Abstract
Dictyostelium contains two guanylyl cyclases, GCA, a 12-transmembrane enzyme, and sGC, a homologue of mammalian soluble adenylyl cyclase. sGC provides nearly all chemoattractant-stimulated cGMP formation and is essential for efficient chemotaxis toward cAMP. We show that in resting cells the major fraction of the sGC-GFP fusion protein localizes to the cytosol, and a small fraction is associated to the cell cortex. With the artificial substrate Mn2+/GTP, sGC activity and protein exhibit a similar distribution between soluble and particulate fraction of cell lysates. However, with the physiological substrate Mg2+/GTP, sGC in the cytosol is nearly inactive, whereas the particulate enzyme shows high enzyme activity. Reconstitution experiments reveal that inactive cytosolic sGC acquires catalytic activity with Mg2+/GTP upon association to the membrane. Stimulation of cells with cAMP results in a twofold increase of membrane-localized sGC-GFP, which is accompanied by an increase of the membrane-associated guanylyl cyclase activity. In a cAMP gradient, sGC-GFP localizes to the anterior cell cortex, suggesting that in chemotacting cells, sGC is activated at the leading edge of the cell.
INTRODUCTION
In the social amoeba Dictyostelium, cGMP is implicated as a second messenger for chemotaxis (van Haastert and Kuwayama, 1997). Dictyostelium cells grow on bacteria. In the absence of the food source, Dictyostelium induces a developmental program leading to a multicellular fruiting body. Growing cells chemotax toward folic acid, a compound secreted by various bacteria, whereas cells starved for ∼5 h respond to cAMP, a compound secreted by other Dictyostelium cells. The later response is necessary for cell aggregation. Recently two guanylyl cyclases (GCs), GCA and sGC, have been cloned in Dictyostelium. GCA consists of 12 putative transmembrane regions and 2 cyclase domains, which is similar to the topology of mammalian membrane-bound adenylyl cyclase (Roelofs et al., 2001b). sGC has two cyclase domains as well, but does not have any transmembrane regions and the topology is similar to sAC, a soluble adenylyl cyclase found in mammals (Roelofs et al., 2001a). A Dictyostelium cell line in which the two genes encoding these guanylyl cyclases were inactivated has no detectable GC activity (Roelofs and Van Haastert, 2002), indicating that these two cyclases are responsible for all cGMP production. This double knockout gca-/sgc- cell line shows strongly reduced chemotaxis (Bosgraaf et al., 2002). Furthermore, this mutant established that cGMP mediates the formation of myosin filaments at the cortex, which is important for retraction of the uropod at the back of the cell and inhibition of pseudopodia at the sides of the cell.
Stimulation of Dictyostelium cells with folic acid or cAMP induces rapid increases of cGMP levels, with a peak at ∼10 s after stimulation. The folic acid and cAMP-induced cGMP responses are maximal during growth and 5-h starvation, respectively. Because only two guanylyl cyclases, GCA and sGC, are present in Dictyostelium, the single knockouts can be used to study the function and biochemical properties of the other guanylyl cyclase. It has been shown that both enzymes contribute to the folic acid and cAMP induced cGMP responses, but maximal stimulation of GCA (2.5-fold) is much smaller than maximal stimulation of sGC (8-fold; Roelofs and Van Haastert, 2002). The mRNA expression of GCA is maximal during growth and decreases during early development, whereas mRNA levels of sGC increase to a maximum at 6 h of cell development. These differences in expression and activation explain why the cGMP response that is mediated by cAMP is stronger than the cGMP response induced by folic acid. Furthermore, it was calculated that sGC contributes ∼60 and 90% to the folic acid– and cAMP-mediated cGMP responses, respectively (Roelofs and Van Haastert, 2002).
After cell fractionation, ∼80% of Mn2+-dependent sGC activity is located in the supernatant and the remaining 20% resides in the pellet. Surprisingly, when measuring the same fractions with Mg2+/GTP as substrate, the activity in the supernatant is extremely low (∼3% of Mn2+-dependent activity), whereas the activity in the pellet is approximately the same as with Mn2+/GTP. For adenylyl cyclases it has been shown that Mn2+/GTP stimulates enzymatic activity in the absence of G proteins, but that coupling of adenylyl cyclase to G proteins requires Mg2+/GTP as substrate (Ross et al., 1978; Tesmer and Sprang, 1998). In accordance with this notion, we observed that activation of G-proteins with GTPγS enhances Dictyostelium sGC activity with Mg2+-GTP as the substrate, but not with Mn2+-GTP. In vivo Mg2+ is most likely the physiological relevant cation, because at the prevailing ion concentration in cells (3.5 mM Mg2+ and 10 μM Mn2+) only Mg2+ supports substantial guanylyl cyclase activity. (Padh and Brenner, 1984). This suggests that in vivo the major fraction of sGC resides in the cytosol where it is uncoupled and in an inactive state, whereas a small fraction is membrane-associated and at least partly active.
Based on the large inactive pool of sGC in the cytosol, we reasoned that translocation of inactive soluble sGC to the membrane may contribute to the observed increase of sGC activity. Therefore, we have investigated the localization of the sGC activity and the sGC protein in resting cells and after chemoattractant stimulation. Our results provide the first evidence that Dictyostelium uses the balance between membrane-associated and cytosolic sGC as a mechanism to regulate its guanylyl cyclase activity in vivo.
MATERIALS AND METHODS
Strain and Culture Conditions
AX3 (“wild-type”), gca- null cells (Roelofs et al., 2001b), gca-/sgc- double-null (Roelofs and Van Haastert, 2002) and sGC-GFPoe cells (see below) were grown in HG5 medium. When grown with selection, HG5 medium was supplemented with 10 μg/ml blasticidine S or 10 μg/ml G418. Previous to cGMP activity assays and fluorescence microscopy, cells were starved for 5 h by shaking in 10 mM phosphate buffer, pH 6.5 (PB) at a density of 107 cells/ml.
Construction of sGC-GFP Expression Vector and Visualization of sGC-GFP
A partial cDNA of sGC, spanning base pairs 1195–6339, was used as starting material to construct the complete 8529-base pair open reading frame of sGC. The missing 5′ and 3′ fragments of the gene were obtained by PCR from genomic AX3 DNA. Immediately preceding the start codon in the 5′ fragment, a BamHI site and a Kozak sequence were introduced, by using the primer GCGCGGATCCAAAATGGAAAATGTATCAAGCC in combination with an internal sGC primer. In the missing 3′ fragment, the stop codon was disrupted and a small linker with a BamHI site was introduced by using the PCR primer GCGCGGATCCTTCTAATTCTTCTTTCCA together with an internal sGC primer. The PCR fragments were purified and ligated to the cDNA using endogenous restriction sites in the overlapping regions. The resulting vector, containing the complete open reading frame of sGC, was named pBK-CMV/sGC. Sequence analysis confirmed that the constructed sgc gene encoded the previously reported protein (entrez protein AAK92097; Roelofs et al., 2001b).
For the expression of the GFP-fusion protein, a new Dictyostelium expression vector, MB74-GFP, was constructed from MB12neo (Linskens et al., 1999). The MB74-GFP vector contains an A15 promoter, subsequently followed by a small multiple cloning site (AGATCTACTAGT), the GFP gene, and an A8 terminator. The complete sgc open reading frame was excised from pBK-CMV/sGC with BamHI and cloned into the BglII site of MB74-GFP. The resulting MB74/sGC-GFP vector was transfected to the gca-/sgc- cell strain, yielding the sGC-GFPoe strain.
Localization of sGC-GFP in sGC-GFPoe cells was visualized with a Zeiss LSM510 (Carl Zeiss, Oberkochen, Germany) confocal fluorescence microscope using a Plan-Neofluar 40× 1.3 NA oil immersion objective and a 73-μm pinhole. For global cAMP stimulation, we settled the starved cells in a custom-made flow chamber and perfused the chamber with 10-6 M cAMP in PB. To study the localization of the sGC-GFP in a cAMP gradient, we settled a droplet of cell suspension on a coverslip and locally stimulated the cells with a femtotip (Eppendorf, Hamburg, Germany) containing 10-6 M cAMP in PB. Fluorescence from 505 to 550 nm was recorded every 2 s in 12-bit grayscale and analyzed using Photoshop (San Jose, CA; www.adobe.com) and Matlab (MathWorks, Natick, MA; www.mathworks.com).
We determined the average cytosolic fluorescence intensity by averaging the pixel values of the cytosol, excluding pixels in the nucleus and internal vesicles with low fluorescence (Postma et al., 2003). The width of the membrane is ∼7 nm. Because the pixel size of the obtained images is 220 nm, even pixels in the membrane region of the cell consist mainly of cytosol. To calculate the membrane-associated fluorescence, we therefore subtracted the average cytosolic fluorescence intensity from each cell pixel and summarized the remaining fluorescence.
In vivo cGMP Formation
After starvation for the indicated times, cells were washed in PB, resuspended to a density of 1 × 108 cells/ml in PB containing 2 mM caffeine, and aerated for at least 10 min. Cells were stimulated by adding 10 μl cAMP solution to 40 μl of cell suspension, yielding a final concentration of 100 nM cAMP. The reaction was stopped by adding 40 μl of stimulated cell suspension to an equal volume of 3.5% perchloric acid at subsequent time points. Samples were neutralized by adding 20 μl 50% saturated KHCO3 and incubated for 5 min at room temperature to allow the CO2 to escape. After checking the correct pH, the samples were centrifuged 5 min at 500 × g and the cGMP levels of the supernatant were determined using the cGMP-[3H] Biotrak Assay kit (Amersham, Amersham, Unite Kingdom) according to manufacturer's instructions as detailed in Snaar-Jagalska and Van Haastert (1994).
In Vitro Guanylyl Cyclase Assay
After starvation, cells were washed and resuspended in 10 mM Tris·Cl, pH 8.0, to a density of 2 × 108 cells/ml. All subsequent steps were performed at 4°C and are described below for the standard procedure; deviations from this protocol are described in the legends to the figures. One volume of cell suspension was mixed with one volume of lysis buffer (LB) yielding 15 mM Tris, 250 mM sucrose, 3 mM [ethylenebis (oxyethylenenitrilo)] tetra-acetic acid (EGTA), pH 8.0. When guanylyl cyclase activity was measured with Mg2+, the LB was always supplemented with 0.1 mM guanosine 5′-O-3-thiotriphosphate (GTPγS) to activate G proteins. After lysis through a nucleopore filter, the lysate was fractionated by centrifugation at 14,000 × g for 1.5 min. Pellets were resuspended in LB or reconstituted with supernatant fractions as described in the legends of the figures. Guanylyl cyclase assays were performed at 22°C with 50 μl cell fraction in a total volume of 100 μl containing 15 mM Tris, pH 8.0, 250 mM sucrose, 0.5 mM GTP, 10 mM 1,4-dithio-threithol (dithiothreitol), 100 μM 3-isobutyl-1-methylxanthine (IBMX), 1.5 mM EGTA, and 2.5 mM MgSO4 or 2.5 mM MnCl2 (final concentrations). When measured with Mg2+, GTPγS was present at a final concentration of 50 μM. GC assays were performed for 20, 40, and 60 s and were terminated by addition of 40-μl assay mixture to an equal volume of 3.5% perchloric acid. Samples were neutralized and assayed for cGMP content as described in the in vivo assay (Snaar-Jagalska and Van Haastert, 1994).
RESULTS
Localization of sGC Enzyme and sGC Activity
To investigate the localization of sGC during chemotaxis we expressed a sGC-GFP fusion protein in gca-/sgc- cells that lack all GC activity. An in vitro assay of the resulting sGC-GFPoe cell strain in the presence of 2 mM Mn2+ showed that expression of the cloned gene restored the ability of gca-/sgc- cells to produce cGMP (Figure 1A). Moreover, the restored activity was stimulated in vivo by extracellular cAMP (Figure 1B). The cGMP-response to osmotic shock and folic acid was recovered in a similar manner (unpublished data). These data confirm that the constructed sGC-GFP fusion protein is active and sensitive to extracellular stimuli.
Figure 1.
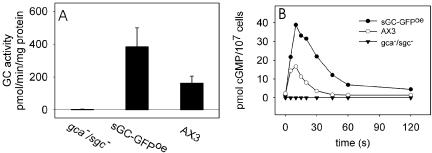
Guanylyl cyclase activity of the sGC-GFP fusion protein. A plasmid encoding GFP fused to the C-terminus of sGC was introduced in gca-/sgc- null cells to obtain the sGC-GFPoe strain. (A) Guanylyl cyclase activity in vitro; gca-/sgc-, sGC-GFPoe, and wild-type AX3 cells were lysed, and Mn2+-dependent activity was measured in the lysates. The mean activity and SD are shown of two independent experiments, each with lysis in triplicate. (B) cGMP formation in vivo; 5-h–starved cells were stimulated with 100 nM cAMP. At the indicated time points, the cells were lysed and the amount of accumulated cGMP was determined. The data shown are the means of three experiments.
We visualized the localization of the sGC-GFP fusion protein using confocal fluorescence microscopy. The expression of this large 343-kDa sGC-GFP protein is rather low when compared with the expression of free GFP using the same expression vector (unpublished data), requiring relative long exposure times to obtain sufficient signal. Nevertheless, the approximately twofold higher cyclase activity of sGC-GFPoe cells compared with wild-type cells suggests the expression levels are somewhat higher than that of endogenous sGC in wild-type cells (Figure 1A). In resting cells, fluorescent sGC-GFP was found partly in the cytosol and partly at the membrane, but fluorescence was excluded from the nucleus (Figure 2A). To obtain the average fluorescence intensity of the cytosol, a large cytosolic area of the cell was selected, excluding the nucleus and vesicles when present (Figure 2B). We obtained the membrane-associated fluorescence by subtracting the value of the average fluorescence intensity of the cytosol from each cell pixel (Figure 2C). By summarizing the total amount of fluorescence of a cell (cf. Figure 2A) and the fluorescence at the membrane (cf. Figure 2C) we calculated for 11 resting cells that in the confocal plane, 13 ± 2% of the cellular fluorescence is membrane-associated and the remaining 87% is cytosolic. A control cell strain expressing free GFP showed no extrafluorescence at the cell membrane (-3 ± 2%; n = 6).
Figure 2.
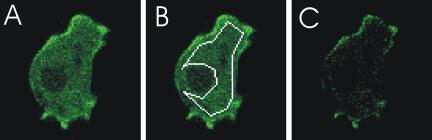
Analysis of sGC-GFP localization in the cytosol and at the membrane. (A) Confocal image of 5-h–starved sGC-GFPoe cells; exposure time is 8 s. The fluorescence intensity of the cytosol is rather homogeneous, but at the membrane fluorescence was nonuniformly distributed and predominantly found in protrusions. (B) The average fluorescence intensity of the cytosol was determined by averaging the pixel values of a large area of the cell containing only cytosol pixels. This value was subtracted from each pixel in A. The remaining fluorescence, that is mainly membrane-associated, is displayed in C.
To investigate whether the localization of sGC-GFP is correlated with the activity, we measured the guanylyl cyclase activity of sGC-GFP in the particulate and supernatant fractions of cell lysates. Mn2+-dependent sGC-GFP activity is present for ∼75% in the supernatant fraction and for ∼25% in the particulate fraction of a cell lysate (Figure 3), which is essentially identical to the distribution of the endogenously expressed sGC (Roelofs and Van Haastert, 2002). This distribution of Mn2+-dependent enzyme activity is similar to the distribution of the sGC-GFP protein, confirming the general notion that Mn2+ uncovers the intrinsic cyclase activity (Ross et al., 1978). Previously we have discussed that the Mn2+ concentration needed for this activity (2–3 mM) is 200 times higher than the Mn2+ concentration in Dictyostelium cells (∼10 μM; Padh and Brenner, 1984), suggesting that this activity is not physiologically relevant. When Mn2+ is replaced by 2.5 mM Mg2+, which is close to the cellular Mg2+ concentration of 3.5 mM (Padh and Brenner, 1984), the activity of endogenous sGC in the particulate fraction remained approximately the same, whereas the activity in the supernatant fraction decreases ∼30-fold (Roelofs et al., 2001a). For the sGC-GFPoe cell strain we observe similar features. When guanylyl cyclase is measured with Mg2+ instead of Mn2+, the activity in the supernatant decreases dramatically, whereas the activity in the membrane fraction increases ∼2–3-fold (Figure 3). This indicates that for both endogenous sGC and ectopically expressed sGC-GFP nearly all the physiologically relevant Mg2+-dependent GC activity is localized at the membrane and a large reservoir of presumably inactive sGC is present in the cytosol.
Figure 3.
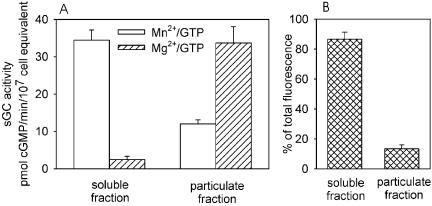
Localization of sGC activity and sGC protein. (A) Distribution of Mn2+- and Mg2+-dependent GC activity in sGC-GFPoe cells. Five-hour–starved cells were lysed and separated in a soluble and particulate fraction by centrifuging for 1.5 min at 14,000 × g. Each fraction was assayed for sGC activity with either 2.5 mM Mn2+ (open bars) or 2.5 mM Mg2+ (diagonally filled bars). Error bars represent the SD of two independent experiments, each with lysis and fractionation in triplicate. (B) The amount of fluorescence in the cytosol and at the membrane of sGC-GFPoe cells, measured in a confocal slice of ∼1 μm thick as described in Figure 2.
In the following paragraphs we wish to determine the effect of known regulators of guanylyl cyclase on the distribution of sGC protein and sGC activity between particulate and soluble fractions. Previously it has been demonstrated that sGC activity measured with Mg2+ is modulated by several compounds, such as cAMP-stimulation, GTPγS, and Ca2+. Therefore, the Mg2+-dependent sGC activity in the pellet fraction may increase by a combination of sGC protein translocated from the cytosol and activation of existing membrane-associated protein. Because the Mn2+-dependent sGC activity is not altered by cAMP stimulation, GTPγS, and Ca2+ (Roelofs et al., 2001b), a shift of Mn2+-dependent activity between supernatant and particulate fraction can be attributed to a shift in protein distribution. Furthermore, a depletion of sGC in the cytosol can only be measured biochemically with Mn2+, because the enzyme in the cytosol shows very low activity with Mg2+.
Uniform cAMP Stimulation Induces Transient Membrane Association of sGC
cAMP induces a transient rise of cGMP levels in vivo with a maximum at 10 s after stimulation and recovery of basal cGMP levels after ∼30 s. To test whether cAMP induces the translocation of sGC to the membrane, we stimulated 5-h starved sGC-GFPoe cells in a custom-made flow chamber with 10-6 M cAMP. After stimulation, a short jump of fluorescent sGC to the membrane was observed (Figure 4A). We visualized the kinetics of the transient membrane association by plotting the average fluorescence intensity of the cytosol after cAMP stimulation (Figure 4B). Results show that during the first 6 s after stimulation, the fluorescence intensity in the cytosol does not change relative to prestimulation levels. Depletion of fluorescence intensity starts at 8 s after stimulation, reaches a maximum at 10 s, and has recovered to prestimulation levels at 14 s after stimulation. At 10 s after stimulation the decrease of cytosolic fluorescence is 21 ± 6% (n = 5) relative to the cytosolic fluorescence before stimulation (Figure 4B). The membrane-associated fluorescence before stimulation and at 10 s after stimulation was calculated as described above. In unstimulated cells, 12% ± 7% of the total sGC was membrane-associated. At 10 s after stimulation the fraction of membrane-associated sGC in the confocal plane increased to 23 ± 5%, whereas the total cellular fluorescence remained the same (102 ± 17%).
Figure 4.
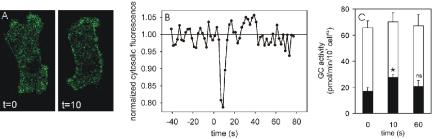
cAMP-dependent translocation of sGC to the membrane. Starved sGC-GFPoe cells were settled in a perfusion chamber, stimulated with 10-6 M cAMP at t = 0 s, and a sequence of images was recorded with an exposure time of 2 s per frame. (A) A cell just before cAMP stimulation and at 10 s after stimulation. (B) The fluorescence intensity of sGC-GFP in the cytosol after cAMP stimulation is presented relative to the cytosolic fluorescence intensity before cAMP stimulation. (C) Translocation of sGC activity. Cells in suspension were stimulated at t = 0 s with 0.1 μM cAMP at 22°C and lysed at 0°C at the indicated time points. The lysate was centrifuged and Mn2+-dependent sGC activity was determined in the pellets (filled bars) and supernatants (open bars on top of filled bars). The activity in the pellet at t = 10 s is significantly higher compared with t = 0 s (* Student's t test at p < 0.05); at t = 60 s the difference is not significant (ns). The data shown are the means and SE of the means of two experiments each performed with lysis in triplicate.
This transient membrane association of sGC-GFP could also be detected for endogenous sGC by measuring the Mn2+-dependent activity of the particulate and supernatant fractions of gca- cell lysates. gca- cells lack the second Dictyostelium guanylyl cyclase GCA, thereby allowing investigation of the remaining sGC activity (Roelofs et al., 2001b). gca- cells stimulated with 0.1 μM cAMP and lysed at 10 s after stimulation showed a 61 ± 10% increase of membrane localized Mn2+-dependent sGC activity compared with unstimulated cells (Figure 4C). Total sGC activity was not significantly different between both conditions, as should be expected for redistribution of Mn2+-dependent activity between cytosol and membrane fractions. Cell lysis at 60 s after cAMP stimulation, when cGMP has returned to basal levels, yielded a distribution of sGC activity between particulate and soluble fraction that was similar to the distribution before cAMP stimulation.
Translocation of sGC-GFP to the leading edge in a cAMP gradient
sGC-GFPoe cells were deposited on a coverslip and stimulated with a micropipette containing 10-6 M cAMP. Phase contrast and confocal fluorescent images were recorded every 2 s (see Supplementary Video). One image is presented in Figure 5A. The migrating cell shows elevated concentrations of sGC-GFP at the leading edge. We analyzed the concentration of sGC-GFP along the direction of movement for this sequence of images. In each image the cell was subdivided in 20 slices of equal width perpendicular to the axis of the cAMP gradient. The average fluorescence intensity of each slice was quantified and is presented relative to the fluorescence intensity of the cytosol (Figure 5B). The plot clearly shows elevated levels of sGC-GFP at the leading edge of the cell. The results imply that cAMP induces membrane localization of sGC, which accumulates at the leading edge in a cAMP gradient.
Figure 5.
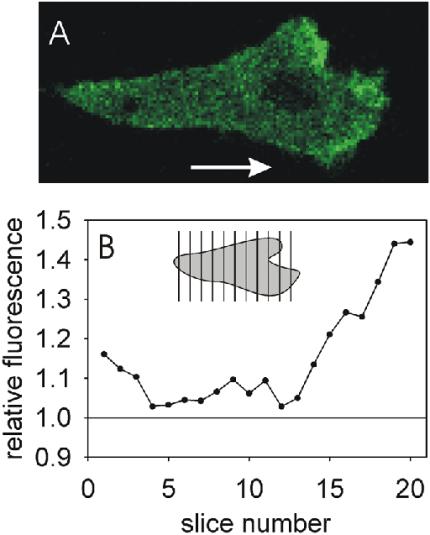
Translocation of sGC-GFP to the leading edge in a cAMP gradient. Five-hour–starved sGC-GFPoe cells were locally stimulated with a micropipette containing 10-6 M cAMP. A movie was recorded with a 2-s exposure of each frame (Supplementary Video). (A) One frame of this movie with a cell moving in the direction of the arrow. (B) The cell was divided in 20 slices of equal width, perpendicular to the direction of the gradient. The average fluorescence intensity of each slice is presented relative to the average fluorescence intensity of the cytosol. Data shown are the average of 15 frames of the chemotaxing cell.
Membrane Association Stimulates sGC Activity
To test if membrane-association induces Mg2+-dependent sGC activity, we mixed the membranes of gca-/sgc- cells that have no background GC activity (<0.25 pmol/min/mg; Figure 6) with soluble sGC from the supernatant fraction of gca- cells that has a small Mg2+-dependent sGC activity (2.2 ± 0.32 pmol/min/107 cell equivalent). After combining this supernatant and particulate fractions, GC activity increased almost fivefold to 9.7 ± 1.4 pmol/min/107 cell equivalent (Figure 6). When this mixture is separated again in a particulate and a supernatant fraction, sGC activity is mainly present in the particulate fraction (6.7 ± 1.6 pmol/min/107 cell equivalent), whereas the activity in the supernatant fraction is reduced to 1.2 ± 0.16 pmol/min/107 cell equivalent. These reconstitution data imply that soluble sGC associates to membranes, which leads to the formation of an Mg2+ active state of the enzyme.
Figure 6.
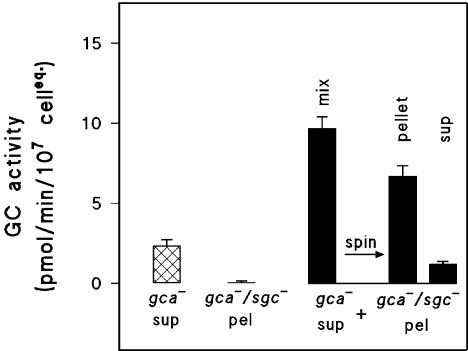
Reconstitution of membrane-associated sGC by mixing the supernatant of a gca- lysate with the membranes from a gca-/sgc- lysate. A supernatant fraction of gca- cells was prepared, and Mg2+-dependent sGC activity was measured (crossed bars). Simultaneously, a membrane fraction of gca-/sgc- cells showed no guanylyl cyclase activity (second bar). The supernatant of gca- lysates was combined with the membrane fraction of gca-/sgc- lysates, which results in an enhanced activity (first filled bar). When this mixture was separated in a pellet and a supernatant, most activity is now present in the pellet and only little in the supernatant (second and third filled bar). Experimental details in addition to the general procedure in Materials and Methods: Cells were lysed at a density of 108 cells/ml. The pellet of the gca-/sgc- lysate was dissolved in either lysis buffer (LB) or in the supernatants of the gca- lysate. The activities are expressed as pmol/min/equivalent of 107 cells, to allow a direct comparison of the cGMP forming activities in the different preparations. The data shown are the means and SE of the means of two independent experiments each performed with lysis and reconstitution in triplicate.
Saturation of Membrane-localized sGC-binding Sites
We investigated the potential of membranes to bind and activate sGC by mixing a dilution series of gca- supernatant with a constant amount of gca-/sgc- membranes. Mg2+-dependent sGC activity was measured in the gca- supernatant, in the reconstituted mixture of gca- supernatant and gca-/sgc- membranes, and in the pelleted fraction of this reconstituted mixture (Figure 7). Increasing amounts of gca- supernatants yielded a linear increase of sGC activity, as can be expected for this nonregulated cytosolic activity. In the reconstituted mixtures a strong increase of Mg2+-dependent sGC activity was observed at low amounts of the supernatant fraction that leveled off at higher amounts. When these mixtures were centrifuged, the enhanced sGC activity was recovered in the pellets. At increasing amounts of cytosolic fraction, the sGC activity in the recovered particulate fraction increased gradually up to a cytosol/membrane ratio of ∼0.5. Higher ratios did not result in a further increase of pelletable sGC activity and may even result in a slight reduction.
Figure 7.
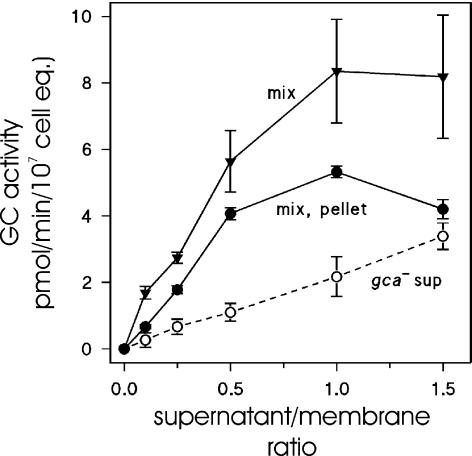
Saturation of sGC-binding sites in membranes from gca-/sgc- cells. gca- cells (1.5 × 108 cells/ml) were lysed and the soluble fraction was obtained. Simultaneously, gca-/sgc- cells were lysed, and the pellet was resuspended in LB to a density equivalent to 5 × 108 cells/ml. Thus, on a cell basis, the particulate fraction is 3.3-fold more concentrated than the soluble fraction. Membranes (25 μl) were incubated with 0–125 μl supernatant in a total volume of 150 μl. After 1 min on ice, half of the mixture was centrifuged and the pellet was resuspended to the original volume in LB. Mg2+-dependent sGC activity was determined in the mixture (mix) and in the obtained pellet. As a control, the Mg2+-dependent sGC activity was measured in the gca- supernatant (sup) at the same dilutions as in the mixtures. The x-axis refers to the ratio of supernatant and membranes on a cell-cell basis; 25 μl membranes was derived from 1.25 × 107 cells, whereas 125 μl supernatant was derived from 1.87 × 107 cells; thus, the supernatant/membrane ratio is 1.5. The data shown are the means and SEs of the means of two experiments with lysis and reconstitutions in triplicate.
The experiment of Figure 7 demonstrates that gca-/sgc- membranes contain a limited number of sGC binding sites that saturate in vitro at a cytosol/membrane ratio of ∼0.5. This suggests that in vivo, at a normal cytosol/membrane ratio of 1.0, the amount sGC protein in the cytosol will saturate sGC-binding sites in the membrane. Therefore, the cytosol contains a large pool of sGC and the creation of extra-sGC binding sites would directly result in additional association of sGC to the membrane and consequently enhanced Mg2+-dependent sGC activity.
Regulation by Ca2+ and GTPγS
Because previous studies have shown a strong inhibition of Mg2+-dependent sGC activity by nanomolar Ca2+ concentrations in Dictyostelium lysates (Janssens and de Jong, 1988; Janssens et al., 1989; Valkema and Van Haastert, 1992), the effect of calcium ions on the distribution of sGC activity was tested in gca- cells. Cells were lysed in the presence of 1 μM free Ca2+ or in the presence of 3 mM EGTA, which reduces free Ca2+ to a concentration below 1 nM. Subsequently, sGC activity was measured in the presence of EGTA or Ca2+ (Figure 8A). The presence of 1 μM Ca2+ during the assay resulted in a 10-fold reduction of sGC activity. This inhibition appears to be reversible because the subsequent addition of sufficient EGTA to chelate this Ca2+ recovered the high sGC activity.
Figure 8.
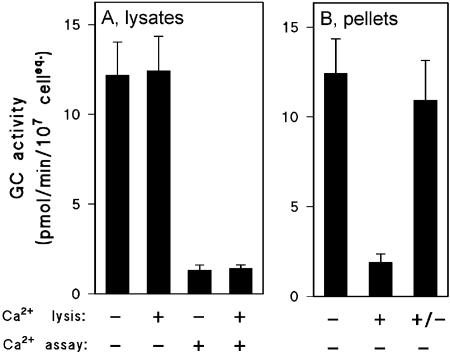
Effect of Ca2+ on the Mg2+-dependent sGC activity. gca- cells were assayed GC activity in either Ca2+-free buffer or at 1 μM free Ca2+. The activity was measured in the lysates (A), or in the pellets (B). Experimental details in addition to the general procedure in Materials and Methods: (A) Cells were resuspended in LB from which EGTA was omitted (see Materials and Methods). Lysis occurred either at 1.5 mM EGTA (-) or at 2 μM added Ca2+ (+). GC assays were performed in the absence of calcium (-, with 0.75 mM EGTA in the assay), or in the presence of 1 μM free calcium (+, achieved by the addition of 1 mM Ca2+ if EGTA was present during lysis). (B) Lysis was performed in the presence (+) or absence (-) of Ca2+ as described above or in the presence of Ca2+ followed by addition of EGTA 1 min after lysis (+/-). Two minutes after lysis samples were centrifuged and pellets were resuspended in LB (which contained the normal level of 1.5 mM EGTA); GC assays were performed with additional EGTA (1.5 mM final concentration) so no free calcium was present. The data shown are the averages and SE of the means of two independent experiments with lysis in triplicate.
The sGC activity in the particulate fraction is presented in Figure 8B. Nearly all Mg2+-dependent activity of a lysate prepared in EGTA was recovered in the particulate fraction. However, the presence of Ca2+ during lysis and subsequent pelleting of the membranes resulted in a nearly complete loss of activity in these particulates, even when EGTA was present during the assay. In contrast, normal enzyme activity in the particulate fraction was obtained when EGTA was added before fractionation of the Ca2+-prepared lysate. This suggests that Ca2+ ions induce the reversible dissociation of sGC from the particulate fraction.
Finally, GTPγS has been shown to be a potent activator of GC activity in Dictyostelium (Janssens et al., 1989; Schulkes et al., 1992). GTPγS stimulates G-proteins thereby inducing a decrease of the Km and an increase of the Vmax of sGC for the substrate GTP; GTPγS itself is a poor substrate for sGC. Furthermore, GTPγS has no effect on Mn2+-dependent sGC activity, and stimulates Mg2+-dependent sGC activity only in the particulate fraction, not in the supernatant (Roelofs et al., 2001a; Roelofs and Van Haastert, 2002). The stimulation of sGC by GTPγS does not reverse upon removal of GTPγS from the particulate fraction (unpublished data). Thus, the Mg2+-dependent sGC activity in the pellet fraction may increase by a combination of translocated protein and activation of existing protein. We used Mn2+/GTP as substrate of sGC to investigate the effect of GTPγS on the distribution of sGC between particulate and supernatant fractions. gca- cells were lysed in the presence or absence of GTPγS; this was done under Ca2+-free conditions as well as in the presence of 1 μM free Ca2+. Mn2+-dependent activity was measured in particulate and soluble fractions (Figure 9). The data show that GTPγS has no significant effect on the distribution of sGC between particulate or soluble fraction, neither in the presence nor in the absence of Ca2+.
Figure 9.
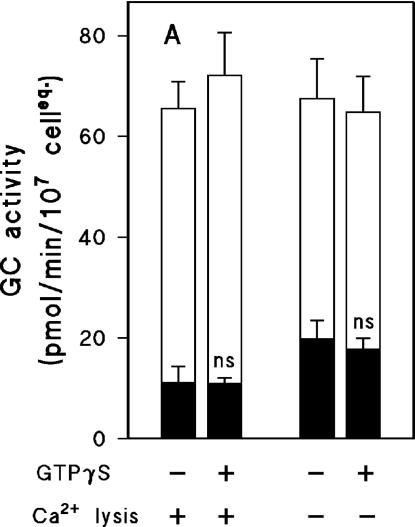
Effect of GTPγS on the Mn2+-dependent sGC activity. gca- cells were lysed in the presence or absence of 100 μM GTPγS. Lysis was performed at either 1 μM free Ca2+ or 1.5 mM EGTA. Lysates were fractionated by centrifugation, and activities were measured in pellets (resuspended in LB, filled bars) and supernatants (open bars on top of filled bars). The activity in the pellet with GTPγS is not significantly different in a Student's t test when compared with the pellet without GTPγS.
DISCUSSION
In Dictyostelium, guanylyl cyclase activity is present in membrane fractions as well as in supernatant fractions of cell lysates. From the two guanylyl cyclases present in Dictyostelium, only GCA has transmembrane regions and enzyme activity is exclusively found in the membrane fraction of cell lysates (Roelofs et al., 2001a; Roelofs and Van Haastert, 2002). Furthermore, GFP fusions to the C- or N-terminus of GCA reveal that the protein is located at the plasma membrane (Roelofs, Bosgraaf, Van Der Ploegh, Van Haastert, unpublished results). GFP fusions to the second guanylyl cyclase, sGC, show that the sGC is predominantly located in the cytosol and a small fraction is membrane associated. sGC activity measurements in vitro using Mn2+/GTP confirm this distribution of the protein, because much more activity is present in the supernatant fraction than in the particulate fraction of cell lysates. However with the more physiological substrate Mg2+/GTP nearly all activity has been detected in the particulate fraction (Roelofs et al., 2001a).
We observed a transient association of sGC to the membrane upon receptor stimulation that presumably provides the cell with more activatable sGC. Using confocal microscopy, we calculated that in unstimulated cells ∼13% of the sGC-GFP is associated to the membrane and the remaining 87% is cytosolic. On stimulation, the amount of membrane-associated sGC-GFP almost doubles to 23%. The cAMP-induced translocation of the sGC-GFP protein is also apparent as an increase of the Mn2+-dependent enzyme activity in the membrane fraction with a concomitant reduction of activity in the soluble fraction of a cell lysate.
The biochemical reconstitution assays demonstrate that soluble sGC isolated from gca- cells does bind to membranes from gca-/sgc- cells and that this interaction leads to an approximately fivefold increase of Mg2+-dependent activity. The assays also show that membranes contain a limited amount of sGC-binding sites. Half-maximal saturation of membrane binding sites takes place at a supernatant/membrane ratio of ∼0.3 on a cell basis. These results suggest that in vivo the binding sites are saturated with sGC and that the excess sGC resides in the cytosol, where it is inactive. Compounds that affect the number of binding sites are therefore expected to modulate the fraction of sGC residing at the membrane and consequently the Mg2+-dependent activity of sGC. GTPγS is known to activate heterotrimeric and small G-proteins and strongly activates Mg2+-dependent sGC activity in vitro. However, GTPγS has no effect on the localization of enzyme activity. The creation of membrane binding sites by the chemoattractant cAMP is therefore probably induced by a G-protein–independent mechanism, as are several other responses in Dictyostelium, such as phosphorylation of the cAMP receptor, opening of calcium channels, activation of MAP and STAT kinases by cAMP, and the activation of sGC by osmotic shock (Milne et al., 1995; Maeda and Firtel, 1997; Araki et al., 1998; Kuwayama and Van Haastert, 1998). cAMP-dependent activation of sGC is therefore proposed to be mediated via two pathways, a G-protein–independent translocation of soluble sGC to the membrane and G-protein–dependent activation of the membrane-localized sGC.
The two Dictyostelium guanylyl cyclases, GCA and sGC, are stimulated by cAMP to a different level, 2.5-fold for GCA and 8-fold for sGC (Roelofs et al., 2001a). Stimulation of both guanylyl cyclases in vivo is presumably mediated by the same heterotrimeric and small G-proteins. In vitro, this stimulation can be mimicked by adding GTPγS to cell lysates, which stimulates both GCA and sGC ∼3-fold via the same mechanism (increasing the Vmax and reducing the Km for Mg2+/GTP). The 2.5-fold stimulation of GCA by cAMP in vivo is consistent with the 3-fold stimulation by GTPγS in vitro. On the basis of our new results, we propose that the observed 8-fold activation of sGC by cAMP is composed of the 3-fold activation by GTPγS and the 2-fold increase of membrane-localized sGC.
A second regulator of sGC is Ca2+, which is known to inhibit cGMP formation in vivo and Mg2+-dependent sGC activity in vitro (Schoen et al., 1996; Roelofs and Van Haastert, 2002). Interestingly, Ca2+ ions induce the dissociation of sGC from the membranes. The mechanism by which Ca2+ inhibits guanylyl cyclases is not understood. We have not detected a segment of sGC that could act as a potential Ca2+-binding domain. The inhibition of sGC by Ca2+ is reminiscent of the inhibition of membrane-bound guanylyl cyclase by GCAP in the vertebrate eye (Gorczyca et al., 1994; Palczewski et al., 1994). Although Dictyostelium contains several genes encoding for GCAP-like proteins with four EF hands, none of these genes has been attributed to Ca2+ regulation of GC activity in knockout studies (Kuwayama and van Haastert, unpublished results).
In a cAMP gradient sGC becomes enriched at the leading edge. Furthermore, membrane-association of sGC leads to activation. This would imply that cGMP formation occurs at the anterior of cells in a cAMP gradient. In contrast, cAMP formation and subsequent secretion occur mainly at the cell posterior (Kriebel et al., 2003). The physiological importance of cGMP formation at the leading edge is intriguing. An attractive hypothesis would be that the local production of cGMP leads to the formation of an intracellular cGMP gradient. However, models predict that on average a cGMP molecule will diffuse 55 μm from its point of synthesis before it is degraded. In a 10-μm cell this rapid diffusion of cGMP would prohibit its potential to store spatial information (Postma and Van Haastert, 2001). Another possibility would be that sGC is present at the leading edge in a complex together with the cGMP target, GbpC. Rapid diffusion of cGMP may be prevented by tunneling cGMP directly from sGC to GbpC. In support of this theory, previous experiments have shown that guanylyl cyclase activity is regulated by a cGMP-binding protein, which are now characterized as sGC and GbpC, respectively (Kuwayama and Van Haastert, 1996). Although we have no direct evidence that GbpC and sGC are present in a complex, preliminary experiments suggest that GbpC-GFP also translocates to the leading edge and this translocation is enhanced by overexpression of sGC (Bosgraaf and van Haastert, unpublished observations).
It has been well established that cGMP mediates myosin filament formation in the cortex of Dictyostelium cells. In contrast to the anterior localization of sGC, the assembly of myosin filaments takes place at the posterior of the cell, where it is involved in retraction of the uropod and suppression of lateral pseudopodia. This spatial separation of activating enzyme and target of its product is not unique for sGC and myosin, because adenylate cyclase A is located at the posterior of the cell, whereas its activator, CRAC, is localized at the leading edge. In a similar manner, PAKa, an enzyme regulating myosin assembly at the back, is activated by Akt that localizes to the front of the cell.
Interestingly, the chemotaxis defect of the gca-/sgc- cell strain is more severe than the defect of the myosin II heavy-chain knockout cell strain, suggesting that gca-/sgc- cells have additional defects (Wessels et al., 1988). However, no other functions for cGMP concerning chemotaxis have been found. It is possible that the sGC protein has an additional role in chemotaxis besides cGMP formation. Because sGC is present at the leading edge, it is tempting to speculate that the sGC protein may be important for the local stimulation of pseudopod extension. This would introduce an interesting new concept, where sGC globally inhibits pseudopod formation via the fast diffusing second-messenger cGMP and locally stimulates pseudopod extension at the leading edge via protein-protein interactions of the sGC protein.
Supplementary Material
Article published online ahead of print in MBC in Press on December 15, 2004 (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-08-0701).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Araki, T., Gamper, M., Early, A., Fukuzawa, M., Abe, T., Kawata, T., Kim, E., Firtel, R. A., and Williams, J. G. (1998). Developmentally and spatially regulated activation of a Dictyostelium STAT protein by a serpentine receptor. EMBO J. 17, 4018-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraaf, L., Russcher, H., Smith, J. L., Wessels, D., Soll, D. R., and Van Haastert, P.J.M. (2002). A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 21, 4560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca, W. A., Gray-Keller, M. P., Detwiler, P. B., and Palczewski, K. (1994). Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc. Natl. Acad. Sci. USA 91, 4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens, P. M., and de Jong, C. C. (1988). A magnesium-dependent guanylate cyclase in cell-free preparations of Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 150, 405-411. [DOI] [PubMed] [Google Scholar]
- Janssens, P. M., De Jong, C. C., Vink, A. A., and Van Haastert, P. J. (1989). Regulatory properties of magnesium-dependent guanylate cyclase in Dictyostelium discoideum membranes. J. Biol. Chem. 264, 4329-4335. [PubMed] [Google Scholar]
- Kriebel, P. W., Barr, V. A., and Parent, C. A. (2003). Adenylyl cyclase localization regulates streaming during chemotaxis. Cell 112, 549-560. [DOI] [PubMed] [Google Scholar]
- Kuwayama, H., and Van Haastert, P.J.M. (1996). Regulation of guanylyl cyclase by a cGMP-binding protein during chemotaxis in Dictyostelium discoideum. J. Biol. Chem. 271, 23718-23724. [DOI] [PubMed] [Google Scholar]
- Kuwayama, H., and Van Haastert, P.J.M. (1998). Chemotactic and osmotic signals share a cGMP transduction pathway in Dictyostelium discoideum. FEBS Lett. 424, 248-252. [DOI] [PubMed] [Google Scholar]
- Linskens, M. H., Grootenhuis, P. D., Blaauw, M., Huisman-de Winkel, B., Van Ravestein, A., Van Haastert, P. J., and Heikoop, J. C. (1999). Random mutagenesis and screening of complex glycoproteins: expression of human gonadotropins in Dictyostelium discoideum. FASEB J. 13, 639-645. [DOI] [PubMed] [Google Scholar]
- Maeda, M., and Firtel, R. A. (1997). Activation of the mitogen-activated protein kinase ERK2 by the chemoattractant folic acid in Dictyostelium. J. Biol. Chem. 272, 23690-23695. [DOI] [PubMed] [Google Scholar]
- Milne, J. L., Wu, L., Caterina, M. J., and Devreotes, P. N. (1995). Seven helix cAMP receptors stimulate Ca2+ entry in the absence of functional G proteins in Dictyostelium. J. Biol. Chem. 270, 5926-5931. [DOI] [PubMed] [Google Scholar]
- Padh, H., and Brenner, M. (1984). Studies of the guanylate cyclase of the social amoeba Dictyostelium discoideum. Arch. Biochem. Biophys. 229, 73-80. [DOI] [PubMed] [Google Scholar]
- Palczewski, K. et al. (1994). Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase-activating protein. Neuron 13, 395-404. [DOI] [PubMed] [Google Scholar]
- Postma, M., Roelofs, J., Goedhart, J., Gadella, T. W., Visser, A. J., and Van Haastert, P. J. (2003). Uniform cAMP stimulation of Dictyostelium cells induces localized patches of signal transduction and pseudopodia. Mol. Biol. Cell 14, 5019-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma, M., and Van Haastert, P.J.M. (2001). A diffusion-translocation model for gradient sensing by chemotactic cells. Biophys. J. 81, 1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs, J., Meima, M., Schaap, P., and Van Haastert, P.J.M. (2001a). The Dictyostelium homologue of mammalian soluble adenylyl cyclase encodes a guanylyl cyclase. EMBO J. 20, 4341-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs, J., Snippe, H., Kleineidam, R.G., and Van Haastert, P.J.M. (2001b). Guanylate cyclase in Dictyostelium discoideum with the topology of mammalian adenylate cyclase. Biochem. J. 354, 697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs, J., and Van Haastert, P.J.M. (2002). Characterization of two unusual guanylyl cyclases from Dictyostelium. J. Biol. Chem. 277, 9167-9174. [DOI] [PubMed] [Google Scholar]
- Ross, E. M., Howlett, A. C., Ferguson, K. M., and Gilman, A. G. (1978). Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J. Biol. Chem. 253, 6401-6412. [PubMed] [Google Scholar]
- Schoen, C. D., Schulkes, C. C., Arents, J. C., and van Driel, R. (1996). Guanylate cyclase activity in permeabilized Dictyostelium discoideum cells. J. Cell. Biochem. 60, 411-423. [DOI] [PubMed] [Google Scholar]
- Schulkes, C. C., Schoen, C. D., Arents, J. C., and Van Driel, R. (1992). A soluble factor and GTP gamma S are required for Dictyostelium discoideum guanylate cyclase activity. Biochim. Biophys. Acta 1135, 73-78. [DOI] [PubMed] [Google Scholar]
- Snaar-Jagalska, B. E., and Van Haastert, P.J.M. (1994). G-protein assays in Dictyostelium. Methods Enzymol. 237, 387-408. [DOI] [PubMed] [Google Scholar]
- Tesmer, J. J., and Sprang, S. R. (1998). The structure, catalytic mechanism and regulation of adenylyl cyclase. Curr. Opin. Struct. Biol. 8, 713-719. [DOI] [PubMed] [Google Scholar]
- Valkema, R., and Van Haastert, P.J.M. (1992). Inhibition of receptor-stimulated guanylyl cyclase by intracellular calcium ions in Dictyostelium cells. Biochem. Biophys. Res. Commun. 186, 263-268. [DOI] [PubMed] [Google Scholar]
- van Haastert, P.J.M., and Kuwayama, H. (1997). cGMP as second messenger during Dictyostelium chemotaxis. FEBS Lett. 410, 25-28. [DOI] [PubMed] [Google Scholar]
- Wessels, D., Soll, D. R., Knecht, D., Loomis, W. F., De Lozanne, A., and Spudich, J. (1988). Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev. Biol. 128, 164-177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


