Abstract
Among nonhuman primates, chimpanzees are well known for their sophistication and diversity of tool use in both captivity and the wild. The evolution of tool manufacture and use has been proposed as a driving mechanism for the development of increasing brain size, complex cognition and motor skills, as well as the population-level handedness observed in modern humans. Notwithstanding, our understanding of the neurological correlates of tool use in chimpanzees and other primates remains poorly understood. Here, we assessed the hand preference and performance skill of chimpanzees on a tool use task and correlated these data with measures of neuroanatomical asymmetries in the inferior frontal gyrus (IFG) and the pli-de-passage fronto-parietal moyen (PPFM). The IFG is the homolog to Broca’s area in the chimpanzee brain and the PPFM is a buried gyrus that connects the pre- and post-central gyri and corresponds to the motor-hand area of the precentral gyrus. We found that chimpanzees that performed the task better with their right compared to left hand showed greater leftward asymmetries in the IFG and PPFM. This association between hand performance and PPFM asymmetry was particularly robust for right-handed individuals. Based on these findings, we propose that the evolution of tool use was associated with increased left hemisphere specialization for motor skill. We further suggest that lateralization in motor planning, rather than hand preference per se, was selected for with increasing tool manufacture and use in Hominid evolution.
Keywords: Tool use, Handedness, Hand skill, Pli-de-passage, Broca’s area, Chimpanzee
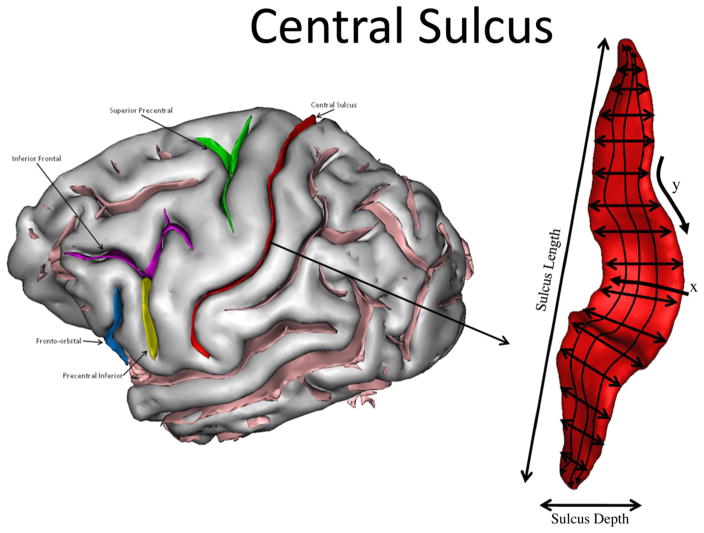
The central sulcus (CS) of the brain divides the pre- and post-central gyri, the area in which all primary motor and sensory processes are represented in the cortex. The pli-de-passage fronto-parietal moyen parietale (PPFM) is a buried gyrus that in centrally located along the CS and connects the pre- and post-central gyri [1]. The presence of the PPFM has resulted in the formation of a neuroanatomical landmark in the CS referred to as the motor-hand region of the precentral gyrus or KNOB [2]. In the axial view, the KNOB is an epsilon or omicron shaped posterior displacement of the CS in the central region. Functional imaging and electrical stimulation studies in humans and chimpanzees have shown that the KNOB corresponds to the region where the fingers and hand are represented in the primary motor and sensory cortex [1–6]. Furthermore, studies in human and nonhuman primates have shown that individual variation in the size of the KNOB is associated with handedness [7–11], as well as specific motor learning experiences, such as playing a musical instrument. For instance, several studies have shown differences in the morphology and connectivity of the KNOB of expert musicians who use their hands, such as guitarists and piano players, compared to novice musicians [12,13]. These collective findings have led some to suggest that the KNOB represents one neurobiological substrate for handedness and variability of motor skills in humans.
The motor-hand area of the precentral gyrus is also present in great ape brains, but not in the brains of more distantly related primates, such as Old and New World monkeys [14,15]. This suggests that the formation of the KNOB is a recent evolutionary development and may reflect increased gyrification and cortical folding in regions of the CS associated with the emergence of greater motor control of the individual digits and possibly handedness. For instance, hand preferences for coordinated bimanual actions are associated with asymmetries of the KNOB in chimpanzees [9,16]. Moreover, PET imaging of reach-and-grasp actions in chimpanzees has been localized to the KNOB region in the hemisphere contralateral to the hand used during responding [17]. These results suggest that the KNOB region in chimpanzees may also serve as the cortical representation of the hand.
In addition to the motor hand area of the precentral gyrus, there are important premotor regions involved in motor planning and execution, notably the inferior frontal gyrus (IFG)[18] or Broca’s area [19–22]. In humans, clinical data and functional imaging studies have identified Broca’s (among several) as an important region involved in the voluntary production of planned motor actions particularly for grasping and tool-use [23–26]. Furthermore, clinical studies have shown that individuals with lesions to the inferior frontal gyrus often exhibit apraxia, a deficit in planned motor actions [27,28]. Previous studies in chimpanzees have shown that chimpanzees that prefer to use the right-hand have larger fronto-orbital sulci than individual who are non-right-handed [18]. The fronto-orbital sulcus is the anterior border of the IFG and is the primary location of Brodmann area 45 cells in the chimpanzee brain [29,30].
In this study, we examined whether individual differences in the motor hand area of the precentral gyrus and IFG regions were associated with asymmetries in hand preference or motor performance for tool use in a sample of chimpanzees. Though many species use tools, chimpanzees are considered the most sophisticated tool users in the animal kingdom, save humans [31,32]. Different forms of tool use are wide spread in chimpanzees from various communities throughout Africa and the diversity in tool use is thought to reflect learning of localized social traditions [33,34]. Some have hypothesized that the selection for increased motor skill associated with tool use was a driving force toward the emergence of increased brain size and lateralization of structure and function in the human brain [35–37]. In humans, functional imaging studies have shown that motor planning associated with tool use for both the left and right hands is controlled by premotor and primary motor cortex in the left hemisphere [38,39]. In nonhuman primates, there is little data on the neurobiological correlates of tool use, though such data would be important for understanding the origin of praxic and motor skill functions in the human brain. There is some evidence that individual difference in hand preferences for tool use are associated with asymmetries and interhemispheric connectivity in the brains of chimpanzees and capuchin monkeys [18,40,41]; however, we know of no studies that have examined neuroanatomical correlates of asymmetries in tool-use skill. Here, we quantified and the volume of the IFG and the cortical folding of the CS in the region corresponding to the pli-de-passage fronto-parietal moyen (PPFM) in a sample of captive chimpanzees. Additionally, we measured hand preference and performance on a tool-use task designed to simulate the termite fishing seen in wild chimpanzees. We then examined the effects whether hand preference or asymmetries in tool use performance were associated with lateralization in the PPFM and IFG. We hypothesized that asymmetries within the PPFM and IFG region would be larger in the hemisphere contralateral to their preferred hand or the hand that performed the task better than the opposite hand.
1. Methods
1.1. Subjects
Magnetic resonance images (MRI) were obtained from a total of 189 chimpanzees (Pan troglodytes) including 116 females and 73 males. All subjects were housed at either the Yerkes National Primate Research Center (YNPRC, n = 68) or the National Center for Chimpanzee Care (NCCC, n = 121) in social groups ranging from 2 to 20 individuals. All of the subjects in this paper had been previously tested for hand preference and performance on a tool use task that had been designed to simulate termite fishing [42]. Institute of Medicine guidelines for the use of chimpanzees in research were adhered to during all aspects of the study.
1.2. Image collection and procedure
All chimpanzees were scanned during their annual physical examination. Magnetic resonance image (MRI) scans followed standard procedures at the YNPRC and NCCC and were designed to minimize stress. Thus, the animals were first sedated with ketamine (10 mg/kg) or telazol (3–5 mg/kg) and were subsequently anaesthetized with propofol (40–60 mg/(kg/h)). They were then transported to the MRI scanning facility and placed in a supine position in the scanner with their head in a human-head coil. Upon completion of the MRI, chimpanzees were singly-housed for 2–24 h to permit close monitoring and safe recovery from the anesthesia prior to return to their home social group. All procedures were approved by the Institutional Animal Care and Use Committees at YNPRC and NCCC and also followed the guidelines of the Institute of Medicine on the use of chimpanzees in research. Sixty-six chimpanzees were scanned using a 3.0 T scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA). T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2300 ms, echo time = 4.4 ms, number of signals averaged = 3, matrix size = 320 × 320, with 0.6 × 0.6 × 0.6 resolution). The remaining 123 chimpanzees were scanned using a 1.5T G.E. echo-speed Horizon LX MR scanner (GE Medical Systems, Milwaukee, Wisconsin, USA). T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged = 8, matrix size = 256 × 256, with 0.7 × 0.7 × 1.2 resolution).
1.3. Image processing
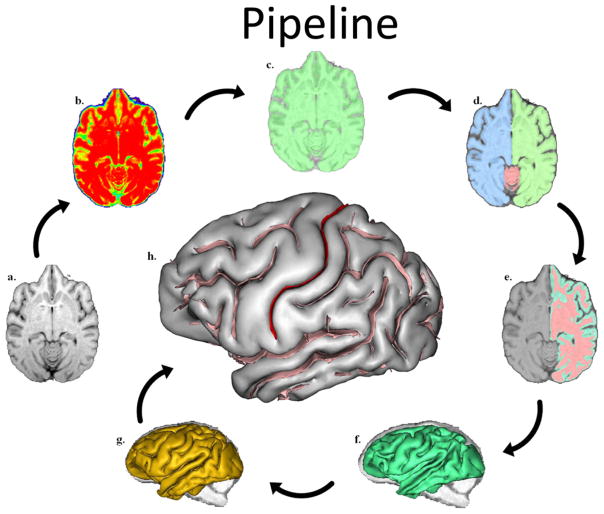
PPFM: The sequence of post-image processing steps performed from the images are shown in Figs. 1a to 1h and have been described in detail elsewhere [43]. The steps used to extract the central sulcus (CS) from the raw T1-weighted image derives from a pipeline initially dedicated to the human brain and freely distributed as a BrainVISA toolbox (http://brainvisa.info) [44]. The human-dedicated pipeline has been used previously for at least 5000 different human subjects. The pipeline processing steps proceeded in the following manner: First, corrections of the spatial inhomogeneities of the signal, which prevent direct association between the signal intensity and the nature of the tissue, were performed (see Fig. 1A). The estimation of the spatially smooth bias field (see Fig. 1B) used to restore the signal intensity was performed via minimisation of the signal entropy [45]. After correction, each tissue intensity distribution was stable across the brain (see Fig. 1C). Second, automatic analysis of the signal histogram and mathematical morphology was used to compute a binary mask of the brain (see Fig. 1C). This approach is built on the fact that the brain is surrounded by dark areas corresponding to skull and cerebrospinal fluid (CSF). Therefore, once the range of intensities corresponding to brain tissue had been defined by histogram analysis, brain segmentation mainly amounts to splitting the connections with external structures, like the optical nerves. For the chimpanzee anatomy, some specific tuning had to be applied relative to the human-dedicated processing performed by BrainVISA [46]. For some chimpanzees, indeed, the largest object in the image after splitting connections turned out to be the muscles. Hence, in order to reliably select the brain, we had to introduce an additional constraint relative to the localization of the brain in the center of the head. Once the brain mask had been defined, the mask was split into three parts corresponding to hemispheres and cerebellum (see Fig. 1D) [47].
Fig. 1.
BrainVISA’s pipeline processing steps. a) MR image of a skull-stripped chimpanzee brain, b) stable tissue intensities after bias field correction, c) binary mask of the brain, d) split mask of left and right hemispheres and cerebellum, e) grey and white interface, f) A negative mould of the white matter, g) skeletonised mould of cortical folding, h) cortical fold graph of chimpanzee sulci with the central sulcus in red.
After a mask had been defined for each hemisphere, a negative mould of the white matter was computed [47]. The outside boundary of this mould results from a 5-mm morphological closing of the masked hemisphere, filling up the folds. The inside boundary is the grey/white interface computed with topology preserving deformations assuring the spherical topology of the mould (see Fig. 1F). The mould is finally skeletonized in order to detect the cortical fold as crest surfaces of the 3D MR image located inside the mould [44]. These surfaces stem from a morphological watershed process iteratively eroding the mould from the lightest intensities to the darkest intensities. Topological constraints guarantee that the resulting surfaces have no holes. The end result is a set of topologically elementary surfaces located along the darkest part of the fold corresponding to CSF (see Fig. 1G). These elementary surfaces are split further when a deformation of the deepest part of the fold indicates the presence of a buried gyrus. The clues allowing the detection of buried gyri are embedded in the curvature of the grey/white interface [44]. Finally, the fold making up the (CS) was selected manually by the user using a 3D visualization interface (see Fig. 1H). The extracted CS from a representative subject in this study is shown in Fig. 2.
Fig. 2.
3-D reconstruction of the sulci of the chimpanzee brain with the central sulcus indicated to the right.
1.4. CS depth measures
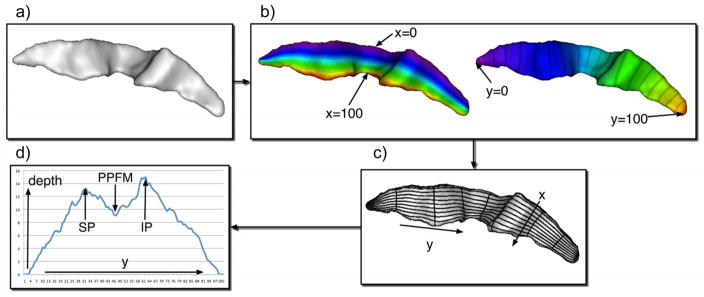
We obtained two measures of CS folding. First, the average depth was computed for the entire CS. Mean depth (mm) is the average depth of the sulcus along its principal axis of projection (i.e., dorsal-ventral) and this is automatically computed by Brain-Visa. In addition, we calculated the depth of the CS in the region corresponding to the PPFM by parameterizing the entire CS and identifying key landmarks used to localize the region. For this measurement, the selected CS is meshed using a triangular mesh, and the resulting surface is parameterized in order to create a normalized x-y coordinate system [48]. The parameterization process is constrained by four features: the bottom ridge of the sulcal mesh (i.e., the sulcal fundus), the top ridge (i.e., at the brain envelope), and the end points of the sulcus where these top and bottom ridges joined. From these features, two coordinate fields (x and y) are extrapolated over the entire mesh surface, by solving the heat equation on the surface, with the constraints behaving as constant heat sources [48]. This results in a smooth x-y coordinate system, with mesh surface points localized in respect to the features. The coordinate system extended along the length of the CS from the superior (y = 1) to the inferior end of the sulcus (y = 100), and from the brain envelope (x = 1) to the fundus (x = 100) of the sulcus (see Fig. 3). Depth was measured at 100 sulcal length positions in a superior-to-inferior progression along the parameterized sulcal mesh surface. Position 1 was adjacent to the interhemispheric fissure and position 100 was adjacent to the sylvian fissure (see Fig. 3). At each position, y, along the length, the depth is computed by measuring a geodesic distance (in millimeters) from x = 1 to x = 100 at constant y.
Fig. 3.
a) Chimpanzee central sulcus b & c) the surface area and depth dimensions are shown in the extracted sulcus, as well as the x and y coordinates used for computing differences in cortical folding of the CS along the dorsal-ventral axis d) outputted data from CS parameterization. Depth of CS is plotted on ordinate and the y coordinate along the abscissa. SP = superior maximum CS depth before y coordinate 50, IP = maximum inferior depth after y coordinate 50, PPFM = pli-de-passage moyen parietale, which is the shallowest CS depth measure between the SP and IP y coordinates.
1.5. Determining the SP, PPFM and IP
Following the previously explained procedure, the individual depth measures for each hemisphere at each of the 100 positions were outputted to a text file. To quantify the region corresponding to the PPFM, we followed procedures that have previously been employed with human and chimpanzee brains [8,49]. Specifically, the largest depth value between positions 1 to 50 was determined to be the maximum superior point (SP) of the CS, while the largest depth value found between positions 51 to 100 was determined to be the maximum inferior point (IP). The point of minimum value was determined to be the PPFM position. From these measures, we computed the maximum depth of the PPFM for the left and right hemisphere following the formula: [PPFM max = ((depth(IP) + depth(SP))/2.0) − depth(PPFM)].
1.5.1. Inferior frontal gyrus (IFG) volume
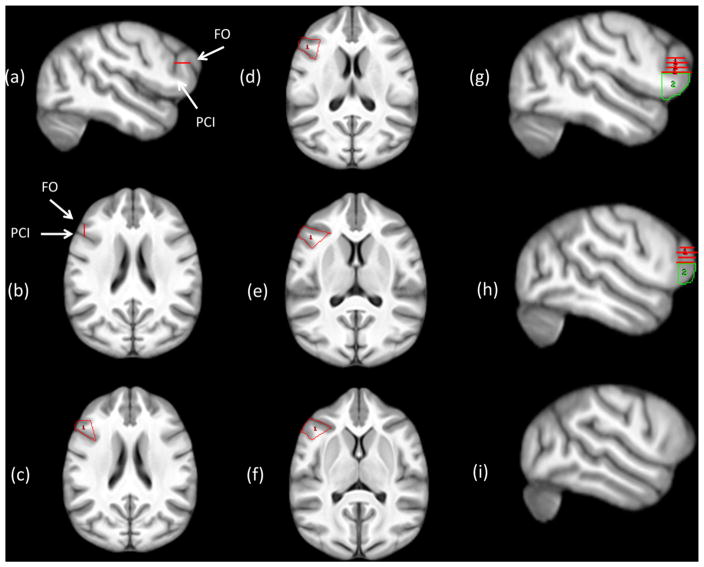
We measured the IFG using methods that have been described in detail elsewhere [50]. At the start, the image was first oriented into the sagittal plane, where the most lateral slice with a fully visible fronto-orbital (FO) sulcus was identified. A perpendicular straight line was then drawn from the most superior edge of the FO to the inferior precentral inferior sulcus (PCI). The image was then flipped into the transverse plane and the image containing the previously drawn line served as the most dorsal border for the IFG. Starting at the most dorsal transverse point, the anterior and posterior borders of IFG were the FO and PCI sulci. On each image moving ventrally, using a mouse drive pointer, the entire gyrus between the most medial points of PCI and FO were drawn. The lateral boundary was the surface of the brain (see Fig. 5). This was repeated for all slices ventral to the first slice traced until the inferior boundary was reached. The inferior boundary was determined by the crossing of the medial boundary by the anterior limb of the insula or the termination of the PCI sulcus. In cases where PCI bifurcation arose, both anterior and posterior limbs were included only if they branched from a single, common origin, which could be confirmed when viewed in the sagittal plane. Following completion of the transverse tracing, the image was flipped into the sagittal plane and the first lateral slice where the insula was no longer visible was located. The ROI was extended from the PCI if it was still apparent or straight down if it was not to the inferior edge of the brain (see Fig. 5). We then traced along the anterior outer contour of the cortex until the line connected with the most superior intersection with FO. This was repeated for all slices lateral to the first closing of the insula for both hemispheres. When tracing was completed, the object map was saved and subsequently applied to the segmented GM and WM volume. The volume of grey and white matter within the IFG object map was then computed for the left (L IFG) and right (R_IFG) hemisphere for each subject. The individuals tracing the brains were blind to the sex and handedness of the chimpanzees.
Fig. 5.
Method used to quantify the volume of the IFG. a) identification of the superior border of the IFG (red horizontal line) b) flip brain into transverse view and identify the superior border (red vertical line) c–f) trace gyrus (red) using the FO and PCI sulci as the anterior and posterior borders g–h) reorient brain into sagittal plane and draw ROI (green) from the inferior point of PCI to the sylvian fissure and along the outer contour of the cortex until meeting the FO sulcus i) neither PCI or FO visible, terminate tracing. PCI = precentral inferior sulcus, FO = fronto-orbital sulcus.
1.6. Behavior
Hand preference and performance data were taken from the previous published study by Hopkins et al. [42]. Briefly, the motor and cognitive demands of termite fishing were simulated by using threaded poly-vinyl-chloride PVC pipes (approx. 4 cm in diameter and approx. 20 cm in length) attached to threaded (PVC) bases that were affixed to the subjects’ home enclosures at multiple locations approximately 60 to 80 cm above the ground or floor. The pipes were fitted with a disc in one end with a 7 mm hole cut out to greatly reduce the size of the opening available for tool insertion, thus increasing the motor demands of the task. The other end was closed with a removable screw on cap. The pipes were filled with a preferred food substance that would adhere to the tool, such as mustard, yogurt, syrup, or applesauce, before being screwed into the bases. The chimpanzees were provided with flexible, thin ‘lollipop’ sticks (approx. 11 cm in length and 4 mm in diameter, like those used to make large lollipops), made out of tightly rolled, thin paper. The animals used the lollipop sticks to dip into the small hole of the pipe and retrieve the food, thereby simulating the actions of termite fishing observed in the wild.
1.7. Procedure
Each subject was tested for hand use and performance on 50 insertions of the lollipop stick into the device. Each PVC pipe was filled with a preferred food substance and then screwed onto a base. After placing the device on the enclosure, each subject was supplied with a tool by either handing it to them directly or by dropping it near them. If the tool became unusable, a new tool was offered to the subject. The experimenter recorded the hand used by the subject (left or right) each time they successfully inserted the tool into the pipe. In addition to hand use, the experimenter recorded the time required to insert the stick into the hole during each attempted probe. Time per dip was measured from the time the subject initiated an attempt to insert the tool with one hand and ended when the chimpanzee successfully inserted and removed the tool. If subjects stopped attempting to use the tool, or switched the tool to their mouth or other hand the response was not counted.
1.8. Data analysis
Hand preferences were characterized two ways. First, we classified subjects as right-, left- or ambiguously-handed by using z-scores based on the frequency in left and right hand use. This is the procedure most frequently used in the non-human primate literature to characterize individual hand preferences [51,52]. Subjects with z-scores greater than 1.96 or less than −1.96 were classified as right- and left-handed, respectively. All other subjects were classified as ambiguously-handed. We also calculated a latency handedness index (LI) for each subject. For each chimpanzee, we averaged the latency to dip for the right and left hands and computed the LI score following the formula LI = (R − L), where R and L represented the mean latency in dipping responses for the right and left hands, respectively. Positive LI values reflected faster performance by the left compared to right hand. Negative LI values indicated faster performance by the right compared to left hand. Thus, based on the sign of their LI score, subjects were classified as left- or right-handed. For the IFG, an asymmetry quotient (IFG AQ) was derived following the formula [AQ = (R − L)/((R + L)*.5))] where R and L represent the right and left hemisphere volumes. Positive AQ values represented right hemisphere asymmetries and negative values reflected left hemisphere biases. For the PPFM, we also computed an asymmetry coefficient (PPFM AQ) following the formula [AQ = (L − R)/((R + L) *0.5)] where R and L indicated the right and left hemisphere PPFM max values. Positive AQ values indicated a leftward asymmetry while negative values indicated a rightward asymmetry. By way of interpretation of the results reported here, it is important to recognize that positive PPFM AQ values actually reflect a leftward asymmetry in the size of the PPFM along the sulcus. This is because the PPFM is a buried gyrus and presumably the larger gyrus would prevent the CS from increased cortical folding or depth values [1]. Thus, a positive AQ values reflects a larger right PPFM and a negative AQ reflects a larger left PPFM gyrus. Fig. 4 illustrates the theoretical relationship between the sulcus shape and the presumed PPFM gyrus size that might underlie the variation. Presumably, a larger PPFM would allow for greater intrahemispheric connectivity between the pre- and post-central gyri in the region corresponding to the motor-hand area. Finally, we also classified subjects as left-, right- or non-lateralized (no bias) based on the sign and strength of their AQ values. Following cut-points used in the human literature [53], we classified chimpanzees with AQ values ≥ 0.025 or AQ ≤ −0.025 as right (R) and left (L) lateralized, respectively. Subjects with AQ scores > −0.025 and < 0.025 were classified as having no bias (NB).
Fig. 4.
Illustration of the buried pli-de-passage within the precentral gyrus (blue lines) and how the CS (red) folds over and around it to form the PPFM region measured from the depth measures. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Results
2.1. Descriptive behavioral data
By way of the descriptive data, for the hand preference measure, there were 78 left-, 44 ambiguously- and 67 right-handed chimpanzees. This distribution did not differ significantly from a predicted random distribution. A chi-square test of independence also failed to reveal a significant association between hand preference and sex X2(2, N = 189) = 0. 360, p = 0.654 (see Table 1). When considering hand preference based on differences in performance, there were 91 left- and 98 right-preferent individuals and this distribution also did not differ from a predicted random distribution X2(1, N = 189) = 0.20, p = 0.99. However, we did find a significant association between sex and hand preferences based on performance differences between the hands X2(2, N = 189) = 4.197, p = 0.040 (see Table 1). There were a significantly higher proportion of females who performed better with their right hand (58%, 67/116) compared to the males (43%, 31/73).
Table 1.
Number of Left-, Ambiguously-, and Right-Handed Chimpanzees Based on z-scores From the Tool Use Task.
| #L | #A | #R | |
|---|---|---|---|
| Hand Preference | |||
| Males | 31 | 18 | 24 |
| Females | 47 | 26 | 43 |
| Total | 78 | 44 | 67 |
| Hand Performance | |||
| Males | 42 | – | 31 |
| Females | 49 | – | 67 |
| Total | 91 | 98 | |
Additionally, we examined the distribution of hand preference within the subsamples of chimpanzees that were classified as left-or right-handed based on the between hand differences in performance (see Table 2). Within the sample that performed better with their right hand, there were 32 left-, 17 ambiguously- and 49 right-handed chimpanzees. The number of right-handed chimpanzees was significantly higher than the number of ambiguously-X2(1, N = 66) = 14.56, p = 0.001 but not left-handed individuals X2(1, N = 81) = 3.16 p =0.076. For those chimpanzees that performed better with their left hand, there were 46 left-, 27 ambiguously-and 18 right-handed individuals. The number of left-handed chimpanzees was significant greater than the number of right-X2(1, N = 64) = 11.40, p = 0.001, and ambiguously-handed X2(1, N = 73) = 4.44, p = 0.035 chimpanzees. Thus, hand preferences generally conformed to performance asymmetries between the hands. This interpretation is further supported by the finding of a significant negative correlation between the HI and LI scores (r = −0.332, df = 187, p = 0.001) showing that more right-handed chimpanzees performed better with the right hand compared to the left. However, it should be noted that the relationship was not perfect, with 50 individuals showing a complete disconcordance between their preferred hand and the hand that performed the task better. Lastly, we examined whether hand preference and sex had a significant effect on overall tool use performance. For this analysis, we used analysis of covariance (ANOVA) with sex and hand preference as between-group factors while the performance standardized z-scores served as the dependent measure. No significant main effects or interactions were found.
Table 2.
Distribution of Hand Preferences for Chimpanzees that Performed the Tool Use Task Better with their Left or Right Hand.
| Left-Handed | Ambiguous | Right-Handed | |
|---|---|---|---|
| Better with Left Hand | 46 | 27 | 18 |
| Better with Right Hand | 32 | 17 | 49 |
2.2. Descriptive neuroanatomical data
The average AQ score and the distribution in laterality for the IFG and PPFM are shown in Table 3. One sample t-tests failed to reveal population-level asymmetries for either the PPFM or IFG and independent samples t-tests revealed no significant differences in AQ scores for either brain region. When considering only the lateralized subjects (there were too few non-lateralized subjects), the number of left- chimpanzees was significantly greater than the number of right-lateralized individuals for the PPFM X2(1, N = 182) = 5.28, p = 0.021 but not the IFG X2(1, N = 177) = 0.02, p = 0.887. Chi-square tests of independence revealed no significant associations between sex and the distribution of laterality for either the PPFM X2(2, N = 189) = 0.51, p = 0.775 or IFG X2(1, N = 189) = 2.326, p = 0.312.
Table 3.
Distribution of Asymmetries in the PPFM and IFG in Male and Female Chimpanzees.
| #L | #NB | #R | Mean AQ | s.e. | t | p | |
|---|---|---|---|---|---|---|---|
| PPFM | |||||||
| Males | 41 | 2 | 31 | −0.034 | 0.068 | −0.494 | 0.623 |
| Females | 66 | 5 | 44 | −0.048 | 0.050 | −0.938 | 0.350 |
| Overall | 107 | 7 | 75 | −0.042 | 0.040 | −1.03 | 0.302 |
| IFG | |||||||
| Males | 29 | 5 | 40 | 0.063 | 0.049 | 1.28 | 0.203 |
| Females | 58 | 7 | 50 | −0.001 | 0.038 | −0.178 | 0.859 |
| Overall | 87 | 12 | 90 | +0.021 | 0.030 | +0.68 | 0.497 |
L = left lateralized, NB = non-lateralized, R = right lateralized.
s.e. = standard error.
2.3. Neuroanatomical correlates in tool use hand preference and performance asymmetries
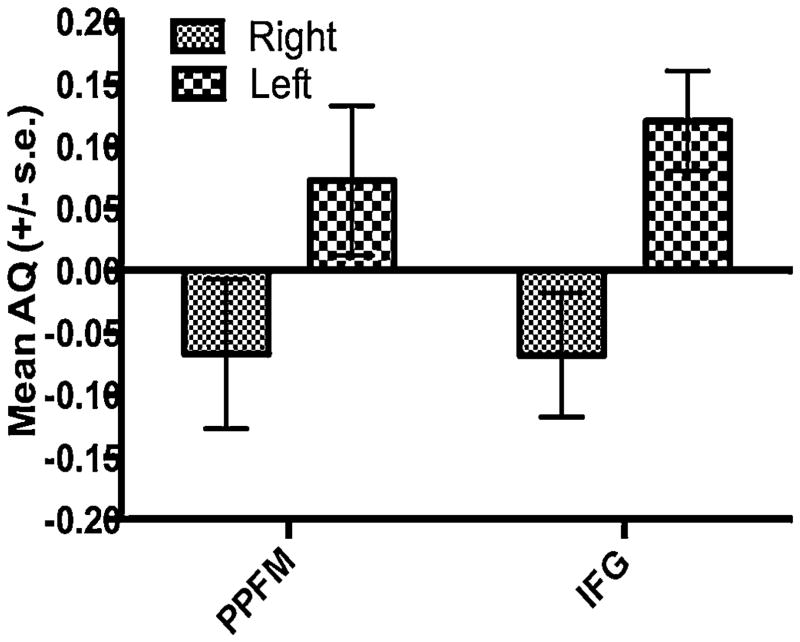
In the initial analysis, we performed a mixed-model analysis of variance (ANOVA) with the PPFM AQ and IFG AQ scores serving as the repeated measures while sex, hand preference (Left, Ambiguous, Right) and hand performance group (Left, Right) were the between-group factors. We found a significant main effect for performance group F(1, 177) = 8.290, p = 0.004. The mean AQ scores for the PPFM and IFG in chimpanzees that performed the tool task better with their right or left hand is shown in Fig. 6. Overall, chimpanzees that performed the tool use task better with their right hand had significantly greater leftward AQ scores (Mean AQ = −0.067, s.e. = 0.041) compared to apes that performed better with their left hand (Mean AQ = 0.095, s.e. = 0.039). The difference in performance groups was consistent across the two brain regions.
Fig. 6.
Mean PPFM AQ and IFG AQ (+/− standard errors) for chimpanzees that performed better with their right or left hands on the tool use task. Positive AQ values indicate a rightward asymmetry, while negative values indicate a leftward asymmetry.
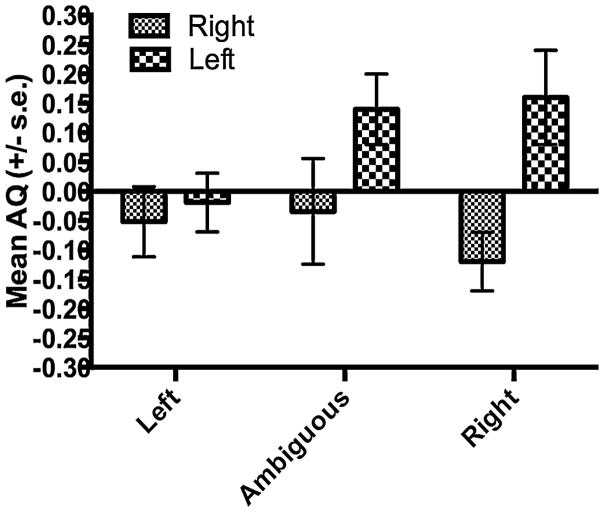
Though the interaction between performance group and handedness was not significant, we performed a follow up analysis to further explore the mediating influence of hand preference on AQ scores in the two performance groups. For this analysis, we performed separate ANOVAs in the left, right and ambiguously-handed chimpanzees subsamples. For each analysis, repeated measure ANOVAs were performed with the PPFM and IFG AQ scores serving as the repeated measures while sex and performance group served as between group factors. The mean AQ scores in chimpanzees that performed the tool task better with their right or left hand within the left- ambiguously- and right-handed subsamples is shown in Fig. 7. No significant main effects or interactions were found for the left- and ambiguously-handed subsamples; however, for the right-handed chimpanzees, individuals that performed the task better with their right hand had significantly greater leftward asymmetries than individuals that performed better with their left hand F(1, 63) = 9.500, p = 0.003.
Fig. 7.
Mean PPFM AQ and IFG AQ values (+/− s.e.) for chimpanzees that performed better with their right or left hands on the tool use task within the left-, ambiguously-or right-handed subsamples.
Lastly, the previous analyses were based on the chimpanzees’ hand preference and performance classification based on the sign and strength of their HI and LI scores. The HI and LI scores were on a continuous scale of measurement; therefore, we performed yet another analysis to examine the relative contributions of hand preference and performance to variation in the PPFM and IFG AQ scores using multiple regression. For this analysis, we averaged the AQ scores for the PPFM and IFG regions to compute a single measure of asymmetry. We subsequently performed step-wise multiple regression entering sex, HI and subsequently LI as predictor variables and computing the significance in change in R2 with each variable. The full model regression was significant R=0.211, F(3, 177) = 2.741, p = 0.045. Entering the variables sex and HI did not account for a significant change in R2 but entering LI did account for a significant change in R2 =0.044, Fchange (1, 177) = 6.935, p = 0.009. Thus, after accounting for sex and hand preference, between hand difference in latency accounts for a significant proportion of variance in the average PPFM and IFG AQ scores.
3. Discussion
Results showed that variability in motor performance on a tool-use task was associated with asymmetries in the motor-hand area of the precentral gyrus and inferior frontal gyrus in chimpanzees. The association between overall tool-use performance, IFG and PPFM asymmetries was modulated, to some extent, by hand preference. That is, differences in AQ scores were chimpanzees that performed the task better with their right compared to left hand were consistent across handedness groups but were particularly robust among chimpanzees that also preferred to use their right hand.
The results of this study suggest that, overall and somewhat independent of hand preference, chimpanzees with better tool using skill have a larger PPFM and IFG gyrus in the contralateral hemisphere (see Fig. 6). This was particularly the case for chimpanzees that had a right hand preference, but less so for those with a left- or no preference (Fig. 7). Though it is unclear how the hand preferences mediate the AQ scores in relation to performance asymmetries, one possible explanation may be related to the neurological distinction between motor execution and motor planning reported in human subjects. Functional imaging and clinical studies suggest that the execution of motor actions by the left and right hands are done by the contralateral hemisphere [54–56]; however, the planning of motor actions, particularly complex actions such as tool use, are largely controlled by premotor regions in the left hemisphere [38]. This seems to be the case for both left- and right-handed individuals [23,25,57]. In the case of the findings in this study, it might be hypothesized that the planning of precise motor actions, such as the tool-use task studied here, is carried out by the left hemisphere and this is independent of the preferred hand for executing the task. However, if the hand preference aligns with the performance asymmetry, the associations with brain asymmetry are more robust.
An alternative explanation is that performance asymmetries in hand use are more strongly linked to the PPFM and IFG asymmetry than to hand preferences per se [58,59]. Recall that performance measures of tool use in the analyses described above were based on the subjects overall performance for the left and right hands. Moreover, most chimpanzees perform better (as reflected in shorter dipping latencies) with their preferred compared to non-preferred hand [42]; however, the relationship between hand preference and performance is anything but perfect (see Table 2). Indeed, though the correlation between HI and LI scores is significant (r = −0.332, df = 187 p < 0.001), the association is not particularly strong. Furthermore, the multiple regression analysis indicated that both LI significantly predicted the average PPFM and IFG AQ scores. One limitation of this analysis is that the LI scores are somewhat confounded with the frequency in left and right hand use between subjects. In other words, the number of responses that went into calculating the mean latency for each hand varied between subjects because they were more or less strongly lateralized in their frequencies of hand use. However, as shown in Fig. 7, the differences in AQ scores between chimpanzees that performed better with the left or right hand were consistent across all handedness groups. This is particularly relevant to the ambiguously-handed group because these individual did not show significant differences in the frequency of right and left hand use.
The implication of the results reported here are significant for both the measurement and interpretation of data on hand preference in extant nonhuman primates, as well as evolutionary theories on handedness and tool use in primates. Specifically, there have been numerous published studies on hand preferences in nonhuman primates in the past 25 years, including data from captive and wild chimpanzees for tool use [60–64]. The general view is that hand preferences for different types of tasks reflect inherent specializations of the left and right cerebral hemispheres [64–66]. Based on the findings reported here, the assumption that hand preferences alone reflect underlying specializations in the contralateral hemisphere seems unwarranted. One way to address this issue would be for researchers to focus on assessing, not just hand preferences, but performance asymmetries for any given task as a means of validating their sensitivity to potential underlying functional asymmetries. Measures of performance asymmetries in hand use is fairly uncommon in research on laterality involving nonhuman primates [67–72] and this could 0be a fruitful area for further investigation.
With respect to evolutionary theory, it has been proposed that increased selection for the motor control involved in tool manufacture and use was an important factor in the emergence of 1) increased cortical expansion and connectivity in the primary and premotor cortex [73] and 2) population-level handedness in primates [36]. Alternatively, others have suggested that increasingly lateralization was associated with selection for increasingly sophisticated communication skills, particularly in the domain of gestural communication [74]. Evidence in support of this theory largely come from data showing that hand preferences, and specifically right-handedness, is more pronounced for gestural communication when compared to hand use for non-communicative actions such as simple reaching or coordinated bimanual actions in human infants, apes and some monkey species [75–78]. At face value, the evidence presented here supports the evolutionary model of the role of tool use, and more generally object manipulation, on potential changes in brain organization and asymmetry [79–81]; however, we did not directly test this framework against lateralization for manual gestures and, to be fair, there is some evidence that chimpanzees that prefer to gesture with their right hand showed larger leftward asymmetries in the IFG compared to non-right-handed individuals [82]. In our view, it is likely that asymmetries in PPFM and IFG are linked to a number of behavioral and cognitive functions in chimpanzees and that more sophisticated approaches will needed to identify whether similar or distinct mechanism underlie their expression.
In terms of behavioral asymmetry for tool use, there are two general findings from this study that warrant some discussion. First, we found no evidence of population-level handedness for this behavior, which differs from findings on hand use for termite-fishing in wild chimpanzees, which is the task our measure was designed to simulate [72,83–86]. The results also differ from findings of population-level handedness for other types of tasks in captive chimpanzees [87]. Thus, for reasons that are not entirely clear, the tool task in this study did not elicit population-level biases and reinforces the view by some that nonhuman primate handedness is task-specific [63]. Second, as we have noted previously [42,88], there were an unusually large number of ambiguously-handed chimpanzees for this tool-use task which differs from findings in these same chimpanzees for other tasks as well as from results in wild chimpanzees. Indeed, nearly 50% of wild chimpanzees show near exclusive hand use for tool use whereas < 18% of our sample preferred their dominant hand on greater than 90% of the responses. Thus, the tool-use task used in this study did not elicit particularly strong preferences at the individual level.
Most theories propose that the emergence of population-level handedness occurred after the split between humans and chimpanzees from their common ancestor [36]. However, there is a growing body of evidence for population-level handedness in tool use in captive and wild great apes and these results raise some questions regarding the time line for the evolution of population-level handedness. For instance, there is evidence of population-level left-handedness for termite fishing in chimpanzees [72,85,89,90], while right-handedness has been reported for wadge dipping, ant dipping, for some motor actions involved in pestle pounding [83,84,91–93] and other forms of tool use [94,95]. One challenge in these results, much like many studies of handedness in nonhuman primates, is the fact that asymmetries vary depending on the type of tool-use task. Based on the findings presented in this study, we suggest what might commonly underlie asymmetries for all of these tasks is the planning of motor actions. Thus, though behaviorally directional biases may manifest themselves differently, what might be consistent across studies is a common lateralized neurobiological basis for the planning of tool use action. In our view, if motor skill in tool use was selected for in the evolution of handedness, then it makes sense that selection acted on a more general lateralized system for motor control and planning, rather than just on a specific type of tool-use task.
In conclusion, the data reported here suggest that asymmetries in motor performance, rather than hand preference, is a better predictor of variation in neuroanatomical asymmetries of the IFG and PPFM size in chimpanzees. Indeed, intermanual differences in motor skill performance, as manifest by faster dipping latencies was associated with larger PPFM and IFG volumes, suggesting a possible functional asymmetry for motor control and/or planning. Whether similar results would be evident for either additional brain regions or other measures of motor skill should be considered in future studies. Specifically, behaviorally, future studies on end-state comfort and grasping behaviors in different primates might also contribute to this topic in meaningful ways [96–99]. End-state comfort refers to the manner in which an individual grasps an object in relation to the intended action to be performed with the object. For instance, in grasping an upside down glass, subjects will typically select the object with the thumb down, then orient the glass 180°, and place the glass down with the thumb oriented up, which is the more comfortable end-state of the thumb orientation. At least one study has suggested that measuring end-state comfort by the left and right hands is a reliable method for assessing asymmetries in motor planning [57] and these methods have and could be further applied to other species. This could, in turn, provide valuable evolutionary data on the interface between motor action and representation in primates, including humans.
HIGHLIGHTS.
Chimpanzee hand preference is only moderately associated with hand skill.
Individual difference in hand skill are associated with asymmetry in premotor and primary cortex.
Hand preference for tool use was not associated with anatomical asymmetries.
Females show increased motor skill for the right hand compared to males.
Acknowledgments
This research was supported in part by NIH grants NS-42867, NS-73134, HD-60563, NSF INSPIRE grant 1542848 and Human Frontiers Science Program grant RGP0044 to W.D.H. O. Coulon and A. Meguerditchian are funded by the French National Research Agency (ANR-09-BLAN-0038-1, BrainMorph; ANR-12-DOC-0014, LangPrimate). The NCCC chimpanzees are supported by Cooperative Agreement U42-OD011197. The Yerkes Center and NCCC are fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study.
References
- 1.Alkadhi H, Kollias SS. Pli de passage fronto-parietal moyen of Broca separates the motor homoculus. Am J Neuroradiol. 2004;25:809–812. [PMC free article] [PubMed] [Google Scholar]
- 2.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 3.Boling W, Olivier A, Bittar R, Reutens D. Localization of hand motor activation in Broca’s pli de passage moyen. J Neurosurg. 1999;91:903–910. doi: 10.3171/jns.1999.91.6.0903. [DOI] [PubMed] [Google Scholar]
- 4.Sastre-Janer FA, Regis J, Belin P, Mangin JF, Dormont D, Masure MC, Remy P, Frouin V, Samson Y. Three-dimensional reconstruction of the human central sulcus reveals a morphological correlate of the hand area. Cereb Cortex. 1998;8:641–647. doi: 10.1093/cercor/8.7.641. [DOI] [PubMed] [Google Scholar]
- 5.Pizzella V, Tecchio F, Romani GL, Rossini PM. Functional localization of the sensory hand area with respect to the motor central gyrus knob. Neuroreport. 1999;10:3809–3814. doi: 10.1097/00001756-199912160-00016. [DOI] [PubMed] [Google Scholar]
- 6.Bailey P, von Bonin G, McCulloch WS. The Isocortex of the Chimpanzee. University of Illinois Press; Urbana-Champaign: 1950. [Google Scholar]
- 7.Kloppel S, Mangin JF, Vongerichten A, Frackowiak RSJ, Siebner HR. Nurture versus nature: long-term inpact of forced right-handedness on structure of pericentral cortex and basal ganglia. J Neurosci. 2010;30(9):3271–3275. doi: 10.1523/JNEUROSCI.4394-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins WD, Coulon O, Mangin JF. Observer-independent characterization of sulcal landmarks and depth asymmetry in the central sulcus of the chimpanzee brain. Neuroscience. 2010;171:544–551. doi: 10.1016/j.neuroscience.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins WD, Cantalupo C. Handedness in chimpanzees is associated with asymmetries in the primary motor but not with homologous language areas. Behav Neurosci. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amunts K, Schlaug G, Schleicher A, Steinmutz H, Drabinghaus A, Roland P, Zilles K. Asymmetry in the human motor cortex and handedness. Neuroimage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Preuss TM, Rilling JK, Hopkins WD, Glasser MF, Kumar B, Nana R, Zhang X, Hu X. Chimpanzee pre-central corticospinal system asymmetry and handedness: a diffusion magnetic reonance imaging study. PLoS One. 2009;5(9):e12886. doi: 10.1371/journal.pone.0012886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaser C, Schlaug G. Brain structures differ between musicians and non-muscians. J Neurosci. 2003;23(27):9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Han Y, Wang D, Wang H, Fan Y, Lv Y, Tang H, Gong Q, Zang Y, He Y. Mapping surface variability of the central sulcus of musicians. Cereb Cortex. 2010;20(1):25–33. doi: 10.1093/cercor/bhp074. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins WD, Pilcher DL. Neuroanatomical localization of the motor hand area with magnetic resonance imaging: the left hemisphere is larger in Great Apes. Behav Neurosci. 2001;115(5):1159–1164. [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins WD, Meguerditchian A, Coulon O, Bogart SL, Mangin JF, Sherwood CC, Grabowski MW, Bennett AJ, Pierre PJ, Fears SC, Woods RP, Hof PR, Vauclair J. Evolution of the central sulcus morphology in primates. Brain Behav Evol. 2014;84:1930. doi: 10.1159/000362431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006;44:2482–2486. doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins WD, Taglialatela JP, Russell JL, Nir T, Schaeffer JA. Cortical representation of lateralized grasping in chimpanzees (Pan troglodytes): A combined MRI and PET study. PLoS One. 2010;5(10):1–10. doi: 10.1371/journal.pone.0013383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins WD, Russell JL, Cantalupo C. Neuroanatomical correlates of handedness for tool use in chimpanzees (Pan troglodytes): Implication for theories on the evolution of language. Psychol Sci. 2007;18(11):971–977. doi: 10.1111/j.1467-9280.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper DL. Broca’s Arrow: evolution, prediction and language in the brain. Anat Rec (Part B: New Anat) 2006;289B:9–24. doi: 10.1002/ar.b.20088. [DOI] [PubMed] [Google Scholar]
- 20.Fazio P, Cantagallo A, Craighero L, D’Ausilio A, Roy AC, Pozzo T, Calzolari F, Granieri E, Fadiga L. Encoding of human action in Broca’s area. Brain Res Rev. 2009;132:1980–1988. doi: 10.1093/brain/awp118. [DOI] [PubMed] [Google Scholar]
- 21.Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca’s area: the pars triangularis and pars opercularis. Brain Lang. 1998;64(3):282. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- 22.Nishitani N, Schurmann M, Amunts K, Hari R. Broca’s region: from action to language. Physiology. 2005;20:60–67. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- 23.Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Phil Trans R Soc B: Biol Sci. 2008;363:1951–1957. doi: 10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12(3):211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- 25.Martin KM, Jacobs S, Frey SH. Handedness-dependent and -independent cerebral asymmetries in the anterior intrparietal sulcus and ventral premotor cortex during grasp planning. Neuroimage. 2011;57(2):502–512. doi: 10.1016/j.neuroimage.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roby-Brami A, Hermsdorfer J, RAC, Jacobs S. A neuropsycholigical perspective on the link between language and praxis in modern humans. Phil Trans R Soc B: Biol Sci. 2012;367:144–160. doi: 10.1098/rstb.2011.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meador KJ, Loring DW, Lee K, Hughes M, Lee G, Nichols M, Heilman KM. Cerebral lateralization: relationship of language and ideomotor apraxia. Neurology. 1999;53(9):2028–2031. doi: 10.1212/wnl.53.9.2028. [DOI] [PubMed] [Google Scholar]
- 28.Meguerditchian A, Phillips KA, Chapelain A, Mahovetz LM, Milne S, Stoinski T, Lonsdorf E, Schaeffer J, Russell JR, Hopkins WD. Handedness for unimanual grasping in 564 great apes: the effect on grip morphology and a comparison with hand use for a bimanual coordinated task. Front Psychol. 2015;23(6):1794. doi: 10.3389/fpsyg.2015.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenker NM, Hopkins WD, Spocter MA, Garrison AR, Stimpson CD, Erwin JM, Hof PR, Sherwood CC. Broca’s area homologue in chimpanzees (Pan troglodytes): probabilistic mapping, asymmetry and comparison to humans. Cereb Cortex. 2010;20:730–742. doi: 10.1093/cercor/bhp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantalupo C, Hopkins WD. Asymmetric Broca’s area in great apes. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck BB. Animal Tool Behavior: The Use and Manufacture of Tools by Animals. Garland, New York: 1980. [Google Scholar]
- 32.Vaesen K. The cognitive bases of human tool use. Behav Brain Sci. 2012;35:203–262. doi: 10.1017/S0140525X11001452. [DOI] [PubMed] [Google Scholar]
- 33.Whiten A, Goodall J, McGrew W, Nishida T, Reynolds V, Sugiyama Y, Tutin C, Wrangham R, Boesch C. Charting cultural variation in chimpanzees. Behaviour. 2001;138:1489–1525. [Google Scholar]
- 34.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 35.Bradshaw JL. Human Evolution: A Neuropsychological Perspective. Psychology Press; Hove U.K: 1997. [Google Scholar]
- 36.Bradshaw JL, Rogers LJ. The evolution of lateral asymmetries, language, tool use and intellect. Academic Press, Inc; San Diego: 1993. [Google Scholar]
- 37.Frost GT. Tool behavior and the origin of laterality. J Hum Evol. 1980;9:447–459. [Google Scholar]
- 38.Johnson-Frey SH. The neural basis of complex tool use in humans. Trends Cogn Sci. 2004;8(2):71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, Hallett M. Gesture subtype-dependen eft lateralization for praxis planning: an event-related fMRI study. Cereb Cortex. 2009;19:1256–1262. doi: 10.1093/cercor/bhn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips KA, Thompson CR. Hand preference for tool-use in capuchin monkeys (Cebus apella) is associated with asymmetry in the primary motor cortex. Am J Primatol. 2013;75(5):435–440. doi: 10.1002/ajp.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips KA, Schaeffer J, Barrett E, Hopkins WD. Performance asymmetries in tool use are associated with corpus callosum integrity in chimpanzees (Pan troglodytes): A diffusion tensor imaging study. Behav Neurosci. 2013;127(1):106. doi: 10.1037/a0031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. Handedness for tool use in captive chimpanzees (Pan troglodytes): Sex differences, performance, heritability and comparion to the wild. Behaviour. 2009;146:1463–1483. doi: 10.1163/156853909X441005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogart SL, Mangin JF, Schapiro SJ, Reamer L, Bennett AJ, Pierre PJ, Hopkins WD. Cortical sulci asymmetries in chimpanzees and macaques: a new look at an old idea. Neuroimage. 2012;61:533–541. doi: 10.1016/j.neuroimage.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangin JF, Riviere D, Cachia A, Duchesnay E, Cointepas Y, Papadopoulos-Orfanos D, Collins DL, Evans AC, Regis J. Object-based morphometry of the cerebral cortex. Med Imaging. 2004;23(8):968–982. doi: 10.1109/TMI.2004.831204. [DOI] [PubMed] [Google Scholar]
- 45.Mangin JF. Entropy Minimization for Automatic Correction of Intensity Nonuniformity, MMBIA. IEEE Press; Hilton Head South Carolina: 2000. pp. 162–169. [Google Scholar]
- 46.Mangin JF, Coulon O, Frouin V. Robust brain segmentation using histogram scale-space analysis and mathematical morphology. In: Colchester A, Delp S, editors. Medical Image Computing and Computer-Assisted Intervention-MICCAI’98. Springer-Verlag; Boston, MA: 1998. pp. 1230–1241. [Google Scholar]
- 47.Mangin JF, Regis J, Frouin V. Shape bottlenecks and conservative flow systems. Workshop on Mathematical Methods in Biomedical Image Analysis; San Francisco, CA: IEEE Press; 1996. pp. 319–328. [Google Scholar]
- 48.Coulon O, Clouchoux C, Operato G, Dauchot K, Sirigu A, Anton J-L. Cortical localization via surface parameterization: a sulcus-based approach. Neuroimage. 2006;31:S46. [Google Scholar]
- 49.Cykowski MD, Coulon O, Kochunov PV, Amunts K, Lancaster JL, Laird AR, Glahn C, Fox PT. The central sulcus: an observer-independent characterization of sulcal landmarks and depth asymmetry. Cereb Cortex. 2008;18:1999–2009. doi: 10.1093/cercor/bhm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hopkins WD, Misiura M, Pope S, Latash E. Behavioral and brain asymmetries in primates: A preliminary evaluation of two evolutionary hypotheses. Annals of the New York Academcy of Sciences. doi: 10.1111/nyas.12936. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopkins WD. Independence of data points in the measurement of handedness: statistical problem or urban myth? Am J Phys Anthropol. 2013;151:151–157. doi: 10.1002/ajpa.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hopkins WD. On the other hand: statistical issues in the assessment and interpretation of hand preference data in non-human primates. Int J Primatol. 1999;20:851–866. [Google Scholar]
- 53.Knaus TA, Corey DM, Bollich AM, Lemen LC, Foundas AL. Anatomical asymmetries of anterior perisylvian speech-language regions. Cortex. 2007;43:499–510. doi: 10.1016/s0010-9452(08)70244-2. [DOI] [PubMed] [Google Scholar]
- 54.Castiello U. The neuroscience of grasping. Nat Rev: Neurosci. 2005;6:726–736. doi: 10.1038/nrn1744. [DOI] [PubMed] [Google Scholar]
- 55.Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neurosci Biobehav Rev. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 56.Hatta T. Handedness and the brain: a review of brain-imaging techniques. Magn Reson Med Sci. 2006;6(2):99–112. doi: 10.2463/mrms.6.99. [DOI] [PubMed] [Google Scholar]
- 57.Janssen L, Meulenbrock GJ, Steenbergen B. Behavioral evidence for left-hemisphere specialization of motor planning. Exp Brain Res. 2011;209:65–72. doi: 10.1007/s00221-010-2519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amunts K, Schlaug G, Jancke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp. 1997;5:206–215. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 59.Barber AD, Srinivasan P, Joel SE, Caffo BS, Pekar JJ, Motfsky SH. Motor dexterity?: Evidence of left hemisphere lateralization of motor circuit connectivity is associated with better motor performance in children. Cereb Cortex. 2012;22(1):51–59. doi: 10.1093/cercor/bhr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychol Bull. 2006;132(4):538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cashmore L, Uomini N, Chapelain A. The evolution of handedness in humans and great apes: a review and current issues. J Anthropol Sci. 2008;86:7–35. [PubMed] [Google Scholar]
- 62.Marchant LF, McGrew WC. Laterality of function in apes: a meta-analysis of methods. J Hum Evol. 1991;21:425–438. [Google Scholar]
- 63.McGrew WC, Marchant LF. On the other hand: current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearb Phys Anthropol. 1997;40:201–232. [Google Scholar]
- 64.Fagot J, Vauclair J. Manual laterality in nonhuman primates: a distinction between handedness and manual specialization. Psychol Bull. 1991;109(1):76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- 65.Rogers LJ, Vallortigara G, Andrew RJ. Divided Brains: the Biology and Behaviour of Brain Asymmetries. Cambridge University Press; New York: 2013. [Google Scholar]
- 66.Hook-Costigan MA, Rogers LJ. Hand preferences in new world primates. Int J Comp Psychol. 1997;9:173–207. [Google Scholar]
- 67.McGrew WC, Marchant LF. Laterality of hand use pays off in foraging success for wild chimpazees. Primates. 1999;40(3):509–513. [Google Scholar]
- 68.Rigamonti MM, Previde EP, Poli MD, Marchant LF, McGrew WC. Methodology of motor skill and laterality: new test of hand preference in Macaca nemestrina. Cortex. 1998;34:693–705. doi: 10.1016/s0010-9452(08)70773-1. [DOI] [PubMed] [Google Scholar]
- 69.Hopkins WD, Washburn DA, Berke L, Williams M. Behavioral asymmetries of psychomotor performance in rhesus monkeys (Macaca mulatta): A dissociation in hand preference and performance. J Comp Psychol. 1992;106:392–397. doi: 10.1037/0735-7036.106.4.392. [DOI] [PubMed] [Google Scholar]
- 70.Hopkins WD, Russell JL. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes) Neuropsychologia. 2004;42:990–996. doi: 10.1016/j.neuropsychologia.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 71.Spinozzi G, Truppa V, Lagana T. Grasping behavior in tufted capuchin monkeys (Cebus apella): grip types and manual laterality for picking up a small food item. Am J Phys Anthropol. 2004;125:30–41. doi: 10.1002/ajpa.10362. [DOI] [PubMed] [Google Scholar]
- 72.Sanz CM, Morgan DB, Hopkins WD. Lateralization, performance asymmetries in termite fishing of wild chimpanzees in the Goualougo Triangle, Republic of Congo. Am J Primatol. doi: 10.1002/ajp.22574. (in press) [DOI] [PubMed] [Google Scholar]
- 73.Stout D, Chaminade T. Stone tools, language and the brain in human evolution. Phil Trans R Soc B : Biol Sci. 2012;367:75–87. doi: 10.1098/rstb.2011.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corballis MC. From Hand to Mouth: The Origins of Language. Princeton University Press; Princeton, NJ: 2002. [Google Scholar]
- 75.Cochet H, Vauclair J. Pointing gestures produced by toddlers from 15 to 30 months: different functions, hand shapes and laterality patterns. Infant Behav Dev. 2010;33:431–441. doi: 10.1016/j.infbeh.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Meguerditchian A, Vauclair J. Baboons communicate with their right hand. Behav Brain Res. 2006;171:170–174. doi: 10.1016/j.bbr.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 77.Meunier H, Vauclair J, Fagard J. Human infants and baboons show the same pattern of handedness for a communicative gesture. PLoS One. 2012;7(3):e33959. doi: 10.1371/journal.pone.0033959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hopkins WD, Gardner M, Mingle M, Reamer L, Schapiro SJ. Within- and between-task consistency in hand use as a means of characterizing hand preferences in captive chimpanzees (Pan troglodytes) J Comp Psychol. 2013;127(4):380–391. doi: 10.1037/a0031071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forrester GS, Leavens DA, Quaresmini C, Vallortigara G. Target animacy influences gorilla handedness. Anim Cogn. 2011;14(4):903–907. doi: 10.1007/s10071-011-0413-6. [DOI] [PubMed] [Google Scholar]
- 80.Forrester GS, Quaresmini C, Leavens DA, Mareschal D, Thomas MSC. Human handedness: an inherited evolutionary trait. Behav Brain Res. 2013;237:200–206. doi: 10.1016/j.bbr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 81.Forrester GS, Quaresmini C, Leavens DA, Spiezio C, Vallortigara G. Target animacy influences chimpanzee handedness. Anim Cogn. 2012;15:1121–1127. doi: 10.1007/s10071-012-0536-4. [DOI] [PubMed] [Google Scholar]
- 82.Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. Neuroreport. 2006;17(9):923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchant LF, McGrew WC. Ant fishing by wild chimpanzees is not lateralised. Primates. 2007;48:22–26. doi: 10.1007/s10329-006-0020-3. [DOI] [PubMed] [Google Scholar]
- 84.Humle T, Matsuzawa T. Laterality in hand use across four tool use behaviors among the wild chimpanzees of Bossou Guinea, West Africa. Am J Primatol. 2009;71:40–48. doi: 10.1002/ajp.20616. [DOI] [PubMed] [Google Scholar]
- 85.Bogart SL, Pruetz JD, Ormiston LK, Russell JL, Meguerditchian A, Hopkins WD. Termite fishing laterality in the Fongoli savanna chimpanzees (Pan troglodytes verus): Further evidence of a left hand preference. Am J Phys Anthropol. 2012;149:591–598. doi: 10.1002/ajpa.22175. [DOI] [PubMed] [Google Scholar]
- 86.McGrew WC, Marchant LF. On which side of the apes? In: McGrew WC, Marchant LF, Nishida T, editors. Great Ape Societies. Cambridge University Press; Cambridge: 1996. pp. 255–272. [Google Scholar]
- 87.Hopkins WD. Behavioral and brain asymmetries in chimpanzees: a case for continuity. Ann N Y Acad Sci. 2013;1288:27–35. doi: 10.1111/nyas.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hopkins WD, Reamer L, Mareno MC, Schapiro SJ. Genetic basis for motor skill and hand preference for tool use in chimpanzees (Pan troglodytes) Proceedings of the Royal Society: Biological Sciences B. doi: 10.1098/rspb.2014.1223. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McGrew WC, Marchant LF. Chimpanzees, tools, and termites: hand preference or handedness? Curr Anthropol. 1992;33:114–119. [Google Scholar]
- 90.Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proc Natl Acad Sci. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34(2):151–159. [Google Scholar]
- 92.Biro D, Sousa C, Matsuzawa T. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: case studies in nut cracking and leaf folding. In: Matsuzawa T, Tomonaga T, Tanaka M, editors. Cognitive Development of Chimpanzees. Springer; New York: 2006. pp. 476–507. [Google Scholar]
- 93.Boesch Handedness in wild chimpanzees. Int J Primatol. 1991;6:541–558. [Google Scholar]
- 94.Bardo A, PE, Meunier H. Do bimanual coordination tool use and body posture contribute equally to hand preferences in bonobos? J Hum Evol. 2015;82:159–169. doi: 10.1016/j.jhevol.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 95.Hopkins WD, Russell JL, Schaeffer JA. The neural and cognitive correlates of aimed throwing in chimpanzees: a magnetic resonance image and behavioural study on a unique form of social tool use. Phil Trans R Soc B: Biol Sci. 2012;367(1585):37–47. doi: 10.1098/rstb.2011.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weiss DJ, Wark JD, Rosenbaum DA. Monkey see, monkey plan monkey do: the end-state comfort effect in cotton-top tamarins (Saguinus oedipus) Psychol Sci. 2007;18(12):1063–1068. doi: 10.1111/j.1467-9280.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- 97.Chapman KM, Weiss DJ, Rosenbaum DA. Evolutionary roots of motor planning: the end-state comfort effect in Lemurs. J Comp Psychol. 2010;124(2):229–232. doi: 10.1037/a0018025. [DOI] [PubMed] [Google Scholar]
- 98.Nelson EL, Berthier NE, Metevier CM, Novak MA. Evidence of motor planning in monkeys: rhesus macaques select efficient grips when transporting spoons. Dev Sci. 2011;14(4):822–831. doi: 10.1111/j.1467-7687.2010.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Frey SH, Povinelli DJ. Comparative investigations of manual action representations: evidence that chimpanzees represent the costs of potential future actions involving tools. Phil Trans R Soc B : Biol Sci. 2012;367(1585):48–58. doi: 10.1098/rstb.2011.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]