Abstract
EB1 is a conserved microtubule plus end tracking protein considered to play crucial roles in microtubule organization and the interaction of microtubules with the cell cortex. Despite intense studies carried out in yeast and cultured cells, the role of EB1 in multicellular systems remains to be elucidated. Here, we describe the first genetic study of EB1 in developing animals. We show that one of the multiple Drosophila EB1 homologues, DmEB1, is ubiquitously expressed and has essential functions during development. Hypomorphic DmEB1 mutants show neuromuscular defects, including flightlessness and uncoordinated movement, without any general cell division defects. These defects can be partly explained by the malfunction of the chordotonal mechanosensory organs. In fact, electrophysiological measurements indicated that the auditory chordotonal organs show a reduced response to sound stimuli. The internal organization of the chordotonal organs also is affected in the mutant. Consistently, DmEB1 is enriched in those regions important for the structure and function of the organs. Therefore, DmEB1 plays a crucial role in the functional and structural integrity of the chordotonal mechanosensory organs in Drosophila.
INTRODUCTION
The microtubule network is one of the major cytoskeletal systems in eukaryotes. Dynamic reorganization of the microtubule network is necessary for many diverse cellular functions such as organelle and protein transport, cell architecture, cell polarity, and division. Microtubules alternate between phases of rapid growth and disassembly. The microtubule dynamics are spatially and temporally regulated within cells, both during the cell cycle and in development. A central question is how microtubule dynamics and organization are controlled and linked to other cellular processes.
A number of microtubule-associated proteins (MAPs) and motors have been shown to modify the behavior of microtubules (Cassimeris and Spittle, 2001). An interesting class of MAPs are the plus end tracking proteins that preferentially bind to plus ends of growing microtubules (for review, see Schuyler and Pellman, 2001). Microtubule plus ends interact with kinetochores or particular regions of the cell cortex. A “search and capture” mechanism is proposed to achieve such interactions, which involves selective stabilization of the microtubules contacting kinetochores or specific molecules at the cell cortex (Kirschner and Mitchison, 1986). Motor-guided mechanisms also may be involved (Yin et al., 2000; Hwang et al., 2003). Molecular mechanisms of interactions between dynamic microtubule ends and kinetochores/the cell cortex are still mysterious, but plus end tracking proteins are considered to play a central role in this process.
One of the most studied family of plus end tracking proteins is the EB1 family. EB1 was first identified as an interacting protein of the adenomatous polyposis coli tumor suppressor protein (Su et al., 1995). EB1 homologues are present in all eukaryotes and preferentially localize to the plus ends of growing microtubules (Tirnauer et al., 1999; Mimori-Kiyosue et al., 2000; Rehberg and Gräf, 2002; Rogers et al., 2002). In mammalian cells, EB1 has been reported to interact with components of the dynein/dynactin complex (Berrueta et al., 1999). RNA interference of a Drosophila homologue, DmEB1, has shown that it affects the dynamics of interphase microtubules, as well as the organization and positioning/orientation of the mitotic spindle (Lu et al., 2001; Rogers et al., 2002). A study using Xenopus egg extracts has described EB1 as an antipause, anticatastrophe factor (Tirnauer et al. 2002a). In addition, a role for EB1 in microtubule–kinetochore attachment has been suggested (Rehberg and Gräf, 2002; Tirnauer et al., 2002b). Studies in budding yeast provide significant functional and mechanistic insights into EB1, which indicate critical roles in the spindle orientation by connecting an astral microtubule to the cell cortex. (Schwartz et al., 1997; Tirnauer et al., 1999; Korinek et al., 2000; Lee et al., 2000; Miller et al., 2000; Hwang et al., 2003; Liakopoulos et al., 2003).
Interactions between microtubules and the cell cortex are considered to be particularly important during development. However, the roles of EB1 in development have not been well investigated. In this study, we examine the role of EB1 in flies, particularly in the chordotonal sensory organs. A chordotonal sensory organ detects a relative position of body parts by acting as an internal stretch sensor as well as making up auditory receptors in flies (Eberl et al., 2000). Chordotonal organs belong to type I mechnoreceptors, which have monodendritic, ciliated neurons associated with supporting cells (Jarman, 2002). These neurons and supporting cells are highly polarized and contain specialized cytoskeletal systems (Dettman et al., 2001), but only limited information is available on the molecular basis of the cell architecture in this sensory organ.
Here, we report the first genetic study of EB1 in higher eukaryotes by using the fruit fly Drosophila melanogaster to elucidate its role in developing organisms. We have found that one of EB1 homologues, DmEB1, has an essential function during development. Loss of DmEB1 disrupts body coordination and compromises the function and structure of the chordotonal sensory organs.
MATERIALS AND METHODS
Drosophila Genetics
Standard techniques of fly manipulation (Ashburner, 1989) were followed throughout. All stocks and crosses were grown at 25°C in cornmeal media. w1118 was used as wild type. Details of balancers and mutations are described in Lindsley and Zimm (1992) or FlyBase (The FlyBase Consortium, 2003). l(2)04524 (DmEB1P) was obtained from The Bloomington Stock Center (Indianapolis, IN). DmEB12 and DmEB15 were a generous gift from John Roote (Cambridge University, Cambridge, United Kingdom) and were studied over DmEB1P or each other because the chromosomes seem to have unrelated background lethal mutations. These mutations were kept over CyO or In(2LR)Gla, Bc Elp.
Wild-type DmEB1 cDNA was cloned into a transformation vector (pUb) under the control of the ubiquitin promotor. The resulting plasmid was used for germline transformation. Transgenes on X and the third chromosome were used for rescue experiments. DmEB1P homozygote carrying one copy of the transgene produced completely viable, fertile, healthy adult flies without behavioral defects.
DNA Manipulation, Protein Preparation, and Immunoblots
Standard molecular techniques (Sambrook et al., 1989; Harlow and Lane, 1988) were followed throughout. Mutation sites in DmEB12 and DmEB15 were determined by direct sequencing of DmEB1 coding regions amplified from mutant genomic DNAs by polymerase chain reaction. Microtubule sedimentation experiments were carried out as described previously (Cullen et al., 1999). A total protein sample from each stage or adult body part of Drosophila was prepared as described previously (Cullen et al., 1999). The DmEB1 antibody used for these studies was generated at Diagnostic Scotland (Midlothian, United Kingdom) by immunization of a rabbit with full-length DmEB1 fused with glutathione S-transferase made in bacteria. The specificity of the antibody against Drosophila extract was confirmed by immunoblots.
Cytological Analysis
Immunostaining of fly tissues was carried out as described in Cullen et al. (1999) and Cullen and Ohkura (2001). Methanol fixation was used for embryos and nonactivated oocytes, and formaldehyde was used for other tissues. Samples were examined and images were collected using an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY) attached with a charge-coupled device camera (Hamamatsu, Bridgewater, NJ) or confocal microscopes TCS (Leica Microsystems, Deerfield, IL) or LSM5.10 (Carl Zeiss). Figures were prepared using Photoshop (Adobe Systems, Mountain View, CA).
Preparation of abdominal chordotonal organs for immunostaining was carried out as follows. Pharate adults were used within 1 d after wings become darkened, to obtain wild-type and DmEB1P homozygotes of roughly the equivalent stage. These pharate adults were dissected from pupae cases and fixed with 3.4% of formaldehyde in phosphate-buffered saline immediately after the abdomen was cut open. Internal tissues were removed and subjected for immunostaining.
Various primary antibodies used in these studies were as follows: anti-Futsch (22C10; Fujita et al., 1982), anti-Msps (Cullen et al., 1999), anti-α-tubulin (TAT1, Woods et al., 1989; DM1A, Sigma-Aldrich, St. Louis, MO; and YOL1/34, SeraLab, Crawley, United Kingdom), and anti-DmEB1 (this study). Secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA) or Molecular Probes (Eugene, OR).
Orcein staining of central nervous systems and calculation of mitotic index and anaphase frequency were carried out as described previously (Cullen et al., 1999).
For electron microcopy of chordotonal organs, Johnston's organs from pharate adults were prepared according to Eberl et al. (2000). Sections of 90-nm thickness were made using a UCT Ultra microtome (Leica Microsystems) and examined using a transmission electron microscope CM120 Biotwin (Philips, The Netherlands).
Neurological Analysis
Electrophysiological measurements of chordotonal neurons in Johnston's organ in the antenna were recorded as described previously (Eberl et al., 2000). Recordings from DmEB1 homozygotes were usually preceded by and alternated with age-matched controls (w or heterozygous siblings). The response of each antenna was averaged to 10 trains of five pulses, and then the maximum peak-peak amplitude was taken from the averaged trace.
The behavior of adult flies was examined after sufficient time was given for recovery if they had been exposed to carbon dioxide. Flies were either directly observed or videorecorded by using a digital camera under a dissection microscope. For measuring the time taken to “get up,” each adult fly was placed on a petri dish and turned over on their back by using a paint brush. Flight test was carried out according to Ashburner (1989) by using a vertical tube 8 cm in width and 40 cm in height.
RESULTS
A Drosophila EB1 Homologue, DmEB1, Is Ubiquitously Expressed during Drosophila Development
To understand the role of EB1 in the context of developing animals, we used D. melanogaster as a model organism. Drosophila genome sequencing has identified three genes (CG3265, CG18190, and CG32371) closely related to human EB1 and a few others with limited similarity. We studied an in vivo role of CG3265 (which is also called DmEB1 or dEB1; Lu et al., 2001; Rogers et al., 2002), because it shows the highest similarity to human EB1 and seems to be most abundantly expressed.
As a first step, we examined protein expression of DmEB1 during Drosophila development. Total protein extracts were prepared from various developmental stages. Immunoblots showed that DmEB1 protein is ubiquitously expressed throughout development (Figure 1A). To see the protein expression profile in different tissue types in adult flies, we prepared total protein samples from head, thorax, and abdomen of adult Drosophila. These body parts are enriched in neural cells, muscle cells, and visceral/reproductive cells, respectively, and therefore serve as populations of contrasting cell types. The immunoblots showed that DmEB1 protein is equally well expressed in all of the body parts (Figure 1B). These results indicated that DmEB1 is expressed ubiquitously during development.
Figure 1.
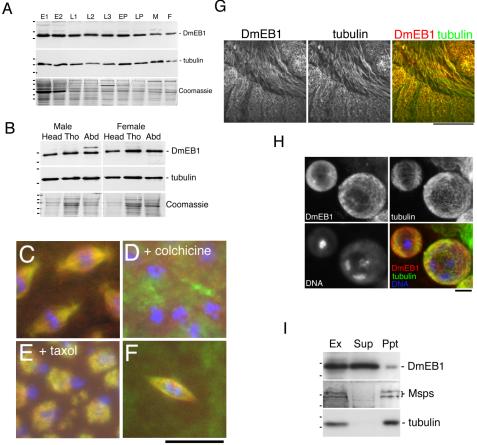
DmEB1 is ubiquitously expressed and associates with microtubules during development. (A) DmEB1 is ubiquitously expressed during Drosophila development. Total protein samples were prepared from each stage of wild-type Drosophila. E1, 0- to 4-h embryos; E2, 4- to 24-h embryos; L1, first instars; L2, second instars; L3, third instars; EP, early (white) pupae; LP, late (dark) pupae; M, adult male; and F, adult female. From the top, an immunoblot probed with a DmEB1 antibody, an immunoblot using α-tubulin antibody, and Coomassie Blue staining. The dots on the left show the positions of molecular mass markers (48, 33, and 25 kDa [top]; 62, 48, and 33 kDa [middle]; and 175, 83, 62, 48, 33, 25, and 16 kDa [bottom]). (B) DmEB1 is ubiquitously expressed in different adult tissues. Protein samples were prepared from head, thorax and abdomen of adult males and females. The dots on the left show the positions of molecular mass markers (33 and 25 kDa [top]; 62 and 48 kDa [middle]; and 83, 62, 48, 33, and 25 kDa [bottom]). (C–G) DmEB1 associates with microtubule structures. Blue, DNA; green, α-tubulin; and red, DmEB1. Bar, 10 μm for C–F and 100 μm for G. (C) DmEB1 colocalizes with spindles during syncytial mitosis. (D) Depolymerization of microtubules by colchicine delocalizes DmEB1. (E) DmEB1 is associated with taxol-stabilized spindle microtubules. (F) DmEB1 colocalizes with spindle microtubules in female meiosis I, which lacks centrosomes. (G) DmEB1 colocalizes with interphase microtubules in cellularized embryos. Lateral view of a stage 10 embryo. (H) DmEB1 colocalizes with a meiotic spindle and interphase microtubules in spermatocytes. (I) A minor proportion of DmEB1 cosedimented with microtubules. A soluble Drosophila embryonic extract (Ex) was incubated with paclitaxel and GTP to polymerize microtubules. Microtubules and associated proteins (Ppt) were separated from soluble proteins (Sup). From the top, the immunoblot probed with DmEB1 antibody, the control immunoblot probed with antibody against Msps (another microtubule-associated protein; Cullen et al., 1999), and immunoblot probed with α-tubulin. The dots on the left show the positions of molecular mass markers (33 and 25 kDa [from the top]; 175, 83, 62, and 48 kDa [from the bottom]).
DmEB1 Is Associated with Microtubule Structures
EB1 and its homologues have been shown to localize to various microtubule structures. However, localization studies have been limited mainly to cultured cells or unicellular systems. We examined subcellular localization of DmEB1 in various tissues during development.
In rapid syncytial embryonic cell cycles, DmEB1 colocalizes with spindle microtubules during mitosis (Rogers et al., 2002; Figure 1C). There is conflicting evidence in other organisms on whether EB1 also can localize to centrosomes without microtubules (Berrueta et al., 1998; Rehberg and Gräf, 2002; Louie et al., 2004). To test whether localization to spindle poles is dependent on microtubules, we depolymerized microtubules during syncytial division by incubation with colchicine. Tubulin staining indicates that microtubules were depolymerized. DmEB1 is diffused throughout the cytoplasm, and no accumulations were detected on centrosomes.
In human cultured cells, it is reported that EB1 fails to localize to taxol-stabilized microtubule structures (Morrison et al., 1998). We incubated Drosophila embryos with taxol and then subjected them to immunostaining. Taxol incubation promotes microtubule assembly mainly from centrosomes but also ectopic sites. DmEB1generally followed microtubule structures, indicating that DmEB1 is able to associate with taxol-stabilized microtubules in vivo (Figure 1E).
To examine the localization of DmEB1 in various cell cycles, we have examined DmEB1 distribution in later stages of development. In cellularized embryos, we found that DmEB1 localizes to spindle structures during mitosis (our unpublished data) and with cytoplasmic microtubules during interphase (Figure 1G). We also observed overlapped localization of DmEB1 with interphase microtubules in male premeiotic cells and meiotic spindles (Figure 1H). We cannot tell whether it is concentrated to plus ends of microtubules because we cannot resolve ends of single microtubules. We also examined female meiotic spindles, which are formed in a chromosome-driven manner without centrosomes or discrete microtubule organizing centers. DmEB1 localizes along the microtubules and no concentration was observed around the spindle poles (Figure 1F).
To assay the association of DmEB1 to microtubules in vitro, microtubule cosedimentation experiments were carried out. Soluble extracts from embryos were incubated with taxol to polymerize microtubules, and then microtubules were pelleted by centrifugation. Immunoblots indicated that a minor fraction of the protein coprecipitates with microtubules, whereas the majority of DmEB1 proteins stays in the supernatant (Figure 1I).
DmEB1 Is Essential during Drosophila Development
Although budding and fission yeast have only one EB1 homologue, it is not essential for viability. In higher eukaryotes, which have multiple genes, no genetic analysis has been reported. We asked questions as to whether DmEB1 is essential for viability during development and, if so, what would be the function.
As part of the genome project, one lethal mutant, l(2)04524, has been identified with a P-element insertion in proximity of the DmEB1 gene. Sequence analysis showed that a P-element of ∼15 kb in length is inserted in the noncoding transcribed region (Figure 2A). Immunoblotting of total protein extracts from late third instars indicated that the amount of DmEB1 protein is greatly reduced in the l(2)04524 mutant (Figure 2C).
Figure 2.
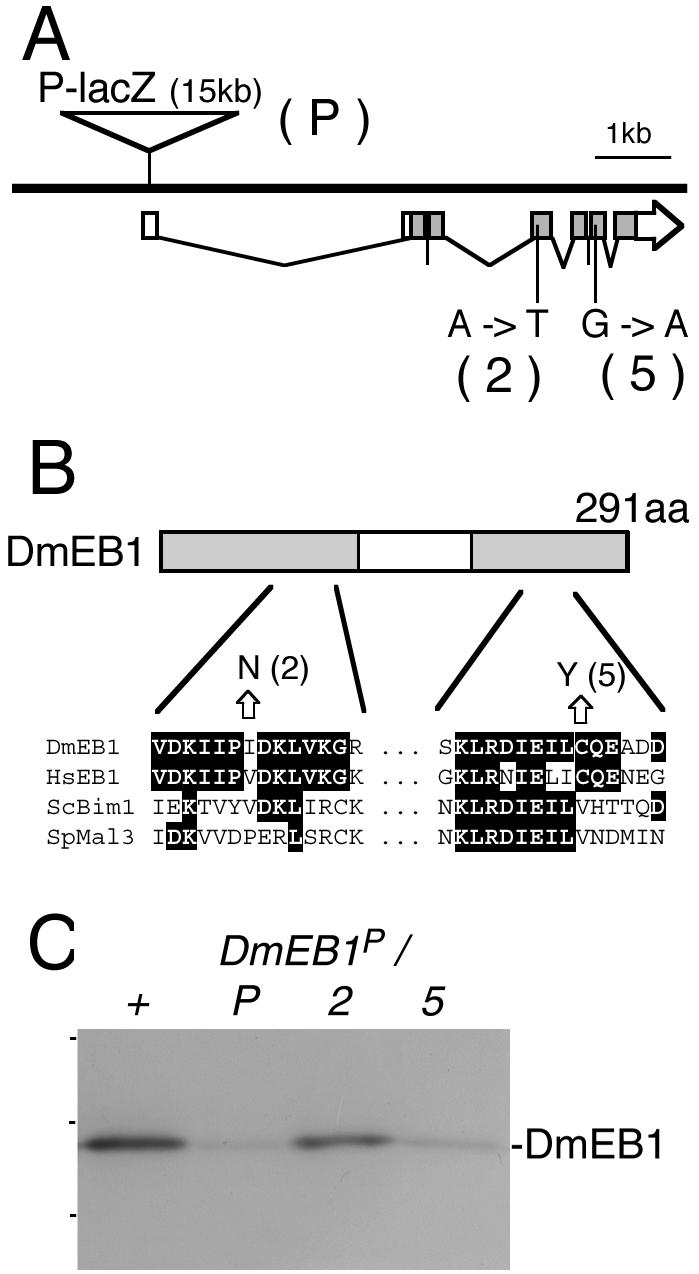
Molecular defects in DmEB1 alleles. (A) Molecular maps of DmEB1 genomic region with mutations in DmEB1 alleles. DmEB1P (P) has an insertion of 15-kb-long P-lacZ in the 5′-noncoding transcribed region, whereas DmEB12 (2) and DmEB15 (5) have a single point mutation (adenine to thymine and guanine to adenine, respectively) in the coding region. (B) Amino acid conversions caused by DmEB12 and DmEB15. The EB1 family of proteins commonly has two conserved regions (gray shadows). DmEB12 and DmEB15 mutations result in conversions I93N andC252Y, respectively. Amino acid alignments of DmEB1, human (Hs) EB1, and Saccharomyces cerevisiae Bim1p, Schizosaccharomyces pombe (Sp) Mal3 are shown below. Identical amino acids to DmEB1 were marked in black. (C) DmEB1 proteins in DmEB1 alleles. Total protein samples were prepared from third instars of wild-type (+), DmEB1P (P), DmEB12 (2), DmEB15 (5), all over DmEB1P. The immunoblot was probed with DmEB1 antibody. The dots on the left show the positions of molecular mass markers (48, 33, and 25 kDa from the top).
The lethality of l(2)04524 is reverted at a high frequency by remobilization of the P-element, indicating that the P-element is responsible for the lethality (our unpublished data). To prove that the lethality is a direct consequence of disrupting DmEB1, we made a transgene of wild-type DmEB1 cDNA under the control of the ubiquitin promotor. This transgene completely rescued the lethality, fertility and any defects of the mutant (our unpublished data). Therefore, we concluded that DmEB1 is essential for viability in Drosophila and renamed l(2)04524 to DmEB1P.
Semilethal mutants that fail to complement l(2)04524 (DmEB1P) have been isolated (Roote, unpublished data). We studied two of the alleles, DmEB12 and DmEB15, at the molecular level. Sequencing of the DmEB1 coding region indicated that each allele has one point mutation resulting in a conversion of amino acid sequences (I93N for DmEB12 and C252Y for DmEB15; Figure 2B). From the primary sequence, DmEB1 can be divided into three domains: the N-terminal conserved region, the central nonconserved region, and the C-terminal conserved region. The mutated amino acids are not conserved in all EB1 homologues and mapped in different conserved regions of the proteins (Figure 2B). Therefore, the mutant proteins may still maintain some molecular functions that lead to the hypomorphic nature of the mutation. Immunoblotting indicated that the DmEB12 mutation does not affect the level of DmEB1 protein, whereas a mutation in DmEB15 reduced the amount of DmEB1 protein significantly (Figure 2C).
Mutations in DmEB1 Cause Neuromuscular Defects
DmEB1P homozygous larvae are fully viable and show normal motility and touch response. Dissection of third instars revealed fully formed imaginal discs and internal organs. To see possible defects at a cellular level, we examined chromosome and microtubule organization in dividing cells of the central nervous systems. The DmEB1P mutant had a similar mitotic index and frequency of anaphase to wild type (Figure 3, A and B) and did not show abnormal mitotic figures. Immunostaining indicated that the organization of mitotic spindles and interphase microtubules were not disrupted in the mutant (our unpublished data). Therefore, the residual amount of DmEB1 protein in the mutant seems to be sufficient for cell division.
Figure 3.
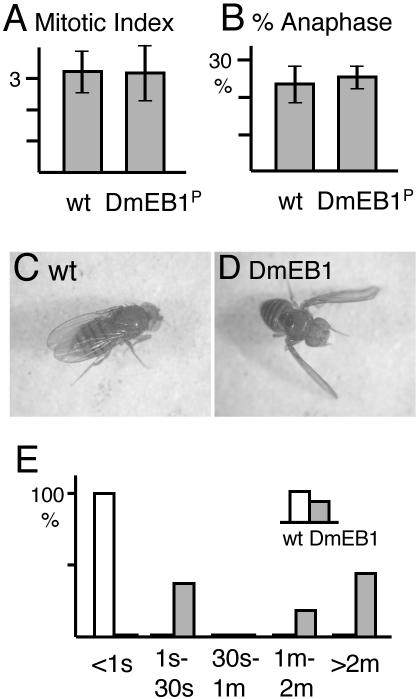
DmEB1 mutants show neuromuscular defects. (A and B) Normal cell division in DmEB1P mutant. Central nervous systems of third instars from wild-type and DmEB1P homozygotes were dissected and stained with aceto-orcein after being squashed. Average mitotic indexes (A) and percentages of anaphase among mitotic cells (B) were shown with standard deviations (bars). Mitotic index was calculated as the number of mitotic cells per microscope field containing typically 200–400 cells. (C) All wild-type (w1118) adult flies hold wings on their backs. (D) All adult DmEB15/DmEB1P flies hold their wings downward. (E) Times taken to get up after being turned over. Each adult fly was turned over on their back by using a paint brush. Wild-type and DmEB12/DmEB1P were represented by open and shaded bars, respectively.
Homozygous larvae are able to pupate and develop into pharate adults but fail to eclose. Dissection of pupae indicated that a complete adult body was formed inside the pupal case. Examination of the external structure did not reveal morphological defects. Defects generally associated with a failure of cell division at a low frequency, such as roughened eyes or missing bristles, were not found. The planar polarity in wing cells or microchaetes was not affected. Visual inspection and immunostaining indicated that external sensory organs correctly formed in the DmEB1P mutant and neuron morphology was indistinguishable to that of wild type (our unpublished data). These results together with the observation from the larval central nervous systems indicated that there were no general cell division defects in this hypomorphic mutant. Cytological observation of testes did not reveal any defects (our unpublished data).
We found that very rare homozygotes of DmEB1P (i.e., escapers) were able to eclose. The escaper adults generally had severe problems in coordinating body movements. Legs shook and often legs of both sides crossed each other. They were barely able to walk and, once they fell upside down, could not coordinate body movements to correct the body position. These escapers usually died within a few days. Homozygotes dissected from the pupal cases showed a wide range of viability and body coordination defects.
Two other alleles, DmEB12 and DmEB15, showed reduced viability over DmEB1P. Heterozygotes between DmEB12 and DmEB15 showed reduced viability as well, whereas we did not examine the alleles as homozygotes due to unrelated background lethal mutations. Although viable escapers from these semilethal allelic combinations had no apparent morphological defects, their wings are held abnormally. All of DmEB15/DmEB1P adults held their wings downward (Figure 3D). In DmEB12/DmEB1P or DmEB12/DmEB15, this phenotype was seen at a lower frequency, and wings were often held up or parted. Such abnormal wing positioning also is seen in some mutants defective in neuronal activity (Kernan et al., 1994; Zhang et al., 2001). A flight test indicated that all of DmEB1 mutant flies were flightless. Even individuals that held their wings in the normal position were flightless, suggesting that the phenotype may be due to neuromuscular defects rather than morphological defects.
The most striking defect of these semilethal flies was a behavioral defect. Although they walked in a coordinated way, once the flies were turned upside down, they failed to recover their correct body position promptly (Figure 3E and Supplemental Movies 1 and 2). In wild type, recovery takes less than one second. In contrast, it took significantly longer and often >2 min in the DmEB12/DmEB1P mutant. This also was observed in individuals that held their wings in the correct position. This defect is not due to paralysis or a general failure of movements, because they could walk and frantically moved their legs and bodies when they were turned over.
Functional Defects in Chordotonal Sensory Organs in a DmEB1 Mutant
Some aspects of DmEB1 mutant behavior are similar to the behavior of mutants with defective chordotonal mechanosensory organs (Eberl et al., 2000), although a strong DmEB1 mutant is less viable than mutants completely lacking chordotonal function. Chordotonal organs are stretch receptors that transduce movement or vibration of limb and body segments. They comprise multiple sensory units or scolopidia, each of which includes one to three ciliated sensory neurons that are enclosed by a scolopale cell and attached to a cap cell (Jarman, 2002). Specialized actin- and microtubule-based cytoskeletal structures are prominent features of these support cells (Dettman et al., 2001). The largest chordotonal organ (Johnston's organ), located in the adult antenna, includes ∼100 scolopidia and responds to sound-induced vibration of distal antennal segments. (Eberl et al., 2000; Caldwell and Eberl, 2002).
To assay the function of chordotonal organs in the DmEB1P mutant, we recorded sound-evoked compound potentials from Johnston's organ. Individual wild-type and mutant flies were exposed to near-field sound pulse trains while extracellular potentials were recorded from their antennae (Figure 4A; Eberl et al., 2000). In wild-type controls, a standard pulse stimulus evoked compound potentials with an average peak-to-peak amplitude of 615 μV (Figure 4, B and C). In DmEB1P mutants, responses to stimuli of the same intensity were reduced to an average of 237 μV (Figure 4, B and C). Although many of the mutants showed reduced activity and severe uncoordination, the reduction in response amplitude is unlikely to be due to general “sickness,” because four especially healthy and vigorous mutants gave similarly reduced responses (average amplitude 220 μV).
Figure 4.
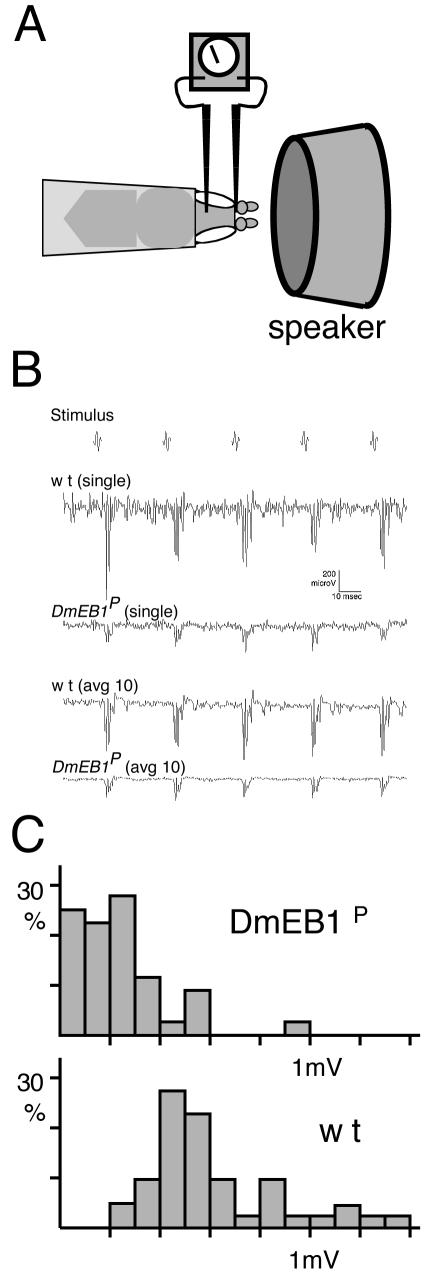
The sound-evoked electrophysiological response of chordotonal neurons is greatly reduced in DmEB1P. (A) Schematic diagram of recording of sound-evoked potentials on adult flies. Electrodes are placed between the first and second antennal segments, and in the head capsule. This records a total potential of neurons located distal to the second segment including chordotonal neurons of a Johnston's organ, which responds to sound. Sound stimuli are delivered through a speaker. (B) Electrophysiological response to sound. Five successive pulse sounds were applied as a stimulus. Examples of single recordings from wild type and DmEB1P mutants are presented together with averages of 10 recordings. (C) Neuronal response is greatly reduced in the DmEB1P mutant. A histogram of average amplitudes from each antenna of the DmEB1P mutant and wild-type (N = 36 and 41). As a control, heterozygous siblings and unrelated w stock were used. Reponses of these two controls show no significant differences (628 ± 228 vs. 594 ± 356 μV), and the data were pooled to compare with DmEB1P mutant (237 ± 189 μV).
Because antennal sound-evoked potentials represent an aggregate response from the many scolopidia in Johnston's organ, the response amplitude reduction may reflect either loss of function in a subset of scolopidia or an overall partial loss of function. Nevertheless, this result indicates that DmEB1 is required for normal electrophysiological function of auditory chordotonal organs.
DmEB1 Is Concentrated in a Subset of Cell Structures of Chordotonal Organs
To understand the role of chordotonal organs, we examined the localization of DmEB1 in the chordotonal sensory organs in wild-type flies. We chose a pair of chordotonal organs located at the anterior ventral part of the abdomen because of its suitability for immunostaining. Fixed samples were immunostained with antibodies against DmEB1 and α-tubulin as well as a monoclonal antibody (mAb) 22C10, which recognizes a neuron-specific marker, the MAP1 homologue Futsch.
Each sensory unit in the chordotonal organs is comprised of a monociliated neuron and support cells (Figure 5C) and aligned in parallel in a highly organized manner (Figure 5B). Cap cell and ligament structures were strongly stained with the anti-α-tubulin antibody (Figure 5A). DmEB1 was concentrated in the distal ends of cap cells, which are connected to body walls, and subregions of scolopale cells and ligament cells. Magnified images surrounding neuron dendrites revealed that DmEB1 was localized to the scolopale, spindle-shaped structure enclosing the cilia (Figure 5D, left), and regions of cells surrounding the inner dendritic segment of the neurons (Figure 5D, right). We do not see significant signals in cap structures or ciliary endings.
Figure 5.
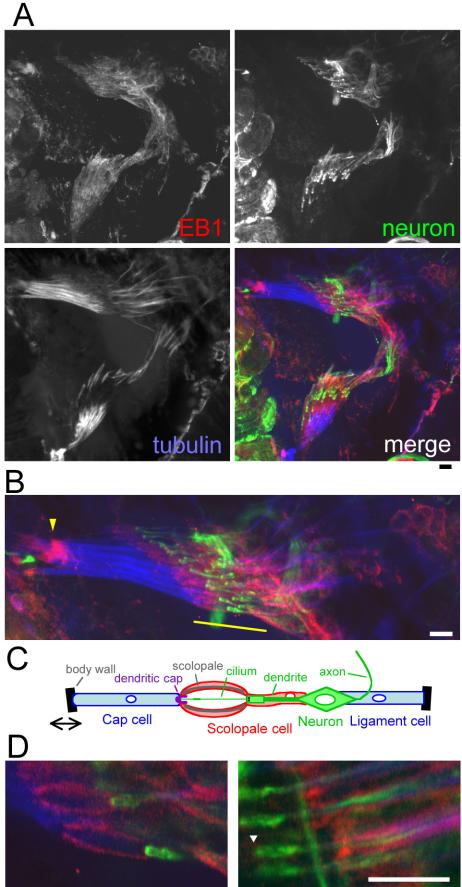
DmEB1 is concentrated in a subset of cell structures in chordotonal organs. (A) Adult abdominal chordotonal mechanosensory organs from wild type. Chordotonal organs were dissected together with the cuticle from the ventral part of a pharate adult abdomen and immunostained using antibodies against DmEB1 (red), α-tubulin (YOL1/34; blue), and the neuron marker Fustch (22C10; green). The z-axis projection of confocal sections is shown here. High DmEB1 signals were observed in the anterior ends of the cap cells connected to body walls and parts of scolopale cells and ligament cells. Bar, 10 μm. (B) Higher magnification of one chordotonal organ. The arrowhead indicates high DmEB1 signals at the distal end of cap cells. The area above the yellow line corresponds to scolopales and inner dendritic segments and is magnified in D. Bar, 10 μm. (C) Schematic diagram of cell organization in each sensory unit of the chordotonal organs. (D) Higher magnification of neuron dendrites that correspond to the area above the yellow line in B. Single confocal sections are shown. DmEB1 is concentrated in the scolopale regions (left) and regions surrounding inner dendritic segments (right). The arrowhead marks the position of the base of the cilium, which is not stained by 22C10. Bar, 10 μm.
The Organization of Chordotonal Organs Is Disrupted in DmEB1 Mutant
To understand the basis of functional defects of chordotonal organs, we compared the morphology of chordotonal organs of the first abdominal segment from wild-type and mutant flies. Fixed samples were immunostained with an α-tubulin antibody and the mAb 22C10.
The structures and overall organization of chordotonal organs were largely unaltered in the mutant (Figure 6, B and D). Quantitative comparison of the overall shape of chordotonal organs did not reveal differences between wild-type and DmEB1P mutant. Lengths and widths of chordotonal organs were not significantly different between wild-type and the mutant. In addition, the number of neurons (represented by the number of dendrites) are not significantly affected by the mutation (Figure 6D), and the number of other cell types seems unaltered. This is consistent with the previous observation that there are no general defects in cell division or fate determination in this allele.
Figure 6.
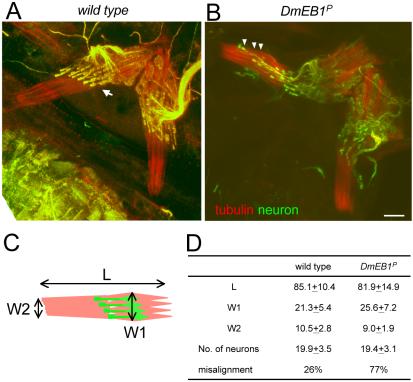
Organization of chordotonal organs is disrupted in DmEB1P mutant. Adult abdominal chordotonal mechanosensory organs from wild type (A) and DmEB1P homozygote (B). Chordotonal organs were dissected as described in Figure 5 and immunstained using antibodies against α-tubulin antibody (YOL1/34; red) and the neuron-specific marker Fustch (22C10; green). The z-axis projection of confocal sections is shown here. The arrow in A indicates the bulged ends of dendrites. The arrowheads in B indicate dendrites with the misaligned end and stretched morphology. Bar, 10 μm. (C) Schematic diagram of a chordotonal organ. Three measurements (L, W1, and W2) were taken to compare the overall morphology of the organs from wild type and the mutant. (D) The mean value (in micrometers) of L, W1, and W2 with standard deviations were shown together with the number of neurons represented by the number of dendrites. No significant differences were found between wild type and the mutant (p > 0.05). At the bottom, the percentages of chordotonal organs that have at least one misaligned dendrite ends were shown. The difference between wild type and the DmEB1P mutant is statistically significant (p < 0.001).
Next, we looked at the organization within the abdominal chordotonal organs. In wild-type flies, each chordotonal neuron, as revealed by the 22C10 antibody, has one extended dendrite with a bulged end (Figure 6A, arrow) to which the cilium is attached (the cilium itself does not stain with 22C10). In wild type, ends of the dendrites are generally well aligned within the organ (Figure 6A). In contrast, they are not well aligned in the chordotonal organs of DmEB1P mutant flies (Figure 6B). For consistent quantification, we looked at the alignment of the most anterior row of dendrites. In the mutant, most chordotonal organs show the ends of at least one dendrite ends is not aligned, whereas this kind of misalignment is much less often seen in wild type (Figure 6D; 77% in the mutant vs. 26% in wild type; p < 0.001). This misalignment is often associated with unusually stretched dendrite morphology (Figure 6A, arrowheads).
In conclusion, our immunostaining results suggest that the DmEB1 mutation does not affect cell division or fate determination to form chordotonal organs, but it disrupts the alignment and structural integrity of neuronal sensory processes within the organs.
Ultrastructures of Auditory Chordotonal Organs in DmEB1 Mutant
To complement the light microscopy analysis, we examined mutant chordotonal organs by electron microscopy. The Johnston's organ in the second antennal segment was used for this study. Each sensory unit in the Johnston's organ contains two monociliated neurons (Figure 7A).
Figure 7.
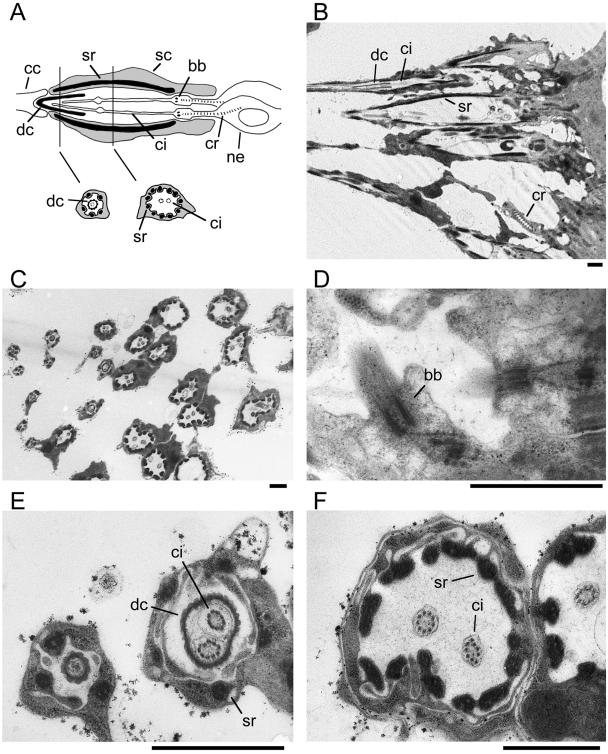
Ultrastructure of chordotonal organs in DmEB1P mutant. Electron micrographs of auditory chordotonal organs in Johnston's organs from DmEB1P mutant. All prominent ultrastructures with normal morphology are observed. Bar, 1 μm. (A) Schematic diagram of each sensory unit of the auditory chordotonal organs, modified from Dubruille et al. (2002). Top, longitudinal section. Bottom, cross sections. bb, basal body; cc, cap cell; ci, cilium; cr, ciliary rootlet; dc, dendritic cap; sc, scolopale cell; sr, scolopale rod; and ne, neuron. (B) Longitudinal section revealing a dendritic cap, cilia, scolopale rods, and ciliary rootlet. (C) Cross section of multiple chordotonal organs. More proximal cross sections of the organs are observed toward the left. (D) Higher magnification of a longitudinal section showing basal bodies. (E and F) Higher magnification of different cross sections. Dendritic caps are visible between cilia and a circle of scolopale rods near the distal ends (E) but absent from more proximal positions (F).
Longitudinal sections of the chordotonal organs from the DmEB1P mutant revealed prominent ultrastructures, including a dendritic cap, scolopale, cilia, and ciliary rootlets (Figure 7B). In each scolopidium, the dome-shaped scolopale surrounds cilia whose tips are connected to the dendritic cap. At the base, a pair of ciliary rootlets with regular repetitive structures is visible. In higher magnification, basal bodies are clearly identifiable at the base of cilia (Figure 7D). Comparison with wild-type organs indicates that these structures are morphologically normal. Cross-sections of a number of organs at different positions revealed regular structures with wild-type morphology (Figure 7C). Near the distal end, the extracellular dendritic cap is visible surrounding the cilia and interior to a circle of scolopale rods (Figure 7E). In a more proximal region, a pair of cilia are observed in the middle of a circle of scolopale rods without the cap (Figure 7F).
In summary, electron microscopy revealed that mutant chordotonal organs have all the basic inner structures with normal morphology.
DISCUSSION
In this study, we investigated the in vivo function of EB1 in Drosophila development. We demonstrated by mutational analysis that one of the EB1 homologues, DmEB1, is essential for viability during development. The mutants showed neuromuscular defects, including flightlessness, uncoordinated movements, and partial deafness. These defects are caused in part by the defects in functional and structural defects in chordotonal mechanosensory organs. Consistently, DmEB1 is concentrated in mechanically important areas of the supporting cells in wild-type chordotonal organs. This is the first genetic study of EB1 homologues in animals and has uncovered a role for EB1 in neuronal function.
Essential Roles of DmEB1 during Drosophila Development
EB1 is a fundamental protein in eukaryotes that is conserved from yeast to humans and plants (Tirnauer and Bierer, 2000). Yeasts have only one EB1 homologue, which has important roles in the organization of cytoplasmic microtubules, but it is not essential for viability (Beinhauer et al., 1997; Schwartz et al., 1997). In contrast, higher eukaryotes, such as humans and Drosophila, have multiple EB1 homologues (Juwana et al., 1999; Rogers et al., 2002). In these organisms, most studies have so far been concentrated on one of the homologues, and functional relationships among the other homologues have not been studied.
In this study, we use Drosophila as a model system to examine in vivo roles of one of the EB1 homologues in a developmental context. In Drosophila, proteins encoded by up to six genes share some homology to EB1 in the N-terminal region (microtubule binding domain; Juwana et al., 1999). The similarity of three of them to EB1 also is extended to the C-terminal region. Among them, DmEB1 is the most similar to human EB1. We showed that the DmEB1 protein is ubiquitously expressed during Drosophila development. Other EB1 homologues also are expressed during development, although their expression is rather limited (our unpublished data). In this study, we identified three mutant alleles of DmEB1, one of which is lethal and the other two are semilethal. Transgenic wild-type DmEB1 genes expressed from the ubiquitin promotor fully rescues these DmEB1 mutants. Therefore, the DmEB1 gene has a unique and essential function that is not complemented by the other homologues during Drosophila development. Inability to complement the DmEB1 defects could be due to differences in expression or protein activity of the other EB1 homologues.
Previous studies using RNA interference (RNAi) or antibody injection indicated that DmEB1 is required for the regulation of microtubule dynamics and spindle organization/positioning (Lu et al., 2001; Rogers et al., 2002). Therefore, it is rather puzzling at first sight that our DmEB1 mutants showed limited defects without significant abnormalities in universal cell functions. However, it should be emphasized that the DmEB1 mutations examined in this study are hypomorphic and a low level of active protein produced in the mutants may be sufficient to support spindle formation. Moreover, DmEB1 protein has a large maternal contribution that may mask the requirement in early development. It is also possible that other EB1 homologues in Drosophila may have redundant functions that compensate for loss of DmEB1. Therefore, it is likely that the phenotype we observed in DmEB1 mutants represents a subset of DmEB1 functions in vivo. Nevertheless, our hypomorphic mutations uncovered the neuromuscular involvement of DmEB1 without interference by more universal cellular roles.
Roles of DmEB1 in Neural Function
Although our DmEB1 alleles do not show significant cell division or morphological defects, studies on semilethal alleles of DmEB1 revealed defective neuromuscular functions. These adult flies are flightless and hold their wings in the wrong position when resting. Moreover, they show uncoordinated body movements when correcting the body position after being overturned. Consistently, rare escapers of a stronger allele show much more severe body coordination defects.
These behavioral phenotypes of DmEB1 mutants share some characteristics with the phenotypes of mutants defective in chordotonal sensory organ function (Eberl et al., 2000). Chordotonal organs are mechanosensory organs that act as stretch receptors and also form the fly's auditory systems. We showed that DmEB1P mutant is indeed defective in the function of auditory chordotonal organs. Therefore, malfunction of chordotonal organs can, in part, explain the DmEB1 mutant defects. However, mutants completely lacking chordotonal organs show better viability than the DmEB1P homozygotes, indicating that the DmEB1 mutants have additional, unidentified defects.
Mechanotransduction typically requires intact mechanical linkages between stimulus-delivering structures and neuronal transducing elements. Each sensory unit of a chordotonal organ is comprised of one or a few ciliated neurons and several supporting cells, including cap cells, ligament cells, and scolopale cells (Eberl et al., 2000; Jarman, 2002). The central structure is the scolopale, a spindle-shaped structure encasing a sensory cilium. Sensory cilia are connected at their tips to the extracellular dendritic cap, at their base to a ciliary rootlet, and via transcellular attachments to the actin-based rods of the scolopale. These structures are connected to other body structures through cap cells and neurons/ligament cells. Disruption of any of these linkages could result in a reduced response.
Our immunolocalization study of DmEB1 indicated that, rather than DmEB1 distributing uniformly to all cells in the chordotonal organs, it is concentrated at high levels in subregions of supporting cells in the chordotonal organs. These coincide with structurally important regions for chordotonal functions. They include 1) anterior ends of cap cells that are in contact with the body walls, 2) scolopale region, 3) regions of cells surrounding the inner segment of neuron dendrites, and 4) part of the ligament cells. These cell structures probably require a high level of DmEB1 and are sensitive to a reduction of the protein level in the mutant, although all cells are likely to express and require it at some level. It also should be noted that DmEB1 does not have significant concentration at ciliary endings, although the EB1 homologue is localized to the flagellar tip and implicated in intraflagellar transport in Chlamydomonas (Pedersen et al., 2003).
Immunostaining of mutant chordotonal organs in the abdomen revealed that, although the overall arrangement of the chordotonal organs and the number of cells are not altered, the alignment of the dendrites is disrupted. This misalignment is often associated with unusually stretched dendrite morphology. Electron microscopy of mutant chordotonal organs revealed ultrastructures with normal morphology, such as cilia, dendritic cap, scolopale structures, basal bodies, and ciliary rootlets. Comparing the mutant phenotype with the immunolocalization results, one possibility is that this misalignment may represent an underlying weakness of cell structures such as the area supporting inner segment of neuron dendrites. In addition, the defects of other DmEB1-rich regions may well contribute functional and structural defects of chordotonal organs in the mutant.
Molecular Function of DmEB1
How then does the loss of DmEB1 cause the defects in function and organization of chordotonal organs at the molecular level? RNAi in Drosophila revealed that DmEB1 plays roles in regulating the mitotic spindle organization and spindle positioning/orientation. Any defects in chromosome segregation may lead to a failure to produce the sufficient number of cells comprising the chordotonal organs. Any defects in spindle positioning or orientation disrupt asymmetric divisions and result in a failure of correct fate determination among cells comprising chordotonal organs. However, we do not observe general cell division defects or significant alteration of the cell numbers in chordotonal organs of the mutant.
Instead, our observation on mutant chordotonal organs is consistent with roles of DmEB1 on microtubule cytoskeleton. DmEB1 and homologues in other organisms are shown to regulate interphase microtubule dynamics and thought to be involved in interactions between microtubule plus ends and the cell cortex. Cells comprising chordotonal organs are highly polarized and have specialized cytoskeletons both molecularly and morphologically (Eberl et al., 2000; Dettman et al., 2001). Furthermore, chordotonal organs require robust mechanical linkages between stimulus-delivering structures and neuronal transducing elements to sense tensions effectively.
The actin-microtubule linker Short stop, which localizes to the cell cortex, interacts with DmEB1 (Subramanian et al., 2003) and is shown to be required for chordotonal organ integrity (Prokop et al., 1998). The chordotonal phenotype of the short stop mutant is similar to defects seen in the DmEB1 mutant. Therefore, it is possible that DmEB1 may link microtubules to the cell cortex via interactions with Short stop. Although the linkage between microtubules and the cell cortex is important for most cell types, chordotonal organs are highly polarized and are required to withstand strong mechanical forces; therefore, they may be particularly sensitive to the reduction in DmEB1 activity. Immunofluorescence microscopy using antibodies against α-tubulin and Futsch (the MAP1 homologue) or our electron microscopy could not resolve the precise defect of the cytoskeleton. Further studies are required to understand exact roles of DmEB1 in chordotonal organ function.
Supplementary Material
Acknowledgments
We thank Luke Harrison, Alejandra Clark, Iain Gallagher, Joanna Vearey, and Duncan Sproul for participating in this study as a part of short-term student projects; Bloomington Stock Center for fly stocks; Institute of Cell and Molecular Biology (Edinburgh, Scotland) facilities for mass spectrometry and DNA sequencing; John Findlay for electron microscopy; Anne Davidson for critical reading of the manuscript; and Petra zur Lage, Hiledegard Tekotte, and Andrew Jarman for technical advice. We especially thank Glynnis Johnson, Terri Morley, and John Roote in Michael Ashburner's laboratory for unpublished mutants and data on DmEB1 semilethal alleles. Special thanks also go to Steve Rogers and Ron Vale for stimulating discussion. The work was supported by The Wellcome Trust (to H. O.), National Institute for Deafness and Communicative Disorders, and the Pew Scholars Program in the Biomedical Sciences (to M.J.K.).
Article published online ahead of print in MBC in Press on December 9, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0633).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Ashburner, M. (1989). Drosophila. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Beinhauer, J. D., Hagan, I. M., Hegemann, J. H., and Fleig, U. (1997). Mal3, the fission yeast homologue of the human APC-interacting protein EB-1 is required for microtubule integrity and the maintenance of cell form. J. Cell Biol. 139, 717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta, L., Kraeft, S. K., Tirnauer, J. S., Schuyler, S. C., Chen, L. B., Hill, D. E., Pellman, D., and Bierer, B. E. (1998). The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc. Natl. Acad. Sci. USA 95, 10596-10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta, L., Tirnauer, J. S., Schuyler, S. C., Pellman, D., and Bierer, B. E. (1999). The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr. Biol. 9, 425-428. [DOI] [PubMed] [Google Scholar]
- Caldwell, J. C., and Eberl, D. F. (2002). Towards a molecular understanding of Drosophila hearing. J. Neurobiol. 53, 172-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris, L., and Spittle, C. (2001). Regulation of microtubule-associated proteins. Int. Rev. Cytol. 210, 163-226. [DOI] [PubMed] [Google Scholar]
- Cullen, C. F., Deak, P., Glover, D. M., and Ohkura, H. (1999). Mini spindles: a gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol. 146, 1005-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, C. F., and Ohkura, H. (2001). Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat. Cell Biol. 3, 637-642. [DOI] [PubMed] [Google Scholar]
- Dettman, R. W., Turner, F. R., Hoyle, H. D., and Raff, E. C. (2001). Embryonic expression of the divergent Drosophila beta3-tubulin isoform is required for larval behavior. Genetics 158, 253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubruille, R., Laurencon, A., Vandaele, C., Shishido, E., Coulon-Bublex, M., Swoboda, P., Couble, P., Kernan, M., and Durand, B. (2002). Drosophila regulatory factor X is necessary for ciliated sensory neuron differentiation. Development 129, 5487-5498. [DOI] [PubMed] [Google Scholar]
- Eberl, D. F., Hardy, R. W., and Kernan, M. J. (2000). Genetically similar transduction mechanisms for touch and hearing in Drosophila. J. Neurosci. 20, 5981-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The FlyBase Consortium. (2003). The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 31, 172-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, S. C., Zipursky, S. L., Benzer, S., Ferrus, A., and Shotwell, S. L. (1982). Monoclonal antibodies against the Drosophila nervous system. Proc. Natl. Acad. Sci. USA 79, 7929-7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Hwang, E., Kusch, J., Barral, Y., and Huffaker, T. C. (2003). Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J. Cell Biol. 161, 483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman, A. P. (2002). Study of mechanosensation using fly. Hum. Mol. Gen. 11, 1215-1218. [DOI] [PubMed] [Google Scholar]
- Juwana, J. P., Henderikx, P., Mischo, A., Wadle, A., Fadle, N., Gerlach, K., Arends, J. W., Hoogenboom, H., Pfreundschuh, M., and Renner, C. (1999). EB/RP gene family encodes tubulin binding proteins. Int. J. Cancer 81, 275-284. [DOI] [PubMed] [Google Scholar]
- Kernan, M., Cowan, D., and Zuker, C. (1994). Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron 12, 1195-1206. [DOI] [PubMed] [Google Scholar]
- Kirschner, M. W., and Mitchison, T. (1986). Beyond self-assembly: from microtubules to morphogenesis. Cell 9, 329-342. [DOI] [PubMed] [Google Scholar]
- Korinek, W. S., Copeland, M. J., Chaudhuri, A., and Chant, J. (2002). Molecular linkage underlying microtubule orientation toward cortical sites in yeast. Science 287, 2257-2259. [DOI] [PubMed] [Google Scholar]
- Lee, L., Tirnauer, J. S., Li, J., Schuyler, S. C., Liu, J. Y., and Pellman, D. (2000). Positioning of the mitotic spindle by a cortical-microtubule capture mechanism. Science 287, 2260-2262. [DOI] [PubMed] [Google Scholar]
- Liakopoulos, D., Kusch, J., Grava, S., Vogel, J., and Barral, Y. (2003). Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell 112, 561-574. [DOI] [PubMed] [Google Scholar]
- Lindsley, D. L., and Zimm, G. G. (1992). The genome of Drosophila melanogaster, New York: Academic Press.
- Louie, R. K., Bahmanyar, S., Siemers, K. A., Votin, V., Chang, P., Stearns, T., Nelson, W. J., and Barth, A. I. (2004). Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J. Cell Sci. 117, 1117-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, B., Roegiers, F., Jan, L. Y., and Jan, Y. N. (2001). Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409, 522-525. [DOI] [PubMed] [Google Scholar]
- Miller, R. K., Cheng, S. C., and Rose, M. D. (2000). Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules. Mol. Biol. Cell 11, 2949-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue, Y., Shiina, N., and Tsukita, S. (2000). The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr. Biol. 10, 865-868. [DOI] [PubMed] [Google Scholar]
- Morrison, E. E., Wardleworth, B. N., Askham, J. M., Markham, A. F., and Meredith, D. M. (1998). EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene 17, 3471-3477. [DOI] [PubMed] [Google Scholar]
- Pedersen, L. B., Geimer, S., Sloboda, R. D., and Rosenbaum, J. L. (2003). The Microtubule plus end-tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr. Biol. 13, 1969-1974. [DOI] [PubMed] [Google Scholar]
- Prokop, A., Uhler, J., Roote, J., and Bate, M. (1998). The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J. Cell Biol. 143, 1283-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg, M., and Gräf, R. (2002). Dictyostelium EB1 is a genuine centrosomal component required for proper spindle formation. Mol. Biol. Cell 13, 2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, S. L., Rogers, G. C., Sharp, D. J., and Vale, R. D. (2002). Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158, 873-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schuyler, S. C., and Pellman, D. (2001). Microtubule “plus-end-tracking proteins”: the end is just the beginning. Cell 105, 421-424. [DOI] [PubMed] [Google Scholar]
- Schwartz, K., Richards, K., and Botstein, D. (1997). BIM1 encodes a microtubule-binding protein in yeast. Mol. Biol. Cell 8, 2677-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, L. K., Burrell, M., Hill, D. E., Gyuris, J., Brent, R., Wiltshire, R., Trent, J., Vogelstein, B., and Kinzler, K. W. (1995). APC binds to the novel protein EB1. Cancer Res. 55, 2972-2977. [PubMed] [Google Scholar]
- Subramanian, A., Prokop, A., Yamamoto, M., Sugimura, K., Uemura, T., Betschinger, J., Knoblich, J. A., and Volk, T. (2003). Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 13, 1086-1095. [DOI] [PubMed] [Google Scholar]
- Tirnauer, J. S., and Bierer, E. E. (2000). EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J. Cell Biol. 149, 761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., O'Toole, E., Berrueta, L., Bierer, B. E., and Pellman, D. (1999). Yeast Bim1p promotes the G1-specific dynamics of microtubules. J. Cell Biol. 145, 993-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., Grego, S., Salmon, E. D., and Mitchison, T. J. (2002a). EB1-microtubule interactions in Xenopus egg extracts: role of EB1 in microtubule stabilization and mechanisms of targeting to microtubules. Mol. Biol. Cell 13, 3614-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirnauer, J. S., Canman, J. C., Salmon, E. D., and Mitchison, T. J. (2002b). EB1 targets to kinetochores with attached, polymerizing microtubules. Mol. Biol. Cell 13, 4308-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A., Sherwin, T., Sasse, R., MacRae, T. H., Baines, A. J., and Gull, K. (1989). Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93, 491-500. [DOI] [PubMed] [Google Scholar]
- Yin, H., Pruyne, D., Huffaker, T. C., and Bretscher, A. (2000). Myosin V orientates the mitotic spindle in yeast. Nature 406, 1013-1015. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. Q., Bailey, A. M., Matthies, H. J., Renden, R. B., Smith, M. A., Speese, S. D., Rubin, G. M., and Broadie, K. (2001). Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107, 591-603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


