Abstract
Background
Young women (≤ 55 years) with acute myocardial infarction (AMI) have higher mortality risk than similarly aged men. Elevated inflammatory markers are associated with an increased risk of cardiovascular outcomes after AMI, but little is known about whether young women have higher inflammatory levels following AMI compared with young men.
Methods and Results
We assessed sex differences in post-AMI inflammatory markers and whether such differences account for sex differences in 12-month health status, using data from 2219 adults with AMI 18 to 55 years of age in the United States. Inflammatory markers including high-sensitivity C-reactive protein (hsCRP) and lipoprotein-associated phospholipase A2 (Lp-PLA2) were measured 1 month after AMI. Overall, women had higher levels of hsCRP and Lp-PLA2 following AMI compared with men, and this remained statistically significant after multivariable adjustment. Regression analyses showed that elevated 1-month hsCRP was associated with poor health status (symptom, function, and quality of life) at 12 months. However, the association between hsCRP and health status became non-significant after adjustment for socio-demographics, comorbidities, and treatment factors. Half of these patients had “residual inflammatory risk” (hsCRP > 3 mg/L) compared with a third who had “residual cholesterol risk” (Low-density lipoprotein cholesterol > 100 mg/dL).
Conclusions
Young women with AMI had higher inflammatory levels compared with young men. Elevated 1-month hsCRP was associated with poor health status at 12 months after AMI, but this was attenuated after adjustment for patient characteristics. Targeted anti-inflammatory treatments are worthy of consideration for secondary prevention in these patients if ongoing trials of anti-inflammatory therapy prove effective.
Keywords: myocardial infarction, inflammation, C-reactive protein, women
Young women (≤55 years) with acute myocardial infarction (AMI) have a significantly higher risk of mortality and morbidity following AMI compared with similarly aged men.1 While the cause of increased risk in young women is not completely understood, one hypothesis is that young women may have a higher inflammatory state after AMI. Inflammation is important in AMI prognosis: elevations of inflammatory markers such as high-sensitivity C-reactive protein (hsCRP) following AMI are associated with an increased risk of recurrent cardiovascular events.2, 3 Lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme produced by inflammatory cells, has also been shown to be associated with cardiovascular events independent of hsCRP.4 However, as inflammatory markers are not routinely measured in post-AMI care, we know little about whether young women have higher inflammatory levels following AMI compared with men and whether the potential sex differences in inflammatory markers account for the sex differences in outcomes.
Prior evidence of sex differences in inflammatory markers is mostly derived from the general population. Studies have shown that hsCRP levels are higher in women compared with men across all ethnic subgroups, even after adjusting for traditional cardiovascular risk factors.5-7 The magnitude of increase is about 30-50%.5-7 However, no studies have compared inflammatory markers after AMI in young women with those in men. Characterizing sex differences in post-AMI inflammatory markers may provide insight into sex differences in long-term outcomes and may provide a target to reduce the excess risk in young women with AMI. Further, the proportion of young women with AMI who are afflicted primarily by “residual cholesterol risk” compared with “residual inflammatory risk” is uncertain, which has important implications for the selection of appropriate secondary prevention treatment to reduce residual risk in these patients.8
We utilized data from the VIRGO study (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients)9 to assess sex differences in post-AMI inflammatory markers and whether such differences account for sex differences in health status (symptom, function, and quality of life) at 12 months after hospital discharge for AMI. We hypothesized that young women would have higher levels of inflammatory markers after AMI compared with men, and that sex differences in inflammatory markers would partially explain women's worse health status after AMI. We compared the sex differences in inflammatory markers in VIRGO with those in a nationally representative sample of the population in the United States. We also examined the proportion of VIRGO patients who have “residual cholesterol risk” compared with “residual inflammatory risk.” Findings from this study may enhance our understanding of inflammatory markers and their potential contribution to sex differences in health status among young women and men with AMI.
Methods
Participants and Study Design
This study is a pre-specified analysis of VIRGO. Details about the design of VIRGO have been described previously.9 In brief, VIRGO is a prospective observational study designed to investigate the demographic, clinical, psychosocial, biological, and behavioral factors associated with the higher mortality in young women with AMI.1 Between August 2008 and May 2012, patients 18 to 55 years of age were recruited into the VIRGO study from 103 United States (US), 24 Spanish, and 3 Australian hospitals. Of the 6538 patients screened at contributing sites, 3572 were eligible and enrolled. These consisted of 2985 from the US, and 516 patients from Spain, and 71 from Australia. Given that the inflammatory markers were measured only in participants enrolled in the US, we limited our study population to the US participants, which represent 84% of the overall VIRGO population. Participants were recruited using a 2:1 female to male enrollment design to enrich the study's inclusion of young women.
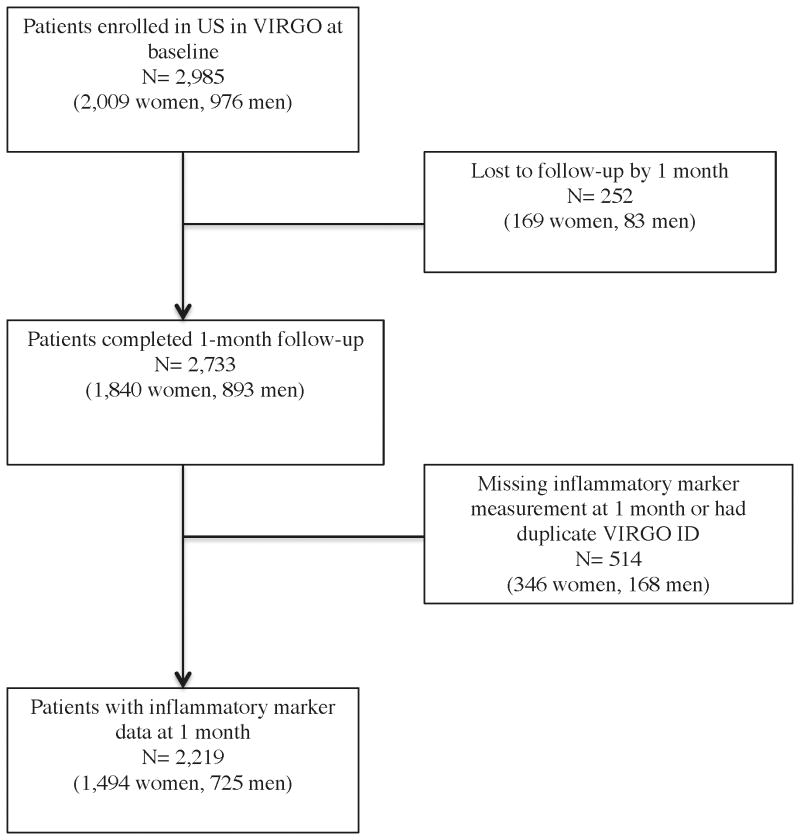
Participants were eligible for the study if they had increased cardiac biomarkers indicative of myocardial necrosis (with at least 1 cardiac biomarker above the 99th percentile of the upper reference limit) within 24 hours of admission and evidence of acute myocardial ischemia, including symptoms of ischemia, electrocardiogram changes indicative of new ischemia, or imaging evidence of infarction. We excluded individuals who met any of the following criteria: died before baseline interview, unable to provide informed consent, previous enrollment in VIRGO, neither English nor Spanish speaking, development of elevated cardiac markers due to elective coronary revascularization, or AMI caused by physical trauma.9 We also excluded participants who did not have laboratory measurements at 1 month after hospital discharge or who were lost to follow-up by 1 month (Figure 1). Institutional Review Board approval was obtained at each participating institution, and participants provided informed consent for study participation including baseline hospitalization and follow-up interviews.
Figure 1. Flowchart of sample selection for post-AMI inflammatory analysis in VIRGO.
Blood Analysis
We measured inflammatory markers by standardized assay at 1 month after hospital discharge. We delayed the measurement to allow the perturbation associated with the acute event to reside. Specifically, concentrations of CRP were determined using fixed-rate nephelometry (Quest Diagnostics, Madison, New Jersey),10 the most sensitive test available at study initiation; the technique sensitively quantifies levels that are > 0.8 mg/L. Levels below this were defined as not detectable. Lp-PLA2 mass concentration was measured by in-house enzyme-linked immunoassays. In addition, a VAP lipid profile, which included total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglyceride levels were measured by a blood draw according to standard procedures. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald equation11 if triglycerides were <400 mg/dL, and was measured directly if triglycerides were ≥400 mg/dL. All inflammatory markers and lipid profiles were measured at Quest Diagnostics, and the laboratory was certified by the National Heart, Lung, and Blood Institute and Centers for Disease Control and Prevention Lipid Standardization Program.
Data Collection and Variables
We collected baseline information on patients' socio-demographics, clinical presentation, cardiac risk factors, non-cardiac comorbidities, and treatment from medical chart abstraction and standardized in-person interviews administered by trained personnel during the index AMI admission. Follow-up telephone interviews at 1 and 12 months were conducted by the Yale Follow-Up Center and in-person blood tests were collected at 1 month.
At 12 months after hospital discharge, we measured angina-specific and overall health status (symptoms, function, and quality of life) for each patient. Angina frequency, angina-related physical limitations, and angina-related quality of life were assessed with the Seattle Angina Questionnaire.12 General health status was measured with the 12-item Short-Form Health Survey (SF-12) physical component summary (PCS) and mental component summary (MCS) scores.13 Health-related quality of life was assessed by the EuroQol (EQ-5D) utility index and visual analog scale.14 The EQ-5D utility index generates scores between 0 and 1, whereas all other scales are scored between 0 and 100. On all measures, a higher value indicated better symptoms, function, or quality of life.
We quantified patients' socioeconomic status by defining marital status, highest level of education, health insurance, employment status, and financial hardship (not having enough money to make ends meet or having just enough money to make ends meet versus having some money left over at the end of the month). We assessed the clinical severity of AMI presentations by final AMI diagnosis (ST-elevation AMI [STEMI]), Global Registry of Acute Coronary Events (GRACE) risk score,15 left ventricular ejection fraction <40%, hemodynamic instability, and present >6 h after symptom onset. We included other cardiac risk factors and comorbidities, such as history of heart disease, diagnosis of hypertension, diabetes, and dyslipidemia extracted from the medical record, obesity (body mass index ≥30 kg/m2), prospectively measured waist circumference (high classified as >88 cm for women, >102 cm for men), smoking, physical activity, and depression. Self-reported physical activity was measured with the Behavioral Risk Factor Surveillance Survey physical activity instrument, which has high reliability and validity among young adults.16 We also assessed reperfusion treatment during hospitalization and use of statin, estrogen, aspirin, beta-blockers, and angiotensin converting enzyme (ACE) inhibitors/ Angiotensin II Receptor Blockers (ARBs) at hospital discharge. High-intensity statins were defined as statins dosed at a level expected to lower LDL-C by at least 50% according to the American College of Cardiology (ACC)/American Heart Association (AHA) recommendation.17
Statistical Analyses
Descriptive statistics of patient characteristics at baseline and 1 month were calculated for the overall study population and compared between men and women. We calculated frequencies for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. We determined statistical differences between women and men using chi-squared, Student's t and Wilcoxon Rank Sum tests, where appropriate.
To describe inflammatory markers by sex, we plotted the distributions of inflammatory markers at 1 month following AMI for men and women separately. We reported the median levels with IQRs of inflammatory markers and also categorized them into clinically meaningful and interpretable groups. HsCRP was categorized into < 1, 1 to 3, 3 to 5, and ≥ 5 mg per liter based on the Centers for Disease Control and Prevention/AHA guideline.18 We compared the distributions of inflammatory markers in VIRGO with those in the National Health and Nutrition Examination Survey (NHANES) 2011-2012. NHANES uses a multi-stage, stratified, clustered probability sampling design and provided a representative sample of the non-institutionalized population in the United States.19 We included individuals aged 40-55 years (median age of 47) in order to have an age distribution similar to that of VIRGO. We accounted for the complex survey design in NHANES to make the estimates representative of the corresponding sex group in the national population. We also used Spearman correlation coefficients to evaluate and compare the relationships between inflammatory marker levels and metabolic risk factor levels in men and women.
We conducted bivariate analyses and multivariable regression analyses to identify factors that differed between men and women and might explain the sex differences in inflammatory markers. We considered continuous hsCRP, Lp-PLA2 mass, and elevated hsCRP (1 to 3, ≥3.0 mg/L versus <1 mg/L) as the dependent variables and developed separate models for each. We transformed continuous hsCRP and Lp-PLA2 to the log scale because their distributions were not normal. For categorical hsCRP, we used proportional odds models to assess different categories of hsCRP elevation. We included patient sex, age, race, current smoking, hypertension, diabetes, body mass index, statin use at discharge, and LDL-C at 1 month as covariates in the models, as these variables were previously known to be associated with elevated inflammatory markers.2, 3
We also conducted multivariable linear regression analyses to assess the relationship between sex, 1-month hsCRP, and 12-month health status. Health status scores (angina frequency, angina-related physical limitation, angina-related quality of life, SF-12 PCS score, SF-12 MCS scores, EQ-5D utility index, and EQ-5D visual analog scale) served as dependent variables in these models. Explanatory variables included patient sex, 1-month hsCRP, 1-month health status score, and other covariates such as age, race, education, current smoking, hypertension, diabetes, body mass index, statin use at discharge, and LDL-C at 1 month. For each dependent variable, 3 models were developed: model 1 included sex only, model 2 included sex and hsCRP, and model 3 included sex, hsCRP and all other covariates. We compared models with and without adjustment for 1-month hsCRP to assess whether controlling for hsCRP reduced estimated sex differences in health status. We calculated the percentage of reduction in the gender effect after adjusting for hsCRP and after additionally adjusting for other covariates. In a secondary analysis, we repeated the analysis among patients with type I AMI classified by the Third Universal Definition of MI because these patients were in relatively severe condition and expected to have high inflammatory risk.20 We also conducted sensitivity analysis in STEMI and NSTEMI patients separately to assess whether the results change by AMI type. Missing covariate data were minimal in our analysis, with 14.7% of patients missing any covariate data. Missing covariates were imputed using a multiple imputation approach in R 3.10 (The R Foundation for Statistical Computing), which allowed incorporation of all patients into multivariable models.
Finally, in this post-AMI population, we assessed the proportion of individuals with “residual cholesterol risk” (i.e., LDLC > 100 mg/dL) compared with the proportion of those with “residual inflammatory risk” (i.e., hsCRP > 3 mg/L). We consider a 2-sided p <0.05 as statistically significant. All analyses were conducted with SAS 9.3 (SAS Institute Inc., Cary, North Carolina) and R 3.10 (The R Foundation for Statistical Computing), using the most recent version of the VIRGO database.
Results
Sample Characteristics
There were 2,219 adults with AMI in VIRGO included in this analysis (725 men and 1,494 women; Figure 1). The median age was similar for both sexes (48 years for men and 49 for women). The majority of patients were white (78%), married (52%), had more than a high school education (59%), and had health insurance (79%). About half presented with ST-elevation AMI (STEMI) and one third had prior heart disease. Cardiovascular risk factors (e.g., hypertension, diabetes, dyslipidemia, and obesity) were common (Table 1). Nearly half of the women were post-menopausal and 2.3% of post-menopausal women were taking estrogen therapy at the time of their AMI. The median LDL-C level was 66 mg/dl (IQR 66 to 106) while the median hsCRP was 2.9 mg/L (IQR 1.2 to 6.4). 2% of patients died during 12 months of follow-up.
Table 1. Baseline characteristics of study participants, stratified by sex.
| Characteristics | Overall (N=2219) |
Men (N=725) |
Women (N=1494) |
P-value |
|---|---|---|---|---|
| Socio-demographics | ||||
| Age, median (IQR), year | 49 (44-52) | 48 (44-52) | 49 (44-52) | 0.05 |
| Race, n (%) | ||||
| White | 1720 (77.7%) | 610 (84.3%) | 1110 (74.4%) | <0.01 |
| Black | 358 (16.2%) | 60 (8.3%) | 298 (20.0%) | |
| Other | 137 (6.2%) | 54 (7.5%) | 83 (5.6%) | |
| Married/living with a partner as if married, n (%) | 1161 (52.4%) | 430 (59.3%) | 731 (49.0%) | <0.01 |
| Education, n (%) | ||||
| < High school | 42 (1.9%) | 11 (1.5%) | 31 (2.1%) | 0.64 |
| High school | 858 (38.9%) | 279 (38.7%) | 579 (39.0%) | |
| >High school | 1304 (59.2%) | 431 (59.8%) | 873 (58.9%) | |
| Ability to pay for medication, n (%) | ||||
| Health insurance | 1738 (78.6%) | 560 (77.7%) | 1178 (79.0%) | 0.47 |
| Employed (work full or part time) | 1419 (63.9%) | 533 (73.5%) | 886 (59.3%) | <0.01 |
| Finances at end of month | <0.01 | |||
| Some money left over | 704 (31.8%) | 291 (40.2%) | 413 (27.7%) | |
| Just enough to make ends meet | 798 (36%) | 251 (34.7%) | 547 (36.6%) | |
| Not enough to make ends meet | 715 (32.3%) | 182 (25.1%) | 533 (35.7%) | |
| AMI severity, n (%) | ||||
| AMI type | ||||
| NSTEMI | 1109 (50.0%) | 312 (43.0%) | 797 (53.3%) | <0.01 |
| STEMI | 1110 (50.0%) | 413 (57.0%) | 697 (46.7%) | |
| GRACE risk score >99 | 194 (8.9%) | 61 (8.5%) | 133 (9.1%) | 0.67 |
| Left ventricular ejection fraction <40% | 214 (10.1%) | 70 (10.0%) | 144 (10.1%) | 0.97 |
| Hemodynamic instability | 166 (7.5%) | 57 (7.9%) | 109 (7.3%) | 0.70 |
| Present >6 h after symptom onset | 959 (43.4%) | 272 (37.6%) | 687 (46.2%) | <0.01 |
| Comorbidities and CVD risk factors, n (%) | ||||
| Prior heart disease (CAD, angina, heart failure) | ||||
| Hypertension | 1441 (64.9%) | 456 (62.9%) | 985 (65.9%) | 0.16 |
| Diabetes | 664 (29.9%) | 153 (21.1%) | 511 (34.2%) | <0.01 |
| Dyslipidemia | 1128 (50.9%) | 397 (54.9%) | 731 (49.0%) | 0.01 |
| Obesity (BMI ≥30 kg/m2) | 1162 (52.4%) | 346 (47.7%) | 816 (54.6%) | <0.01 |
| High waist circumference (women >88 cm, men >102 cm) | 1270 (71.1%) | 321 (53.3%) | 949 (80.1%) | <0.01 |
| Current smoking | 1211 (54.6%) | 391 (54.0%) | 820 (54.9%) | 0.73 |
| Physical activity | ||||
| Recommended | 849 (38.5%) | 313 (43.4%) | 536 (36.1%) | <0.01 |
| Insufficient | 654 (29.6%) | 192 (26.6%) | 462 (31.1%) | |
| Inactive | 703 (31.9%) | 216 (30%) | 487 (32.8%) | |
| Depression | 6 (2-12) | 5 (2-9) | 7 (3-13) | <0.01 |
| Baseline health status, mean (SD) | ||||
| SF-12 PCS score | 44 (12) | 46 (11.6) | 43 (12) | <0.01 |
| SF-12 MCS score | 46 (12.2) | 48 (11.4) | 45 (12.3) | <0.01 |
| Angina frequency score | 84 (20.5) | 86 (18.1) | 82 (21.4) | <0.01 |
| Angina-related physical limitation score | 82 (25) | 87 (20.8) | 79 (26.5) | <0.01 |
| Angina-related quality of life score | 58 (24.6) | 62 (22.5) | 56 (25.4) | <0.01 |
| EQ-5D utility index score | 0.8 (0.22) | 0.8 (0.2) | 0.7 (0.23) | <0.01 |
| EQ-5D visual analog scale | 65 (21.1) | 67 (19.8) | 65 (21.7) | 0.05 |
| Reperfusion in-hospital, n (%) | ||||
| None | 859 (42.1%) | 227 (34.1%) | 632 (45.9%) | <0.01 |
| Fibrinolytic therapy | 98 (4.8%) | 47 (7.1%) | 51 (3.7%) | |
| PCI | 1085 (53.1%) | 391 (57.8%) | 694 (50.4%) | |
| Discharge medication, n (%) | ||||
| Statin use | ||||
| High intensity statin | 868 (39.1%) | 310 (42.8%) | 558 (37.3%) | 0.15 |
| Low and moderate intensity statin | 1167 (52.6%) | 381 (52.5%) | 786 (52.6%) | |
| None | 184 (8.3%) | 34 (4.7%) | 150 (10.1%) | |
| Aspirin | 2142 (98.2%) | 712 (98.9%) | 1430 (97.9%) | 0.13 |
| Beta-blockers | 2031 (97.1%) | 638 (98.5%) | 1348 (96.4%) | 0.01 |
| ACE inhibitors or ARBs | 1416 (70.8%) | 512 (77.3%) | 904 (67.5%) | <0.01 |
| Estrogen use | - | - | 35 (2.3%) | - |
All percentages are calculated by excluding missing, don't know and patient refused. AMI= acute myocardial infarction.
IQR= interquartile range; SD= standard deviation; AMI= acute myocardial infarction; BMI= body mass index; CAD= coronary artery disease; CVD= cardiovascular disease; NSTEMI= non ST-elevation AMI; STEMI= ST-elevation AMI; PCS= physical component summary; MCS= mental component summary; GRACE= Global Registry of Acute Coronary Events; ACE = angiotensin converting enzyme; ARBs =Angiotensin II Receptor Blockers.
Compared with men, women were more likely to be black, single, have diabetes mellitus, prior stroke, and congestive heart failure. In particular, women were much more likely than men to have obesity (54.6% vs. 47.7%) and a high waist circumference (80.1% vs. 53.3%). However, women were less likely to have dyslipidemia and present with STEMI. Women were also less likely to receive in-hospital reperfusion treatment, as well as statin, beta-blockers, and ACE inhibitors or ARBs at discharge. Women had significantly lower observed generic and disease-specific health status scores than men at baseline, 1- and 12-months post AMI. The rates of angina-free in women were 44.1%, 59.7%, and 6.7% at baseline, 1- and 12-months post AMI. The corresponding rates in men were 48.4%, 67.0%, and 73.8%.
Overall, the proportion of these post-MI patients with “residual cholesterol risk” (i.e., LDL-C > 100 mg/dL) was 32.5% (34.6% in women vs. 28.2% in men), while the proportion of these post-MI patients with “residual inflammatory risk” (i.e., hsCRP > 3 mg/L) was 49.1% (53.6% in women vs. 39.5% in men). Among all patients, 15% had only the hsCRP goal achieved (LDL > 100 mg/dL and hsCRP ≤3 mg/L), 31% had only the LDL goal achieved (LDL ≤ 100 mg/dL and hsCRP >3 mg/L), 36% had both goals achieved (LDL ≤ 100 mg/dL and hsCRP ≤3 mg/L), and 18% had neither goal achieved (LDL > 100 mg/dL and hsCRP >3 mg/L).
Sex Differences in Inflammatory Markers at 1 Month after AMI
Women had significantly higher levels of inflammatory markers at 1 month after AMI compared with men (Figure 2, Table 2). The median (IQR) of hsCRP was 3.4 (1.3-7.3) mg/L for women and 2.2 (1.0-5.0) mg/L for men (P <0.01). These values were substantially higher than those in the similarly aged general population captured in NHANES (1.9 (0.7-4.4) mg/L for women and 1.3 (0.6-3.0) mg/L for men, Supplemental Table 1). When hsCRP was categorized into groups, there were more women (35.8%) with very high hsCRP (≥ 5 mg/L) compared with men (25.2%, P <0.01). The percentage of high hsCRP (3-5 mg/L) was also larger in women (15.8% vs. 14.3%). In addition, women had higher levels of Lp-PLA2 (4.8 (3.1-7.8) vs 3.0 (2.1-4.7) ng/mL, P<0.01). Both hsCRP and Lp-PLA2 have significantly stronger correlations with body mass index and waist circumference in women compared with those in men (Table 3).
Figure 2.
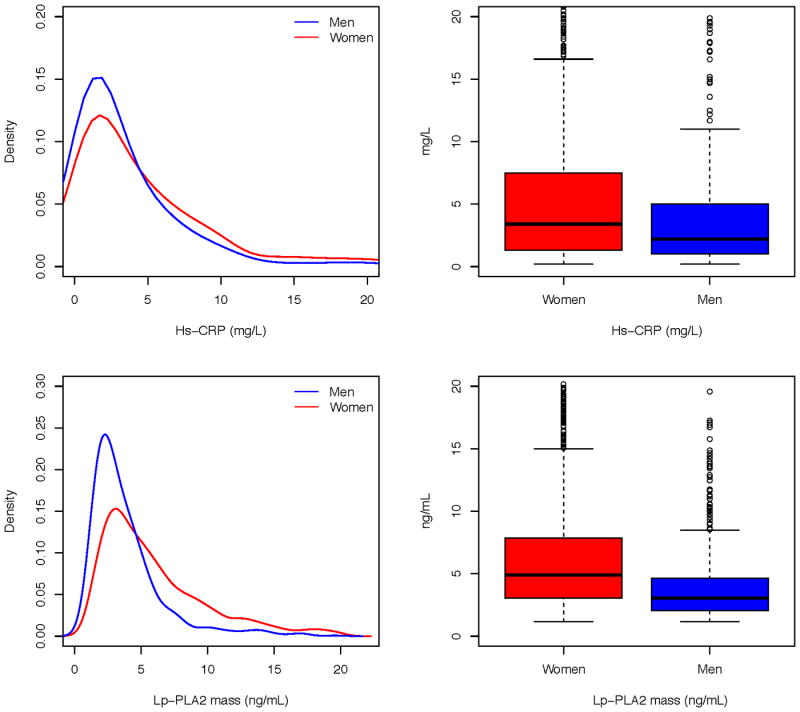
Distributions of inflammatory markers at 1 month after discharge for AMI, by sex (men = blue, women = red). AMI= acute myocardial infarction; VIRGO = Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients; HsCRP = high-sensitivity C-reactive protein; Lp-PLA2 = lipoprotein-associated phospholipase A2.
Table 2. Sex differences in inflammatory markers and lipid profiles at 1 month after discharge.
| Overall (N=2219) |
Men (N=725) |
Women (N=1494) |
P-value | |
|---|---|---|---|---|
| HsCRP, median (IQR), mg/L | 2.9 (1.2-6.4) | 2.2 (1.0-5.0) | 3.4 (1.3-7.3) | <0.01 |
| HsCRP category, n (%) | ||||
| Very high (≥ 5 mg/L) | 709 (33.7%) | 172 (25.2%) | 537 (37.8%) | <0.01 |
| High (3-5 mg/L) | 323 (15.4%) | 98 (14.3%) | 225 (15.8%) | |
| Moderate (1 -3 mg/L) | 676 (32.1%) | 262 (38.2%) | 414 (29.1%) | |
| Low (<1 mg/L) | 395 (18.8%) | 153 (22.3%) | 242 (17.1%) | |
| Lp-PLA2 mass, median (IQR), ng/mL | 4.1 (2.6-6.7) | 3.0 (2.1-4.7) | 4.8 (3.1-7.8) | <0.01 |
| Lipid profile, median (IQR), mg/dL | ||||
| Total cholesterol | 153 (129-182) | 145 (126-174) | 157 (132-185) | <0.01 |
| LDL cholesterol | 85 (66-106) | 81 (65-104) | 86 (67-107) | 0.01 |
| HDL cholesterol | 37 (30-46) | 33 (28-39) | 39 (32-48) | <0.01 |
| Triglyceride | 122 (86-181) | 130 (91-193) | 119 (83.5-173) | <0.01 |
All percentages are calculated by excluding missing, don't know, and patient refused.
IQR= interquartile range; HsCRP = high-sensitivity C-reactive protein; Lp-PLA2 = lipoprotein-associated phospholipase A2; LDL= low-density lipoprotein, HDL= high-density lipoprotein.
Table 3. Spearman correlations between inflammatory markers and metabolic risk factors at 1 month after discharge.
| HsCRP | Lp-PLA2 mass | |||||
|---|---|---|---|---|---|---|
| Men | Women | P-value * | Men | Women | P-value * | |
| Body mass index † | 0.24 | 0.40 | <0.01 | 0.18 | 0.32 | <0.01 |
| Waist circumference † | 0.25 | 0.40 | <0.01 | 0.21 | 0.31 | 0.02 |
| Total cholesterol | 0.13 | 0.05 | 0.08 | 0.08 | 0.04 | 0.38 |
| LDL cholesterol | 0.18 | 0.11 | 0.11 | 0.12 | 0.10 | 0.66 |
| HDL cholesterol | -0.24 | -0.32 | 0.06 | -0.10 | -0.20 | 0.02 |
| Triglyceride | 0.14 | 0.25 | 0.01 | 0.06 | 0.11 | 0.27 |
The P-value shows the significance of the difference in correlation coefficients between men and women.
Body mass index and waist circumference were measured at baseline and carried over to 1 month.
HsCRP = high-sensitivity C-reactive protein; Lp-PLA2 = lipoprotein-associated phospholipase A2; LDL= low-density lipoprotein, HDL= high-density lipoprotein.
The regression analyses demonstrated the independent effect of gender on inflammatory markers (Table 4). Adjustment for patient characteristics in the multivariable regression models reduced the sex differences in hsCRP and in Lp-PLA2, but the effect of gender remained statistically significant. On the log scale, women had 0.21 mg/L higher hsCRP and 0.35 ng/mL higher Lp-PLA2 than men in the fully adjusted models (both P values <0.01). When hsCRP was used as a categorical variable, women were 1.41 times more likely than men to have high or moderate hsCRP (P <0.01). In particular, older age, smoking, having diabetes, and higher body mass index at baseline, and higher 1-month LDL-cholesterol were all associated with high or moderate hsCRP at 1 month after AMI. These variables were also associated with a significantly higher hsCRP and Lp-PLA2 levels.
Table 4. Patient characteristics associated with inflammatory markers at 1 month after hospital discharge.
| Characteristics | Categorical hsCRP (High [≥3 mg/L], Moderate [1-3 mg/L], Low [<1 mg/L]) |
Continuous hsCRP on log scale, mg/L | Continuous Lp-PLA2 mass on log scale, ng/mL | |||
|---|---|---|---|---|---|---|
| Unadjusted Odds Ratio (P value) | Adjusted Odds Ratio (P value)† | Unadjusted Coefficient (P value) | Adjusted Coefficient (P value)† | Unadjusted Coefficient (P value) | Adjusted Coefficient (P value)† | |
| Women (vs. men) | 1.65 (<0.01) | 1.41 (<0.01) | 0.36 (<0.01) | 0.21 (<0.01) | 0.41 (<0.01) | 0.35 (<0.01) |
| Age at baseline (per 5 year increase) | - | 1.10 (0.01) | - | 0.07 (<0.01) | - | 0.04 (<0.01) |
| White race (vs. non-white) | - | 0.83 (0.1) | - | -0.14 (0.02) | - | -0.18 (<0.01) |
| Current smoking at baseline | - | 1.91 (<0.01) | - | 0.38 (<0.01) | - | 0.15 (<0.01) |
| Hypertension at baseline | - | 1.09 (0.38) | - | 0.15 (<0.01) | - | 0.02 (0.41) |
| Diabetes at baseline | - | 1.71 (<0.01) | - | 0.29 (<0.01) | - | 0.14 (<0.01) |
| Body mass index at baseline | - | 1.1 (<0.01) | - | 0.05 (<0.01) | - | 0.02 (<0.01) |
| Statin use at discharge | ||||||
| Non-statin | - | Reference category | - | Reference category | - | Reference category |
| Low or moderate intensity | - | 0.87 (0.26) | - | -0.17 (0.01) | - | -0.01 (0.69) |
| High intensity | - | 1.08 (0.51) | - | -0.04 (0.50) | - | 0.04 (0.30) |
| LDL-cholesterol at 1 month (per 10 mg/dl increase) | - | 1.09 (<0.01) | - | 0.04 (<0.01) | - | 0.02 (<0.01) |
HsCRP = high-sensitivity C-reactive protein; Lp-PLA2 = lipoprotein-associated phospholipase A2; LDL= low-density lipoprotein.
Model was also adjusted for patient age, race, current smoking, hypertension, diabetes, body mass index, statin use at discharge, and LDL-cholesterol at 1 month.
Sex, Inflammatory Markers, and 12-Month Health Status
At 12 months after AMI, women had significantly worse angina, more disease-specific physical limitations, and a poorer quality of life than men as measured by the SAQ (P<0.01 for all, Table 5). In addition, women had a significantly worse physical and mental generic health status than men as measured by the SF-12 (P<0.01 for all). Women also had lower EQ-5D index and visual analog scale scores (P<0.01 for all).
Table 5. Adjusted 12-month health status associated with sex and hsCRP at 1 month, coefficient (p value).
| Health status at 12 months of follow up | Unadjusted, Women (vs Men) | After adjustment for 1-month hsCRP | After adjustment for 1-month hsCRP and other covariates* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Women (vs Men) | 1-month hsCRP | Reduction in gender effect after adjustment for CRP | Women (vs Men) | 1-month hsCRP | Reduction in gender effect after adjustment for CRP and other covariates | ||||
| Moderate (vs Low) | High (vs Low) | Moderate (vs Low) | High (vs Low) | ||||||
| Angina frequency | -2.25 (<0.01) | -2.05 (0.01) | -1.40 (0.20) | -2.18 (0.03) | 8.9% | -0.87 (0.24) | 0.04 (0.96) | -0.42 (0.68) | 61.3% |
| Angina-related physical limitation | -2.24 (<0.01) | -1.97 (0.01) | -0.55 (0.60) | -2.12 (0.04) | 12.1% | -0.96 (0.22) | 0.02 (0.98) | 0.10 (0.92) | 57.1% |
| Angina-related quality of life | -6.51 (<0.01) | -6.09 (<0.01) | -1.96 (0.19) | -3.84 (<0.01) | 6.5% | -3.66 (<0.01) | -0.37 (0.77) | -0.63 (0.64) | 43.8% |
| SF-12 PCS score | -3.72 (<0.01) | -3.07 (<0.01) | -1.77 (0.02) | -5.35 (<0.01) | 17.5% | -1.36 (<0.01) | 0.19 (0.77) | -0.06 (0.92) | 63.4% |
| SF-12 MCS score | -3.31 (<0.01) | -3.05 (<0.01) | 0.05 (0.92) | -1.62 (0.01) | 7.9% | -1.69 (<0.01) | 0.05 (0.93) | -0.65 (0.31) | 48.9% |
| EQ-5D utility index score | -0.05 (<0.01) | -0.04 (<0.01) | -0.01 (0.33) | -0.06 (<0.01) | 20.0% | -0.02 (0.01) | 0.003 (0.77) | -0.01 (0.27) | 60.0% |
| EQ-5D visual analog scale | -4.22 (<0.01) | -3.21 (<0.01) | -2.70 (0.03) | -7.60 (<0.01) | 23.9% | -1.59 (0.07) | -0.83 (0.47) | -2.19 (0.09) | 62.3% |
Results are reported as coefficients and p values. EQ-5D indicates EuroQol; MCS, Mental Component Summary; PCS, Physical Component Summary; and SF-12, Short Form-12.
Model was also adjusted for patient age, race, education, current smoking, hypertension, diabetes, body mass index, statin use at discharge, 1-month LDL-cholesterol, and the corresponding 1-month value of the outcome variable.
Adjustment for 1-month hsCRP levels attenuated these sex differences, but they remained statistically significant (Table 5). The reductions in gender effect after adjustment for hsCRP ranged from 6.5% for SF-12 MCS to 23.9% EQ-5D visual analog scale score. Compared with patients with low hsCRP values, those with high hsCRP values had significantly worse scores in angina frequency, angina-related physical limitation, angina-related quality of life, SF-12 PCS, SF-12 MCS, EQ-5D utility index, and EQ-5D visual analog scale (Table 5). For those with moderate hsCRP values, the scores were significantly worse for SF-12 PCS and EQ-5D visual analog scale, but not statistically different for others.
Additional adjustment of all other covariates further reduced the sex differences in health status. The reductions in gender effect after adjustment for hsCRP and all other covariates ranged from 43.8% for angina-related quality of life score to 63.4% SF-12 PCS score. There were no significant sex differences in angina frequency, angina-related physical limitation, and the EQ-5D visual analog scale. However, women still had a significantly poorer quality of life, worse physical and mental generic health status as measured by the SF-12, and lower EQ-5D index score than men. The association of hsCRP with health status was also attenuated in the fully adjusted model. Compared with patients with low hsCRP levels, patients with high or moderate hsCRP levels were not significantly different with regard to all health status scores except for the EQ-5D visual analog scale (P=0.04). Interaction terms between sex and hsCRP levels (high and moderate versus low) were not statistically significant in any of the fully adjusted models for 12-month health status, indicating that the association of 1-month hsCRP with health status did not vary between men and women. Our sensitivity analysis among type I AMI patients, STEMI, or NSTEMI patients showed results consistent with the main analysis (Supplemental Tables 2-7).
Discussion
Among young and middle-aged AMI patients, women had significantly higher levels of inflammatory markers following AMI compared with their male counterparts. There were 53.6% and 37.8% of women with hsCRP ≥3 and ≥5 mg/L at 1 month after AMI compared with only 39.5% and 25.2% in men. The higher hsCRP level in women with AMI was partially explained by a higher prevalence of smoking, diabetes, and obesity in that group, as well as higher 1-month LDL-C level. Post-AMI inflammatory marker was associated with poor health status in young and middle-aged AMI patients, and adjustment for 1-month hsCRP partially attenuated the observed sex difference in health status 12 months after AMI. However, the association between hsCRP and health status became non-significant after adjustment for socio-demographics, comorbidities, and treatment factors, suggesting that hsCRP may be a marker of other factors associated with poor health status.
Our study extends prior literature in several important ways. First, it demonstrates a significant sex difference in inflammatory markers at 1 month after an AMI. Previous studies have focused on the general population and data on AMI patients are limited. Assessing sex difference in inflammatory markers among AMI patients is important, not only because it is essential for monitoring the rise in acute phase reactants after an AMI, but also because it helps identify potential factors that may account for the excess adverse outcomes in women. Second, our study shows that sex differences in post-AMI inflammatory markers are partially explained by the differences in comorbidities between men and women. For instance, compared with men, women had a much higher rate of obesity and a stronger correlation between obesity and inflammatory markers. Women also had higher rates of smoking and diabetes, and experienced greater stress and depression, but were less likely to receive high-intensity statins, all of which contribute to higher levels of inflammatory markers in women. Hence, interventions that recognize and address these predictors of inflammatory markers would be beneficial for inflammation management in men and women with AMI.
Third, our study shows that elevated hsCRP is associated with poor health status (symptoms, function, and quality of life) among young and middle-aged patients with AMI. Earlier analyses of VIRGO documented worse physical functioning, mental health status, and quality of life in women than in men at 12 months post-AMI.21 The current study found this gap to be partially attenuated after adjustment for differential levels of 1-month hsCRP between men and women. It was additionally attenuated after adjustment for other comorbidities, indicating that hsCRP is correlated with factors associated with poor health status. Although the association of hsCRP with health status may not involve a causal relationship, the strong association suggests that post-AMI hsCRP may be a useful prognostic marker for predicting health outcomes in young and middle-aged AMI patient. Further studies that examine the underlying pathways responsible for post-AMI health status and potential sex differences may help identify novel therapeutic interventions.
Finally, our study is also among the first to address the population prevalence of “residual cholesterol risk” compared with “residual inflammatory risk.” 8 Consistent with prior data,22 the proportion of individuals in whom post-MI risk is predominantly on the basis of a persistent pro-inflammatory response was higher than the proportion in whom post-MI risk is predominantly on the basis of persistently elevated levels of LDL-C. These data have implications for the use of further LDL-lowering agents (such as PCSK9 inhibitors) compared with potential anti-inflammatory treatment strategies such as low-dose methotrexate or canakinumab.
This study has several limitations. First, VIRGO was an observational study that required patients to be healthy enough at baseline to participate; thus, we were unable to enroll those who were too ill to be enrolled. Second, about 9% of patients were lost to follow-up at 1 month after AMI. If they were less healthy than the remaining patients and were disproportionately distributed between men and women, our estimated sex difference in inflammatory markers may be biased. However, our data suggest that the numbers of men and women who were lost to follow-up were comparable, and baseline socio-demographic and clinical attributes were generally similar between those who were lost to follow-up and those who remained in the sample. Third, we did not measure inflammatory markers at baseline and were unable to examine change in inflammatory markers during follow-up.
In conclusion, there was a significantly higher level of post-AMI inflammatory markers in young women compared with similarly aged men. An elevated level of hsCRP at 1 month was associated with poor health status at 12 months after AMI, but this was attenuated after adjusting for socio-demographics, comorbidities, and treatment factors. In half of the men and women with AMI, the post-AMI risk is predominantly on the basis of a persistent pro-inflammatory response. Targeted anti-inflammatory treatments are worthy of consideration for secondary prevention in these patients if the ongoing clinical trials of anti-inflammatory therapy prove effective at cardiovascular event reduction.
Supplementary Material
What is known.
Young women (≤ 55 years) with acute myocardial infarction (AMI) have higher mortality risk than similarly aged men.
Elevated inflammatory markers are associated with an increased risk of cardiovascular outcomes after AMI.
What the study adds.
Young women with AMI had higher inflammatory levels compared with young men.
Elevated 1-month hsCRP was associated with poor health status at 12 months after AMI, but this was attenuated after adjustment for patient characteristics.
Half of the young patients had “residual inflammatory risk” (hsCRP > 3 mg/L) compared with a third who had “residual cholesterol risk” (Low-density lipoprotein cholesterol > 100 mg/dL).
Acknowledgments
We thank Quest Diagnostics for providing financial support for inflammatory marker measurements of study participants.
Funding Sources: The VIRGO study (NCT00597922) was supported by grant R01 HL081153 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Disclosures: Dr. Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson (Janssen), through Yale University, to develop methods of clinical trial data sharing; is the recipient of a grant from the Food and Drug Administration and Medtronic to develop methods for post-market surveillance of medical devices; works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; chairs a cardiac scientific advisory board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; and is the founder of Hugo, a personal health information platform. Dr. Spertus is supported by grants from Gilead, Genentech, Lilly, Amorcyte, and EvaHeart, and has a patent for the Seattle Angina Questionnaire with royalties paid. He also owns the copyright to the SAQ. Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to Seimens and AstraZeneca, and has received investigator-initiated research funding from Novartis, Amgen, Pfizer, Kowa, AstraZeneca, and the National Institutes of Health.
References
- 1.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E, Pravastatin or Atorvastatin E and Infection Therapy-Thrombolysis in Myocardial Infarction I C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 4.Lp-PLA2 Studies Collaboration. Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, Kaptoge S, Ballantyne C, Cannon CP, Criqui M, Cushman M, Hofman A, Packard C, Thompson SG, Collins R, Danesh J. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr, Herrington DM. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J. 2006;152:593–598. doi: 10.1016/j.ahj.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 7.Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4:109–114. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM. Residual inflammatory risk: addressing the obverse side of the atherosclerosis prevention coin. Eur Heart J. 2016;37:1720–1722. doi: 10.1093/eurheartj/ehw024. [DOI] [PubMed] [Google Scholar]
- 9.Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65:168–172. doi: 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 13.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 15.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA, GRACE Investigators A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 16.Yore MM, Ham SA, Ainsworth BE, Kruger J, Reis JP, Kohl HW, 3rd, Macera CA. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007;39:1267–1274. doi: 10.1249/mss.0b013e3180618bbe. [DOI] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson JG, Lichtenstein AH, Goff DC, Jr, Lloyd-Jones DM, Smith SC, Jr, Blum C, Schwartz JS, ACC/AHA Cholesterol Guideline Panel Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med. 2014;160:339–343. doi: 10.7326/M14-0126. [DOI] [PubMed] [Google Scholar]
- 18.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention and American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. US National Health and Nutrition Examination Survey. [November 17, 2016]; http://www.cdc.gov/nchs/nhanes.htm.
- 20.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ES, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 21.Dreyer RP, Wang Y, Strait KM, Lorenze NP, D'Onofrio G, Bueno H, Lichtman JH, Spertus JA, Krumholz HM. Gender differences in the trajectory of recovery in health status among young patients with acute myocardial infarction: Results from the VIRGO Study. Circulation. 2015;131:1971–1980. doi: 10.1161/CIRCULATIONAHA.114.014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E. Achievement of dual low-density lipoprotein cholesterol and high-sensitivity C-reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in IMPROVE-IT. Circulation. 2015;132:1224–1233. doi: 10.1161/CIRCULATIONAHA.115.018381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.