Abstract
Transferring genetic molecules into the peripheral sensory nervous system to manipulate nociceptive pathophysiology is a powerful approach for experimental modulation of sensory signaling and potentially for translation into therapy for chronic pain. This can be efficiently achieved by the use of recombinant adeno-associated virus (rAAV) in conjunction with nociceptor-specific regulatory transgene cassettes. Among different routes of delivery, direct injection into the dorsal root ganglia (DRGs) offers the most efficient AAV-mediated gene transfer selectively into the peripheral sensory nervous system. Here, we briefly discuss the advantages and applications of intraganglionic microinjection, and then provide a detailed approach for DRG injection, including a list of the necessary materials and description of a method for performing DRG microinjection experiments. We also discuss our experience with several adeno-associated virus (AAV) options for in vivo transgene expression in DRG neurons.
Keywords: Dorsal root ganglion, Chronic pain, Microinjection, Gene therapy, Adeno-associated virus
1 Introduction
Chronic neuropathic pain is common and largely resistant to pharmacological treatments. Gene therapy targeting the peripheral nervous system is an approach that shows great promise for the treatment of chronic pain [1–4]. The intent is to deliver analgesic genetic molecules to the pathophysiological area and affect a positive therapeutic outcome. Disordered cellular mechanisms underlying chronic pain, such as that which follows nerve injury, reside at diverse sites, including in receptive fields in peripheral tissues, in the somata of the injured sensory neurons, and in the dorsal horn of the spinal cord. Many maladaptive gene expressions upon nerve damage are mainly synthesized in the primary sensory neurons and trafficked to central presynaptic terminals as well as peripheral axons to induce central or peripheral hypersensitivity. The dorsal root ganglia (DRGs), which harbor the somata of primary sensory neurons that transmit sensory signals from the peripheral organs toward the appropriate integral sites in the central nervous system, are thus an optimal target for therapeutic gene transfer [5–7].
Vector selection plays a critical role for the success of gene therapy. Currently, the most successful gene therapy strategies rely on recombinant viral vectors (e.g., adeno-associated virus, adenovirus, lentivirus, retrovirus, and herpes simplex virus), although the utility of nonviral vectors is continuing to emerge [8]. Enthusiasm for the recombinant adeno-associated virus (AAV) vector system for in vivo viral gene transfer to the DRG neurons has grown in recent years since this vector provides highly efficient gene transfer into the post-mitotic primary sensory neurons and long-term control of neuropathic pain with minimal toxicity [1, 6, 7, 9–11].
Genetic molecules can be transferred to DRG neurons through direct AAV intraganglionic injection [5, 7, 12], or by intrathecal delivery of AAV vectors into cerebrospinal fluid (CSF) [13–16], as well as retrograde transfer by injection of AAV into sciatic nerve and skeletal muscles [11]. Each delivery route has features that make them applicable to certain pain therapies. Among the various routes, direct intraganglionic injection offers the most efficient AAV-mediated gene transfer selectively into the peripheral sensory nervous system, including sensory neuron somata and their central and peripheral axonal terminals [7, 17, 18]. The loose and porous structure of DRG surrounded by a connective tissue capsule renders it well suited to injection. Due to the skeletal elements surrounding the DRG, a foraminotomy (minimal laminectomy) is necessary to expose the distal pole of the DRG for a reliable and consistent successful intraganglionic injection [19]. The small size of the adult rodent DRG (approximately 1 cm long and 0.5 cm in diameter in rat) determines the injected volumes be kept quite small [5]. Slow and sustained injection through a pulled small-tip glass micropipette attached to a microprocessor-controlled injector enables continuous and predictable filling of the DRG with 2 μl of AAV particle suspension without significant leakage and damage to the DRG [5]. Since this approach targets only the peripheral sensory neurons, some of the problems associated with other pain therapies that also affect the central nervous system can be avoided. The injection procedure produces only transient hypersensitivity to threshold mechanical stimulation without any significant changes in hyperalgesia or other sensory modalities or motor behavior [5]. Direct injection into the DRG has been proven well tolerated in both humans and rodents [5, 7, 12, 20, 21] and is a simple outpatient procedure in humans.
Intrathecal delivery of AAV through an intrathecal pump or by lumbar puncture has been demonstrated to produce effective gene transfer to the DRG and spinal cord. This method also has the advantage of allowing for repeated injections, which can be problematic for DRG injection because scar tissue formation caused by the initial surgery can make subsequent surgical exposure difficult. However, intrathecal delivery requires far more AAV vector [22], which creates a costly biomanufacturing challenge compared to intraganglionic injection. More importantly, intrathecal delivery cannot achieve a localized effect owing to the spread of the vectors within the CSF of the subarachnoid space, and robust transduction occurs in multiple spinal cord tissues including spinal motor neurons. This paradigm indicates that intrathecal AAV delivery has more therapeutic potential for widespread neurological disease processes that require diffuse gene delivery to spinal motoneurons as well as sensory neurons [23]. Intraneural AAV injection into the sciatic nerve can potentially transfer genes to primary sensory neurons, but this route of AAV delivery also shows preferential transduction of motor neurons, and injections into peripheral nerve carry the risk of damage from intrafasicular injection [24, 25]. Intramuscular AAV causes highly efficient transduction of skeletal muscle fibers, but does not efficiently transduce sensory neurons by retrograde axonal transport [11, 26]. In neonate rodents, intraperitoneal administration of AAV has been shown to efficiently target transgene expression in the DRG neurons [27].
In the subsequent material, we provide a detailed approach for DRG injection in adult rats using AAV vectors carrying GFP reporter as the injectate, including a list of the necessary materials and detailed description of a method for performing DRG microinjection experiments. Development of these techniques would likewise be suitable for intraganglionic application of various other therapeutic injectates, such as pharmacological agents and nonviral vectors.
2 Materials
2.1 Animals
Male Sprague-Dawley rats (see Note 1). We generally use rats weighing between 100 and 150 g to allow easier handling over extended post-injection observation. The surgery can be successfully performed on rats as large as 300 g or more if necessary, although the surgery can become more challenging as the size of the animal increases.
2.2 Reagents
High titer and high-pure AAV particles (titer should ideally be >1012 GC/ml) should be stored in small aliquots at −80 °C to prevent repeated freeze/thaw cycles, which significantly reduce infection efficiency (see Note 2).
Volatile anesthetic (isoflurane, sevoflurane) and delivery system.
Anesthesia chamber.
Anesthesia nose cone.
Betadine.
70 % (v/v) ethanol in spray bottle.
Sterile cotton-tipped applicators.
Biohazard bin.
2.3 Equipment
Dissecting microscope.
Glass micropipette puller.
Glass Pasteur pipettes.
Oxygen tank.
Vaporizer.
Induction box.
Electric razor.
Betadine or other skin disinfectant.
Injection system: Micromanipulator-mounted, piston-driven injector employing positive displacement of a noncompressible fluid (i.e., mineral oil) under control of a microprocessor-controlled micro-stepping motor such as Nanoliter 2000 (World Precision Instruments, Sarasota, FL, USA) or equivalent.
Three-axis micromanipulator.
Magnetic Stand.
Surgical tools: #3 Scalpel handle, #10 scalpel blade, No. 2 forceps ×2, No. 5 forceps, microrongeur, spring scissors, small animal retraction kit, absorbable 6-0 suture, skin stapler.
Glass bead sterilizer.
Method to control bleeding (Gauze, Gelfoam, bone wax).
Rolled paper towel or wooden dowel of sufficient diameter to support the animal during surgery.
Heating pad to maintain the animal’s body temperature.
3 Methods
3.1 Setup
Pulled glass pipettes for injection: Pulled to produce a long tip with a sharp point, with minimal regard to initial tip diameter. The tip should be pressed through a tautly held laboratory wipe (e.g., Kimwipe). This reliably produces a final tip diameter between 40 and 60 μm and a slightly “jagged” appearance of the tip under microscopic viewing (see Note 3). The exact procedure can be different based on the personal preference and on the different equipment for glass needle pulling for injection. Store glass needles upright with the tips pointed down in an electrode storage jar after pulling.
Spinal stabilization clamp: a spring-loaded or screw-driven clamp affixed to metal rods that attach to a magnetic base positioned caudal of the animal as it lies in position for surgery. The rods should be long enough to accommodate the injection system. The clamp should be large enough to grasp the spinous processes of the lumbar vertebra, and should grip with enough force to lift the rat slightly, but not tightly enough to damage the bone or overlying fascia.
Microinjection rig setup: The microinjector should be mounted on a horizontal bar attached to the same magnetic base as the stabilization clamp. Sufficient distance should be present to allow the injector to approach the animal from the caudal direction at a shallow angle (approximately 20° from horizontal), as close to parallel with the spine of the animal as the anatomy will allow.
3.2 Anesthetize the Rat
Anesthetize the rat in a suitable chamber. We routinely use the volatile anesthetic isoflurane (4–5 % for induction, in O2 ≈ 2 L/min). Sevoflurane (with 50 % higher dosing) is also suitable.
Confirm achievement of a surgical plane of anesthesia by lack of withdrawal to plantar pinch. Anesthetic depth should be monitored by vigilant observation of spontaneous breathing with lowering anesthetic levels if tidal volumes diminish.
When immobility is achieved, shave the surgical area of the back, leaving a wide margin around the planned incision to prevent contamination of the surgical field with hair.
Disinfect the surgical area, i.e., with Betadine and rinse three times with 70 % ethanol.
3.3 Surgical Exposure of DRG and Injection
Place the rat in ventral recumbency, with rolled paper towels or a wooden rod beneath the hips, to arch the back and elevate the lumbar spine. The animal’s face is placed in the anesthetic cone to maintain anesthesia and the isoflurane concentration reduced to 1.5–2.5 % (in O2 ≈ 2 L/min).
Confirm achievement of a surgical plane of anesthesia by lack of withdrawal to plantar pinch. Anesthetic depth should be monitored by vigilant observation of spontaneous breathing with lowering anesthetic levels if tidal volumes diminish.
Make a 3–3.5 cm skin incision just lateral of the dorsal midline, extending rostral from just caudal to the iliac crest (Fig. 1).
Make an underlying incision in the fascia of the paravertebral muscles just lateral to the midline.
Use forceps to separate two layers of the paravertebral musculature by blunt dissection to expose the lateral aspect of the L6-L4 vertebrae, applying retraction using retractors mounted on magnetic holders as needed to maintain a clear view of the surgical field.
Position the rat partially on to its side to achieve a better view of the lateral aspect of the L6-L4 vertebrae.
Clean the lateral aspect of the vertebrae by blunt dissection to expose the L4/L5 and L5/L6 intervertebral foramina (IVFs), overlaid by a membrane of connective tissue, to reveal the L4 and L5 spinal nerves just distal (approximately 2 mm) from their DRGs, which are covered by laminar bone (Fig. 1a).
Gently separate the membrane from the vertebral bone, exposing the entry of the spinal nerves into the IVFs. Pause as appropriate to control bleeding by compression with small balls of sterilized absorbent tissue paper.
Use rongeurs to slightly enlarge the IVF and remove a 1 mm-deep crescent of laminar bone, exposing the distal third of the L4 and L5 DRGs (Fig. 1b) (see Note 4). Avoid compression of the DRG by using the smallest rongeur, taking small bites with only the tip of the rongeur, and maintaining a lifting force against the bone with the portion of the rongeur that is in contact with the DRG.
Remove one aliquot of virus from −80 °C freezer. Thaw, flick the tube to mix, and then do a quick spin-down in a microcentrifuge (<2100 × g) to ensure no liquid is in the cap or on the walls of the tube.
Load the pipette with injectate by drawing injectate back through the tip, taking care to avoid introducing air bubbles or cavitation, which can affect the accuracy of injection volume.
Mount the rat in the clamp (Fig. 1c) and lift it such that the abdomen is just barely touching the table (to reduce venous bleeding). Physical support can then be provided under shoulder and hip with paper towel to stabilize the position.
Approach the ganglion at as shallow an angle as possible (i.e., tangential to the surface of the capsule) to facilitate entry into the DRG without compression against the underlying bone.
Slowly advance the pipette into the DRG to a depth of approximately 100–150 μm.
Wait 2–3 min before beginning injection to allow the DRG capsule to close around the pipette tip.
Gradually expel injectate (2 μl of AAV) in small boluses, carefully watching for backflow from around the pipette tip (see Note 5).
Injection of 2 μl of solution per DRG should take approximately 5 min.
Once injection is complete, wait 5 min before withdrawing pipette, to allow pressure within the ganglion to equalize and minimize backflow.
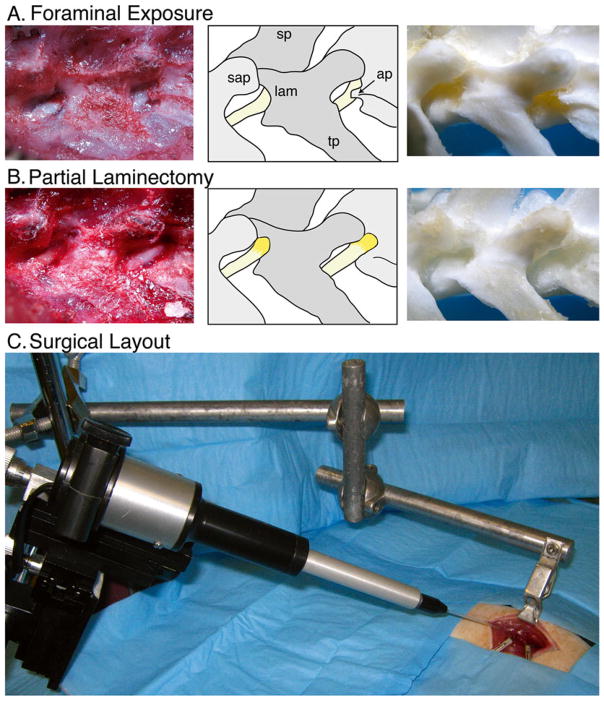
Fig. 1.
Intraganglionic injection. Paravertebral surgical exposure for intraganglionic injection, illustrating the operative field (left panel), reference diagram (middle panel), and cleaned vertebral bones (right panel). (a) Initial dissection of soft tissues at the level of the fourth lumbar (L4) and L5 spinal nerves (yellow) shows the superior articular processes (sap), spinous processes (sp), and transverse processes (tp), as well as the laminar bone (lam) and accessory process on L4 (ap). The dorsal root ganglia are covered by laminar bone. (b) Removal of laminar bone superior to the foramen and the L4 accessory process reveals the distal dorsal root ganglion, recognized by its broader diameter and brownish-orange color. (c) The motorized injection system is mounted on a magnetic stand via a manual micromanipulator. The rat vertebral column is stabilized by clamping the spinous process of L5 using a clip mounted on an articulated arm that is attached to the same magnetic stand. The head of the rat is to the right. Reproduced with minor modification from Fischer et al. [5] with permission
3.4 Suture and Recovery
After injections are completed, disengage the clamp and lower the rat to the table.
After ensuring hemostasis, close the muscle fascia using absorbable suture.
Close the skin with removable skin staples or wound clips.
Once the incision is closed, discontinue the anesthetic, but continue to deliver oxygen.
Keep the rat on the heating pad and monitor it continuously until the righting reflex is regained, at which point the animal can be transferred back to its cage.
3.5 Representative Results
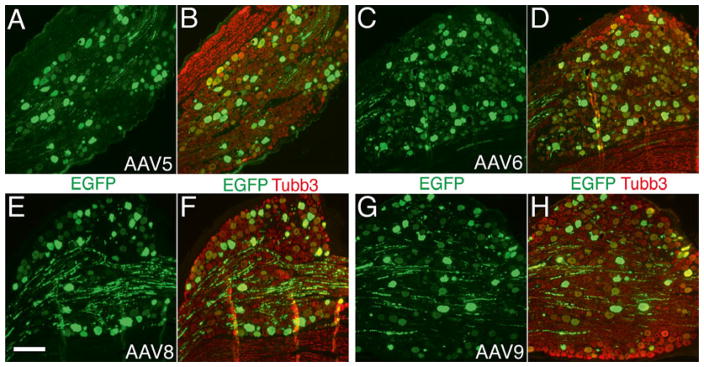
When following this protocol, a very precise injection with consistently successful transgene expression mediated by AAV is obtained. As a representative result of this method, we injected self-complimentary AAVs of various serotypes (5, 6, 8, and 9) into the L4 and L5 DRGs. Variable levels of EGFP expression were detected in the primary sensory neurons and their axons at 1 week following vector administration. A robust EGFP expression for all vectors tested was observed at 2–4 weeks after injection. EGFP expression was restricted to the sensory neuronal somata and projections, and no EGFP could be identified in satellite glia or other nonneuronal cells (Fig. 2).
Fig. 2.
Typical sensory neuron transduction after DRG injection of AAV5-, 6-, 8-, and 9-EGFP. Paraffin-sections from L5 DRGs were double-immunolabeled with the antibodies against GFP (green color) and neuron-specific β3-tubulin (Tubb3, red color). High-efficient transgene (EGFP) expression with predominant neuronal tropism is demonstrated in DRGs 4-week after intraganglionic injection of AAV5 (a and b), AAV6 (c and d), AAV8 (e and f), and AAV9 (g and h). Scale bar for all images: 200 μm
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs Rehabilitation Research and Development (3690-03), the Advancing a Healthier Wisconsin (FP00005706), and the National Institute of Neurological Disorders and Stroke (R01NS079626-01), to Q.H.H.
Footnotes
All protocols using live animals must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must be performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
A highly purified AAV vector with a titer of at least 1 × 109 GC viral particles (injected) and >90 % purity by silver stain is recommended. Injecting less than 108 viral particles with low-purity per DRG may not lead to a desired in vivo transduction. AAV is generally considered nonpathogenic and regarded as a Biosafety Level 2 (BSL-2) material, and appropriate practices must be followed during AAV application.
A 40–60 μm tip is suitable for dissolved compounds and suspensions of very small particles, such as viruses and nanoparticles. Larger openings may be necessary for suspensions of larger particles, i.e., cell suspensions.
Cauterization is not recommended due to the risk of thermal injury to the spinal nerve. In the case of the L4 DRG, foraminotomy will also involve the removal of the small accessory process that projects caudally and partially overlays the foramen.
Increasing injectate volume over 2 μl per DRG does not necessarily increase the transduction but will lead to significant leakage [5, 12]. Use of a visible dye (i.e., Fast Green) is especially helpful at this stage to ensure that no leakage occurs. Fast Green at a concentration of 0.1 % w/v is an effective method to track the spread of injectate in real time. DAPI can be used at a concentration of 0.25 μg/μl to measure injectate spread in fixed tissue sections for at least 2 weeks after injection without toxicity [5].
Contributor Information
Hongwei Yu, Medical College of Wisconsin, Milwaukee WI.
Gregory Fischer, Medical College of Wisconsin, Milwaukee WI.
Quinn H. Hogan, Medical College of Wisconsin and Zablocki VA Medical Center, Milwaukee WI.
References
- 1.Beutler AS. AAV provides an alternative for gene therapy of the peripheral sensory nervous system. Mol Ther. 2010;18(4):670–673. doi: 10.1038/mt.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glorioso JC, Fink DJ. Gene therapy for pain: introduction to the special issue. Gene Ther. 2009;16(4):453–454. doi: 10.1038/gt.2009.18. [DOI] [PubMed] [Google Scholar]
- 3.Goins WF, Cohen JB, Glorioso JC. Gene therapy for the treatment of chronic peripheral nervous system pain. Neurobiol Dis. 2012;48(2):255–270. doi: 10.1016/j.nbd.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handy CR, Krudy C, Boulis N. Gene therapy: a potential approach for cancer pain. Pain Res Treat. 2011;2011:987597. doi: 10.1155/2011/987597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer G, Kostic S, Nakai H, Park F, Sapunar D, Yu H, Hogan Q. Direct injection into the dorsal root ganglion: technical, behavioral, and histological observations. J Neurosci Methods. 2011;199(1):43–55. doi: 10.1016/j.jneumeth.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer G, Pan B, Vilceanu D, Hogan QH, Yu H. Sustained relief of neuropathic pain by AAV-targeted expression of CBD3 peptide in rat dorsal root ganglion. Gene Ther. 2013 doi: 10.1038/gt.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Fischer G, Ferhatovic L, Fan F, Light AR, Weihrauch D, Sapunar D, Nakai H, Park F, Hogan QH. Intraganglionic AAV6 results in efficient and long-term gene transfer to peripheral sensory nervous system in adult rats. PLoS One. 2013;8(4):e61266. doi: 10.1371/journal.pone.0061266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sloane EM, Soderquist RG, Maier SF, Mahoney MJ, Watkins LR, Milligan ED. Long-term control of neuropathic pain in a non-viral gene therapy paradigm. Gene Ther. 2009;16(4):470–475. doi: 10.1038/gt.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler AS, Reinhardt M. AAV for pain: steps towards clinical translation. Gene Ther. 2009;16(4):461–469. doi: 10.1038/gt.2009.23. [DOI] [PubMed] [Google Scholar]
- 10.Eaton MJ, Blits B, Ruitenberg MJ, Verhaagen J, Oudega M. Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated over-expression of BDNF in the rat spinal cord. Gene Ther. 2002;9(20):1387–1395. doi: 10.1038/sj.gt.3301814. [DOI] [PubMed] [Google Scholar]
- 11.Towne C, Pertin M, Beggah AT, Aebischer P, Decosterd I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain. 2009;5:52. doi: 10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A, Timmermans E, Blits B, Verhaagen J. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther. 2010;18(4):715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beutler AS, Banck MS, Walsh CE, Milligan ED. Intrathecal gene transfer by adeno-associated virus for pain. Curr Opin Mol Ther. 2005;7(5):431–439. [PubMed] [Google Scholar]
- 14.Wang X, Wang C, Zeng J, Xu X, Hwang PY, Yee WC, Ng YK, Wang S. Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther. 2005;12(2):314–320. doi: 10.1016/j.ymthe.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Vulchanova L, Schuster DJ, Belur LR, Riedl MS, Podetz-Pedersen KM, Kitto KF, Wilcox GL, McIvor RS, Fairbanks CA. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain. 2010;6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS, Morrison JH, Beutler AS. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A. 2008;105(3):1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glatzel M, Flechsig E, Navarro B, Klein MA, Paterna JC, Bueler H, Aguzzi A. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci U S A. 2000;97(1):442–447. doi: 10.1073/pnas.97.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Gu Y, Wu P, Li GW, Huang LY. Efficiencies of transgene expression in nociceptive neurons through different routes of delivery of adeno-associated viral vectors. Hum Gene Ther. 2003;14(9):897–906. doi: 10.1089/104303403765701187. [DOI] [PubMed] [Google Scholar]
- 19.Puljak L, Kojundzic SL, Hogan QH, Sapunar D. Targeted delivery of pharmacological agents into rat dorsal root ganglion. J Neurosci Methods. 2009;177(2):397–402. doi: 10.1016/j.jneumeth.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Tang Z, Zhang H, Atianjoh FE, Zhao JY, Liang L, Wang W, Guan X, Kao SC, Tiwari V, Gao YJ, Hoffman PN, Cui H, Li M, Dong X, Tao YX. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 2013;16(8):1024–1031. doi: 10.1038/nn.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samad OA, Tan AM, Cheng X, Foster E, Dib-Hajj SD, Waxman SG. Virus-mediated shRNA knockdown of Na(v)1.3 in rat dorsal root ganglion attenuates nerve injury-induced neuropathic pain. Mol Ther. 2012 doi: 10.1038/mt.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagoe ND, Eggers R, Verhaagen J, Mason MR. A compact dual promoter adeno-associated viral vector for efficient delivery of two genes to dorsal root ganglion neurons. Gene Ther. 2013 doi: 10.1038/gt.2013.71. [DOI] [PubMed] [Google Scholar]
- 23.Federici T, Taub JS, Baum GR, Gray SJ, Grieger JC, Matthews KA, Handy CR, Passini MA, Samulski RJ, Boulis NM. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19(8):852–859. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 24.Lu YY, Wang LJ, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. Intramuscular injection of AAV-GDNF results in sustained expression of transgenic GDNF, and its delivery to spinal motoneurons by retrograde transport. Neurosci Res. 2003;45(1):33–40. doi: 10.1016/s0168-0102(02)00195-5. [DOI] [PubMed] [Google Scholar]
- 25.Boulis NM, Noordmans AJ, Song DK, Imperiale MJ, Rubin A, Leone P, During M, Feldman EL. Adeno-associated viral vector gene expression in the adult rat spinal cord following remote vector delivery. Neurobiol Dis. 2003;14(3):535–541. doi: 10.1016/j.nbd.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Qiao C, Wang CH, Li J, Yuan Z, Zhang C, Xiao X. Efficient retrograde transport of adeno-associated virus type 8 to spinal cord and dorsal root ganglion after vector delivery in muscle. Hum Gene Ther. 2010;21(1):87–97. doi: 10.1089/hum.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machida A, Kuwahara H, Mayra A, Kubodera T, Hirai T, Sunaga F, Tajiri M, Hirai Y, Shimada T, Mizusawa H, Yokota T. Intraperitoneal administration of AAV9-shRNA inhibits target gene expression in the dorsal root ganglia of neonatal mice. Mol Pain. 2013;9:36. doi: 10.1186/1744-8069-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]