Recent studies from the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Trans-catheter Valve Therapy (TVT) Registry have reported 30-day and 1-year mortality after transcatheter aortic valve replacement (TAVR).(1) While there has been rapid uptake of TAVR, little is known about hospital-level variation in volume and outcomes.
We studied hospital performance on TAVR using data from all Medicare fee-for-service (FFS) beneficiaries ≥65 years of age who underwent TAVR from January 1, 2011 to December 31, 2013, identified using International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes 35.05 and 35.06. For each hospital that performed at least 1 TAVR during the study period, we calculated risk-standardized 30-day mortality (30-day RSMR), 1-year mortality (1-year RSMR), and 30-day all-cause readmission (30-day RSRR) using the Centers for Medicare & Medicaid Services (CMS) risk-standardized method(2,3), which employs 2-level (patient and hospital) hierarchical logistic regression models that account for the clustering of patients within the same hospital as well as patient-specific information on age and sex and a number of comorbidities identified from secondary discharge diagnosis codes in the index hospitalization as well as principal or secondary diagnosis codes of all inpatient hospitalizations up to 1 year prior.
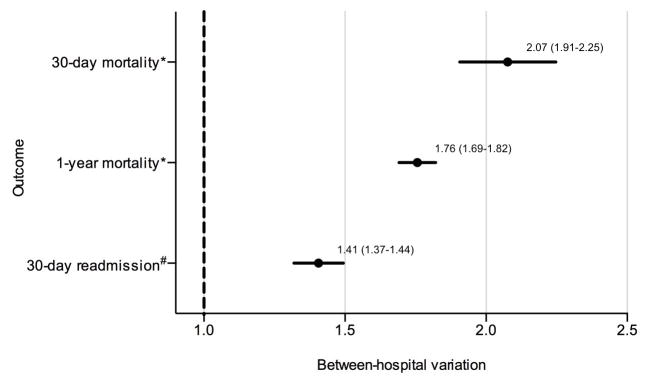
To study the effect of the variation in hospital performance on an individual patient’s outcome, we quantified the between-hospital variation by fitting a mixed model at the patient level with hospital random effects to estimate the odds ratio (OR) of death within 30 days for a Medicare FFS patient undergoing TAVR at a hospital that is 1 standard deviation (SD) above the national average 30-day mortality rate relative to undergoing TAVR at a hospital that is 1 SD below the national average, adjusting for patient characteristics from the CMS models; we made similar models for 1-year mortality and 30-day readmission. If the difference in outcome at a hospital 1 SD above is not significantly different than that at a hospital 1 SD below, then the OR estimate will cross 1.0.
During 2011–2013, 417 hospitals performed a total of 14,722 TAVR procedures for Medicare FFS beneficiaries. The median (Interquartile Range [IQR]) number of TAVRs performed per hospital during the study period was 17 (2–46). The median (IQR) 30-day RSMR was 6.0% (5.2 – 6.9) ranging from 3.8% to 10.2%, 1-year RSMR was 17.5% (16.2 – 19.1) with range 11.8% to 25.6%, and 30-day RSRR was 20.9% (20.2 – 22.1) with range 17.1% to 24.4%. The top 7 principal diagnoses for these readmissions were heart failure (4.8% of all readmissions), post-operative complications such as shock, hematoma, wound dehiscence and infection (1.4%), arrhythmias (1.1%), sepsis (0.9%), pneumonia (0.8%), gastrointestinal bleed (0.6%). and mechanical device complications (0.5%). Adjusting for patient characteristics, the odds of each adverse outcome for a patient treated at a hospital 1 SD above the national average relative to that of a patient treated at a hospital 1 SD below the national average was statistically significant (Figure 1).
Figure 1. Between-hospital variation for 30-day mortality, 1-year mortality, and 30-day readmission.
The odds (95% CI) of each outcome if a Medicare FFS patient is treated at a hospital 1 SD above the national average for that outcome relative to treatment at a hospital 1 SD below the national average is shown on the x-axis.
CI: confidence interval. FFS: fee-for-service. SD: standard deviation.
*Adjusted for age, sex, hypertension, diabetes or its complications, renal failure, acute myocardial infarction, location of myocardial infarction, chronic atherosclerosis, other acute/subacute forms of ischemic heart disease, history of coronary artery bypass graft, history of percutaneous transluminal coronary angioplasty, congestive heart failure, valvular and rheumatic heart disease, cerebrovascular disease, stroke, peripheral vascular disease, cardio-respiratory failure and shock, chronic liver disease, chronic obstructive pulmonary disease, dementia, hemiplegia/paraplegia/paralysis/functional disability, major psychiatric disorders, metastatic cancer, acute leukemia/other severe cancers, pneumonia, protein-calorie malnutrition, trauma in the last year.
#In addition to variables in the mortality model, adjusted for unstable angina, asthma, cancer, decubitus ulcer or chronic skin ulcer, depression, disorders of fluid, electrolyte, acid-base, drug/alcohol abuse/dependence/psychosis, end stage renal disease or dialysis, fibrosis of lung or other chronic lung disorders, history of infection, iron deficiency or other anemias and blood disease, biliary disease, nephritis, other gastrointestinal disorders, other or unspecified heart disease, other psychiatric disorders, other urinary tract disorders, peptic ulcer, hemorrhage, other specified gastrointestinal disorders, severe hematological disorders, arrhythmias, vascular or circulatory disease.
Since the Food and Drug Administration approval of TAVR in November 2011, there has been rapid expansion in the number of hospitals performing TAVR. Our results show marked variation in hospital performance with TAVR, with an IQR of 1.8% for 30-day RSMR. For perspective, the IQR for 30-day RSMR for isolated coronary artery bypass grafting, a commonly performed invasive cardiac procedure, is 1%.(4)
We found that for an individual patient, the between-hospital variation translates to a >2-fold higher risk of dying within 30 days for a patient undergoing TAVR at a hospital 1 SD above the national average compared with undergoing TAVR at a hospital 1 SD below. The between-hospital variation was lower for 1-year mortality and 30-day readmission, but remained substantial. Some of this between-hospital variation can be attributed to clinical factors insufficiently captured by our adjustment model, but hospital and system factors are likely also important drivers of this variation. In addition, TAVR volume and duration of center experience were not assessed and could influence outcomes. As the importance of hospital and system factors was not investigated in this paper henceforth, the conclusions of this paper reflect the authors’ opinion.
This study serves as an important benchmark for quality measurement and future performance improvement efforts for TAVR. Moving forward, as more centers and operators begin performing TAVR, and existing centers and operators become more proficient, it will be important to continue to monitor the extent of hospital variation to ensure the delivery of optimal outcomes for patients.
Acknowledgments
Grant support: This project was supported by grant 1U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The sponsor did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
During the time the work was conducted, Dr. Murugiah and Mr. Nuti were affiliated with the Center for Outcomes Research and Evaluation, Yale-New Haven Hospital, New Haven, CT and Mr. Nuti was affiliated with the Section of Cardiovascular Medicine, Department of Internal Medicine, Yale School of Medicine, New Haven, CT, USA.
Disclosures: Dr. Krumholz is the recipient of research agreements from Medtronic and from Johnson & Johnson (Janssen), through Yale University, to develop methods of clinical trial data sharing and is chair of a cardiac scientific advisory board for UnitedHealth. The other authors report no conflicts of interest.
References
- 1.Holmes DR, Jr, Brennan JM, Rumsfeld JS, et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–28. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 2.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–92. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed 10 September 2015];Medicare Hospital Quality Chartbook 2014: Performance Report on Outcome Measures. 2014 Prepared by Yale New Haven Health Services Corporation Center for Outcomes Research and Evaluation for the Centers for Medicare and Medicaid Services. http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Medicare-Hospital-Quality-Chartbook-2014.pdf.