Abstract
Transplantation of macroencapsulated tissue-engineered grafts (TEGs) is being investigated as a treatment for type 1 diabetes, but there is a critical need to measure TEG viability both in vitro and in vivo. Oxygen deficiency is the most critical issue preventing widespread implementation of TEG transplantation and delivery of supplemental oxygen (DSO) has been shown to enhance TEG survival and function in vivo. In this study, we demonstrate the first use of oxygen-17 magnetic resonance spectroscopy (17O-MRS) to measure the oxygen consumption rate (OCR) of TEGs and show that in addition to providing therapeutic benefits to TEGs, DSO with 17O2 can also enable measurements of TEG viability. Macroencapsulated TEGs containing βTC3 murine insulinoma cells were prepared with three fractional viabilities and provided with 17O2. Cellular metabolism of 17O2 into nascent mitochondrial water (H217O) was monitored by 17O-MRS and, from the measured data, OCR was calculated. For comparison, OCR was simultaneously measured on a separate, but equivalent sample of cells with a well-established stirred microchamber technique. OCR measured by 17O-MRS agreed well with measurements made in the stirred microchamber device. These studies confirm that 17O-MRS can quantify TEG viability noninvasively.
Graphical Abstract
Techniques to measure tissue-engineered graft (TEG) viability are limited, but a 17O-MRS technique is proposed to quantify the viability of macroencapsulated cells. Cells metabolized 17O2 gas into H217O water and this oxygen consumption rate (OCR) was measured with 17O-MRS. The 17O-MRS measurements agreed well with those of a stirred microchamber, a well-established technique, and it was found that 17O-MRS offers a reliable, accurate, and noninvasive approach to TEG viability assessment.
Transplantation of pancreatic islets is an emerging treatment for type 1 diabetes, but intrahepatic delivery is not optimal and long-term outcomes are inconsistent (Emamaullee and Shapiro, 2007). Macroencapsulated tissue-engineered islet grafts (TEGs) may mitigate or eliminate many limitations associated with intraportal transplantation, but graft dysfunction and failure can occur due to various adverse factors (Colton, 2014; Papas et al., 2016). Because of the possibility of graft failure, there is a critical need to measure TEG viability both in vitro and in vivo. Because islet viability and graft success have been correlated to oxygen consumption rate (OCR), this can be accomplished by measuring the OCR of the TEG (Papas et al., 2007a; Papas et al., 2007b; Suszynski et al., 2011). These OCR-based viability measurements, therefore, can be used to optimize cell culture techniques, improve surgical protocols, predict post-transplant outcomes, monitor graft function in vivo, and monitor cell proliferation, but current techniques to directly measure TEG OCR in vitro and in vivo are limited.
Many methods to measure tissue oxygenation (both invasively and noninvasively) have been developed, including magnetic resonance spectroscopy (MRS)-based techniques (Einstein et al., 2016; Kodibagkar et al., 2008). This oximetry can be used to estimate OCR, but yields indirect measurements (Suszynski et al., 2011). Oxygen-17 MRS (17O-MRS) allows for the noninvasive, direct measurement of in vitro and in vivo OCR. 17O is a stable isotope possessing a quadrupolar nucleus with a spin of 5/2 (Gordji-Nejad et al., 2014; de Graaf et al., 2008; Wiesner et al., 2016). This quadrupole moment leads to short relaxation times (T1 ≈ 7 ms) (Gordji-Nejad et al., 2014; de Graaf et al., 2008; Wiesner et al., 2016), allowing for numerous signal averages per unit time. This averaging can compensate for the inherent low sensitivity of 17O (Mateescu et al., 1987). While this isotope has a low natural abundance (Mateescu and Cabrera, 1997), it is possible to enrich oxygen gas preparations so that the majority of the oxygen molecules are 17O2. As 17O2 is supplied to tissue using various delivery techniques, the mitochondria in the cells metabolize the 17O2 into H217O. By monitoring the generation of this nascent mitochondrial H217O with 17O-MRS, the OCR of the tissue can be determined and quantified. This technique has primarily been utilized to characterize cerebral oxygen metabolism, both through direct measurement with 17O-MRS as well as through the indirect effect of 17O on 1H relaxation (Gordji-Nejad et al., 2014). 17O-MRS has also been applied successfully to measure the OCR of invertebrates (Mateescu and Cabrera, 1997), but this technique has not been extended to viability measurements of cell- and tissue-based therapeutic products such as TEGs.
In addition to enabling measurements of viability, delivery of supplemental oxygen (DSO) during 17O-MRS can also provide therapeutic benefits to TEGs. Hypoxia is the most significant limitation preventing widespread utilization of macroencapsulated TEG transplantation (Colton, 2014; Papas et al., 2016) and DSO has been shown to enhance TEG survival and function in vivo (Colton, 2014). In this study, we validated noninvasive 17O-MRS measurements of TEG OCR and demonstrated that monitoring the cellular metabolism of delivered 17O2 into H217O can quantify TEG viability.
TEGs were prepared by loading a macroencapsulation device with a matrix impregnated with insulin-secreting cells (Figure 1A). βTC3 murine insulinoma cells were used as they have been previously utilized in bioartificial pancreata and their OCR characteristics have been previously studied (Colton, 2014; Mukundan et al., 1995; Skelin et al., 2010). TEGs were prepared with three controlled increments of fractional viability (FV); (0% [N=3], 50% [N=3], and 100% [N=3]) by mixing various quantities of 100% viable cells with nonviable cells to obtain the desired FV, while maintaining the same total cell number in each TEG.
Figure 1.
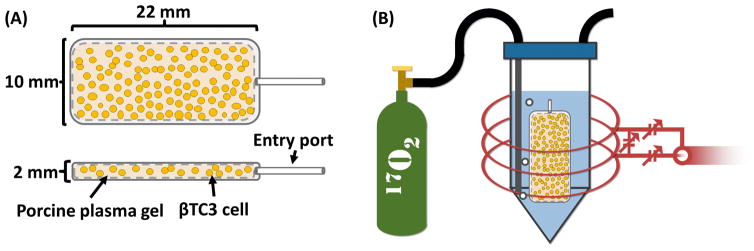
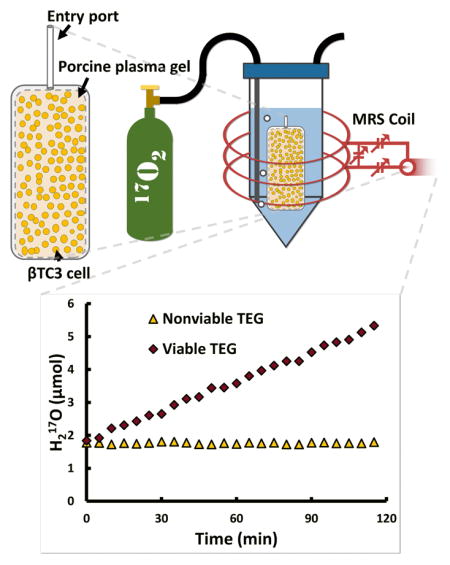
Illustration of (A) top and side view of a TEG showing a macroencapsulation device loaded with βTC3 cells (not to scale) and plasma gel and (B) the TEG inserted into a vial filled with perfluorodecalin (blue) and bubbled with 17O2 gas (green tank) while scanned with a 17O-MRS solenoid coil (red).
TEGs were prepared and immediately placed into a sealed, intubated vial filled with perfluorodecalin (PFD) and pre-warmed to 37 °C. The vial was placed into a custom-built three-loop solenoid coil (Figure 1B) and the coil was tuned to the 17O resonance frequency (94.6 MHz). The coil was then inserted into the center of a 16.4 T MRI scanner and the magnet was shimmed (off-resonance) to 1H (which was localized within the TEG). Initial 17O-MRS scans determined the baseline H217O peak integral (background). Following baseline acquisition, the PFD was bubbled with 17O2 gas using a syringe pump located outside the magnet room with tubes running into the bore. Gas was initially pumped at a rate of 5 ml per minute for 10 minutes (50 ml bolus) and then pumped at a slower rate of 0.2 ml per minute for at least 2 hours. MRS data were acquired continuously throughout the experiment. Temperature within TEGs was maintained at 37 ± 1 °C, as measured with a fiber-optic temperature probe (SAII, Stony Brook, NY, USA), throughout the experiment with a forced-air heater and water circulation system (carefully placed as to not be in the sensitive volume of the coil). 17O-MRS OCR measurements were simultaneously validated using a stirred microchamber (SM) system as previously described in detail (Papas et al., 2007a; Papas et al., 2007b) on a matched sample of equivalent cells as prepared for each TEG.
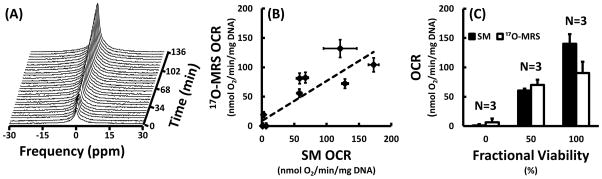
The H217O signal evolved as expected throughout the experiment as measured with 17O-MRS. For the TEGs with no viable cells, the integral of the H217O peak remained constant and unchanged throughout the entire experiment. For TEGs with viable cells, however, the integral of the H217O peak remained constant throughout the baseline acquisition and then increased linearly with time after the introduction of the 17O2 gas (Figure 2A). These 17O-MRS data were successfully quantified and agreed well with SM measurements (Figure 2B). The techniques were positively correlated (Pearson’s r = 0.85) and least-squares-weighted linear regression yielded a slope and intercept of 0.68 ± 0.05 and 8.7 ± 2.9 nmol O2/min/mg DNA, respectively.
Figure 2.
Results of OCR measurements and validation showing (A) the evolution of the intensity of the H217O peak over time as measured with 17O-MRS during a typical experiment with a 100% viable TEG, (B) a comparison of OCR measurements made with 17O-MRS and stirred microchamber (SM) techniques, and (C) the least-squares-weighted means of the OCR measured with the SM and 17O-MRS techniques for the three different fractional viabilities (FV; 0%, 50%, and 100%). For TEGs with viable cells, the integral of the H217O peak increased linearly with time after introduction of 17O2 gas. Measurements made by monitoring the accumulation of nascent mitochondrial water with 17O-MRS correlated well with those made with an SM device (Pearson’s r=0.85) and were not statistically different for any FV. Least-squares-weighted linear regression yielded a slope of 0.68 ± 0.05 and intercept of 8.7 ± 2.9 nmol O2/min/mg DNA.
Additionally, there were no statistically significant differences between the 17O-MRS and SM OCR measurements for any individual FV (Figure 2C). Least-squares-weighted means of the 17O-MRS OCR of TEGs with 0%, 50%, and 100% FV were 6.4 ± 6, 70.0 ± 9, and 90.2 ± 19 nmol O2/min/mg DNA, respectively. SM OCR for TEGs with 0%, 50%, and 100% FV were measured to be 1.2 ± 2, 60.7 ± 3, and 140.3 ± 16 nmol O2/min/mg DNA, respectively.
While no FV exhibited a statistically significant difference between the 17O-MRS and SM OCR measurements, the 100% viable TEGs appeared to have diminished OCR as measured by 17O-MRS. This is possibly due to loss of viable cells as a result of lack of nutrients, hypoxia (prior to the delivery of 17O2), or pH changes; especially considering the aggravating factors of high-cell density and diffusion limitations presented by the macroencapsulation device (Papas et al., 2016). Additionally, the accuracy of 17O-MRS OCR quantification would likely be improved by the inclusion of a 17O standard, which should be feasible as metabolites have been detected with distinct 17O chemical shifts (de Graaf et al., 2008). Nevertheless, 17O-MRS OCR was quantified successfully and independently of SM measurements.
High cell densities were used in these experiments to mirror therapeutically-relevant cell densities (Papas et al., 2016), but this technique is expected to be applicable to lower densities due to the high precision of the technique (evidenced by relatively small measurement errors). One potential barrier to large-scale application is the current cost of 17O2, but economies of scale may permit practical translation if a suitable application is discovered.
This technique could be expanded by coupling phosphorus-31 MRS with 17O-MRS, which would provide quantitative information concerning defects in oxidative phosphorylation which have been implicated in beta cell dysfunction in addition to numerous degenerative diseases (Mateescu et al., 1998). Future studies will also adapt this technique for in vivo measurements, conceivably using TEGs with an integrated 17O-MRS coil (such as a meander-line surface coil (Nakada et al., 1987)). Proper coil design will be essential in minimizing background H217O signal from surrounding tissue that can limit the sensitivity of 17O-MRS OCR measurements.
In conclusion, 17O-MRS appears to offer a valuable tool to study the progression of cellular viability within TEGs and to evaluate the success of therapeutic interventions to improve TEG survival and function.
Materials and Methods
Cell culture, maintenance, and preparation
βTC3 cells were obtained from the laboratory of Dr. Shimon Efrat, (Tel Aviv University). In preparation for the study, the cells were cultured and passaged according to standard protocols in supplemented Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA), which contained 4 mM L-glutamine, 4.5 g/L glucose, 1 mM sodium pyruvate (all from Invitrogen) and 10% heat-inactivated fetal bovine serum. They were passaged using a ratio of 1:3 every 2–4 days. To collect cells for each experiment, the adherent cells were detached using 0.25%-(w/v)-trypsin-0.53 mM EDTA solution, washed, and then re-suspended in fresh medium. The cells were manually counted with trypan blue exclusion staining using a hemocytometer and then allocated for loading into the macroencapsulation device or SM as needed.
TEG construction
30 million βTC3 cells were counted and collected into a centrifuge tube on the day of experiment. The cells were gently centrifuged to settle them at the bottom of the tube (forming a loose pellet approximately 30 μl in volume) and the supernatant was carefully removed. The cells were then re-suspended in 50 μl of porcine plasma (Sigma Aldrich, St. Louis, MO, USA) and an additional 20 μl of plasma was initially drawn into a 250 μl precision syringe (Hamilton Company, Reno, NV, USA) to assist with flushing all the cells into the main chamber of the macroencapsulation device. The re-suspended cells were then drawn into the syringe and the entire contents of the syringe were injected into a well-established (Scharp and Marchetti, 2014) 20 μl immunoisolation device (TheraCyte, Inc. Laguna Hills, CA, USA). A fibrin gel was created by soaking the loaded device in a 5% v/v bovine thrombin solution. The thrombin solution was made by diluting concentrated topical thrombin solution (GenTrac Inc., Middleton, WI, USA) in PBS++ (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Cells were rendered nonviable as needed by pelletizing them and heating them to 60 °C for 1 hour. TEGs were then immediately placed into a sealed, intubated 2 ml vial filled with PFD (FluoroMed, L.P., Round Rock, TX, USA). PFD was used because of its high oxygen solubility (Riess, 2005) and lack of oxygen atoms in its chemical structure (which could contribute to the background 17O-MRS signal). Additionally, measurements of the PFD T1 allowed for verification of successful 17O2 (70.2% isotopic purity; Cambridge Isotope Laboratories, Inc., Tewksbury, MA, USA) delivery (Einstein et al., 2016).
17O-MRS
17O-MRS spectra were acquired with a 16.4 T MR system (Agilent Technologies, Santa Clara, CA, USA). A basic pulse and acquire sequence was used to acquire spectra. An adiabatic half-passage pulse (90°) was used to ensure a uniform flip angle and maximum signal. Pulse length (3 ms) and pre-acquisition delay times (<1 ms total) were minimized to decrease H217O relaxation during the sequence, while maintaining adiabaticity. Repetition time was set at 35 ms to allow for full relaxation after excitation. 8570 signal averages (5 min) were combined for each spectrum.
17O-MRS OCR measurement quantification
17O-MRS OCR measurements were quantified (OCR/DNA; nmol O2/min/mg DNA) using the following assumptions: 1 mole of 17O2 gas produced 2 moles of H217O water (Mateescu and Cabrera, 1997), plasma was composed of 95% water (Morrison and Fleck, 1973), cells were composed of 70% water (Morrison and Fleck, 1973), the natural abundance of 17O was 0.0373% (Gordji-Nejad et al., 2014; de Graaf et al., 2008), and each cell contained 6.7 pg DNA (Papas et al., 2007b).
Validation of 17O-MRS OCR measurements
5000 cells were counted, collected, centrifuged, and re-suspended in serum-free medium. The cells were split into 2–3 samples and placed into pre-calibrated, water-jacketed, 120 μl titanium chambers outfitted with a fiber-optic oxygen sensor (OXY-4 mini, PreSens Precision Sensing GmbH, Regensburg, Germany). The chambers were capped with a glass plug and the consumption of oxygen over time was measured at 37 °C. Following SM OCR measurements, the cells were removed from the chambers with multiple washes of medium and DNA quantity was assessed for normalization (OCR/DNA; nmol O2/min/mg DNA) using a dsDNA fluorescent dye (Quant-iT PicoGreen dsDNA Assay Kit, Invitrogen, Life Technologies Corporation, Grand Island, NY, USA).
Acknowledgments
This work was supported in part by the Minnesota Lions Diabetes Foundation, the Schott Family Foundation, the JDRF [grant 5-2013-141], and the NIH [grants P41 EB015894, S10 RR025031, R01 NS041262, and R01 NS070839]. The authors would like to thank all members of the Center for Magnetic Resonance Research, especially Drs. Wei Chen and Xiao-Hong Zhu. Additional thanks to Liberty Kirkeide, Dr. Thomas Suszynski, and Dr. Meri Firpo.
References
- Colton CK. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev. 2014;67–68:93–110. doi: 10.1016/j.addr.2014.02.007. http://dx.doi.org/10.1016/j.addr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Einstein SA, Weegman BP, Firpo MT, Papas KK, Garwood M. Development and validation of noninvasive magnetic resonance relaxometry for the in vivo assessment of tissue-engineered graft oxygenation. Tissue Eng Part C Methods. 2016 doi: 10.1089/ten.tec.2016.0106. Ahead of print. http://online.liebertpub.com/doi/10.1089/ten.TEC.2016.0106. [DOI] [PMC free article] [PubMed]
- Emamaullee JA, Shapiro AMJ. Factors Influencing the Loss of β-Cell Mass in Islet Transplantation. Cell Transplant. 2007;16:1–8. doi: 10.3727/000000007783464461. http://www.ncbi.nlm.nih.gov/pubmed/17436849. [DOI] [PubMed] [Google Scholar]
- Gordji-Nejad A, Möllenhoff K, Oros-Peusquens AM, Pillai DR, Shah NJ. Characterizing cerebral oxygen metabolism employing oxygen-17 MRI/MRS at high fields. Magn Reson Mater Physics, Biol Med. 2014;27:81–93. doi: 10.1007/s10334-013-0413-4. http://link.springer.com/10.1007/s10334-013-0413-4. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Brown PB, Rothman DL, Behar KL. Natural abundance 17O NMR spectroscopy of rat brain in vivo. J Magn Reson. 2008;193:63–67. doi: 10.1016/j.jmr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodibagkar VD, Wang X, Mason RP. Physical principles of quantitative nuclear magnetic resonance oximetry. Front Biosci. 2008;13:1371–84. doi: 10.2741/2768. http://www.bioscience.org/2008/v13/af/2768/list.htm. [DOI] [PubMed] [Google Scholar]
- Mateescu GD, Cabrera ME. In Vivo 17O Magnetic Resonance Spectroscopy. Oxyg Transp to Tissue XVIII. 1997:585–590. http://link.springer.com/10.1007/978-1-4615-5865-1_72. [PubMed]
- Mateescu GD, Cabrera ME, Fercu D. Spat Resolv Magn Reson. Weinheim, Germany: Wiley-VCH Verlag GmbH; 1998. 17O and 31P Magnetic Resonance Imaging and Spectroscopy: In Vivo Investigations of Cell Bioenergetics; pp. 421–429. http://doi.wiley.com/10.1002/9783527611843.ch39. [Google Scholar]
- Mateescu GD, Yvars GM, Dular T. Oxygen-17 Magnetic Resonance Imaging. Sixth Annu Meet SMRM. 1987:929. [Google Scholar]
- Morrison B, Fleck A. Plasma or whole blood glucose? Clin Chim Acta. 1973;45:293–297. doi: 10.1016/0009-8981(73)90441-5. http://linkinghub.elsevier.com/retrieve/pii/0009898173904415. [DOI] [PubMed] [Google Scholar]
- Mukundan NE, Flanders PC, Constantinidis I, Papas KK, Sambanis A. Oxygen Consumption Rates of Free and Alginate-Entrapped βTC3 Mouse Insulinoma Cells. Biochem Biophys Res Commun. 1995;210:113–118. doi: 10.1006/bbrc.1995.1634. http://linkinghub.elsevier.com/retrieve/pii/S0006291X85716348. [DOI] [PubMed] [Google Scholar]
- Nakada T, Kwee IL, Miyazaki T, Iriguchi N, Maki T. 31P NMR spectroscopy of the stomach by zig–zag coil. Magn Reson Med. 1987;5:449–455. doi: 10.1002/mrm.1910050506. http://doi.wiley.com/10.1002/mrm.1910050506. [DOI] [PubMed] [Google Scholar]
- Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE, Wildey GM, Pisania A, Weir GC, Hering BJ. Human Islet Oxygen Consumption Rate and DNA Measurements Predict Diabetes Reversal in Nude Mice. Am J Transplant. 2007a;7:707–713. doi: 10.1111/j.1600-6143.2006.01655.x. http://doi.wiley.com/10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007b;98:1071–1082. doi: 10.1002/bit.21486. http://onlinelibrary.wiley.com/doi/10.1002/bit.21486/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas KK, Avgoustiniatos ES, Suszynski TM. Effect of oxygen supply on the size of implantable islet-containing encapsulation devices. Panminerva Med. 2016;58:72–7. http://www.ncbi.nlm.nih.gov/pubmed/26837777. [PubMed] [Google Scholar]
- Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:47–63. doi: 10.1081/bio-200046659. [DOI] [PubMed] [Google Scholar]
- Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. http://www.ncbi.nlm.nih.gov/pubmed/23916992. [DOI] [PubMed] [Google Scholar]
- Skelin M, Rupnik M, Cencič A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX. 2010;27:105–13. doi: 10.14573/altex.2010.2.105. http://www.ncbi.nlm.nih.gov/pubmed/20686743. [DOI] [PubMed] [Google Scholar]
- Suszynski TM, Avgoustiniatos ES, Stein SA, Falde EJ, Hammer BE, Papas KK. Assessment of Tissue-Engineered Islet Graft Viability by Fluorine Magnetic Resonance Spectroscopy. Transplant Proc. 2011;43:3221–3225. doi: 10.1016/j.transproceed.2011.09.009. http://www.ncbi.nlm.nih.gov/pubmed/22099762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner HM, Balla DZ, Shajan G, Scheffler K, Uğurbil K, Chen W, Uludağ K, Pohmann R. 17 O relaxation times in the rat brain at 16.4 tesla. Magn Reson Med. 2016;75:1886–1893. doi: 10.1002/mrm.25814. http://doi.wiley.com/10.1002/mrm.25814. [DOI] [PMC free article] [PubMed] [Google Scholar]