Abstract
Alzheimer’s disease (AD) is a common, chronic expensive debilitating neurodegenerative disease with no current treatments to prevent the physical deterioration of the brain and the consequent cognitive deficits. The current pathophysiology of Alzheimer’s disease is the accumulation of neurofibrillary tangles (NFTs) of hyperphosphorylated tau protein and amyloid-beta (Aβ) plaques. Antibody therapy of Tau and Amyloid beta, vaccines and other methods to decrease Tau and or Amyloid have not been successful after considerable pharmaceutical and biotech efforts. For example, Eli Lilly announced a major change to its closely watched clinical trial for the Alzheimer’s drug solanezumab which failed to reach statistical significance. Recently, a report on animal models using photomodulation with near infrared light to treat AD pathology in K369I tau transgenic model (K3) l engineered to develop neurofibrillary tangles, and the APPs/PSEN1dE9 transgenic model (APP/PS1) to develop amyloid plaques. Mice were treated with NIR 20 times over a four-week period and NIR treatment (600–1000 nm) was associated with a reduction in the size and number of amyloid-β plaques in the neocortex and hippocampus. We now report a small pilot double blind, placebo-controlled trial (n=11) 6 active, 3 controls and 2 dropouts assessing the effect of 28 consecutive, sixminute transcranial sessions of near infrared (NIR) stimulation using 1060–1080 nm light emitting diodes. Subjects were independently diagnosed with dementia conducted in an outpatient behavioral healthcare clinic. IRB approval was obtained through the Quietmind Foundation’s institutional review Board (IRB). Results showed changes in executive functioning; clock drawing, immediate recall, praxis memory, visual attention and task switching (Trails A&B) as well as a trend of improved EEG amplitude and connectivity measures. Neuroplasticity has also been reported with NIR light stimulation and mitochondrial enhancement.
Keywords: Alzheimer’s disease, Photobiomodulation, Memory, Cognition
Introduction
Alzheimer’s disease (AD) is a common, chronic progressive, expensive neurodegenerative disorder slowly resulting in dementia. Its etiology and pathogenesis is complex, with many genetic and environmental risk factors including stress and insulin resistance. The expression of many genes, and upregulation of multiple pathogenic pathways result in amyloid β peptide (Aβ) deposition, tau hyperphosphorylation, inflammation, reactive oxidative stress (ROS), mitochondrial disorders, insulin resistance, methylation defects and down regulation of neuroprotective factors [1].
Antibody therapy of Tau and Amyloid beta, vaccines and other methods to decrease Tau and or Amyloid have not been successful after considerable pharmaceutical and biotech efforts [2].
For example, Eli Lilly in Indianapolis announced a major change to its closely watched clinical trial for the Alzheimer’s drug solanezumab which failed to reach statistical significance. “A major challenge of such trials is how to measure the drug’s benefits,” says Dennis Selkoe, a Neurologist at Brigham and Women’s Hospital in Boston, who is not involved in the Lilly trial. “Although people with early Alzheimer’s may show mild memory impairment and problems with attention and focus, they can often follow recipes, make a cup of coffee, or drive a car,” Selkoe reiterates. Such abilities are unlikely to change much over the course of an 18-month clinical trial.” [3].
Recently, a report on animal models using photomodulation with near infrared light to treat AD pathology in K369I tau transgenic model (K3) l engineered to develop neurofibrillary tangles, and the APPs/PSEN1dE9 transgenic model (APP/PS1) to develop amyloid plaques. Mice were treated with NIR 20 times over a four-week period and NIR treatment (600–1000 nm) was associated with a reduction in the size and number of amyloid-β plaques in the neocortex and hippocampus [4].
The role of emerging pathogens such as dental spirochetes, Borrelia Bd, with bacterial biofilms and is also gaining traction [5–7]. Fungal and even viral infections have been implicated [8]. Infections induce potent immune responses, too, and they likely worsen the problem says Rudy Tanzi. “Normally, brain immune cells called microglia clear amyloid proteins from the brain. But when these cells get fired up in response to infection, they stop, causing the proteins to build up even faster.” Tanzi’s team at Harvard showed in a 2014 Nature paper, that the amyloid proteins that fill up the brain then spark the creation of tau tangles, which cause more brain cell death [9]. Mitochondrial dysfunction has important roles in the neurodegenerative cascade [10]. Amyloid β can interact with the mitochondria and cause mitochondrial dysfunction [11]. Finally, misfolded proteins of Tau and Amyloid β by proteasomes have been elucidated in AD [12]. Therefore, it has been proposed that targeting the mitochondria, increasing ATP in proteasomes for ubiquitination of misfolded proteins, decreasing inflammation and even antibacterial and anti-viral effect of NIR light [13] could prove valuable for AD therapeutics and is a safe, simple and effective approach to treat early to mid-dementia in Alzheimer’s and other related neurodegenerative disorders.
The study objective was to determine if intensive near-infrared treatment (INIRT) using 1072 nm IR will affect significant positive changes in mood, behavior, and cognitive functioning of people with dementing illness.
Neuroplastic effects of transcranial NIR stimulation (tNIRS) as a tool on the motor cortex to modulate cortical excitability in the corticospinal pathway and intracortical circuits was recently published by Chaleb and his lab at the University of Bonn. They used tNIRS at wavelength of 810 nm for 10 min over the hand area of the primary cortex and transcranial magnetic stim at 2.2 Tesla to assess levels of magnetic evoked motor-evoked- potentials of the dorsal interosseous in human brains in 55 healthy volunteers. They concluded that tNIRS is suitable as a tool for influencing cortical excitability and activity [14].
Study hypothesis
The provision of brief, repeated exposure to 1072 nm infrared stimulation of the cortex surface improves cognitive and behavioral functioning as indicated by normalization of EEG activity, increased cerebral oxygenation, and demonstrated improvement on standardized neuropsychological measures.
Endpoints
This single-center, double-blind, randomized, placebo-controlled clinical trial of the 1072 nm Infrared Light Stimulation Helmet was analyzed by the following endpoints: see below.
Materials and Methods
Participants
Patients were recruited from several local continuing care communities and using print and online media. All subjects were independently diagnosed with probable Alzheimer’s dementia by a neurologist by means of the criteria of NIA-OA [15]. Patients were excluded if there was diagnosis of multi-infarction dementia or Parkinson’s disease. MCI subjects (n=11, 6 active and 3 placebo and 2 withdrawals) under the Quietmind Foundation’s IRB approved randomized, double blind, placebo controlled design. The protocol was also approved by Baylor Scott and White IRB. Informed consent was obtained prior to initiation of treatment. Compliance with this standard provides public assurance that the rights, safety, and well-being of trial subjects are protected, consistent with the principles that have their origin in the Declaration of Helsinki.
Exclusion criteria
Uncontrolled or unstable chronic illness, e.g., hypertension, COPD
Diagnosed actively growing intracranial pathology (tumors etc.).
An associated psychotic illness.
Misusing illegal substances or alcohol.
On regular systemic steroids or anti-metabolites.
Systemic malignancies or space occupying head and/or neck lesions
Fluent in English.
Moderate to severe depression as assessed by Beck Depression Inventory score.
Epilepsy or other seizure disorder.
Previous history of stroke or heart attack.
History of aggression or violence.
Inability to travel to the research venue for 28 multiple assessments
Inclusion criteria
Age 40 – 85 years.
Have established cognitive decline, Mini Mental Status Examination (MMSE) between 15–25 (out of a possible score of 30).
Generally healthy as indicated by recent physical examination.
Subjects should have had a CT MRI scan in the previous 12 months which was consistent with a dementia diagnosis.
Have a caregiver/informant who has cared for the patient at least 5 days a week and is willing to attend study visits and provide information about the patient.
If taking any psychotropic medication should have been stable for the previous 3 months.
Must have had B12, folic acid, full blood count, ferritin screen within 6 months or be on B12 and/or folic acid replacement.
Subject assessment
Testing included Mini Mental Status Exam MMSE, Quantitative EEG (QEEG), Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog) that were administered the first day of treatment and within 3 days of completing the required 28 consecutive exposure sessions. Surface cortical perfusion was measured before and after each treatment session using infrared spectroscopy. A two minute baseline was recorded using the Biocomp Research Hemoencephalography recording system and Bioexplorer software (Tables 1 and 2).
Table 1.
Subject demographics (UM- upper middle, MC-middle class, WC-working class).
| ID Code - Gender | Age | SES - class | EDUC- years |
|---|---|---|---|
| ETBM - F | 88.51 | UM | 16 |
| ARBN - M | 86.91 | UM | 18 |
| ARVI- M | 78.04 | MC | 12 |
| BSMN F | 81.61 | UM | 16 |
| EHUL- F | 75.89 | UM | 18 |
| GWSN-M | 77.42 | WC | 18 |
| HJLK-M | 95.52 | UM | 18+ |
| LDMCE-F | 80.36 | WC | 12 |
| BSMN- F | 80.64 | UM | 16 |
| MCLN- F | 74.01 | MC | 12 |
| RDYR-M | 80.92 | UM | 18 |
| Average | 81.80 | 14.9 | years |
Table 2.
ADAS- Cog (11) Improvement (SEM).
| Delayed Word Recall | Pre=0.77 (0.43) | Post = 1.6 (0.37) | p=0.10 |
|---|---|---|---|
| TX Group mean PRE-test (0.5 score) was lower than Controls pre-test mean score (1.333), but at POST testing the TX Group Mean rose to 1.83 and Ctrl Grp mean post-test score remained at 1.333. | |||
| Trail Making A | Pre=3.55 (1.73) | Post 1.33 (0.87) | p=0.044 |
| Omission Errors - More active and successful engagement with the task. | |||
| Ideational Praxis | Pre= 3.55 (0.38) | Post= 4.33 (0.33) | p=0.03 |
| Multi step instructions to address and seal envelope. | |||
| Boston Naming | Pre=11.75 (2.91) | Post 13.00 (2.43) | p=0.035 |
| Recall names of familiar objects when shown them on cards. | |||
| Worsened | |||
| Auditory Verbal Learning | Pretest 2.44 (0.53) | Post 1.66 (0.58) | p=0.08 |
| Auditory Verbal Learning Test Trial 1 | |||
| (first exposure to auditory word list- immediate verbal memory) | |||
| Pre-Test mean TX = 3.00 (0.67) and CTRL | Pre-Test mean = 1.33 (1.53) | ||
| Post-Test mean TX= 2.167 (0.60) and CTRL Post-Test mean = .667 (0.67) | |||
| Trail Making-A | Pretest 2.00 (1.64) | Post Test 2.88 (1.81) p=.034 | |
| Commission Errors = More attempts = > effort = > potential errors. | |||
| Cortical Perfusion | |||
| ARVI* (Treatment): 1.77% | |||
| BSMN (Treatment): 6.86% | |||
| EGKY (Treatment): 4.93% | |||
| MCLN (Treatment) : 2.72% | |||
| EHUL (Control): −0.19% | |||
| HJLK (Control): 0.86% | |||
| LDMC (Control): 3.32%** | |||
Study subject identifier codes
This placebo group subject came to study having had not left her small town urban row home where she lived with her husband and over the previous 18months had not been outside except for doctor appointments. Her response was clearly tied to the intensive positive interpersonal stimulation of the treatment context.
Experimental device and procedure
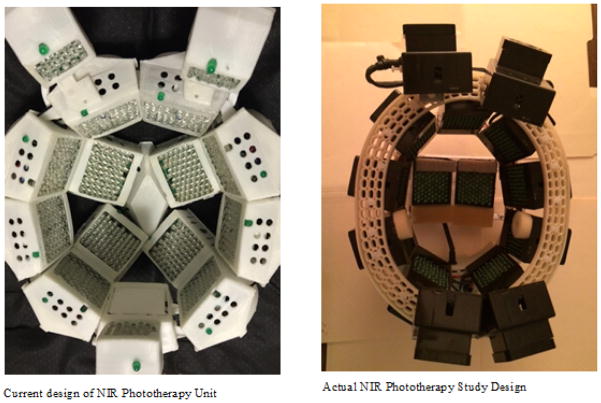
The experimental device used 1100 LEDs set in 15 arrays of 70 LEDs/array with all matched to 1060–1080 nm and pulsed at 10 hz with a 50% duty cycle. Stimulation was administered for 6 minutes daily over 28 consecutive days. (Figure 1 is inside view of the current version of Cognitolite Transcranial Photomodulation System.)
Figure 1.
NIR LED Helmet (inside view).
All subjects were reassessed after the treatment cycle. Then was reexamined with MME and ADAS-Cog.
Data analysis
Cohorts consisted of active treatment group and controls (N=11). The pre/post design allows improvement or arrested decline in mood, behavior, and cognitive functions to be established with relatively powerful statistical analyses. Repeated-measures ANOVAs will be used with each measure to determine overall improvement in the treatment group relative to the placebo controls. Additional categorical variables such as gender and/or covariates such as age will be included in ANOVAs to determine whether the overall effect is consistent across these factors. Multiple regressions will be performed to determine the effects of combinations of factors on the effectiveness of the treatment, and a canonical correlation will assess how the outcome measures interact. Finally, parallel analyses were conducted with the 30 daily near-infrared cerebral oxygenation (HEG) measures. This enabled an attribution of the expected improvements in mood, behavior, and cognitive function to the mediating variable of cerebral blood flow.
Results
Delta power increase = improved alertness, attention. Alpha decrease = less anxiety
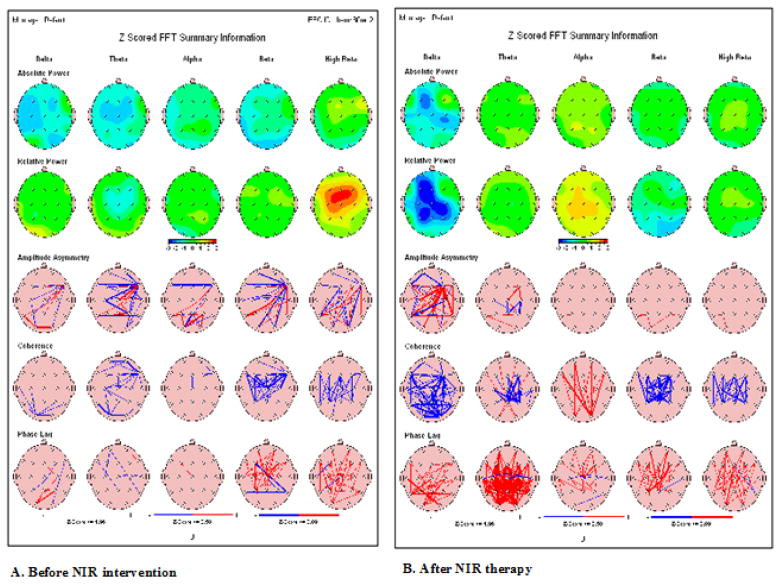
Similar QEEG improvements before and after NIR Helmet intervention was seen in the other 5 patients, but not in control patients (Figure 2). Supplement files for these QEEG may be obtained on line at www.Quitemindfnd.org.
Figure 2.
Quantitative EEG changes before NIR helmet and after as seen below; Green color indicates regions where values of power were between 0 and 1SD; Yellow between 1 and 2SD; Red between 2 and 3 SD. Pink represents Pre- and postcoherence summary maps.
Research in Context
Systemic review
A search of the world’s medical literature of significant therapy in Alzheimer’s and dementia fails to reveal any published long term improvement to date, whether medical or by devices. NIR trials in Traumatic Brain Injury (TBI) have been published by Naesar and Hamblin. NIR light passes readily through the scalp and skull and arrive at the cortical surface of the human brain. The primary photoreceptors for NIR light and red light are mitochondria [16]. Cortical neurons are rich in mitochondria with increased biochemical pathways such as increased ATP and signaling pathways activated by ROS. Photobiomodulation is based upon the ability of the light to alter cell metabolism as it is absorbed by general hemoproteins and cytochrome c oxidase (COX) in particular [17].
Naesar reported eleven chronic TBI patients whose cognition improved following treatment with red and NIR light emitting diodes (LEDs) applied transcranially to forehead and scalp at 10 minutes per area and red light nasally with 18 outpatient sessions. Neuropsychological testing at 1, 2 and after 18 treatments of LED treatments demonstrated improvement in the Stroop test for Executive Function [18].
Interpretation
This is the first published report that has shown a trend of improvement in executive functioning in patients with MCI in Alzheimer’s and dementia with NIR 1072 nm light. Even though this pilot control study was very small, n=11 with 8 patients treated with NIR helmet for 28 days and 3 placebo control and 2 dropouts which often occurs in Alzheimer’s clinical trials, there was some trend of improvement in the MMSE (especially clock drawing, ACOG (especially on attention and digit span forward-verbal recall), and Quantitative QEEG; in other words, executive functioning, our objective! [19].
Statistical analysis
Any statistical analysis of a small pilot study in MCI patients is problematic when n=11, 6 treated who completed the study, 3 placebo controls and 2 dropouts ; the most revealing was to graph the result of the data of each ADAS Cog 11 and the clock drawing. The value of clock drawing has been found to be moderately sensitive and specific for detecting executive cognitive dysfunction in people even with normal MMSE [20].
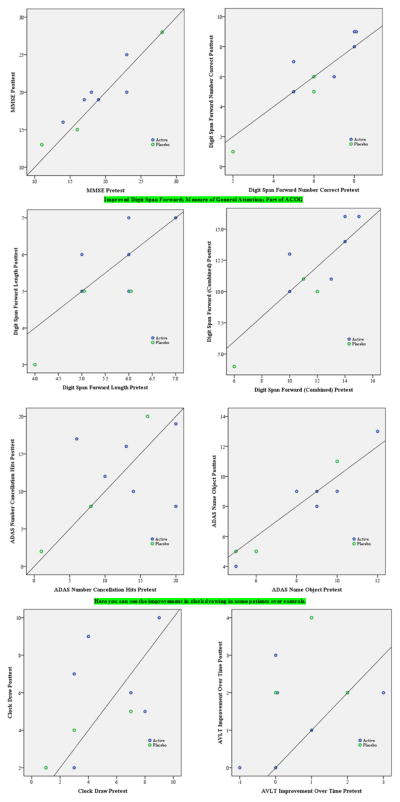
Graphs comparing active and placebo scores
Any dot above the line indicates improvement (that is, a higher score, so the reverse for measures in which a high score is bad) pre to post. The upper right blue dot is a little darker, indicating that it is actually two active subject dots laid on top of each other (Figure 3).
Figure 3.
Graphs comparing active and placebo scores.
So in the best case scenario, no placebo subject improved, but 3 of the 6 active group subjects did improve. This was the analysis with repeated measures ANOVA that was p= 0.12.
However, these were variables selected because they were close to or marginally significant.
Discussion
Although this was a small placebo controlled pilot study of PBT in which there was no statistical improvement in treated patients over controls, 28 days is a very short period of time in Alzheimer’s and dementia time line. Quietmind Foundation has had experience now in several Alzheimer’s patients treated with PBM adding Neurobiofeedback (NBB) with marked improvement in executive function and memory using a two year treatment protocol. Patients are being observed over 4–5 years who are showing continued cognitive and functional improvement with no medications other than vitamins and supplements for improve gut flora and chelation of heavy metals and other neurotoxins.
Recently a large clinical trial of monthly IV Aducanumab for early Alzheimer’s disease at 6 months found that at higher the dose of drug, the less people with Alzheimer’s declined on tests of memory and thinking skills. Headaches in some patients and brain scans some brain swelling was detected in those taking the higher doses [21]. PBM and NBB have virtually no side effects.
The addition of PBM and NBB in combination with novel medications in Alzheimer’s may be another potential therapeutic strategy. Saltmarche and associates had a poster recently in July 2016 on significant improvement in cognition after transcranial and intranasal photomodulation (Table 3) [22].
Table 3.
Socio-economic information.
| ID Code - Gender | Age | SES - class | EDUC- years |
|---|---|---|---|
| ETBM - F | 88.51 | UM | 16 |
| ARBN - M | 86.91 | UM | 18 |
| ARVI- M | 78.04 | MC | 12 |
| BSMN F | 81.61 | UM | 16 |
| EHUL- F | 75.89 | UM | 18 |
| GWSN-M (AFAM) | 77.42 | WC | 18 |
| HJLK-M | 95.52 | UM | 18+ |
| LDMCE-F | 80.36 | WC | 12 |
| BSMN- F | 80.64 | UM | 16 |
| MCLN- F | 74.01 | MC | 12 |
| RDYR-M | 80.92 | UM | 18 |
| Average | 81.8 | - | 14.9 years |
Furthermore the neuroplastic effects of transcranial stimulation on brain motor cortex in combination with TCMS provided tools for studies of human cortical neuroplasticity. Recent human and animal studies have demonstrated the NIR light applied over the cortex has beneficial effect on stroke rehabilitation [23] and may minimize the cognitive deficits in traumatic brain injury [24].
Conclusion
Our study investigated the potential effect of Photobiomodulation on Alzheimer’s dementia with results suggestive a trend of improvement in executive functioning; clock drawing, immediate recall, praxis memory, visual attention and task switching (Trails A&B) as well as improved EEG amplitude and connectivity measures. Although this very small pilot clinic study using low level light at 1070 nm to increase ATP in the mitochondria and proteasomes and induce neural plasticity did not reach statistical significance, the short duration of therapy and small number of participants should not defer further clinical trials.
Future Directions
A larger placebo controlled, IRB helmet approved trial of MCI in Alzheimer’s and dementia is now ongoing with a much larger sample size at Scott and White Clinic in Texas. Hopefully this clinical trial will usher in photomodulation therapy in Alzheimer’s dementia with MCI and a future trial in TBI in patients in the near future is also planned.
Acknowledgments
This study was supported by contributions from Cognitolite, LLC and Quietmind Foundation’s Dementia Research Initiative. This study was also supported, in part, by NIH/NINDS R01 NS067435 (to Jason H. Huang).
Abbreviations
- AD
Alzheimer’s Disease
- NFT
Neurofibrillary Tangles
- Aβ
Amyloid Beta
- APPS
Amyloid Precursor Protein Mouse Model
- PSEN
Presenilin
- NIR
Near Infra-Red
- ROS
Reactive Oxygen Species
- IRB
Institutional Review Board
- EEG
Electroencephalogram
- INIRT
Intensive Near-Infrared Treatment
- TNIRS
Transcranial NIR Stimulation
- NIA-OA
National Institute On Aging- Office Of Acquisition
- MCI
Multi Cerebral Infarction
- MMSE
Mini Mental Status Examination
- LED
Light Emitting Dioide
- ADAS-COG
Alzheimer Disease Assessment Scale-Cognitive
- ANOVA
Analysis of Variance
- HEG
Hemoencephalography
- TBI
Traumatic Brain Injury
- COX
Cytochrome C Oxidase
- NBB
Neurobiomodulation
Footnotes
Competing Interest
Marvin H. Berman is a stockholder in Noothera, and Trent W. Nichols is a stockholder in Lumineu. Both corporations have US Patents applications in Low Level Light therapy.
Supplementary Data
For the Excel spreadsheet of patient data on ADAS Cog11 and MMSE go to www.quietmindfnd.org/suppl_data_photomod.
References
- 1.Nichols TW. Hyperphosphorylation of tau protein in Down’s dementia and Alzheimer’s disease, Methylation and implications in prevention and therapy. J Alzheimer’s Dis Parkinsonism. 2014;4:5. [Google Scholar]
- 2.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6:2–7. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Underwood E. Why the big change to Lilly’s Alzheimer’s trial is not evidence its drug has failed again. Science 2016 [Google Scholar]
- 4.Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex - evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6:2. doi: 10.1186/alzrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miklossy J. Alzheimer’s disease - A neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation. 2011;8:90. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen HB, Morales D, Jones K, Joshi S. Alzheimer’s disease: A novel hypothesis integrating spirochetes, biofilm, and the immune system. J Neuroinfect Dis. 2016;7:1. [Google Scholar]
- 7.Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L. Different brain regions are infected with fungi in Alzheimer’s disease. Sci Rep. 2015;5:15015. doi: 10.1038/srep15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moyer MW. Controversial new push to tie microbes to Alzheimer’s disease. Sci America 2016 [Google Scholar]
- 9.Choi SH, Kim YH, Hebisch M, Sliwinski C. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimer’s Dis. 2010;20:265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picone P, Nuzzo D, Cauana L, Scafidi V, Di Carlo M. Mitochondrial dydsfunction; different routes to Alzheimer’s therapy. Ox Med Cell Long. 2014 doi: 10.1155/2014/780179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonati E, Sala G, Bulbarelli A. Protein misfolding and accumulation as root cause in neurodegeneration. Austin Alzheimers J Parkinsons Dis. 2014;1:10. [Google Scholar]
- 13.Landthaler M, Haima D, Waidelich W. Treatment of zoster, post -zoster pain and herpes simple recidivans in loco with laser light. Fortschr Med. 1983;101:1039–1412. [PubMed] [Google Scholar]
- 14.Chaleb L, Antal A, Masurat F, Paulus W. Neuroplastic effects of transcranial near-infrared stimulation (tNIRS) on the motor cortex. Front Behav Neurosci. 2012;9:147. doi: 10.3389/fnbeh.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman BT, Phelps CH, Beach TG. National Institute on Aging-Alzheimer’s Association: Guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naeser MA, Hamblin MA. Potential for transcranial lase or LED therapy to treat stroke, traumatic brain injury and neurodegenerative disease. Photo Med Laser. 2011;29:443–446. doi: 10.1089/pho.2011.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HP. Lightening up light therapy: Activation of retrograde signaling pathway by photobiomodulation. Biomol Ther. 2014;22:491–496. doi: 10.4062/biomolther.2014.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naeser MA, Zafonte R, Krengel MH, Martin PI. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J Neurotrauma. 2014;31:1008–1017. doi: 10.1089/neu.2013.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman M. Clinical Trial; Efficacy of 1072 nm infrared stimulation on executive functioning in dementia 2010 [Google Scholar]
- 20.Angela J, Tench S, Baker V. The value of clock drawing in identifying executive cognitive dysfunction in people with normal Mini-Mental State Examination score. Can Med Assoc J. 2002;167:859–864. [PMC free article] [PubMed] [Google Scholar]
- 21.Biogen. 12th International Conference on Alzheimer’s and Parkinson’s Diseases and Related Neurological Disorders in Nice; France. 2016. [Google Scholar]
- 22.Saltmarche A. Significant improvement in cognition after transcranial and intranasal photobiomodulation: A controlled, single-blind pilot study in participants with dementia. Poster presented at Alzheimer’s Association International Conference; Toronto. 2016. [Google Scholar]
- 23.Hashmi JT, Huang YY, Osmani BZ, Sharma SK. Role of low-level laser therapy in neurorehabilitation. PMR. 2010:S292–S305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including pooled analysis of NEST-1 and NEST-2. Curr Cardiol Rep. 2010;12:29–33. doi: 10.1007/s11886-009-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]