Abstract
Background
Prior claims analyses suggest that the use of intravenous inotropic therapy for patients hospitalized with heart failure varies substantially by hospital. Whether differences in the clinical characteristics of the patients explain observed differences in the use of inotropic therapy is not known.
Methods and Results
We sought to characterize institutional variation in inotrope use among patients hospitalized with heart failure before and after accounting for patients’ clinical factors. Hierarchical generalized linear regression models estimated risk-standardized hospital-level rates of inotrope use within 209 hospitals participating in Get With The Guidelines-Heart Failure (GWTG-HF) registry between 2005–2011. The association between risk-standardized rates of inotrope use and clinical outcomes were determined. Overall, an inotropic agent was administered in 7,691 of 126,564 (6.1%) HF hospitalizations: dobutamine 43%, dopamine 24%, milrinone 17%, or a combination 16%. Patterns of inotrope use were stable over the 7-year study period. Use of inotropes varied significantly between hospitals even after accounting for patient and hospital characteristics (median risk-standardized hospital rate 5.9%, IQR 3.7–8.6%, range 1.3–32.9%). After adjusting for case mix and hospital structural differences, model intra-class correlation indicated that 21% of the observed variation in inotrope use was potentially attributable to random hospital effects (i.e. institutional preferences). Hospitals with higher risk-standardized inotrope use had modestly longer risk-standardized length of stay (p=0.005) but had no difference in risk-standardized inpatient mortality (p=0.12)
Conclusions
Use of intravenous inotropic agents during hospitalization for heart failure varies significantly among U.S. hospitals, even after accounting for patient and hospital factors.
Key Words (MeSH Headings): Heart failure, cardiotonic agents [entry term: inotropic agents, positive cardiac], outcome assessment (health care), physician’s practice patterns [entry term: variations, clinical practice]
Subject Codes: [110] Heart Failure, Congestive, [100] Ethics and Policy, Health policy and outcome research, [33] Other diagnostic testing
INTRODUCTION
Pharmacological options for treating acute decompensated heart failure have changed little over the past few decades. Moreover, few positive studies exist of acute treatments for heart failure. As a result, in-hospital management is dictated largely by expert opinion.1–3 While most patients receive intravenous (IV) loop diuretic therapy to relieve congestion as the primary inpatient intervention4 followed by transition back to a stable oral regimen, some patients receive more intensive approaches including IV inotropic therapy.
For hemodynamically stable heart failure outpatients, the use of inotropic therapies, other than digoxin, has been associated with increased mortality.5,6 For hospitalized heart failure patients without evidence of end-organ hypoperfusion, randomized trials of IV milrinone and levosimendan compared with placebo both found an increased risk of adverse events.7,8 For unstable heart failure patients, few high-quality data exist to guide care decisions.9 As would be expected from treatment selection biases, observational data show that patients hospitalized with heart failure who receive an IV inotrope tend to have greater heart failure severity and higher subsequent mortality.10,11
Based on these limited data and expert opinion, the American Heart Association/American College of Cardiology (AHA/ACC) clinical practice guidelines provide the following Level IIb recommendation (i.e. may be considered): “Short-term, continuous intravenous inotropic support may be reasonable in those hospitalized patients presenting with documented severe systolic dysfunction who present with low blood pressure and significantly depressed cardiac output to maintain systemic perfusion and preserve end-organ performance.”1 The European Society of Cardiology (ESC) guidelines make a similar recommendation, although with at a Level IIa (i.e. it is reasonable to consider).3 Both guidelines also explicitly state (Level III) that IV inotropic agents are contraindicated in hospitalized patients with heart failure who do not have evidence of decreased organ perfusion.
Analyses of billing data12 and randomized trial data11 suggest that inotrope use varies significantly across hospitals. However, billing data lack the clinical detail to enable robust adjustment for case mix and trial data may not adequately reflect non-protocol controlled community practice. Accordingly, we sought to characterize hospital patterns of IV inotrope use using a large contemporary U.S. registry of patients hospitalized with heart failure. The objectives were to 1) quantify current day hospital variation in IV inotropic agent use; 2) determine the phenotypes of the hospitals with high and low use rates; 3) assess whether case mix or structural characteristics explain observed differences; and 4) characterize associations between risk-standardized rates of hospital inotrope use and performance measures, length of stay, and in-hospital mortality.
METHODS
Data Source
We conducted a cross-sectional study using data from the Get With The Guidelines–Heart Failure (GWTG-HF) voluntary quality improvement initiative. The design and validity of this program’s methods and data capture have been published previously.13–15 Briefly, trained personnel at each site abstract clinical data for all patients admitted with heart failure in compliance with The Joint Commission and Centers for Medicare and Medicaid Services standards for quality indicators. Variables collected include demographic and clinical characteristics, medical history, previous treatments, admission medications, in-hospital treatments, in-hospital outcomes, and discharge medications, including contraindications to evidence-based therapies. The data collection form specifically includes a section on in-hospital “parenteral therapies” including individual yes/no responses for “dobutamine”, “dopamine”, “milrinone”, and “none”. Dopamine, dobutamine, and milrinone are the IV inotropic agents highlighted in the AHA/ACC heart failure guidelines.1 Clinical data use the point-of-service, interactive, Internet-based Patient Management Tool (Outcome Sciences, Inc, Cambridge, Massachusetts). The Internet-based system performs checks to ensure the completeness of the reported data. Additionally, data quality is monitored independently and reports are generated to confirm the completeness and accuracy of submitted data. Hospital data elements are collected for all enrolling hospitals from the American Hospital Association database.
Patient data are de-identified in accordance with the Health Insurance Portability and Accountability Act and a random hospital identifier is used to identify the various hospitals. All participating institutions are required to comply with local regulatory and privacy guidelines and to submit the program protocols for review and approval by their institutional review boards. Because data are used primarily at the local site for quality improvement, sites are granted a waiver of informed consent under the common rule. The Duke Clinical Research Institute (Durham, NC) serves as the data analysis center.
Patients and Hospitals
We confined the current analysis to hospital admissions between January 1, 2005 and December 31, 2011 at a hospital fully participating in the GWTG-HF program. Fully participating hospitals were considered to be those with no more than 25% of history panel forms incomplete and no more than 20% of parental therapies data missing. We then excluded hospitalizations for whom discharge data or gender was missing, hospitalizations resulting in transfer to another facility, or hospitalizations that did not have a response for in-patient parental therapies recorded. Finally, we excluded hospitals with less than 25 patients (Appendix 1).
Statistical Analysis
Observed hospital rates of inotrope use were calculated as percent of total patients receiving one or more inotropes during hospitalization. Hospital risk-standardized inotrope use rates were computed as the ratio of predicted to expected rates obtained from hierarchical generalized linear models (HGLMs), with patient and hospital structural characteristics as model fixed effects, multiplied by the overall observed inotrope use rate.12,16 In the hierarchical model the numerator is what is “predicted” for that hospital based on its rate and sample size if it had the average case mix and the denominator is what is “expected” if its performance was the average for all the hospitals. Patient and hospital-level characteristics were selected based upon previous literature17 and clinical criteria.1,3
Categorical variables with missing observations (all <5% missing) were imputed to the most common category. Insurance status was imputed to Medicare if age>=65 and to none otherwise. Race and insurance status were excluded in a sensitivity analysis. For continuous variables, missing observations ranged from 2–16% and were imputed with group-specific medians based on inotrope use. For left ventricular ejection fraction (LVEF) we also considered gender for the imputation. We used cubic spline plots to explore the functional form of continuous variables. As a result, age, systolic blood pressure, LVEF, blood urea nitrogen, hemoglobin, and hospital bed size were modeled as linear continuous, with LVEF truncated at 60% and hemoglobin truncated at 11 g/dL. Heart rate was modeled with 3 linear splines with knots at 75 and 105 beats per minute. Serum creatinine and sodium were modeled with 2 linear splines, with knots at 2.5 mg/dl and 140 mmol/L, respectively.
Patient and hospital characteristics, overall and by quartiles of risk-standardized hospital rates of inotrope use, were summarized using frequencies and proportions for categorical data and medians with inter-quartile ranges (IQRs) for continuous variables. P-values for comparison across quartiles of risk-standardized hospital inotrope rates were computed with Wilcoxon or Kruskal-Wallis test for categorical variables and chi-square rank correlation statistics for continuous variables.
Hierarchical generalized linear models (HGLMs) were also performed to evaluate factors associated with inotrope use. First, patient-level factors were modeled.16 Subsequently, hospital structural characteristics were added to the model. Odds ratios (OR) and corresponding 95% confidence intervals (95%CI) were reported for all factors in the models. To further assess the contribution of hospital preferences (i.e. random institutional effects) to the variation in use of positive inotropic agents, we calculated the intra-class correlation coefficient for the HGLM model after adding a hospital random effect to the fixed patient and hospital structural effects.18
The outcomes of in-hospital mortality and length of stay > 4 days were summarized in the same fashion as patient and hospital characteristics. Risk-standardized hospital mortality and length of stay were calculated using the same methodology to risk-standardized hospital inotrope rates. Performance measures collected by GWTG-HF (discharge to home, documentation of LVEF, use of angiotensin converting enzyme inhibitor [ACEI] or angiotensin receptor blocker [ARB] for left ventricular systolic dysfunction [LVSD], use of beta-blocker for LVSD, and smoking cessation counseling) were reported at the hospital level as percent of eligible patients treated; because all patients deemed eligible for a measure were presumed to merit its application, the performance measures were not risk standardized.
Due to the potential use of low-dose dopamine to theoretically “improve diuresis and better preserve renal function and renal blood flow” (Class IIb, Level of Evidence B)1 and the absence of dosing data in GWTG, analyses were repeated with hospitalizations involving dopamine alone treated as no inotrope.
A p value ≤0.05 was considered statistically significant for all tests. All analyses were performed with SAS software version 9.2 (SAS Institute, Cary, NC). The HGLMs were estimated using the GLIMMIX macro in SAS. Figures were created with R (version 2.11.1).19
RESULTS
Hospital and Patient Characteristics
The final study population consisted of 126,564 patients hospitalized with heart failure at 209 sites (from a starting population of 520,434 patients from 726 sites, Appendix 2). Median annual volume of heart failure admissions per hospital over the 7-year enrollment period was 268 (IQR 94–692; range 25–5995), median hospital size was 240 beds (IQR 121–369), 20% were located in a rural area, 35% were teaching hospitals, and 5.3% performed heart transplants. Summarized at the hospital level, median hospital patient age was 77 years (IQR of hospital median ages 73–80 years); median hospital gender was 51.0% women (IQR 46.2–56.2%); median hospital race was 82.5% white (IQR 59.4–92.7%) and 6.3% black (IQR 0.9–20.8%); median hospital payer was private insurance 20.7% of patients (IQR 9.7–38.2%), Medicaid 6.0% (IQR 2.7–13.3%), Medicare 55.0% (IQR 29.5–75.1%), and no insurance 1.8% (IQR 0.5–4.2%); median LVEF 44% (IQR 37%–46%), and comorbidities were common (Appendix 2). At the hospital level, median length of stay was 4 days (hospital median IQR 4–5 days) and median in-hospital mortality was 3.1% (hospital median IQR 1.6–4.3%).
Observed and Risk-Standardized Hospital Use of IV Inotropes
In total, IV inotropes were administered in 7,691 (6.1%) of heart failure hospitalizations. Inotrope use was divided between dobutamine alone in 43% of cases, dopamine alone in 24% of cases, milrinone alone in 17% of cases, and a combination of these agents in 16%.
Among individual hospitals, the observed rate of IV inotrope use ranged from 0–34.4% (median 3.9%, IQR 1.8–7.2%; Table 1). After adjustment for patient-level factors (listed in Table 2) and hospital-level factors (listed in Table 3), variation was observed in institutional risk-standardized rates of IV inotrope use (median rate 5.9%, IQR 3.7–8.6%, range 1.3–32.0%; Table 1, Figure 1).
Table 1.
Hospital-level inotrope use rates, stratified by quartiles of risk-standardized hospital rates of inotrope use.
| Variable | Overall | Q1 | Q2 | Q3 | Q4 |
|---|---|---|---|---|---|
| Low Use Hospitals | High Use Hospitals | ||||
| N=52 | N=52 | N=53 | N=52 | ||
|
| |||||
| Inotrope use, OBSERVED, at the hospital level | |||||
|
| |||||
| Overall: | |||||
| median | 3.9% | 1.0 % | 2.7% | 4.7% | 10.4% |
| IQR | 1.8–7.2% | 0.0–1.9% | 1.6–4.0% | 3.2–6.9% | 7.1–15.4% |
| Range | 0.0–34.4% | 0.0–8.4% | 0.0–7.5% | 1.7–22.1% | 3.6–34.4% |
|
| |||||
| Dopamine: | |||||
| median | 1.5% | 0.6% | 1.2% | 2.2% | 2.9% |
| IQR | 0.6–2.7% | 0.0–1.1% | 0.1–1.8% | 1.1–3.6% | 1.9–4.8% |
| Range | 0.0–27.9% | 0.0–2.4% | 0.0–3.9% | 0.0–7.4% | 0.0–27.9% |
|
| |||||
| Dobutamine: | |||||
| median | 1.9% | 0.4% | 1.4% | 2.4% | 5.5% |
| IQR | 0.6–4.0% | 0.0–0.8% | 0.6–2.3% | 1.0–3.8% | 3.3–10.0% |
| Range | 0.0–28.1% | 0.0–3.9% | 0.0–5.6% | 0.0–15.2% | 0.8–28.1% |
|
| |||||
| Milrinone: | |||||
| median | 0% | 0% | 0% | 0.5% | 1.0% |
| IQR | 0.0–1.0% | 0.0–0.2% | 0.0–0.5% | 0.0–1.2% | 0.0–2.8% |
| Range | 0.0–21.7% | 0.0–5.3% | 0.0–3.5% | 0.0–11.3 | 0.0–21.7% |
|
| |||||
| Inotrope use, RISK-STANDARDIZED for patient and hospital factors, at the hospital level | |||||
|
| |||||
| Overall: | |||||
| median | 5.9% | 3.1% | 4.7% | 7.0% | 11.5% |
| IQR | 3.7–8.6% | 2.3–3.5% | 4.1–5.3% | 6.6–7.8% | 9.8–18.5% |
| Range | 1.3–32.9% | 1.3–3.7% | 3.7–5.8% | 5.9–8.6% | 8.6–32.9% |
Q=quartile; IQR=interquartile range.
Table 2.
Patient characteristics summarized at the hospital level and stratified by quartiles of risk-standardized hospital rates of inotrope use.
| Patient Variable summarized at Hospital Lever | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|
| Low Use Hospitals | High Use Hospitals | ||||
| N=52 (Patients 29,336) | N=52 (Patients 31,582) | N=53 (Patients 34,551) | N=52 (Patients 31,095) | ||
| Patient Demographics | |||||
| Age, hospital median of mean years (IQR) | 77 (73–80) | 77 (73–80) | 78 (74–81) | 75 (72–78) | 0.11 |
| Female, median hospital percent (IQR) | 52.0% (48.5–58.9) | 51.8% (47.3–58.2) | 51.2% (46.2–55.2) | 48.4% (43.3–52.0)% | 0.0021 |
| Race, hospital median percent (IQR) | |||||
| White | 85.6% (59.2–95.8)% | 85.1% (61.6–93.8) | 82.5% (57.7–93.7) | 74.7% (57.8–86.6) | 0.081 |
| Black | 4.6% (1.2–30.5) | 8.5% (0.8–19.7) | 4.4% (0.9–12.0) | 8.6% (1.0–26.6) | 0.77 |
| Other | 3.6% (1.5–11.2) | 2.9% (0.9–7.0) | 5.2% (1.2–15.6) | 6.5% (1.6–14.4) | 0.15 |
| Health Insurance Status, median hospital percent (IQR) | |||||
| Medicare | 56.3% (29.7–79.6) | 53.4% (35.9–74.5) | 48.9% (20.6–67.6) | 59.3% (26.2–76.7 | 0.64 |
| Medicaid | 5.3% (1.9–11.4) | 7.0% (2.8–14.6) | 6.0% (3.0–14.0) | 5.4% (3.3–12.6) | 0.89 |
| Private/other | 21.4% (8.1–41.1) | 19.1% (8.3–38.9) | 20.8% (12.0–32.4) | 20.8% (10.3–35.7) | 0.98 |
| None or undetermined | 2.1% (0.5–4.2) | 1.7% (0.3–4.6) | 1.8% (0.5–3.6) | 1.9% (0.8–5.1) | 0.82 |
| Cardiac History, median hospital percent (IQR) | |||||
| Heart failure, prior diagnosis | 55.5% (32.3–66.2) | 62.2% (43.4–73.7) | 58.9% (36.5–70.3) | 63.7% (45.9–74.2) | 0.34 |
| Ischemic heart disease | 52.8% (45.4–58.0) | 55.0% (47.5–66.5) | 55.4% (50.4–60.9) | 54.6% (47.5–59.6) | 0.47 |
| Valve disease | 5.4% (3.2–15.0) | 10.2% (4.0–17.0) | 12.4% (6.7–21.4) | 12.3% (4.7–19.5) | 0.018 |
| ICD or CRT-D | 7.4% (4.6–14.2) | 9.3% (5.5–12.6) | 10.6% (5.2–14.4) | 11.1% (7.3–17.9) | 0.087 |
| Atrial fibrillation or flutter | 28.3% (22.6–39.2) | 35.5% (24.8–42.2) | 36.1% (27.5–41.2) | 33.2% (23.2–42.9) | 0.31 |
| Beta-blocker at admission | 38.8% (0.0–57.6) | 49.6% (11.0–66.3) | 49.0% (20.8–66.0) | 56.6% (10.8–67.6) | 0.34 |
| Medical History, median hospital percent (IQR) | |||||
| Diabetes | 41.5% (36.9–47.9) | 43.0% (37.3–47.7) | 40.7% (38.1–45.4) | 41.0% (34.5–45.2) | 0.81 |
| Hypertension | 71.7% (66.9–77.7) | 76.1% (69.7–81.5) | 76.8% (65.4–81.5) | 72.0% (63.4–78.6) | 0.084 |
| Hyperlipidemia | 36.5% (27.4–44.4) | 43.2% (33.5–52.7) | 41.7% (34.6–49.1) | 38.8% (25.3–47.4) | 0.065 |
| Peripheral vascular disease | 8.7% (4.3–12.9) | 13.0% (7.9–18.2) | 12.1% (7.7–17.2) | 9.1% (5.2–13.2) | 0.0059 |
| CVA/TIA | 13.4% (9.7–16.2) | 15.3% (10.6–19.4) | 13.8% (10.7–16.2) | 12.2% (7.8–15.3) | 0.054 |
| Renal insufficiency, no dialysis | 16.3% (12.4–21.0) | 16.5% (12.5–22.3) | 19.1% (15.6–23.0) | 16.3% (11.4–22.5) | 0.33 |
| Renal insufficiency, dialysis | 2.3% (0.3–4.0) | 1.8% (0.0–4.2) | 2.2% (0.0–4.4) | 2.2% (1.1–3.5) | 0.94 |
| Anemia | 15.7% (7.5–23.2) | 21.0% (13.4–28.0) | 16.1% (11.1–24.1) | 16.2% (10.1–21.8) | 0.071 |
| COPD or asthma | 28.3% (23.7–32.4) | 32.2% (25.8–36.3) | 29.2% (22.4–36.6) | 29.4% (22.1–24.9) | 0.25 |
| Smoking | 14.6% (11.4–19.7) | 16.8% (107–19.9) | 12.3% (7.7–17.7) | 16.7% (10.5–18.7) | 0.062 |
| Depression | 7.3% (4.3–13.7) | 10.7% (6.5–15.8) | 9.1% (4.9–12.3) | 7.8% (3.9–11.3) | 0.042 |
| Measurements Closest to Admission, median hospital median (IQR) | |||||
| Systolic blood pressure, mmHg | 138 (135–142) | 139 (135–142) | 139 (134–140) | 138 (133–140) | 0.59 |
| Heart rate, bpm | 83 (80–85) | 82 (80–85) | 82 (80–84) | 82 (80–85) | 0.83 |
| Body mass index, kg/m2 | 28.3 (24.0–34.3) | 28.3 (24.0–34.0) | 28.2 (24.0–34.0) | 28.3 (24.2–34.3) | 0.024 |
| Left ventricular ejection fraction | 0.45 (0.36–0.50) | 0.45 (0.40–0.45) | 0.45 (0.38–0.47) | 0.40 (0.35–0.45) | 0.80 |
| Serum sodium, mmol/L | 138 (138–139) | 138 (137–139) | 138 (138–139) | 138 (137–139) | 0.26 |
| Serum creatinine, mg/dL | 1.3 (1.2–1.4) | 1.3 (1.3–1.4) | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) | 0.99 |
| Blood urea nitrogen, mg/dL | 25 (23–26) | 25 (23–26) | 26 (24–28) | 26 (23–27) | 0.0058 |
| Hemoglobin, g/dL | 11.9 (11.7–12.2) | 11.8 (11.6–12.3) | 11.9 (11.7–12.1) | 12.0 (11.6–12.3) | 0.89 |
Q=quartile; IQR=interquartile range; ICD=implantable cardioverter-defibrillator; CRT-D=cardiac resynchronization therapy with implantable cardioverter-defibrillator; CVA=cerebrovascular accident; TIA=transient ischemic attack; COPD=chronic obstructive pulmonary disease; mmHg=millimeters mercury; bpm=beats per minute; BNP=b-type natriuretic peptide.
P-values calculated by comparing only non-missing values. All tests treat the column variable as ordinal.
Missing data: body mass index 18.9%; left ventricular ejection fraction 4.1%; sodium 15.2%; Cr 13.6%; BUN 15.0%; HgB 16.2%; BNP 31.8%.
Table 3.
Hospital characteristics stratified by quartiles of risk-standardized hospital rates of inotrope use.
| Hospital Variable | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|
| Low Use Hospitals | High Use Hospitals | ||||
| N=52 | N=52 | N=53 | N=52 | ||
| Number of heart failure hospitalizations 2005–2011, median (IQR) | 255 (97–634) | 195 (77–606) | 269 (72–788) | 344 (116–732) | 0.83 |
| Hospital size, inpatient beds, median (IQR) | 219 (128–369) | 227 (61–83) | 258 (107–326) | 286 (174–359) | 0.31 |
| Region | 0.88 | ||||
| West | 15.4% | 7.7% | 13.2% | 15.4% | |
| South | 44.2% | 34.6% | 37.7% | 42.3% | |
| Midwest | 22.1% | 30.8% | 24.5% | 23.1% | |
| Northeast | 19.2% | 26.9% | 24.5% | 19.2% | |
| Rural location | 15.4% | 23.1% | 28.3% | 13.5% | 0.21 |
| Teaching hospital | 36.5% | 38.5% | 24.5% | 42.3% | 0.26 |
| Heart transplants performed | 7.7% | 1.9% | 5.7% | 5.8% | 0.61 |
Q=quartile; IQR=interquartile range.
All tests treat the column variable as ordinal.
Figure 1.
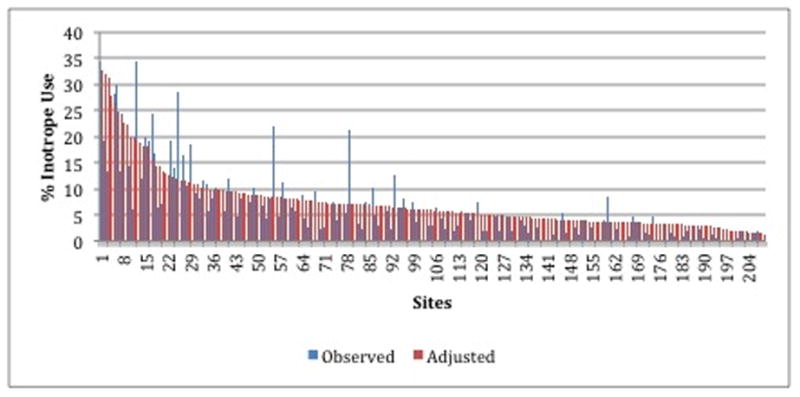
Hospital rates of inotrope use among patients hospitalized with heart failure, with observed (blue) and risk-standardized (red) rates paired for each hospital site. Sites ordered from highest (left) to lowest (right) by hospital risk-standardized rates.
Overall patterns of inotrope use changed relatively little in absolute terms over the 7-year study period (Figure 2, Panel A). Among the 24 hospitals participating continuously from 2005–2011, the risk-standardized hospital median percent of IV inotrope use ranged from 2.0–3.3%, with stable use over time. Dobutamine was the most commonly used agent with stable use over time, whereas dopamine use trended downward and milrinone use trended upward (p for annual trend <0.0001; Figure 2, Panel B).
Figure 2.
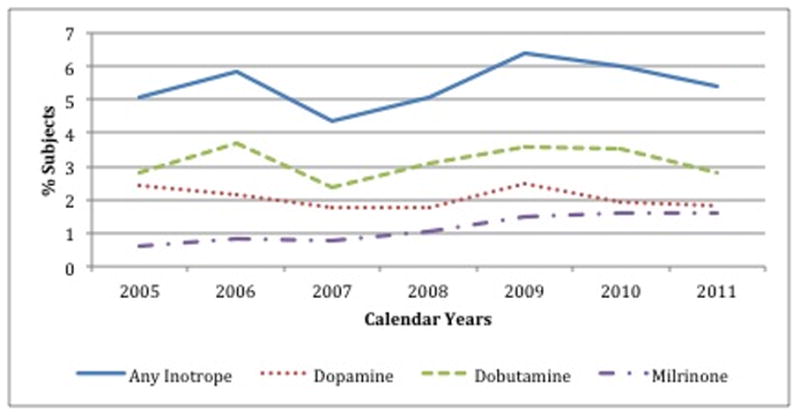
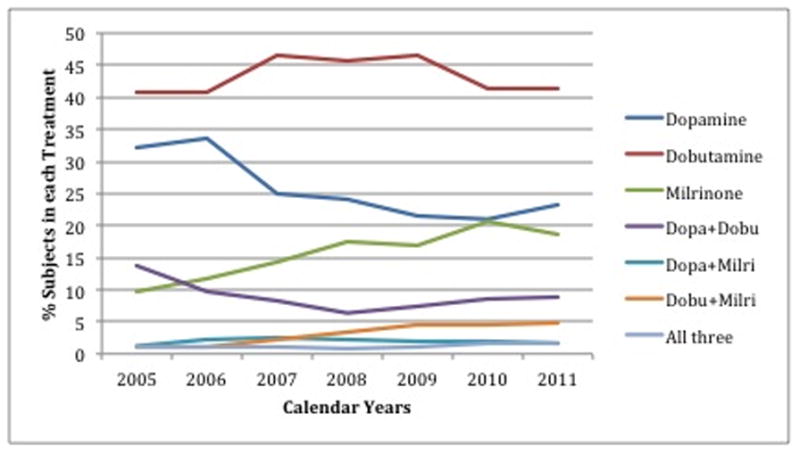
Temporal trends in use of intravenous inotropes. Panel A shows the hospital-level mean percent of patients hospitalized with heart failure treated with any inotrope (solid blue line), and treated with individual agents (dashed lines), in each of the years studied. Panel B shows a detailed breakdown of the agent(s) used among patients treated with an inotrope.
Patient Characteristics and the Use of Inotropic Agents
We assessed the observed (unadjusted) relationship between patient characteristics and IV inotrope use, showing that patients treated with inotropes were more likely to be male, have prior heart failure, have ischemic etiology, have renal dysfunction, and have lower systolic pressure but notably similar diastolic pressure (difference isolated to pulse pressure) (Appendix 3). Of note, although patients receiving IV inotropes had lower median LVEF, more than a quarter of inotrope-treated patients had a LVEF >35% (Appendix 3). Likewise, although patients receiving IV inotropic agents had lower admission systolic blood pressures, three quarters had an admission SBP >100 mm Hg (noting that blood pressures throughout the course of hospitalization were not available).
In a model accounting for hospital clustering and adjusting for patient-level factors only, the patient factors that were most strongly associated with IV inotrope use were lower systolic blood pressure, followed by lower LVEF, elevated serum creatinine, hyponatremia, advancing age, and elevated blood urea nitrogen (Table 4). The c-statistic for the patient-level model of inotrope use was 0.84.
Table 4.
Predictors of inotrope use as determined by hierarchical generalized linear regression.
| Variable | Model Fixed Effects Patient + Hospital Characteristics C Index=0.8428 |
Model Fixed Effects ONLY Patient Characteristics C Index=0.8427 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR | Lower 95% CI | Upper 95% CI | T-Value | P-value | Global Test CHi-Sq | P-Value Chi-Sq | Adjusted OR | Lower 95% CI | Upper 95% CI | T-Value | P-value | Global Test Hi-Sq | P-Value Chi-Sq | |
| Age, per 5 year | 0.92 | 0.91 | 0.93 | −14.18 | <.0001 | 201.13 | <.0001 | 0.92 | 0.91 | 0.93 | −14.29 | <.0001 | 204.27 | <.0001 |
| Female | 1.04 | 0.98 | 1.10 | 1.27 | 0.2057 | 1.60 | 0.2057 | 1.04 | 0.98 | 1.10 | 1.26 | 0.2071 | 1.59 | 0.2071 |
| Race: Other | 0.82 | 0.74 | 0.91 | −3.75 | 0.0002 | 20.25 | <.0001 | 0.82 | 0.74 | 0.91 | −3.66 | 0.0003 | 19.08. | <.0001 |
| Race: Black | 0.88 | 0.81 | 0.95 | −3.21 | 0.0013 | 0.88 | 0.81 | 0.95 | −3.09 | 0.0020 | ||||
| Race: White | 1.00 | Reference | . | . | 1.00 | Reference | . | |||||||
| No Insurance | 0.80 | 0.70 | 0.92 | −3.12 | 0.0018 | 13.45 | 0.0037 | 0.80 | 0.70 | 0.92 | −3.13 | 0.0017 | 13.33 | 0.0040 |
| Medicare | 1.01 | 0.94 | 1.08 | 0.19 | 0.8455 | 1.00 | 0.94 | 1.07 | 0.12 | 0.9055 | ||||
| Medicaid | 1.06 | 0.96 | 1.16 | 1.12 | 0.2632 | 1.05 | 0.96 | 1.16 | 1.07 | 0.2843 | ||||
| Private Insurance | 1.00 | Reference | . | . | 1.00 | Reference | . | |||||||
| MHX:Ischemic Heart Disease | 1.03 | 0.97 | 1.09 | 1.06 | 0.2906 | 1.12 | 0.2906 | 1.03 | 0.97 | 1.09 | 1.08 | 0.2801 | 1.17 | 0.2801 |
| MHX:Atrial fibrillation or flutter | 1.06 | 1.00 | 1.12 | 2.04 | 0.0416 | 4.15 | 0.0416 | 1.06 | 1.00 | 1.12 | 2.03 | 0.0428 | 4.10 | 0.0428 |
| MHX:Valve Disease | 1.42 | 1.33 | 1.52 | 10.20 | <.0001 | 104.11 | <.0001 | 1.42 | 1.33 | 1.52 | 10.20 | <.0001 | 104.13 | <.0001 |
| MHX Diabetes | 0.98 | 0.93 | 1.04 | −0.68 | 0.4949 | 0.47 | 0.4949 | 0.98 | 0.93 | 1.04 | −0.67 | 0.5013 | 0.45 | 0.5013 |
| MHX Hypertension | 0.86 | 0.81 | 0.92 | −4.86 | <.0001 | 23.58 | <.0001 | 0.86 | 0.81 | 0.92 | −4.84 | <.0001 | 23.38 | <.0001 |
| MHX Dislipidemia | 1.03 | 0.98 | 1.09 | 1.14 | 0.2523 | 1.31 | 0.2523 | 1.03 | 0.98 | 1.09 | 1.15 | 0.2511 | 1.32 | 0.2511 |
| MHX Peripheral vascular disease | 1.14 | 1.05 | 1.23 | 3.23 | 0.0012 | 10.45 | 0.0012 | 1.14 | 1.05 | 1.23 | 3.23 | 0.0013 | 10.41 | 0.0013 |
| MHX Renal insufficiency, dialysis | 0.54 | 0.44 | 0.66 | −5.99 | <.0001 | 39.70 | <.0001 | 0.54 | 0.44 | 0.66 | −5.99 | <.0001 | 39.62 | <.0001 |
| MHX Renal insufficiency, no dialysis | 1.02 | 0.95 | 1.10 | 0.56 | 0.5724 | 1.02 | 0.95 | 1.10 | 0.57 | 0.5718 | ||||
| MHX Anemia | 0.97 | 0.90 | 1.04 | −0.81 | 0.4172 | 0.66 | 0.4172 | 0.97 | 0.90 | 1.04 | −0.89 | 0.3748 | 0.79 | 0.3748 |
| MHX COPD | 1.00 | 0.94 | 1.06 | −0.04 | 0.9663 | 0.00 | 0.9663 | 1.00 | 0.94 | 1.06 | −0.05 | 0.9574 | 0.00 | 0.9574 |
| MHX Smoking | 0.87 | 0.81 | 0.94 | −3.60 | 0.0003 | 12.97 | 0.0003 | 0.87 | 0.81 | 0.94 | −3.61 | 0.0003 | 13.04 | 0.0003 |
| MHX Depression | 1.03 | 0.95 | 1.12 | 0.67 | 0.5007 | 0.45 | 0.5007 | 1.03 | 0.94 | 1.12 | 0.63 | 0.5262 | 0.40 | 0.5262 |
| SBP, per 5 mmHg | 0.90 | 0.90 | 0.91 | −37.47 | <.0001 | 1404.16 | <.0001 | 0.90 | 0.90 | 0.91 | −37.49 | <.0001 | 1405.53 | <.0001 |
| HR,<=75 bpm | 1.00 | 0.99 | 1.00 | −1.15 | 0.2482 | 1.33 | 0.2482 | 1.00 | 0.99 | 1.00 | −1.16 | 0.2478 | 1.34 | 0.2478 |
| HR,75–105 bpm | 1.01 | 1.01 | 1.01 | 7.11 | <.0001 | 50.58 | <.0001 | 1.01 | 1.01 | 1.01 | 7.10 | <.0001 | 50.35 | <.0001 |
| HR,>=105 bpm | 1.00 | 1.00 | 1.00 | 0.53 | 0.5990 | 0.28 | 0.5990 | 1.00 | 1.00 | 1.00 | 0.52 | 0.6013 | 0.27 | 0.6013 |
| LVEF, per 5% decrease from 60% | 1.18 | 1.17 | 1.19 | 33.70 | <.0001 | 1135.45 | <.0001 | 1.18 | 1.17 | 1.19 | 33.73 | <.0001 | 1137.75 | <.0001 |
| Serum sodium, >140 mmol/L | 1.01 | 0.99 | 1.03 | 0.97 | 0.3311 | 0.94 | 0.3311 | 1.01 | 0.99 | 1.03 | 0.99 | 0.3221 | 0.98 | 0.3221 |
| Serum sodium, <=140 mmol/L | 0.95 | 0.94 | 0.95 | −15.87 | <.0001 | 251.76 | <.0001 | 0.95 | 0.94 | 0.95 | −15.89 | <.0001 | 252.43 | <.0001 |
| Blood urea nitrogen, per mg/dL increase | 1.01 | 1.01 | 1.01 | 13.99 | <.0001 | 195.66 | <.0001 | 1.01 | 1.01 | 1.01 | 13.99 | <.0001 | 195.83 | <.0001 |
| Serum creatinine, > 2.5 mg/dL | 0.84 | 0.80 | 0.87 | −8.72 | <.0001 | 76.06 | <.0001 | 0.84 | 0.81 | 0.87 | −8.72 | <.0001 | 75.98 | <.0001 |
| Serum creatinine, <=2.5 mg/dL | 1.83 | 1.70 | 1.97 | 16.41 | <.0001 | 269.30 | <.0001 | 1.83 | 1.70 | 1.97 | 16.41 | <.0001 | 269.19 | <.0001 |
| HgB, per g/dL increase up to 11 | 1.04 | 1.00 | 1.07 | 2.05 | 0.0400 | 4.22 | 0.0400 | 1.04 | 1.00 | 1.07 | 2.01 | 0.0445 | 4.04 | 0.0445 |
| Time: per 1 yr | 0.97 | 0.96 | 0.99 | −2.73 | 0.0064 | 7.44 | 0.0064 | 0.97 | 0.96 | 0.99 | −2.67 | 0.0075 | 7.15 | 0.0075 |
| Teaching hospital | 0.84 | 0.63 | 1.14 | −1.10 | 0.2727 | 1.20 | 0.2727 | |||||||
| Hospital beds, per 200 | 1.22 | 1.06 | 1.42 | 2.72 | 0.0066 | 7.39 | 0.0066 | |||||||
| Heart transplants performed | 1.25 | 0.68 | 2.27 | 0.72 | 0.4706 | 0.52 | 0.4706 | |||||||
| Rural-(ref. urban) | 0.74 | 0.51 | 1.09 | −1.50 | 0.1335 | 2.25 | 0.1335 | |||||||
| Region West | 1.57 | 1.00 | 2.48 | 1.95 | 0.0510 | 11.11 | 0.0112 | |||||||
| Region South | 1.80 | 1.27 | 2.55 | 3.30 | 0.0010 | |||||||||
| Region Midwest | 1.57 | 1.06 | 2.32 | 2.25 | 0.0247 | |||||||||
| Region Northeast | 1.00 | Reference | . | . | ||||||||||
OR=odds ratio; LVEF=left ventricular ejection fraction; HgB=hemoglobin; HR=hart rate; bpm=beats per minute; MHX=medical history; COPD=chronic obstructive pulmonary disease; SBP=systolic blood pressure; LVEF=left ventricular ejection fraction; BUN=blood urea nitrogen.
Hospital Characteristics and the Use of Inotropic Agents
Although many of the measured hospital characteristics were crudely associated with inotrope use (Table 3), only hospital region and larger hospital size were statistically associated with inotrope use in multivariable HGLM; hospital rural location, academic status, and capacity to perform heart transplantation had no significant association with IV inotrope use after accounting for other patient and hospital factors (Table 4). The addition of hospital-level factors (structural characteristics) to patient-level factors in the HGLM did not improve model discrimination (patient model only c-statistic 0.84; patient and hospital model c-statistic 0.84).
Variation Attributable to Hospital Preferences
The intra-class correlation was 0.21 after adding a hospital random effect to patient characteristics and hospital structure fixed effects in HGLM models. This suggests that 21% of the observed variation in inotrope use may be explained by individual hospital preferences rather than patient case-mix and hospital structural differences.
Association between Outcomes and Use of IV Inotropic Agents
When comparing hospitals across quartiles of risk-standardized inotrope use, risk-standardized in-hospital mortality rates were not different between high- and low-use hospitals (Table 5). Length of stay greater than 4 days was statistically more common among high-use hospitals, although absolute differences were small after risk-standardization. Process performance measures were not significantly different between hospitals by inotrope use rates (Table 5).
Table 5.
Outcomes, stratified by quartiles of risk-standardized hospital rates of inotrope use.
| Variable | Overall | Q1 Low Use Hospitals N=52 |
Q2 N=52 |
Q3 N=53 |
Q4 High Use Hospitals N=52 |
P value |
|---|---|---|---|---|---|---|
| In-hospital mortality, % patients by hospital, median (IQR) | ||||||
| Observed | 3.1 (1.6–4.3) | 2.4 (0.8–3.7) | 3.3 (1.7–4.2) | 3.3 (1.9–5.3) | 3.0 (1.7–4.3) | 0.12 |
| Adjusted | 3.1 (2.5–3.8) | 2.9 (2.3–3.5) | 3.2 (2.6–3.7) | 3.2 (2.6–4.1) | 3.5 (2.6–4.1) | 0.13 |
| Length of stay, > 4 days, % patients by hospital, median (IQR) | ||||||
| Observed | 43.3 (35.3–50.7) | 39.5 (33.9–48.7) | 41.8 (34.0–48.1) | 43.6 (33.7–48.3) | 46.5 (40.8–56.5) | 0.0040 |
| Adjusted | 45.4 (39.6–51.4) | 42.7 (38.1–47.6) | 42.4 (39.0–48.4) | 43.6 (39.9–51.8) | 49.9 (47.1–55.2) | <.0001 |
| Performance Measures, % of eligible | ||||||
| Discharged home, among patients eligible, % by hospital, median (IQR) | 89.6 (75.4–95.5) | 87.0 (67.0–95.4) | 90.0 (77.9–96.6) | 88.5 (73.0–95.3) | 91.0 (81.4–95.5) | 0.51 |
| Documentation of LVEF, % by hospital, median (IQR) | 97.7 (93.9–99.7) | 97.5 (93.2–99.6) | 97.6 (94.3–99.8) | 97.5 (92.7–99.5) | 98.6 (95.7–99.8) | 0.50 |
| Discharged on ACEI/ARB, among patients with LVSD and no contraindication, % by hospital, median (IQR) | 90.0 (77.7–95.0) | 89.3 (78.2–96.1) | 89.4 (76.9–94.3) | 89.4 (76.0–94.5) | 91.2 (80.9–95.3) | 0.86 |
| Discharged on beta-blocker, among patients with LVSD and no contraindication, % by hospital, median (IQR) | 94.5 (87.0–97.8) | 93.4 (85.6–97.9) | 94.7 (89.4–96.9) | 95.2 (88.0–98.0) | 94.0 (89.3–97.2) | 0.92 |
| Smoking cessation counseling, among patients with smoking history, % by hospital, median (IQR) | 97.3 (85.7–100) | 95.3 (80.3–100) | 96.6 (85.5–100) | 97.1 (83.3–100) | 99.0 (88.8–100) | 0.30 |
| Composite for 100% compliance, % by hospital, median (IQR) | 82.7 (64.9–90.8) | 78.5 (56.5–90.0) | 84.2 (64.1–89.8) | 79.6 (64.5–91.0) | 84.8 (67.6–91.5) | 0.59 |
Q=quartile; IQR=interquartile range; LVEF=left ventricular ejection fraction; ACEI=angiotensin converting enzyme inhibitor; ARB=angiotensin receptor blocker; LVSD=left ventricular systolic dysfunction.
All tests treat the column variable as ordinal.
Adding insurance status and race to the adjustment process made no significant change on any of the adjusted results.
Secondary Analysis Excluding Dopamine
When the 1,865 hospitalizations involving dopamine only were redefined as no inotrope, observed median institutional rates of IV inotrope use was 2.3% (IQR 0.7–5.3%, range 0–28.1%) and risk-standardized median rate 4.2% (IQR 2.8–6.8%, range 1.0–40.5%). This change in definition of inotrope use caused 76 hospitals (36%) to reclassify to a different quartile of risk-standardize inotrope use rate, although only 2 hospitals changed more than 1 quartile. With dopamine excluded, model C index was 0.87 (for both the patient only and patient plus hospital factor models) and the intra-class correlation was 0.26. With exclusion of dopamine form the inotrope definition, hospitals in the higher quartiles of risk-standardize inotrope use continued to show a higher adjusted length of stay (p=0.29) but no association with adjusted in-hospital mortality (p=0.86).
DISCUSSION
In this large clinical registry of a diverse group of hospitals in the United States, we found marked variation in the rates of use of these agents between hospitals. Using state of the art methods to adjust for patient case mix and hospital structural differences, we found that a substantial portion of variation is attributable to practice patterns at individual hospitals. It is increasingly appreciated that hospital culture can exert a strong influence on discretionary therapies. Although existing data do not clearly define ideal rates of IV inotrope use for patients hospitalized with heart failure, these agents can have negative consequences. The manifold variation in use even after risk standardization highlights the need for additional research into the optimal use of the agents.
Our findings are consistent with and extend an analysis that was recently conducted with billing data.12 In that cross-sectional study of 376 hospitals participating in Perspective, a fee-supported database developed by Premier, Inc., the risk-standardized rates of inotrope use ranged across hospitals from 0.9% to 44.6% (median 6.3%, IQR 4.3–9.2%). When adjusting for patient case mix and an individual hospital effect, model c-statistics ranged from 0.77 to 0.88 and intraclass correlation coefficient was 0.113. In that study, hospital rates or patterns of use were not associated with differences in length of stay or risk-standardized mortality rates.
The GWTG-HF registry data include detailed clinical data not present in administrative billing data—in particular LVEF, vital signs and laboratory test results—that guidelines recommend as the basis for deciding when to initiate IV inotropic support (“patients presenting with severe systolic dysfunction, low blood pressure and evidence of low cardiac output”).1 Despite the addition of this clinical information in the current analysis, we found relatively similar variation in risk-standardized hospital rates of inotrope use and a greater degree to which hospital treatment preferences were responsible for these differences (11% versus 21%). Our study is also consistent with data from the ESCAPE randomized trial which reported that the most significant predictor of inotrope use in multivariable modeling was the study site (hospital).11 The external validity of findings derived from narrow ESCAPE eligibility criteria within select academic institutions guided by trial treatment protocols had been in question. Additionally, the authors did not quantify the degree to which inotrope use varied across these 26 hospitals nor the proportion of variation that was not explained by differences in patient factors. Therefore, the GWTG-HF analysis reported here provides the most representative analysis of inotropic support in the hospitalized adult with heart failure to include detailed clinical data. Outside the scope of hospitalized adults with heart failure, few data exist looking at rates and variation in inotrope use.20
Despite evidence of marked hospital-level variation, it is difficult to determine to what extent hospitals may be over- or under-using inotropic agents. It is known that for hemodynamically stable patients, these agents can increase short and long-term risk. The newest recommendations from the ESC provide the most specific recommendation to date, reserving IV inotropes (and/or intra-aortic balloon pump) for patients with “systolic blood pressure <85 mmHg or shock” evidenced by “reduced peripheral and vital organ perfusion.”3 Registries suggest that this group, i.e. those with hypotension and shock, represents about 3% of all patients hospitalized with heart failure.4 Our findings from GWTG-HF and those from other studies suggest that many more patients are being treated with these agents, particularly among certain hospitals, and that a significant portion of this high use is attributable to institutional culture rather than medical indications.10,12
A hospital’s propensity to use inotropes was largely independent of outcomes. Risk-standardized hospital inotrope use was not associated with risk-standardized in-hospital mortality rates. A statistically significant but small absolute increase in length of stay at hospitals with higher rates of inotrope use, although may represent incomplete adjustment for case mix. Due to the nature of GWTG-HF data, we were not able to look at events after discharge.
There are several limitations to consider. First, hospitals voluntarily participating in GWTG-HF may not be representative of all hospitals in the United States, although prior study has shown that GWTG-HF hospitals have characteristics similar to hospitals nationwide. We found lower rates of overall IV inotrope use in GWTG-HF registry than in cross-sectional studies, suggesting that, if anything, the general findings here may understate the use and variation that do exist. Second, the GWTG-HF case report form only captured dichotomous use of IV inotropes, without consideration for timing, duration, or dose. Our sensitivity analysis around dopamine use suggest that institutional variation in inotrope use is not explained by low-dose dopamine. Third, physician reasoning and hemodynamic monitoring at the time of inotropic therapy initiation were not available. However, detailed clinical information, including laboratory and vital sign data, that form the foundation of guideline recommendations around inotrope use were available from the time of admission. Fourth, we included many patient and hospital factors during the process of adjustment that are not obviously used in medical decisions around inotrope use. We felt that a liberal approach to covariate inclusion in the process of rate standardization was preferable, but may over-adjust for hospital differences in inotrope use. Fifth, the analysis is at the hospital level, whereas decisions for inotrope use are typically physician driven. We did not have data on prescribing at the physician level. We would expect even greater variation if hospital rates of inotrope use were further divided by individual clinician. Sixth, GWTG collects data by site and hospitalization event, not by patient. The effect of recurrent hospitalizations for individual patients are not specifically accounted for in the analysis. Finally, only the 3 listed inotropes were included, without data on other inotropic agents such as norepinephrine and epinephrine. Although we are not able to provide information on use of these other inotropes, guidelines continue to favor dobutamine, dopamine, and milrinone in their recommendations.1–3
Conclusion
Among U.S. hospitals participating in GWTG-HF, marked differences were observed in the rates of inotrope use for the treatment of patients hospitalized with heart failure. Significant variation persisted even after accounting for measured differences in case mix and hospital type, suggesting that additional data are needed to clarify optimal indications for inotrope use. This study heralds an urgent need for randomized clinical trials to define the proper role of inotropic agents in this high-risk patient population.
Supplementary Material
Acknowledgments
Funding Sources: Get With The Guidelines–Heart Failure is a program of the American Heart Association and is also supported by unrestricted educational grants from GlaxoSmithKline and Medtronic. Dr. Allen is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL105896. Dr. Krumholz is supported by grant U01 HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Pamela Peterson is supported by grant K08 HS019814-01 from the Agency for Healthcare Research and Quality.
Footnotes
Conflict of Interest Disclosures: Dr. Allen reports receiving research grants from the National Institutes of Health and the American Heart Association; and serving as a consultant for Amgen, Inc, Johnson & Johnson, and Novartis. Dr. Fonarow reports receiving research grants or other research support from National Institutes of Health, and the Agency for Healthcare Research and Quality; consulting for Bayer, Gambro, Novartis, Medtronic, and Johnson & Johnson; Dr. Fonarow holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and is also supported by the Ahmanson Foundation (Los Angeles, California). Dr. Hernandez reported receiving research support from Johnson and Johnson, Medtronic, and Merck and Co.; serving on the speakers’ bureau for Novartis; and receiving honoraria from AstraZeneca and Medtronic. Dr. Heidenreich reported no conflicts. Dr. Bhatt reports the following relationships - Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology); Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), WebMD (CME steering committees); Data Monitoring Committees: Duke Clinical Research Institute; Harvard Clinical Research Institute; Mayo Clinic; Population Health Research Institute; Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda. Dr. Eric Peterson reported receiving research support from Bristol-Myers Squibb, Sanofi-Aventis, Merck and Co., and Eli Lilly; and serving as the principal investigator of the analytic center for the American Heart Association Get With the Guidelines Program. Dr. Krumholz reports that he is the recipient of a research grant from Medtronic, through Yale University, to develop methods of clinical trial data sharing and is chair of a cardiac scientific advisory board for UnitedHealth.. Drs. Hernandez and E. Peterson have made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp).
References
- 1.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, Casey DE, Jr, McMurray JJV, Drazner MH, Mitchell JE, Fonarow GC, Peterson PN, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2013;128:e240–319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Cardiac Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, Gottlieb SO, McGrew F, DeMets DL, White BG The Vesnarinone Trial Investigators. A Dose-Dependent Increase in Mortality with Vesnarinone among Patients with Severe Heart Failure. New Engl J Med. 1998;339:1810–1816. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 6.Moran JF, Rad N, Scanlon PJ. Long term survival of class IV heart failure patients treated with oral amrinone. J Clin Pharm. 1989;29:494–499. doi: 10.1002/j.1552-4604.1989.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 7.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M for the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure I. Short-term Intravenous Milrinone for Acute Exacerbation of Chronic Heart Failure: A Randomized Controlled Trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 8.Packer M. Randomized multicenter evaluation of intravenous levosimendan efficacy versus placebo in the short-term treatment of decompensated heart failure study (REVIVE-2). Paper presented at: Proceedings of the American Heart Association Scientific Sessions; November 13–16, 2005; Dallas, TX. [Google Scholar]
- 9.Goldhaber JI, Hamilton MA. Role of inotropic agents in the treatment of heart failure. Circulation. 2010;121:1655–1660. doi: 10.1161/CIRCULATIONAHA.109.899294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, Young JB, Rayburn BK, Rogers JG, DeMarco T, Leier CV. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Partovian C, Gleim SR, Mody PS, Li SX, Wang H, Strait KM, Allen LA, Lagu TC, Normand SL, Krumholz HM. Hospital Patterns of Use of Positive Inotropic Agent in Patients With Heart Failure. J Am Coll Cardiol. 2012;60:1402–1409. doi: 10.1016/j.jacc.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Int Med. 2004;164(2):203–209. doi: 10.1001/archinte.164.2.203. [DOI] [PubMed] [Google Scholar]
- 14.Smaha LA. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148(5 Suppl):S46–48. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O’Connor CM, Yancy CW, Young J. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Krumholz HM, Wang Y, Mattera JA, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 17.Fonarow GC. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008;155:200–207. doi: 10.1016/j.ahj.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 20.Lasky T, Greenspan J, Ernst FR, Gonzalez L. Dopamine and dobutamine use in preterm or low birth weight neonates in the premier 2008 database. Clin Ther. 2011;33:2082–2088. doi: 10.1016/j.clinthera.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. New Engl J Med. 2010;362:779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


