Abstract
Knowledge on molecular systems involved in myogenic precursor cell (mpc) fusion into myotubes is fragmentary. Previous studies have implicated the a disintegrin and metalloproteinase (ADAM) family in most mammalian cell fusion processes. ADAM12 is likely involved in fusion of murine mpc and human rhabdomyosarcoma cells, but it requires yet unknown molecular partners to launch myogenic cell fusion. ADAM12 was shown able to mediate cell-to-cell attachment through binding α9β1 integrin. We report that normal human mpc express both ADAM12 and α9β1 integrin during their differentiation. Expression of α9 parallels that of ADAM12 and culminates at time of fusion. α9 and ADAM12 coimmunoprecipitate and participate to mpc adhesion. Inhibition of ADAM12/α9β1 integrin interplay, by either ADAM12 antisense oligonucleotides or blocking antibody to α9β1, inhibited overall mpc fusion by 47–48%, with combination of both strategies increasing inhibition up to 62%. By contrast with blockade of vascular cell adhesion molecule-1/α4β1, which also reduced fusion, exposure to ADAM12 antisense oligonucleotides or anti-α9β1 antibody did not induce detachment of mpc from extracellular matrix, suggesting specific involvement of ADAM12–α9β1 interaction in the fusion process. Evaluation of the fusion rate with regard to the size of myotubes showed that both ADAM12 antisense oligonucleotides and α9β1 blockade inhibited more importantly formation of large (≥5 nuclei) myotubes than that of small (2–4 nuclei) myotubes. We conclude that both ADAM12 and α9β1 integrin are expressed during postnatal human myogenic differentiation and that their interaction is mainly operative in nascent myotube growth.
INTRODUCTION
Adult skeletal muscle regeneration after injury results from activation, proliferation, and fusion of mononucleated myogenic precursor cells (mpc) (Hawke and Garry, 2001). The mpc fusion results from an ordered sequence of events, including clustering and alignment of cells, establishment of close cell-to-cell contacts, and plasma membrane merging (Doberstein et al., 1997; Taylor, 2003). Various membrane proteins have been implicated in myotube formation, including N- and M-cadherins, neural cell adhesion molecule (NCAM), and vascular cell adhesion molecule (VCAM), α4β1 and other integrins, and a disintegrin and metalloproteinases (ADAMs) (Abmayr et al., 2003). ADAMs form a family of >30 transmembrane glycoproteins with a unique domain organization, including a prodomain, a proteolytic domain (metalloprotease), an adhesion integrin-binding site formed by both disintegrin and cysteine-rich domains, an epidermal growth factor-like domain, a transmembrane domain, and a signaling cytoplasmic tail (Huovila et al., 1996; Primakoff and Myles, 2002; White, 2003). In addition, some ADAMs contain a hydrophobic sequence in a cysteine-rich region that may represent a fusion peptide, suggesting that this subclass of ADAMs might participate to plasma membrane merging (Huovila et al., 1996). Some ADAMs have been implicated in most mammalian cell fusion processes, including ADAM1, 2, and 3 in fertilization (Blobel et al., 1992; Huovila et al., 1996; Wolfsberg and White, 1996; Hooft, 1998) and ADAM12 in osteoclast (Abe et al., 1999; Choi et al., 2001) and macrophage-derived multinucleated giant cell formation (Abe et al., 1999), in trophoblast syncytialization (Huovila et al., 1996; Gilpin et al., 1998; Shi et al., 2000), and in myogenesis (Yagami-Hiromasa et al., 1995).
Several ADAMs are expressed by adult and developing skeletal muscles, including ADAM1, 4, 9, and 15 that are ubiquitous, and ADAM12, also called meltrin-α, whose expression is less widespread (Yagami-Hiromasa et al., 1995; Loechel et al., 2000; Kratzschmar et al., 1996; Weskamp et al., 1996; Kurisaki et al., 1998). In rodents, constitutive muscle expression of ADAM12 starts at the embryonic stage when myotubes are formed (Kurisaki et al., 1998), persists at the neonatal stage (Yagami-Hiromasa et al., 1995; Borneman et al., 2000; Kronqvist et al., 2002), and ceases in adulthood (Borneman et al., 2000; Kronqvist et al., 2002). Adult muscle regeneration after experimental injury is associated with ADAM12 reexpression by fusing myogenic cells and newly formed fibers (Galliano et al., 2000; Kronqvist et al., 2002). Consistently, ADAM12 expression parallels fusion and myogenin expression of C2C12 myogenic cells (Yagami-Hiromasa et al., 1995). Transfection experiments have demonstrated that the recombinant disintegrin and cysteine-rich domains of ADAM12 are crucially involved in myoblast fusion (Yagami-Hiromasa et al., 1995), although other, as yet unknown, molecular partners are likely necessary to achieve plasma membrane merging (Yagami-Hiromasa et al., 1995).
Firm cell-to-cell adhesion is required before plasma membrane merging. Both the cysteine-rich and the disintegrin domains of ADAM12 provide molecular information for cell attachment (Iba et al., 1999, 2000; Zolkiewska, 1999; Thodeti et al., 2003). A model has been proposed in which initial low-affinity binding of the cysteine-rich domain by syndecan 4 triggers a conformational change of ADAM12-cys, allowing its binding to integrins (Iba et al., 2000). Both adhesion experiments to ADAM12 disintegrin domain by using cells expressing α2, α3, α4, α5, α6, and α9 integrins and cell-to-cell attachment between ADAM12 and α9-expressing cells have shown that α9 subunit is a preferred integrin partner for ADAM12, among integrins tested (Eto et al., 2000, 2002). The integrin α9 subunit forms a single heterodimer α9β1 (Palmer et al., 1993). Thus, this integrin constitutes a choice candidate as a privileged partner of ADAM12 during postnatal mpc fusion process (White, 2003).
Unlike α4β1, an integrin closely related to α9β1 and expressed by differentiating myogenic cells (Rosen et al., 1992), the role of α9β1 in postnatal myogenesis has not been investigated (Mayer, 2003) despite previous mention of its expression at onset of myotube formation in the mouse embryo (Wang et al., 1995).
In the present article, we show that both ADAM12 and α9 integrin are constitutively expressed during human myogenic cell differentiation and mediate a cell–cell interaction selectively involved in fusion of mononucleated myoblasts to preformed myotubes.
MATERIALS AND METHODS
Cell Culture
Unless indicated, culture media components were from Invitrogen (Paisley, Scotland) and culture plastics were from TPP AG (Trasadingen, Switzerland). Human mpc were cultured from muscle samples as described previously, in Ham's-F12 medium containing 15% fetal calf serum (FCS) (growth medium, GM) and antibiotics (Authier et al., 1999). Human mpc seeded at 5000 cells/cm2 undergo proliferation and at day 4 of culture (nearly confluence), the medium was replaced with Ham's-F12 medium containing 5% FCS (differentiation medium, DM) to induce myogenic differentiation characterized by mpc fusion into myotubes (Baroffio et al., 1993). By using this procedure, mpc undergo standardized myogenic differentiation, including proliferation phase up to day 4, and then withdrawal of cell cycle to enter differentiation and formation of small myotubes (day 7), and growth of preformed myotubes through accretion of additional mpc (day 14). Only cultures presenting >95% cells expressing CD56/neural cell adhesion molecule (NCAM), a marker of myogenic cells (De Rossi et al., 2000) were used (clone 123C3, 1:20; Sanbio/Monosan, Uden, The Netherlands) (Chazaud et al., 2003).
mpc Growth and Differentiation
mpc growth curves are established by counting the cells with trypan blue dye exclusion (Authier et al., 1999). Myogenic differentiation is assessed by expression of myogenin as recommended previously (Fujio et al., 1999). Myogenin immunoblotting was carried out using 10 μg of mpc protein extract and M-225 antibody (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA). mpc fusion was estimated by light microscopy examination of culture dishes after May-Grünwald Giemsa staining (Authier et al., 1999). Cultures were evaluated at the following stages: 1) proliferation stage (day 4 of culture, GM); 2) early differentiation stage (day 7 of culture, 3 d in DM); and 3) late differentiation stage (day 14 of culture, 10 d in DM). Experiments were run for each point at least on cultures from three individuals at passage 2.
The fusion index that helps assessment of ability of cells to fuse into multinucleated cells was calculated as the ratio of nuclei in myotubes to the total number of nuclei (Hirsch et al., 1998; Authier et al., 1999). To evaluate separately both formation of small nascent myotubes (2–4 nuclei) and growth of preformed myotubes, we performed nuclear number assays as described previously (Horsley et al., 2001). The number of nuclei in individual myotubes was counted for 50–200 myotubes. Myotubes were grouped in two categories: those with two to four nuclei and those with five or more nuclei. The percentage of myotubes in each category was calculated.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total mpc RNA (1 μg) prepared using the RNeasy mini kit (QIAGEN, Hilden, Germany) was reversely transcribed and amplified using OneStep RT-PCR kit (QIAGEN) and specific primers. Human ADAM12 is particular in that alternative splicing of the mRNA results in expression of two forms, a membrane-anchored form (ADAM12-L) and a shorter secreted form (ADAM12-S) (Gilpin et al., 1998). For ADAM12-S (GenBank accession no. AF23477), primers were 5′-GTT TGG CTT TGG AGG AAG CAC AG-3′ (sense) and 5′-GAA GCT CAA CCA GGA AGT CG-3′ (antisense). For ADAM12-L (GenBank accession no. AF23476), the sense primer was the same as for ADAM12-S and the antisense primer was 5′-GCA GCA ATC TCC TGG GAT TG-3′ (Gilpin et al., 1998). Amplifications were performed at 94, 60, and 72°C for 30, 45, and 45 s, respectively, for 40 cycles. For detection of α9 and β1 integrins mRNAs, we designed primers using Oligo 5.0 software. For α9 integrin (GenBank accession no. NM_002207), the primers were 5′-GAT GAG TGG ATG GGG GTG AG-3′ (sense) and 5′-CAA TGG TGG ACG GGT GAG AG-3′ (antisense). For β1 integrin (GenBank accession no. NM_033668), the primers were 5′-AGG ATT ACT TCG GAC TTC AGA-3′ (sense) and 5′-CTT TGG CAT TCA CAT TCA-3′ (antisense). Forty cycles of amplification were performed at 94, 60 (for α9), or 55°C (for β1) and at 72°C for 30, 45, and 45 s, respectively. An endogenous human β2-microglobulin cDNA was amplified in parallel. Amplification products (10 μl) were subjected to electrophoresis on 2% agarose gel stained with ethidium bromide (5 μg/ml) for visualization.
Electrophoresis and Immunoblotting
Cells were detached mechanically from culture flasks as described previously (Authier et al., 1999) and stored at -80°C in protease inhibitor cocktail for mammalian tissues (P8340; Sigma-Aldrich, St. Louis, MO). Cell pellet was incubated with lysis buffer [1% CHAPS (wt/vol), 150 mM NaCl, 50 mM Tris, 5 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride] and gently shaken for 10 min on ice. The homogenate was centrifuged at 4000 × g for 10 min at 4°C. To detect secreted ADAM12-S, complete culture medium was replaced by Ham's-F12 without serum, and supernatants were collected after 24 h and concentrated using Vivaspin 15R concentrator (Vivascience, Hannover, Germany). Protein concentration was determined using bicinchoninic acid protein assay kit from Pierce Chemical (Rockford, IL). Aliquots corresponding to 20–50 μg of proteins were subjected to SDS-PAGE, followed by transfer to nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Ponceau Red staining was used to ensure loading of equal amounts of proteins. All antibodies were diluted in phosphate-buffered saline (PBS)-0.1% Tween 20 (PBST). Staining of the blotted membranes was performed as follows: 1) blockade of the nonspecific binding by incubation with PBST-5% dry nonfat milk for 2 h at room temperature; 2) overnight incubation at 4°C with the primary antibody; 3) link-step either with a rabbit horseradish peroxidase-conjugated anti-rat Ig (1:1000; 1 h) in case of monoclonal primary antibody, or with goat horseradish peroxidase-conjugated anti-rabbit Ig (1:1000; 1 h) in case of polyclonal primary antibody. Revelation was performed by using the enhanced chemiluminescence kit (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom). Detection of ADAM12 was ensured by using a rat monoclonal antibody (mAb) (2F7) recognizing the disintegrin domain (Kawaguchi et al., 2002) and goat-polyclonal antibody directed toward cytoplasmic tail of ADAM12-L (sc-16527; Santa Cruz Biotechnology, Santa Cruz, CA) that of α9 integrin by a rabbit anti-α9 antiserum raised against cytoplasmic domain (#1057; generous gift from Dean Sheppard, University of California, San Francisco, San Francisco, CA) and that of myogenin by a rabbit polyclonal anti-myogenin (M-225; Santa Cruz Biotechnology). Controls ensured the lack of endogenous binding of the secondary antibody and the absence of peroxidase.
Immunofluorescence
For immunofluorescence procedure, mpc were cultured on glass coverslips and fixed in 1% paraformaldehyde. Primary antibodies included rabbit polyclonal antibodies to hADAM12 cysteine-rich domain (rb122) (Kawaguchi et al., 2002) (1/100; 37°C; 60 min), mouse mAb to α9β1 integrin IgG1 (Y9A2; Chemicon International, Temecula, CA) (20 μg/ml; 4°C; overnight), and mouse mAb to myogenin (F5D; DakoCytomation Denmark A/S, Glostrup, Denmark) (1/20; 37°C; 60 min). Secondary antibody were fluorescein isothiocyanate (FITC)- or tetramethylrhodamine B isothiocyanate (TRITC)-conjugated secondary antibody (The Jackson ImmunoResearch Laboratories, Bar Harbor, ME; 1/250; 37°C; 60 min). For double staining, unpermeabilized mpc were first incubated with anti-α9β1 antibody and then with anti-ADAM12 antibody. All antibodies were diluted in 2% FCS PBS. The specificity of the immunostaining was investigated by replacing primary antibodies with normal rabbit, goat, or mouse IgG fraction (10 or 20 μg/ml). Coverslips were mounted on slides with Vectashield medium containing 4,6-diamidino-2-phenylindole (DAPI) or propidium iodide (Vector Laboratories, Burlingame, CA). A Zeiss AXIOPHOT2 microscope was used for conventional fluorescence microscopy, and digital photographs were taken using Hammamatsu ORCA-ER camera.
Antisense Oligonucleotides
Antisense oligonucleotides and controls directed to ADAM12 were designed and manufactured by Biognostik (Göttingen, Germany). Sequences for ADAM12 antisenses were chosen to inhibit both isoforms: CTC TCT TTT ATG CCT TCT (position 909 in the prodomain) and CCC CAT TCC TTT CTC C (position 1512 in the disintegrin domain). Sequences were chosen because they were short enough to penetrate living cells and long enough to be sequence specific. Nucleotides in the 3′ and -5′ ends are modified oligonucleotides with phosphorothioate backbone modification conferring exonuclease resistance. Cross-examination of GenBank showed neither autohybridization between these oligonucleotides nor a complementary sequence in any other endogenous gene thus far entered into the database and especially with other ADAMs expressed in myogenic cells (ADAM9, ADAM17, and ADAM19). Random control oligos (nonsense in our legend) have been designed thoroughly to avoid toxic motifs and to be nonhomologous to any known sequence (ACT ACT ACA CTA GAC TAC and GCT CTA TGA CTC CCA G). In a first step, cellular uptake is monitored with FITC-labeled control oligonucleotides according to manufacturer's recommendations. In a second step, the translation of the targeted protein is inhibited specifically. ADAM12 antisense oligos were added at 2 μM to culture medium at time of switch in DM and are reapplied at the same concentration 2 d later in case of cultures evaluated at day 14. Inhibition of protein ADAM12 synthesis was quantified by immunoblotting on cell extracts for ADAM12-L and 24-h supernatants for ADAM12-S at day 14. Effect on fusion index was evaluated at day 5 and day 14 for each sample: untreated cells, antisense-treated cells, and nonsense-treated cells. For each experiment, 10 randomly chosen fields were evaluated for calculation of fusion index.
Blocking Antibodies
When GM was replaced with DM, the following mouse monoclonal blocking antibodies were added at saturating concentrations (10 μg/ml): anti-α9β1 (clone Y9A2; Chemicon International), anti-VCAM-1/CD106 (clone 1G11), and anti-α4/CD49d (clone HP2/1). Controls included cells grown in DM only and in DM with mouse isotype control (Vector Laboratories). Blocking antibodies were added to culture medium at time of switch in DM and are reapplied at the same concentration 2 d later in case of cultures evaluated at day 14. Effect on fusion index was evaluated at days 5 and 14: untreated cells, anti-α9β1–treated cells, and mouse IgG-treated cells. For each experiment, 10 randomly chosen fields were evaluated for calculation of fusion index.
Coimmunoprecipitation Assay
To assess interaction between ADAM12 and α9β1 during mpc fusion, we performed coimmunoprecipitation assays by using anti-ADAM12 and anti-α9β1 antibodies prebound on protein G beads. Cells were scraped and homogenized in Tris-buffered saline (20 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1 mM EDTA, and 1 mM EGTA) containing protease inhibitors (P8340; Sigma-Aldrich). Protein extraction was done on ice by 10-min incubation with an equal volume of a modified radioimmunoprecipitation assay (RIPA) buffer (20 mM HEPES-NaOH, pH 7.4, 150 mM NaCl, 0.05% Nonidet P-40, 10% glycerol, 10 mM EDTA, and protease inhibitors) and gently passaged through a 22-gauge needle. Cell extracts were centrifugated at 4°C to remove debris. Protein concentration was determined as described previously.
Coimmunoprecipitation was performed using protein G beads (Immunoprecipitation Starter Pack; Amersham Biosciences UK) because of mouse IgG1 affinity was higher for protein G than for protein A. Protein extracts (500 μg–1 mg) were precleared by incubation with protein G beads-Sepharose 4B (60 min at 4°C in an end-over-end mixer) followed by a centrifugation (10 min at 10,000 × g; 4°C). Equal volumes of cleared supernatants were used for different immunoprecipitations. Protein G beads were first prebound with anti-ADAM12 antibodies (6E6, 8F8, and 6C10 ascites mix; 10 μl), anti-α9β1 antibody (Y9A2; 20 μg), or irrelevant mouse Ig (20 μg) (60 min at 4°C in an end-over-end mixer), and then immunoprecipitation was conducted overnight at 4°C in an end-over-end mixer. Immunocomplexes on beads were centrifuged (250 × g; 2 min), washed twice with RIPA buffer; and resuspended in an appropriate volume of 2× SDS sample buffer (1% SDS, 100 mM dithiothreitol, and 50 mM Tris, pH 7.5) and boiled (10 min) to detach precipitated proteins. After centrifugation at 17,000 × g, samples were subjected to gel migration in denaturing conditions (SDS-PAGE), transfer on nitrocellulose membrane, and after rouge ponceau staining, immunoblotting with antibodies different from those used for immunoprecipitation: rabbit polyclonal anti-α9 antibody (#1057) and rabbit polyclonal anti-ADAM12 (rb122).
Cell Adhesion Assays
A first set of experiments was designed to evaluate the ability of α9β1 expressed at mpc surface to bind ADAM12. Ninety-six well plates were first incubated overnight at 4°C with recombinant full-length human ADAM12 (Loechel et al., 2000). Recombinant full-length human ADAM12 (10 μg/ml in coating buffer [6.22 g/l NaHCO3, 1.70 g/l Na2CO3, and H2O, pH 9.5]) and then saturated with 1% bovine serum albumin in PBS (37°C; 60 min). mpc were seeded (0.6 × 106 cells/ml) and allowed to attach 60 min at 37°C in presence of Y9A2, mAb to α9β1 (20 μg/ml in Ham's-F12) or O26, monoclonal blocking antibody to α7 (20 μg/ml; generous gift from S. J. Kaufman, University of Illinois, Urbana, IL) (Crawley et al., 1997). Controls were ensured by replacement of Y9A2 and O26 by normal IgG fraction (20 μg/ml in Ham's-F12). mpc adhesion was evaluated using crystal violet staining (Chazaud et al., 2002).
A second set of experiments was designed to assess involvement of different molecules in natural adhesion to their support of cultured mpc. Mpcs were seeded in 96-well plates (10,000/cm2 in GM) and allowed to attach overnight. At subconfluence, GM was replaced by DM supplemented with different effectors (10 μg/ml), including RGD peptide (600 μg/ml, G5646; Sigma-Aldrich) and monoclonal blocking antibodies to α9β1 (Friday et al., 2003), α4 (Sanchez-Madrid et al., 1986), or VCAM-1 (Thornhill et al., 1991). mpc deadhesion was evaluated after 4 h and 3 d by using crystal violet staining (Chazaud et al., 2002).
A third set of experiments was designed to evaluate the ability of ADAM12, α9β1, and α7β1 expressed at mpc surface to mediate cell-cell adhesion. Cultured cells were separated in two parts. A part of cells was seeded at 50,000 cells/cm2 in 96-wells plates and treated or not with antisense or nonsense (2 μM) during 72 h and allowed to differentiate in small myotubes. During the same time, the second part of cells was seeded at 5000 cells/cm2 in 75-cm2 flasks with 5-bromo-2-deoxyuridine (BrdU) (1 μM, referenced as mpc-BrdU) and with antisense or nonsense (2 μM; referenced, respectively, as mpc-AS-BrdU and mpc-NS-BrdU). At day of assay, these cells were detached from culture flasks by a nonenzymatic solution to preserve membrane adhesion molecules (cell dissociation solution; Sigma-Aldrich Chemie, Steinheim, Germany), incubated as described previously with blocking antibodies (Y9A2, O26, or IgG fraction), and allowed to attach 90 min on cell layer in 96-wells plates. Adherent cells were detected by the measurement of BrdU incorporation during DNA synthesis by using the cell proliferation enzyme-linked immunosorbent assay, BrdU (colorimetric) (Roche Diagnostics, Manheim, Germany). Optical density was read at 450 nm.
Statistics
All experiments were performed using at least three different cultures. Statistical analyses were achieved with paired t test and Kruskal–Wallis test, by using InStat 3.0 (GraphPad Software, San Diego, CA). A p value <0.05 was considered significant.
RESULTS
Human mpc Fusion
Human mpc were cultured in GM until subconfluence, and at day 4 DM was used to boost myogenesis. mpc density and differentiation were evaluated at the stage of proliferation (day 4), early differentiation (day 7), and late differentiation (days 14 and 21). Myogenesis was assessed by both fusion index and myogenin expression. Shift from GM to DM allows to increase fusion index, reaching 66 ± 0.43% at day 14 and finally 67 ± 2% at day 21 (Figure 1A), whereas spontaneously it does not exceed an average of 30%. As assessed by myogenin expression, mpc truly undergo differentiation program in these conditions (Figure 1B). As assessed by plateauing of both cell density from day 7 to day 14 and fusion index from day 14 to day 21, the increase of fusion index observed in this time lapse (7–14) likely reflected elongation of existing myotubes rather than appearance of newly formed myotubes (Figure 1C).
Figure 1.

In vitro differentiation of human mpc. (A) mpc growth is expressed in number of cells per square centimeter (closed circles), and mpc differentiation is estimated by the fusion index (open circles). There is no significant increase of fusion index from day 14 to day 21. Results are means ± SD of eight independent cultures. (B) Myogenin immunoblot at days 4, 7, and 14 of mpc culture. (C) May-Grünwald Giemsa stain of mpc at day 4 (d4), 7 (d7), and 14 (d14) of culture. Bar, 40 μm.
Human mpc Constitutively Express ADAM12 during In Vitro Myogenesis
RT-PCR showed expression of both short (S) and long (L) isoforms of ADAM12 by human mpc, at the three stages of differentiation (Figure 2A). Immunoblots assessed the production of both ADAM12-L and -S proteins at the three stages of differentiation (Figure 2B). In mpc extracts, ADAM12-L was revealed as two immunoreactive bands corresponding to ADAM12-L proform (110 kDa) and mature ADAM12-L (90 kDa) (Figure 2B). In mpc supernatants, ADAM12-S was detected as ADAM12-S proform (92 kDa) and mature ADAM12-S (68 kDa) (Figure 2B). Cell-associated ADAM12-L expression and release of ADAM12-S from mpc is similar to that observed in several other cell types expressing ADAM12 (Gilpin et al., 1998; Cao et al., 2002). Note that a fraction of the ADAM12-S proform does not get processed by human mpc and is secreted in culture medium as shown previously (Loechel et al., 1998). Immunostaining of ADAM12 in mpc after plasma membrane permeabilization revealed both intracellular and plasma membrane localization of ADAM12 (Figure 2C). Such a distribution in the murine myogenic cell line C2C12 (Cao et al., 2002) and other cell types (Hougaard et al., 2000; Kadota et al., 2000) has been shown to reflect association to the organelles composing the secretory pathway, i.e., endoplasmic reticulum, Golgi apparatus, and post-Golgi components. Moreover, plasma membrane labeling showed as discrete and elongated spots distributed throughout the cell body and plasma membrane projection as lamellipodiae (Hougaard et al., 2000; Kadota et al., 2000) (Figure 2C). ADAM12 immunostaining on unpermeabilized cell showed extensive membrane labeling (Figure 2D). Coexpression of the myogenic transcription factor myogenin and ADAM12 in single myotubes confirmed that differentiating myogenic cells also express ADAM12 (Figure 2E).
Figure 2.

ADAM12 expression by human mpc. (A) RT-PCR analysis of ADAM12-L, ADAM12-S, and β2-microglobulin mRNAs in mpc at days 4, 7, and 14 of culture (expected sizes of PCR products were 314, 371, and 335 base pairs, respectively). (B) Immunoblot analysis of pro-(100-kDa) and mature (90-kDa) ADAM12-L (top) in mpc lysates with goat polyclonal anti-cytoplasmic tail of ADAM12-L, and of pro-(92-kDa) and mature (68-kDa) ADAM12-S (bottom) in mpc supernatants with mouse monoclonal 2F7 directed toward disintegrin domain of ADAM12, at day 4, 7, and 14. (C) ADAM12 localization (FITC) in Triton X-100 permeabilized mpc by using rb122 antibody. (D) ADAM12 localization (FITC) in unpermeabilized mpc. (E) After permeabilization, differentiated mpc (day 7) were labeled with rb122 antibody revealed by FITC-conjugated secondary antibodies (green) and with myogenin antibody revealed by TRITC-conjugated secondary antibodies (red). Nuclei staining with DAPI. Bars, 20 μm.
α9β1 Integrin Is Also Expressed during mpc Differentiation
It is well established that β1 integrin subunit is expressed by both myoblasts and myotubes (Dickson et al., 1992; Hirsch et al., 1998); and consistently, β1 mRNAs were detected by RT-PCR (Figure 3A). RT-PCR also showed expression of α9 integrin subunit mRNAs at the three stages of myogenesis (Figure 3A). Immunoblots performed with antiserum #1057 assessed the production of α9 integrin protein at the same time points in the form of immunoreactive band corresponding to the mature α9 (150 kDa) (Figure 3B). Immunofluorescence on unpermeabilized cells confirmed expression of α9β1 integrin at the membrane throughout in vitro myogenesis (Figure 3C). Double immunostaining showed that coexpression of both ADAM12 and α9β1 was mainly observed when cells have entered differentiation. Colocalization of ADAM12 and α9β1 could be observed at membrane cell surface and at close cell-cell contact (Figure 4).
Figure 3.

α9β1 expression by human mpc. (A) α9 and b1 integrin subunits in mpc at days 4, 7, and 14 of culture, analyzed by RT-PCR. Expected size of PCR products was, respectively, 424 and 881 base pairs. (B) Detection by immunoblot analysis of mature α9 (150 kDa) in cultured cell lysate at days 4, 7, and 14. (C) Unpermeabilized mpc exhibited membrane immunolabeling of α9β1 integrin with monoclonal Y9A2 antibody (directed toward α9β1) by using FITC-conjugated secondary antibody. Propidium iodide stain of nuclei. Bars, 20 μm.
Figure 4.

Membrane expression of ADAM12 and α9β1 integrin and colocalization at cell-cell contact. Double immunolabeling on unpermeabilized mpc of ADAM12 (rb122) and α9β1 integrin (Y9A2) by using TRITC- or FITC-conjugated secondary antibodies, respectively. DAPI stain of nuclei. Bar, 20 μm.
α9β1 Integrin Acts as a Ligand for mpc Membrane-bound ADAM12
Coimmunoprecipitation experiments using mpc extracts incubated with antibodies to ADAM12 and subjected to immunoblotting with anti-α9 antibody disclosed an expected immunoreactive band at 150 kDa, corresponding to α9, thus confirming binding of α9 to ADAM12. Inverse experiments using anti-α9β1 antibody followed by immunoblotting with anti-ADAM12 antibody disclosed an immunoreactive band at 90 kDa, corresponding to mature membrane-bound ADAM12 (Figure 6A).
Figure 6.
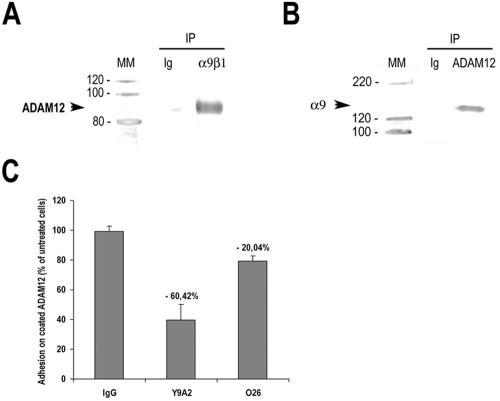
α9β1 integrin acts as a ligand for mpc membrane-bound ADAM12. (A) Immunoblot analysis of ADAM12 expression (with rb122, anti-disintegrin domain) after coimmunoprecipitation with irrelevant mouse immunoglobulins or monoclonal anti-Y9A2 antibody (left), and of α9 expression (with #1057, anti-cytoplasmic domain) after coimmunoprecipitation with irrelevant mouse immunoglobulins or monoclonal anti-ADAM12 antibodies (6E6, 8F8, and 6C10 mix) (right). Example of one blot. (B) Percentage of bound cells to plate coated with purified full length ADAM12 and incubated with either irrelevant mouse Ig, anti-α9β1 (Y9A2), or anti-a7b1 (O26) blocking antibodies. Results are means ± SD of three experiments run in triplicate (p < 0.05).
Inhibition of ADAM12/α9β1 Integrin Interplay Inhibits mpc Fusion
To investigate the role of ADAM12–α9β1 integrin interplay in human mpc differentiation, we incubated mpc cultures with ADAM12 antisense oligonucleotides or α9β1 blocking antibodies at time of differentiation induction. At day 14, cells cultured in the presence of ADAM12 antisense oligonucleotides showed dramatic expression drop of both ADAM12-S and -L isoforms as assessed by immunoblotting (inhibition by 91 ± 8.5%, p < 0.001 and 85 ± 1.5%, p <0.001, respectively) (Figure 5A). When subjected to ADAM12 antisense oligonucleotides or α9β1 blocking antibody, morphology of cultures changed, with myotubes being less numerous and showing a thinner diameter, and a lower number of nuclei than controls, despite a subnormal length (Figure 5B). When incubated with either nonsense oligonucleotides or isotypic immunoglobulins, cultures displayed normal morphology with similar myotube size and nuclei content (Figure 5B).
Figure 5.

ADAM12 and α9β1 integrin in mpc fusion. (A) Immunoblot quantification of ADAM12 isoforms expression by mpc-treated or not with an ADAM12 antisense and nonsense. Graph shows densitometric analysis of three different cultures, and immunoblot presents an example of one culture. (B) May-Grünwald Giemsa stain of mpc at day 14 showing morphological appearance of myotubes after different treatments. Bar, 40 μm. (C) Fusion index was measured for each situation and normalized as percentage of the control (untreated cells). Results are means ± SD of three experiments run in duplicate (*p < 0.05, **p < 0.01, ***p < 0.001). (D) Percentage of nuclei included in mononucleated cells, in cells with two to four nuclei and in cells with five or more nuclei under the total number of nuclei (**p < 0.01, ***p < 0.001). (E) Percentage of nuclei included in myotubes with two to four nuclei (left) or five or more nuclei (right) under the total number of nuclei in myotubes with two or more nuclei (*p < 0.05, ***p < 0.01, ***p < 0.001).
Significant inhibition of fusion was assessed by fusion index decrease at day 14 (inhibition by 46.7 ± 4.3% at day 14, p < 0.01) compared with untreated cells (Figure 5C). Similarly, α9β1 blocking antibodies used at saturating concentrations (Eto et al., 2000) induced fusion index decrease by 48.2 ± 1% at day 14 (p < 0.01). When both ADAM12 antisense oligonucleotides and α9β1 blocking antibodies were used, a further decrease of mpc fusion was observed: 62.2 ± 2.4% at day 14 (p < 0.001) (Figure 5C).
ADAM12 and α9β1 Are Mainly Operative in Myotube Growth
To assess the role of ADAM12 and α9β1 integrin in formation and growth of myotubes that represent two distinct phases of myogenesis, we analyzed the effects of ADAM12 antisense and anti-α9β1 antibody with regard to the size of myotubes. These effectors were added at time of shift (day 4) and 2 d later (day 6). Evaluation of early differentiation was done at day 5, when first small nascent myotubes (no cell with more than 4 nuclei are formed. Both effectors induced a decrease of nuclei included in small myotubes (2–4 nuclei) by 18% (p < 0.0001), suggesting delayed early differentiation.
Evaluation at day 14 (late differentiation) showed an increased proportion of mononucleated cells and a twofold decrease of the proportion of nuclei included in large myotubes (≥5 nuclei), whereas a proportion of nuclei included in small myotubes (containing 2–4 nuclei) was similar to controls (Figure 5D). Then, we used a previously described nuclear number assay, in which the number of nuclei inside myotubes according to their size is established. Myotubes were separated into two groups: small myotubes with two to four nuclei and large myotubes with five or more nuclei. Then, we calculated the percentage of nuclei belonging to each group. This assay showed that the number of nuclei incorporated into small myotubes was significantly higher and that of nuclei incorporated into large myotubes significantly lower than in controls after exposure of cultures to ADAM12 antisense oligonucleotides and/or anti-α9β1 antibody (Figure 5E). This result indicates that both effectors inhibit growth of myotubes (by accretion of additional nuclei to small preformed myotubes) more than formation of nascent myotubes.
ADAM12–α9β1 Constitutes a Potent Adhesion System
To further explore significance of ADAM12–α9β1 interaction, we seeded mpc on full-length ADAM12-coated plates. Mpc were adherent on this support and anti-α9β1 blocking antibody (Y9A2) induced an inhibition of mpc adherence to ADAM12-coated plates by 60.4 ± 10.6% (p < 0.05) (Figure 6B). Because the disintegrin domain of ADAM12 was shown to bind to α7β1 integrin (Zhao et al., 2004), we compared the respective inhibitory effects of both Y9A2 and anti-α7 blocking antibody (O26) on mpc adhesion on ADAM12-coated plates. In the same conditions, blockade of α7 integrin inhibited mpc adhesion at lower level (21 ± 3.6%) than Y9A2 (p < 0.05) (Figure 6B). Together, these results strongly support actual α9β1 integrin binding to full-length ADAM12, the membrane-bound isoform of ADAM12.
α9β1 Integrin Binding to ADAM12 Induces Intercellular mpc Adhesion
Because both ADAM12 and α9β1 integrin may induce cell-to-cell adhesion (Eto et al., 2000), we also evaluated whether inhibition of fusion could be related to a nonspecific alteration of mpc attachment to their self-produced extracellular matrix. Detachment of mpc was assessed under the treatments used in fusion inhibition assays. Antibodies to either VLA-4 or VCAM-1 induced significant mpc detachment after 4 h or 3 d (p < 0.03 for each molecule at each time point) (Figure 7A). By contrast, no mpc detachment was observed after exposure to either α9β1 blocking antibody (at 4 h and 3 d) or ADAM12 antisense oligonucleotides (3 d) (Figure 7A). Accordingly, treated and untreated cell cultures looked similar. These results indicate that ADAM12 and α9β1 interact predominantly at the intercellular level.
Figure 7.

α9β1 integrin binding to ADAM12 induces intercellular mpc adhesion. (A) Cell deadhesion was measured for each situation and normalized as percentage of the control (untreated cells). Results are means ± SD of three experiments run in triplicate (*p < 0.03). (B) Adhesion of cells treated or not with nonsense or antisense oligonucleotides on untreated cell layer after incubation with mouse immunoglobulins, anti-α9β1 (Y9A2), or anti-a7b1 (O26) blocking antibodies. (C) Adhesion of cells treated or not with nonsense or antisense oligonucleotides on nonsense treated cell layer after incubation with mouse immunoglobulins, anti-α9β1 (Y9A2), or anti-α7β1 (O26) blocking antibodies. (D) Adhesion of cells treated or not with nonsense or antisense oligonucleotides on antisense treated cell layer after incubation with mouse immunoglobulins, anti-α9β1 (Y9A2), or anti-a7b1 (O26) blocking antibodies. Results are means ± SD of three experiments run in triplicate.
The role of ADAM12 and α9β1 in prefusional mpc adhesion was evaluated using adhesion assays on a cell layer. Treatment of mpc-BrdU by ADAM12 antisense oligonucleotides (mpc-AS-BrdU) or incubation of mpc-BrdU with anti-α9β1 (Y9A2) inhibited their adhesion on untreated differentiating mpc by 51.6 ± 1.3% (p < 0.05) and 52.4 ± 7% (p < 0.05) respectively (Figure 7, B and C). Combination of both inhibition strategies (mpc-AS-BrdU + Y9A2) further decreased mpc adhesion by 65.7 ± 2.5% (p < 0.01) (Figure 7D). Interestingly, the observed inhibition level of mpc-mpc adhesion was comparable with that of mpc fusion. Because α7β1 integrin may interact with disintegrin and cysteine-rich domains of ADAM12 to mediate cell adhesion (Zhao et al., 2004), we evaluated its implication in our model. The anti-α7 antibody O26 did not induce significant inhibition of mpc adhesion regardless of what mpc-BrdU was used: antisense, nonsense, or controls (Figure 7). In a second set of experiments, we replaced the untreated differentiating mpc by a layer of mpc previously subjected to ADAM12 antisense oligonucleotides. When mpc-BrdU was put on to adhere, inhibition of adhesion was increased: 57.4 ± 2.6, 61.2 ± 1.3, and 70.1 ± 0.2% for mpc-BrdU + Y9A2, mpc-AS-BrdU, and mpc-As-BrdU + Y9A2, respectively (Figure 7). As expected, O26 did not induce any significant effect (Figure 7, B–D). Together, our results confirmed that ADAM12 binding to α9β1 mediates mpc adhesion to differentiating mpc. Moreover, interaction of ADAM12 and α9β1 in mpc adhesion to nascent myotubes seems to be bidirectional, as suggested by expression analyses showing that mpc similarly express ADAM12 and α9β1 during their differentiation.
DISCUSSION
In the present study, we show that ADAM12 and α9β1 integrin are coexpressed during in vitro differentiation of human myogenic cell, that ADAM12 and α9 coimmunoprecipitate and are instrumental in intercellular mpc adhesion, and that both molecules participate to mpc fusion to myotubes.
ADAM12 is a multidomain protein that could promote myogenic differentiation by exerting several different activities at different times, either individually or through multimolecular interactions (Engvall and Wewer, 2003). For example, ADAM12 cell signaling may positively influence myogenesis, because membrane-bound ADAM12 activates phosphatidylinositol (PI) 3-kinase by mediating its recruitment to the membrane (Kang et al., 2001), active PI 3-kinase being crucially involved in terminal differentiation of myogenic cells (Jiang et al., 1998; Li et al., 2000). Moreover, the ADAM12 cytoplasmic tail binds α-actinin 1 and 2 (Galliano et al., 2000; Cao et al., 2001), this binding being required for full-blown fusion of C2C12 myogenic cells (Galliano et al., 2000).
ADAM12 also may exert protease activity, leading to growth factor release (Asakura et al., 2002). The secreted form ADAM12-S can cleave insulin-like growth factor (IGF)-binding protein-3 and -5 (Loechel et al., 2000; Shi et al., 2000), and in so doing promotes bioactivity of IGF-1 and -2, two positive regulators of muscle growth, survival, and regeneration (Husmann et al., 1996; Kaliman et al., 1996; Kronqvist et al., 2002).
In addition, ADAM12 may influence integrin-mediated cell adhesion functions. In mouse, ADAM12 positively modulates expression of integrin α7 (Cao et al., 2003; Moghadaszadeh et al., 2003), which has functions in skeletal muscle development and disease (Burkin and Kaufman, 1999). α7β1 integrin is mainly involved in mpc adhesion and migration on the matrix component laminin (Yao et al., 1996; Crawley et al., 1997; Blanco-Bose and Blau, 2001) and in myotendinous junction formation (Nawrotzki et al., 2003). Murine α7β1 integrin can bind ADAM12 disintegrin and cysteinerich domain and induce cell adhesion (Zhao et al., 2004). In our model however, ADAM12–α7 interaction was not crucially involved in cell-cell adhesion as assessed by α7 blockade experiments.
Our data indicate that ADAM12 binding to α9β1 is involved in mpc fusion. mpc fusion results from an ordered sequence of events, including cell migration, alignment, recognition, adhesion, and membrane merging. Any effector hindering one of these steps decreases the fusion rate. In our model, differentiation was induced when mpc were at subconfluence state to minimize the role of cellular motility in myotube formation. mpc adhesion to extracellular matrix also was considered, because both ADAM12 and α9β1 integrin may bind directly or indirectly extracellular matrix components such as fibronectin (Kawaguchi et al., 2003) or tenascin-C (Marcinkiewicz et al., 2000). In our experiments, inhibition of ADAM12–α9β1 interactions induced marked decrease of mpc fusion, without altering their adhesion to their own matrix. This contrasted with inhibition of VCAM1–α4β1 integrin interactions that decreased both mpc adhesion to support and fusion.
The key role of β1 integrins in mpc fusion was recently stressed by using β1-deficient transgenic murine cell lines (Schwander et al., 2003). In vitro, β1-deficient mpc can establish cell-to-cell adhesion, but they are unable to proceed into myotube formation (Schwander et al., 2003). β1 integrins may form a complex with the tetraspanin CD9 (Hemler, 2001), a molecule involved in murine mpc fusion and myotube maintenance (Tachibana and Hemler, 1999), and, in this way, play a part in mpc fusion (Schwander et al., 2003). However, fusion defects are rescued when β1-deficient and wild-type mpc are mixed, indicating that β1 integrins have to establish heterophilic interactions with another cell surface receptor, which may belong to ADAMs (Hirsch et al., 1998; Schwander et al., 2003). The concept that ADAMs may launch plasma membrane merging originated from the implication of the sperm surface protein fertilin in gamete fusion (Primakoff and Myles, 2002). Of note, however, none of ADAMs currently known to be expressed on sperm surface has been shown to directly act as a sperm-egg membrane merger (He et al., 2003). Therefore, it seems likely that ADAM12 and α9β1 integrin are primarily involved in cell-cell adhesion processes.
In mammals, most molecules known to participate to muscle cell fusion were simply regarded as mediating myotube formation (Rosen et al., 1992; Yagami-Hiromasa et al., 1995; Barnoy et al., 1996; Gorza and Vitadello, 2000). Evidence emerges, however, that formation of small nascent myotubes is distinct from the subsequent accretion of mpc to performed myotubes, as initially shown in Drosophila (Rau et al., 2001) and confirmed in mammals (Horsley et al., 2001; Horsley and Pavlath, 2003). Our results suggest that ADAM12 and its ligand α9β1 integrin constitute a molecular system that is predominantly, but not exclusively, involved in myotube elongation. Molecular mechanisms that regulate mpc fusion with preformed myotubes in mammals include activation of the nuclear factor of activated T cells (NFAT)c2 pathway and interleukin (IL)-4/IL–4Rα interactions (Horsley et al., 2003; Pavlath and Horsley, 2003). Interestingly, the ADAM12 signaling pathway includes activation of PI3-kinase, a molecule involved in expression of both NFAT (Jascur et al., 1997) and IL-4 (Hirasawa et al., 2000). It could be appropriate to further investigate relationships of the different systems at work in myotube elongation and growth.
Cell therapy using transplantation of exogenous mpc aimed at fusing with mature muscle fibers may help treating devastating muscle diseases. Unfortunately, inability of mpc to fuse efficiently with host myofibers represents a major limitation of cell therapy in this setting (Skuk and Tremblay, 2000). Further understanding of the role of ADAM12 and α9β1 integrin in mpc fusion is needed with expectance that manipulation of the system aimed at increasing mpc fusion to preexisting muscle cells could improve future therapeutic strategies.
Acknowledgments
We thank S. J. Kaufman (University of Illinois) and D. Sheppard (University of California, San Francisco) for the kind gift of α7 and α9 antibodies, respectively. We greatly appreciated the invaluable assistance of Dean Sheppard. This study was supported by a Fellowship to P. L. from Association Française contre les Myopathies.
Article published online ahead of print in MBC in Press on December 1, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0226).
References
- Abe, E., Mocharla, H., Yamate, T., Taguchi, Y., and Manolagas, S. C. (1999). Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif. Tissue Int. 64, 508-515. [DOI] [PubMed] [Google Scholar]
- Abmayr, S. M., Balagopalan, L., Galletta, B. J., and Hong, S. J. (2003). Cell and molecular biology of myoblast fusion. Int. Rev. Cytol. 225, 33-89. [DOI] [PubMed] [Google Scholar]
- Asakura, M., et al. (2002). Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-epidermal growth factor: metalloproteinase inhibitors as a new therapy. Nat. Med. 8, 35-40. [DOI] [PubMed] [Google Scholar]
- Authier, F. J., Chazaud, B., Plonquet, A., Eliezer-Vanerot, M. C., Poron, F., Belec, L., Barlovatz-Meimon, G., and Gherardi, R. K. (1999). Differential expression of the IL-1 system components during in vitro myogenesis: implication of IL-1beta in induction of myogenic cell apoptosis. Cell Death Differ. 6, 1012-1021. [DOI] [PubMed] [Google Scholar]
- Barnoy, S., Glasner, T., and Kosower, N. S. (1996). The role of calpastatin (the specific calpain inhibitor) in myoblast differentiation and fusion. Biochem. Biophys. Res. Commun. 220, 933-938. [DOI] [PubMed] [Google Scholar]
- Baroffio, A., Aubry, J. P., Kaelin, A., Krause, R. M., Hamann, M., and Bader, C. R. (1993). Purification of human muscle satellite cells by flow cytometry. Muscle Nerve 16, 498-505. [DOI] [PubMed] [Google Scholar]
- Blanco-Bose, W. E., and Blau, H. M. (2001). Laminin-induced change in conformation of preexisting alpha7beta1 integrin signals secondary myofiber formation. Dev. Biol. 233, 148-160. [DOI] [PubMed] [Google Scholar]
- Blobel, C. P., Wolfsberg, T. G., Turck, C. W., Myles, D. G., Primakoff, P., and White, J. M. (1992). A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356, 248-252. [DOI] [PubMed] [Google Scholar]
- Borneman, A., Kuschel, R., and Fujisawa-Sehara, A. (2000). Analysis for transcript expression of meltrin alpha in normal, regenerating, and denervated rat muscle. J. Muscle Res. Cell Motil. 21, 475-480. [DOI] [PubMed] [Google Scholar]
- Burkin, D. J., and Kaufman, S. J. (1999). The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 296, 183-190. [DOI] [PubMed] [Google Scholar]
- Cao, Y., Kang, Q., Zhao, Z., and Zolkiewska, A. (2002). Intracellular processing of metalloprotease disintegrin ADAM12. J. Biol. Chem. 277, 26403-26411. [DOI] [PubMed] [Google Scholar]
- Cao, Y., Kang, Q., and Zolkiewska, A. (2001). Metalloprotease-disintegrin ADAM 12 interacts with alpha-actinin-1. Biochem. J. 357, 353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y., Zhao, Z., Gruszczynska-Biegala, J., and Zolkiewska, A. (2003). Role of metalloprotease disintegrin ADAM12 in determination of quiescent reserve cells during myogenic differentiation in vitro. Mol. Cell Biol. 23, 6725-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud, B., Ricoux, R., Christov, C., Plonquet, A., Gherardi, R. K., and Barlovatz-Meimon, G. (2002). Promigratory effect of plasminogen activator inhibitor-1 on invasive breast cancer cell populations. Am. J. Pathol. 160, 237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud, B., Sonnet, C., Lafuste, P., Bassez, G., Rimaniol, A. C., Poron, F., Authier, F. J., Dreyfus, P. A., and Gherardi, R. K. (2003). Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J. Cell Biol. 163, 1133-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. J., Han, J. H., and Roodman, G. D. (2001). ADAM 8, a novel osteoclast stimulating factor. J. Bone Miner. Res. 16, 814-822. [DOI] [PubMed] [Google Scholar]
- Crawley, S., Farrell, E. M., Wang, W., Gu, M., Huang, H. Y., Huynh, V., Hodges, B. L., Cooper, D. N., and Kaufman, S. J. (1997). The alpha7beta1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp. Cell Res. 235, 274-286. [DOI] [PubMed] [Google Scholar]
- De Rossi, M., Bernasconi, P., Baggi, F., de Waal, M. R., and Mantegazza, R. (2000). Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int. Immunol. 12, 1329-1335. [DOI] [PubMed] [Google Scholar]
- Dickson, G., Azad, A., Morris, G. E., Simon, H., Noursadeghi, M., and Walsh, F. S. (1992). Co-localization and molecular association of dystrophin with laminin at the surface of mouse and human myotubes. J. Cell Sci. 103, 1223-1233. [DOI] [PubMed] [Google Scholar]
- Doberstein, S. K., Fetter, R. D., Mehta, A. Y., and Goodman, C. S. (1997). Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 136, 1249-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall, E., and Wewer, U. M. (2003). The new frontier in muscular dystrophy research: booster genes. FASEB J. 17, 1579-1584. [DOI] [PubMed] [Google Scholar]
- Eto, K., Huet, C., Tarui, T., Kupriyanov, S., Liu, H. Z., Puzon-McLaughlin, W., Zhang, X. P., Sheppard, D., Engvall, E., and Takada, Y. (2002). Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9beta 1, implications for sperm-egg binding and other cell interactions. J. Biol. Chem. 277, 17804-17810. [DOI] [PubMed] [Google Scholar]
- Eto, K., Puzon-McLaughlin, W., Sheppard, D., Sehara-Fujisawa, A., Zhang, X. P., and Takada, Y. (2000). RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J. Biol. Chem. 275, 34922-34930. [DOI] [PubMed] [Google Scholar]
- Friday, B. B., Mitchell, P. O., Kegley, K. M., and Pavlath, G. K. (2003). Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation 71, 217-227. [DOI] [PubMed] [Google Scholar]
- Fujio, Y., Guo, K., Mano, T., Mitsuuchi, Y., Testa, J. R., and Walsh, K. (1999). Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 19, 5073-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano, M. F., Huet, C., Frygelius, J., Polgren, A., Wewer, U. M., and Engvall, E. (2000). Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha-actinin-2, is required for myoblast fusion. J. Biol. Chem. 275, 13933-13939. [DOI] [PubMed] [Google Scholar]
- Gilpin, B. J., Loechel, F., Mattei, M. G., Engvall, E., Albrechtsen, R., and Wewer, U. M. (1998). A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J. Biol. Chem. 273, 157-166. [DOI] [PubMed] [Google Scholar]
- Gorza, L., and Vitadello, M. (2000). Reduced amount of the glucose-regulated protein GRP94 in skeletal myoblasts results in loss of fusion competence. FASEB J. 14, 461-475. [DOI] [PubMed] [Google Scholar]
- Hawke, T. J., and Garry, D. J. (2001). Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91, 534-551. [DOI] [PubMed] [Google Scholar]
- He, Z. Y., Brakebusch, C., Fassler, R., Kreidberg, J. A., Primakoff, P., and Myles, D. G. (2003). None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Dev. Biol. 254, 226-237. [DOI] [PubMed] [Google Scholar]
- Hemler, M. E. (2001). Specific tetraspanin functions. J. Cell Biol. 155, 1103-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa, N., Sato, Y., Fujita, Y., and Ohuchi, K. (2000). Involvement of a phosphatidylinositol 3-kinase-p38 mitogen activated protein kinase pathway in antigen-induced IL-4 production in mast cells. Biochim. Biophys. Acta 1456, 45-55. [DOI] [PubMed] [Google Scholar]
- Hirsch, E., Lohikangas, L., Gullberg, D., Johansson, S., and Fassler, R. (1998). Mouse myoblasts can fuse and form a normal sarcomere in the absence of beta1 integrin expression. J. Cell Sci. 111, 2397-2409. [DOI] [PubMed] [Google Scholar]
- Hooft van Huijsduijnen, R. (1998). ADAM 20 and 21; two novel human testis-specific membrane metalloproteases with similarity to fertilin-alpha. Gene 206, 273-282. [DOI] [PubMed] [Google Scholar]
- Horsley, V., Friday, B. B., Matteson, S., Kegley, K. M., Gephart, J., and Pavlath, G. K. (2001). Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J. Cell Biol. 153, 329-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley, V., Jansen, K. M., Mills, S. T., and Pavlath, G. K. (2003). IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113, 483-494. [DOI] [PubMed] [Google Scholar]
- Horsley, V., and Pavlath, G. K. (2003). Prostaglandin F2(alpha) stimulates growth of skeletal muscle cells via an NFATC2-dependent pathway. J. Cell Biol. 161, 111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard, S., Loechel, F., Xu, X., Tajima, R., Albrechtsen, R., and Wewer, U. M. (2000). Trafficking of human ADAM 12-L: retention in the trans-Golgi network. Biochem. Biophys. Res. Commun. 275, 261-267. [DOI] [PubMed] [Google Scholar]
- Huovila, A. P., Almeida, E. A., and White, J. M. (1996). ADAMs and cell fusion. Curr. Opin. Cell Biol. 8, 692-699. [DOI] [PubMed] [Google Scholar]
- Husmann, I., Soulet, L., Gautron, J., Martelly, I., and Barritault, D. (1996). Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 7, 249-258. [DOI] [PubMed] [Google Scholar]
- Iba, K., et al. (2000). The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading. J. Cell Biol. 149, 1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba, K., Albrechtsen, R., Gilpin, B. J., Loechel, F., and Wewer, U. M. (1999). Cysteine-rich domain of human ADAM 12 (meltrin alpha) supports tumor cell adhesion. Am. J. Pathol. 154, 1489-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jascur, T., Gilman, J., and Mustelin, T. (1997). Involvement of phosphatidylinositol 3-kinase in NFAT activation in T cells. J. Biol. Chem. 272, 14483-14488. [DOI] [PubMed] [Google Scholar]
- Jiang, B. H., Zheng, J. Z., and Vogt, P. K. (1998). Anessentialroleofphosphatidylinositol 3-kinase in myogenic differentiation. Proc. Natl. Acad. Sci. USA 95, 14179-14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota, N., Suzuki, A., Nakagami, Y., Izumi, T., and Endo, T. (2000). Endogenous meltrin alpha is ubiquitously expressed and associated with the plasma membrane but exogenous meltrin alpha is retained in the endoplasmic reticulum. J. Biochem. (Tokyo) 128, 941-949. [DOI] [PubMed] [Google Scholar]
- Kaliman, P., Vinals, F., Testar, X., Palacin, M., and Zorzano, A. (1996). Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J. Biol. Chem. 271, 19146-19151. [DOI] [PubMed] [Google Scholar]
- Kang, Q., Cao, Y., and Zolkiewska, A. (2001). Direct interaction between the cytoplasmic tail of ADAM 12 and the Src homology 3 domain of p85alpha activates phosphatidylinositol 3-kinase in C2C12 cells. J. Biol. Chem. 276, 24466-24472. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, N., et al. (2003). ADAM12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating {beta}1 integrin function. J. Cell Sci. 116, 3893-3904. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, N., Xu, X., Tajima, R., Kronqvist, P., Sundberg, C., Loechel, F., Albrechtsen, R., and Wewer, U. M. (2002). ADAM 12 protease induces adipogenesis in transgenic mice. Am. J. Pathol. 160, 1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzschmar, J., Lum, L., and Blobel, C. P. (1996). Metargidin, a membrane-anchored metalloprotease-disintegrin protein with an RGD integrin binding sequence. J. Biol Chem. 271, 4593-4596. [DOI] [PubMed] [Google Scholar]
- Kronqvist, P., Kawaguchi, N., Albrechtsen, R., Xu, X., Schroder, H. D., Moghadaszadeh, B., Nielsen, F. C., Frohlich, C., Engvall, E., and Wewer, U. M. (2002). ADAM12 alleviates the skeletal muscle pathology in mdx dystrophic mice. Am. J. Pathol. 161, 1535-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisaki, T., Masuda, A., Osumi, N., Nabeshima, Y., and Fujisawa-Sehara, A. (1998). Spatially- and temporally-restricted expression of meltrin alpha (ADAM12) and beta (ADAM19) in mouse embryo. Mech. Dev. 73, 211-215. [DOI] [PubMed] [Google Scholar]
- Li, Y., Jiang, B., Ensign, W. Y., Vogt, P. K., and Han, J. (2000). Myogenic differentiation requires signalling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell Signal. 12, 751-757. [DOI] [PubMed] [Google Scholar]
- Loechel, F., Fox, J. W., Murphy, G., Albrechtsen, R., and Wewer, U. M. (2000). ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem. Biophys. Res. Commun. 278, 511-515. [DOI] [PubMed] [Google Scholar]
- Loechel, F., Gilpin, B. J., Engvall, E., Albrechtsen, R., and Wewer, U. M. (1998). Human ADAM 12 (meltrin alpha) is an active metalloprotease. J. Biol. Chem. 273, 16993-16997. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz, C., Taooka, Y., Yokosaki, Y., Calvete, J. J., Marcinkiewicz, M. M., Lobb, R. R., Niewiarowski, S., and Sheppard, D. (2000). Inhibitory effects of MLDG-containing heterodimeric disintegrins reveal distinct structural requirements for interaction of the integrin alpha 9beta 1 with VCAM-1, tenascin-C, and osteopontin. J. Biol. Chem. 275, 31930-31937. [DOI] [PubMed] [Google Scholar]
- Mayer, U. (2003). Integrins: redundant or important players in skeletal muscle? J. Biol. Chem. 278, 14587-14590. [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh, B., et al. (2003). Compensation for dystrophin-deficiency: ADAM12 overexpression in skeletal muscle results in increased alpha 7 integrin, utrophin and associated glycoproteins. Hum. Mol. Genet. 12, 2467-2479. [DOI] [PubMed] [Google Scholar]
- Nawrotzki, R., Willem, M., Miosge, N., Brinkmeier, H., and Mayer, U. (2003). Defective integrin switch and matrix composition at alpha 7-deficient myotendinous junctions precede the onset of muscular dystrophy in mice. Hum. Mol. Genet. 12, 483-495. [DOI] [PubMed] [Google Scholar]
- Palmer, E. L., Ruegg, C., Ferrando, R., Pytela, R., and Sheppard, D. (1993). Sequence and tissue distribution of the integrin alpha 9 subunit, a novel partner of beta 1 that is widely distributed in epithelia and muscle. J. Cell Biol. 123, 1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath, G. K., and Horsley, V. (2003). Cell fusion in skeletal muscle–central role of NFATC2 in regulating muscle cell size. Cell Cycle 2, 420-423. [PubMed] [Google Scholar]
- Primakoff, P., and Myles, D. G. (2002). Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296, 2183-2185. [DOI] [PubMed] [Google Scholar]
- Rau, A., Buttgereit, D., Holz, A., Fetter, R., Doberstein, S. K., Paululat, A., Staudt, N., Skeath, J., Michelson, A. M., and Renkawitz-Pohl, R. (2001). Rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development 128, 5061-5073. [DOI] [PubMed] [Google Scholar]
- Rosen, G. D., Sanes, J. R., LaChance, R., Cunningham, J. M., Roman, J., and Dean, D. C. (1992). Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 69, 1107-1119. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid, F., De Landazuri, M. O., Morago, G., Cebrian, M., Acevedo, A., and Bernabeu, C. (1986). VLA-3, a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur. J. Immunol. 16, 1343-1349. [DOI] [PubMed] [Google Scholar]
- Schwander, M., Leu, M., Stumm, M., Dorchies, O. M., Ruegg, U. T., Schittny, J., and Muller, U. (2003). Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev. Cell 4, 673-685. [DOI] [PubMed] [Google Scholar]
- Shi, Z., Xu, W., Loechel, F., Wewer, U. M., and Murphy, L. J. (2000). ADAM 12, a disintegrin metalloprotease, interacts with insulin-like growth factor-binding protein-3. J. Biol. Chem. 275, 18574-18580. [DOI] [PubMed] [Google Scholar]
- Skuk, D., and Tremblay, J. P. (2000). Progress in myoblast transplantation: a potential treatment of dystrophies. Microsc. Res. Tech. 48, 213-222. [DOI] [PubMed] [Google Scholar]
- Tachibana, I., and Hemler, M. E. (1999). Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J. Cell Biol. 146, 893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, M. V. (2003). Muscle differentiation: signalling cell fusion. Curr. Biol. 13, R964-R966. [DOI] [PubMed] [Google Scholar]
- Thodeti, C. K., et al. (2003). ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J. Biol. Chem. 278, 9576-9584. [DOI] [PubMed] [Google Scholar]
- Thornhill, M. H., Wellicome, S. M., Mahiouz, D. L., Lanchbury, J. S., Kyan-Aung, U., and Haskard, D. O. (1991). Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J. Immunol. 146, 592-598. [PubMed] [Google Scholar]
- Wang, A., Patrone, L., McDonald, J. A., and Sheppard, D. (1995). Expression of the integrin subunit alpha 9 in the murine embryo. Dev. Dyn. 204, 421-431. [DOI] [PubMed] [Google Scholar]
- Weskamp, G., Kratzschmar, J., Reid, M. S., and Blobel, C. P. (1996). MDC9, a widely expressed cellular disintegrin containing cytoplasmic SH3 ligand domains. J. Cell Biol. 132, 717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. M. (2003). ADAMs: modulators of cell-cell and cell-matrix interactions. Curr. Opin. Cell Biol. 15, 598-606. [DOI] [PubMed] [Google Scholar]
- Wolfsberg, T. G., and White, J. M. (1996). ADAMs in fertilization and development. Dev. Biol. 180, 389-401. [DOI] [PubMed] [Google Scholar]
- Yagami-Hiromasa, T., Sato, T., Kurisaki, T., Kamijo, K., Nabeshima, Y., and Fujisawa-Sehara, A. (1995). A metalloprotease-disintegrin participating in myoblast fusion. Nature 377, 652-656. [DOI] [PubMed] [Google Scholar]
- Yao, C. C., Ziober, B. L., Squillace, R. M., and Kramer, R. H. (1996). Alpha7 integrin mediates cell adhesion and migration on specific laminin isoforms. J. Biol. Chem. 271, 25598-25603. [DOI] [PubMed] [Google Scholar]
- Zhao, Z., Gruszczynska-Biegala, J., Cheuvront, T., Yi, H., von der, M. H., von der, M. K., Kaufman, S. J., and Zolkiewska, A. (2004). Interaction of the disintegrin and cysteine-rich domains of ADAM12 with integrin alpha7beta1. Exp. Cell Res. 298, 28-37. [DOI] [PubMed] [Google Scholar]
- Zolkiewska, A. (1999). Disintegrin-like/cysteine-rich region of ADAM 12 is an active cell adhesion domain. Exp. Cell Res. 252, 423-431. [DOI] [PubMed] [Google Scholar]


