Abstract
An integral part of cell division is the separation of daughter cells via cytokinesis. There is now good evidence that the completion of cytokinesis requires coordinated membrane trafficking to deliver new membrane to the tip of the furrow and to complete the abscission. Here we have examined membrane traffic in cytokinesis and describe several novel observations. First, we show that Rab11- and FIP3-containing recycling endosomes accumulate near the cleavage furrow and are required for successful completion of cytokinesis. Second, we demonstrate that the Rab11-FIP3 protein complex is intimately involved in the delivery of endosomes to the cleavage furrow. Significantly, although FIP3 recruitment to endosomes is Rab11 dependent, we find that the targeting of FIP3 to the midbody is independent of Rab11. Third, we show that the Rab11-FIP3 complex is required for a late stage of cytokinesis, possibly abscission. Finally, we demonstrate that localization of FIP3 is subject to substantial spatial and temporal regulation. These data provide the first detailed analysis of recycling endosomes in cell division and provide a new model for membrane traffic to the furrow. We propose that the dynamic Rab11-FIP3 interaction controls the delivery, targeting, and fusion of recycling endosomes with furrow during late cytokinesis and abscission.
INTRODUCTION
An integral part of cell division is the physical separation of two daughter cells via a process known as cytokinesis (Scholey et al., 2003). At least two distinct processes are required for successful cytokinesis: formation and constriction of an acto-myosin contractile ring and the delivery of new membrane to the progressing cleavage furrow (O'Halloran, 2000; Scholey et al., 2003). Both of these steps are tightly controlled and crucial for cell abscission, the final separation of the two cells. Although the function of the acto-myosin ring in cell division is well understood, we are only beginning to understand the role of membrane transport during cytokinesis. Evidence suggests that insertion of new membrane at the apex of cleavage furrow is crucial for the successful completion of cellularization in Drosophila embryos (Rothwell et al., 1999; Zhang et al., 2000). Similar requirements for membrane transport and fusion were also observed in Xenopus laevis eggs (Byers and Armstrong, 1986; Bieliavsky et al., 1992).
The plasma membrane of the cleavage furrow is distinct in its lipid and protein composition from the rest of the plasma membrane (Emoto et al., 1996; Umeda and Emoto, 1999; Emoto and Umeda, 2000). The unique composition of cleavage furrow plasma membrane may underscore its ability to be deformed during ingression, as a cell is pinched in two, as well as possibly generating the signals that regulate progression of cytokinesis. Thus, in addition to the delivery of the membrane to compensate for the expanding plasma membrane surface, membrane traffic during cytokinesis could also mediate the delivery of proteins that control the ingression of the cleavage furrow as well as cell-cell abscission. However, the source of this new membrane and the mechanisms of its targeting to cleavage furrow remains obscure.
One hypothesis is that telophase microtubules serve as tracks for motor-mediated delivery of vesicles to the cleavage furrow during cell abscission (Finger and White, 2002; Shuster and Burgess, 2002). Consistent with this idea, recent work in Xenopus suggests that membrane in the cleavage furrow is not derived from preexisting plasma membrane, but is delivered from internal stores (Byers and Armstrong, 1986; Bieliavsky et al., 1992). Endosomes are perfectly suited to be a membrane source during cytokinesis, and a role for the endocytic machinery in cytokinesis has recently been suggested. Dynamin, a protein involved in clathrin coat–dependent endocytosis, is known to localize to the midbody, and mutations in dynamin have been shown to inhibit ingression of the cleavage furrow (Swanson and Poodry, 1980). Furthermore, α-adaptin, a component of coat proteins involved in receptor-mediated endocytosis, is localized near the cleavage furrow during cellularization of Drosophila embryo (Dornan et al., 1997). Syntaxin 1, a known plasma membrane SNARE, was also shown to be present in the cleavage furrow and is required for cell division (Conner and Wessel, 1999; Jantsch-Plunger and Glotzer, 1999). Although the dynamics of endosomes during cell division remain to be understood, it has been shown that most of the components that regulate endocytic membrane traffic remain associated with endosomes during mitosis (Hobdy-Henderson et al., 2003).
Rab11 is small GTPase that plays a key role in regulating the trafficking of plasma membrane receptors through endosomes. Rab11 has recently been implicated in regulating membrane delivery to the cleavage furrow. In Caenorhabditis elegans embryos, depletion of Rab11 by RNAi causes cytokinesis defects, including furrow regression and failure in cell scission (Skop et al., 2001). Furthermore, it has been demonstrated that Rab11-containing vesicles are required for Drosophila furrow formation during cellularization (Pelisser et al., 2003; Riggs et al., 2003). Thus, it was proposed that Rab11 GTPase may regulate the delivery of endocytic vesicles to the cleavage furrow during cellularization (Riggs et al., 2003, Strickland and Burgess, 2004). However, the mechanisms involved remain unclear. Furthermore, whether there is a similar requirement for Rab11 in mammalian cytokinesis remains untested.
The cycling between GTP- and GDP-bound forms of Rab proteins regulates the recruitment of various effector proteins to cellular membranes. These effector proteins regulate the targeting and fusion of transport vesicles (Gonzalez and Scheller, 1999). Recently, we and others have identified a novel family of Rab11-interacting proteins (FIP; Shin et al., 1999; Prekeris et al., 2000, 2001; Hales et al., 2001; Hickson et al., 2003), which all share a highly conserved, 20-amino acid motif at the C-terminus of the protein, known as the Rab11-binding domain (RBD; Prekeris et al., 2001; Meyers and Prekeris, 2002). Interestingly, the C-termini of FIP3 and FIP4 (also known as arfophilin1 and arfophilin2) have homology to the Drosophila protein nuclear fallout (Nuf). Nuf was originally identified as a protein required for cellularization (Rothwell et al., 1998; Hickson et al., 2003; Riggs et al., 2003). However, it remains unclear whether FIP3 and FIP4 are actually mammalian homologues of Nuf because the N-terminal half of FIP3 and FIP4 have no homology to Nuf. The N-terminus of FIP3 contains several protein motifs such as a proline-rich region, an ARF-binding domain, and EF-hands. It is likely that FIP3/FIP4 and Nuf may play different roles reflecting crucial functional distinctions between cellularization and cytokinesis.
In this study we investigated the importance of endocytic membrane traffic during cytokinesis in mammalian cells and characterized the regulatory interactions that control membrane targeting to the cleavage furrow. First, we show that Rab11-containing recycling endosomes accumulate near the cleavage furrow and are required for successful completion of cytokinesis in mammalian cells. Second, using a combination of dominant negative mutants and RNAi, we demonstrate that Rab11 is a key component involved in the delivery of endosomes to the cleavage furrow. Consistent with this we show that both FIP3 and FIP4 accumulate on endosomes in the furrow and the midbody. Significantly, although FIP3 recruitment to endosomes is Rab11 dependent, we find that the targeting of FIP3 to the midbody during late cytokinesis in independent of Rab11. Third, we demonstrate that Rab11, complexed with FIP3 and possibly FIP4, but not other FIP proteins, is required for correct targeting of endocytic vesicles to the cleavage furrow. Moreover, we show that the Rab11-FIP3 complex is required for completion of cytokinesis. Finally, using FIP3-GFP, we show that the localization of FIP3 is subject to substantial spatial and temporal regulation. FIP3 localizes strongly to centrosomes during early anaphase before rapidly moving to the furrow at the onset of cytokinesis. After abscission, FIP3 returns to the centrosome. Together, these data suggest that accumulation of FIP3 in the midbody and its interaction with Rab11-containing endosomes allows the docking and subsequent fusion of endocytic vesicles with the apex of the cleavage furrow. These data provide the first detailed analysis of recycling endosomes in mammalian cell division and provide a new model for membrane traffic to the furrow. We propose that the dynamic Rab11-FIP3 interaction controls the delivery, targeting, and subsequent fusion of vesicles derived from recycling endosomes with the growing furrow. The dynamic redistribution of FIP3 during mitosis serves to couple recycling endosome-derived membrane vesicle traffic with the cell cycle, thus regulating furrowing and abscission.
MATERIALS AND METHODS
Reagents and Plasmids
Cell culture reagents were obtained from Life Technologies (Rockville, MD) unless otherwise specified. Most chemicals were obtained from Sigma (St. Louis, MO). Mouse monoclonal anti-myc antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). DAPI and transferrin conjugated to Texas Red (Tf-TxR) were purchased from Molecular Probes (Eugene, OR). Fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG and Texas Red–labeled anti-mouse IgG antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Mouse monoclonal anti-EEA1 antibody was purchased from BD Biosciences (San Diego, CA). Mouse monoclonal antitransferrin receptor and rabbit polyclonal anti-Rab11a antibodies were obtained from Zymed Laboratories (South San Francisco, CA). Mouse monoclonal anti-Rab4 and anti-Rab5 antibodies were purchased from Transduction Laboratories (San Diego, CA). Mouse antitubulin antibody was purchased from BD Biosciences. Rabbit polyclonal anti-Rab11a/b antibodies were described previously (Meyers et al., 2002). Goat polyclonal anti-FIP4 antibody was described previously (Hickson et al., 2003). Anti-C-Nap1 was described previously (Fry et al., 1998). Polyclonal rabbit anti-FIP3 antibody was prepared by immunization with recombinant FIP3, expressed and purified from Escherichia coli as a GST-fusion protein. cDNAs encoding FIP3 were cloned in pEGFP-N1 or pEYFP-N1. cDNA encoding CFP-Rab11a was a generous gift from Dr. Alexander Sorkin (UCHSC).
Cell Culture and Immunofluorescence Microscopy
HeLa and NIH-3T3 cells were cultured as described previously (Prekeris et al., 2001; Meyers et al., 2002). For immunofluorescence microscopy, cells were fixed either with 4% paraformaldehyde or with ice-cold methanol (for FIP3 staining), and nonspecific sites were blocked with phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin and 1% fetal bovine serum. After incubation with specific antibodies, samples were extensively washed and mounted in VectaShield (Vector Laboratories, Burlingame, CA). For localization of Rab11, EEA1, Tf, transferrin receptor (TfR), and FIP3, cells were imaged with an inverted Zeiss Axiovert 200M deconvolution microscope (Thornwood, NY) and images processed using Intelligent Imaging Innovations (Denver, CO) three-dimensional (3D) rendering and exploration software. High-resolution FIP4 imaging was performed either using MicroMax cooled CCD camera (5 MHz; Roper Scientific, Tucson, AZ) on a DeltaVision Restoration Microscope (Applied Precision, LLC, Issaquah, WA) built around a Nikon TE200 stand (Garden City, NY), or using a CoolSnap HQ cooled CCD camera (Photometrics, Tucson, AZ) on a DeltaVision Spectris Restoration microscope (Applied Precision Life Science, Issaquah, WA) built around Olympus IX70 stand (Lake Success, NY). 3D data set were acquired with 100×/1.4 NA lens with an optical section every 0.2 μm. All the images were deconvolved using the constrained iterative algorithm (Swedlow et al., 1997; Walace et al., 2001). TIFF or Photoshop images (Adobe, San Jose, CA) were derived from single optical sections unless otherwise stated in the figure legends.
Time-lapse Microscopy
HeLa cells were plated on collagen-coated coverslips and mounted on the heater platform PH2 with TC-344B dual automatic temperature controller (Warner Instruments, Hamden, CT). Cells were imaged at 37°C using phase illumination on an inverted microscope (Zeiss Axiovert 200M) using 63× oil immersion lens. Images were acquired and analyzed using Intelligent Imaging Innovations 3D rendering and exploration software.
Fluorescence Resonance Energy Transfer Analysis
For fluorescence resonance energy transfer (FRET) analysis, cells were cotransfected with proteins tagged with YFP and CFP. Cells were imaged and corrected FRET (cFRET) was calculated using Intelligent Imaging Innovations 3D rendering and exploration software as described previously using the following equation: cFRET = FRET - 0.4 × CFP - 0.037 × YFP (Sorkin et al., 2000). Normalized FRET (NFRET) was calculated using the following equation: NFRET = cFRET/CFP. Only cells expressing similar amounts of CFP- and YFP-tagged proteins (YFP/CFP = 0.5–2.0) were included in the FRET analysis.
RNA Interference Analysis
Rab11a and Rab11b isoforms were depleted with siRNA oligonucleotides based on human Rab11a and Rab11b sequences (Rab11a, 5′-aatgtcagacagacgcgaaaa-3′; Rab11b, 5′-aagcacctgacctatgagaac-3′). FIP3 was depleted with siRNA oligonucleotides based on human FIP3 sequence (5′-aaggcagtgaggcggagctgt-3′). siRNAs were cotransfected into HeLa cells using Lipofectamine 2000 (Life Technologies). Transfected cells were incubated for either 48 or 72 h and analyzed for Rab11a/b or FIP3 expression by Western blotting. Remaining cells were used for either flow cytometry or fluorescence microscopy studies.
For subcellular fractionation studies, mock- or siRNA-transfected HeLa cells were trypsinized and lysed using a glass homogenizer. Cells were centrifuged at 2000 × g for 5 min to remove nuclei and intact cells. Post-nuclear supernatant was then centrifuged at 100,000 × g for 1 h to separate cytosolic from membrane fractions. Equal amounts of cytosol and membrane proteins (as determined by Bradford assay) were separated on SDS/PAGE and immunoblotted with anti-Rab11, anti-Rab5, and anti-FIP3 antibodies.
Cell Cycle Analysis by Flow Cytometry
HeLa cells were either mock-transfected or transfected overnight with siRNAs against Rab11A and B (12 μg of siRNA, 5 μl Lipofectamine 2000 for 1 well of a 6-well plate). The next day the cells were split into T25 cm2 flasks. Two days after splitting, the cells were trypsinized. In control experiments, HeLa cells were also incubated with 80 μM blebbistatin in dimethyl sulfoxide (Calbiochem, La Jolla, CA) for 16 h at 37°C before being trypsinized. The media from the cells and the PBS from the wash step were collected and added back to the trypsinized cells to ensure no cells were lost in the trypsinization process. The cells were pelleted and fixed by resuspending in 1 ml of prechilled 70% methanol in PBS for 1 h at -20°C. The cells were pelleted and washed once with 1 ml of PBS. The cells were pelleted and resuspended in 1 ml of 1 mg/ml propidium iodide, 0.5 mg/ml RNAse in PBS for 30 min at 37°C. Approximately 15,000 cells were then analyzed on a BD FACSCalibur (San Jose, CA). Cell doublets were excluded by plotting pulse width versus pulse area and gating on the single cells. The collected data were analyzed using the software package Flowjo using the “Dean-Jett-Fox” or “Watson Pragmatic” algorithm (Fox, 1980; Watson et al., 1987).
RESULTS
Rab11-containing Recycling Endosomes Are Localized to the Cleavage Furrow
The insertion of membrane at the cleavage furrow is an important step during cytokinesis (O'Halloran, 2000). Because endosomes mediate recycling of proteins to the plasma membrane, we tested whether endosomes may mediate membrane delivery to the cleavage furrow. After endocytosis, proteins are transferred to early endosomes (EE), from where they can be transported back to the cell surface either directly or via a centrally localized compartment known as recycling endosomes (RE; Mellman, 1996). To determine the localization of EE or RE during mitosis, we stained HeLa cells with the EE marker EEA1 and the RE marker Rab11 (Figure 1, A–D). During metaphase and anaphase EE and RE become distributed throughout the cytoplasm (Figure 1, A and B). Interestingly, these endocytic compartments remained as distinct nonoverlapping structures, suggesting that EE and RE retain their identity during mitosis. As cells progressed into telophase, Rab11-containing RE became highly concentrated around the cleavage furrow (Figure 1C and Supplementary Figure 2, D–F). Furthermore, weak Rab11 staining could also be observed inside the midbody (Figure 1C, asterisk). In contrast, EEA1-containing EE remained scattered through the cytoplasm and were never observed concentrated close to or within the midbody/furrow (Figure 1D). During interphase, the majority of Rab11 is associated with RE in the pericentrosomal region (Ullrich et al., 1996). To test whether Rab11-containing endosomes during mitosis represents bona fide RE rather then a specialized endocytic pathway, we costained HeLa cells with Rab11 and another well-characterized RE marker, TfR. As shown in Figure 1, E–I, Rab11 and TfR largely colocalized (interphase 60.5 ± 4.7% colocalization; metaphase 53.2 ± 5.2% colocalization; anaphase 45.1 ± 5.2 colocalization; telophase 66 ± 6.2% colocalization) during mitosis. Furthermore, endosomes present at the cleavage furrow were also positive for both Rab11 and TfR (Figure 1, J–L, asterisk), suggesting that RE rather then EE mediates membrane delivery to the cleavage furrow/midbody.
Figure 1.
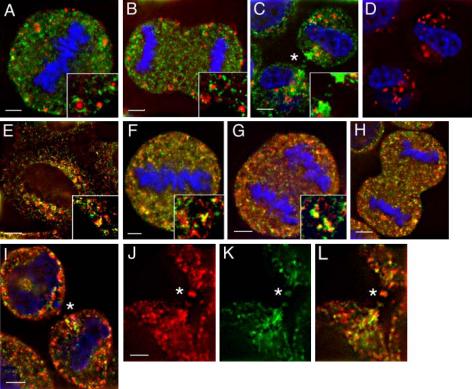
Rab11-containing endosomes are localized to the cleavage furrow. HeLa cells at different stages of mitosis were fixed and stained with anti-Rab11 (A–L, green), anti-EEA1 (A–D, red), or anti-TfR (E–L, red) antibodies and DAPI DNA stain (A–D, F–H, blue). Asterisks mark the cleavage furrow/midbody. Yellow in A–C, E–I, and L represents the degree of colocalization. Scale bars, 2 μm (A–I), 1 μm (J–L).
Two previous studies have shown that during metaphase and early anaphase, endocytic traffic is markedly reduced in mammalian cells (Sager et al., 1984; Warren et al., 1984). Indeed, we have also found that the uptake of transferrin is reduced during these stages of the cell cycle (unpublished data). Interestingly, we and others have found that endocytosis resumes during late anaphase. Moreover, we have found that the majority of transferrin internalized during telophase accumulated in recycling endosomes in close proximity to the furrow (unpublished data). These data support the notion that recycling endosomes are involved in active membrane transport to the growing furrow.
Rab11 Is Required for Cytokinesis
Despite evidence supporting a role for Rab11 in cellularization in Drosophila embryos, the role of Rab11 in cytokinesis in mammalian cells remains untested. This is particularly important because the mechanisms that regulate cellularization are distinct to those that regulate cytokinesis. We therefore sought to test the hypothesis that Rab11 is involved in mammalian cell cytokinesis by disrupting its function using three distinct approaches. First, HeLa cells were transfected with a myc-tagged dominant negative Rab11a mutant (S25N) that was previously shown to inhibit Rab11-dependent protein recycling (Ullrich et al., 1996). Cells were then fixed and stained with anti-myc (Figure 2A) and anti-tubulin antibodies (Figure 2B). As shown in Figure 2C, overexpression of Rab11-S25N significantly increased the number of binucleate cells, an indication of increased failure in cytokinesis. Second, the depletion of Rab11 with Rab11 siRNA oligonucleotides also increased the number of binucleate cells (Figure 2, D and E). In addition to binucleate cells, a number of cells also exhibited multiple nuclei as well as chromatin bridges between daughter cells (unpublished data). The Rab11 RNAi was specific, because it had no effect on the expression level of Rab5 GTPase (Figure 2D). Furthermore, transfection of HeLa cells with scrambled Rab11 siRNA did not have any effect on the number of binucleate cells (unpublished data). Interestingly, Rab11 depletion resulted in increased levels of Rab4 GTPase (Figure 2D). Because Rab4 is also known for its role in endocytic recycling, it is likely that this represents a compensatory increase as a result of Rab11 depletion.
Figure 2.
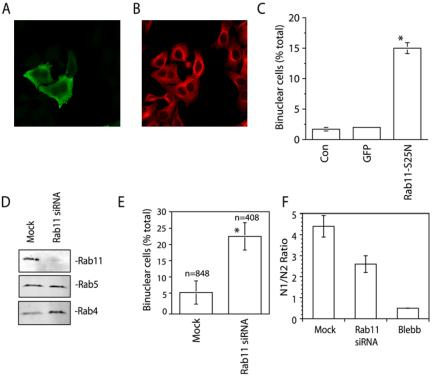
Rab11 is required for cytokinesis. (A–C) In A and B, HeLa cells were transfected with myc-Rab11-S25N and stained with antimyc (A) and antitubulin (B) antibodies. The number of binucleate cells in untransfected (Con), GFP only (GFP), or myc-Rab11-S25N-transfected cells is quantitated in C. (D–F) HeLa cells were transfected with Rab11a and Rab11b siRNA. The specificity and extent of Rab11a/b knockdown is shown in D. The effects of Rab11a/b siRNA on cytokinesis were evaluated by either counting binucleate cells (E) or performing a flow cytometry analysis to measure DNA content (F). Blebb is blebbistatin, a known inhibitor of cytokinesis. In E, n is the number of cells counted. In C, E, and F data are the means ± SE. *Statistically significant difference at p < 0.01. The profiles of flow cytometry data are shown in Supplementary Figure 1.
To confirm this data, we used flow cytometry to measure the DNA content in untreated or Rab11 siRNA-treated HeLa cells. The data were expressed as a N1/N2 ratio, with N2 representing binuclear cells. Consistent with our immunofluorescence data, Rab11 siRNA increased the number of N2 cells and decreased number of N1 cells compared with untreated cells (Figure 2F and Supplementary Figure 1), although the Rab11 siRNA effect was not as dramatic as treatment by blebbistatin, a small-molecule inhibitor of myosin known to block cytokinesis (Straight et al., 2003).
Third, we injected HeLa cells with either anti-Rab11 or anti-Rab5 antibodies. Injection of anti-Rab11 antibody resulted in almost fivefold enhancement of binucleate HeLa cells compared with IgG or anti-Rab5a injected cells (unpublished data). Collectively, all these data argue that Rab11 is essential for the completion of general cytokinesis and support the notion that recycling endosomes may be intimately involved in membrane traffic to the growing furrow.
Rab11-binding Proteins FIP3 and FIP4 Are Recruited to the Cleavage Furrow and Midbody during Cytokinesis
Because the Rab GTPases work by recruiting effector proteins (Gonzalez and Scheller, 1999), we next sought to identify the Rab11 effectors involved in cytokinesis. Several novel Rab11-interacting proteins have been recently identified and are often referred to as FIP proteins (Prekeris et al., 2000, 2001; Hales et al., 2001). Based on sequence homology, FIPs can be divided into two main subfamilies: C2-domain containing class I FIPs and EF-hands containing class II FIPs (Prekeris, 2003). To determine whether FIPs, and in particular which class of FIP proteins, may mediate Rab11's role in cytokinesis, we investigated the subcellular localization of class I (Rip11, RCP, and FIP2) and class II (FIP3 and FIP4) FIPs in the cell cycle. Class I FIPs did not localize to the RE present in the cleavage furrow (Supplementary Figure 2, A and B); thus, they are not likely to play a direct role in cytokinesis (Hobdy-Henderson et al., 2003). More importantly, RNAi knockdown of RCP and Rip11 had no effect on the ratio between mono-nucleate and binucleate cells as measured by flow cytometry (Supplementary Figure 2C). In marked contrast, FIP3 was highly enriched in the proximity of the late cleavage furrow (Figure 3, F–J, and Horgan et al., 2004). During interphase, metaphase and early anaphase, FIP3 was present on endosomes scattered throughout the cytoplasm (Figure 3, A–C), a distribution highly reminiscent of Rab11 (Figure 1). It is noteworthy that a proportion of FIP3 is associated with the centrosome throughout the cell cycle (Figure 3, A–C and Supplementary Figure 3) and on the spindle midzone in anaphase (Figure 3D). In telophase FIP3 started accumulating at the minus ends of midzone microtubules (Figure 3E) and remained localized to the developing midzone during telophase until the completion of cytokinesis (Figure 3, F–J). The staining at the midbody was specific because it could be blocked by preincubation of anti-FIP3 antibodies with purified GST-FIP3 protein (Supplementary Figure 4). Although some FIP3 staining could be observed on vesicular structures in the cytoplasm, the large fraction of FIP3 in telophase colocalized with midzone microtubules (Figure 3, I and J). Furthermore, a significant fraction of FIP3 localized within the midbody itself (Figure 3, I and J). Consistent with this, FIP3 in the cleavage furrow colocalized with components of the Exocyst (Supplementary Figure 5, A–C), a protein complex thought to play a role in vesicle targeting to the cleavage furrow in yeast (TerBush et al., 1996). FIP4, the close homologue of FIP3 (Hickson et al., 2003), also exhibited a similar pattern of staining during cytokinesis (Figure 3, K–M). Again the staining was specific because it could be blocked by purified GST-FIP4 (unpublished data). Interestingly, like FIP3, FIP4 has been shown to be localized to the centrosome (Hickson et al., 2003).
Figure 3.
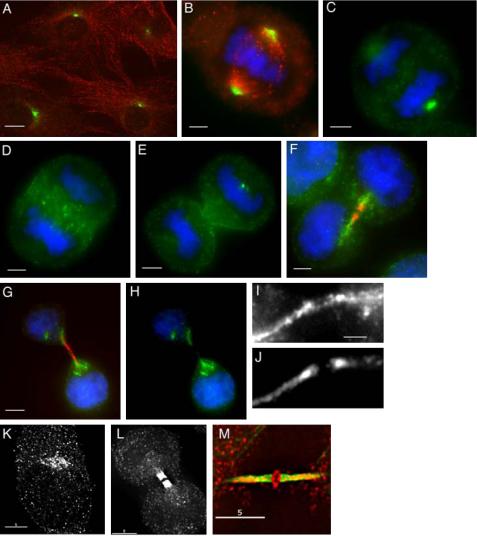
FIP3 and FIP4 localizes to the midbody during cytokinesis. HeLa cells were fixed at different stages of mitotic cell division and stained with anti-FIP3 (A–H, green, and I), anti-FIP4 (K–L, and M, red), or antitubulin (A, B, F, G, red, and J and M, green) antibodies. DNA was visualized with DAPI (B–H, blue). Scale bars, 5 μm (A), 2 μm (B–H), 1 μm (I).
Rab11 Mediates FIP3 Association with Endosomes
Classical Rab effector proteins are recruited to the membranes via their interaction with Rab GTPases. To test whether FIP3 and Rab11 are localized to the same organelles during cytokinesis, we costained HeLa cells with anti-Rab11, anti-FIP3, and antitubulin antibodies. As shown in Figure 4, A–D, organelles that accumulated in the close proximity of the cleavage furrow contained both Rab11 and FIP3. Interestingly, the centrosomal FIP3 also colocalized with Rab11 (Figure 4, A–D, asterisk).
Figure 4.
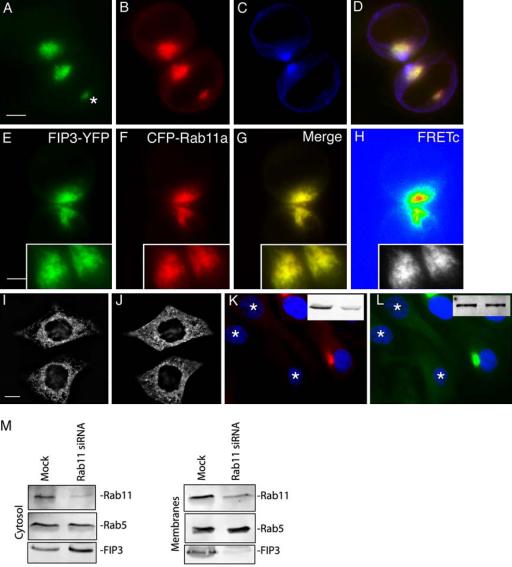
FIP3 is recruited to endosomes by binding to Rab11. (A–D) HeLa cells were fixed and stained with anti-FIP3 (A and D), anti-Rab11 (B and D), and antitubulin (C and D) antibodies. (E–H) HeLa cells were cotransfected with FIP3-YFP (E) and CFP-Rab11A (F), fixed, and imaged. cFRET image (H) was generated as described in Materials and Methods. NFRET was calculated using the equation NFRET = cFRET/CFP and is an average from at least five randomly picked cells. The yellow signal in G represents the overlap between CFP and YFP. (I and J) myc-Rab11-S25N–transfected HeLa cells were fixed and stained with anti-FIP3 (J) and antimyc (I) antibodies. (K and L) A stable HeLa cell line expressing FIP3-GFP (L) was transfected with Rab11 siRNAs. After 72 h, cells were fixed and stained with anti-Rab11 (K) antibodies. An asterisk marks a cell with depleted Rab11. The total amount of Rab11 (K) and FIP3-GFP (L) are shown in insets. Left lane is mock and right lane is Rab11 siRNA transfected cells. (M) HeLa cells were either mock-transfected or transfected with Rab11 siRNAs. After a 72-h incubation, cells were fractionated into cytosol and membranes, followed by immunoblotting with anti-Rab11, anti-Rab5, and anti-FIP3 antibodies.
To test whether Rab11 and FIP3 actually interact with each other, we measured fluorescence resonance energy transfer (FRET) between FIP3-YFP and CFP-Rab11a. We have previously reported that FIPs and CFP-Rab11A can exhibit FRET if YFP is attached to the C-terminus of FIP proteins (Peden et al., 2004). Previously published data suggest that a C-terminal tag does not affect the ability of FIPs to interact with Rab11 (Prekeris et al., 2001; Peden et al., 2005). Furthermore, the localization of FIP3-YFP was similar to the localization of endogenous FIP3 (unpublished data). As shown in Figure 4, E–H, we could detect FRET between FIP3-YFP and CFP-Rab11a (also see Supplementary Figure 6), suggesting that FIP3 and Rab11 form a complex during cell division. The FRET was specific to Rab11A, because CFP-Rab5a and CFP-Rab4a did not elicit any FRET with FIP3-YFP (Supplementary Figure 6). Furthermore, FRET was also not observed if FIP3-YFP was coexpressed with CFP-Rab11-CC, a mutant lacking a geranylgeranylation site and incapable of associating with endosomes (Supplementary Figure 6).
These FRET studies indicate that FIP3 and Rab11 form a complex on endosomes associated with the cleavage furrow, suggesting that FIP3 may be recruited to the membranes via its interaction with Rab11. To directly test this hypothesis, we transfected HeLa cells with dominant negative Rab11-S25N and examined the distribution of FIP3. As shown in Figure 4, I and J, overexpression of Rab11-S25N resulted in translocation of FIP3 from endosomes to the cytosol, although some punctuate FIP3 staining could still be observed (Figure 4J).
To confirm that Rab11 is required for FIP3 recruitment to the membranes, we depleted Rab11 in HeLa cells (Figure 4K, asterisk) expressing FIP3-GFP (Figure 4L). In agreement with Rab11-S25N data, Rab11 knockdown resulted in loss of both membrane- and centrosome-associated FIP3-GFP (Figure 4L). Overall FIP3-GFP fluorescence levels were also decreased. This is likely due to the relocation of FIP3-GFP to cytosol because the cellular levels of FIP3-GFP did not change, as determined by Western blotting (Figure 4L, inset). To test whether endogenous FIP3 also depends on binding to Rab11 to be recruited to the membranes, we fractionated HeLa cells 72 h after either mock transfection or transfection with Rab11 siRNA. As shown in Figure 4M, Rab11 knockdown resulted in translocation of FIP3 from the membranes to the cytosol. Thus, association of FIP3 with endocytic membranes and the centrosome is dependent on its binding to Rab11.
Rab11 and FIP3 Act at a Late Stage of Cytokinesis
The data presented above suggest that FIP3 may act in concert with Rab11 to regulate membrane targeting to the cleavage furrow during cytokinesis. Consistent with this, we found that depletion of FIP3 using siRNA resulted in a significant increase in the number of binucleate as well as multinucleate cells (Figure 5, A and B). Such experiments do not, however, reveal what stage of cytokinesis Rab11 and FIP3 regulate. Binucleate cells may arise either from a failure to furrow or by regression of the furrow if abscission fails. Interestingly, in both Rab11 siRNA and FIP3 siRNA-treated cells we could observe cells with clear midbody structures (Figure 5, C–E). One interpretation of this is that furrowing and midbody formation do not require Rab11 and FIP3, suggesting that they play a role at a later stage of cytokinesis, possibly abscission.
Figure 5.
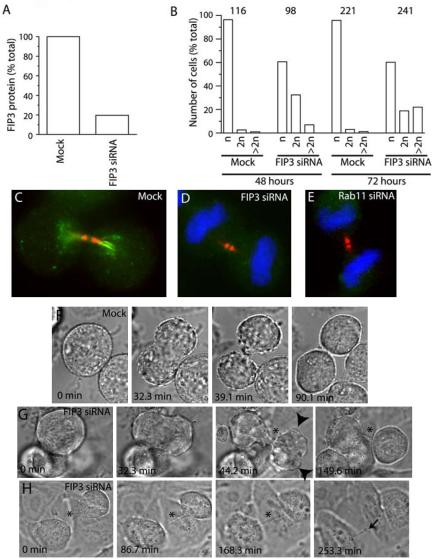
FIP3 and Rab11 regulate the late stage of cytokinesis. (A) HeLa cells were transfected with FIP3 siRNA and incubated for 48 h. Cells were then harvested and levels of FIP3 protein determined by immunoblotting. (B) HeLa cells were transfected with FIP3 siRNA and incubated for 48 or 72 h before they were fixed and stained with anti-FIP3 antibodies and DAPI stain. The number of cells with one, two, or more than two nuclei were then counted and expressed as a percentage of the total number of cells. The numbers on top of the figure indicate the total number of cells counted for each condition. (C–E) HeLa cells were either mock-transfected (C) or transfected with FIP3 (D) or Rab11 (E) siRNAs. Cells were incubated for 72 h, fixed, and stained with anti-FIP3 (C and D, green), anti-Rab11 (E, green), or antitubulin (C–E, red) antibodies. Nuclei were visualized using DAPI stain (D and E, blue). (F) Mock-transfected HeLa cells (also see Supplementary Movie 1) were imaged using phase-contrast time-lapse microscopy. Cells in the metaphase were chosen for analysis and images were collected every 1.4 min for 2.5 h. (G) HeLa cells transfected with FIP3 siRNA were incubated for 72 h and then imaged using time-lapse microscopy (also see Supplementary Movie 2). Cells in metaphase were chosen for analysis and images were collected every 1.4 min for 2.5 h. Arrowheads mark plasma membrane protrusions during telophase. An asterisk marks a cytoplasmic bridge between daughter cells. (H) HeLa cells transfected with FIP3 siRNA were incubated for 72 h and then imaged using time-lapse microscopy (also see Supplementary Movie 3). Cells attempting cytokinesis were imaged using phase-contrast time-lapse microscopy. Images were collected every 1.4 min for 4.5 h. During most of time-lapse series cells remained connected by a narrow cytoplasmic bridge (asterisk) and did not undergo abscission. At the end of the time-lapse series, cells broke the cytoplasmic bridge (arrow) and separated.
To test this model we performed phase-contrast time-lapse imaging experiments. These data (Figure 5, F, G, and H, Supplementary Movies 1, 2, and 3) revealed that Rab11 and FIP3 are required for a late stage of cytokinesis. In mock-transfected HeLa cells cytokinesis is essentially complete and midbody no longer visible within 93 ± 12.2 min (n = 4; Figure 5F, Supplementary Movie 1). In contrast, HeLa cells treated for 72 h with FIP3 siRNA were defective in late cytokinesis and abscission. FIP3 siRNA-treated cells were capable of initiating furrowing (Figure 5G). Interestingly, furrowing cells continuously formed and retracted multiple membrane protrusions (Figure 5G, arrowheads, Supplementary Movie 2). Daughter cells remained connected by a long cytoplasmic bridge during the duration of the time-lapse series (Figure 5, G and H, Supplementary Movies 2 and 3). Often, daughter cells never separated, but rather became detached and appeared to undergo apoptosis. Consistent with this, transfection of HeLa cells with FIP3 (or Rab11) siRNA for 72 h resulted in significantly lower number of cells compared with a control or mock-transfected HeLa cells. A portion of the cells continued pulling on the cytoplasmic bridge until eventually it broke, separating daughter cells (Figure 5H, arrow, Supplementary Movie 3). This type of separation explains why RNAi of cytokinesis regulating proteins (including Rab11 and FIP3) usually gives only a partial phenotype (Low et al., 2003; Neef et al., 2003; Tomas et al., 2004).
FIP3 Associates with the Midbody in a Rab11-independent Manner
Our data suggest that FIP3 recruitment to the endosomes is dependent on Rab11 binding and that cytokinesis requires both Rab11 and FIP3 function. To explore this possibility, we transfected HeLa cells with a mutant of FIP3 defective in Rab11 binding, GFP-FIP3-I737E (Figure 6, A–H). Consistent with a role for Rab11 in FIP3 association with endosomes, GFP-FIP3-I737E exhibited predominately cytosolic and plasma membrane staining (Figure 6, B and D). Interestingly, although endosome-associated GFP-FIP3 staining was reduced significantly, GFP-FIP3-I737E was still recruited to the midbody during cytokinesis (Figure 6, B and C, asterisk). In experiments not shown here, we also found that FIP4 recruitment to the midbody was not blocked by abrogation of Rab11 function or by similar mutation that abolished FIP4 and Rab11 interaction (our unpublished work). This suggests that FIP3 and FIP4 association with the midbody is independent of its binding to Rab11. Furthermore, the I737E mutation appears to increase the amount of FIP3 in the midbody, perhaps because of loss of FIP3 targeting to the endosomes.
Figure 6.
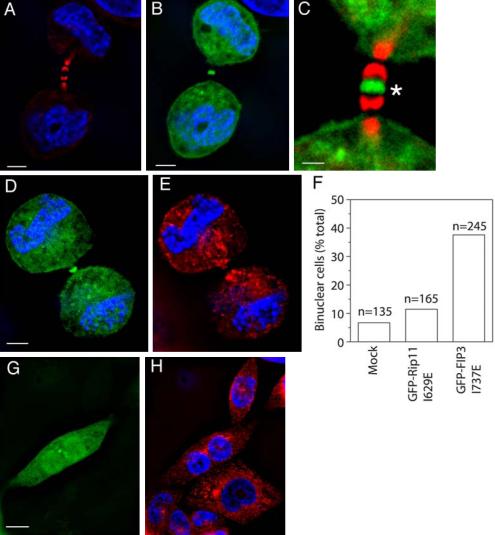
FIP3 and Rab11 interactions are required for cytokinesis. (A–E) HeLa cells were transfected with GFP-FIP3-I737E (B–D, green) and stained with antitubulin antibodies (A and C, red) and anti-Rab11 (E, red) antibodies and DAPI (A, B, D, and E, blue). Asterisk marks GFP-FIP3-I737E present in the midbody. (F–H) HeLa cells were transfected with GFP-FIP3-I737E (G, green), fixed, and stained with anti-Rab11 (H, red) antibodies and DAPI (H, blue). In F is the quantitation of the number of binucleate cells in untransfected HeLa cells or in cells transfected with GFP-Rip11-I629E or GFP-FIP3-I737E. n is the number of counted cells.
Rab11 and FIP3 Complex Formation Is Required for Completion of Cytokinesis
We next sought to determine whether the functional interaction of Rab11 and FIP3 was required for completion of cytokinesis. Because FIP3 localized to both endocytic vesicles in the furrow and the midbody, it is possible that FIP3 may function to control docking of Rab11-positive membranes in the furrow region. To test this, we overexpressed GFP-FIP3-I737E and analyzed its role in cell division. Because GFP-FIP3-I737E can still be recruited to the midbody, but cannot bind to Rab11, it should act as a dominant negative inhibitor, preventing Rab11-positive vesicle docking to the midbody and subsequent fusion with the cleavage furrow. As shown in Figure 6, D–H, overexpression of GFP-FIP3-I737E dramatically increased the number of binucleate cells, while having no effect on overall distribution of Rab11-containing RE. An equivalent mutation in Rip11 (a class I FIP) had no effect on the number of binucleate cells (Figure 6F), suggesting that the effect is specific to class II FIPs (FIP3 and FIP4).
FIP3 Traffics between Centrosomes and Furrow
The data presented above suggest a model in which Rab11 interacts with FIP3 and that this interaction regulates membrane traffic between RE and the cleavage furrow. If this model is correct, then the prediction would be that FIP3 traffic and/or localization will be under strict spatial and temporal control during the cell cycle. The data presented above (Figures 2 and 3) reveal that the distribution of FIP3 and FIP4 in interphase cells is quite distinct to that observed in mitosis. To more clearly define the dynamics of FIP3 redistribution, we expressed FIP3-GFP in cells and examined its movement in real time (Figure 7, Supplementary Movies 4 and 5). During metaphase and early anaphase FIP3-GFP is predominately localized to endosomal structures in the cytosol (Figure 7A). By contrast, during late anaphase, just after furrow initiation, FIP3-GFP strikingly redistributes to the centrosome within ∼5 min (Figure 7A). During the late phase of cytokinesis, FIP3 relocates to the cleavage furrow where it stays until abscission (Figure 7, A and B). Interestingly, translocation of FIP3 from centrosomes to the furrow coincides with formation of the midbody (unpublished data). Finally, upon separation of the daughter cells, FIP3 moves back to the centrosome (Figure 7B, Supplementary Movie 5). Such data argue that Rab11-FIP3 complex regulates transport of endosomes from centrosomes to the cleavage furrow, possibly via midzone microtubules.
Figure 7.
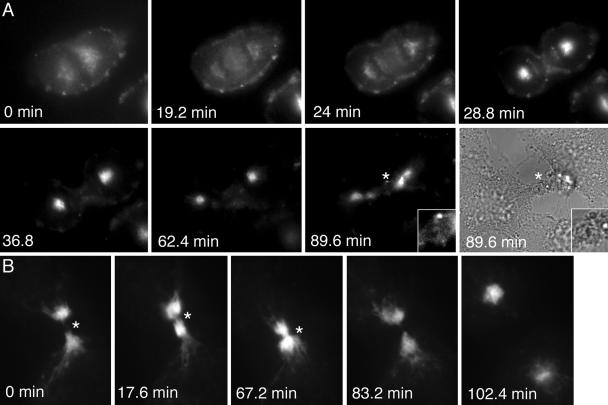
Temporal and spatial dynamics of FIP during mitosis. HeLa cells stably expressing FIP3-GFP were plated on collagen-coated coverslips and imaged using time-lapse microscopy (see Supplementary Movies 4 and 5). Images were collected every 1.4 min for 2.5 h. (A) A cell in metaphase was chosen for analysis. An asterisk marks midbody. (B) A cell in the late phase of cytokinesis was chosen for analysis. An asterisk marks the midbody.
DISCUSSION
Membrane traffic to the cleavage furrow plays a vital role during the progression of cell division and cytokinesis. However, the mechanisms regulating the targeting and source of these membranes remain to be elucidated. Here we show that Rab11-positive endosomes accumulate in the cleavage furrow of dividing cells. Furthermore, we demonstrate that Rab11 recruits FIP3 to these endosomes and that a Rab11-FIP3 protein complex is required for the late phase of cytokinesis. Finally, we show that FIP3 is subject to dynamic spatial and temporal regulation during mitosis, perhaps coupling cytokinetic events with the end of the cell cycle.
Endosomes are known to mediate recycling of proteins to the plasma membrane. Hence as a starting point for these studies, we tested whether endosomes may mediate the membrane delivery to the cleavage furrow known to accompany cytokinesis. Our data are consistent with the view that Rab11-containing RE became concentrated around the cleavage furrow during late telophase (Figure 1C and Supplementary Figure 1, D–F). Furthermore, Rab11 staining could also be observed inside the cleavage furrow. In contrast, EEA1-containing EE remained scattered throughout the cytoplasm and were never observed concentrated juxtaposed to the midbody/furrow. These data argues that RE rather then EE mediate membrane delivery to the cleavage furrow. Consistent with previous reports, we found that endocytic recycling dramatically decreases during metaphase and early anaphase, but noted that endosomes retain their compartmentalized distribution, with EEA1-positive EE and TfR-positive RE exhibiting distinct staining patterns. Furthermore, upon resumption of endocytic recycling during cytokinesis, TfR-containing RE were found to redistribute to the furrow, whereas EE remain scattered throughout the cell, suggesting that recycling through RE plays an important role in cell division. Collectively, these data make a compelling case that membrane traffic from recycling endosomes to the furrow plays a crucial role in cytokinesis.
Given the localization of Rab11-positive endosomes to and near the furrow, we sought to determine whether Rab11 is required for completion of cytokinesis. Although Rab11 is clearly required in specialized examples of cytokinesis, most notably Drosophila embryo cellularization, no studies have addressed the role of Rab11 in general cytokinesis. Using RNAi, dominant negative Rab11 mutants, and anti-Rab11 antibody injections, we demonstrate that depletion of Rab11 or inhibition of its function results in defective cytokinesis. This is likely to be a consequence of an inability of the cells to recruit FIP3 to endocytic membranes (see below).
Rab GTPases work by recruiting effector proteins that regulate the targeting and fusion of transport vesicles. FIP proteins have been shown to interact with Rab11 and regulate several membrane traffic pathways (for review see Prekeris, 2003); hence, we sought to determine whether class I or class II FIPs regulate cytokinesis. Mammalian cells have two closely related isoforms of class II FIPs, FIP3 and FIP4 (Shin et al., 1999; Hickson et al., 2003; Prekeris, 2003). We show that class II (FIP3 and FIP4) but not class I FIPs (Rip11, RCP, and FIP2) redistribute during cytokinesis to the furrow and midbody. Moreover, we show that Rab11 is required for association of FIP3 with RE and their traffic to the furrow. Interestingly, FIP3 appears to be recruited to the midbody in a Rab11-independent manner. The mechanisms regulating the targeting of FIP3 to the midbody remain to be determined. One interesting possibility is a putative FIP3 interaction with ARF6 GTPases (Shin et al., 2001; Hickson et al., 2003). Recent reports demonstrate that activated (GTP-bound) ARF6 is enriched in the cleavage furrow (Schweitzer and D'Souza-Schorey, 2002). Because ARF6 also has been reported to interact with the Exocyst complex (Prigent et al., 2003), one attractive hypothesis is that it could play a role in FIP3 recruitment, regulating the fusion of incoming membrane vesicles with the plasma membrane.
It remains to be determined why cells require two closely related isoforms of a vesicle tethering protein. One possibility is that FIP3 and FIP4 may regulate cell division in different tissues or cell types. Alternatively, FIP3 and FIP4 may play distinct, although overlapping, roles in cytokinesis. Indeed, unlike FIP3, FIP4 lacks the proline-rich domain present at the N-terminus of the protein. In HeLa cells FIP4 also localizes to the mitotic spindle, suggesting that it may play a role in spindle function, perhaps regulating earlier trafficking steps in furrow formation. Sequence comparison revealed that FIP3/4 share some homology with Nuf, a protein originally identified for its role in cellularization in Drosophila embryos (Hickson et al., 2003). Furthermore, Nuf was also shown to interact with Rab11 (Riggs et al., 2003). It remains unclear, however, whether Nuf is a true FIP3/4 homologue. Unlike FIP3/4 it does not have EF-hands motifs. Furthermore, in our hands, Nuf does not interact with Drosophila Arf proteins (unpublished data). Thus, it is likely that Nuf is a specialized version of FIP3/4 that is designed to regulate Drosophila cellularization. Indeed, midbody formation is not required for Drosophila cellularization, but it plays a central role in general cytokinesis. Interestingly, the recruitment of FIP3 to the midbody appears to be a key step in regulating the targeting of Rab11-containing endosomes to the cleavage furrow.
The data described above suggested the hypothesis that Rab11 acting in complex with FIP3 plays a key role in cytokinesis. To directly test the role of FIP3, we utilized RNAi and mutant protein approaches. Depletion of FIP3 by RNAi consistently gave a clear increase in the number of binucleate and multinucleate cells. Analysis of furrow formation and abscission in real time in cells depleted of FIP3 or Rab11 revealed that furrow formation and ingression does not appear to be compromised. Rather, these cells remain connected by a long cytoplasmic bridge and appear to be defective in abscission. Furthermore, the cells often became detached from the plate or appeared apoptotic. Consistent with this, we note that both Rab11 and FIP3 RNAi reduced the number of cells on the dish. Hence, we suggest that the 20–40% binucleate cells represent the population of cells that are able to regress the furrow and not apoptose, whereas many cells with cytokinesis defects are lost to analysis. Our time-lapse imaging also demonstrates that a portion of the cell can eventually brake the cytoplasmic bridge pulling it and thus completing mitosis, although several hours later. Thus, a cytokinesis defect in Rab11- and FIP3-depleted cells may be more prevalent than the 20–40% reported here. Interestingly, a similar partial block (20–60%) was also observed after down-regulation or inhibition of other proteins known to regulate late cytokinesis, namely syntaxin 2, anexin 11, VAMP8, and MKlp2 (Neef et al., 2003; Low et al., 2003; Tomas et al., 2004), suggesting that the ability of cells to brake the cytoplasmic bridge is a common consequence of a late cytokinesis block. Regardless of actual numerical value, the data clearly support a central role of Rab11 and FIP3 at the late stage of cytokinesis.
Expression of FIP3-GFP in cells in cells revealed that FIP3 undergoes dynamic regulation, localizing first to the centrosome in anaphase and then dramatically relocating to the furrow in late cytokinesis. The mechanism that mediates the association of FIP3-containing endosomes with centrosomes remains unclear. It has been recently demonstrated that a subpopulation of the Golgi can also associate with centrosomes in a very dynamic matter via binding of GMAP-210 to γ-tubulin and/or the centrosome matrix (Rios et al., 2004). Thus, it is tempting to speculate that FIP3/Rab11 complex may interact with the centrosomal matrix to target endosomes to the centrosome. Indeed, overexpression of FIP3 often results in the relocalization of Rab11 endosomes from the cell periphery to the centrosome (Hickson et al., 2003). The function of centrosome association of FIP3-containing endosomes also remains unclear. Interestingly, movement of endosomes from centrosome to the cleavage furrow coincides with the final stages of furrowing and formation of the midbody. It is possible that the release of FIP3-containing organelles from the centrosome at late telophase allow them to be targeted to the cleavage furrow via movement on the midzone microtubules.
The mechanism allowing the “activation” and targeting of FIP3 to the centrosome and later to the cleavage furrow and midbody remain unclear. One possibility is a role for phosphorylation (possibly by Aurora B and/or Polo kinases, because these kinases are known to be involved in regulation of protein targeting to the spindle microtubules and the midbody; Kaitna et al., 2002; Neef et al., 2003). Interestingly, in this context, the type 1 protein phosphatase (PP1) is localized to the ingressing cleavage furrow and the midbody in HeLa cells (Trinkle-Mulcahy et al., 2003). In budding yeast, PP1 has also been shown to be required for the last step in membrane fusion (Peters et al., 1999). Alternatively, changes in Ca2+ concentration may play a role triggering FIP3 translocation or function. Although the role of Ca2+ in mammalian cell cytokinesis remains poorly understood, Ca2+ is known to be involved in the exocytosis required for cytokinesis in sea urchin eggs (Shuster and Burgess, 2002). Intriguingly, FIP3 contains EF-hand motifs that bind Ca2+ in other proteins (Prekeris, 2003). Whatever the mechanism, the recruitment of FIP3 to the midbody appears to play a major role in the successful completion of cytokinesis. Further experiments will be needed to establish the mechanism of FIP3 regulation.
In summary, we have demonstrated that recycling endosomes accumulate in the region of the furrow during cytokinesis and that Rab11, a resident protein of recycling endosomes, is required for general cytokinesis. We further show that class II FIPs, notably FIP3, also traffic with Rab11 positive endosomes into the furrow where they associate with the midbody. The association of FIP3 with Rab11 is required both for localization of FIP3 to endosomes and for completion of a late stage of cytokinesis. Because the FIP3-Rab11 complex acts at a late stage of cytokinesis, it is unlikely that it is required for the initiation and initial progression of the cleavage furrow. Perhaps the insertion of additional membranes is required only at the late stage of furrow ingression when the plasma membrane tension would be expected to increase. Alternatively, Rab11-dependent endocytic transport could be involved in delivering proteins and/or lipids that regulate cleavage furrow ingression. Finally, the Rab11-FIP3 complex may play a role in cell abscission, a process that remains poorly understood. We postulate a model in which the interaction of FIP3 with Rab11 allows docking and subsequent fusion of endocytic vesicles with the plasma membrane at the apex of the cleavage furrow, thus allowing the delivery of new membranes and/or proteins necessary to allow daughter cell separation.
Supplementary Material
Acknowledgments
We thank Dr. Tom Evans for the critical reading of the manuscript and Drs. Richard Pagano and Julie Donaldson for constructs and antibodies. This work was supported in part by Howard Hughes Medical Institute Junior Faculty Award (to R.P.) and by grant 17/C13723 from the Biotechnology and Biological Sciences Research Council (to G.W.G.). A.B.F. and X.Y. thank The Welcome Trust for PhD studentships. G.W.G. thanks The Welcome Trust for a Research Leave Award. P.D.A. thanks Jason Swedlow and The Wellcome Trust for support and purchase of the DeltaVision Restoration Microscope.
Article published online ahead of print in MBC in Press on December 15, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0927).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Bieliavsky, N., Geuskens, M., Goldfinger, M., and Tencer, R. (1992). Isolation of plasma membranes, Golgi bodies and mitochondria of Xenopus laevis morulae. Identification of plasma membrane proteins. J. Submicrosc. Cytol. Pathol. 24, 335-349. [PubMed] [Google Scholar]
- Byers, T. J., and Armstrong, P. B. (1986). Membrane protein redistribution during Xenopus first cleavage. J. Cell Biol. 102, 2176-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, S. D., and Wessel, G. M. (1999). Syntaxin is required for cell division. Mol. Biol. Cell 10, 2735-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornan, S., Jackson, A. P., and Gay, N. J. (1997). Alpha-adaptin, a marker for endocytosis, is expressed in complex patterns during Drosophila development. Mol. Biol. Cell 8, 1391-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, K., Kobayashi, T., Yamaji, A., Aizawa, H., Yahara, I., Inoue, K., and Umeda, M. (1996). Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA 93, 12867-12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, K., and Umeda, M. (2000). An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J. Cell Biol. 149, 1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger, F. P., and White, J. G. (2002). Fusion and fission: membrane trafficking in animal cytokinesis. Cell 108, 727-730. [DOI] [PubMed] [Google Scholar]
- Fox, M. H. (1980). A model for the computer analysis of synchronous DNA distributions obtained by flow cytometry. Cytometry 1, 71-77. [DOI] [PubMed] [Google Scholar]
- Fry, A. M., Mayor, T., Meraldi, P., Stierhof, Y.-D., Tanaka, K., and Nigg, A. E. (1998). C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulating kinase Nek2. J. Cell Biol. 141, 1563-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, L., Jr., and Scheller, R. H. (1999). Regulation of membrane trafficking: structural insights from a Rab/effector complex. Cell 96, 755-758. [DOI] [PubMed] [Google Scholar]
- Hales, C. M., Griner, R., Hobdy-Henderson, K. C., Dorn, M. C., Hardy, D., Kumar, R., Navarre, J., Chan, E. K., Lapierre, L. A., and Goldenring, J. R. (2001). Identification and characterization of a family of Rab11-interacting proteins. J. Biol. Chem. 276, 39067-39075. [DOI] [PubMed] [Google Scholar]
- Hickson, G.R.X., Matheson, J., Riggs, B., Maier, V. H., Fielding, A. B., Prekeris, R., Sullivan, W., Barr, F. A., and Gould, G. W. (2003). Arfophilins are dual Arf/Rab11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell 14, 2908-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobdy-Henderson, K. C., Hales, C. M., Lapierre, L. A., Cheney, R. E., and Goldenring, J. R. (2003). Dynamics of the apical plasma membrane recycling system during cell division. Traffic 4, 681-693. [DOI] [PubMed] [Google Scholar]
- Horgan, C. P., Walsh, M., Zurawski, T. H., and M. W. McCaffrey. (2004). Rab11-FIP3 localizes to a rab11-positive pericentrosomal compartment during interphase and to the cleavage furrow during cytokinesis. BBRC 319, 83-94. [DOI] [PubMed] [Google Scholar]
- Jantsch-Plunger, V., and Glotzer, M. (1999). Depletion of syntaxins in the early Caenorhabditis elegans embryo reveals a role for membrane fusion events in cytokinesis. Curr. Biol. 9, 738-745. [DOI] [PubMed] [Google Scholar]
- Kaitna, S., Pasierbek, P., Jantsch, M., Loidl, J., and M. Glotzer. (2002). The AuroraB kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 12, 798-812. [DOI] [PubMed] [Google Scholar]
- Low, S. H., Li, X., Miura, M., Kudo, N., Quinones, B., and Weimbs, T. (2003). Syntaxin 2 and Endobrevin are required for the treminal step of cytokinesis in mammalian cells. Dev. Cell 4, 753-759. [DOI] [PubMed] [Google Scholar]
- Mellman, I. (1996). Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575-625. [DOI] [PubMed] [Google Scholar]
- Meyers, J. M., and Prekeris, R. (2002). Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J. Biol. Chem. 277, 49003-49010. [DOI] [PubMed] [Google Scholar]
- Neef, R., Preisinger, C., Sutcliffe, J., Kopajtich, R., Nigg, E. A., Mayer, T. U., and Barr, F.A. (2003). Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran, T. J. (2000). Membrane traffic and cytokinesis. Traffic 1, 921-926. [PubMed] [Google Scholar]
- Peden, A. A., Schonteich, E., Chun, J., Junutula, J. R., Scheller, R. H., and Prekeris, R. (2005). The RCP-Rab11 complex regulates endocytotic protein sorting. Mol. Biol. Cell 15, 3530-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, C., Andrews, P. D., Stark, M. J., Cesaro-Tadic, S., Glatz, A., Podtelejnikov, A., Mann, M., and Mayer, A. (1999). Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 285, 1084-1087. [DOI] [PubMed] [Google Scholar]
- Pelisser, A., Chauvin, J. P., and Lecuit, T. (2003). Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr. Biol. 13, 1848-1857. [DOI] [PubMed] [Google Scholar]
- Prekeris, R. (2003). Rabs, Rips, FIPs, and endocytic membrane traffic. Sci. World J. 3, 870-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., Davies, J. M., and Scheller, R. H. (2001). Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J. Biol. Chem. 276, 38966-38970. [DOI] [PubMed] [Google Scholar]
- Prekeris, R., Klumperman, J., and Scheller, R. H. (2000). A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol. Cell 6, 1437-1448. [DOI] [PubMed] [Google Scholar]
- Prigent, M., Dubois, T., Raposo, G., Derrien, V., Tenza, D., Rosse, C., Camonis, J., and Chavrier, P. (2003). ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J. Cell Biol. 163, 1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs, B., Rothwell, W., Mische, S., Hickson, G. R., Matheson, J., Hays, T. S., Gould, G. W., and Sullivan, W. (2003). Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J. Cell Biol. 163, 143-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios, R. M., Sanchis, A., Tassin, A. M., Fedriani, C., and Bornens, M. (2004). GMAP-210 recruits γ-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118, 323-335. [DOI] [PubMed] [Google Scholar]
- Rothwell, W. F., Fogarty, P., Field, C. M., and Sullivan, W. (1998). Nuclear-fallout, a Drosophila protein that cycles from the cytoplasm to the centrosomes, regulates cortical microfilament organization. Development 125, 1295-1303. [DOI] [PubMed] [Google Scholar]
- Rothwell, W. F., Zhang, C. X., Zelano, C., Hsieh, T. S., and Sullivan, W. (1999). The Drosophila centrosomal protein Nuf is required for recruiting Dah, a membrane associated protein, to furrows in the early embryo. J. Cell Sci. 112(Pt 17), 2885-2893. [DOI] [PubMed] [Google Scholar]
- Sager, P. R., Brown, P. A., and Berlin, R. D. (1984). Analysis of transferrin recycling in mitotic and interphase HeLa cells by quantitative fluorescence microscopy. Cell 39, 275-282. [DOI] [PubMed] [Google Scholar]
- Scholey, J. M., Brust-Mascher, I., and Mogilner, A. (2003). Cell division. Nature 422, 746-752. [DOI] [PubMed] [Google Scholar]
- Schweitzer, J. K., and D'Souza-Schorey, C. (2002). Localization and activation of the ARF6 GTPase during cleavage furrow ingression and cytokinesis. J. Biol. Chem. 277, 27210-27216. [DOI] [PubMed] [Google Scholar]
- Shin, O. H., Couvillon, A. D., and Exton, J. H. (2001). Arfophilin is a common target of both class II and class III ADP-ribosylation factors. Biochemistry 40, 10846-10852. [DOI] [PubMed] [Google Scholar]
- Shin, O. H., Ross, A. H., Mihai, I., and Exton, J. H. (1999). Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors. J. Biol. Chem. 274, 36609-36615. [DOI] [PubMed] [Google Scholar]
- Shuster, C. B., and Burgess, D. R. (2002). Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc. Natl. Acad. Sci. USA 99, 3633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skop, A. R., Bergmann, D., Mohler, W. A., and White, J. G. (2001). Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr. Biol. 11, 735-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin, A., McClure, M., Huang, F., and Carter, R. (2000). Interaction of epidermal growth factor receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 10, 1395-1398. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., Cheung, A., Limouze, J., Chen, I., Westwood, N. J., Sellers, J. R., and Mitchison, T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299, 1743-1747. [DOI] [PubMed] [Google Scholar]
- Strickland, L. I., and Burgess, D. R. (2004). Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 14, 115-118. [DOI] [PubMed] [Google Scholar]
- Swanson, M. M., and Poodry, C. A. (1980). Pole cell formation in Drosophila melanogaster. Dev. Biol. 75, 419-430. [DOI] [PubMed] [Google Scholar]
- Swedlow, J. R., Sedat, J. W., and Agard, D. A. (1997). Deconvolution in optical microscopy. In: Deconvolution of Images and Spectra, New York: Academic Press, 284-309.
- TerBush, D. R., Maurice, T., Roth, D., and Novick, P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483-6494. [PMC free article] [PubMed] [Google Scholar]
- Tomas, A., Futter, C., and Moss, S. E. (2004). Anexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J. Cell Biol. 165, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkle-Mulcahy, L., Andrews, P. D., Wickramasinghe, S., Sleeman, J., Prescott, A., Lam, Y. W., Lyon, C., Swedlow, J. R., and Lamond, A. I. (2003). Time-lapse imaging reveals dynamic relocalization of PP1gamma throughout the mammalian cell cycle. Mol. Biol. Cell 14, 107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbe, S., Zerial, M., and Parton, R. G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda, M., and Emoto, K. (1999). Membrane phospholipid dynamics during cytokinesis: regulation of actin filament assembly by redistribution of membrane surface phospholipid. Chem. Phys. Lipids 101, 81-91. [DOI] [PubMed] [Google Scholar]
- Walace, W., Schaefer, L. H., and Swedlow, J. R. (2001). A working person's guide to deconvolution in light microscopy. Biotechniques 31, 1076-1097. [DOI] [PubMed] [Google Scholar]
- Warren, G., Davoust, J., and Cockcroft, A. (1984). Recycling of transferrin receptor in A431 cells is inhibited during mitosis. EMBO J. 3, 2217-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, J. V., Chambers, S. H., and Smith, P. J. (1987). A pragmatic approach to the analysis of DNA histograms with a definable G1 peak. Cytometry 8, 1-8. [DOI] [PubMed] [Google Scholar]
- Zhang, C. X., Rothwell, W. F., Sullivan, W., and Hsieh, T. S. (2000). Discontinuous actin hexagon, a protein essential for cortical furrow formation in Drosophila, is membrane associated and hyperphosphorylated. Mol. Biol. Cell 11, 1011-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


