Abstract
The laminin family of proteins is critical for managing a variety of cellular activities including migration, adhesion, and differentiation. In bone, the roles of laminins in controlling osteogenic differentiation of human mesenchymal stem cells (hMSC) are unknown. We report here that laminin-5 is found in bone and expressed by hMSC. hMSC isolated from bone synthesize laminin-5 and adhere to exogenous laminin-5 through α3β1 integrin. Adhesion to laminin-5 activates extracellular signal-related kinase (ERK) within 30 min and leads to phosphorylation of the osteogenic transcription factor Runx2/CBFA-1 within 8 d. Cells plated on laminin-5 for 16 d express increased levels of osteogenic marker genes, and those plated for 21 d deposit a mineralized matrix, indicative of osteogenic differentiation. Addition of the ERK inhibitor PD98059 mitigates these effects. We conclude that contact with laminin-5 is sufficient to activate ERK and to stimulate osteogenic differentiation in hMSC.
INTRODUCTION
Human mesenchymal stem cells (hMSC) are multipotent cells found within the bone marrow and periosteum (Barry and Murphy, 2004). Typically they differentiate into chondrogenic, adipogenic, or osteogenic lineages, but recent evidence suggests that hMSC can also express phenotypic characteristics of endothelial, neural, smooth muscle, skeletal myoblast, and cardiac myocyte cells (Pittenger and Martin, 2004). The mechanisms governing hMSC differentiation are not well understood, but the ability of these cells to self renew and develop into numerous tissues makes their potential use in clinical applications quite promising.
Extracellular matrix (ECM) proteins are well-known regulators of multiple cellular functions, including differentiation. The laminin (Ln) family of ECM proteins are ubiquitously expressed but are especially abundant in the basement membrane of many epithelial and endothelial tissues, where they mediate cell attachment, migration, and tissue organization in conjunction with other ECM proteins (Malinda and Kleinman, 1996). Each laminin molecule is a heterotrimer, composed of an α-, β-, and γ-subunit. The subunits share homology with one another and upon combining through disulfide bonds form an asymmetric cross-like structure with one long and three short arms (Colognato and Yurchenco, 2000). The Ln-5 isoform is composed of α3, β3, and γ2 subunits. Expression of the γ2 subunit has only been found in Ln-5, whereas the α3 subunit is found in both Ln-6 and Ln-7. Ln-5 is bound by α2β1, α3β1, α6β1, and α6β4 integrin receptors (Decline and Rousselle, 2001), all of which are found in hMSC (Pittenger et al., 1999). The role of Ln family members in osteogenic differentiation is not known (Roche et al., 1999), though expression of the γ2 chain has been previously detected in bone marrow (Siler et al., 2002).
Ln-5 is expressed in distinct temporal and spatial patterns in developing epithelial tissues and influences tissue compartmentalization and cellular phenotypes from early embryonic development onward (Aberdam et al., 1994; Timpl and Brown, 1996). These tissues include the oral, nasal, and olfactory epithelium, intestine and stomach, bronchi and bronchioli, and breast (Virtanen et al., 1995; Virtanen et al., 1996; Orian-Rousseau et al., 1996; Stahl et al., 1997; Thorup et al., 1997). In these tissues Ln-5 has been shown to promote cellular growth, morphogenesis, and wound healing.
Ln-5 is typically found in tissues derived from ectoderm and endoderm. We recently discovered the expression of Ln-5 in the intimal layer of the vasculature, where it is expressed by vascular smooth muscle cells and plays a role in controlling the growth and migration of these cells in response to peptide growth factors (Kingsley et al., 2001, 2002a, 2002b). The fact that we found this Ln isoform in a mesodermally derived tissue implies that Ln-5 may play a role in regulating the growth and differentiation of other mesodermal tissues as well, including bone.
Recently it has been discovered that the ECM proteins collagen-I (COLL-I) and vitronectin (VN), found in bone, induce osteogenic differentiation comparable to that observed with soluble osteogenic supplements (Salasznyk et al., 2004). This suggests that other bone ECM proteins, and their associated integrin receptors, may also play a similar role in the differentiation of hMSC. Integrins stimulate intracellular signaling pathways via short cytoplasmic tails that act as docking sites for interactions with downstream signaling molecules (Schwartz, 2001). There are at least six classes of signaling molecules stimulated by integrin activation: protein tyrosine kinases, serine/threonine kinases, lipid kinases, lipid phosphatases, protein phosphatases, and ion fluxes (Schwartz and Ginsberg, 2002). The roles that these molecules play in the differentiation of hMSC are for the most part unknown.
One of the potential signal transduction pathways that might direct the differentiation of hMSC is the mitogen-activated protein (MAP) kinase pathway. Extracellular signal-related kinase (ERK) is a member of the MAP kinase family that stimulates the differentiation of hMSC into osteoblasts via phosphorylation of the osteogenic transcription factor runx2/CBFA-1 (Jaiswal et al., 2000). However, the cellular pathways linking ECM contact with ERK activation in hMSC are unknown. The goal of this study was to examine the role of Ln-5-associated ERK signaling in osteogenic differentiation of hMSC. We hypothesized that Ln-5 stimulates osteogenic differentiation of hMSC by α3β1 integrin-mediated activation of ERK, with subsequent activation of runx2/CBFA-1 and expression of osteogenic genes.
MATERIALS AND METHODS
Tissue culture media (DMEM) and penicillin G-streptomycin sulfate (GPS) were purchased from Mediatech (Cellgro, Herndon, VA). Fetal bovine serum (FBS) was purchased from Gemini Bio-Products (Woodland, CA). Trypsin-EDTA and purified bovine COLL-I were obtained from Sigma Chemical Co. (St. Louis, MO). Laminin-1 G4 domain was cloned into a pET vector, expressed in BL21DE3 bacteria, and purified using a nickel column. Purified mouse collagen IV and Ln-1 were purchased from Collaborative Research (Bedford, MA) Purified laminin-5 was generously provided by Desmos (San Diego, CA). Purified human plasma VN and human plasma fibronectin (FN) were purchased from Chemicon International (Temecula, CA). The αvβ3 (catalogue no. MAB1976) and the α1–6 and β1–4 integrin function-blocking antibodies (Alpha integrin blocking and IHC kit, catalogue no. ECM 430; Beta integrin screening kit, catalogue no. ECM 440) were purchased from Chemicon International (Temecula, CA). Rabbit polyclonal IgG Anti-g-actin (catalogue no. AAM01-A) antibody was from Cytoskeleton (Denver, CO). Rabbit polyclonal IgG antibodies against anti-ERK1/2 (catalogue no. AB3053), osteopontin (catalogue no. AB1870), osteocalcin (catalogue no. AB1857), and phosphoserine (catalogue no. AB1603) were obtained from Chemicon International. Mouse monoclonal IgG antibodies for α6 integrin (catalogue no. CBL458), α3 integrin (catalogue no. MAB2056), and Ln-5 (catalogue no. MAB1947) were obtained from Chemicon International. Rabbit polyclonal IgG phosphospecific antibodies against anti-ERK 1/2 (pTpY185/187) (catalogue no. 44–680) was from Biosource International (Camarillo, CA). Mouse monoclonal IgG antibodies against anti-Runx2/Cbfa1 were purchased from MBL International (Watertown, MA). Rabbit polyclonal IgG antibody for Ln-5 was provided by Vito Quaranta (Vanderbilt University, Nashville, TN). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). Protein A/G-agarose beads and the MEK1 inhibitor PD98059 were purchased from Calbiochem (San Diego, CA). RT-PCR primers listed in Table 1 were purchased from IDT Technologies (Coralville, Iowa). The protein assay kit was purchased from Pierce (Rockford, IL). Unless otherwise specified, the other standard reagents were obtained from Fisher Scientific (Fair Lawn, NJ).
Table 1.
RT-PCR primers
| Gene name | Primer sequences | Product size (bp) |
|---|---|---|
| ALPL (alkaline phosphatase) | Forward 5′-GGGGGTGGCCGGAAATACAT-3′ | 543 |
| Reverse 5′-GGGGGCCAGACCAAAGATAGAGTT-3′ | ||
| SSP1 (osteopontin) | Forward 5′-AGACCCCAAAAGTAAGGAAGAAGA-3′ | 564 |
| Reverse 5′-GACAACCGTGGGAAAACAAATAAG-3′ | ||
| BGLAP (osteocalcin) | Forward 5′-CGCAGCCACCGAGACACCAT-3′ | 400 |
| Reverse 5′-AGGGCAAGGGGAAGAGGAAAGAA-3′ | ||
| CBFA1 (core binding factor alpha 1) | Forward 5′-ATGGCGGGTAACGATGAAAAT-3′ | 421 |
| Reverse 5′-ACGGCGGGGAAGACTGTGC-3′ | ||
| GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) | Forward 5′-ATGGAAATCCCATCACCATCT-3′ | 1000 |
| Reverse 5′-GGTTGAGCACAGGGTACTTTATT-3′ | ||
| Laminin alpha 1 chain | Forward 5′-ATTGCCTTCCAGCGAAACCGG-3′ | 780 |
| Reverse 5′-TTGGACACATTTATCGTCCTG-3′ | ||
| Laminin beta 1 chain | Forward 5′-TTGGACCAAGATGTCCTGAG-3′ | 659 |
| Reverse 5′-CAATATATTCTGCCTCCCCG-3′ | ||
| Laminin-5 alpha 3 chain | Forward 5′-GTGGTTACCTCACTTACCAAGCCA-3′ | 269 |
| Reverse 5′-GGTGAGCCTTTGAGTCTCTGTGAA-3′ | ||
| Laminin-5 beta 3 chain | Forward 5′-CCAATATCATGCCCTGGTGAGCTA-3′ | 374 |
| Reverse 5′-TGCAGAACAGTAGCTGAGTCTGTG-3′ | ||
| Laminin-5 gamma 2 chain | Forward 5′-TGGTGATTACAGAAGCCAGAAGG-3′ | 436 |
| Reverse 5′-GTCAGTTGACCTGAGCATACCCAT-3′ |
Cell Culture
Cryopreserved hMSC were purchased from Cambrex (Walkersville, MD) and were grown according to the manufacturer's instructions. Briefly, cells were plated at 5 × 103 cells/cm2 in a T75 flask (75 cm2) for continuous passaging in DMEM medium supplemented with 10% FBS, 1% l-glutamine (29.2 mg/ml), penicillin G (10,000 U/ml) and streptomycin sulfate (10,000 μg/ml). Medium was changed twice weekly and cells were detached by trypsin-EDTA and passaged into fresh culture flasks at a ratio of 1:3 upon reaching confluence. Cultures were incubated at 37°C in a humidified atmosphere containing 95% air and 5% CO2.
Laminin-5 matrix was isolated from rat bladder carcinoma 804G cells as described previously (Plopper et al., 1996)
For in vitro osteogenic assays, hMSC were passaged three times before they were inducted and plated at densities of 3.1 × 103 cells/cm2 in 0.2 ml/cm2 of medium on 100-mm Falcon dishes (78.5 cm2). The following day (day 0), we replaced the culture medium with fresh control medium in the absence or presence of osteogenic supplements (OS) containing 0.1 μM dexamethasone, 0.05 mM ascorbic acid-2-phosphate, and 10 mM β-glycerophosphate (Cambrex). In each experiment, control and OS media–treated cells were processed in parallel. For inhibition of MEK1, PD98059 in dimethyl sulfoxide (DMSO) was added to control and OS media at a final concentration of 50 μM twice a week during media changes unless otherwise specified. The final concentration of DMSO never exceeded 0.1%, and the same amount of DMSO vehicle was added to control conditions. For all other assays, suspensions of cells were incubated with PD98059 (50 μM) 15 min before plating. At days 8, 16, and 21, cultures were assayed as described below.
Immunological Detection of Ln-5 in Bone
Timed pregnancy rats were handled according to the University of Nevada, Las Vegas Protocol for Animal Care and Use, Protocol Number R701-1297-136: Developmental studies in rats and mice. Twelve-, 14-, 16-, and 18-d timed-pregnancy embryos were harvested from pregnant rats under general anesthesia and the mothers were killed using intracardial injection of Nembutal (sodium pentabarbital) ∼100 mg/kg.
Embryos were fixed at 4°C for 24 h in each of three successive paraformaldehyde solutions (4% wt/vol) containing 5, 10, and 15% sucrose, respectively. Embryos were then halved using standard dissection tools and frozen in Miles Tissue-TEK OCT 4583 embedding medium (Naperville, IL) in standard cryomolds using liquid freon. Tissue sections (8 μm thick) were cut using a Microm Cyostat HM 505E (Microm GmbH, Walldorf, Germany) and adhered to poly-l-lysine (0.8 mg/ml)-coated glass slides for photobleaching and immunostaining.
Immunostaining
Tissues were maintained at 27°C in a tinoxid-filter greenhouse at constant oxygen (20%) and CO2 (5%) to remove autofluoresence. Tissues were then sectioned using the cryotome and stained suing the Molecular Probes (Eugene, OR) Enzyme-Linked-Fluorescence ELF-97 Immunohistochemistry Protocol, MP 06600 using TR1 or CM6 monoclonal antibody (mAb), for the gamma and alpha chains of laminin-5, respectively (Plopper et al., 1996a). Stained tissues were visualized using a Leica DM LB immunofluorescence microscope (Deerfield, IL) with DAPI and FITC filter sets. ELF-substrate excitation and emission wavelengths are 360 and 535 ± 18 nm, respectively, and images were collected using SPOT diagnostic digital imaging equipment.
Mineralized Bone: Fourier Transform Infrared Analysis
The presence of apatite in cell matrix was detected by Fourier transform infrared (FTIR) analysis of ground powders. Cell layers, collected in 50 mM ammonium bicarbonate (pH 8.0) after 21 d, were lyophilized and analyzed as potassium bromide (KBr) pellets on a Bio-Rad FTS 40-A spectrometer (Bio-Rad Microscience, Cambridge, MA). The data were collected under nitrogen purge, and the spectral baseline was corrected and analyzed using GRAMS/386 software (Galactic Industries, Salem, NH) as previously described (Kato et al., 2001). The mineral content was calculated based on the spectrally derived mineral-to-matrix ratio (the integrated areas of the phosphate absorbance [900–1200 cm-1] and protein amide I band [1585–1720 cm-1]).
Immunoprecipitation of Runx2 and Western Blotting
Whole cell extracts were prepared by harvesting overnight serum-deprived cells or cells after 8 and 16 d in culture (DMEM + 0.1% FBS) with ice-cold radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 50 mM Tris, 1% Triton X-100, 0.3 mM sodium vanadate, 1% deoxycholic acid, 0.2% SDS, pH 7.4). For immunoprecipitation, samples were precleaned twice with 20 μl of protein A/G-agarose beads for 30 min followed by pelleting of beads. Mouse monoclonal anti-Runx2, 4 μl, was added and incubated for 2 h at 4°C with shaking. The immune complexes were collected on the addition of 20 μl of protein A/G-agarose beads and subsequently incubated for 1 h at 4°C, followed by centrifugation at 12,000 × g for 10 min. Precipitates were washed thoroughly and suspended three times in ice-cold RIPA buffer, centrifuged at 2500 × g for 10 s, and resuspended in Laemmli sample buffer. Proteins were denatured at 100°C for 5 min, resolved by 8% SDS-PAGE, and electrophoretically transblotted to Trans-Blot nitrocellulose membranes (0.2 μm; Bio-Rad Laboratories, Hercules, CA). The membranes were incubated with blocking solution (5% nonfat dried milk in 1× phosphate-buffered saline [PBS] + 0.2% Tween-20 [PBST]) for 1 h and then probed with various primary antibodies (1:500) overnight at 4°C. After three washes with PBST, membranes were incubated with HRP-conjugated secondary IgG (1:25,000) for 1 h, followed by another three washes with PBST. Immunoreactive bands were detected using the SuperSignal Chemiluminescent reagent (Pierce) and quantitatively analyzed by normalizing band intensities to the controls on scanned films by IMAGEJ software (National Institutes of Health, Bethesda, MD).
Adhesion Assays
Cell adhesion assays were performed as previously described using Sarstedt 96-well suspension cell culture plates (Newton, NC; Plopper et al., 1998). Tissue culture plates were coated with purified ECM proteins at a concentration of 20 μg/ml for 1 h at room temperature. Wells were washed twice with PBS and incubated with nd-blotto (5% nondairy creamer in PBS + 0.2% Tween 20) for 30 min before the assay. Cells were allowed to attach for 30 min at 37°C and were subsequently fixed with 3% paraformaldehyde, washed twice in PBS, and incubated in crystal violet dye for 15 min. Wells were washed thoroughly with water and the violet dye was extracted with 10% SDS solution. Absorbance was measured using a Tecan SPECTAFluor spectrophotometer (Hillsborough, NC) at 595 nm, and relative adhesion was compared with cells attached to nd-blotto.
Integrin-blocking adhesion assays were performed according to the procedure above, but the cells were incubated with a functional integrin-blocking antibody for 30 min at 37°C, with vortexing every 5 min before plating.
Migration Assays
Cell migration assays were performed using 8-μm MIC plates from Millipore (Danvers, MA). Filters were coated with purified ECM proteins (COLL-I, VN, Ln-5, or FN) at a concentration of 20 μg/ml or nd-blotto for 1 h at room temperature before assay. Basal chambers for the nd-blotto wells were filled with migration medium (DMEM + 1% sodium pyruvate + 1× GPS), whereas the basal chambers for the ECM containing wells were filled with control medium. Cell suspensions in migration medium were seeded at a density of 5 × 103 cells per well. Migrations were allowed to run for 18 h at 37°C. Filters were then incubated for 30 min with 5 μM calcein AM (Molecular Probes, Eugene, OR) and washed thoroughly with PBS. Residual cells were swabbed from the top of the wells to avoid false readings. To quantitate migration, plates were read at 485Ex/535Em with a Tecan SPECTAFluor spectrophotometer. Relative fluorescence values for each experimental condition were expressed relative to FN and nd-blotto controls.
RT-PCR
RNA was isolated from 10 × 108 hMSC cultured for 16 d in the presence or absence of PD98059 (50 μM) on tissue culture plastic (±OS media) or on Ln-5 in control media. Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA). RT-PCR was performed with the OneStep RT-PCR Kit (Qiagen) and a 96-well thermal cycler (MJ Research, Waltham, MA) using the primers listed in Table 1, which were designed by the Lasergene v5.0 program (DNASTAR, Madison, WI). One microgram of template RNA was used per reaction. The reverse transcription step ran for 30 min at 50°C, followed by PCR activation for 15 min at 95°C. Thirty amplification cycles were run, consisting of 1-min denaturation at 94°C, 1 min of annealing at 58°C, and 1 min of extension at 72°C. Final extension was allowed to run 10 min at 72°C. Reaction products were separated by gel electrophoresis using a 1% agarose gel. Bands were visualized by UV illumination of ethidium-bromide–stained gels and captured using a ChemiImager 4400 Gel imaging system (Alpha Innotech, San Leandro, CA). Band intensity was quantitatively analyzed by IMAGEJ software for each gene and was normalized to corresponding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) values.
Immunohistochemistry
hMSC were grown on glass coverslips for 16 d, fixed with acetone, and blocked with PBS/1% bovine serum albumin (BSA) for 30 min. Primary mouse antibodies for Ln-5, α3 integrin, and α6 integrin were diluted 1:200 in 1× PBS + 1% BSA and added to cells for 1 h. Double staining was done with a rabbit polyclonal for Ln-5 with either of the integrin antibodies. FITC and TRITC secondary antibodies were added for 1 h and cover slips were mounted using Prolong antifade medium (Molecular Probes). Cells were visualized with a Nikon TE2000-S inverted fluorescence/phase contrast microscope (Garden City, NY) equipped with a digital SPOT camera.
Calcium Assay
Cultured monolayers of hMSC cultured for 16 days were washed twice with PBS and extracted with 0.5 N HCl, and the resulting lysate was shaken for 5 h at 4°C, followed by centrifugation at 2000 × g for 10 min. The supernatant was utilized for calcium determination, according to the manufacturer's instructions contained in Sigma Kit 587. Total calcium was calculated from absorbance at 575 nm using standard solutions prepared in parallel as calibration points.
Statistical Analysis
All experiments were repeated a minimum of two times, and the representative data were presented as mean ± SE. Statistical analyses were preformed using Student's unpaired t test, and a p value < 0.05 was considered significant.
RESULTS
Laminin-5 Is Expressed in Bone and hMSC
We utilized enzyme-linked fluorescence (ELF)-based immunohistochemistry and RT-PCR analysis to detect Ln-5 in adult bone tissue and hMSC. Positive staining for Ln-5 α3 and γ2 chains were detected in rat rib periosteum (Figure 1, E and F, respectively), whereas no visible staining was observed in the negative controls (Figure 1, A–D). Similar staining was seen in all adult and embryonic bone tissues (unpublished data).
Figure 1.
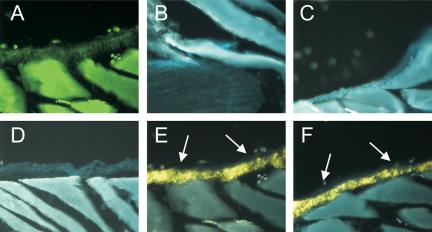
Immunolocalization of Ln-5 subunits to the periosteum of rat rib. Enzyme-linked fluorescence technology was used to detect immunostaining (arrows) of Ln α3 and γ2 chains with CM6 and TR1 antibodies in frozen sections of adult rat rib (E and F, respectively). Controls include autofluorescence (A), reduced autofluorescence following exposure to UV light (B, bone-cartilage junction; and D, rib synovium), background staining from uv-treated section stained with normal mouse IgG (C). All images taken at 10× magnification. Cortical bone shattered during frozen sectioning.
Because mesenchymal stem cells are found in the periosteum (Barry and Murphy, 2004), we hypothesized that they were the source of the Ln-5 in bone. After routine fixation and Triton X-100 permeabilization of cells plated for 16 d on glass coverslips, staining for Ln-5 appeared to be largely intracellular, in the endoplasmic reticulum and Golgi complex (Figure 2B). Intense staining was also visible for the α3 integrin as punctate dots after 16 d (Figure 2A), confirming that hMSC express this integrin. Both the α3 integrin and Ln-5 were detected in double-labeled cells (Figure 2, C and D). Ln-5 was also colocalized with the α6 integrin but with weaker intensity for the α6 integrin (unpublished data). Extraction of hMSC with 20 mM HN4OH, which solubilizes virtually all cellular proteins but leaves behind the insoluble ECM (Gospodarowicz, 1984), removed all evidence of Ln-5 staining (unpublished data). No staining was observed in the negative control (unpublished data). RT-PCR confirmed the presence of the three Ln-5 chains in hMSC cultured for 16 d on tissue culture plastic (Figure 2E). Ln-1 chains, α1 and β1, were also detected as a positive control. Adhesion and migration of hMSC was greater on Ln-5 than with other ECM proteins tested, including the G4 domain of Ln-1 in adhesion assays (Figure 3). In 30-min adhesion assays, integrin chain-specific blocking antibodies against α3 and β1 integrin subunits reduced adhesion to Ln-5 by ∼50 and 60%, respectively, whereas antibodies against α6 and β4 reduced adhesion by 20–25% (Figure 4). Control antibodies targeting integrin subunits that do not bind Ln-5 (α1, α2, αv) did not reduce adhesion.
Figure 2.
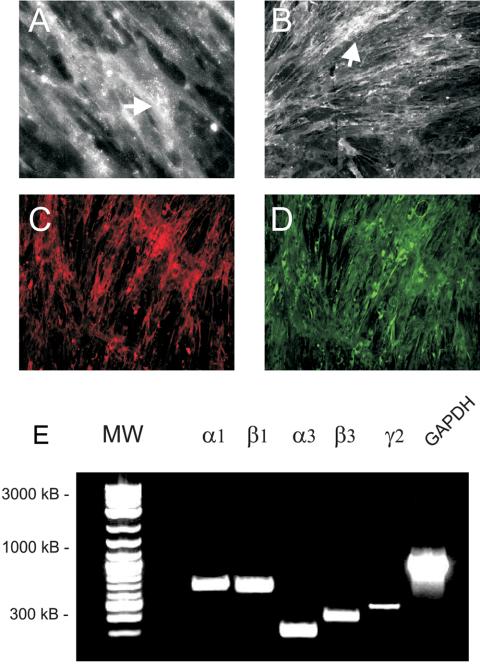
hMSC express Ln-5 in culture. (A–D) Cells were grown on glass coverslips for 8 d and then were fixed and stained for α3 integrin (A and C) and human Ln-5 (B and D). (A) Magnification, 40×. (B–D) Magnification, 10×. Images in C and D were taken of the same microscopic field. (E) RT-PCR was used to amplify the indicated mRNA transcripts from hMSC grown on tissue culture plastic. The α1 and β1 chains, found in Ln-1, were included as a positive control. GAPDH was amplified as a positive control.
Figure 3.
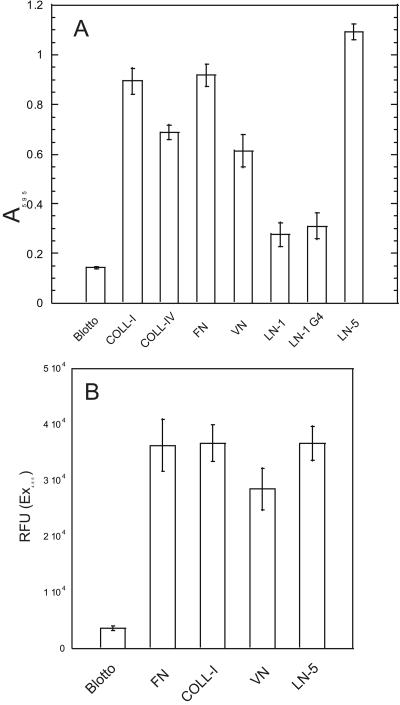
hMSC bind to and migrate on Ln-5. (A) Static 30-min assay of hMSC adhesion to purified ECM proteins. Adherent cells were stained with crystal violet and then solubilized in SDS, and absorbance was determined at 595 nm. Values represent mean ± SD (n = 5). (B) Cells were plated in the upper chamber of a MIC filter migration plate (Millipore) and allowed to migrate toward the lower chamber through a 8-μm filter coated with Ln-5. Controls had filters coated with COLL-I, VN, FN, or nd-blotto. Each condition was repeated in 12 wells.
Figure 4.
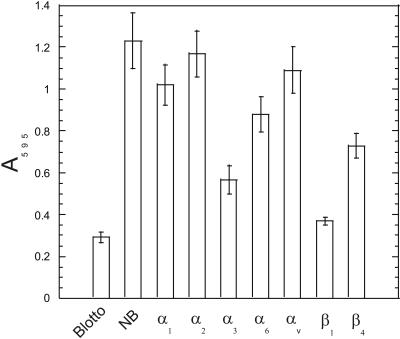
hMSC use α3β1 integrin to bind Ln-5. Adhesion assays as per Figure 3A were performed in the presence of integrin blocking antibodies.
ERK as a Mediator of Osteogenic Differentiation in hMSC Plated on Ln-5
ERK is a common component of many integrin signaling pathways. To test our hypothesis that adhesion to Ln-5 induces osteogenic differentiation of hMSC via ERK signaling, we plated hMSC on Ln-5 and observed the levels of phosphorylated ERK (pERK). Cells plated on Ln-5 for 30 min contained four and six times more phosphorylated ERK 1 and ERK 2, respectively, than cells plated on poly-l-lysine–coated control surfaces (Figure 5). Cells plated on tissue culture plastic and treated with osteogenic supplement (OS) media also had more phosphorylated ERK 1 and ERK 2 than that of controls. Cells kept in suspension exhibited no detectable pERK1 and only a trace amount of pERK2. Addition of the MAP Kinase Kinase 1 (MEK1) inhibitor PD98059, which blocks ERK activation, eliminated pERK1 and drastically reduced the amount of pERK2 detected in both the Ln-5– and OS-treated cultures.
Figure 5.
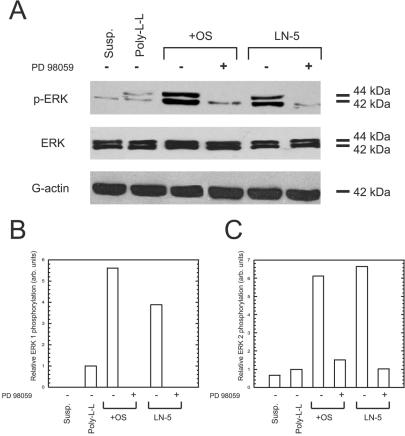
Adhesion to Ln-5 activates ERK in hMSC. (A) Cells kept in suspension (susp.), or plated for 30 min on poly-l-lysine (Poly-L-L), tissue culture plastic with OS media (+OS), or on Ln-5 were probed by immunoblot for phosphorylated ERK (top row), total ERK (middle row), or actin as a loading control (bottom row). Where indicated by a “+” symbol, 50 μM PD98059 was added to the culture conditions. (B and C) Densitometric measurements of band intensities for phospho-ERK 1 and phospho-ERK 2, respectively.
Activation of the transcription factor Runx2 was detected by immunoprecipitation and immunoblotting for phosphoserine residues. Cells grown on Ln-5 for 8 d exhibited a threefold increase in phospho-runx2/CBFA-1 (pRunx2) compared with control cells plated on tissue culture plastic. Cells treated with OS media exhibited a 2.5-fold increase in pRunx2 over the same time course (Figure 6). Addition of PD98059 reduced the amount of detectable pRunx2 by ∼50% in OS and Ln-5 conditions, even as early as 1 d after plating.
Figure 6.
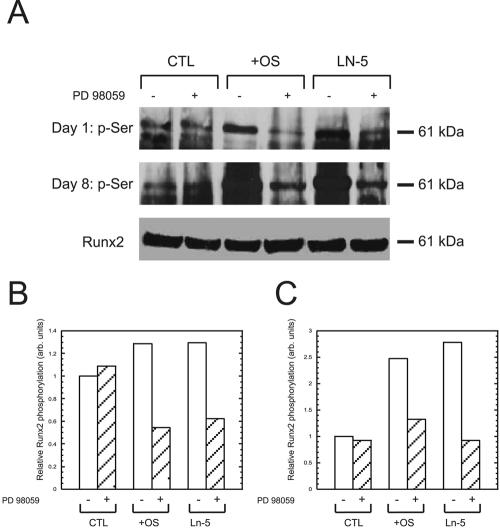
Adhesion to Ln-5 stimulates runx2/CBFA-1 phosphorylation. Cells were plated for 1 d (top row) or 8 d (middle and bottom rows) on tissue culture plastic in the absence (CTL) or presence of OS media (+OS), or on Ln-5, and then lysed, and runx2/CBFA-1 was immunoprecipitated. The immunoprecipitated proteins were separated by SDS-PAGE and probed by immunoblot for phosphoserine (top two rows) or total runx2/CBFA-1 as a loading control (bottom row). Where indicated by a “+” symbol, 50 μM PD98059 was added to the culture conditions. (B and C) Densitometric measurements of bands for pRunx2 on days 1 and 8, respectively.
To measure the effects of Ln-5 plating on hMSC osteogenic differentiation, we performed RT-PCR for three osteogenic marker genes (osteopontin, osteocalcin, and alkaline phosphatase [ALP]), which are downstream targets of Runx2, as well for Runx2 itself (Figure 7). We found that adhesion to Ln-5 for 16 d activated expression of all three marker genes, greater than that found in hMSC grown in OS media over the same time course. Cells plated on poly-l-lysine control expressed only trace amounts of each marker gene. Addition of PD98059 to the experimental conditions reduced the expression of all three marker genes to levels below or equal to that of the control. Runx2 levels were comparable across all controls and conditions, regardless of the presence of the ERK inhibitor.
Figure 7.
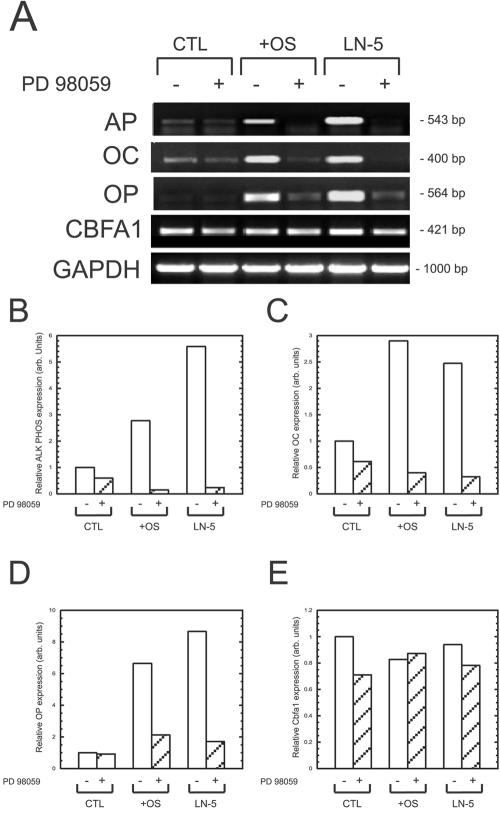
Ln-5 induces expression of osteopontin and osteocalcin in hMSC. (A) RT-PCR was performed on hMSC grown for 16 d on tissue culture plastic in the absence (CTL) or presence of OS media (+OS), or on Ln-5. Where indicated by a “+” symbol, 50 μM PD98059 was added to the culture conditions. Indicated genes were amplified using primers listed in Table 1. GAPDH was amplified as a loading control. (B–E) Densitometric analysis of bands in A for (B) ALP, (C) osteocalcin, (D) osteopontin, and (E) runx2/CBFA-1.
To confirm the RT-PCR data, Western blots were performed for osteopontin and osteocalcin in cultured hMSC (Figure 8). Control cultures expressed no detectable levels of these proteins in the presence or absence of PD98059. However, both proteins were detected in OS media and Ln-5 samples, and the expression of these proteins was greatly diminished when PD98059 was added to the cultures.
Figure 8.
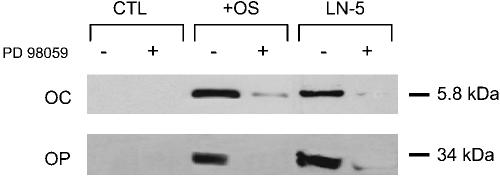
hMSC plated on Ln-5 express osteocalcin and osteopontin. Cells were plated for 16 d under the same conditions as in Figure 7 and probed by immunoblot for osteocalcin (OC) and osteopontin (OP).
The presence of hydroxyapatite in the cell matrix is a reliable sign of osteogenic differentiation. Hydroxyapatite formation was measured using FTIR analysis of insoluble matrix washed with ammonium bicarbonate. The results, expressed as the mineral:matrix ratio of secreted ECM, were 2.0 and 2.8 for cultures grown for 21 d on poly-l-lysine and Ln-5, respectively (Figure 9). The very broad band in the nu4 region (500–650 cm-1) is indicative of very poorly crystalline apatite or of apatite of very small crystal size. Fully differentiated adult osteoblasts produce a matrix that yields a ratio of 5.4 over the same time course (Salasznyk et al., 2004). Thus, cells grown on Ln-5 contain more hydroxyapatite than cells grown on tissue culture plastic, even after only 21 d.
Figure 9.
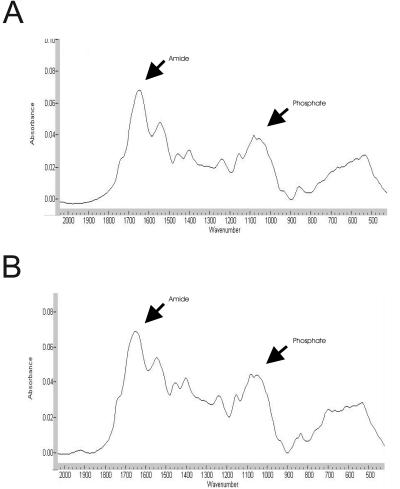
Ln-5 induces matrix mineralization. FTIR analysis of hMSC plated on tissue culture plastic (A) or Ln-5 (B) for 21 d. The mineral:matrix is computed by comparing the area of the phosphate peak (mineral) to the amide peak (protein) shown.
Fibronectin is found in the periosteum (Nilsson et al., 1998) and supports hMSC adhesion (Figure 3A), but is thought to play a role in adipogenic differentiation rather than osteogenic differentiation of stromal cells (Urs et al., 2004). Adhesion to fibronectin for 30 or 60 min induced phosphorylation of ERK 2 (42 kDa), but not ERK 1 (44 kDa; Figure 10A). Similarly, plating cells on fibronectin failed to induce calcium deposition in hMSC cultured for 16 d (Figure 10B). Thus, fibronectin is not an osteogenic stimulus of hMSC under our culture conditions.
Figure 10.
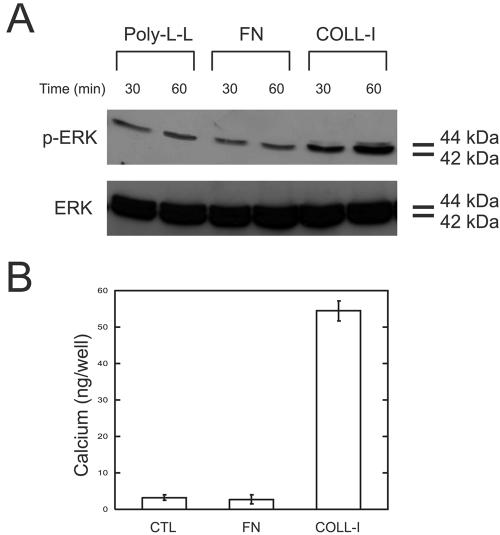
Fibronectin is not an osteogenic stimulus for hMSC. (A) hMSC were plated on poly-l-lysine, fibronectin, and collagen-I for 30 and 60 min and assayed for ERK activation as in Figure 5. (B) Calcium deposition of hMSC plated on poly-l-lysine, fibronectin, and collagen-I for 16 d. Calcium was solublized in HCl, and absorbance was determined at 575 nm. Values represent mean ± SD (n = 4).
DISCUSSION
Ln-5 is typically found in tissues derived from ectoderm and endoderm, such as skin, gut, lung, and other epithelia (Yamamoto et al., 2001). Our data presented here, combined with our recent discovery of Ln-5 in vascular smooth muscle cells (Kingsley et al., 2001, 2002a, 2002b), suggests that Ln-5 is also expressed in mesoderm-derived tissues as well. Why has Ln-5 gone undetected in these tissues previously? We believe we were able to uncover its presence in rat periosteum because of our high level of detection sensitivity. ELF (enzyme-labeled fluorescence) is a powerful tool in immunohistochemistry because it provides significant signal amplification over conventional indirect immunofluorescence techniques (Paragas et al., 2002). In addition, we developed a method for significantly reducing autofluorescence in mesodermal tissues (Kingsley et al., 2001). This has allowed us to generate a very high signal-to-noise ratio, allowing the detection of both the α and γ chains of Ln-5, even in tissues where it is expressed in relatively low levels.
Our immunolocalization data raise an important issue regarding ECM synthesis in hMSC. That Ln-5 is expressed in low abundance in situ (Figure 1) and is not incorporated into an insoluble matrix in hMSC cultured on 2D surfaces (Figure 2) suggests that hMSC may not assemble their own ECM. This is somewhat surprising for most cell types, but virtually all studies involving ECM effects on 2D cultures of hMSC utilize exogenous ECM proteins rather than hMSC matrix. A recent study demonstrated that these cells synthesize ECM proteins when grown in 2D, but that the proteins are localized to the cytoplasm; only when the cells are cultured in a 3D environment are the proteins deposited as a matrix (Grayson et al., 2004).
Our findings are also consistent with what is known about Ln-5 assembly. Ln-5 assembly into epithelial basement membranes requires direct cell contact (Plopper et al., 1996b) and requires the formation of disulfide bonds (Falk-Marzillier et al., 1998). It is quite possible that hMSC lack the machinery to perform this assembly.
2D tandem mass spectrometry (120 μg total protein, detection threshold ≥200 pmol) of whole-cell hMSC lysate grown in 2D fails to identify any ECM proteins (Salasznyk, Westcott, Klees, Ward, Xiang, Vandenberg, Bennet, Plopper, unpublished results). Likewise, mass spectrometry of the NH4OH-insoluble protein from 60 × 106 cultured hMSC fails to detect any ECM proteins (Klees, unpublished data). This leads us to conclude that, although it is clear that hMSC synthesize the mRNA for the Ln-5 constituent chains and produce immunologically detectable levels of the γ2 chain in Ln-5, hMSC are not likely responsible for assembling this Ln-5 into bone matrix. Instead, this function may be provided during development by chondrocytes and in adults by committed osteoblasts. Osteoblasts colocalize with hMSC in the periosteum and bone marrow and are well known to synthesize and assemble both collagenous and noncollagenous elements of bone matrix (Marks and Odgren, 2002).
Mesenchymal stem cells have been found in many locations, including the bone marrow, trabecular bone, adipose tissue, skeletal muscle, and periosteum (Barry and Murphy, 2004). Our detection of Ln-5 in the periosteum suggests the possibility that hMSC may be the source of this protein. Ln-5 has been shown to support the adhesion of primary osteoprogenitor cells (Roche et al., 1999). Our RT-PCR and immunohistochemistry data demonstrate that Ln-5 is expressed by hMSC in culture, supporting our supposition. The RT-PCR data also confirm that hMSC express the α1 and β1 chains of Ln-1, which are also found in the periosteum (Nilsson et al., 1998) but which do not support adhesion or osteogenic differentiation of hMSC (Salasznyk et al., 2004). Expression of the α3 laminin chain also indicates that Laminin-6 and Laminin-7 may be present in hMSC, because they share this α chain with Ln-5 (Champliaud et al., 1996). To date, these isoforms have not been reported in bone.
In 30-min adhesion assays, hMSC interact with Ln-5 through known Ln-5 receptors, primarily α3β1. All four Ln-5 integrin receptors (α2β1, α3β1, α6β1, α6β4) are expressed by hMSC (Pittenger et al., 1999) and may be utilized at different times during hMSC maturation. For example, Nguyen et al. (2000) have suggested that in human foreskin keratinocytes, these receptors function cooperatively to mediate cellular response to Ln-5 binding. Specifically, they suggest that initial adhesion to Ln-5 is mediated by α3β1, and later, more stable contact is established through α6β4 integrins, which form hemidesmosomes. During wound repair, this process is reversed: cells switch from a RhoGTPase-dependent adhesion via α6β4 integrin to a PI3K-dependent adhesion and spreading via α3β1 integrin (Nguyen et al., 2000). Our data suggest that stem cells establish early contact with Ln-5 through α3β1; it is not yet known what role the other integrins may play in hMSC contact with Ln-5.
In addition to engaging at least three different integrin receptors, Ln-5 is also known to promote several integrin-associated signaling pathways. Our data demonstrate that adhesion to Ln-5 stimulates ERK 1 and ERK 2, primarily through α3β1 integrin. α3β1 is thought to be the primary receptor for initial adhesion of most epithelial cells to Ln-5, and this binding affects the ability of other receptors to bind Ln-5 at later times (Belkin and Stepp, 2000). Members of the transmembrane 4 superfamily of proteins (tetraspanins) are known to associate tightly with α3β1 integrin and form a link between the receptor and protein kinase C (PKC; Zhang et al., 2001). PKC activity is dependent on the intracellular calcium concentration in developing osteoblasts (Matsumoto, 1995). Tetraspanins can also modulate other integrin signaling and may function in reorganization of the actin cytoskeleton (Berditchevski and Odintsova, 1999). We have noted a significant rearrangement of F-actin in differentiating hMSC (Salasznyk et al., 2004). Integrins also stimulate ERK activity (Carloni et al., 2004), which we hypothesize is crucial for osteogenic differentiation. In breast cells, Ln-5/α6β4 integrin complexes are believed to be conduits for signals from the extracellular environment to intracellular signaling pathways including MAP kinase (Giancotti, 1996; Gonzales et al., 1999). Collectively, these data suggest that hMSC use pathways similar to those found in other cells to transduce Ln-5 binding into intracellular signaling activity.
How, then, does hMSC adhesion to Ln-5 result in osteogenic gene expression? Osteoblast differentiation from bone marrow progenitor cells has been described as a series of up to seven steps, each defined by a change in gene expression (Aubin, 1998). More recent studies suggest that these steps are a continuum, rather than distinct events (Ryoo et al., 1997; Hou et al., 1999; Smith et al., 2000). This in turn explains why gene expression patterns are somewhat heterogeneous, making true osteoblast differentiation patterns difficult to define. The most critical of these events is the activation/phosphorylation of the master bone gene runx2 (Lian et al., 2004). Runx2 is responsible for expression of osteogenic marker genes, including osteopontin, osteocalcin, and ALP. Runx2/CBFA-1 is a substrate of ERK, which can be activated by soluble osteogenic supplements (Bruder et al., 1997; Jaiswal et al., 2000).
Though it is expressed in relatively low abundance, Ln-5 influences responsiveness to other extracellular stimuli in vascular smooth muscle (Kingsley et al., 2002a, 2002b). Osteoprogenitor cells adhere better to laminins than to other ECM proteins (Roche et al., 1999). What is clear is that Ln-5 plays more than a simple adhesive role during hMSC differentiation: FN supports adhesion and migration of these cells but does not induce expression of an osteoblastic phenotype (Salasznyk et al., 2004). Although adhesion to Ln-5 does induce ERK activation, which is common with adhesion to many ECM proteins, this binding also induces expression of osteogenic genes, which is observed upon binding to only a subset of bone ECM proteins (COLL-I, VN). Our finding that fibronectin fails to activate ERK 1 and does not induce calcium deposition suggests that ERK 1–specific signals may be necessary for osteogenic differentiation. That this differentiation occurs in the absence of any other extracellular stimulus strongly suggests that, although ERK 1 activation is involved, additional signaling pathways likely play a role in controlling osteogenic gene expression. In fact, ERK is known to control activation of SMADs and the AP-1 transcription complex, both of which play a role in regulating osteogenic gene expression (Franceschi, 1999; Franceschi and Xiao, 2003).
Initiation of these signaling changes must begin with an external stimulus. In addition to stimulating integrin receptors, Ln-5 may act as a repository for soluble osteogenic factors. It is well known that the bone ECM is a repository for bone morphogenic proteins (BMPs), which induce differentiation of osteoprogenitor cells through regulation of osteoblast-specific transcription factors. BMPs bind to chordin like cysteine-rich repeats found in several ECM proteins (O'leary et al., 2004). Both the γ2 and α3 chains of Ln-5 contain potential cysteine-rich regions that may bind BMPs. Other growth factors may interact with Ln-5 and/or its associated proteins within the bone matrix.
To our knowledge, this report is the first demonstration that Ln-5 controls differentiation of a mesodermally derived tissue. Our data suggest a model whereby adhesion to Ln-5 via α3β1 integrin stimulates ERK activation. This in turn leads to phosphorylation of runx2/CBFA-1 and subsequent expression of osteopontin, osteocalcin, and ALP. The fact that Runx2/CBFA-1 activation and osteogenic gene expression are sensitive to PD98059 supports our model that adhesion to Ln-5 alone, in the absence of any exogenous soluble stimulants, is sufficient to induce osteogenic differentiation and that this is mediated by ERK 1. We have observed significant increases in matrix mineralization in cells plated on Ln-5 for only 21 d, though achieving mineralization comparable to that found with fully mature osteoblasts will likely take much longer. By necessity, we have focused on the earlier, inductive stages of osteogenic differentiation in this study.
No in vitro culture conditions can recreate the complex microenvironment and intracellular communication necessary for successful osteogenesis (Fox et al., 2000). Successful bone formation is a highly complicated process that clearly requires careful integration of signals from multiple stimuli, including many ECM proteins. Our data demonstrate that Ln-5 is capable of inducing osteogenic gene expression and matrix mineralization in vitro, but do not suggest that Ln-5 alone is the osteogenic stimulus for hMSC, nor that Ln-5 is necessary for bone formation. Ln-5 and the other noncollagenous molecules found in bone collectively make up no more than 5% of bone protein, and it is quite likely that many of these 5% are functionally redundant with regard to assisting in bone formation and maintenance.
Acknowledgments
This work was supported by Public Health Service Grant 5R01EB002197-02 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB; to G.E.P.) and by SEED funds from Rensselaer Polytechnic Institute.
Article published online ahead of print in MBC in Press on December 1, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0695).
Abbreviations used: BMP, bone morphogenic protein; COLL-I, collagen-I; ELF, enzyme-linked fluorescence; ERK, extracellular-related kinase; FN, fibronectin; FTIR, Fourier transform infrared; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hMSC, human mesenchymal stem cells; Ln, Laminin; MEK1, MAPK kinase; nd-blotto, 5% non-dairy creamer in PBS + 0.2% Tween 20; OS, osteogenic supplement; pERK, phosphorylated ERK; pRunx2, phospho-runx2/CBFA-1; RIPA, radioimmunoprecipitation assay; VN, vitronectin.
References
- Aberdam, D., Aguzzi, A., Baudoin, C., Galliano, M. F., Ortonne, J. P., and Meneguzzi, G. (1994). Developmental expression of nicein adhesion protein (laminin-5) subunits suggests multiple morphogenic roles. Cell Adhes. Commun. 2, 115-129. [DOI] [PubMed] [Google Scholar]
- Aubin, J. E. (1998). Advances in the osteoblast lineage. Biochem. Cell Biol. 76, 899-910. [PubMed] [Google Scholar]
- Barry, F. P., and Murphy, J. M. (2004). Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36, 568-584. [DOI] [PubMed] [Google Scholar]
- Belkin, A. M., and Stepp, M. A. (2000). Integrins as receptors for laminins. Microsc. Res. Technol. 51, 280-301. [DOI] [PubMed] [Google Scholar]
- Berditchevski, F., and Odintsova, E. (1999). Characterization of integrintetraspanin adhesion complexes: role of tetraspanins in integrin signaling. J. Cell Biol. 146, 477-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder, S. P., Jaiswal, N., and Haynesworth, S. E. (1997). Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J. Cell Biochem. 64, 278-294. [DOI] [PubMed] [Google Scholar]
- Carloni, V., Mazzocca, A., and Ravichandran, K. S. (2004). Tetraspanin CD81 is linked to ERK/MAPKinase signaling by Shc in liver tumor cells. Oncogene 23, 1566-1574. [DOI] [PubMed] [Google Scholar]
- Champliaud, M. F., Lunstrum, G. P., Rousselle, P., Nishiyama, T., Keene, D. R., and Burgeson, R. E. (1996). Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J. Cell Biol. 132, 1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., and Yurchenco, P. D. (2000). Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213-234. [DOI] [PubMed] [Google Scholar]
- Decline, F., and Rousselle, P. (2001). Keratinocyte migration requires alpha2beta1 integrin-mediated interaction with the laminin 5 gamma2 chain. J. Cell Sci. 114, 811-823. [DOI] [PubMed] [Google Scholar]
- Falk-Marzillier, J., Domanico, S. Z., Pelletier, A., Mullen, L., and Quaranta, V. (1998). Characterization of a tight molecular complex between integrin alpha 6 beta 4 and laminin-5 extracellular matrix. Biochem. Biophys. Res. Commun. 251, 49-55. [DOI] [PubMed] [Google Scholar]
- Fox, S. W., Fuller, K., and Chambers, T. J. (2000). Activation of osteoclasts by interleukin-1, divergent responsiveness in osteoclasts formed in vivo and in vitro. J. Cell Physiol. 184, 334-340. [DOI] [PubMed] [Google Scholar]
- Franceschi, R. T. (1999). The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit. Rev. Oral Biol. Med. 10, 40-57. [DOI] [PubMed] [Google Scholar]
- Franceschi, R. T., and Xiao, G. (2003). Regulation of the osteoblast-specific transcription factor, Runx2, responsiveness to multiple signal transduction pathways. J. Cell Biochem. 88, 446-454. [DOI] [PubMed] [Google Scholar]
- Giancotti, F. G. (1996). Signal transduction by the alpha 6 beta 4 integrin: charting the path between laminin binding and nuclear events. J. Cell Sci. 109(Pt 6), 1165-1172. [DOI] [PubMed] [Google Scholar]
- Gonzales, M., Haan, K., Baker, S. E., Fitchmun, M., Todorov, I., Weitzman, S., Jones, J. C. (1999). A cell signal pathway involving laminin-5, alpha3beta1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol. Biol. Cell 10, 259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz, D. (1984). Preparation of extracellular matrices produced by cultured bovine corneal endothelial cells and PF-HR-9 endodermal cells: their use in cell culture. In: Methods for Preparation of Media, Supplements, and Substrata, ed. D. W. Barnes, D. A. Sirbasku, G. H. Stau, New York: Alan R. Liss, 275-293.
- Grayson, W. L., Ma, T., and Bunnell, B. (2004). Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnol. Prog. 20, 905-912. [DOI] [PubMed] [Google Scholar]
- Hou, Z., Nguyen, Q., Frenkel, B., Nilsson, S. K., Milne, M., Van Wijnen, A. J., Stein, J. L., Quesenberry, P., Lian, J. B., Stein, G. S. (1999). Osteoblast-specific gene expression after transplantation of marrow cells: implications for skeletal gene therapy. Proc. Natl. Acad. Sci. USA 96, 7294-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal, R. K., Jaiswal, N., Bruder, S. P., Mbalaviele, G., Marshak, D. R., Pittenger, M. F. (2000). Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 275, 9645-9652. [DOI] [PubMed] [Google Scholar]
- Kato, Y., Boskey, A., Spevak, L., Dallas, M., Hori, M., and Bonewald, L. F. (2001). Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. J. Bone Miner. Res. 16, 1622-1633. [DOI] [PubMed] [Google Scholar]
- Kingsley, K., Carroll, K., Huff, J. L., and Plopper, G. E. (2001). Photobleaching of arterial autofluorescence for immunofluorescence applications. Biotechniques 30, 794-797. [DOI] [PubMed] [Google Scholar]
- Kingsley, K., Huff, J. L., Rust, W. L., Carroll, K., Martinez, A. M., Fitchmun, M., Plopper, G. E. (2002a). ERK1/2 mediates platelet-derived growth factor (PDGF)-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem. Biophys. Res. Commun. 293, 1000-1006. [DOI] [PubMed] [Google Scholar]
- Kingsley, K., Rust, W. L., Huff, J. L., Smith, R. C., and Plopper, G. E. (2002b). PDGF-BB enhances expression of, and reduces adhesion to, laminin-5 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 294, 1017-1022. [DOI] [PubMed] [Google Scholar]
- Lian, J. B., Javed, A., Zaidi, S. K., Lengner, C., Montecino, M., Van Wijnen, A. J., Stein, J. L., and Stein, G. S. (2004). Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev. Eukaryot. Gene Expr. 14, 1-41. [PubMed] [Google Scholar]
- Malinda, K. M., and Kleinman, H. K. (1996). The laminins. Int. J. Biochem. Cell Biol. 28, 957-959. [DOI] [PubMed] [Google Scholar]
- Marks, S. C., and Odgren, P. R. (2002). Structure and development of the skeleton. In: Principles of Bone Biology, ed. J. P. Bilezikian, L. G. Raisz, G. A. Rodan, San Diego: Academic Press, 3-15.
- Matsumoto, A. (1995). The effect of cell environment on osteoblastic function. Nippon Yakurigaku Zasshi 105, 273-283. [DOI] [PubMed] [Google Scholar]
- Nguyen, B. P., Gil, S. G., and Carter, W. G. (2000). Deposition of laminin 5 by keratinocytes regulates integrin adhesion and signaling. J. Biol. Chem. 275, 31896-31907. [DOI] [PubMed] [Google Scholar]
- Nilsson, S. K., Debatis, M. E., Dooner, M. S., Madri, J. A., Quesenberry, P. J., and Becker, P. S. (1998). Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J. Histochem. Cytochem. 46, 371-377. [DOI] [PubMed] [Google Scholar]
- O'leary, J. M., Hamilton, J. M., Deane, C. M., Valeyev, N. V., Sandell, L. J., Downing, A. K. (2004). Solution structure and dynamics of a prototypical chordin-like cysteine-rich repeat (VWC module) from collagen IIA. J. Biol. Chem. (in press). [DOI] [PubMed]
- Orian-Rousseau, V., Aberdam, D., Fontao, L., Chevalier, L., Meneguzzi, G., Kedinger, M., Simon-Assmann, P. (1996). Developmental expression of laminin-5 and HD1 in the intestine: epithelial to mesenchymal shift for the laminin gamma-2 chain subunit deposition. Dev. Dyn. 206, 12-23. [DOI] [PubMed] [Google Scholar]
- Paragas, V. B., Kramer, J. A., Fox, C., Haugland, R. P., and Singer, V. L. (2002). The ELF-97 phosphatase substrate provides a sensitive, photostable method for labelling cytological targets. J. Microsc. 206, 106-119. [DOI] [PubMed] [Google Scholar]
- Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., Moorman, M. A., Simonetti, D. W., Craig, S., and Marshak, D. R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284, 143-147. [DOI] [PubMed] [Google Scholar]
- Pittenger, M. F., and Martin, B. J. (2004). Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 95, 9-20. [DOI] [PubMed] [Google Scholar]
- Plopper, G., Falk-Marzillier, J., Glaser, S., Fitchmun, M., Giannelli, G., Romano, T., Jones, J. C., and Quaranta, V. (1996). Changes in expression of mAb epitopes on laminin-5r induced by cell contact. J. Cell Sci. 109(Pt 7), 1965-1973. [DOI] [PubMed] [Google Scholar]
- Plopper, G. E., Domanico, S. Z., Cirulli, V., Kiosses, W. B., and Quaranta, V. (1998). Migration of breast epithelial cells on Laminin-5, differential role of integrins in normal and transformed cell types. Breast Cancer Res. Treat. 51, 57-69. [DOI] [PubMed] [Google Scholar]
- Roche, P., Rousselle, P., Lissitzky, J. C., Delmas, P. D., and Malaval, L. (1999). Isoform-specific attachment of osteoprogenitors to laminins: mapping to the short arms of laminin-1. Exp. Cell Res. 250, 465-474. [DOI] [PubMed] [Google Scholar]
- Ryoo, H. M., Hoffmann, H. M., Beumer, T., Frenkel, B., Towler, D. A., Stein, G. S., Stein, J. L., Van Wijnen, A. J., and Lian, J. B. (1997). Stage-specific expression of Dlx-5 during osteoblast differentiation: involvement in regulation of osteocalcin gene expression. Mol. Endocrinol. 11, 1681-1694. [DOI] [PubMed] [Google Scholar]
- Salasznyk, R. M., Williams, W. A., Boskey, A., Batorsky, A., and Plopper, G. E. (2004). Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Biotechnol. 2004, 24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. A. (2001). Integrin signaling revisited. Trends Cell Biol. 11, 466-470. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A., and Ginsberg, M. H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65-E68. [DOI] [PubMed] [Google Scholar]
- Siler, U., Rousselle, P., Muller, C. A., and Klein, G. (2002). Laminin gamma2 chain as a stromal cell marker of the human bone marrow microenvironment. Br. J. Haematol. 119, 212-220. [DOI] [PubMed] [Google Scholar]
- Smith, E., Redman, R. A., Logg, C. R., Coetzee, G. A., Kasahara, N., and Frenkel, B. (2000). Glucocorticoids inhibit developmental stage-specific osteoblast cell cycle. Dissociation of cyclin A-cyclin-dependent kinase 2 from E2F4–p130 complexes. J. Biol. Chem. 275, 19992-20001. [DOI] [PubMed] [Google Scholar]
- Stahl, S., Weitzman, S., and Jones, J. C. (1997). The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J. Cell Sci. 110(Pt 1), 55-63. [DOI] [PubMed] [Google Scholar]
- Thorup, A. K., Dabelsteen, E., Schou, S., Gil, S. G., Carter, W. G., and Reibel, J. (1997). Differential expression of integrins and laminin-5 in normal oral epithelia. APMIS 105, 519-530. [DOI] [PubMed] [Google Scholar]
- Timpl, R., and Brown, J. C. (1996). Supramolecular assembly of basement membranes. Bioessays 18, 123-132. [DOI] [PubMed] [Google Scholar]
- Urs, S., Smith, C., Campbell, B., Saxton, A. M., Taylor, J., Zhang, B., Snoddy, J., Jones, V. B., and Moustaid-Moussa, N. (2004). Gene expression profiling in human preadipocytes and adipocytes by microarray analysis. J. Nutr. 134, 762-770. [DOI] [PubMed] [Google Scholar]
- Virtanen, I., Laitinen, A., Tani, T., Paakko, P., Laitinen, L. A., Burgeson, R. E., and Lehto, V. P. (1996). Differential expression of laminins and their integrin receptors in developing and adult human lung. Am. J. Respir. Cell Mol. Biol. 15, 184-196. [DOI] [PubMed] [Google Scholar]
- Virtanen, I., Tani, T., Back, N., Happola, O., Laitinen, L., Kiviluoto, T., Salo, J., Burgeson, R. E., Lehto, V. P., and Kivilaakso, E. (1995). Differential expression of laminin chains and their integrin receptors in human gastric mucosa. Am. J. Pathol. 147, 1123-1132. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, H., Itoh, F., Iku, S., Hosokawa, M., and Imai, K. (2001). Expression of the gamma(2) chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin. Cancer Res. 7, 896-900. [PubMed] [Google Scholar]
- Zhang, X. A., Bontrager, A. L., and Hemler, M. E. (2001). Transmembrane-4 superfamily proteins associate with activated PKC and link PKC to specific beta(1) integrins. J. Biol. Chem. 276, 25005-25013. [DOI] [PubMed] [Google Scholar]


