Abstract
Bile acids constitute a group of structurally closely related molecules and represent the most abundant constituents of human bile. Investigations of bile acids have garnered increased interest owing to their recently discovered additional biological functions including their role as signaling molecules that govern glucose, fat and energy metabolism. Recent NMR methodological developments have enabled single-step analysis of several highly abundant and common glycine- and taurine- conjugated bile acids, such as glycocholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, taurocholic acid, taurodeoxycholic acid, and taurochenodeoxycholic acid. Investigation of these conjugated bile acids in human bile employing high field (800 MHz) 1H-NMR spectroscopy reveals that the ratios between two glycine-conjugated bile acids and their taurine counterparts correlate positively (R2 = 0.83–0.97; p = 0.001 × 10−2–0.006 × 10−7) as do the ratios between a glycine-conjugated bile acid and its taurine counterpart (R2 = 0.92–0.95; p = 0.004 × 10−3–0.002 × 10−10). Using such correlations, concentration of individual bile acids in each sample could be predicted in good agreement with the experimentally determined values. These insights into the pattern of bile acid conjugation in human bile between glycine and taurine promise useful clues to the mechanism of bile acids’ biosynthesis, conjugation and enterohepatic circulation, and may improve our understanding of the role of individual conjugated bile acids in health and disease.
Keywords: Human gallbladder bile, Conjugation pattern, Glycine-conjugated bile acids, Taurine-conjugated bile acids, 1H-NMR, Single-step analysis, Metabolite profiling
Introduction
Bile acids constitute a group of structurally closely related molecules. Their steroid-like moieties consist of mono-, di-, or tri-hydroxy groups. Tri- and di-hydroxy bile acids, such as cholic acid and chenodeoxycholic acid, respectively, are known as primary bile acids because they are biosynthesized from cholesterol in hepatocytes. The others are known as secondary bile acids. Secondary bile acids are thought to be the products of gut bacterial deconjugation during enterohepatic circulation. In human bile, both primary and secondary bile acids are normally conjugated to glycine or taurine. Conjugated bile acids are the major components of human bile, with their total concentration exceeding that of any other metabolite in bile.
Bile acids have long been known to play an important, but less appreciated, biological role in lipid absorption and cholesterol catabolism. Recently, they have also been recognized as signaling molecules with systemic endocrine functions [1]. In addition, it is shown that increased bile acid levels facilitate liver regeneration and decreased levels inhibit re-growth [2]. The role of bile acids in maintaining energy homeostasis has been recently highlighted [3]. Strong evidence for a previously unknown mechanism by which conjugated bile acids facilitate their antimicrobial effects in the small intestine was recently presented [4]. Bile acids are also shown to have close links with human diseases including hepatocellular carcinoma and cholangiocarcinoma [5–8]. Key differences in bile acid metabolism observed in diabetes based on investigations of human subjects, suggest yet another important role of bile acid metabolism [9, 10].
The link between bile acids and a multitude of deleterious effects in humans has increased the interest in better understanding of the bile acid physiology [11, 12]. Although, mass spectrometry based methods are highly useful for analyzing bile acids, establishing the relationship between glycine- and taurine-conjugation has not been possible so far [13–16]. This may be due to the tedious and often not so reproducible separation procedures associated with such analysis methods. In contrast, NMR spectroscopy is highly reproducible and quantitative. Hence, it is an attractive technique for analyzing a large number of bile acids in a single-step, particularly when the concentration is not a limiting factor. Recently, from a series of comprehensive NMR studies of human bile and authentic bile acids, we reported simple NMR methodologies for detecting and quantifying major bile metabolites including a number of conjugated bile acids in human bile [17–20]. Subsequently, using these methods we have shown significant differences in the concentrations of bile metabolites between liver disease patients and controls [21]. Such developments would be useful for discovering biomarkers in light of the emerging area of ‘metabolomics’, which is based on single-step analysis of multiple metabolites in biological systems [22–27]. In the present study, we hypothesized that understanding of the conjugation pattern of bile acids is important to explore its pathophysiological significance in human health and disease. Accordingly, continuing our studies of human bile, we have investigated bile acids conjugation pattern using NMR spectroscopy at high magnetic fields (800 MHz). The investigations reveal that glycine- and taurine-conjugation of bile acids in human bile is not random, but follows a highly correlative pattern. Such findings open new avenues for exploring physiology of bile acids in health and understanding the possible links between conjugation pattern and liver diseases.
Materials and Methods
Chemicals
Deuterium oxide (D2O), and sodium salt of trimethylsilylpropionic acid-d4 (TSP) were purchased from Sigma-Aldrich (Milwaukee, WI, USA).
Human Gallbladder Bile
Gallbladder bile was obtained from 44 patients (22 male; 22 female; mean age 50.7 years). These included both liver disease (n = 27) and non-liver disease patients (n = 17). Liver disease included both malignant, hepatocellular carcinoma (n = 10) and cholangiocarcinoma (n = 7), and non-malignant (n = 9) disease patients. Nonmalignant liver disease included patients with alcoholic cirrhosis (n = 3); cirrhosis due to hepatitis C infection (n = 3); non-alcoholic steatohepatitis cirrhosis (n = 1); choledochal cyst (n = 1) and primary sclerosing cholangitis (n = 1).
Bile Collection and Storage
Gallbladder bile was taken at the beginning of the case, prior to any devascularization of the gallbladder. A purse string suture was placed in the fundus of the gallbladder. Through an 18-gauge needle, 10 mL of bile was removed and placed immediately into a red top tube treated with 0.1% sodium azide. The suture was tied down to eliminate any bile leakage and the gallbladder taken off the liver. Bile specimen was then divided into 1-mL aliquots and stored at −80 °C until analysis. An Institutional Review Board protocol, approved at both Indiana University School of Medicine and Purdue University, was in place for the collection, storage, and analysis of human bile for research purposes.
NMR Experiments
All 1H-NMR experiments were performed on a Bruker Biospin Avance 800 MHz NMR spectrometer using a 5-mm CHN inverse probehead equipped with automatic tune and match, and shielded triple axis (x, y, z) gradients.
Bile solutions were prepared for NMR analysis by diluting 100 μL of bile to 600 μL using doubly distilled water. A reusable co-axial capillary tube containing TSP in D2O was inserted into the NMR tube before recording 1H-NMR spectra. While D2O served as a field-frequency locking solvent, TSP served as chemical shift as well as a quantitative reference. Recently, from the comprehensive analysis of bile 1H-NMR spectra, we have shown that the characteristic amide signals constituting three glycine-conjugated bile acids, glycocholic acid (GCA), glycodeoxycholic acid (GDCA), glycochenodeoxycholic acid (GCDCA), and their taurine counterparts, taurocholic acid (TCA), taurodeoxycholic acid (TDCA) and taurochenodeoxycholic acid (TCDCA), invariably appear in the region 7.8–8.1 ppm [20]. We have also shown that these bile acids signals are better distinguished when the coupling between amide protons and the attached methylene protons is removed by decoupling. Hence, one dimensional 1H-NMR spectra were obtained using a one pulse sequence with (and without) homonuclear decoupling of the methylene protons. Simultaneous decoupling of the amide protons of all the conjugated bile acids was achieved by setting the decoupling frequency at 3.65 ppm, in between the chemical shifts of methylene protons of conjugated taurine (3.56 ppm) and conjugated glycine (3.75 ppm).
It is shown from the experiments performed at different pH that the amide signals of individual conjugated bile acids represent more quantitatively over the pH range 6 ± 0.5 [18, 20]. Hence, the pH of each bile solution was adjusted to this range by the addition of 1–2 μL of 1 N hydrochloric acid. Typical parameters used were: spectral width: 12,000 Hz; time domain data points: 32 K; flip angle: 45°; acquisition time: 1.4 s; relaxation delay: 3 s; number of transients: 64; spectrum size: 32 K points. This resulted in an acquisition time of 4.6 min for each NMR experiment. Water signal suppression was achieved by presaturation during the relaxation delay while homonuclear decoupling was performed during the data acquisition. The recycle delay was chosen based on the optimization studies performed earlier using delays varying from 1 to 10 s [20]. Thus, we rule out any change in intensity of the amide signals either due to relaxation or NOE buildup during decoupling of the adjacent methylene protons.
Quantitative Estimation of Individual Conjugated Bile Acids
Peak areas for the characteristic bile acids’ amide signals (between 7.8 and 8.1 ppm; see Figs. 1, 2) and the TSP reference were obtained by integration. The total bile acid concentration in each bile sample was first determined using these integrals and the concentration of the reference compound after taking into account the number of protons contributed from the bile acids as well as the reference compound. Subsequently, the relative area under each conjugated bile acid signal was determined by signal deconvolution using Bruker xwinnmr software. For the deconvolution of the spectra, Lorentzian line shape was used. The concentration of individual bile acids was then determined using the relative integrals of individual bile acids and the amount of total bile acids as determined above. Decoupled spectra were used for the analysis of bile acids.
Fig. 1.
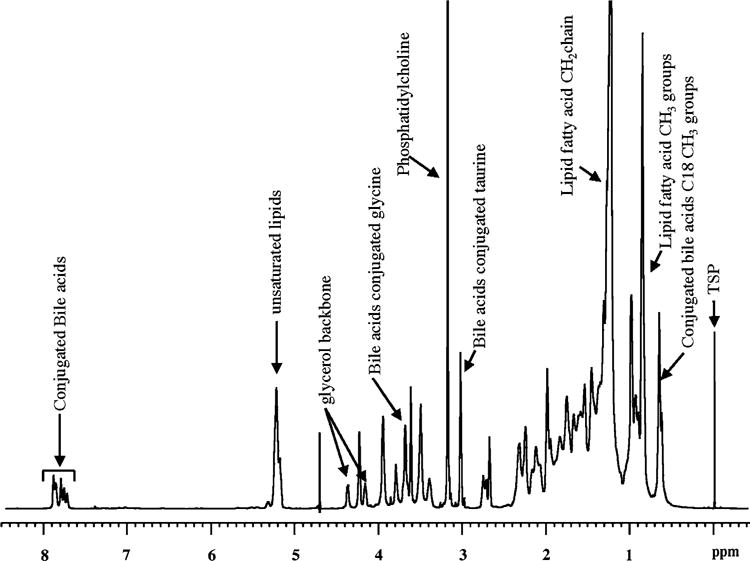
Typical 1H-NMR spectrum at 800 MHz of human gallbladder bile (100 μL bile diluted to 600 μL)
Fig. 2.
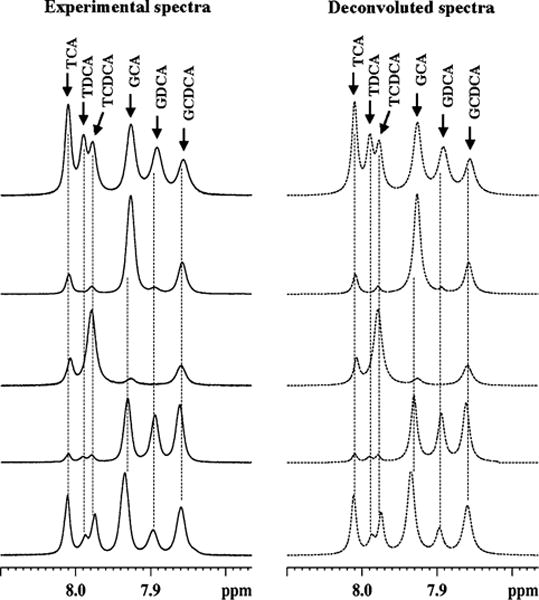
Left Portions of the 800 MHz 1H-NMR spectra of gallbladder bile from five different patients (four from non-liver disease controls and one from hepatitis C patient). The spectra were obtained by decoupling methylene protons of glycine-/taurine-conjugated bile acids. Right The corresponding deconvoluted spectra obtained for estimating the integrals. GCA glycocholic acid; GCDCA glycochenodeoxycholic acid; GDCA glycodeoxycholic acid; TCA taurocholic acid; TCDCA: taurochenocholic acid and TDCA taurodeoxycholic acid
Assessment of Bile Acids Conjugation
Glycine- and taurine-conjugation in human bile samples was assessed by comparing the bile acids individually and in the following combinations. (1) The ratio between glycine- and the corresponding taurine-conjugated bile acid in different bile samples was assessed by comparison. (2) The ratio between two glycine-conjugated bile acids was compared with the ratio between the corresponding taurine-conjugated counterparts. (3) The ratio between a glycine-conjugated bile acid and its taurine-conjugated counterpart was compared with the ratio between another glycine-conjugated bile acid and its taurine-conjugated counterpart. Linear regression analysis was performed for the ratios obtained in the latter two steps (steps 2 and 3) to estimate the inter-relationship between the ratios of the bile acids.
Finally, concentration of each conjugated bile acid was predicted using experimentally determined values of the other three bile acids. This prediction was based on the results of the regression analysis as described below which indicate that the ratio between two glycine-conjugated bile acids linearly correlates with the ratio between the corresponding taurine-conjugated bile acids or, alternatively, the ratio between a glycine-conjugated bile acid and its taurine-conjugated counterpart linearly correlates with the ratio between another glycine-conjugated bile acid and its taurine-conjugated counterpart. The predicted bile acids concentrations were then compared with the experimentally determined values from the NMR spectra for the six conjugated bile acids, individually.
Statistical Analysis
All linear regression analyses were made using Microsoft Excel and R statistical package (version 2.8.1). The Excel software was first used to calculate the ratios of various combinations of the glycine- and taurine-conjugated bile acids (TCA/TCDCA, GCA/GCDCA, TCA/TDCA, GCA/GDCA, TDCA/TCDCA, GDCA/GCDCA, GCA/TCA, GCDCA/TCDCA, GCA/TCA, GDCA/TDCA, GCDCA/TCDCA and GDCA/TDCA) for each patient using the concentration of individual bile acids obtained from 1H-NMR spectra. The plots of the ratio of TCA/TCDCA versus GCA/GCDCA, TCA/TDCA versus GCA/GDCA, TDCA/TCDCA versus GDCA/GCDCA, GCA/TCA versus GCDCA/TCDCA, GCA/TCA versus GDCA/TDCA and GCDCA/TCDCA versus GDCA/TDCA were then made to assess the correlation of the ratios. Subsequently, using the Excel software, the linear regression analysis was made and, the equations for the line-fitting and the correlation coefficient (R2) were determined. Further, the statistical significance of the correlations was determined using the R program using Pearson product moment correlation analysis.
Results
This investigation aims at understanding the conjugation pattern of glycine- and taurine-conjugated bile acids in humans. Considering the fact that the glycine and taurine conjugation depends on a number of factors and the amount of bile acids varies widely between the two types even across the non-liver disease patients (as shown later), it will not be possible to trace possible relationship between the two types of bile acids in humans from a glycine- and a taurine-conjugated bile acids. It thus necessitated comparison of the ratios of a pair of glycine- conjugated bile acids with the corresponding pair of taurine-conjugated bile acids. Hence, all the bile samples that showed less than a pair of glycine and the corresponding taurine-conjugated bile acids were omitted from the analysis (see for example, supplementary Fig. S1). In addition, four bile samples in which individual bile acids could not be quantified accurately due to excessive line broadening were also omitted to avoid possible error in the measurement of the signal integrals adversely influencing the outcome of the study (see for example, supplementary Fig. S2). Of the 44 bile samples, 24 met the minimum requirement which were used for evaluating the bile acids conjugation pattern. Table 1 shows the patient profile and the number of conjugated bile acids detected in each category of patients considered in this study.
Table 1.
Patient profile and the number of bile acids observed by NMR
| Disease type | Number of patients | Number of patients in which all six conjugated bile acids were detecteda | Number of patients in which only four conjugated bile acids were detectedb |
|---|---|---|---|
| Hepatocellular carcinoma | 6 | 2 | 4 |
| Cholangio-carcinoma | 3 | 1 | 2 |
| Hepatitis C | 2 | 0 | 2 |
| Alcoholic cirrhosis | 1 | 0 | 1 |
| Non-alcoholic steatohepatitis cirrhosis | 1 | 0 | 1 |
| Non-liver disease | 11 | 7 | 4 |
Six bile acids detected were GCA, GCDCA, GDCA, TCA, TCDCA, and TDCA
Secondary bile acids, GDCA, and TDCA, were not detected in these patients
Figure 1 shows a typical one-dimensional 1H-NMR spectrum of intact human gallbladder bile obtained at 800 MHz. Owing to the amphipathic nature of major bile metabolites such as phospholipids, cholesterol and bile acids which exist in aggregated form, the spectral lines appear somewhat broad as seen in the figure. All major metabolites which contribute to the bulk of the complex NMR spectra of bile have recently been individually identified by exhaustive NMR studies at different magnetic fields [20]. Six of the metabolites were bile acids conjugated to glycine or taurine (GCA, GCDCA, GDCA, TCA, TCDCA, and TDCA). In the present study, all the six conjugated bile acids were observed in 14 of 24 bile samples, while only four (GCA, GCDCA, TCA, and TCDCA) were observed in the remaining 10 (Table 1). It may be noted that, in the entire set of 24 bile samples, all the glycine- conjugated bile acids were the counterparts of the taurine-conjugated bile acids or vice versa.
Not surprisingly, the concentration of the individual bile acids varied significantly across the sample set as shown in Figs. 2 and 3. Even the ratios between the total glycine-and taurine-conjugated bile acids (Fig. 4a) or between a glycine-conjugated bile acid and its taurine counterpart varied significantly (Fig. 4b–d). Interestingly, however, the ratios between two glycine-conjugated bile acids and between their taurine counterparts linearly correlated (Fig. 5) and the significance of the correlations was very high for all the ratios. The correlation coefficients and p values were, R2 = 0.83 and p = 0.006 × 10−7 for the correlation of ratio of GCA and GCDCA with the ratio of TCA and TCDCA (Fig. 5a); 0.97 and 0.004 × 10−4 for the correlation of the ratio of GCA and GDCA with the ratio of TCA and TDCA (Fig. 5b) and 0.92 and 0.001 × 10−2 for the correlation of the ratio of GDCA and GCDCA with the ratio of TDCA and TCDCA (Fig. 5c). Similarly the ratios between glycine-conjugated bile acid and its taurine counterpart, and those between another glycine-conjugated bile acid and its taurine counterpart were also highly linearly correlated: R2 = 0.92 and p = 0.002 × 10−10 for the correlation of the ratio of GCA and TCA with that of GCDCA and TCDCA (Fig. 5d); 0.95 and 0.003 × 10−3 for the correlation of the ratio of GCA and TCA with that of GDCA and TDCA (Fig. 5e) and 0.94 and 0.004 × 10−3 for the correlation of the ratio of GCDCA and TCDCA with that of GDCA and TDCA (Fig. 5f). The regression equations of the correlations show a slope of close to one for the ratios of bile acids as shown in Fig. 5.
Fig. 3.
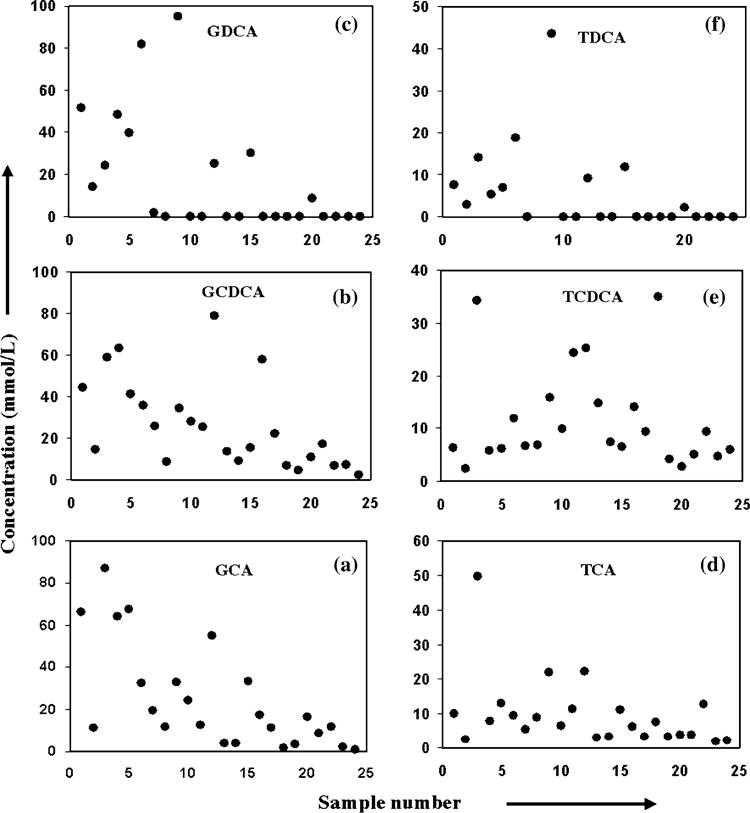
Concentration of individual bile acids (in mmol/L) for different bile samples. Abbreviations are as described before in Fig. 2 caption
Fig. 4.
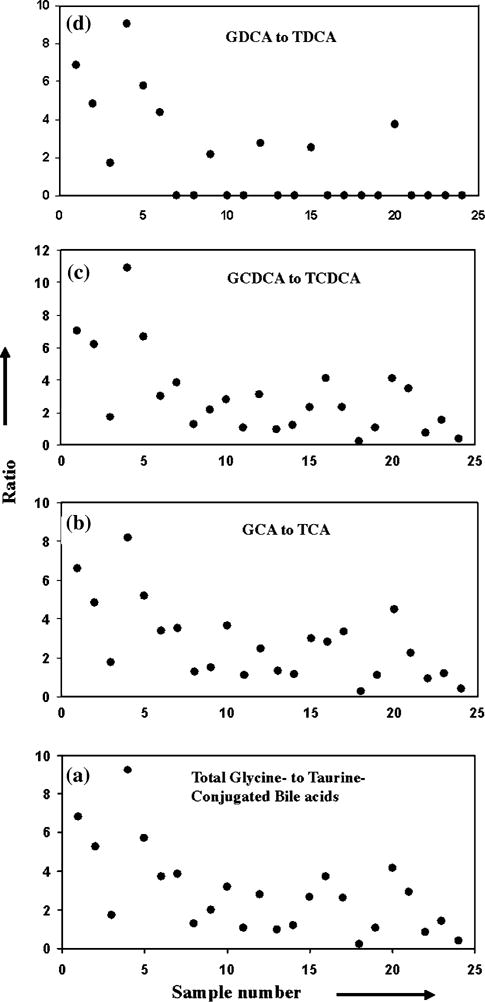
Ratios of a glycine- to taurine-conjugated bile acids and b–d glycine to the corresponding taurine-conjugated bile acid. Abbreviations are as described before in Fig. 2 caption
Fig. 5.
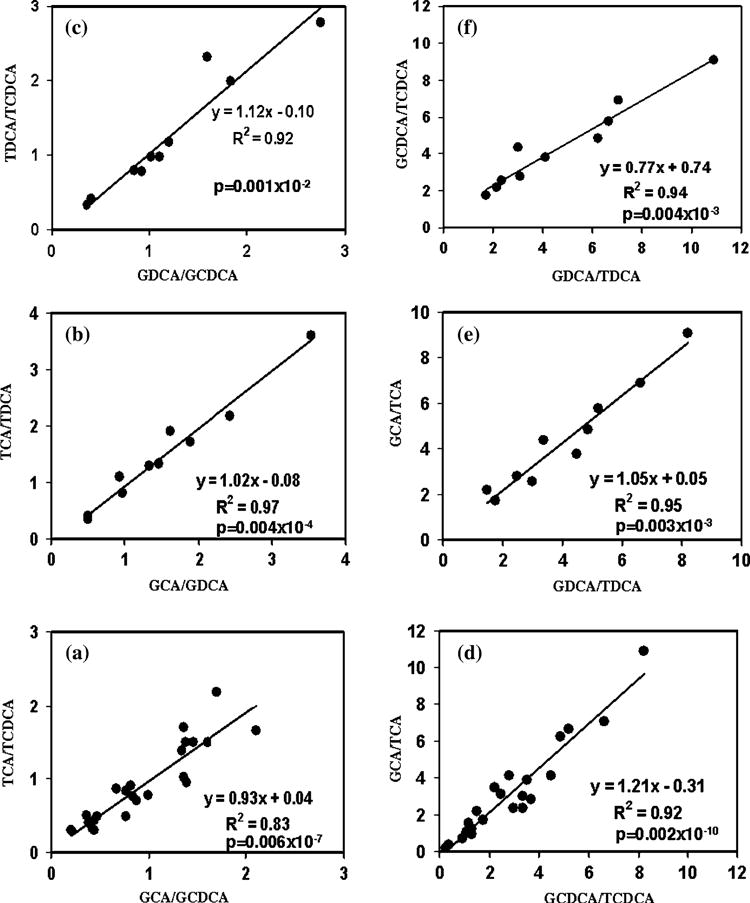
Plots showing the linear correlation of the ratios a–c between a pair of glycine-conjugated bile acids and between the corresponding taurine-conjugated bile acids; and d–f between a glycine-conjugated bile acid and its taurine counterpart and between another glycine-conjugated bile acid and its taurine counterpart. Abbreviations are as described before in Fig. 2 caption
The concentration of individual bile acids in each bile sample was predicted using such correlations and experimentally determined values of the other three bile acids as follows. The concentration of GCA was predicted using the experimental values of TCA, GCDCA, and TCDCA; the concentration of GDCA using GCA, TCA, and TDCA; the concentration of GCDCA using GCA, TCA, and TCDCA; the concentration of TCA using GCA, GCDCA, and TCDCA; the concentration of TDCA using GCA, TCA, and GDCA and the concentration of TCDCA using GCA, TCA, and GCDCA. The correlations between the experimental and predicted values for the six conjugated bile acid are shown in the supplementary Fig. S30; as excepted from the results shown in Fig. 5, the experimental and predicted values for all the bile acids were highly correlated (supplementary Fig. S3).
Discussion
This study takes advantage of recent methodological advancements in NMR which enabled identification of major bile metabolites in intact human bile [19, 20]. Employing these methodologies and using 1H-NMR spectroscopy at 800 MHz, we have studied human bile to investigate glycine and taurine conjugation patterns of bile acids.
Generally, in human bile, six conjugated bile acids can be detected using a simple one-dimensional NMR experiment (Fig. 1). These constitute two primary bile acids, cholic acid and chenodeoxycholic acid, and a secondary bile acid, deoxycholic acid, each of which is conjugated to glycine, resulting in GCA, GCDCA, and GDCA, or taurine, resulting in TCA, TCDCA, and TDCA. Depending upon the status of the liver, concentration of these bile acids vary in individual patients so much that, in the present study, bile acids in 16 patients were either undetectable or fewer than four. Since our aim was to investigate the conjugation pattern by comparing and correlating the ratios between two glycine-conjugated bile acids and their taurine counterparts or the ratios between glycine-conjugated bile acid and its taurine counterpart, we considered only those bile samples which showed at least a pair of glycine-conjugated bile acids and their taurine counterparts.
Significant variation in each of these bile acids concentration across the sample set (Figs. 2, 3) may be attributed to a number of factors such as altered biosynthesis, cholestatic liver disease, bile acid malabsorption or disturbed enterohepatic circulation arising from the heterogeneity of the patients used in this study. Interestingly, from the systematic comparison of the variation in glycine- and taurine-conjugated bile acids concentrations within a bile sample as well as across a set of bile samples, a fundamental relationship between glycine- and taurine-conjugated bile acids levels in human bile could be established (Figs. 2, 5). Despite many factors that can affect individual bile acid concentration and the heterogeneity of the human subjects involved, who varied from non-liver disease patients to patients with liver diseases including hepatocellular carcinoma and cholangiocarcinoma, the ratios between glycine-conjugated bile acids and between taurine-conjugated bile acids did not vary randomly. This is particularly true for the six conjugated bile acids, GCA, GCDCA, GDCA, TCA, TCDCA, and TDCA commonly detected by NMR in a single step. The slope of nearly one for the line-fitting obtained from linear regression analysis and a very low p value clearly shows the linearity of correlation of bile acids ratios. An earlier investigation of bile using high pressure liquid chromatography reported somewhat constant proportion of the three glycine-conjugated bile acids in healthy humans (GCA, GCDCA, and GDCA) [28]. These results are in accordance with the relationship between glycine- and taurine-conjugated bile acids observed in the present study. Further, these results indicate that it is even possible to predict the concentration of a bile acid utilizing the relationship of co-variation of the bile acids ratios (supplementary Fig. S3). Considering the fact that numerous factors affect individual bile acid’s concentration and that it is not yet known whether damage to bile acids during enterohepatic circulation is corrected in the hepatocytes, either partially or completely [29], the findings of the present study that the individual bile acids do not vary randomly may provide clues to the mechanism of the regulatory make up of bile acids in the hepatocytes.
Interest in better understanding bile acids metabolism and their role in a variety of diseases has increased since the recent discovery of a nuclear receptor for bile acids, which regulates bile acids synthesis and controls glucose, fat and energy metabolism. A very recent study on bile acids kinetics showed significantly higher cholic acid synthesis in type 2 diabetes patients [9]. Although bile acids have common structural features, the number and the position of the hydroxyl groups, and the nature of conjugation is anticipated to make each of the bile acids exhibit widely differing biological functions. For example, taurodeoxycholic acid, but not glycodeoxycholic acid, has inhibitory potential against apoptosis [30], and chenodeoxycholic acid levels in blood exceeding 15 μmol/L are indicative of end stage liver disease [31]. Hence, understanding the metabolism and physiology of individual bile acid conjugation becomes an important aspect of the study of bile acids. Most of the literature about bile acids to date deals with bile acids as a whole, such as the primary bile acids, cholic acid and chenodeoxycholic acid, and the secondary acids, deoxycholic acid and lithocholic acid. Specific roles for the individual taurine- or glycine-conjugated bile acids have not been extensively explored.
Present investigation was based on the six conjugated bile acids, GCA, GCDCA, GDCA, TCA, TCDCA, and TDCA that are commonly found in human bile at high concentrations. Conjugates of ursodeoxycholic acid and lithocholic acid that are also thought to be present commonly in bile were not detected by NMR in this study, probably, owing to their low concentrations. It is also quite likely that such undetected low concentration conjugated bile acids overlap with the signals of the abundant bile acids. In our earlier investigation, we have shown that all the bile acids’ signals are resolved even at low magnetic fields (400 MHz) except the two, TCDCA and TDCA, and at 800 MHz, all the signals get resolved [20]. Now that the relationship between glycine- and taurine-conjugated bile acids is established, it would be possible to predict the concentrations of TCDCA and TDCA, even if they overlap, provided the integrals of the other four are known as described in the supplementary Fig. S3. Thus, the analysis of individual bile acids can be performed even using instruments with lower magnetic fields (for example 400 MHz) as long as the correlation of the ratios is valid.
Conclusions
Bile acids have been intensively studied over the last several decades; however, investigations with an emphasis on bile acids conjugation are sparse. With a multitude of new roles for bile acids in several diseases coming to light, the need to investigate and understand the specific role of each member of the bile acid family has gained significance. Utilizing the latest methodological developments in bile acids analysis, we have made a detailed correlative analysis of individual glycine- and taurine-conjugated bile acids, which normally occur abundantly in human bile. We show here that glycine- and taurine-conjugated bile acids do not vary randomly but follow a definite correlative relationship, despite significantly altered levels of individual bile acids across the sample set. Although, this investigation involves a small sample set and only six conjugated bile acids, the correlations between the two classes of conjugated bile acids are highly consistent and striking. Further studies using a greater number of bile samples targeting additional number of bile acids that are undetected in the present study are needed to substantiate the findings and to further our understanding of the physiology of bile acids’ conjugation. Further, it will be interesting and useful to understand the pathophysiological circumstances which contribute to the deviation of such a correlation, although present study on a small sample set has not detected any difference between liver diseases and non-liver disease controls.
Supplementary Material
Acknowledgments
This work was supported by the Walther Cancer Institute Multi-Institution Cancer Research Seed Project, the Purdue Oncological and Cancer Centers, and a collaborative research grant between Purdue University/Discovery Park and the Indiana University School of Medicine.
List of abbreviations
- GCA
Glycocholic acid
- GDCA
Glycodeoxycholic acid
- GCDCA
Glycochenodeoxycholic acid
- TCA
Taurocholic acid
- TDCA
Taurodeoxycholic acid
- TCDCA
Taurochenodeoxycholic acid
- NMR
Nuclear magnetic resonance
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11745-009-3296-4) contains supplementary material, which is available to authorized users.
Contributor Information
G. A. Nagana Gowda, Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA
Narasimhamurthy Shanaiah, Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
Amanda Cooper, Department of Surgery, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Mary Maluccio, Department of Surgery, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Daniel Raftery, Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
References
- 1.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25(7):1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159(22):2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 6.Jansen PL. Endogenous bile acids as carcinogens. J Hepatol. 2007;47(3):434–435. doi: 10.1016/j.jhep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 9.Brufau G, Kuipers F, Prado K, Abbey S, Jones M, Schwartz S, Stellaard F, Murphy E. California altered bile salt metabolism in type 2 diabetes mellitus. 68th scientific sessions of the American Diabetes Association; San Francisco. June 6–10; 2008. abstract No. 1553-P. [Google Scholar]
- 10.Meinders AE, Van Berge Henegouwen GP, Willekens FL, Schwerzel AL, Ruben A, Huybregts AW. Biliary lipid and bile acid composition in insulin-dependent diabetes mellitus. Arguments for increased intestinal bacterial bile acid degradation. Dig Dis Sci. 1981;26(5):402–408. doi: 10.1007/BF01313581. [DOI] [PubMed] [Google Scholar]
- 11.Scotti E, Gilardi F, Godio C, Gers E, Krneta J, Mitro N, De Fabiani E, Caruso D, Crestani M. Bile acids and their signaling pathways: eclectic regulators of diverse cellular functions. Cell Mol Life Sci. 2007;64:2477–2491. doi: 10.1007/s00018-007-7280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimber A, Gespach C. Bile acids and derivatives, their nuclear receptors FXR, PXR and ligands: role in health and disease and their therapeutic potential. Anticancer Agents Med Chem. 2008;8(5):540–563. doi: 10.2174/187152008784533008. [DOI] [PubMed] [Google Scholar]
- 13.Burkard I, von Eckardstein A, Rentsch KM. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry J Chrom B Anal. Technol Biomed Life Sci. 2005;826(1–2):147–159. doi: 10.1016/j.jchromb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Ding M, Ye L, Wang M, Shan Y. Simultaneous determination of free and conjugated bile acids in serum by high performance liquid chromatography–tandem mass spectrometry. Chin J Anal Chem. 2007;35(10):1506–1508. [Google Scholar]
- 15.Kandrac J, Kevresan S, Gu JK, Mikov M, Fawcett JP, Kuhajda K. Isolation and determination of bile acids. Eur J Drug Metab Pharm. 2006;31(3):157–177. doi: 10.1007/BF03190712. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Griffiths WJ. Modern methods of bile acid analysis by mass spectrometry: a view into the metabolome. Cur Anal Chem. 2007;3(2):103–126. [Google Scholar]
- 17.Ijare OB, Somashekar BS, Jadegoud Y, Nagana Gowda GA. 1H and 13C NMR characterization and stereochemical assignments of bile acids in aqueous media. Lipids. 2005;40:1031–1041. doi: 10.1007/s11745-005-1466-1. [DOI] [PubMed] [Google Scholar]
- 18.Ijare OB, Somashekar BS, Nagana Gowda GA, Sharma A, Kapoor VK, Khetrapal CL. Quantification of glycine and taurine conjugated bile acids in human bile using 1H NMR spectroscopy. Magn Reson Med. 2005;53(6):1441–1446. doi: 10.1002/mrm.20513. [DOI] [PubMed] [Google Scholar]
- 19.Nagana Gowda GA, Somashekar BS, Ijare OB, Sharma A, Kapoor VK, Khetrapal CL. One step analysis of major bile metabolites in human bile using 1H-NMR spectroscopy. Lipids. 2006;41:577–589. doi: 10.1007/s11745-006-5007-8. [DOI] [PubMed] [Google Scholar]
- 20.Nagana Gowda GA, Ijare OB, Somashekar BS, Sharma A, Kapoor VK, Khetrapal CL. Single step analysis of individual conjugated bile acids in human bile using 1H-NMR Spectroscopy. Lipids. 2006;41:591–603. doi: 10.1007/s11745-006-5008-7. [DOI] [PubMed] [Google Scholar]
- 21.Nagana Gowda GA, Shanaiah N, Cooper A, Maluccio M, Raftery D. Visualization of bile homeostasis using 1H-NMR spectroscopy as a route for assessing liver cancer. Lipids. 2009;44:27–35. doi: 10.1007/s11745-008-3254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005;18(3):143–162. doi: 10.1002/nbm.935. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Pan Z, Talaty N, Raftery D, Cooks RG. Combining desorption electrospray ionization mass spectrometry and nuclear magnetic resonance for differential metabolomics without sample preparation. Rapid Commun Mass Spectrom. 2006;20:1577–1584. doi: 10.1002/rcm.2474. [DOI] [PubMed] [Google Scholar]
- 24.Lindon JC, Holmes E, Nicholson JK. Metabonomics and its role in drug development and disease diagnosis. Expert Rev Mol Diagn. 2004;4(2):189–199. doi: 10.1586/14737159.4.2.189. [DOI] [PubMed] [Google Scholar]
- 25.Nagana Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics Expert Rev. Mol Diagn. 2008;8(5):617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Z, Raftery D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal Bioanal Chem. 2007;387(2):525–527. doi: 10.1007/s00216-006-0687-8. [DOI] [PubMed] [Google Scholar]
- 27.Shanaiah N, Desilva A, Nagana Gowda GA, Raftery MA, Hainline BE, Raftery D. Metabolite class selection of amino acids in body fluids using chemical derivatization and their enhanced 13C NMR. Proc Natl Acad Sci USA. 2007;104(28):11540–11544. doi: 10.1073/pnas.0704449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi SS, Converse JL, Hofmann AF. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J Lipid Res. 1987;28(5):589–595. [PubMed] [Google Scholar]
- 29.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65(16):2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirano F, Haneda M, Makino I. Chenodeoxycholic acid and taurochenodexycholic acid induce anti-apoptotic cIAP-1 expression in human hepatocytes Source. J Gastro Hepatol. 2006;21(12):1807–1813. doi: 10.1111/j.1440-1746.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 31.Azer SA, Coverdale SA, Byth K, Farrell GC, Stacey NH. Sequential changes in serum levels of individual bile acids in patients with chronic cholestatic liver disease. J Gastroenterol Hepatol. 1996;11(3):208–215. doi: 10.1111/j.1440-1746.1996.tb00064.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


