA mixture were added into the culture and incubated for 3–4 h at 37°C/5% CO2. The culture was washed with sterile PBS once and added back complete medium 1640 with 10% fetal bovine serum (FBS) and 1% PS/AMs and incubated for 60 h at 37°C in 5% CO2.
PAK1 Protein Phosphorylation Detection
For PAK-1 protein and phosphorylated protein detection, AMs were incubated for 24 h in low FBS (0.4%) complete medium and then incubated with LPS for up to 60 min, or zymosan or Pneumocystis for 15 min. Cytoplasmic proteins were isolated and resolved by Western blot using appropriate specific antibodies. Results were compared with unstimulated AMs. Pneumocystis-mediated PAK phosphorylation was examined in the presence and absence of a general small GTPase inhibitor (C. diff B toxin) or specific Rho-associated protein kinase (ROCK) inhibitor, Y-27632 (Uehata et al., 1997).
Statistical Analysis
Experimental conditions were performed in duplicate or triplicate and repeated with AMs from at least three different individual. Data were analyzed with an Apple G3 Power PC computer utilizing StatView (SAS Institute, Cary, NC) and INSTAT2 (GraphPad Software, San Diego, CA) statistical software. Nonparametric data were analyzed by Fisher Exact Test or ANOVA. Statistical significance was accepted for p < 0.05.
RESULTS
Study Subjects
Bronchoscopy was performed on 24 subjects, including 13 healthy individuals (mean age 37 ± 11 y, including 4 females, 5 smokers) and 11 asymptomatic HIV+ subjects (mean age 34 ± 12 y, including 4 female, 9 smokers). None of the individuals had evidence for active respiratory disease. For the HIV+ subjects, peripheral blood CD4 lymphocytes counts were 150-1000 cells/mm3, HIV risk factors included IVDU and homosexual exposures, all were prescribed highly active antiretroviral therapy (HAART), all had undetectable serum viral load (<50 HIV-1 RNA copies/ml), and none experienced a prior opportunistic pneumonia.
AM F-actin Polymerization during Phagocytosis of Unopsonized Pneumocystis Organisms
Actin polymerization is a critical component of phagocytosis (Aderem, 2002), but the role in Pneumocystis phagocytosis has not been previously demonstrated. To determine whether Pneumocystis phagocytosis induces focal F-actin polymerization, AMs from healthy individuals were incubated with unopsonized Pneumocystis organisms, and macrophages were stained with rhodamine-phalloidin (which stains polymerized actin). As expected, after incubation with IgG-opsonized Pneumocystis, focal F-actin–rich cytoplasmic membrane was evident at points of contact with the opsonized Pneumocystis organisms (Figure 1). In comparison, unopsonized Pneumocystis also induced reorganization of the actin-based cytoskeleton at points of contact (Figure 1C). No focal F-actin polymerization was evident in response to Pneumocystis in the presence of cytocholasin B (an inhibitor of actin polymerization), C. difficile toxin (a general inhibitor of RhoGTPases), or Y27632 (specific inhibitor for Rho kinase, ROCK). These data demonstrate that incubation of unopsonized Pneumocystis organisms with AMs from healthy individuals induced focal F-actin polymerization, and Pneumocystis-induced F-actin assembly was dependent on small RhoGTPases, especially Rho.
Figure 1.
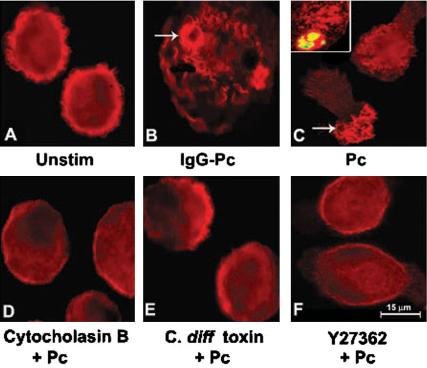
Focal F-actin polymerization during Pneumocystis phagocytosis by AMs from healthy individuals. Immunofluorescence microscopy images of isolated AMs after incubation with Pneumocystis organisms (ratio 10:1 organisms to macrophages) for 20 min at 37°C in 5% CO2. F-actin was detected by rhodamine-phalloidin (red color) as described in Materials and Methods. (A) Unstimulated AMs demonstrate cortical distribution of F-actin. (B) Focal accumulation of F-actin (i.e., phagocytic cup) was evident in AMs at points of contact with IgG-opsonized Pneumocystis (IgG-Pc; positive control for F-actin polymerization; arrow). (C) Focal accumulation of F-actin was evident in AMs at points of contact with unopsonized Pneumocystis (Pc; arrow). Inset demonstrates F-actin polymerization on the macrophage surface surrounding FITC-labeled Pneumocystis organism (yellow color). No F-actin polymerization was observed in the presence of cytocholasin B (D; an inhibitor of actin polymerization), C. difficile toxin B (E; a general inhibitor of small GTPases), or Y27632 (F; a specific inhibitor of Rho kinase, Rock). Depicted images from one healthy individual are representative of AMs from at least four individuals examined.
Pneumocystis Phagocytosis Activates Cdc42, Rac1, and Rho in AMs from Healthy Individuals
Experiments were next conducted to determine whether Pneumocystis-mediated F-actin polymerization was associated with small RhoGTPase activation, with focus on Cdc42, Rac1, and Rho (Caron and Hall, 1998). AMs were incubated with unopsonized Pneumocystis organisms up to 60 min, cell lysates prepared, and Western blot determinations were performed to detect both total and GTP-(activated) forms of Cdc42, Rac1, and Rho proteins. Cdc42, Rac1, and Rho proteins were detected in all cell lysates examined (Figure 2). Unstimulated AMs demonstrated low constitutive activation of Cdc42 and Rho and absent constitutive phosphorylation of Rac1. Incubation with unopsonized Pneumocystis resulted in activation of AM Cdc42, Rac1, and Rho. Cdc42 was detected within 1 min of incubation with Pneumocystis and peaked by 10 min. Rac1 activation was detected at 3 min and peaked by 15 min. Rho activation demonstrated a bimodal response, with early detection and peak by 1 min, followed by decline, and another peak by 15 min. These data demonstrate that unopsonized Pneumocystis organisms induce AM Cdc42, Rac1, and Rho activation in a time-dependent manner, with early activation of Cdc42 and Rho, and relatively delayed activation of Rac1.
Figure 2.
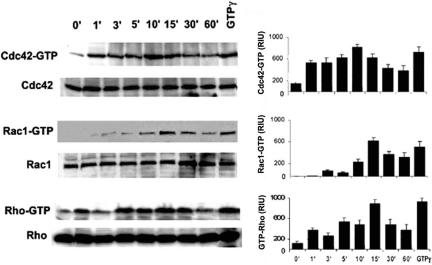
Pneumocystis activates Cdc42, Rac1, and Rho in AMs from healthy individuals. AMs are incubated with unopsonized Pneumocystis organisms (10:1) up to 60 min at 37°C in 5% CO2, and Western blot on cell lysates was performed to detect both total and activated (-GTP) form of Cdc42, Rac1, and Rho. The lane for GTP-γ is a positive control for the assay. Western blots are shown for AMs from one healthy individual and are representative of experiments from three different individuals. The corresponding bar graphs represent densitometry quantitative analysis for Cdc42-GTP, Rac1-GTP, and Rho-GTP (n = 3) expressed as relative intensity units (RIU).
Phagocytosis of Pneumocystis Organisms Impaired by Dominant Negative Constructs for Cdc42 and RhoB
Next, experiments investigated whether AM phagocytosis of Pneumocystis organisms was dependent on functional RhoGTPases.
AMs from healthy individuals were transfected with functional inhibitors of specific RhoGTPases, including GTP-binding–deficient alleles of Cdc42 (Cdc42T17N), Rac1 (Rac1T17N), RhoA (RhoAT19N), or RhoB (RhoBT19N). Transfection of human AMs with these dominant negative constructs did not alter cell morphology (Figure 3A), and individual dominant negative proteins were readily detected by immunoprecipitation in AMs transfected with individual dominant-negative alleles (Figure 3B). Incubation of AMs with unopsonized Pneumocystis resulted in activation of Cdc42, Rac1 and Rho (Figure 3C), whereas incubation of dominant-negative transfected AMs with unopsonized Pneumocystis did not result in activation of Cdc42, Rac1, or Rho. These data demonstrate successful transfection of human AMs with functional inhibitors of Cdc42, Rac1, and Rho.
Figure 3.
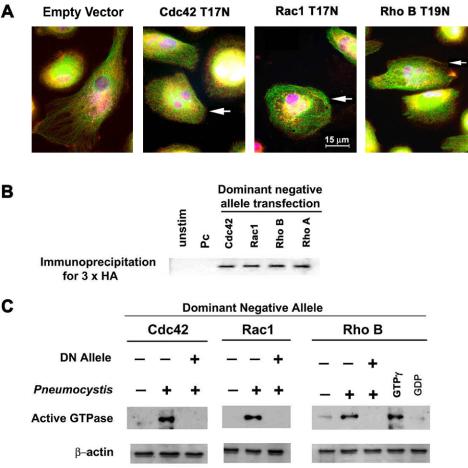
Transfection of AMs from healthy individuals with GTP-binding–deficient alleles of Cdc42 (Cdc42T17N), Rac (Rac1T17N), RhoA (RhoAT19N), or RhoB (RhoBT19N). (A) Immunofluorescence microscopy images illustrate AMs morphology after transfection with empty vector or dominant-negative alleles. Red color indicates 3× HA antibody staining of vector used for transfection (arrows indicate transfected cells stained red). Green staining demonstrates intact cellular tubulin lattice structure, and oval blue-magenta DAPI stain indicates macrophage nuclei. (B) Immunoprecipitation of DN proteins in AMs transfected with individual DN alleles detected by anti-3× HA antibody. (C) Detection of AM Cdc42, Rac1, and Rho protein activation by Western blot after incubation with unopsonized Pneumocystis (15 min at 37°C in 5% CO2) in the presence and absence of dominant-negative (DN) alleles. The lanes marked GTPγ and GDP served as positive and negative controls for the assay.
Next, to determine the role of specific GTPases in Pneumocystis phagocytosis, AMs from healthy individuals transfected with individual dominant negative constructs were incubated with FITC-labeled Pneumocystis organisms and phagocytosis was determined by confocal microscopy as previously described (Koziel et al., 1998a). AMs from healthy individuals could bind and phagocytose Pneumocystis organisms (Figure 4A), and phagocytosis was markedly reduced in the presence of cytocholasin B (inhibitor of actin polymerization). Transfection of AMs with an empty construct (pcDNA3.1+) did not alter Pneumocystis binding and phagocytosis. In comparison, Pneumocystis binding and phagocytosis was reduced in the presence of functional inhibitors of Cdc42 and RhoB by qualitative assessment (Figure 4A). Quantitative analysis revealed a significant reduction in Pneumocystis phagocytosis in AMs transfected with Cdc42 and RhoB, but not Rac1 (Figure 4B) or RhoA (unpublished data). In contrast, Pneumocystis binding was not significantly influenced by the dominant-negative alleles (Figure 4C). These data demonstrate that phagocytosis of unopsonized Pneumocystis organisms by human AMs was dependent on functional Cdc42 and RhoB.
Figure 4.
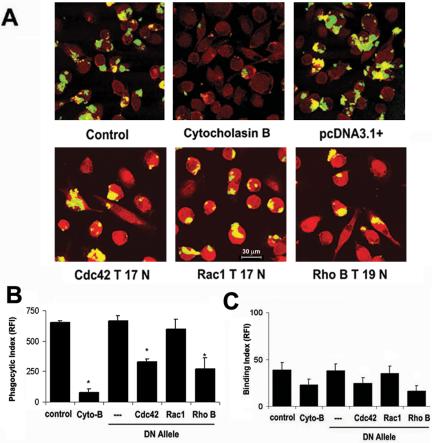
Pneumocystis phagocytosis mediated by macrophage Cdc42 and RhoB. AMs were incubated with FITC-labeled Pneumocystis organisms for 60 min at 37°C in 5% CO2, and phagocytosis was determined as described in Materials and Methods. (A) Representative confocal microscopy images of adherent AMs from healthy individuals in the absence and presence of cytocholasin B (cyto-B), pcDNA3.1+ (empty vector), or individual DN RhoGTPase constructs. Pneumocystis organisms appear yellow-green. Quantitative analysis for Pneumocystis phagocytosis (B) and binding (C) by AMs from healthy individuals. Phagocytic Index and Binding Index expressed as relative fluorescence intensity (RFI; values are mean ± SEM; n = 3) *p < 0.05 compared with control.
Cdc42 and RhoB Activation Mediated through AM Mannose Receptors
Prior studies demonstrated that transfection of nonphagocytic COS cells with cDNA for human mannose receptor allowed Pneumocystis phagocytosis, and mannose receptor inhibition markedly reduced Pneumocystis binding and phagocytosis by AMs (Ezekowitz et al., 1991). To determine the contribution of AM mannose receptors to Pneumocystis phagocytosis and RhoGTPase activation, mannose receptor functional “knock-down” experiments were performed by sequence-specific, posttranslational gene silencing using short interfering RNA (siRNA) in human AMs.
In general, targeted gene silencing (functional “knock-down”) of the human AM mannose receptors reduced cellular mannose receptor protein (Figure 5A), but did not influence the expression of Cdc42, Rac1, and Rho proteins, and constitutive activation of Cdc42, Rac1, and Rho were low in unstimulated cells (Figure 5). Incubation with unopsonized Pneumocystis did not increase AM Cdc42 activation above unstimulated conditions (Figure 5A). As control conditions, incubation with IgG-opsonized Pneumocystis (recognized through Fc-γ receptors) increased Cdc42 activation compared with unstimulated cells. In comparison, Rac1 activation was increased in response to both unopsonized Pneumocystis and IgG-opsonized Pneumocystis (Figure 5B). Similar to the Cdc42 response, Rho activation was not increased in response to unopsonized Pneumocystis, but was increased in response to IgG-opsonized Pneumocystis (Figure 5C). These data demonstrated that human AM Cdc42 and RhoB activation in response to unopsonized Pneumocystis organisms is mediated predominately through mannose receptors.
Figure 5.
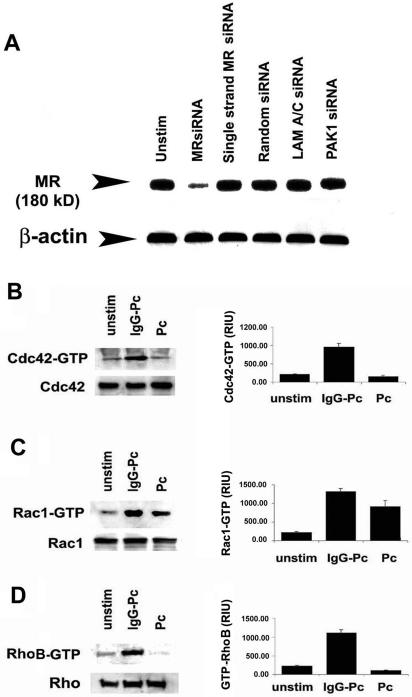
Cdc42 and RhoB activation mediated through AM mannose receptors. Targeted AM mannose receptor gene silencing by siRNA followed by incubation with unopsonized Pneumocystis for 15 min at 37°C in 5% CO2. (A) Reduced mannose receptor total cellular protein in AMs after siRNA gene silencing. Mannose receptor protein was not influenced by single-stranded MR siRNA, random siRNA, or LAM A/C siRNA (served as negative controls). PAK1 siRNA represents gene silencing of AM PAK1 (which did not reduce mannose receptor and demonstrates specificity). (B–D) Influence of Pneumocystis on AM Rho GTPase activation after mannose receptor gene silencing. Western blot of cell lysates performed to detect both total and activated (-GTP) forms of (B) Cdc42, (C) Rac1, and (D) RhoB. Western blots are shown for AMs from one healthy individual and are representative of experiments from at least three different individuals. Corresponding bar graphs represent densitometry quantitative analysis for Cdc42-GTP, Rac1-GTP, and RhoB-GTP (mean ± SEM; n = 3) expressed as relative intensity units (RIU).
HIV-1 Infection Impairs Focal F-actin Polymerization in Response to Unopsonized Pneumocystis Organisms
To consider the clinical relevance of these findings, experiments next examined the role of F-actin polymerization in response to nonopsonized Pneumocystis using AMs from HIV+ persons (recognized clinical risk factor for Pneumocystis pneumonia). Compared with AMs from healthy individuals, incubation of unopsonized Pneumocystis organisms with AMs from asymptomatic HIV+ persons did not induce focal F-actin polymerization at points of contact (Figure 6). Similarly, unopsonized Pneumocystis organisms did not induce F-actin polymerization in AMs from healthy individuals after in vitro HIV infection. These data demonstrate that Pneumocystis-induced F-actin assembly is impaired in AMs from HIV+ persons, and HIV infection is sufficient to impair Pneumocystis-mediated F-actin polymerization in AMs.
Figure 6.
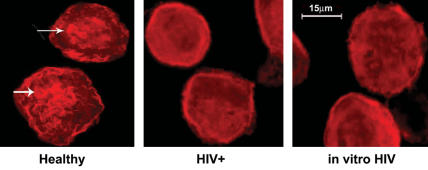
HIV infection impairs AM F-actin polymerization in response to unopsonized Pneumocystis organisms. Immunofluorescence microscopy images of isolated AMs after incubation with Pneumocystis organisms (ratio 10:1 organisms to macrophages) for 20 min at 37°C in 5% CO2. Polymerized F-actin was detected by rhodamine-phalloidin (red color) as described in Materials and Methods. Focal F-actin polymerization was evident in AMs at points of contact with organisms (arrows) in healthy individuals, but not in AMs from HIV+ subjects (HIV+) or AMs from healthy individuals after in vitro HIV infection (in vitro HIV). Representative images of at least three individuals from each group.
HIV Infection Impairs Cdc42 and Rho Activation in AMs in Response to Pneumocystis Organisms
To test the hypothesis that altered mannose receptor expression may influence Pneumocystis-mediated small GTPase activation, experiments next examined Cdc42, Rac1, and Rho activation in AMs from HIV+ subjects, and in AMs from healthy individuals after in vitro HIV infection (conditions associated with reduced mannose receptor expression and function).
Cdc42 protein was detected in the cytoplasm of intact cells and cytosolic extracts from all three groups of AMs studied (Figure 7). For AMs from healthy individuals, Cdc42 activation was increased in response to unopsonized Pneumocystis organisms, in addition to zymosan (alternative ligand for mannose receptors) and PMA (mannose receptor-independent ligand). In comparison, AMs from HIV+ persons demonstrated reduced Cdc42 activation in response to Pneumocystis organisms (and also reduced in response to zymosan and PMA). After in vitro HIV infection, Cdc42 activation was completely inhibited in response to Pneumocystis organisms and zymosan and markedly reduced in response to PMA.
Figure 7.
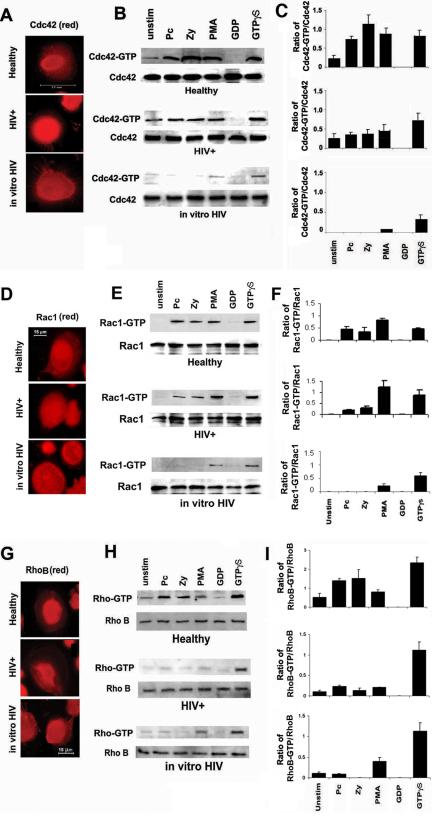
HIV infection impairs AM Cdc42 and Rho activation in response to unopsonized Pneumocystis organisms. AMs were incubated with unopsonized Pneumocystis organisms (Pc), zymosan (Zy), or PMA for 15 min at 37°C in 5% CO2. AMs were from healthy individuals, asymptomatic HIV-infected persons (HIV+), or healthy individuals after in vitro HIV infection (in vitro HIV). Immunofluorescence microscopy images demonstrated similar cellular localization for (A) Cdc42, (D) Rac1, and (G) Rho with representative cells for healthy, HIV+, and in vitro HIV. Western blot of cell lysates was performed to detect both total and activated (-GPT) forms of Cdc42 (B), Rac1 (E), and Rho (H) for healthy, HIV+, and in vitro HIV. The corresponding bar graphs represent densitometry quantitative analysis for Cdc42-GTP (C), Rac1-GTP (F), and Rho-GTP (I) for each group of healthy, HIV+, and in vitro HIV. Data are normalized as a ratio of the active (-GTP) RhoGTPase over the total RhoGTPase and are expressed as relative units (mean ± SEM). Data are representative of experiments from three different individuals in each group (n = 3).
In comparison, Rac1 activation was not significantly impaired in response to Pneumocystis, zymosan, or PMA in AMs from HIV+ persons, although Rac1 activation was completely impaired in response to Pneumocystis and zymosan (but not PMA) after in vitro HIV infection. In contrast, Rho activation was not increased in response to Pneumocystis organisms (or zymosan and PMA) in AMs from HIV+ persons or after in vitro HIV infection. These data demonstrated that Cdc42 and Rho activation was reduced in AMs from HIV+ persons in response to unopsonized Pneumocystis organisms, whereas Rac1 activation was not significantly reduced. Furthermore, in vitro HIV infection of AMs (a condition associated with marked reduction in mannose receptor expression and function; Koziel et al., 1998a) was sufficient to impair Pneumocystis-mediated Cdc42, Rac1, and Rho activation.
HIV Infection Impairs p21-activated Kinase Phosphorylation in Response to Pneumocystis Organisms
The p21-activated kinases (PAK) are important serine/threonine signaling molecules regulating a variety of cellular function (including cytoskeletal dynamics), and PAK are stimulated by activated Cdc42 and Rac (Bokoch, 2003). To determine the downstream effects of Pneumocystis-mediated Cdc42, Rac1, and Rho activation, experiments next measured PAK phosphorylation in response to unopsonized Pneumocystis. Incubation of AMs from healthy individuals with unopsonized Pneumocystis organisms increased PAK-1 phosphorylation (activated PAK) in a time-dependent manner, detected at 10 min and peaked at 15 min (Figure 8A). Pretreatment of the AMs with C. difficile toxin B (general inhibitor of Rho family GTPases) or Y-27632 (specific inhibitor for Rho kinase, ROCK) markedly reduced PAK phosphorylation in response to Pneumocystis (Figure 8B). These data demonstrated that unopsonized Pneumocystis organisms promote AM PAK-1 phosphorylation, and Pneumocystis-mediated PAK-1 phosphorylation was dependent on Rho GTPases, especially Rho.
Figure 8.
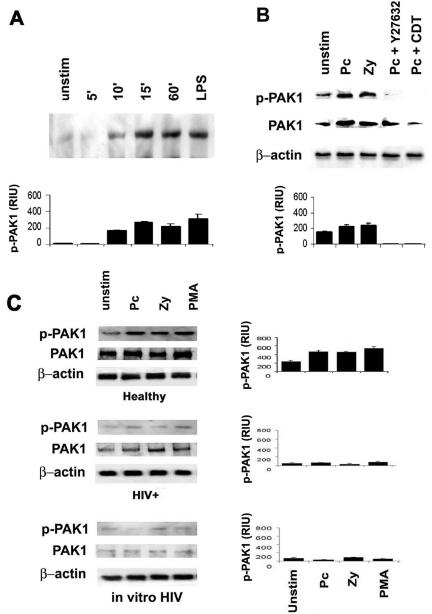
HIV infection impairs PAK-1 activation in response to Pneumocystis organisms. (A) AMs from healthy individuals were incubated with unopsonized Pneumocystis (5:1) up to 60 min at 37°C in 5% CO2, and cell lysates were examined for phosphorylated PAK1 (p-PAK1) by Western blot. LPS served as a positive control for PAK1 phosphorylation. (B) AMs from healthy individuals were incubated with unopsonized Pneumocystis (5:1) or zymosan (5:1) in the presence of absence of Y26371 (the specific inhibitor of Rho kinase, Rock) or C. diff toxin B (CDT; general RhoGTPase inhibitor) for 15 min at 37°C in 5% CO2, and cell lysates were examined for total (PAK1) and phosphorylated PAK1 (p-PAK1) by Western blot using specific antibody. (C) AMs were incubated with Pneumocystis (Pc), zymosan (Zy), or PMA for 15 min at 37°C in 5% CO2, and Western blots on cell lysates were performed to detect both total (PAK1) and phosphorylated PAK1 (p-PAK1) in AMs from healthy, HIV+, and healthy after in vitro HIV. For all Western blots, the corresponding bar graphs represent densitometry quantitative analysis for p-PAK1 for each group of healthy, HIV+, and in vitro HIV expressed as relative intensity units (RIU; mean ± SEM). The depicted Western blots represent cells from one healthy individual and are representative of at least three different subjects examined.
In marked contrast to AMs from healthy individuals, PAK-1 phosphorylation was markedly reduced or absent in AMs from asymptomatic HIV+ persons or in AMs after in vitro HIV infection (Figure 8C). PAK-1 protein levels were also markedly reduced in AMs from HIV+ subjects and healthy individuals after in vitro HIV infection. Taken together, these data demonstrate that impaired Pneumocystis-mediated Cdc42 and Rho phosphorylation in AMs from HIV+ persons was associated with reduced PAK phosphorylation (an effector molecule for Cdc42), and in vitro HIV infection was sufficient to reduce Pneumocystis-mediated PAK phosphorylation.
Role of RhoGTPase Effector Molecules, PAK1, and ROCK on Pneumocystis Phagocytosis by Human AMs
To further define the role of the Cdc42 effector, PAK1, and the Rho effector, ROCK, on Pneumocystis phagocytosis, experiments used siRNA functional gene silencing of AM PAK1 and mannose receptors, and the specific Rho kinase inhibitor Y27632. Functional gene silencing of AM PAK1 reduced PAK cellular protein (Figure 9A), but only partially reduced Pneumocystis phagocytosis (Figure 9B). Functional gene silencing of mannose receptors or inhibition of the Rho kinase ROCK significantly reduced Pneumocystis phagocytosis (Figure 9B). Pneumocystis binding was not influenced by gene silencing of PAK or mannose receptors, but was significantly reduced in the presence of the ROCK inhibitor, Y27632 (Figure 9C). These data suggest that Pneumocystis phagocytosis is only partially dependent on PAK1 activation, but is dependent on the Rho effector molecule, ROCK.
Figure 9.
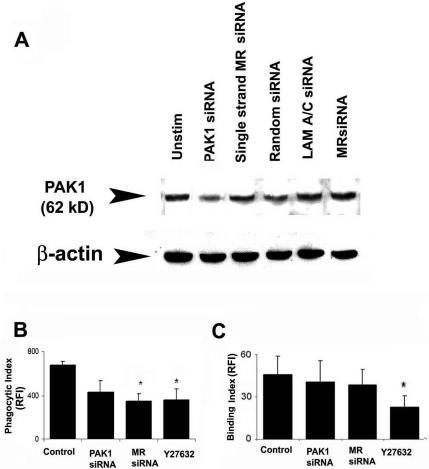
The role of RhoGTPase effector molecules, PAK1 and ROCK, on Pneumocystis phagocytosis by human AMs. (A) Western blot analysis of cellular PAK1 protein in human AMs after siRNA gene silencing. PAK1 protein was not influenced by single-stranded PAK siRNA, random siRNA, or LAM A/C siRNA (served as negative controls). MR siRNA represents gene silencing of AM mannose receptor (which did not reduce PAK1 and demonstrates specificity). Pneumocystis phagocytic index (B) and binding index (C) of AMs after functional siRNA gene silencing of PAK1 (PAK1 siRNA), mannose receptor (MR siRNA), or specific pharmacological inhibition of the Rho kinase ROCK (by Y27632). Phagocytic Index and Binding Index expressed as relative fluorescence intensity (RFI; values are mean ± SEM; n = 3) *p < 0.05 compared with control.
DISCUSSION
These data demonstrate that in AMs from healthy individuals, phagocytosis of unopsonized Pneumocystis was associated with focal F-actin polymerization and small Rho GTPase activation. Phagocytosis of unopsonized Pneumocystis was mediated predominantly through mannose receptors (as determined by targeted mannose receptor siRNA gene silencing) and was critically dependent on Cdc42 and RhoB activation. Unopsonized Pneumocystis also promoted PAK-1 phosphorylation, an important downstream effector molecule, and PAK phosphorylation was dependent in part on RhoGTPase activation, especially Rho. In contrast, HIV infection of AMs (a condition associated with reduced mannose receptor expression and function) was associated with impaired focal F-actin polymerization, reduced Cdc42 and Rho activation, and markedly reduced PAK-1 phosphorylation in response to unopsonized Pneumocystis organisms. These data provide a molecular mechanism for mannose receptor-mediated phagocytosis of unopsonized Pneumocystis organisms by human AMs. Furthermore, impaired AM Cdc42 and Rho activation may provide the molecular basis for impaired Pneumocystis phagocytosis in the context of HIV infection (Koziel et al., 1998a), which may contribute to the pathogenesis of opportunistic pneumonia.
The current study represents the first to examine the molecular mechanism of human AM phagocytosis of unopsonized Pneumocystis organisms. Phagocytosis of Pneumocystis requires focal F-actin polymerization, Cdc42 and Rho activation, promotes PAK1 activation and requires the Rho effector molecule ROCK. A recent study in a murine model identified the dectin-1 receptor for β-glucan receptor as the critical receptor mediating Pneumocystis phagocytosis and killing by macrophages (Steele et al., 2003), although the role of RhoGTPases was not investigated. Although macrophage β-glucan receptors may represent an important component of an effective host response to Pneumocystis in the rodent model, for human AM Pneumocystis recognition is mediated predominantly via mannose receptors. However, defining the specific role of human AM dectin-1 receptor in phagocytosis and signaling requires further investigation.
The current study suggests that the molecular mechanism for opsonin-independent macrophage phagocytosis may be distinct from opsonin-dependent phagocytosis. FcγR-mediated phagocytosis of appropriately opsonized particles may be mediated predominantly by Cdc42 and Rac1, whereas C3R-mediated phagocytosis of appropriately opsonized particles may be mediated primarily by Rho (Caron and Hall, 1998). The distinction, however, may not be absolute, as Rho may also contribute to FcγR-mediated phagocytosis (Hackam et al., 1997). Other investigations of opsonin-independent phagocytosis demonstrated that phagocytosis of unopsonized Borrelia burgdorferi by human macrophages was mediated predominantly by Cdc42 and in part by Rac1, but not Rho (Linder et al., 2001). Macrophage phagocytosis of unopsonized Pseudomonas aeruginosa was mediated by Cdc42 and Rac (although Rho was not specifically investigated; Lee et al., 2000). In the current study, Pneumocystis phagocytosis was mediated via Cdc42 and Rho, but not Rac1. Taken together, although Cdc42 may be an important component to opsonin-independent phagocytosis, the role of other specific RhoGTPases in opsonin-independent phagocytosis of pathogens may depend on multiple factors, including the nature of the pathogen, nature and species origin of the cells, and specific phagocytic receptor(s) engaged. Further studies are required to completely define the role of specific RhoGTPases in opsonin-independent phagocytosis.
The current study represents the first to demonstrate phagocytic signal transduction pathways mediated by mannose receptors. Mannose receptors belong to a family of type I transmembrane C-type lectin receptors that mediate both endocytosis and phagocytosis (Ezekowitz, 1992). Mannose receptors contain multiple C-type lectin-like domains each capable of binding specific monosaccharides such as mannose, fucose, and N-acetylglucosamine. Although endocytosis requires signaling through a conserved tyrosine-based FENTLY motif in the cytoplasmic tail of the receptor (East and Isacke, 2002), the signaling molecules mediating phagocytosis and the downstream cell signaling pathways remain undefined (Fraser et al., 1998). The current study demonstrates that mannose receptor-mediated phagocytosis was associated with focal F-actin polymerization, dependent on Cdc42 and Rho activation, and dependent on the Rho effector molecule, Rock. Rac1 activation was not critical for Pneumocystis phagocytosis (as introduction of nonfunctional Rac1 dominant-negative allele did not influence Pneumocystis phagocytosis). The relative importance of mannose receptors in mediating Pneumocystis phagocytosis by human AMs was supported by targeted siRNA gene silencing experiments and the observations in AMs from HIV-infected persons (a disease state characterized by reduced macrophage mannose receptor surface expression; Koziel et al., 1998a).
Findings in the current study suggests that the initial interaction of unopsonized Pneumocystis organisms with AMs promotes rapid Cdc42 activation, in addition to Rho activation (early portion of biphasic response), followed by a relatively delayed Rac1 activation, and further Rho activation (delayed portion of biphasic response). The data suggest the early activation of Cdc42 and Rho are mediated predominantly through mannose receptors (as supported by the siRNA gene silencing experiments) and that the delayed responses of Rac and Rho were mannose receptor independent (and perhaps mediated by other macrophages receptors such as dectin-1; Brown and Gordon, 2001; Steele et al., 2003), although additional experiments are required to further define these specific events.
The mechanism for impaired RhoGTPase activation in HIV infection was not completely defined. HIV infection was sufficient to impair RhoGTPase activation in response to Pneumocystis. Impaired Rho GTPase activation may in part be attributed to reduced mannose receptor surface expression, based on reduced mRNA (Koziel et al., 1998a) or through increased release of the mannose receptor ectodomain (Fraser et al., 2000). Alternatively, impaired RhoGTPase activation may be due to direct influence by HIV gene products that can influence cellular receptor expression and signal transduction pathways (Emerman and Malim, 1998). HIV-1 tat may down-regulate mannose receptor transcription (Caldwell et al., 2000). HIV-1 vpu impedes NF-κB activation by inhibiting I-κB degradation (Bour et al., 2001) in T-cell lines. HIV-1 nef may alter ASK1 signal transduction pathways (Geleziunas et al., 2001) and down-regulate NF-κB signaling in T-cell lines (Bandres and Ratner, 1994), reduce mannose receptor surface expression on dendritic cells (Quaranta et al., 2002), and down-modulate Fcγ-R expression on monocyte-derived macrophages (De et al., 1998). The influence of specific HIV-1 gene products on AM RhoGTPase signal transduction pathways has not been previously investigated. The observed alteration in Cdc42, Rac1, and Rho in the current study may thus reflect the influence of one or more HIV-related factors and represents an area of active investigation.
The role of other receptors implicated in the AM recognition of Pneumocystis, including fibronectin (Pottratz and Martin, 1990) and immunoglobulin receptors (Masur and Jones, 1978; von Behren and Pesanti, 1978) were not specifically investigated. The observed Cdc42 and Rac phosphorylation in response to IgG-opsonized Pneumocystis in AMs with targeted gene silencing of the mannose receptor suggests that the FcγR pathway was intact. Other limitations of these studies include the possibility that the observed response of human AMs to rodent-derived Pneumocystis may reflect in part the origin of the organisms. Unfortunately, in the absence of reliable culturing methods, Pneumocystis of human origin are not available (Sloand et al., 1993). However, the recent observation that rat-derived Pneumocystis elicit similar chemokine and NF-κB responses comparing human AMs to rat AMs in part validates the use of this model (Zhang et al., 2004). Contaminating rat-derived adherent proteins may be in part responsible for the observed RhoGTPase activity, although the Pneumocystis organisms used in these studies are relatively free of host proteins such as surfactant protein-A (Koziel et al., 1998b). Because the Pneumocystis preparation is composed primarily of the trophic forms, the current findings may represent the predominant macrophage response to the Pneumocystis trophic form and not the cyst or other form. Finally, the possibility that in vitro observations may not accurately reflect events in vivo should be considered.
In conclusion, these data demonstrate that in AMs from healthy individuals, phagocytosis of unopsonized Pneumocystis was mediated predominantly through mannose receptors and was critically dependent on Cdc42 and especially RhoB activation. These data provide a molecular mechanism for mannose receptor-mediated phagocytosis of unopsonized Pneumocystis organisms, and the mechanism is distinct from opsonin-dependent phagocytic receptors. Furthermore, impaired AM Cdc42 and Rho activation may provide the molecular basis for impaired Pneumocystis phagocytosis in AMs from HIV+ persons (Koziel et al., 1998a). Recognizing the spectrum of pathogens recognized by mannose receptors (including Mycobacterium tuberculosis, Cryptococcus neoformans, and Pneumocystis), impaired AM mannose receptor-mediated phagocytosis in the context of HIV infection may contribute to the pathogenesis of opportunistic pneumonia.
Acknowledgments
We acknowledge the participation of all persons who consented to bronchoscopy and the technical assistance of Robert Garland, Rene Andwood, and Lorraine Gryniuk. We acknowledge the generous gift of Martine Armstrong and Frank Richards, who provided Pneumocystis organisms for initial studies. We also are indebted to Naimish Patel and Souvenir Tachado for their critical review of the manuscript and valuable suggestions. None of the authors have conflict of interest disclosures regarding the work in this study. This study was supported by National Institutes of Health research grants RO1 HL63655 (H.K.) and F32 HL71372 (J.Z.).
Article published online ahead of print in MBC in Press on December 1, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-06-0463).
Abbreviations used: GTPases, guanosine triphosphatases; HIV, human immunodeficiency virus; PAK-1, p21-activated kinases; ROCK, Rho-associated coiled-coil kinase.
References
- Aderem, A. (2002). How to eat something bigger than your head. Cell 110, 5-8. [DOI] [PubMed] [Google Scholar]
- Armstrong, M.Y.K., Koziel, H., Rose, R. M., Arena, C., and Richards, F. F. (1991). Indicators of Pneumocystis carinii viability in short-term culture. J. Protozool. 38, 88S-90S. [PubMed] [Google Scholar]
- Bandres, J. C., and Ratner, L. (1994). Human immunodeficiency virus type 1 nef protein down-regulates transcription factors NF-kB and AP-1 in human T cells in vitro after T-cell receptor stimulation. J. Virol. 68, 3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard, V., Bohl, B. P., and Bokoch, G. M. (1999). Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198-13204. [DOI] [PubMed] [Google Scholar]
- Bokoch, G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743-781. [DOI] [PubMed] [Google Scholar]
- Bour, S., Perrin, C., Akari, H., and Strebel, K. (2001). The human immunodeficiency virus type 1 protein inhibits NF-kB activation by interfering with beta-TrCP-mediated degradation of IkB. J. Biol. Chem. 276, 15920-15928. [DOI] [PubMed] [Google Scholar]
- Brown, G. D., and Gordon, S. (2001). A new receptor for beta-glucans. Nature 413, 36-37. [DOI] [PubMed] [Google Scholar]
- Caldwell, R. L., Egan, B. S., and Shephard, V. L. (2000). HIV-1 tat represses transcription from the mannose receptor promoter. J. Immunol. 165, 7035-7041. [DOI] [PubMed] [Google Scholar]
- Caron, E., and Hall, A. (1998). Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717-1721. [DOI] [PubMed] [Google Scholar]
- Chen, F., and Cushion, M. (1994). Use of an ATP bioluminescent assay to evaluate viability of Pneumocystis carinii from rats. J. Clin. Microbiol. 32, 2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De, S. K., Venkateshan, C.N.S., Seth, P., Gajdusek, D. C., and Gibbs, C. J. (1998). Adenovirus-mediated human immunodeficiency virus-1 nef expression in human monocyte-macrophages and effect of nef on downmodulation of Fc-gamma receptors and expression of monokines. Blood 91, 2108-2117. [PubMed] [Google Scholar]
- East, L., and Isacke, C. M. (2002). The mannose receptor family. Biochim. Biophys. Acta 1572, 364-386. [DOI] [PubMed] [Google Scholar]
- Emerman, M., and Malim, M. H. (1998). HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280, 1880-1884. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629-635. [DOI] [PubMed] [Google Scholar]
- Ezekowitz, R.A.B. (1992). The mannose receptor and phagocytosis. In: Mono-nuclear Phagocytes, ed. R.v. Furth, Netherlands: Kluwer Academic Publishers, 208-213.
- Ezekowitz, R.A.B., Williams, D. J., Koziel, H., Armstong, M.Y.K., Warner, A., Richards, F. F., and Rose, R. M. (1991). Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351, 155-158. [DOI] [PubMed] [Google Scholar]
- Fraser, I. P., Koziel, H., and Ezekowitz, R.A.B. (1998). The serum mannose-binding protein and the macrophage mannose receptor are pattern recognition molecules that link innate and adaptive immunity. Semin. Immunol. 10, 363-372. [DOI] [PubMed] [Google Scholar]
- Fraser, I. P., Takahashi, K., Koziel, H., Fardin, B., Harmsen, A., and Ezekowitz, R.A.B. (2000). Pneumocystis carinii enhances the production of soluble mannose receptor by macrophages. Microbes Infect. 2, 1305-1310. [DOI] [PubMed] [Google Scholar]
- Geleziunas, R., Xu, W., Takeda, K., Ichijo, H., and Greene, W. C. (2001). HIV-1 Nef inhibits ASK1-dependent death signaling providing a potential mechanism for protecting the infected host cell. Nature 410, 834-838. [DOI] [PubMed] [Google Scholar]
- Greenberg, S., and Grinstein, S. (2002). Phagocytosis and innate immunity. Curr. Opin. Immunol. 14, 136-145. [DOI] [PubMed] [Google Scholar]
- Hackam, D. J., Rotstein, O. D., Schreiber, A., Zhang, W. J., and Grinstein, S. (1997). Rho is required for the initiation of calcium signaling and phagocytosis by Fc-gamma receptors in macrophages. J. Exp. Med. 186, 955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, J. A., Kafatos, F. C., Janeway, C. A., and Ezekowitz, R.A.B. (1999). Phylogenetic perspectives in innate immunity. Science 284, 1313-1318. [DOI] [PubMed] [Google Scholar]
- Koziel, H., Eichbaum, Q., Kruskal, B. A., Pinkston, P., Rogers, R. A., Armstrong, M.Y.K., Richards, F. F., Rose, R. M., and Ezekowitz, R.A.B. (1998a). Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J. Clin. Invest. 102, 1332-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel, H., Phelps, D. S., Fishman, J. A., Armstrong, M.Y.K., Richards, F. F., and Rose, R. M. (1998b). Surfactant protein-A reduces binding and phagocytosis of Pneumocystis carinii by human alveolar macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 18, 834-843. [DOI] [PubMed] [Google Scholar]
- Lee, D. J., Cox, D., Li, J., and Greenberg, S. (2000). Rac1 and Cdc42 required for phagocytosis, but not NF-kB dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. J. Biol. Chem. 275, 141-146. [DOI] [PubMed] [Google Scholar]
- Limper, A. H., Hoyte, J. S., and Standing, J. E. (1997). The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Invest. 99, 2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, S., Heimerl, C., Fingerle, V., Aepfelbacher, M., and Wilske, B. (2001). Coiling phagocytosis of Borrelia burgdorferi by primary human macrophages is controlled by Cdc42Hs and Rac1 and involves recruitment of Wiskott-Aldrich Syndrome Protein and Arp2/3 complex. Infect. Immun. 69, 1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur, H., and Jones, T. C. (1978). The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J. Exp. Med. 147, 157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralem, T., and Avraham, H. K. (2003). Extracellular matrix enhances heregulin-dependent BRCA1 phosphorylation and suppresses BRCA1 expression through its C terminus. Mol. Cell. Biol. 23, 579-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottratz, S. T., and Martin, W. J. (1990). Mechanism of Pneumocystis carinii attachment to cultured rat alveolar macrophages. J. Clin. Invest. 86, 1678-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta, M. G., Tritarelli, E., Giordani, L., and Viora, M. (2002). HIV-1 nef induces dentritic cell differentiation: a possible mechanism of uninfected CD4+ T cell activation. Exp. Cell Res. 275, 243-254. [DOI] [PubMed] [Google Scholar]
- Ren, X. D., Kiosses, W. B., and Schwartz, M. A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin, S. Z., Rose, R. M., Groopman, J. E., Markham, P. D., and Gallo, R. C. (1986). Human T lymphotropic virus type III infection of human alveolar macrophages. Blood 68, 281-284. [PubMed] [Google Scholar]
- Sloand, E. et al. (1993). The challenge of Pneumocystis carinii culture. J. Euk. Microbiol. 40, 188-195. [DOI] [PubMed] [Google Scholar]
- Steele, C., Marrero, L., Swain, S., Harmsen, A. G., Zheng, M., Brown, G. D., Gordon, S., Shellito, J. E., and Kolls, J. K. (2003). Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the dectin-1 beta-glucan receptor. J. Exp. Med. 198, 1677-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer, J. R., Beard, C. B., Miller, R. F., and Wakefield, A. E. (2002). A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerging Infect. Dis. 8, 891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata, M. et al. (1997). Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990-994. [DOI] [PubMed] [Google Scholar]
- von Behren, L. A., and Pesanti, E. L. (1978). Uptake and degradation of Pneumocystis carinii by macrophages in vitro. Am. Rev. Respir. Dis. 118, 1051-1059. [DOI] [PubMed] [Google Scholar]
- Zhang, J. M., Zhu, J., Imrich, A., Cushion, M. T., Kinane, B. T., and Koziel, H. (2004). Pneumocystis activates human alveolar macrophage NF-kB signaling through mannose receptors. Infect. Immun. 72, 3147-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]


