Abstract
Background
We previously reported evidence of reduced cortical gray matter (GM), white matter (WM), and hippocampal volume in Gulf War (GW) veterans with predicted exposure to low-levels of nerve agent according to the 2000 Khamisiyah plume model analysis. Because there is suggestive evidence that other nerve agent exposures may have occurred during the Gulf War, we examined the association between the self-reported frequency of hearing chemical alarms sound during deployment in the Gulf War and regional brain volume in GW veterans.
Methods
Ninety consecutive GW veterans (15 female, mean age: 52±8 years) participating in a VA-funded study underwent structural magnetic resonance imaging (MRI) on a 3 T scanner. Freesurfer (version 5.1) was used to obtain regional measures of cortical GM, WM, hippocampal, and insula volume. Multiple linear regression was used to determine the association between the self-reported frequencies of hearing chemical alarms during the Gulf War and regional brain volume.
Results
There was an inverse association between the self-reported frequency of hearing chemical alarms sound and total cortical GM (adjusted p = 0.007), even after accounting for potentially confounding demographic and clinical variables, the veterans’ current health status, and other concurrent deployment-related exposures that were correlated with hearing chemical alarms. Post-hoc analyses extended the inverse relationship between the frequency of hearing chemical alarms to GM volume in the frontal (adjusted p = 0.02), parietal (adjusted p = 0.01), and occipital (adjusted p = 0.001) lobes. In contrast, regional brain volumes were not significantly associated with predicted exposure to the Khamisiyah plume or with Gulf War Illness status defined by the Kansas or Centers for Disease Control and Prevention criteria.
Conclusions
Many veterans reported hearing chemical alarms sound during the Gulf War. The current findings suggest that exposure to substances that triggered those chemical alarms during the Gulf War likely had adverse neuroanatomical effects.
Keywords: Brain imaging, Cerebral volume, Chemical warfare agents, Gulf War veterans
1. Introduction
Military personnel of the 1990–1991 Persian Gulf War (GW) encountered numerous potentially hazardous substances during deployment, including oily black smoke generated by burning oil well fires, low-levels of chemical nerve agents such as sarin and cyclosarin, pyridostigmine bromide (PB) pills taken by U.S. and some coalition forces to protect against the acute effects of nerve agents, excessive use of pesticides and insect repellants, munitions containing depleted uranium, receipt of numerous vaccines, chemical resistant coating paint, and other potential hazards (Steele et al., 2012; White et al., 2015). Although exposures to these substances have been suspected of contributing to long-term ill health effects in GW veterans, it has proved difficult to thoroughly evaluate the consequences of these exposures because of the lack of measured data about who was exposed to what during the GW, and at what levels. Despite this ambiguity, we know that at least some GW veterans were exposed to low levels of chemical nerve agents when a munitions storage site at Khamisiyah, Iraq was destroyed in early March 1991. This is because a United Nations Special Commission inspection team that inspected the site in October 1991 found samples that tested positive for sarin and cyclosarin (Haley and Tuite, 2013).
After the GW ended, the Presidential Advisory Committee and the National Security Council requested the Department of Defense (DOD) and Central Intelligence Agency (CIA) to model potential chemical warfare agent release events during the GW, including those associated with detonation of the munitions storage site at Khamisiyah (Presidential Advisory Committee on Gulf War Veterans’ Illnessess, 1996). This lead to simulations that: (1) approximated the direction and extent of these releases using meteorological data and estimates of atmospheric transport and diffusion, (2) approximated the exposure dose across the three days in March 1991 when exposure was deemed likely, and (3) attempted to identify U.S. troops whose units may have been exposed by overlaying the geographical coordinates of the modeled plume with the geographical positions of U.S. military units in the theater on the dates in question. The simulations indicated that no military units were in the “first noticeable effect” area, defined as 3 exposure at levels equal to or greater than 1 mg min/m3 by the US Army (McNamara and Leitnaker, 1971) and the Centers for Disease Control and Prevention (CDC, 1988). However, 98,910 military personnel were identified to have been in the “low level exposure” area, defined as exposure at levels equal to or greater than the general population limit (i.e., 0.01296 mg min/m3) (Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness, 1997). In 2000, the exposure plume data were re-analyzed and refined using additional meteorological modeling information, updated estimates of the total number of rockets destroyed, consideration of agent removal mechanisms, updated unit-level location and personnel data, exposure thresholds for sarin and cyclosarin and combined toxicity aspects of sarin and cyclosarin (Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness, and Military Deployments, 2002). This second plume model identified an additional 2942 military personnel, for a total of 101,752 GW veterans with potential low-level exposure to sarin/cyclosarin.
In a review of the Khamisiyah plume models, the United States General Accounting Office (2004) cited a number of problems, including inaccuracies in the source terms (e.g., quantity and purity of nerve agent), underestimation of explosion plume heights, unrepresentative conditions of field tests, and wide divergence of plume patterns from the various computer plume. This lead the U.S. General Accounting Office to conclude that the plume models did not reliably indicate which troops were exposed to nerve agent, if any was released from the demolitions.
Despite the uncertainty associated the Khamisiyah plume models, we and others have reported evidence of impaired neurobehavioral function (Chao et al., 2010; Proctor et al., 2006; Toomey et al., 2009) as well as reduced total brain gray matter (GM) (Chao et al., 2010, 2011) and white matter (WM) (Chao et al., 2011; Heaton et al., 2007) volume in Khamisiyah-exposed veterans compared to non-exposed GW veterans. We have also found evidence of reduced total hippocampal (Chao et al., 2010) and hippocampal subfield (i.e., CA2, CA3, and dentate gyrus) volumes (Chao et al., 2014) as well as WM microstructural changes (Chao et al., 2015) as measured by diffusion tensor imaging (DTI) in GW veterans with predicted Khamisiyah plume exposure compared to matched, unexposed GW veterans. These findings in GW veterans with predicted Khamisiyah plume exposure are reminiscent of the reports of long-term neurobehavioral (Miyaki et al., 2005; Nishiwaki et al., 2001) and brain (e.g., hippocampal, insula and neighboring white matter atrophy and WM microstructural abnormalities (Yamasue et al., 2007)) changes that have been described in victims of the 1995 Tokyo subway sarin attack, a terrorist attack that exposed more than 5500 civilians to sarin gas (Suzuki et al., 1995).
The DOD/CIA plume models only focused on exposures associated with the Khamisiyah demolitions. However, it has been suggested that other nerve agent exposures may have occurred during the Gulf War (Committee on Banking, 1994; Haley and Tuite, 2013; Office of the Special Assistant for Gulf War Illnesses, 1996; Tucker, 1997; Tuite and Haley, 2013). Therefore, the present study sought to examine whether there are associations between the frequency of hearing chemical alarms in theater and regional brain volumes in GW veteran. Due to the dearth of information on the types and doses of exposures experienced by GW veterans in theater, we relied on self-reports to evaluate possible exposure to chemical nerve agents, as previous epidemiologic studies have done (e.g., Haley and Tuite, 2013; Steele et al., 2012). Based on our findings of reduced hippocampal (Chao et al., 2010, 2014) and total brain GM volume (Chao et al., 2010, 2011) and our (Chao et al., 2011) and Heaton et al. (2007) finding of reduced total brain WM volume among Khamisiyah-exposed veterans, we hypothesized there would be an inverse association between the self-reported frequency of hearing chemical alarms sound during deployment and hippocampal, total brain GM and WM volumes. We also investigated the association between self-reports of hearing chemical alarms sound and the volume of the insula because Yamasue et al. (2007) had reported reduced insular volumes in victims of the 1995 Tokyo subway sarin attack. Finally, because Haley and Tuite (2013) reported a dose-response relationship between the number of times nerve agent alarms sounded in a veteran’s immediate area and the risk of having Gulf War illness (GWI), we examined the frequency of GWI cases among GW veterans who did and did not reports of hearing chemical alarms sound during deployment.
2. Methods
2.1. Participants
We examined the neuroimaging data of 90 consecutive GW veterans who were recruited from 2014–2015 at the San Francisco Veterans Affairs Medical Center (VAMC) as part an on-going, VA-funded study on the effects of predicted exposure to sarin and cyclosarin from the Khamisiyah plume exposure on brain structure and brain function. All participants gave written informed consent, approved by the Institutional Review Boards of the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center.
2.2. Study protocol and measures
The complete study protocol included self-report questionnaires about physical and mental health status, Gulf War military history, a psychological diagnostic interview, a battery of neuropsychological tests, magnetic resonance imaging (MRI) on a 3 T scanner, and optional saliva sampling for apolipoprotein E (APOE) genotyping. Results of the neuropsychological assessments will be reported elsewhere. The current report focuses on evaluation of associations between self-reports of hearing chemical alarms during deployment and volumetric MRI data.
2.2.1. Exposure measures
We used the Kansas Military History and Health Questionnaire (Steele, 2000) to query veterans about 19 specific experiences or exposures of interest during their deployment in the Gulf War. The questions emphasized the veterans’ experiences rather than their impressions of their exposures. For example, rather than asking veterans if they had been exposed to depleted uranium, which many are unlikely to know, the questionnaire asked if veterans had contact with destroyed enemy vehicles, an experience required for nearly all personnel directly exposed to depleted uranium. The following question used to assess exposure to chemical nerve agents during deployment: “While you were in the Persian Gulf region, did you hear chemical alarms sound?” If veterans responded “yes,” they were asked to indicate how many days they heard chemical alarms sound (e.g., 1–6, 7–30, or ≥31 days).
The 2000 Khamisiyah plume analyses produced four modeled hazard areas, one for each day in March 1991 between the 10th to the 13th when exposure to sarin/cyclosarin was considered possible following the detonations of the munitions storage site in Khamisiyah (Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness, and Military Deployments, 2002). We requested and received the estimated days of exposure and dosages assigned to each GW veterans classified as exposed by the 2000 Khamisiyah modeling from the Directorate of Health Risk Management, US Army Center for Health Promotion and Preventive Medicine. Cumulative dosage estimates were provided in mg min/m3 for each of the units considered to be in any of the four hazard areas.
2.2.2. Gulf War illness (GWI) case definition
We used the Kansas Military History and Health Questionnaire (Steele, 2000) to determine whether GW veterans met the case definition for CDC “chronic multisymptom illness” (CMI) (Fukuda et al., 1998) and the Kansas GWI (Steele, 2000). The CDC CMI criteria required veterans to endorse one or more symptoms that had been ongoing for at least six months in two of three symptom categories. The symptom categories include musculoskeletal pain (e.g., joint pain/stiffness, muscle pain), mood-cognition problems (e.g., feeling depressed, moody, anxious, having trouble sleeping, difficulty remembering or concentrating, trouble with word finding), and fatigue. In addition, CDC CMI can be categorized as “severe” or “mild-moderate” depending on how the veteran rates each defining symptom. The Kansas GWI criteria required veterans to have multiple and/or moderate-to-severe chronic symptoms in at least three of six symptom domains. Qualifying symptoms must have first been a problem during or after the Gulf War and persisted over the 6-month period preceding the study. The Kansas GWI symptom domains include: (i) fatigue/sleep problems, (ii) somatic pain, (iii) neurologic/cognitive/mood problems, (iv) gastrointestinal problems, (v) respiratory problems, and (vi) skin problems. The Kansas GWI case criteria also excludes veterans who report being diagnosed with medical (e.g., diabetes, cancer, rheumatoid arthritis) or psychiatric (e.g., bipolar disorder, schizophrenia) conditions that could either explain their symptoms or interfere with their ability to report them. We used the Kansas GWI case definition in our primary analyses because the Institute of Medicine (IOM, 2014) recently recommended that this case definition be used for research purposes. The current study did not obtain the data necessary for determining Haley syndrome criteria (Haley et al., 1997), which uses a complex algorithm to determine symptom scores.
2.2.3. Clinical assessments
All subjects were evaluated by a Ph.D. level psychologist using the Structured Clinical Interview for DSM-IV Diagnosis (SCID, Spitzer et al., 1992), the Clinician Administered PTSD Scale (CAPS, Blake et al., 1995) and an interview version of the Life Stressor Checklist-Revised (Wolfe et al., 1996). The Life Stressor Checklist-Revised assesses 21 stressful life events (e.g., experiencing or witnessing serious accidents, illnesses, sudden death, and physical and sexual assault) to determine exposure to traumatic events. The CAPS was used to diagnose current posttraumatic stress disorder (PTSD). The SCID was used to diagnose current major depressive disorder (MDD) and to rule out individuals with a lifetime history of psychotic or bipolar disorders and alcohol or drug abuse or dependence within the previous 12 months. Other exclusion criteria were neurological illness, head trauma with loss of consciousness greater than 10 min, medical disorders affecting brain function, and conditions contradictory for MRI.
2.2.4. Determination of apolipoprotein E (APOE) ε4 allele status
Because the apolipoprotein E (APOE) ε4 allele, the strongest genetic risk factor for Alzheimer’s disease (AD, Harold et al., 2009; Lambert et al., 2009), has demonstrated a particular phenotype of middle temporal lobe atrophy (Lehtovirta et al., 2000), we controlled for APOE genotype in our analyses to rule out the possibility that any associations between hearing chemical alarms and regional brain volume was independent of the genetic risk for AD. APOE genotype data was available for 69/90 participants. Subjects with 2/3 or 3/3 combined alleles were classified as APOE ε4-negative while those with 3/4 or 4/4 combined alleles were classified as APOE ε4-positive. Because the 2/4 combined allele has been associated with a lower risk of AD (Corder et al., 1994), these subjects were excluded from the APOE ε4-positive group.
2.2.5. Image acquisition
All subjects underwent MRI of the brain on a Siemens 3 Tesla Skyra system at the San Francisco Veterans Affairs Medical Center. A 3D T1-weighted multi-echo Magnetization Prepared Rapid Gradient Echo (ME-MPRAGE) protocol was used to obtain images with optimal contrast between GM, WM, and cerebral spinal fluid (CSF) (TR/TE/TI = 2500/2.98/1100 ms; 1 mm3 isotropic imaging voxels). A 3D T2-weighted turbo spin-echo sequence (TR/TE = 3400/402 ms; 1 mm3 isotropic imaging voxels) was acquired for calculation of intracranial volume (ICV).
2.2.6. Image processing
We used Freesurfer Version 5.1 to label cortical and subcortical tissue classes and derive quantitative estimates of regional brain volume (Dale et al., 1999; Desikan et al., 2006; Fischl et al., 1999). Briefly, Freesurfer processing of the volumetric T1-weighted images included motion correction, brain extraction and removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated spatial transformation and WM segmentation of subcortical volumetric structures (Fischl et al., 2004a), intensity normalization, tessellation of GM/WM boundary, automated topology correction (Segonne et al., 2007), and surface deformation following intensity gradients to optimally place GM/WM and CM/CSF borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999).
Freesurfer spatially normalizes each cortical surface to a template cortical surface, allowing for the automatic parcellation of the cortical surface (Fischl et al., 2004b). Freesurfer also performs an automatic subcortical segmentation of the non-cortical voxels in the normalized brain volume to subcortical labels (e.g., hippocampus). To protect against type I error, we combined the parcels to examine volumes of the entire cortical gray and white matter. Because we had no a priori hypotheses about laterality, volumes of the hippocampus and insula were summed across hemispheres to further reduce the number of measurements.
Because we observed a significant inverse association between the frequency of hearing chemical alarms sound and total cortical GM volume, this prompted us to further investigate the association between the frequency of hearing chemical alarms and GM volume in the frontal, temporal, parietal, and occipital lobes in post-hoc analyses. In the Desikan nomenclature, the frontal lobe parcel included the precentral gyrus, superior frontal, caudal and rostral middle frontal, pars opercularis, pars triangularis, pars orbitalis, lateral and medial orbitofrontal cortex, frontal pole, and caudal and rostral anterior cingulate gyri. The parietal lobe parcel included the postcentral gyrus, inferior parietal, superior parietal, supramarginal, paracentral, precuneus, posterior cingulate, and isthmus of the cingulate. The temporal lobe parcel included the transverse temporal, bank of the superior temporal sulcus, superior, middle, and inferior temporal gyri, temporal pole, entorhinal cortex, fusiform, and parahippocampal gyri. The occipital lobe parcel included the lateral occipital, cuneus, pericalcarine, and lingual gyri. The reconstructed cortical surface models of each participant were manually inspected to ensure segmentation accuracy. Because cortical parcellations were combined to derive total and regional lobar GM volumes, subjects who had poor segmentation (i.e., underestimation of GM) due to poor image quality or misregistration in any parcellation were excluded from statistical analyses. Thus, the current analysis included artifact-free data structural MRI data from 90 consecutive GW veterans.
2.3. Statistical analysis
IBM SPSS Statistics version 23 was used for all analyses. We used multiple linear regressions to examine the association between frequencies of hearing chemical alarms and measures of cortical GM, WM, hippocampal, and insular volume. The analyses were adjusted for age, male gender, ICV, diagnoses of current PTSD and/or MDD, current use of psychotropic medication (i.e., antidepressants, benzodiazepine, and/or antiepileptic medication used to control pain), history of alcohol and/or substance abuse/dependence, predicted exposure to the 2000 Khamisiyah plume, and Kansas-GWI case status. The analyses were corrected for multiple comparisons according to the number of regions of interest (ROIs) (i.e., cortical GM, cortical WM, hippocampus, insula) and the average intercorrelations among the ROIs (Sankoh et al., 1997). With an average intercorrelation r = 0.65, a 2-sided adjusted p < 0.031 was considered statistically significant. We also calculated Cohen’s f2 (Cohen, 1988) to estimate the effect size for a single variable (i.e., hearing chemical alarms) within the regression models.
2.3.1. Post-hoc analyses
The significant inverse association between the frequency of hearing chemical alarms sound and total cortical GM volume prompted us to further investigate the association between the frequency of hearing chemical alarms and GM volume in the frontal, temporal, parietal, and occipital lobes in post-hoc analyses. Adjustment for multiple comparisons was made according to the number of ROIs (i.e., frontal, parietal, temporal, and occipital lobes) and the average intercorrelations among the ROIs (r = 0.85). A 2-sided adjusted p < 0.041 was considered statistically significant for the post-hoc analyses.
Because 78% of the current sample met the CDC CMI case definition, in a second post-hoc analysis we attempted to control for the veterans’ current health status as a causal mechanism of the observed neuroanatomical findings. Specifically, we used hierarchical linear regression to examine the ability of hearing chemical alarms (entered in the last step of the regression model) to predict total cortical, frontal, parietal, and occipital lobe GM volume (the dependent variables) over and above the independent variables considered in the original analysis (i.e., age, male gender, ICV, diagnoses of current PTSD and/or MDD, current use of psychotropic medication, history of alcohol and/or substance abuse/dependence, predicted exposure to the 2000 Khamisiyah plume, and Kansas-GWI case status, forced into the first step of the regression model) and health variables, which were forced into the second step of the regression model. The health variables included whether or not the veterans had a Kansas-GWI exclusionary criterion (i.e., diabetes, heart disease other than hypertension, stroke, lupus, multiple sclerosis, rheumatoid arthritis, Parkinson’s disease, AD, amyotrophic lateral sclerosis, cancer, liver and/or kidney disease, schizophrenia, bipolar disorder, any current infections disease lasting six months or longer) and the severity scores of the 6 Kansas GWI symptom domains (i.e., fatigue/sleep, somatic pain, neurologic/cognitive/mood, gastrointestinal, respiratory, and skin) derived from the Kansas Gulf War Military History and Health Questionnaire.
In a third post-hoc analysis, we attempted to control for other concurrent GW deployment-related exposures. Because research suggests that deployment-related exposures can be highly inter-correlated (Cherry et al., 2001; Fricker et al., 2000; Steele et al., 2012), we first used Spearman’s Rank Order Correlation to determine which of the 18 other deployment-related experiences/exposures queried by the Kansas Military History and Health Questionnaire were significantly correlated with hearing chemical alarms. Next, we used hierarchical linear regression to examine the ability of hearing chemical alarms (entered in the last step of the regression model) to predict total cortical GM, frontal, parietal, and occipital lobe GM volume (the dependent variables) over and above the independent variables considered in the original analysis, which were forced into the first step of the regression model, and the other GW deployment-related exposures that were significantly correlated with hearing chemical alarms, which were forced into the second step of the regression model.
3. Results
Table 1 summarizes the demographic, military, and clinical data of 90 GW veterans studied. Forty-six (51%) veterans had GWI according to the Kansas case definition (Steele, 2000) while 70 (77%) veterans fulfilled the CDC CMI criteria (Fukuda et al., 1998). Fifty-two (58%) GW veterans had predicted exposure to the 2000 Khamisiyah plume, while 73 (81%) GW veterans reported hearing chemical alarms sound in theater. There were 30 GW veterans not identified as having predicted exposure to the 2000 Khamisiyah plume who reported hearing chemical alarms sound in theater.
Table 1.
Demographic, clinical, and military characteristics of study sample.
| N | 90 |
|---|---|
| Age at time of study, mean (SD) | 52.3 (8.3) |
| Years of education, mean (SD) | 15.5 (2.3) |
| Female, N (%) | 15 (17%) |
| Race | |
| White, N (%) | 63 (70%) |
| Black, N (%) | 10 (11%) |
| Other, N (%) | 17 (19%) |
| Military status during Gulf War | |
| Active duty, N (%) | 73 (81%) |
| Reserves, N (%) | 12 (13%) |
| National Guard, N (%) | 5 (6%) |
| Rank | |
| Enlisted, N (%) | 69 (77%) |
| Officer, N (%) | 21 (23%) |
| Branch of service | |
| Army, N (%) | 68 (76%) |
| Marines, N (%) | 14 (16%) |
| Air Force, N (%) | 5 (6%) |
| Navy, N (%) | 3 (3%) |
| Location in Theater | |
| Kuwait, N (%) | 51 (57%) |
| Iraq, N (%) | 48 (53%) |
| Bahrain, N (%) | 46 (51%) |
| Saudi Arabia, N (%) | 89 (99%) |
| At sea, N (%) | 12 (13%) |
| Other, N (%) | 6 (7%) |
| Heard chemical alarms sound, N (%) | 73 (81%) |
| 1–6 days, N (%) | 30 (33%) |
| 7–30 days, N (%) | 30 (33%) |
| ≥31 days, N (%) | 13 (14%) |
| Predicted Khamisiyah exposure,a N (%) | 52 (58%) |
| CDC Chronic Multisymptom Illness case, N (%) | 70 (77%) |
| Kansas Gulf War Illness case, N (%) | 46 (51%) |
| Current PTSD diagnosis, N (%) | 11 (12%) |
| Clinician Administered PTSD Scale, mean (SD) | 21.1 (20.8) |
| Current MDD diagnosis, N (%) | 10 (11%) |
| Beck Depression Inventory score, mean (SD) | 9.1 (7.6) |
| Using psychotropic medication, N (%) | 17 (19%) |
| History of alcohol abuse/dependence, N (%) | 27 (30%) |
| History of substance abuse/dependence, N (%) | 8 (9%) |
| APOE4 allele,b N (%) | 19 (21%) |
According to the DoD.
Data unavailable for 21 veterans.
3.1. Association between the frequency of hearing chemical alarms and brain volume
Table 2 shows the association between the frequencies of hearing chemical alarms sound in theater and measures of cortical GM, WM, hippocampal, and insula volume. All fits were significant (all model p’s < 0.0001, 0.42 ≤ R2’s ≤ 0.77). There was a significant inverse association between the frequency of hearing chemical alarms and total cortical GM volume (standardized coefficient β = −0.17, t = −2.77, p = 0.007; Cohen’s f2 = 0.09; see Fig. 1A). In contrast there were no significant association between hearing chemical alarms and cortical WM (Cohen’s f2 = 0.01), hippocampal (Cohen’s f2 = 0.03), or insula (Cohen’s f2 = 0.03) volume. Although there was an association between predicted exposure to the Khamisiyah plume and hippocampal volume (standardized coefficient β = −0.19, t = −2.03, p < 0.05), this association was no longer significant after adjustments for multiple comparisons. Using the CDC CMI case definition instead of the Kansas GWI cases did not significantly alter the inverse association between the frequency of hearing chemical alarms sound in theater and total cortical GM volume. There were no significant associations between regional brain volume and the days of predicted exposure to the Khamisiyah plume or the estimated dose of exposure to the Khamisiyah plume.
Table 2.
Relationships between self-reported frequencies of hearing chemical alarms to measures of total cortical GM, WM, hippocampal, and insula volume reported as B (SE).
| Unstandardized coefficients B(SE) | Cortical GM | Cortical WM | Hippocampus | Insula |
|---|---|---|---|---|
| Intercept | 141,573 (36,086) | 64,216 (47,985) | 6109 (1,017) | 2619 (1822) |
| Hearing chemical alarms | −8113 (2926) | −511 (3906) | −116 (82) | −220 (153) |
| Age | −1496 (340) | −2240 (460) | −29 (10) | −9 (17) |
| Male sex | −9503 (8446) | 2856 (11,364) | 156 (238) | 291 (427) |
| ICV | 0.255 (0.022) | 0.342 (0.029) | 0.003 (0.001) | 0.00/(0.001) |
| Kansas GWI case | 6411 (5847) | 14,337 (7824) | −65 (165) | −198 (299) |
| 2000 Khamisiyah plume exposure | −9217 (5412) | −7274 (7287) | −310 (165)a | −335 (275) |
| Current MDD | 1794 (8861) | −9034 (11,728) | 258 (250) | 439 (447) |
| Current PTSD | −14,322 (9142) | −40,396 (12,098) | −361 (258) | 236 (462) |
| Current psychotropic medication use | −4193 (7235) | 15,791 (9594) | −134 (204) | −699 (366) |
| Past ETOH abuse/dependence | 6998 (6565) | 2139 (8700) | −43 (185188) | −174 (332) |
| Past substance abuse/dependence | 9134 (10,138) | 4910 (13,430) | 17 (286) | 197 (513) |
GM, gray matter; WM, white matter; ICV, intracranial volume; GWI, Gulf War Illness; MDD, Major Depressive Disorder; PTSD, Posttraumatic Stress Disorder; ETOH, alcohol. Bold: adjusted p < 0.031.
p < 0.05.
Fig. 1.
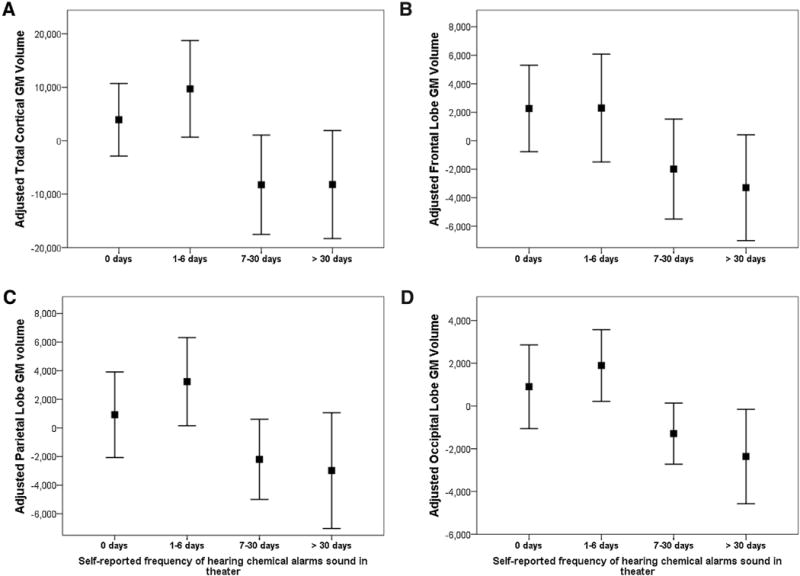
Adjusted total cortical (A), frontal (B), parietal (C), and occipital (D) lobe gray matter (GM) volume plotted as a function of the self-reported frequencies of hearing chemical alarms sound in theater. The y-axis represents the unstandardized residuals that resulted from regressing the regional brain volumes (dependent variables) on age, sex intracranial volume, Kansas-GWI status, predicted Khamasiyah exposure, current PTSD and/or major depressive disorder, current use of psychotropic medication, history of alcohol and/or substance abuse/dependence (independent variables). The square represents the mean value of the unstandardized residuals and the error bars represent the 95% confidence interval.
To determine if the association between the frequency of hearing chemical alarms and total cortical GM volume was independent of the genetic risk for AD, we re-ran the model adjusting for the presence of the apoE4 genotype, which was available for 69 subjects. The regression coefficients for hearing chemical alarms were of similar magnitude and direction as the models without APOE. Moreover, total cortical GM was not significantly associated with the APOE ε4 genotype.
3.2. Post-hoc analyses of the association between the frequency of hearing chemical alarms and lobar GM volume
The significant, inverse association between the self-reported frequency of hearing chemical alarms and total cortical GM volume prompted us to further examine the relationship between hearing chemical alarms and lobar GM volumes in post-hoc analyses. The results of this post-hoc analysis are summarized in Table 3.
Table 3.
Relationships between self-reported frequencies of hearing chemical alarms to measures of cortical volume reported as B (SE).
| Unstandardized coefficients B(SE) | Frontal GM | Parietal GM | Temporal GM | Occipital GM |
|---|---|---|---|---|
| Intercept | 45,930 (14,120) | 41,254 (12,157) | 34,496 (10,770) | 15,549 (6564) |
| Hearing chemical alarms | −2914 (1182) | −2558 (995) | −852 (873) | −1846 (520) |
| Age | −551 (134) | −428 (113) | −355 (101) | −186 (61) |
| Male sex | −7764 (3274) | −2392 (2812) | 1425 (2521) | −2110 (1530) |
| ICV | 0.099 (0.008) | 0.072 (0.007) | 0.056 (0.006) | 0.028 (0.004) |
| KS-GWI | 2062 (24,316) | 2265 (1951) | 1758 (1745) | 1268 (1073) |
| 2000 Khamisiyah plume exposure | −3217 (2142) | −3090 (1814) | −1744 (1615) | −1381 (1005) |
| Current MDD | 2449 (3432) | 693 (2993) | −814 (2645) | −1436 (1566) |
| Current PTSD | −4882 (3543) | −6578 (3079) | −4069 (2728) | −860 (1571) |
| Current psychotropic medication use | −1108 (2805) | −301 (2464) | −2238 (2159) | 724 (1372) |
| Past ETOH abuse/dependence | 3031 (2547) | 2753 (2186) | 964 (1959) | 958 (1127) |
| Past substance abuse/dependence | 2868 (3943) | 3273 (3561) | 1763 (3026) | 1234 (1724) |
Bold: adjusted p < 0.04.
GM, gray matter; ICV, intracranial volume; GWI, Gulf War Illness; MDD, Major Depressive Disorder; PTSD, Posttraumatic Stress Disorder; ETOH, alcohol.
All fits in the post-hoc regression models were significant (all model p’s <0.0001, 0.51 ≤ R2’s ≤ 0.75). There was a significant, inverse association between the frequency of hearing chemical alarms and GM volume in the frontal lobe (standardized coefficient β = −0.16, t = −2.46, p = 0.02, Cohen’s f2 = 0.08), parietal lobe (standardized coefficient β = −0.18, t = −2.57, p = 0.01, Cohen’s f2 = 0.08), and occipital lobe (standardized coefficient β = −0.30, t = −3.55, p = 0.001, Cohen’s f2 = 0.16; see Fig. 1B–D). There was no significant association between hearing chemical alarms and temporal lobe GM volume (Cohen’s f2 = 0.01). As with the primary analysis, re-running the models with CDC CMI case definition instead of Kansas GWI case definition did not significantly alter the inverse association between hearing chemical alarms and GM volumes in the frontal, parietal, and occipital lobes. Similarly, rerunning the models to adjust for the presence of the APOE ε4 genotype did not significantly alter the magnitude or the direction of the inverse relationship between the frequency of hearing chemical alarms and GM volumes in the frontal, parietal, and occipital lobes. There were no significant association between regional lobar GM volume measures and APOE ε4 genotype.
Tables 2 and 3 also show the association between the other independent variables and brain volume. As expected, smaller head size (as indicated by smaller ICVs) and older age were significantly associated with decreased brain volume. Females had smaller frontal lobe volumes than males and veterans with current PTSD had smaller cortical WM and parietal GM volumes.
3.3. Post-hoc analyses controlling for potentially confounding health variables
Because 78% of the GW veterans in the current study sample fulfilled the CDC CMI case definition, we attempted to control for the veterans’ diminished health status as a causal mechanism of the observed neuroanatomical findings in a second set of post-hoc analysis. Specifically, we used hierarchical linear regression to examine the ability of hearing chemical alarms (independent variable entered in the last step of the regression) to predict total cortical, frontal, parietal, and occipital lobe GM volume over and above the independent variables from the original analysis (entered in the first step of the regression) and “health” variables, forced into the second step of the regression. The “health” variables included whether or not the veterans had a Kansas GWI exclusionary criterion and the severity scores of the six Kansas GWI symptom domains. As shown in Table 4, Model 1, hearing chemical alarms significantly predicted total cortical GM volume, frontal, parietal, and occipital lobe GM volume even after accounting for demographic, clinical, and health variables in the regression models.
Table 4.
Post-hoc regression analyses accounting for the effects of adverse health symptoms (model 1) and concurrent GW exposures (in model 2) on the dependent variables.
| Dependent Variables
|
||||
|---|---|---|---|---|
| Cortical GM | Frontal GM | Parietal GM | Occipital GM | |
| Model 1 | ||||
| Step 1a | ||||
| R2 | 0.735 | 0.726 | 0.684 | 0.543 |
| F10,78 | 21.67g | 20.16g | 16.65g | 8.21g |
| Step 2b | ||||
| R2 | 0.760 | 0.759 | 0.694 | 0.610 |
| ΔR2 | 0.025 | 0.033 | 0.010 | 0.066 |
| ΔF7,71 | 1.06 | 1.34 | 0.33 | 1.50 |
| Step 3c | ||||
| R2 | 0.780 | 0.776 | 0.716 | 0.659 |
| ΔR2 | 0.019 | 0.017 | 0.022 | 0.050 |
| ΔF1,70 | 6.18e | 5.08e | 5.43e | 8.93f |
| Model 2 | ||||
| Step 1a | ||||
| R2 | 0.725 | 0.723 | 0.674 | 0.537 |
| F10,69 | 18.17g | 17.45g | 14.09g | 7.08g |
| Step 2d | ||||
| R2 | 0.744 | 0.739 | 0.700 | 0.579 |
| ΔR2 | 0.019 | 0.017 | 0.025 | 0.042 |
| ΔF7,62 | 0.65 | 0.55 | 0.74 | 0.76 |
| Step 3c | ||||
| R2 | 0.768 | 0.753 | 0.734 | 0.657 |
| ΔR2 | 0.025 | 0.013 | 0.034 | 0.078 |
| ΔF1,61 | 6.51e | 3.21 | 7.73f | 12.12f |
Variables entered: age, male sex, ICV, KS GWI case, predicted Khamisiyah exposure, diagnosis of current MDD and/or PTSD, current use of psychotropic medication, history of alcohol and/or substance abuse/dependence.
Variables entered: KS GWI exclusionary criteria, fatigue, pain, neurological, mood, cognitive, skin, gastrointestinal, and respiratory symptom severity scores.
Variable entered: Frequency of hearing chemical alarm.
Variables entered: seeing smoke from oil well fires, living area being fogged or sprayed with pesticides; being within one mile of SCUD missile explosion; coming into direct contact with destroyed enemy vehicles; using pesticide cream or liquid on skin; wearing pesticide-treated uniform; living/sleeping in tent heated by a fuel-burning heater.
p < 0.05.
p < 0.01.
p < 0.001.
3.4. Post-hoc analyses accounting for concurrent deployment-related exposures and experiences
Because many other deployment-related exposures have been suspected of contributing to long-term ill health in GW veterans, we attempted to control for these concurrent exposures in a third post-hoc analysis. We found 7 other deployment-related experiences to be significantly correlated with hearing chemical alarms sound in theater. These include: exposure to smoke from oil well fires (Spearman’s r = 0.28, p = 0.008), being within 1 mile of a SCUD missile explosion (Spearman’s r = 0.28, p = 0.009), contact with destroyed enemy vehicles (Spearman’s r = 0.26, p = 0.02), using cream or liquid pesticide on the skin (Spearman’s r = 0.24, p = 0.03), wearing pesticide-treated uniforms (Spearman’s r = 0.29, p = 0.007), witnessing living area being sprayed with pesticides (Spearman’s r = 0.22, p = 0.04), and living/sleeping in a tent heated by a fuel-burning stove (Spearman’s r = 0.26, p = 0.02). Next, we used hierarchical linear regression to examine the ability of hearing chemical alarms sound (entered in the last step of the regression) to predict total cortical, frontal, parietal, and occipital lobe GM volume over and above the independent variables from the original analysis, entered in the first step of the regression, and these 7 correlated, deployment-related exposures, forced into the second step of the regression. As shown in Table 4, Model 2, the self-reported frequency of hearing chemical alarms significantly predicted total cortical, parietal, and occipital lobe GM volume even after accounting for demographic and clinical variables and correlated, concurrent GW-related exposures/experiences. Only frontal lobe GM volume was not significantly associated with the frequency of hearing chemical alarms after accounting for the correlated concurrent exposure in the regression.
3.5. Association between hearing chemical alarms and GWI cases
When we dichotomized the self-reports of hearing chemical alarms (i.e., yes/no), we found that there were significantly more Kansas GWI cases (56%) among veterans who reported hearing chemical alarms compared to veterans who did not report hearing chemical alarms (25%; Fisher’s exact test p = 0.03). In contrast, there was no difference in the incidence of CDC CMI cases between veterans who did (79%) and did not (69%) report hearing chemical alarms sound in theater (Fisher’s exact test p = 0.34). When we treated self-reports of hearing chemical alarms as a categorical variable (i.e., did not hear chemical alarms, heard chemical alarms for 1–6 days, 7–30 days, or ≥31 days), we found more Kansas GWI cases among veterans who reported hearing chemical alarms sound most frequently (77%) compared to veterans who heard the chemical alarms sound less frequently (i.e., 53% among those who heard alarms 7–30 days, 50% among those who heard alarms 1–6 days, and 25% among veterans who did not report hearing the alarms at all; χ2 linear-by-linear association = 6.85, df = 1, p = 0.009). There was no significant difference in the incidence of CDC CMI cases among veterans who heard chemical alarms ≥31 days (85%), 7–30 days (73%),1–6 days (83%) or among veterans who did not report hearing the alarms (69%; χ2 linear-by-linear association = 0.28, df = 1, p = 0.60). There was no significant difference in the incidence of CDC CMI cases among veterans with (75%) or without (82%) predicted exposure to the Khamisiyah plume (Fisher’s exact test p = 0.61). There was no significant difference in the incidence of Kansas GWI cases among veterans with (46%) or without (58%) predicted exposure to the Khamisiyah plume (Fisher’s exact test p = 0.29).
4. Discussion
The major finding of this study is a significant, inverse association between the self-reported frequency of hearing chemical alarms sound during deployment in the Gulf War and total cortical, frontal, parietal, and occipital lobe GM volume in GW veterans. These inverse associations were independent of predicted exposure to the Khamisiyah plume, demographic and clinical variables, GWI status, and the veterans’ current health status. The inverse association between the self-reported frequency of hearing chemical alarms and total cortical GM, parietal, and occipital lobe GM volumes were also independent of correlated, concurrent deployment-related exposures. The local effect sizes (i.e., Cohen’s f2) of the associations between hearing chemical alarms and regional brain volume ranged from 0.09 for total cortical GM to 0.15 for occipital GM volume, which represent medium effect sizes (Cohen, 1988).
When U.S. troops first arrived in the Gulf region, there were uncertainties about whether they would be exposed to chemical weapons because Iraq had used such weapons in fighting Iran and in attacks on the Kurdish minority. For this reason, chemical alarms were distributed throughout the region to warn of such attacks. The alarms were sensitive and sounded frequently, prompting military personnel to don protective gear and ingest PB pills as an antidote to the effects of nerve agents (IOM, 2014).
Because the chemical alarms were extremely sensitive, it has been suggested that they may have been triggered by substances other than chemical nerve agents, such as organic solvents and pesticides (IOM, 2014). However, other sources have described the sudden sounding of tens of thousands of chemical alarms on the same day the morning after coalition bombing of huge nerve agent storage sites in Iraq (Committee on Banking, 1994; Haley and Tuite, 2013; Office of the Special Assistant for Gulf War Illnesses, 1996; Tucker, 1997; Tuite and Haley, 2013). Moreover, the phenomenon of the sudden, simultaneous sounding of multiple chemical alarms recurred several times during the air war (Tuite and Haley, 2013; Haley and Tuite, 2013). For this reason, it is unlikely that the widespread sounding of chemical weapon alarms during the Gulf War were all due to organic solvents and pesticides, even if it may have occurred on some occasions.
If we assume that the veterans’ self-reports of hearing chemical alarms sound during deployment is a proxy for possible exposure to chemical nerve agents, then our finding of a significant inverse association between the frequency of hearing chemical alarms sound in theater and total cortical GM volume is consistent with our prior reports of reduced cortical GM volume in GW veterans with predicted exposure to the Khamisiyah plume (Chao et al., 2010, 2011). In post-hoc analyses, we extended this finding to cortical GM volume in the frontal, parietal, and occipital lobes. We also show that the significant inverse association between hearing chemical alarms and total cortical, frontal, parietal, and occipital lobe GM volumes was independent of the veterans’ current health status/GWI symptoms and that the inverse relationship between hearing chemical alarms and total cortical, parietal, and occipital lobe GM volumes was independent other correlated, concurrent deployment-related exposures.
We previously used voxel-based morphometry (VBM) to examine regional differences in GM density between GW veterans with and without predicted exposure to the Khamisiyah plume (Chao et al., 2010). Although none of the regional differences in GM density in the VBM analysis survived corrections for multiple comparisons, it is noteworthy that the VBM analysis identified clusters located in the frontal, parietal, and occipital lobes, where we found significant inverse relationships between the frequency of hearing chemical alarms sound and GM volume in the current study.
There is evidence that exposure to low doses of organophosphorus (OP) chemicals (e.g., chemical nerve agents such as sarin and cyclosarin and many of the pesticides used during the GW) insufficient to cause frank poisoning due to acetylcholinesterase inhibition can still have chronic ill-health effects. For example, one in vitro study showed that cellular processes can be altered by exposure to concentrations of OPs insufficient to cause acetyl-cholinesterase inhibition (Eaton et al., 2008). Low-level and/or chronic exposure to OPs have also been linked to lasting neuropsychological deficits such as decreased processing speed and increased mood complaints in agricultural workers and professional pesticide applicators (Bazylewicz-Walczak et al., 1999; Kamel et al., 2007; Mackenzie Ross et al., 2010; Roldan-Tapi et al., 2005; Steenland et al., 1994; Stephens et al., 1995; Toomey et al., 2009). Two recent meta-analyses that examined data from nearly 2000 cases of low-level occupational OP exposures reported significant decrements in working memory, visual memory, attention, psychomotor speed, executive function, and visuospatial function relative to non-exposed controls (Ismail et al., 2012; Ross et al., 2013). Notably, these cognitive processes have been linked with integrity of the frontal (Alvarez and Emory, 2006; Beyer and Krishnan, 2002; Corbetta and Shulman, 2002; Ester et al., 2015; Kochunov et al., 2010), parietal (Corbetta and Shulman, 2002; Ester et al., 2015), and occipital (Knopman et al., 2014) cortex, where we found significant, inverse associations between GM volume and the frequency of hearing chemical alarms sound in theater.
Heaton et al. (2007) were the first to report a significant association between higher levels of estimated Khamisiyah plume exposure and reduced WM volume. Although we also detected an effect of predicted Khamisiyah exposure on total brain WM volume in our previous 4 Tesla imaging study of GW veterans, we did not observe an effect of predicted Khamisiyah exposure on WM volume in our initial investigation of 1.5 T imaging data. Similarly, we did not find an association between self-reports of hearing chemical alarms and total brain WM volume in the current study. However, in line with a prior report by Villarreal et al. (2002), we did find a significant effect of current PTSD diagnosis on total brain WM volume in the present study. Therefore, it may be possible that exposure to low-levels of OP chemicals has a more subtle effect on WM volume than on GM volume, which makes it more difficult to differentiate from other clinical confounds (such as current PTSD). Some support for this comes from the very small local effect size (f2 = 0.01) that hearing chemical alarms had on cortical WM volume.
It is noteworthy that self-reports of hearing chemical alarms had a significant, inverse relationship with brain volumetric data in the current study while predicted Khamisiyah plume exposure did not. One possible explanation for the discordant associations that we observed between regional brain volumes and predicted Khamisiyah plume exposure versus self-reports of hearing chemical alarms in the current study may be that the two methods (i.e., plume modeling and self-reports) identified different subsets of GW veterans. For example, 81% of the current study sample reported hearing chemical alarms whereas only 58% had predicted Khamisiyah plume exposure. Also, there were 30 GW veterans who reported hearing chemical alarms who had not been identified as having predicted Khamisiyah plume exposure. Furthermore, it has been suggested that there were other nerve agent exposures in the Gulf War besides the one associated with the Khamisiyah demolitions (Committee on Banking, 1994; Haley and Tuite, 2013; Office of the Special Assistant for Gulf War Illnesses, 1996; Tucker, 1997; Tuite and Haley, 2013). Because the DOD/CIA plume models only focused on exposures associated with the Khamisiyah demolitions, which occurred after the end of the ground war (Presidential Advisory Committee on Gulf War Veterans’ Illnessess, 1996; Haley and Tuite, 2013; Tuite and Haley, 2013), whereas the chemical alarms sounded mostly in the early phase of the war (Haley and Tuite, 2013; Tuite and Haley, 2013), it follows that the two methods would not necessarily identify an overlapping group of GW veterans.
Although we initially made the assumption that the veterans’ self-reports of hearing chemical alarms sound during deployment was a proxy for possible exposure to chemical nerve agents, there is some evidence that there were also blister agents (i.e., mustard gas) in the Gulf War milieu. For example, Czech chemical experts contracted by the Saudi government to provide chemical weapons surveillance for their troops detected both nerve and blister agents during the Gulf War (Agency, 1993; Tuite and Haley, 2013).Therefore, it may be possibile that veterans who heard chemical alarms sound during deployment were exposed to a different combination of substances than the veterans with predicted exposure to the Khamisiyah plume. With this respect, it is interesting to note that we observed an inverse association between hippocampal volume and predicted exposure to the Khamisiyah plume in the present study but not between hippocampal volume and self-reports of hearing chemical alarms. Although the inverse association between hippocampal volume and predicted Khamisiyah exposure did not survive corrections for multiple comparisons, it is, nevertheless reminiscent of our previous findings in GW veterans with predicted Khamisiyah exposure (Chao et al., 2010, 2014) and of a previous neuroimaging study of victims of the 1995 Tokyo subway sarin attack (Yamasue et al., 2007). If the hippocampus is more sensitive to sarin/cyclosarin exposure, indicated by predicted exposure to the Khamisiyah plume, than exposures to other types of agents that may have triggered some of the chemical alarms during the Gulf War, this could, at least in part, explain why we observed a different relationship between hippocampal volume and predicted Khamisiyah plume exposure compared to the self-reports of hearing chemical alarms. However, there is no way to discern whether a GW veteran was exposed to chemical nerve agents, blisteragents, or both.
Based on Haley and Tuite’s (2013) findings of a dose-response relationship between the number of times nerve agent alarms sounded in a veteran’s immediate area and the risk of having GWI, we examined the frequency of GWI cases among veterans who did and did not report of hearing chemical alarms in theater in the current study. There are a number of different definitions of GWI (e.g., CDC CMI (Fukuda et al., 1998)), Kansas GWI (Steele, 2000), and Haley syndrome criteria (Haley et al., 1997). Haley and Tuite (2013) found a significant a dose–response relationship between the number of times nerve agent alarms sounded and both Haley syndrome and CDC CMI criteria. Contrary to Haley and Tuite (2013), we did not find more CDC CMI cases among GW veterans who reported hearing chemical alarms. However, Haley and Tuite examined a larger sample (N = 8020) than we did. Therefore, it is possible that our failure to replicate their finding is due to a lack of power in the current study.
Haley and Tuite (2013) did not examine Kansas GWI cases in their study. However, we used the Kansas GWI case definition in the current study based on the recommendations of the 2014 IOM report on GWI case definitions. We found more Kansas GWI cases among veterans who reported hearing chemical alarms sound in theater (56%) compared to veterans who did not report hearing chemical alarms (25%). When we treated self-reports of hearing chemical alarms as a categorical variable, we found more Kansas GWI cases among veterans who reported hearing the chemical alarms most frequently (77%) compared to veterans who reported hearing the alarms less frequently (53% among those who heard alarms 7–30 days, 50% among those who heard alarms 1–6 days, and 25% among veterans who did not report hearing the alarms at all). However, it is important to point out that this analysis did not take into account the veterans’ branch of service or deployment, which Steele et al. (2012) found interacted significantly with GWI in a stratified analysis. We also did not account for other concurrent deployment-related exposures in this analysis because the size of our sample did not afford us the ability to evaluate precisely defined subgroups. When they used a stratified analysis that accommodated differences among veteran subgroups and logistic modeling to control for confounding concurrent exposures, Steele et al. (2012) found that Kansas GWI was most strongly associated with use of PB pills and proximity to exploded SCUD missiles among personnel who were in Iraq or Kuwait. For personnel who remained in support areas, Kansas GWI was most strongly associated with wearing pesticide-treated uniforms and using skin pesticides. Although Steele et al. (2012) did not find self-reports of hearing chemical alarms to be significantly associated with risk for Kansas GWI in that study, in a preliminary case-control study of a group of forward deployed, enlisted Army personnel, Dr. Steele reported that GW veterans with a particular genotype of the paraoxonase 1 (PON1) enzyme were at significantly increased risk for Kansas GWI if they also reported hearing chemical alarms sound in theater (Dr. Lea Steele’s presentation to the 2015 RAC-GWVI Meeting on April 20–21, 2015, pp. 50–73 of the meeting minutes). PON1 is an enzyme that plays a significant role in the breakdown of various kinds of OP chemicals (Costa et al., 2013). Different PON1 genotypes can affect an individual’s resistance to the effects of OP-containing pesticides and nerve agents (Clerx et al., 2012; Costa et al.,1990; Li et al.,1993). Thus, it is interesting that Dr. Steele found that GW veterans who were carriers of the PON1 allele that is less effective at metabolizing chemical nerve agents were at increased risk for Kansas GWI if they also reported hearing chemical alarms in theater. We did not examine PON1 genotype in the current study. However, Dr. Steele’s preliminary findings of interactions between PON1 genotype and GW-related exposures hint at the possibility that similar interactions may contribute to the reduced regional brain volumes that we observed in the present study. Future research will be needed to more carefully evaluate this hypothesis.
This study had several limitations: first, we did not query for exposures and experiences that may have occurred in the years since the end of the Gulf War. For this reason, we cannot rule out the possibility that non-GW related exposures and/or experiences (e.g., occupational chemical exposures, excessive pesticide use, etc.) that took place in the 25 years since the end of the Gulf War may have contributed to the current findings. Second, we did not have a group of healthy, control GW veterans for comparison purposes (i.e., only 8 veterans did not have predicted Khamisiyah exposure, Kansas GWI, or Kansas GWI exclusionary criteria). However, we did attempt to control for potentially confounding demographic, clinical, and health-related variables the main and post-hoc analyses. Third, our reliance on the veterans’ own reports of their wartime experiences could have introduced errors due to inaccurate reporting or recall of exposures. However, the lack of measured data on the types and doses of exposures experienced by GW veterans in theater severely limits the number of methods that investigators can use to evaluate the consequences of deployment-related exposures (e.g., Haley and Tuite, 2013; Steele et al., 2012). A final limitation of this study is that we did not examine PON1 genotype, which may have significant interactions with certain GW-related exposures and may have contributed to the reduced regional brain volumes that we observed in the present study. These limitations notwithstanding, the current findings suggest that exposure to substances that triggered chemical alarms during the Gulf War may have had adverse neuroanatomical effects on more GW veterans than just those with predicted exposure to the Khamisiyah plume.
Acknowledgments
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the Army, Department of Defense, or Department of Veterans Affairs. This study supported by VA grant No. CX000798 entitled ‘Longitudinal Assessment of Gulf War Veterans with Suspected Sarin Exposure’. This material is the result of work supported with resources and the use of facilities at the San Francisco Veterans Affairs Medical Center. The authors would like to thank the Gulf War veterans who participated in these studies.
Footnotes
Disclosure statement
The opinions or assertions contained herein are the private views of the author(s) and are not to be construed as official or reflecting the views of the Army, Department of Defense, or Department of Veterans Affairs. Dr. Chao receives grant support from the Department of Veteran’s Affairs.
Conflicts of interest
None.
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicology. 1999;20:819–826. [PubMed] [Google Scholar]
- Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinican-administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Final recommendations for protecting health and safety against potential adverse effects of long-term exposure to low doses of agents GA, GB, VX, mustard agent (H, HD, T), and Lewisite (L) Fed Regist. 1988;1988 [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, Weiner MW. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31:493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Abadjian L, Hlavin J, Meyerhoff DJ, Weiner MW. Effects of low-level sarin and cyclosarin exposure and Gulf War Illness on brain structure and function: a study at 4 Tesla. Neurotoxicology. 2011;32:814–822. doi: 10.1016/j.neuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Chao LL, Kriger S, Buckley S, Ng P, Mueller SG. Effects of low-level sarin and cyclosarin exposure on hippocampal sub fields in Gulf War Veterans. Neurotoxicology. 2014;44:263–269. doi: 10.1016/j.neuro.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Zhang Y, Buckley S. Effects of low-level sarin and cyclosarin exposure on white matter integrity in Gulf War Veterans. Neurotoxicology. 2015;48:239–248. doi: 10.1016/j.neuro.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry N, Creed F, Silman A, Dunn G, Baxter D, Smedley J, Taylor S, Macfarlane GJ. Health and exposures of United Kingdom Gulf war veterans. Part II: the relation of health to exposure. Occup Environ Med. 2001;58:299–306. doi: 10.1136/oem.58.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerx L, Visser PJ, Verhey F, Aalten P. New MRI markers for Alzheimer’s disease: a meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J Alzheimers Dis. 2012;29:405–429. doi: 10.3233/JAD-2011-110797. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Erlbaum Associates; Hilsdale, NJ: 1988. [Google Scholar]
- Committee on Banking, Housing, and Urban Affairs. U.S. chemical and biological warfare-related dual use exports ot Iraq and their possible impact on the health consequences of the Persian Gulf War. :1994. http://gulfweb.org/bigdoc/report/riegle1.html. (accessed 16/10/15).
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Costa LG, McDonald BE, Murphy SD, Omenn GS, Richter RJ, Motulsky AG, Furlong CE. Serum paraoxonase and its in fluence on paraoxon and chlorpyrifos-oxon toxicity in rats. Toxicol Appl Pharmacol. 1990;103:66–76. doi: 10.1016/0041-008x(90)90263-t. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Cole TB, Marsillach J, Furlong CE. Paraoxonase 1 (PON1) as a genetic determinant of susceptibility to organophosphate toxicity. Toxicology. 2013;307:115–122. doi: 10.1016/j.tox.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Defense Intelligence Agency. Detection of Chemical Warfare Agents by Czechoslovak Unit during Desert Storm, Part III (U), IIR 6 284 0008 94. Defense Intelligence Agency; Washington, D.C.: 1993. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness, and Military Deployments. Potential exposure to sarin from the demolitions at Khamisiyah, Iraq onMarch 10, 1991. Falls Church, VA: Aug, 1997. http://www.gulflink.osd.mil. (accessed 16.10.15). [Google Scholar]
- Directorate for Deployment Health Support of the Special Assistant to the Under Secretary of Defense (Personnel and Readiness) for Gulf War Illness Medical Readiness and Military Deployments. US demolition operations at the Khamisiyah ammunition point (case narrative) 2002 Apr; http://www.gulflink.osd.mil/khamisiyahiii. (accessed 16.10.15).
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa L, Coyle J, McKhann G, Mobley WC, Nadel L, et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38:1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Ester EF, Sprague TC, Serences JT. Parietal and frontal cortex encode stimulus-specific mnemonic representations during visual working memory. Neuron. 2015;19:893–905. doi: 10.1016/j.neuron.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fricker RD, Reardon ESKC, Pace JE, Hosek SD. Pesticide Use During The Gulf War: A Survey of Gulf War Veterans. Vol. 12. RAND; Santa Monica, CA: 2000. A review of the scientific literature as it pertains to Gulf War Illnesses. [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–988. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Haley RW, Tuite JJ. Epidemiologic evidence of health effects from long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology. 2013;40:178–189. doi: 10.1159/000345124. [DOI] [PubMed] [Google Scholar]
- Haley RW, Kurt TL, Hom J. Is there a Gulf War Syndrome? JAMA. 1997;277:215–222. [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF. Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin. Neurotoxicology. 2007;28:761–769. doi: 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. National Academies Press; Washington DC: 2014. [PubMed] [Google Scholar]
- Ismail AA, Bodner TE, Rohlman DS. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup Environ Med. 2012;69:457–464. doi: 10.1136/oemed-2011-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- Knopman AA, Wong CH, Stevenson RJ, Homewood J, Mohamed A, Somerville E, Eberl S, Wen L, Fulham M, Bleasel AF. The cognitive profile of occipital lobe epilepsy and the selective association of left temporal lobe hypometabolism with verbal memory impairment. Epilepsia. 2014;55:e80–84. doi: 10.1111/epi.12623. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin D, Hardies J, Kochunov V, Bartzonkis G, Stanley J, Royall D, Schlosser A, et al. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010;49:1190–1199. doi: 10.1016/j.neuroimage.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lehtovirta M, Laakso MP, Frisoni GB, Soininen H. How does the apolipoprotein E genotype modulate the brain in aging and in Alzheimer’s disease? A review of neuroimaging studies. Neurobiol Aging. 2000;21:293–300. doi: 10.1016/s0197-4580(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Li WF, Costa LG, Furlong CE. Serum paraoxonase status: a major factor in determining resistance to organophosphates. J Toxicol Environ Health. 1993;40:337–346. doi: 10.1080/15287399309531798. [DOI] [PubMed] [Google Scholar]
- Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010;32:452–459. doi: 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara B, Leitnaker F. Toxicological Basis for Controlling Emission of Gb into the Environment, Aberdeen Proving Grounds. Edgewood Arsenal, MD: 1971. [Google Scholar]
- Miyaki K, Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Yoshimura K, Etoh N, Masumoto Y, Kikuchi Y, Kumagai N, et al. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. J Occup Health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- Nishiwaki Y, Maekawa K, Ogawa Y, Asukai N, Minami M, Omae K. Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Envrion Health Perspect. 2001;109:1169–1173. doi: 10.1289/ehp.011091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Special Assistant for Gulf War Illnesses. Coalition Chemical Detections and Health of Coalition Troops in Detection Area. Department of Defense; Washington, DC: 1996. [Google Scholar]
- Presidential Advisory Committee on Gulf War Veterans’ Illness. Final Report. U.S. Government Printing Office; Washington, DC: 1996. [Google Scholar]
- Proctor SP, Heaton KJ, Heeren T, White RF. Effects of chemical warfare agent exposure on central nervous system functioning in US army veterans of the 1991 Gulf War. Neurotoxicology. 2006;27:931–939. doi: 10.1016/j.neuro.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Roldan-Tapi L, Leyva A, Laynez F, Santed FS. Chronic neuropsychological sequelae of cholinesterase inhibitors in the absence of structural brain damage: two cases of acute poisoning. Environ Health Perspect. 2005;113:762–766. doi: 10.1289/ehp.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013;43:21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imag. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. The Structured Clinical Interview for DSM-IV Diagnosis (SCID) American Psychiatric Press; Washington, DC: 1992. [Google Scholar]
- Steele L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol. 2000;152:992–1002. doi: 10.1093/aje/152.10.992. [DOI] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR. Complex Factors in the Etiology of Gulf War Illness: wartime exposures and risk factors in veteran subgroups. Environ Health Perspect. 2012;120:112–118. doi: 10.1289/ehp.1003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Jenkins B, Ames RG, O’Malley M, Chrislip D, Russo J. Chronic neurological sequelae to organophosphate pesticide poisoning. Am J Public Health. 1994;84:731–736. doi: 10.2105/ajph.84.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345:1135–1139. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Morita H, Ono K, Maekawa K, Nagai R, Yazaki Y. Sarin poisoning in Tokyo subway. Lancet. 1995;345:980. [PubMed] [Google Scholar]
- Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, Lyons MJ, Henderson WG, Kang HK, Eisen SA, Murphy FM. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. J Int Neuropsychol Soc. 2009;15:717–729. doi: 10.1017/S1355617709990294. [DOI] [PubMed] [Google Scholar]
- Tucker JB. Evidence Iraq used chemical weapons during the persian Gulf War. Nonproliferation Rev. 1997 Spring-Summer;:114–122. [Google Scholar]
- Tuite JJ, Haley RW. Meteorological and intelligence evidence of long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology. 2013;40:160–177. doi: 10.1159/000345123. [DOI] [PubMed] [Google Scholar]
- United States General Accounting Office. DoD’ Conclusions abou US Troops’ Exposure Cannot Be Adequately Supported, Report to the Congressional Requesters. 2004 ( http://www.gao.gov/new.items/d04159.pdf)
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2015;74:449–475. doi: 10.1016/j.cortex.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J, Kimerling R, Brown P, Chresman K, Levin K. Psychometric review of the life stressor checklist-revised. In: Stamm BH, editor. Instrumentation in Stress, Trauma, and Adaptation. Sidran Press; Lutherville, MD: 1996. pp. 144–151. [Google Scholar]
- Yamasue H, Abe O, Kasai K, Suga M, Iwanami A, Yamada H, Tochigi M, Ohtani T, Rogers M, Sasaki T, et al. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]


