Abstract
BLZ-100 is a single intravenous use, fluorescent imaging agent that labels tumor tissue to enable more complete and precise surgical resection. It is composed of a chlorotoxin peptide covalently bound to the near-infrared fluorophore indocyanine green. BLZ-100 is in clinical development for intraoperative visualization of human tumors.
The nonclinical safety and pharmacokinetic (PK) profile of BLZ-100 was evaluated in mice, rats, canines, and non-human primates (NHP). Single bolus intravenous administration of BLZ-100 was well tolerated and no adverse changes were observed in cardiovascular safety pharmacology, PK, and toxicology studies in rats and NHP. The single-dose no-observed-adverse-effect-levels (NOAELs) were 7 mg (28 mg/kg) in rats and 60 mg (20 mg/kg) in NHP, corresponding to peak concentration values of 89,400 ng/mL and 436,000 ng/mL and area-under-the-curve exposure values of 130,000 hr*ng/mL and 1,240,000 hr*ng/mL, respectively. Based on a human imaging dose of 3 mg, dose safety margins are > 100 for rat and monkey.
BLZ-100 produced hypersensitivity reactions in canine imaging studies (lethargy, pruritus, swollen muzzle, etc.). The severity of the reactions was not dose-related. In a follow-up study in dogs, plasma histamine concentrations were increased 5 to 60 minutes after BLZ-100 injection; this coincided with signs of hypersensitivity, supporting the conclusion that the reactions were histamine-based. Hypersensitivity reactions were not observed in other species or in BLZ-100 human clinical studies conducted to date.
The combined imaging, safety pharmacology, PK, and toxicology studies contributed to an extensive initial nonclinical profile for BLZ-100, supporting first-in-human clinical trials.
Keywords: Intraoperative imaging, fluorescence-guided surgery, tumor resection, chlorotoxin, indocyanine green, near-infrared
Introduction
For many types of cancer, surgery is a primary treatment modality and the extent of surgical resection is directly related to survival1–6. Failure to surgically remove a cancer-affected lymph node or residual cancer at the surgical margin may impact a patient’s chance for a successful outcome, often necessitating more aggressive postsurgical treatments such as repeat surgeries and more aggressive chemo/radiotherapy regimens. Alternatively, taking too much tissue can lead to significant morbidity or other health complications. Recent efforts have focused on methods to highlight tumor tissue in real-time in the operating room to facilitate more accurate tumor excision. One such approach is fluorescence-guided surgery, in which contrast between tumor and normal tissue is achieved by using fluorescent agents. These agents are administered to patients prior to surgery and rely on properties of the tumor, such as differential uptake of the dye or enzymatic activation, to achieve differences in fluorescent signal between tumor and normal tissue. Herein we describe nonclinical safety and imaging data with a fluorescent peptide-dye molecule known as BLZ-100.
BLZ-100 is composed of a modified form of the chlorotoxin (CTX) peptide covalently bound to the near-infrared (NIR) fluorescent dye indocyanine green (ICG). CTX peptides were originally isolated from scorpion venom7 and have subsequently been shown to bind specifically to many types of tumor cells8–14, allowing for potential broad applicability to a wide range of cancers including glioma and other brain tumors, prostate, colorectal, lung, breast, head and neck cancers, and sarcomas. CTX was shown to facilitate blood-brain barrier (BBB) penetration of nanoparticles15, suggesting that the peptide may be able to reach brain tumors that do not have compromised BBB function. A synthetic version of the native CTX peptide (TM-601) has previously been studied and shown to be well-tolerated in mice and marmosets16, and an iodine-131-labeled form underwent Phase 1 clinical testing as a therapeutic for targeted delivery of radioactivity to gliomas and other cancers17–19. The peptide component of BLZ-100 is similar to native CTX with modifications made to facilitate consistent attachment of the dye component. CTX binds directly to a phosphorylated form of Annexin A220, which exists on the cell surface as a heterotetramer with S100A1021,22 known as Calpactin. In normal cells, Annexin A2 is primarily intracellular and not phosphorylated. Matrix metalloproteinase 2 has also been proposed as a cell-surface CTX target23. Each proposed target is >90% identical between humans and the species commonly used for toxicology studies, with highest identity (>99%) between human and cynomolgus monkey.
ICG emits NIR fluorescent light that can be monitored during surgery using an appropriate detection device. ICG itself has been used safely for decades as a retinal angiography imaging agent. It has a reputation as a nontoxic and nonionizing agent, it binds efficiently to blood lipoproteins, and it does not leak from circulation24. Singlet oxygen produced from ICG is immediately bound to its own decomposition products, decreasing the likelihood of phototoxicity or photomutagenicity25. ICG does not have any known metabolites, and is removed quickly and without modification from circulation into bile by glutathione S-transferase in the liver25.
Here we show the initial safety pharmacology, PK, and toxicology profile of BLZ-100 based on studies conducted in CD-1 mice, Sprague Dawley rats, beagle dogs, and cynomolgus monkeys. Safety observations from an imaging pharmacology efficacy study conducted in mixed-breed dogs11 are also discussed. These studies achieved a safety profile supporting first-in-human clinical trials for BLZ-100 as a human product candidate.
Materials and Methods
Studies were designed based on current International Conference on Harmonisation (ICH) guidelines, notably ICH S6(R1), S9 and M3(R2), and US Food and Drug Administration (FDA) Guidance for medical imaging drugs26. Since BLZ-100 is intended to target tumor tissue which will not be present in normal animal safety studies, dose selection and inter-species scaling initially relied on tumor imaging data in mice to estimate a minimum effective imaging dose. In this context, an effective imaging dose was estimated by observing sufficient signal strength and contrast in mouse models to distinguish tumor from surrounding normal tissue. Furthermore, since BLZ-100 is intended to be given as a fixed IV bolus dose, the dosages in the animal studies were given as fixed dose levels with adjustments for the body surface area of each species used to scale doses across the species. The appropriateness of this approach was confirmed in the canine imaging efficacy study11. BLZ-100 was chemically synthesized by Blaze Bioscience by covalently attaching the ICG moiety to the chlorotoxin peptide. It was supplied as a lyophilized powder (purity ~95%) and was formulated in an iso-osmotic neutral pH Tris-mannitol solution. Dosing solutions were analyzed for purity using HPLC and for concentration using absorbance at 786 nm and comparison with a standard curve. Validated liquid chromatography–mass spectrometry (LC/MS) methods were used to measure BLZ-100 serum levels. Phoenix WinNonlin (Certara, Princeton, NJ) software was used to estimate pharmacokinetic (PK) parameters for BLZ-100.
Animal studies were approved and conducted in accordance with each institution’s Institutional Animal Care and Use Committee (IACUC) and in compliance with the guidelines and principles listed in the American College of Toxicology Policy on the Use of Animals in Toxicology. For the GLP studies, the Sprague Dawley rats were supplied by Charles River and purpose-bred monkeys of Cambodian origin were supplied by SNBL USA.
Safety pharmacology studies
In vitro hERG assay (non-GLP)
Current amplitude was measured by the manual patch clamp technique at 0.2, 0.6, and 2 μM BLZ-100 (0.2 μM is equivalent to peak serum concentrations from the minimum imaging dose) in stably transfected human embryonic kidney (HEK 293) cells expressing hERG mRNA and incubated at 37°C. E- 4031 (Sigma-Aldrich) was used as a positive control for the assay. Quadruplicate measurements were made of the current amplitude.
Cardiovascular safety study (GLP)
Four male non-naïve cynomolgus monkeys (8.00±0.17 years of age; 7.50±0.39 kg) were surgically implanted with telemetry transducers to assess arterial blood pressure (ABP), heart rate (HR), respiratory rate (breaths/minute), and Lead II electrocardiogram (ECG) parameters (PR interval, QRS duration, RR interval, and QT interval). Each animal was injected with ascending doses of vehicle control, 0.6, 6, and 60 mg BLZ-100, with a minimum 3-day washout period between administrations. The 3-day washout period was supported by pharmacokinetic data showing BLZ-100 blood levels were less than 1% of peak values 72 hours post dose. Hemodynamic and ECG data were collected from 2 hours pre-dose to 24 hours post-dose. Lead II QT interval data were corrected for variations in heart rate using the Fridericia QTc (QTcF) and an individual animal correction factor (QTc1). Data points for analysis were 1 hour pre-dose and 0.5, 1, 2, 3, 4, 5, 6, 12, and 24 hours post-dose.
Studies in rodents
Pilot toxicology study in mice (non-GLP)
Vehicle control, 0.01, 0.1 or 1 mg BLZ- 100 were injected in the tail vein of CD-1 mice (6 females/group), and mice were euthanized 3 or 14 days post-dose (3 females/group/timepoint). Measured parameters included clinical observations, body weight, abbreviated serum chemistry panel (including blood urea nitrogen [BUN], creatinine, alanine aminotransferase [ALT], aspartate aminotransferase [AST] and gamma-glutamyltransferase [GGT]), and histopathology of major organs (including brain, heart, kidney, liver, lungs, intestines, skin, and spleen). Doses of 0.008, 0.08 or 0.8 mg (molar equivalent of BLZ-100 conjugate) of unconjugated peptide were injected into an additional group of female CD-1 mice (3 females/group) for monitoring clinical observations up to 1 hour after injection.
Definitive toxicology study in rats (GLP)
Vehicle, 0.07, 0.7 or 7 mg BLZ-100 was injected intravenously in Sprague Dawley rats (10/sex/group) on one occasion (day 1). Rats were euthanized on day 3 (5/sex/group) or day 15 (5/sex/group). Study parameters included clinical observations, neurological observations (including piloerection, respiratory abnormalities, posture, involuntary motor movements [clonic or tonic], stereotypy, bizarre behavior, gait abnormalities, and vocalization), body weight, food consumption, clinical pathology (serum chemistry, hematology, coagulation, and urinalysis), ophthalmology, gross pathology, organ weights, and histopathology. Animals for toxicokinetic (TK) analyses (6/sex/group) were bled for serum using a staggering sampling scheme (pre-dose, and 15 minutes post-dose, and 1, 3, 6, 12, 24, and 48 hours post-dose).
Studies in dogs
Pilot study (non-GLP)
1 mg BLZ-100 was injected intravenously in the cephalic vein of beagle dogs as a bolus (1 male) or 15 minute infusion (1 male). An molar equivalent amount of unconjugated CTX peptide was bolus injected in a third male dog. Blood was collected from the jugular, cephalic, or saphenous veins for evaluation of serum PK at pre-dose and at 5, 15, and 30 minutes and 1, 2, 4, 6, 24, 48, 72, and 96 hours postdose. Clinical observations were recorded at the same timepoints.
Efficacy study (non-GLP)
Mixed breed dogs with spontaneous solid tumors were treated with IV BLZ-100 and imaged as described11. A follow-up study was conducted with subcutaneous administration of BLZ-100 at 1 to 3 mg/m2 and surgery at 1 or 2 days post-dose. Subcutaneous doses were administered at sites contralateral to tumor location. Monitoring of safety endpoints and intraoperative imaging were performed as described11.
Hypersensitivity study (non-GLP)
1 mg BLZ-100 was injected intravenously in the cephalic vein of 3 male beagle dogs. Pre- and post-dose blood samples were collected at 0, 5, 15, and 30 minutes, and 1, 2, and 24 hours post-dose. Plasma samples (8 aliquots/animal/timepoint) were evaluated for histamine concentration. Activation of complement results in cleavage of C3 into bioactive split products. Therefore, the total amount of intact C3 present in the serum samples is inversely proportional to the degree of complement activation. Post-dose serum samples (4 aliquots/animal/timepoint) were incubated with complement matrix and converter reagent (Quidel) for 60 minutes at 37°C to convert intact C3 to sC5b-9 as a stable end product. The sC5b-9 was then quantified using a commercial ELISA kit (Quidel).
Definitive study in non-human primates (GLP)
Vehicle, 0.6, 6, or 60 mg BLZ-100 were injected intravenously in the peripheral vein of naïve cynomolgus monkeys (3/sex/group, 2 to 3 years of age; 2.3 to 3.7 kg). Due to the wide dose range, the BLZ-100 concentration in the dosing solutions was varied (0.2, 2, and 5 mg/mL) so that dose volumes were feasible to measure and deliver at a dose rate <6 mL/min. Monkeys were euthanized on day 15. Study parameters included clinical observations (neurological and musculoskeletal), body weight, clinical pathology (serum chemistry, hematology, coagulation, and urinalysis), ophthalmology, gross pathology, organ weights, and histopathology. Urine samples collected at days 3 and 15 were analyzed using the Odyssey® CLx near-infrared scanner (LI-COR Biosciences) and the level of BLZ-100 estimated by comparison to a standard curve. Blood was collected and processed to serum for TK evaluations at pre-dose, and 5 and 15 minutes, and 1, 2, 4, 8, 12, 24, 36, 48, 72, 96, and 120 hours post day 1 dose.
Results
Doses for toxicology and safety pharmacology studies were selected to provide at least a 100-fold safety margin for a starting human clinical dose of 3 mg BLZ-100. Dose ranges for human clinical trials were estimated based on results of imaging studies in mice12 and dogs11. Fluorescence intensity and contrast sufficient for effective imaging were obtained with IV bolus doses of 0.1 to 0.3 mg (~ 1–3 mg/m2) in the mouse and confirmed in the dog at a dose of approximately 1 mg (~1 mg/m2). Adjusted for body surface area, these animal dose levels suggested an appropriate starting dose level in human cancer patients with peripheral tumors would be approximately 3 mg. At this level, evidence of tumor uptake of BLZ-100 is expected to be seen, although higher doses may be warranted depending on the tumor type and goals of the surgery (for example, brain cancer may require higher dose levels).
Safety pharmacology studies
An in vitro assay was used to assess the impact of BLZ-100 on hERG current amplitude at concentrations up to the estimated human peak blood concentration following the highest planned clinical dose (30 mg). The effect of BLZ-100 on hERG current amplitude (% change ± SEM) in HEK 293 cells was minimal at the tested concentrations, with the only possibly significant change occurring at the highest concentration (+0.2% ± 0.6 at 0.2 μM, −0.9% ± 0.8 at 0.6 μM, and −12.1% ± 1.0 at 2.0 μM, relative to the pre-drug value). In contrast, the positive control, E-4031, a potent and selective hERG blocker27,28, reduced current amplitude by over 80% at a 0.1 μM concentration. The BLZ-100 concentrations tested (0.2, 0.6 and 2.0 μM), were equivalent to estimated human peak blood concentrations after bolus injection of 3, 9, and 30 mg BLZ-100.
In the in vivo cardiovascular study, there were no changes in clinical signs or in hemodynamic or electrocardiogram parameters (including ABP, HR, breaths/minute, PR interval, QRS duration, RR interval and QT interval) after ascending doses of 0.6, 6, and 60 mg BLZ-100 were administered to conscious male non-human primates. Mean serum levels of BLZ-100 (Cmax) were 436,000 ng/mL (91.5 μM) at the 60 mg dose level in the definitive TK analysis (see below).
Studies in rodents
In mice (administered 0.01, 0.1, or 1 mg BLZ-100), transient decreases in spontaneous motor activity, somnolence, and prostration were noted approximately 1 to 3 minutes post-dose at 0.1 mg (N=4) and 1 mg BLZ-100 (N=6). The hypoactivity lasted 30 to 60 minutes and was completely resolved by 4 hours post-dose. Similar findings were observed in mice injected with molar equivalent doses of the unconjugated CTX peptide.
In the definitive toxicology study at 0, 0.07, 0.7, and 7 mg BLZ-100 in rats, there were no adverse changes up to 14 days post-dose, including no laboratory abnormalities or changes noted by microscopic pathology assessment of tissues. Gross findings at necropsy, presumably from the green colored ICG dye component of BLZ-100, included green discolored kidneys at 7 mg BLZ-100 on day 3 (4 males and 4 females) and day 15 (1 female). However, there were no changes in functional urinary clinical pathology parameters or renal pathology. The NOAEL was considered 7 mg BLZ-100. Exposures based on Cmax and C0 increased in an approximately dose-proportional manner across all dose groups. Exposure based on AUC0-t increased in an approximate dose-proportional manner between 0.07 and 0.7 mg BLZ-100, and increased in a higher than dose-proportional manner between the 2 lower dose groups and 7 mg BLZ-100 (Table 1; Figure 1). There was no obvious influence of gender on exposure.
Table 1.
Mean Non-compartmental BLZ-100 PK Parameters after Single Bolus IV Doses in Male and Female Rats
| Parameter | Dose Group
|
||
|---|---|---|---|
| 0.07 mg (1.8 mg/m2) (N=6)a |
0.7 mg (18 mg/m2) (N=6)a |
7 mg (184 mg/m2) (N=6)a |
|
| Cmax (ng/mL) | 665 | 7510 | 89400 |
| C0 (ng/mL) | 1200 | 13400 | 136000 |
| AUC0-t (h*ng/mL) | 794 | 8070 | 130000 |
3/sex/time point
Cmax = maximum observed concentration; C0 = back-extrapolated concentration at time = 0; AUC0-t = area under the concentration-time curve from time = 0 to the timepoint with the last measurable concentration
Figure 1.

Mean BLZ-100 serum concentrations after single bolus IV doses in rats. The maximal concentration was observed at the first measured timepoint (0.25 hours [hr] post-dose) in all groups (0.07, 0.7, and 7 mg BLZ-100). Mean serum concentrations were measurable out to 12 hr post-dose (lower limit of quantification [LLOQ] = 10 ng/mL) in the 0.07 and 0.7 mg dose groups and out to 48 hr post-dose (the last measured timepoint) in the 7 mg dose group.
Studies in dogs
A pilot study in male dogs revealed pseudoallergic reactions shortly after administration of 1 mg BLZ-100. One dog given a bolus injection began scratching and biting its back 30 seconds post-dose, and was given diphenhydramine (25 mg IV) to ease the severity of the reactions. At 15 to 30 minutes post-dose, the dog was still shaking vigorously, itching, and its ears were warm-to-touch. A second dog given a slower administration via a 15-minute IV infusion of BLZ-100 also demonstrated pseudoallergic reactions during the infusion (including red, warm ears and puffy, upper eyelids, and itching). Diphenhydramine was also administered to this dog. A third dog given 1 mg of the unconjugated CTX peptide by bolus injection showed no clinical signs of note. Pseudoallergic reactions also occurred in 27 of 28 mixed breed dogs during a tumor imaging study that included safety evaluation endpoints11. All the dogs had spontaneous tumors and were undergoing solid tumor resection. The dogs were given IV injections of BLZ-100 at doses ranging from 0.1 to 1.5 mg. The hypersensitivity reactions were observed 5 to 10 minutes post-dose, ranging from mild to severe pruritus, erythema, and swelling of the muzzle and distal extremities (Table 2). The severity of the reactions was not dose-related. Pre-medication with diphenhydramine (1 mg/kg SC) ameliorated symptoms and additional diphenhydramine was given post-dose as needed. The reactions abated 30 minutes to 4 hours after BLZ-100 injections.
Table 2.
Pseudoallergic Reactions in Mixed Breed Dogs with Spontaneous Tumors
| AE Gradea | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Pruritus | No Event | Mild or localized | Intense or widespread | Intense, widespread and Interfering with ADLb | – | – |
| No. animalsc | 2 | 11 | 9 | 5 | 0 | 0 |
| Erythema | No Event | Limited to localized site | Generalized, but requires close examination | Generalized and easily visible | – | – |
| No. animals | 3 | 6 | 14 | 4 | 0 | 0 |
| Edema (extremities and muzzle) | No Event | Mild swelling, skin pliable | Moderate swelling, skin pliable | Severe swelling, skin firm | Function Limited/Disabling | – |
| No. animals | 11 | 6 | 7 | 3 | 0 | 0 |
| Urticaria (hives, welts, wheals) | No Event | – | Transient | Intervention <24H | Intervention >24H | – |
| No. animals | 21 | 0 | 5 | 1 | 0 | 0 |
Adverse event (AE) grades described in Vail et al33
Activities of daily living (ADL) include eating, sleeping, defecating, and urinating.
Animals were counted once at their worst severity. Total N=27; dog 28 had a mild reaction but symptoms were not characterized in detail.
In a follow-up mechanistic study, histamine release was observed following IV injection of 1 mg BLZ-100 to beagle dogs (N=3). Histamine release was increased at the first timepoint of 5 minutes, which coincided with the first clinical signs (including scratching, erythema of muzzle and ears, increased respiration, and lethargy). Histamine release remained increased up to 1 hour post-dose (Figure 2). Histamine plasma concentrations decreased by 2 hours post-dose and returned to baseline concentrations by 24 hours post-dose. Clear evidence of complement activation, denoted by decreased levels of sC5b-9, was not observed. (Figure 3).
Figure 2.

Histamine levels in plasma from canines after bolus IV administration of 1 mg BLZ-100. Data are plotted separately for each animal (N=3). T=0 values were from baseline samples collected prior to dosing. Timepoints listed for post-dose samples are measured from the actual time of dose administration for each animal.
Figure 3.
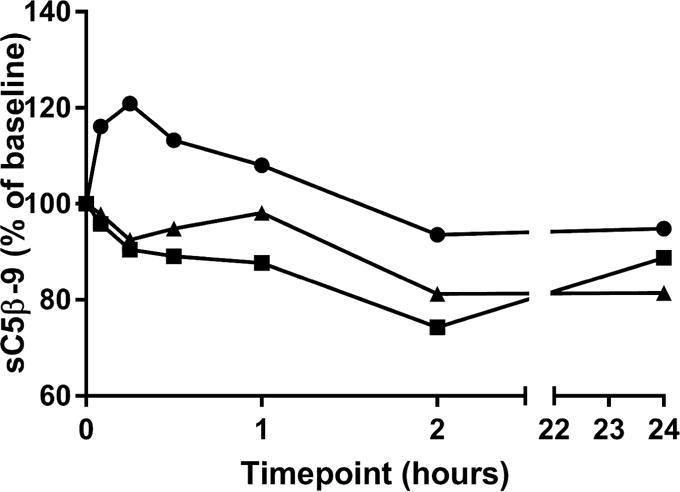
Complement in serum from canines after bolus IV administration of 1 mg BLZ-100. The assay measures sC5b-9 as a stable end product of C3 cleavage. Levels of sC5b-9 in the assay are inversely proportional to complement activation in the serum. Data are plotted for individual animals (N=3).
Subcutaneous administration was evaluated for imaging efficacy and tolerability in 14 dogs. There were no obvious reactions similar to those observed after IV dosing of BLZ-100 in the dogs given BLZ-100 SC, including 2 dogs treated at 3 mg/m2 SC. Furthermore, fluorescence contrast comparable to that seen with IV administration was observed (Figure 4).
Figure 4.

Imaging results following BLZ-100 administration in dogs. (A) Imaging of a mammary carcinoma following an IV dose of 1.24 mg/m2 BLZ-100. (B) Imaging of a soft tissue sarcoma following a subcutaneous dose of 3.0 mg/m2 BLZ-100. Primary masses and satellite lesions were distinguished from surrounding normal tissue in both cases. Imaging was performed as described11. Near-infrared signal is pseudocolored green and shown overlaid on the white light images.
Definitive toxicology study in non-human primates
In the definitive study (0, 0.6, 6 and 60 mg BLZ-100) in non-human primates, there were no pseudoallergic reactions or other adverse findings up to 4 and 14 days postdose, respectively. The only treatment-related finding was green-colored urine on day 3 at 60 mg BLZ-100 (1 male and 3 females). An exploratory fluorescence assay showed that the urine of most but not all treated animals had an increased fluorescent signal intensity on days 3 and 15 at both 6 and 60 mg BLZ-100. There were no clinical or anatomic pathology findings in the study. Select hematology and clinical chemistry parameters highlighting the lack of systemic toxicity of BLZ-100 are listed in Table 3. The NOAEL was considered 60 mg BLZ-100.
Table 3.
Selected hematology and clinical chemistry parameters in non-human primates following single IV bolus doses of BLZ-100
| Vehicle | 0.6 mg BLZ-100 | 6 mg BLZ-100 | 60 mg BLZ-100 | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| White Blood Cells (103/μL) | ||||||||
| Baseline | 11.06 (1.06)1 | 11.17 (3.63) | 11.88 (2.41) | 10.50 (2.81) | 12.03 (2.04) | 9.86 (2.23) | 9.42 (1.92) | 11.74 (2.65) |
| Day 3 | 12.79 (3.87) | 10.47 (0.65) | 11.32 (3.17) | 10.86 (2.35) | 11.19 (1.35) | 7.37 (1.75) | 10.10 (1.36) | 9.55 (3.98) |
| Day 15 | 11.15 (2.52) | 7.44 (0.66) | 14.32 (5.28) | 10.20 (2.63) | 12.46 (5.45) | 6.99 (1.92) | 14.88 (9.25) | 11.07 (2.48) |
| Red Blood Cells (106/μL) | ||||||||
| Baseline | 5.85 (0.27) | 5.69 (0.43) | 6.02 (0.31) | 5.69 (0.18) | 6.47 (0.35) | 5.75 (0.21) | 6.02 (0.36) | 5.81 (0.22) |
| Day 3 | 4.78 (0.40) | 4.60 (0.49) | 4.99 (0.15) | 4.66 (0.19) | 5.40 (0.41) | 4.84 (0.21) | 5.20 (0.44) | 4.70 (0.16) |
| Day 15 | 5.55 (0.20) | 5.43 (0.24) | 5.56 (0.25) | 5.26 (0.30) | 6.00 (0.53) | 5.44 (0.65) | 5.57 (0.55) | 5.29 (0.40) |
| Hematocrit | ||||||||
| Baseline | 46.8 (3.6) | 45.1 (4.4) | 46.8 (2.3) | 43.5 (2.6) | 48.4 (1.2) | 43.7 (1.0) | 46.7 (1.3) | 45.5 (2.6) |
| Day 3 | 38.0 (3.5) | 36.4 (3.5) | 38.9 (1.9) | 35.6 (2.2) | 40.0 (1.3) | 36.5 (1.3) | 40.7 (2.5) | 36.6 (2.2) |
| Day 15 | 44.6 (2.6) | 43.9 (3.8) | 43.3 (2.7) | 41.4 (3.4) | 44.9 (2.2) | 41.8 (4.0) | 44.1 (2.5) | 41.7 (4.2) |
| Platelets (103/μL) | ||||||||
| Baseline | 423 (83) | 376 (54) | 456 (66) | 443 (40) | 449 (52) | 396 (80) | 393 (23) | 528 (174) |
| Day 3 | 384 (97) | 312 (43) | 373 (82) | 399 (20) | 405 (23) | 356 (69) | 294 (19) | 479 (156) |
| Day 15 | 523 (145) | 435 (48) | 473 (57) | 497 (72) | 504 (17) | 426 (92) | 365 (64) | 551 (220) |
| Albumin (g/dL) | ||||||||
| Baseline | 4.4 (0.2) | 4.4 (0.2) | 4.7 (0.3) | 4.3 (0.4) | 4.5 (0.3) | 4.2 (0.3) | 4.5 (0.2) | 4.5 (0.2) |
| Day 3 | 4.3 (0.1) | 4.3 (0.2) | 4.5 (0.3) | 4.2 (0.3) | 4.4 (0.1) | 4.2 (0.3) | 4.5 (0.2) | 4.5 (0.1) |
| Day 15 | 4.3 (0.2) | 4.3 (0.2) | 4.6 (0.2) | 4.2 (0.3) | 4.4 (0.3) | 4.4 (0.2) | 4.3 (0.2) | 4.5 (0.1) |
| Aspartate transaminase (AST) (U/L) | ||||||||
| Baseline | 33 (10) | 34 (4) | 35 (6) | 31 (4) | 25 (7) | 27 (6) | 28 (3) | 29 (5) |
| Day 3 | 81 (44) | 133 (94) | 73 (24) | 51 (8) | 52 (12) | 50 (31) | 69 (35) | 50 (21) |
| Day 15 | 37 (10) | 38 (2) | 42 (5) | 36 (5) | 27 (5) | 32 (6) | 31 (5) | 30 (10) |
| Alanine transaminase (ALT) (U/L) | ||||||||
| Baseline | 43 (25) | 29 (10) | 48 (16) | 24 (5) | 30 (2) | 38 (15) | 24 (8) | 47 (18) |
| Day 3 | 71 (35) | 92 (47) | 78 (23) | 36 (5) | 61 (10) | 66 (34) | 57 (32) | 85 (42) |
| Day 15 | 47 (18) | 40 (8) | 54 (13) | 30 (7) | 41 (9) | 57 (38) | 30 (12) | 51 (14) |
| Bilirubin (mg/dL) | ||||||||
| Baseline | 0.2 (0.1) | 0.2 (0.0) | 0.2 (0.1) | 0.2 (0.0) | 0.1 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.1 (0.1) |
| Day 3 | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.0) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
| Day 15 | 0.2 (0.0) | 0.3 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.4 (0.2) | 0.2 (0.0) | 0.3 (0.1) |
| Gamma glutamyltransferase (GGT) (U/L) | ||||||||
| Baseline | 96 (14) | 68 (16) | 101 (8) | 49 (10) | 88 (35) | 65 (13) | 111 (21) | 90 (44) |
| Day 3 | 78 (15) | 57 (17) | 80 (4) | 43 (11) | 72 (25) | 60 (17) | 92 (11) | 79 (41) |
| Day 15 | 77 (15) | 58 (19) | 78 (7) | 44 (12) | 73 (25) | 65 (22) | 91 (9) | 81 (42) |
| Blood urea nitrogen (mg/dL) | ||||||||
| Baseline | 19 (2) | 22 (2) | 22 (4) | 22 (2) | 21 (2) | 21 (2) | 21 (5) | 18 (2) |
| Day 3 | 18 (2) | 21 (1) | 23 (4) | 19 (3) | 19 (2) | 21 (2) | 19 (1) | 17 (1) |
| Day 15 | 20 (5) | 22 (3) | 24 (1) | 22 (3) | 19 (4) | 24 (5) | 22 (2) | 20 (4) |
| Creatinine (mg/dL) | ||||||||
| Baseline | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.2) | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.7 (0.1) |
| Day 3 | 0.7 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.7 (0.1) | 0.9 (0.1) | 0.7 (0.0) | 0.8 (0.0) | 0.6 (0.1) |
| Day 15 | 0.7 (0.1) | 0.9 (0.2) | 0.8 (0.1) | 0.8 (0.1) | 1.0 (0.1) | 0.8 (0.0) | 0.9 (0.0) | 0.7 (0.1) |
Values listed are mean (SD) for N=3 animals per group
Exposure based on Cmax and C0 was approximately dose proportional across the 100 fold dose range (0.6 to 60 mg). Exposure based on AUC0-t, however, increased in a greater than dose-proportional manner between the 6 and 60 mg doses (Table 4). No clear gender-related differences based on C0 and AUC0-t were observed. Additional noncompartmental TK parameters estimated at 60 mg BLZ-100 (Table 4) included an overall mean t1/2 of 33.7 hours, clearance (CL) of 50.6 mL/hour, and volume of distribution (Vss) of 211 mL (approximately equal to the plasma volume in a 5 kg monkey). In addition, examination of the LC/MS chromatograms from serum samples did not reveal the presence of major metabolites of BLZ-100.
Table 4.
Mean (SD) Non-compartmental BLZ-100 PK Parameters after Single IV Bolus Doses in Male and Female Non-Human Primates
| Parameter | Dose
|
||
|---|---|---|---|
| 0.6 mg (2.4 mg/m2) (N=6) |
6 mg (24 mg/m2) (N=5) |
60 mg (240 mg/m2) (N=6) |
|
| Cmax (ng/mL) | 4440 (796) | 60300 (8410) | 436000 (28900) |
| C0 (ng/mL) | 5260 (807) | 74300 (1300) | 447000 (4100) |
| AUC0-t (hr*ng/mL) | 3800 (1080) | 88300 (8450) | 1240000 (303000) |
| t1/2 (hr) | ND | ND | 33.7 (5.68) |
| CL (mL/hr) | ND | ND | 50.6 (11.2) |
| Vss (mL) | ND | ND | 211 (32.0) |
Cmax = maximum observed concentration; C0 = back-extrapolated concentration at time = 0; AUC0-t = area under the concentration-time curve from time = 0 to the timepoint with the last measurable concentration; t1/2 = terminal half-life; CL = clearance; Vss = steady-state volume of distribution.
ND = not determinable
Discussion
Single bolus IV doses of BLZ-100 were well-tolerated in mice, rats, and monkeys. Treatment-related changes observed in mice included transient decreased spontaneous motor activity, somnolence, and prostration, at 0.1 and 1 mg BLZ-100. An equivalent amount of the CTX peptide produced the same effect, suggesting this response was likely peptide-related. Peptides from scorpions and other insects have ion channel blocking properties and it is possible high doses of the BLZ-100 and CTX in mice resulted in the transient effects seen. These changes have not been observed to-date in the BLZ-100 human clinical trials or in nonclinical species other than mice. In addition to the toxicity evaluations, no effects on heart rate, blood pressure, respiration rate, or ECG tracings were observed in a safety pharmacology study in conscious monkeys.
In a pilot tolerability study in dogs, pseudoallergic/hypersensitivity reactions (itching/scratching, warm ears, etc.) were observed during or immediately after dosing in 2/2 dogs at 1 mg BLZ-100 IV, but were not observed in a third dog given unconjugated peptide. Similar signs of hypersensitivity were observed in most dogs given BLZ-100 in a tumor imaging pharmacology study11. These hypersensitivity reactions have only been observed in dogs and were consistent with a systemic release of histamine, which has been reported for a wide variety of drugs29,30. In an in vivo follow-up study in the dog, clear evidence of a rapid rise in plasma histamine levels was observed while complement levels appeared unaffected. These data supported the concept that the clinical signs observed in the dog were likely a result of direct mast cell/basophil activation by BLZ-100. Subcutaneous administration of BLZ-100 appears to be one method to ameliorate this potential response in the dog.
In the definitive rat and non-human primate studies, the only toxicology findings were green discolored kidneys and green colored urine, respectively, presumably from the ICG dye in BLZ-100. There were no functional or pathologic correlates. A fluorescent assay revealed that the urine of most non-human primates at the two highest doses (6 and 60 mg BLZ-100) had increased fluorescent signal intensity, suggesting BLZ-100 in urine likely accounted for the green color. There were no adverse treatment-related changes in either study (including clinical and neurological observations, body weight, food consumption, ophthalmology, clinical and anatomic pathology). NOAELs were 7 mg (~28 mg/kg) and 60 mg (~20 mg/kg) in rats and non-human primates, respectively. Compared to the estimated minimal imaging dose in patients of 3 mg, the resulting dose safety margins (ratio of NOAEL dose in animal to human dose) are 560 for the rat and 400 for the monkey on a mg/kg basis. Converting the rat and monkey doses on a mg/m2 results in lower but still acceptable margins of 98 and 128 respectively. Finally using AUC pharmacokinetic estimates, the safety margins are 470 for the rat and 4500 for the monkey.
The peptide component of BLZ-100 is similar to native CTX. A synthetic version of the CTX peptide (TM-601) has been studied in mice and marmosets and it was well- tolerated16. The NOAEL for TM-601 after a single IV dose were the highest doses tested in the mouse (6.4 mg/kg) and marmoset (2.0 mg/kg). Repeated-dosing for 7 weeks in mice at 2 and 5 mg/kg IV resulted in clinical signs of transient ptosis and hypoactivity within 1 hour post-dose. No effects on hematology or tissue pathology were observed. These results appear consistent with the BLZ-100 data presented here. ICG itself has been used safely for decades in ophthalmology as a retinal angiography imaging agent. In a review article on the use of ICG in choroidal angiography, Weichel et al31 reported 7 reactions out of 1923 ICG angiograms performed in 1226 patients. The reactions included nausea and vomiting in 2 cases, urticaria in 2 cases, vasovagal reactions in 2 cases and acute hypotension in 1 case (0.3% adverse reaction rate). Olsen et al32 highlighted there was only 1 severe reaction among the 1226 patients (0.05%) and only 2 severe reactions among 2820 patients receiving ICG angiograms from Japanese ophthalmologists. It is also worth noting that the typical doses of ICG vary by indication, but generally range from 25 to 50 mg. By comparison, BLZ-100 contains roughly 0.15 mg dye/mg of drug product. BLZ-100 imaging doses for peripheral tumors are currently estimated to range from 3 to 12 mg, or 0.45 to 1.8 mg equivalents of ICG. Doses for imaging of brain cancer appear to be higher, ranging from 18 to 30 mg, or 2.7 to 4.5 mg equivalents of ICG.
Typical clinical use and exposure to BLZ-100 will be of short duration, consisting of a single injection prior to undergoing surgical excision of cancer. Second or repeat doses are possible in some subjects, possibly due to incomplete excision/tumor regrowth or a second cancer. However, it is likely the time interval between additional doses is months to years. The nonclinical safety profile presented here supported initiation of Phase I human clinical trials.
Figure 5.

Mean BLZ-100 serum concentrations after single bolus IV doses in nonhuman primates. The mean maximal concentration of BLZ-100 was observed at the first measured timepoint (0.083 hr [5 min] post-dose) in all BLZ-100 dose groups (0.6, 6, and 60 mg). Mean serum concentrations were measurable (> 5 ng/mL) out to 8, 48, and 120 hr post-dose (the last measured timepoint) in the 0.6, 6, and 60 mg dose groups, respectively.
Acknowledgments
We thank Carolyn Gombotz, Tori Pinkerton, Gordon Brandt, Claudia Jochheim, Jennifer Zimmer, Mark Stroud, Natalie Nairn, Sandra D. Love, and Devendra Dandekar for administrative and technical support. Portions of this project were funded with Federal funds from National Cancer Institute, NIH, Department of Health and Human Services, under contract no. HHSN261201200054C.
Abbreviations
- CTX
chlorotoxin
- ICG
indocyanine green
- NHP
non-human primate
- NIR
near-infrared
- NOAEL
no-observed-adverse-effect-level
Footnotes
Conflicts of interest
KBB and KST are consultants for Blaze Bioscience Inc. and Blaze Bioscience Australia Pty Ltd. JMO is a co-founder of Blaze Bioscience Inc., holds equity in Blaze and serves on the BOD.
References
- 1.Ramakrishna R, Barber J, Kennedy G, et al. Imaging features of invasion and preoperative and postoperative tumor burden in previously untreated glioblastoma: Correlation with survival. Surgical Neurology International. 2010;1:40. doi: 10.4103/2152-7806.68337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JS, Chang EF, Lamborn KR, et al. Role of the extent of resection in the long-term outcome of low-grade hemispheric gliomas. Journal of Clinical Oncology. 2008;26(8):1388–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 3.Zacharoulis S, Moreno L. Ependymoma: An update. Journal of Child Neurology. 2009;24:1431–1438. doi: 10.1177/0883073809339212. [DOI] [PubMed] [Google Scholar]
- 4.Atean I, Pointreau Y, Rosset P, Garaud P, De-Pinieux G, Calais G. Prognostic factors of extremity soft tissue sarcoma in adults. A single institutional analysis. Cancer radiotherapie: journal de la Societe francaise de radiotherapie oncologique. 2012 Dec;16(8):661–666. doi: 10.1016/j.canrad.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Kouzminova NB, Aggarwal S, Aggarwal A, Allo MD, Lin AY. Impact of initial surgical margins and residual cancer upon re-excision on outcome of patients with localized breast cancer. Am J Surg. 2009 Dec;198(6):771–780. doi: 10.1016/j.amjsurg.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Campos FG, Calijuri-Hamra MC, Imperiale AR, Kiss DR, Nahas SC, Cecconello I. Locally advanced colorectal cancer: results of surgical treatment and prognostic factors. Arquivos de gastroenterologia. 2011 Oct-Dec;48(4):270–275. doi: 10.1590/s0004-28032011000400010. [DOI] [PubMed] [Google Scholar]
- 7.DeBin JA, Maggio JE, Strichartz GR. Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol Cell Physiol. 1993;264:C361–C369. doi: 10.1152/ajpcell.1993.264.2.C361. [DOI] [PubMed] [Google Scholar]
- 8.Ojeda PG, Wang CK, Craik DJ. Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Biopolymers. 2016 Jan;106(1):25–36. doi: 10.1002/bip.22748. [DOI] [PubMed] [Google Scholar]
- 9.Stroud MR, Hansen SJ, Olson JM. In vivo bio-imaging using chlorotoxin-based conjugates. Curr Pharm Des. 2011;17(38):4362–4371. doi: 10.2174/138161211798999375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X-S, Jian X-C, Yin B, He Z-J. Development of the research on the application of chlorotoxin in imaging diagnostics and targeted therapies for tumors. Chinese Journal of Cancer. 2010;29(6):626–630. doi: 10.5732/cjc.009.10359. [DOI] [PubMed] [Google Scholar]
- 11.Fidel J, Kennedy KC, Dernell WS, et al. Preclinical Validation of the Utility of BLZ-100 in Providing Fluorescence Contrast for Imaging Spontaneous Solid Tumors. Cancer Res. 2015 Oct 15;75(20):4283–4291. doi: 10.1158/0008-5472.CAN-15-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baik FM, Hansen S, Knoblaugh SE, et al. Fluorescence Identification of Head and Neck Squamous Cell Carcinoma and High-Risk Oral Dysplasia With BLZ- 100, a Chlorotoxin-Indocyanine Green Conjugate. JAMA otolaryngology– head & neck surgery. 2016 Apr 1;142(4):330–338. doi: 10.1001/jamaoto.2015.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butte PV, Mamelak A, Parrish-Novak J, et al. Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurgical Focus. 2014;36(2):E1. doi: 10.3171/2013.11.FOCUS13497. [DOI] [PubMed] [Google Scholar]
- 14.Kittle DS, Mamelak A, Parrish-Novak J, et al. Fluorescence-guided tumor visualization using the Tumor Paint BLZ-100. Cureus. 2014 Sep 22;6(9):e2014. 2014. [Google Scholar]
- 15.Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill A, Inventor; TransMolecular, Inc., assignee Systemic administration of chlorotoxin agents for the diagnosis and treatment of tumors. 2010 Aug 26; 2010. [Google Scholar]
- 17.Gribbin T, Senzer N, Raizer J, et al. A phase I evaluation of intravenous (IV) ^131 I-chlorotoxin delivery to solid peripheral and intracranial tumors. J Clin Oncol. 2009;27(suppl) abstr e14507. [Google Scholar]
- 18.Hockaday DC, Shen S, Fiveash J, et al. Imaging glioma extent with131 I-TM- 601. The Journal of Nuclear Medicine. 2005;46(4):580–586. [PubMed] [Google Scholar]
- 19.Mamelak AN, Rosenfeld S, Bucholz R, et al. Phase I single-dose study of intracavitary-administered iodine-131-TM-601 in adults with recurrent highgrade glioma. Journal of Clinical Oncology. 2006;24(22):3644–3650. doi: 10.1200/JCO.2005.05.4569. [DOI] [PubMed] [Google Scholar]
- 20.Kesavan K, Ratliff J, Johnson EW, et al. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem. 2010;285(7):4366–4374. doi: 10.1074/jbc.M109.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo M, Hajjar KA. Annexin A2 system in human biology: cell surface and beyond. Seminars in thrombosis and hemostasis. 2013 Jun;39(4):338–346. doi: 10.1055/s-0033-1334143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLeod TJ, Kwon M, Filipenko NR, Waisman DM. Phospholipid-associated Annexin A2-S100A10 heterotetramer and its subunits. J Biol Chem. 2003;278(28):25577–25584. doi: 10.1074/jbc.M301017200. [DOI] [PubMed] [Google Scholar]
- 23.Deshane J, Garner CC, Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003 Feb 7;278(6):4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 24.Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engel E, Schraml R, Maisch T, et al. Light-induced decomposition of indocyanine green. Investigative ophthalmology & visual science. 2008 May;49(5):1777–1783. doi: 10.1167/iovs.07-0911. [DOI] [PubMed] [Google Scholar]
- 26.Developing Medical Imaging Drug and Biological Products. Part I: Conducting Safety Assessments. 2004:1–18. [Google Scholar]
- 27.Herzberg IM, Trudeau MC, Robertson GA. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. The Journal of physiology. 1998 Aug 15;511(Pt 1):3–14. doi: 10.1111/j.1469-7793.1998.003bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imamura H, Furukawa Y, Kasama M, Hoyano Y, Yonezawa T, Chiba S. Inhibition of delayed rectifier K+ current by dofetilide and E-4031 differentially affects electrical cardiac responses to vagus stimulation in anesthetized dogs. Jpn J Pharmacol. 1998 Jan;76(1):31–37. doi: 10.1254/jjp.76.31. [DOI] [PubMed] [Google Scholar]
- 29.Szebeni J. Complement activation-related pseudoallergy: a new class of drug- induced acute immune toxicity. Toxicology. 2005 Dec 15;216(2–3):106–121. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Wang HS, Liu ZP. Agents that induce pseudo-allergic reaction. Drug discoveries & therapeutics. 2011 Oct;5(5):211–219. doi: 10.5582/ddt.2011.v5.5.211. [DOI] [PubMed] [Google Scholar]
- 31.Weichel E, Regillo C, Maguire J. Indocyanine Green Angiography. In: Tasman W, Jaeger E, editors. Duane’s Ophthalmology. Vol. 3. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 32.Olsen TW, Lim JI, Capone A, Jr, Myles RA, Gilman JP. Anaphylactic shock following indocyanine green angiography. Archives of ophthalmology (Chicago, Ill: 1960) 1996 Jan;114(1):97. doi: 10.1001/archopht.1996.01100130093018. [DOI] [PubMed] [Google Scholar]
- 33.Vail DM, Veterinary Co-operative Oncology Group Vet Comp Oncol. 2004 Dec;2(4):194. doi: 10.1111/j.1476-5810.2004.0053a.x. [DOI] [PubMed] [Google Scholar]


