Abstract
Background
Availability of oral disease-modifying therapy (DMT) for relapsing–remitting multiple sclerosis (RRMS) may affect injectable DMT (iDMT) treatment patterns.
Objective
The objective of this paper is to evaluate iDMT persistency, reasons for persistency lapses, and outcomes among newly diagnosed RRMS patients.
Methods
Medical records of 300 RRMS patients initiated on iDMT between 2008 and 2013 were abstracted from 18 US-based neurology clinics. Eligible patients had ≥3 visits: pre-iDMT initiation, iDMT initiation (index), and ≥1 visit within 24 months post-index. MS-related symptoms, relapses, iDMT treatment patterns (i.e. persistency, discontinuation, switching, and restart), and reasons for non-persistency were tracked for 24 months.
Results
At 24 months, iDMT persistency was 61.0%; 28.0% of patients switched to another DMT, 8.0% discontinued, and 3.0% stopped and restarted the same iDMT. The most commonly identified reasons for non-persistency were perceived lack of efficacy (22.2%), adverse events (18.8%), and fear of needles/self-injecting (9.4%). At 24 months, 38.0% of patients had experienced a relapse and 11.0% had changes in MRI lesion counts. Patients without MS-related symptoms at index reported increases in the incidence of these symptoms at 24 months.
Conclusions
Non-persistency with iDMT remains an issue in the oral DMT age. Many patients still experienced relapses and disease progression, and should consider switching to more effective therapies.
Keywords: Multiple sclerosis, disease-modifying therapy, injection, persistence, discontinuation, relapse, disease progression, MRI
Introduction
Injectable disease-modifying therapies (iDMTs) used in the treatment of relapsing–remitting multiple sclerosis (RRMS) include the intramuscular (IM) or subcutaneous (SC) beta-interferons (IFN) 1a or 1b and glatiramer acetate.1 These agents have been shown to reduce the annualized relapse rate by approximately 30%, and have a modest benefit in the prevention of disability progression.2–6 Small differences in efficacy have been demonstrated among these iDMT agents.7
Previous research suggests that some patients receiving iDMT may have poor adherence and/or persistency. In a retrospective cohort pharmacy claims study of 1891 MS patients, approximately half of patients discontinued iDMT within one year of treatment initiation.8 In a systematic review of iDMT observational and randomized controlled trials, adverse events and lack of efficacy were the most commonly cited reasons for discontinuation.9 Another common reason for discontinuation was injection site reactions.9 Notably, psychological issues related to frequent injections, such as needle phobia, also appeared to play a role in the decision to discontinue treatment. In a prospective, randomized, open-label study comparing persistency and patient satisfaction between fingolimod and iDMT, 90.5% of patients randomized to iDMTs switched treatments within one year, mainly for injection-related reasons.10 Retention to randomized treatment with iDMT was 29.2% and with fingolimod was 81.3% after one year.10
Several “real-world” observational studies or retrospective database analyses have examined the persistence and discontinuation of iDMTs.11–23 However, most of these studies were conducted using data from single-centers and/or the studied time periods were before the availability of oral DMTs (2010). The approval of oral DMTs has provided patients with RRMS a viable alternative route of administration for DMT beyond infusions or injections. The availability of these agents has been shown to influence iDMT treatment patterns;24 therefore, studies to examine iDMT persistency, switching, and discontinuation that include the time period after the introduction of oral DMTs are warranted. The objective of this analysis was to provide insight into iDMT treatment patterns (persistency, discontinuation, switching, and restarts), identify reasons for therapy change, and identify RRMS-specific outcomes based on a retrospective review of the medical records of a subset of patients in the United States (US) with RRMS.
Methods
Study overview
This study was a retrospective medical record review of a sample of patients from neurology practice sites across the US. The full timeframe for this study was from January 2008 through August 2015, with the patient identification period occurring between August 2008 and August 2013. The first patient visit for which treatment initiation with iDMT was recorded was defined as the index visit. The iDMTs evaluated were IM IFN β-1a (Avonex®, Biogen, Cambridge, MA), SC IFN β-1a (Rebif®, EMD Serono Inc, Rockland, MA), SC IFN β-1b (Betaseron®, Bayer AG, Leverkusen, Germany), and glatiramer acetate (Copaxone®, Teva Neuroscience, Kansas City, MO). Patients were followed for 24 months after iDMT initiation even if they discontinued treatment with iDMT agents. All data were de-identified. This study was approved by the Sterling centralized institutional review board (Atlanta, GA), and patient consent waivers were obtained before the study was initiated.
Site selection
Study sites were chosen based on a review of a prescriber report provided by IMS Health (Danbury, CT), study sponsor recommendations, and prior RRMS study participating sites as identified by the medical record abstraction data vendor. The criteria for site selection were based on the geographic distribution of disease prevalence, level of specialization (i.e. general neurology, academic-affiliated institutions, and MS specialty neurologists), and results from a pre-qualification/screening questionnaire. Sites were selected to ensure geographic balance based on prevalence, a mix of levels of specialization, and ability/willingness to respond to chart requests. Each site was invited to complete a detailed questionnaire to better understand site characteristics and RRMS treatment experience as well.
Patient selection
Eligible patients were those with RRMS who initiated iDMT within six months of diagnosis, who had at least one pre-index visit, and had subsequent post-index visits (≥1 visit) for up to 24 months after the index visit. Patients were required to have at least 24 months of follow-up post-index, irrespective of treatment status with iDMT. Patients initiating treatment with any DMT other than those specified for this analysis or with any evidence of prior iDMT use in the six months before the index visit were excluded. Patients with missing age or sex information were also excluded.
Information collected from medical records
The medical record reviewers were nurses or pharmacists with medical record experience in neurology. The reviewers were trained on the study’s design, intent, key variable definitions, data collection process/reporting, and adverse event reporting. Standardized data collection forms were used. Baseline patient demographics, comorbidities, and MS disease status were tracked in the pre-index period. Treatment patterns for each patient were tracked at distinct time windows of 3, 6, 9, 12, 18, and 24 months post-index. The tracked treatment patterns were discontinuation, switching to another DMT agent, or a restart of the original iDMT after a short hiatus. Assignment to switch, discontinuation, and restarts were mutually exclusive. Discontinuation was defined as medical record documentation of index iDMT discontinuation within the cumulative time window, with no subsequent recorded use of the discontinued agent. Switching was defined as medical record documentation of index iDMT discontinuation, followed by another documentation indicating the start of a different DMT for RRMS within the cumulative time window. Both forward and backward switch events were captured. Forward switching was defined as the switch to another DMT for RRMS within the cumulative time window, whereas backward switching was a forward switch to another DMT, followed by switching back to the index iDMT within that same time window. A restart was defined as medical record documentation of index iDMT stop or discontinuation within the cumulative time window, with subsequent documentation of restarting the index iDMT after at least 30 days.
Reasons for any of the treatment pattern changes as recorded in the physician notes in the medical record were tracked over the same time period. Captured reasons are listed in Supplemental Table E1. Indicators of disease progression were also tracked at the pre-index and index visits to serve as a baseline, as well as over the distinct time windows of 3, 6, 9, 12, 18, and 24 months post-index. These indicators included magnetic resonance imaging (MRI) evidence of new lesions or more extensive demyelination, and physician narratives of worsening visual disturbance, bowel incontinence, neurological symptoms, and gait or mobility impairment based on physical exam. Relapse episodes were tracked over the distinct time windows of 3, 6, 9, 12, 18, and 24 months post-index and were defined as any record or an office visit, emergency department/urgent care visit, or hospitalization followed by medical record documentation of corticosteroid use within seven days of the event. In addition, any notation by the physician in the medical record of a confirmed relapse event, regardless of corticosteroid intervention, was counted as a relapse episode.
Data analysis
This study was primarily descriptive. Raw counts or percentages were calculated for categorical variables. Means, standard deviations, and medians were calculated for continuous variables. Data were analyzed cumulatively (0–3 months, 0–6 months, etc.) and by time interval (0–3 months, 4–6 months, etc.). Overall persistency was determined by the proportion of patients still being treated with the index iDMT at post-index cumulative time windows. The median censor-adjusted time to switch and median censor-adjusted time to discontinuation were computed. All analyses were conducted using SAS version 9.4 statistical software (Cary, NC).
Results
Study sites and patients
A total of 300 distinct medical records were abstracted from 18 US-based neurology clinics. The majority of the prescribing physicians were general neurologists in small or solo practices; most had ≥10 years of experience. The proportion of patients initiating each drug on the index date was 34% for glatiramer acetate, 31% for IM IFN β-1a, 21% for SC IFN β-1a, and 14% for SC IFN β-1b. The average age of the patients was 42 years and 74% were women (Table 1). The largest proportion of patients were from the Midwest region (44%), whereas only 4% of all patients were from the Western region of the US.
Table 1.
Demographics of iDMT patients.
| Characteristic | Total | Glatiramer acetate | IM IFN β-1a | SC IFN β-1a | SC IFN β-1b |
|---|---|---|---|---|---|
| Number of patients, (%) | 300 (100) | 103 (34) | 94 (31) | 62 (21) | 41 (14) |
| Female, % | 74 | 77 | 77 | 66 | 71 |
| Mean age, years ± SD | 42 ± 12 | 44 ± 12 | 41 ± 12 | 40 ± 11 | 39 ± 13 |
| Region, % | |||||
| Midwest | 44 | 39 | 44 | 53 | 42 |
| Northeast | 17 | 14 | 26 | 18 | 2 |
| South | 36 | 44 | 29 | 26 | 46 |
| West | 4 | 4 | 2 | 3 | 10 |
iDMT: injectable disease-modifying therapy; IFN: interferon; IM: intramuscular; SC: subcutaneous.
Treatment patterns
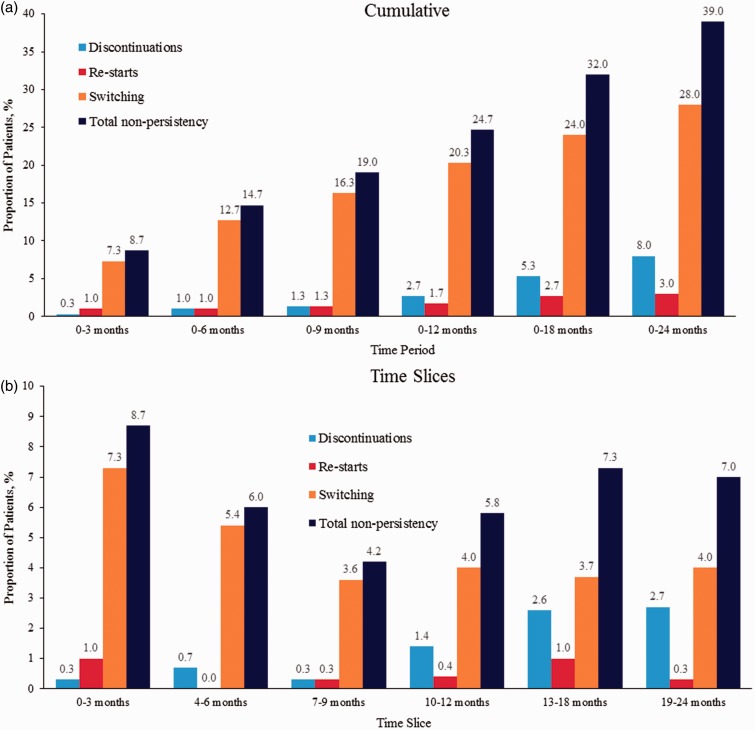
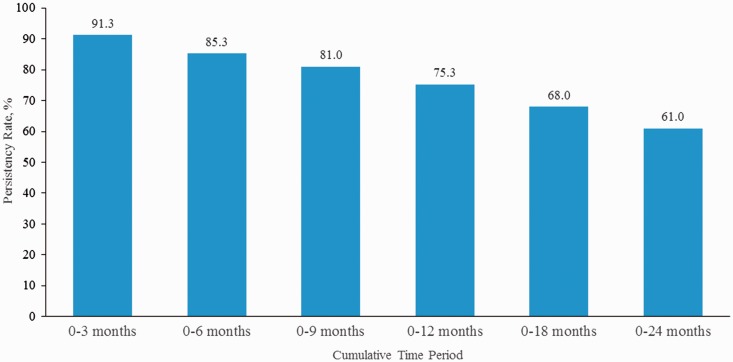
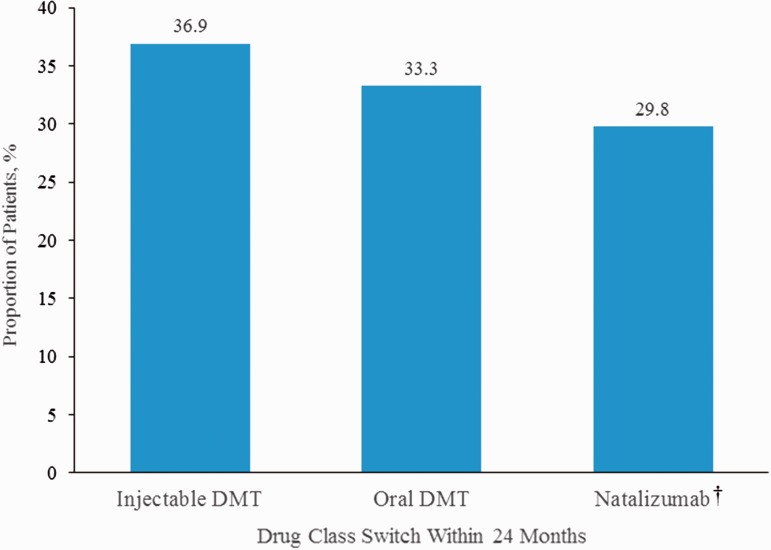
During the 24 months of follow-up, nearly one-third (28.0%) of patients had switched treatments; 8.0% had discontinued treatment, and 3.0% stopped and restarted the same iDMT, for a total non-persistency rate of 39.0% (Figure 1(a)). The overall persistency rate on iDMT medications declined from 91.3% at 3 months to 61.0% at 24 months from the time of initiation (Figure 2). Treatment switches occurred most frequently in the first three months of treatment, whereas discontinuations occurred later (Figure 1(b)). The median time to switch was 288 days and median time to discontinuation was 401 days. When patients switched treatment from the index iDMT, the switch was relatively evenly distributed to iDMTs, oral DMTs, and natalizumab (Figure 3).
Figure 1.
Proportion of patients with injectable disease-modifying therapy who were non-persistent, broken down into discontinuations, restarts, or switches (a) cumulatively over time and (b) over distinct time slices.
Figure 2.
Persistency rate of index injectable disease-modifying therapy over time.
Figure 3.
Proportion of patients who switched from iDMT (n = 84) by DMT drug class to which they switched. †There were no switches to infusion DMTs other than natalizumab. DMT: disease-modifying therapy; iDMT: injectable disease-modifying therapy.
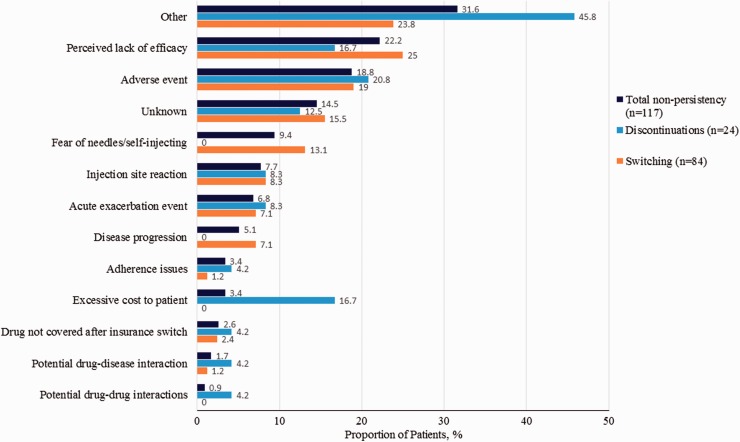
The most commonly identified reasons for non-persistency over the 24-month period were perceived lack of efficacy (22.2%), adverse events (18.8%), and fear of needles/self-injecting (9.4%; Figure 4). These same reasons were the most common reasons for non-persistency over the 0- to 6-month and 0- to 12-month periods. Although the study was not designed or powered to detect differences between persistent and non-persistent patients, the baseline characteristics of non-persistent patients were generally similar to persistent patients. However, non-persistent patients tended to be younger (mean age 39.9 vs 42.9 years, respectively; p = 0.04), require mobility assistance (crutch/cane/walker; p = 0.05), and have a greater incidence of tremors (12.8% vs 4.9%, respectively; p = 0.01).
Figure 4.
Reasons for injectable disease-modifying therapy total non-persistency, switching, and discontinuations among patients with these treatment changes over 24 months.
Clinical outcomes
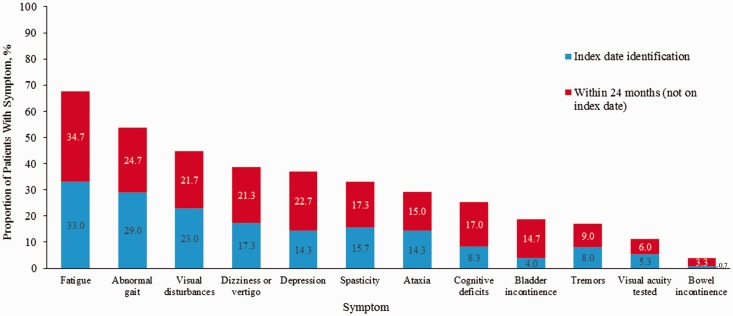
Physician-reported MRI-verified disease progression by month 24 was common. In all, 20.7% of patients had disease progression based on MRI evidence of new/enlarged lesions, 11.0% had a change in lesion count, and 8.3% had a change in level of brain atrophy. The percentage of patients experiencing a relapse increased over time from 30.7% at 12 months to 38.0% at 24 months. The average number of relapses increased from 0.42 (standard deviation = 0.73) at 12 months to 0.54 (standard deviation = 0.88) at 24 months. Patients who did not have MS-related symptoms at index reported increases in the incidence of these symptoms at 24 months (Figure 5).
Figure 5.
Incidence of symptoms at index date and within 24 months in patients who did not have symptoms at index.
Although the study was not powered to determine differences between non-persistent and persistent patients, a greater percentage of non-persistent patients had experienced a relapse compared with persistent patients at 24 months. Furthermore, non-persistent patients had a higher relapse rate, a higher percentage of patients with MRI disease progression (e.g. new/enlarged lesions, changes in lesion count, and a change in level of brain atrophy), and had a greater increase in the incidence of symptoms compared with persistent patients (Supplemental Table E2).
Discussion
This real-world medical chart review revealed that after initiating iDMT for RRMS, overall persistency decreased over time with 75% of patients persistent after 12 months and 61% persistent after 24 months. The most common reason for loss of persistency was switching to another DMT, which occurred much earlier than discontinuation. The most commonly documented reasons for non-persistency were perceived lack of efficacy, adverse events, and fear of needles/self-injecting. Although 61% of patients were persistent with their index treatment, in the overall study population, relapses, MRI disease progression, and incidence of MS-related symptoms all increased by the end of 24 months.
Non-persistency in the form of switching or discontinuation can have a notable negative impact on RRMS. Patients who switch may have a lapse between treatments and it can take several months for a DMT to reach full effectiveness,25 during which time disease may become more active. Lack of persistency to DMTs has been shown to significantly increase the likelihood of hospitalizations and emergency room visits.26 Furthermore, patients who are non-adherent to DMTs have a higher risk of relapse.27,28
In some patients, influenza-like symptoms associated with the IFN DMTs and injection-related tolerability issues may be barriers to persistency with iDMTs.9 In this study, adverse events and fear of needles/self-injecting were among the most common reasons for switching or discontinuing treatment. Oral DMTs may be a suitable treatment option for patients who are non-persistent to iDMT because of injection-related adverse events. However, clinicians and patients must weigh the respective benefit/risk profiles of oral DMTs when making treatment decisions. The timeline of this study was mainly after the introduction of the first oral DMT (fingolimod, 2010), which may have affected patient expectations regarding iDMT tolerability and physician prescribing. Other than the current study, few studies have investigated the persistency of iDMT since the introduction of oral DMTs. Warrender-Sparkes et al.24 conducted an international, observational cohort study of patients in the MSBase registry to assess the effect of the introduction of fingolimod on iDMT persistency. The study found that the likelihood of discontinuation increased significantly after fingolimod became available (hazard ratio = 1.64, p < 0.001 vs pre-fingolimod). The study also found that, consistent with the current analysis, persistency was approximately 80% one year after iDMT initiation, which decreased to 65% by year 2. In a retrospective cohort pharmacy claims study designed to compare compliance between fingolimod and iDMT, persistence with iDMT after one year was somewhat lower than in the current study, at approximately 50%. Another retrospective US claims database designed to compare the persistency of fingolimod with iDMT determined that the persistency of iDMTs was approximately 58% after one year.11 Based on “real-world” observational studies or retrospective database analyses, the two-year persistence of iDMT before the availability of oral DMTs ranged from 32% to 92%.12–14,17,20,22 Given the broad range of reported persistency with iDMT in the pre-oral DMT era, it is difficult to make any conclusions based on the current study regarding any change in the persistence rate since the introduction of oral DMTs. However, the data in this study indicate that persistence with iDMTs remains an issue.
In the current study, approximately one-third of the patients switched to an oral DMT, whereas 37% switched to another iDMT and 30% switched to natalizumab. These results are similar to those found by Warrender-Sparkes et al.,24 wherein 42% of patients switched to fingolimod and 37% switched to another iDMT. Many factors need to be considered when switching DMTs, including comorbidities, convenience, pregnancy, ability to comply with treatment, tolerability, and disease severity. In addition, because of formulary and insurance mandates, a step-wise approach to therapy may be required, with evidence of “failure” of an iDMT needed before a switch to an oral DMT or natalizumab will be covered.
Research indicates that pathologic damage occurs early in the course of RRMS, and early treatment reduces disease progression.29–33 As such, it seems prudent to consider a switch to a more efficacious treatment if patients are not responding well to initial treatment.34 The observed increases in the relapse rate and MRI changes in the current study may be indicators of natural disease progression, and may account for the 30% of patients who switched to natalizumab. Natalizumab has been shown to be more effective than iDMTs in patients with more active disease who switched from an iDMT,35 and may therefore be more often prescribed to these patients. In addition to natural disease progression, it is possible that the increases in the relapse rate and MRI changes could be reflective of the observed iDMT non-persistency. In the subgroup comparison of non-persistent vs persistent patients, the non-persistent patients had higher relapse rate, more symptoms, and more MRI disease progression. However, the study was not designed or powered to evaluate a comparison between non-persistent and persistent patients and the results should be interpreted with caution. The study could not determine if persistency led to better outcomes, or if non-persistent patients had more severe disease and thus discontinued their initial iDMT.
This study was subject to a number of limitations, many of which are related to the retrospective chart review study design. For example, there was a potential bias in the recording of observations since a number of different abstractors collected the information. However, an abstraction manual was developed and used for training, after which an inter-rater reliability assessment was completed by each abstractor in order to reduce this bias. The medical records may have been missing data as clinician input can be variable and pharmacy claims were not confirmed for refills, switches, restarts, or discontinuations. Thus, without actual prescription fill data for verification, persistency may have been over-reported. The neurology practices and medical records were not randomly selected and may not be representative of national prescription and neurology practice patterns. Finally, the sample size was small and under-represented the Western region of the US. Subsequently, there was low power for more robust comparative analyses of the iDMT cohorts identified and followed in this study.
Conclusions
Over 24 months, 39% of patients were non-persistent on their initial iDMT, indicating that persistency of iDMTs in the age of oral DMTs remains an issue. Among the 61% of patients who remained persistent after 24 months, many still experienced relapses, MRI disease progression, and MS-related symptoms. The fairly high rate of disease progression as evidenced by reported relapses and changes in adverse MRI findings, combined with a higher MS-related symptom burden and low persistency rate for iDMT, suggests that treatment with DMTs that are more efficacious should be considered in some patients. More efficacious treatments with a higher persistency rate may have more profound effects on prevention of disease progression, and therefore, decrease MS-related symptom burden. A greater understanding of reasons for non-persistency such as treatment-related tolerability, adverse events, and effectiveness of iDMT agents at distinct time points may also inform appropriate treatment selection and management.
Supplementary Material
Acknowledgments
The study sponsor was involved in the study design, interpretation of the data, and writing of the manuscript. The decision to submit the manuscript was made by the authors. The authors would like to thank Tonya Green, Jennifer Welstead, Peri Barr, and Riad Elmor of Indegene for their efforts with site recruitment, data abstraction, and data cleaning. Medical writing and editorial assistance was provided by Erin P. Scott, PhD, of Scott Medical Communications, LLC. This assistance was funded by Novartis.
Conflicts of interest
J. Nicholas, P. Navaratnam, H.S. Friedman, and F. Ernst are paid consultants for Novartis Pharmaceuticals Corporation. J.J. Ko, J. Park, and V. Herrera are employees of Novartis Pharmaceuticals Corporation.
Funding
This work was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ.
References
- 1.Wingerchuk DM, Carter JL. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc 2014; 89: 225–240. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen PS. New management algorithms in multiple sclerosis. Curr Opin Neurol 2014; 27: 246–259. [DOI] [PubMed] [Google Scholar]
- 3.Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998; 352: 1498–1504. [PubMed]
- 4.Interferon beta-1b is effective in relapsing–remitting multiple sclerosis: I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993; 43: 655–661. [DOI] [PubMed]
- 5.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996; 39: 285–294. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing–remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995; 45: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 7.Kalincik T, Jokubaitis V, Izquierdo G, et al. Comparative effectiveness of glatiramer acetate and interferon beta formulations in relapsing–remitting multiple sclerosis. Mult Scler 2015; 21: 1159–1171. [DOI] [PubMed] [Google Scholar]
- 8.Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol 2013; 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: Tolerability and adherence. Mult Scler 2012; 18: 932–946. [DOI] [PubMed] [Google Scholar]
- 10.Crayton H, Steingo B, Huang D, et al. Real-world patient retention and satisfaction on fingolimod versus platform injectable disease-modifying therapies in early relapsing–remitting multiple sclerosis: Results from PREFERMS. Congress of the American Academy of Neurology. Vancouver, Canada, 2016.
- 11.Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: A retrospective US claims database analysis. J Med Econ 2014; 17: 696–707. [DOI] [PubMed] [Google Scholar]
- 12.Evans C, Tam J, Kingwell E, et al. Long-term persistence with the immunomodulatory drugs for multiple sclerosis: A retrospective database study. Clin Ther 2012; 34: 341–350. [DOI] [PubMed] [Google Scholar]
- 13.Hansen K, Schüssel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: A retrospective cohort study. PLoS One 2015; 10: e0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jokubaitis VG, Spelman T, Lechner-Scott J, et al. The Australian Multiple Sclerosis (MS) immunotherapy study: A prospective, multicentre study of drug utilisation using the MSBase platform. PLoS One 2013; 8: e59694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalincik T, Spelman T, Trojano M, et al. Persistence on therapy and propensity matched outcome comparison of two subcutaneous interferon beta 1a dosages for multiple sclerosis. PLoS One 2013; 8: e63480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinman NL, Beren IA, Rajagopalan K, et al. Medication adherence with disease modifying treatments for multiple sclerosis among US employees. J Med Econ 2010; 13: 633–640. [DOI] [PubMed] [Google Scholar]
- 17.Oleen-Burkey M, Cyhaniuk A, Swallow E. Retrospective US database analysis of persistence with glatiramer acetate vs. available disease-modifying therapies for multiple sclerosis: 2001–2010. BMC Neurol 2014; 14: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portaccio E, Zipoli V, Siracusa G, et al. Long-term adherence to interferon beta therapy in relapsing–remitting multiple sclerosis. Eur Neurol 2008; 59: 131–135. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds MW, Stephen R, Seaman C, et al. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin 2010; 26: 663–674. [DOI] [PubMed] [Google Scholar]
- 20.Río J, Porcel J, Téllez N, et al. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler 2005; 11: 306–309. [DOI] [PubMed] [Google Scholar]
- 21.Syed M, Rog D, Parkes L, et al. Patient expectations and experiences of multiple sclerosis interferon beta-1a treatment: A longitudinal, observational study in routine UK clinical practice. Patient Prefer Adherence 2014; 8: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong J, Gomes T, Mamdani M, et al. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci 2011; 38: 429–433. [DOI] [PubMed] [Google Scholar]
- 23.Zhornitsky S, Greenfield J, Koch MW, et al. Long-term persistence with injectable therapy in relapsing–remitting multiple sclerosis: An 18-year observational cohort study. PLoS One 2015; 10: e0123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warrender-Sparkes M, Spelman T, Izquierdo G, et al. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Mult Scler 2016; 22: 520–532. [DOI] [PubMed] [Google Scholar]
- 25.Rich SR, Coleman IC, Cook R, et al. Stepped-care approach to treating MS: A managed care treatment algorithm. J Manag Care Pharm 2004; 10(3 Suppl B): S26–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas NP, Curkendall S, Farr AM, et al. The impact of persistence with therapy on inpatient admissions and emergency room visits in the US among patients with multiple sclerosis. J Med Econ 2016; 19: 497–505. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg SC, Faris RJ, Chang CF, et al. Impact of adherence to interferons in the treatment of multiple sclerosis: A non-experimental, retrospective, cohort study. Clin Drug Investig 2010; 30: 89–100. [DOI] [PubMed] [Google Scholar]
- 28.Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011; 28: 51–61. [DOI] [PubMed] [Google Scholar]
- 29.Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–285. [DOI] [PubMed] [Google Scholar]
- 30.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: A randomised study. Lancet 2001; 357: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 31.Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): A randomised, double-blind, placebo-controlled trial. Lancet 2009; 374: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 32.Comi G, Martinelli V, Rodegher M, et al. Effects of early treatment with glatiramer acetate in patients with clinically isolated syndrome. Mult Scler 2013; 19: 1074–1083. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med 2000; 343: 898–904. [DOI] [PubMed] [Google Scholar]
- 34.Ziemssen T, De Stefano N, Pia Sormani M, et al. Optimizing therapy early in multiple sclerosis: An evidence-based view. Mult Scler Relat Disord 2015; 4: 460–469. [DOI] [PubMed] [Google Scholar]
- 35.Spelman T, Kalincik T, Zhang A, et al. Comparative efficacy of switching to natalizumab in active multiple sclerosis. Ann Clin Transl Neurol 2015; 2: 373–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.