Abstract
Background
Most studies of sex and race differences after acute myocardial infarction (AMI) have not taken into account differences in life expectancy in the general population. Years of potential life lost (YPLLs) is a metric that takes into account the burden of disease and can be compared by sex and race.
Objectives
We sought to determine sex and race differences in long-term survival after AMI using life expectancy and YPLL to account for differences in population-based life expectancy.
Methods
Using data from the Cooperative Cardiovascular Project, a prospective cohort study of Medicare beneficiaries hospitalized for AMI between 1994–1995 (n = 146,743), we calculated life expectancy and YPLLs using Cox proportional hazards regression with extrapolation using exponential models.
Results
Of the 146,743 patients with AMI, 48.1% were women and 6.4% were black; the average age was 75.9 years. Post-AMI life expectancy estimates were similar for men and women of the same race but lower for black patients than white patients. On average, women lost 10.5% (SE 0.3%) more of their expected life than men, and black patients lost 6.2% (SE 0.6%) more of their expected life than white patients. After adjustment, women still lost an average of 7.8% (0.3%) more of their expected life than men, but black race became associated with a survival advantage, suggesting that racial differences in YPLLs were largely explained by differences in clinical presentation and treatment between black and white patients.
Conclusions
Women and black patients lost more years of life after AMI, on average, than men and white patients, an effect that was not explained in women by clinical or treatment differences.
Keywords: acute coronary syndromes, population perspective, survival
Eliminating sex and race differences in health requires an understanding of whether these differences are disease-specific or reflect differences in the broader population. Although numerous studies have examined sex and race differences in outcomes associated with particular conditions, most have not taken into account differences in life expectancy in the general population, leaving uncertainty as to whether the observed differences reflect broader differences in the general population or are specific to the condition. Differences in life expectancy by sex and race in the general population are essential to consider when investigating the sex and race differences in the burden of a particular condition.
Acute myocardial infarction (AMI) has received significant attention regarding sex and race differences, but uncertainty remains. Studies of sex differences in AMI have generally reported no differences in long-term survival between older men and women after risk adjustment (1–7), but these analyses have not considered women’s longer life expectancy in the general population.(8). Similarly, studies of racial differences among patients with AMI have consistently reported poorer long-term survival for black patients compared with white patients (9–11), but it is unclear whether these differences in mortality are disease-specific or simply reflect the shorter life expectancy of blacks in the general population (8).
Understanding the distinction between disease-specific and broader health differences is critical for targeting efforts to reduce sex and racial differences among patients with AMI. If the observed differences are specific to patients with AMI, then efforts should target factors related to the AMI such as sex and race differences in presentation and care. Alternatively, if the observed differences are due to differences in life expectancy in the general population, then efforts should target factors contributing to health disparities in the general population, such as socioeconomic status or access to care.
Years of potential life lost (YPLLs) can incorporate information about the general population experience when investigating the burden of a particular condition. YPLLs measure the difference in life expectancy between patients with a particular condition and persons with similar characteristics in the general population. Thus, YPLLs provide a direct assessment of disease-related mortality and allow us to separate sex and race differences in post-AMI mortality from background sex and race differences in the general population (12). To date, no large national studies have examined YPLLs due to AMI, because such analyses require massive studies of patients with AMI and the general population, each with long-term follow-up. Although a few studies have accounted for sex or race differences in population-based mortality using methods other than YPLLs (13–16), their results have been limited by small sample sizes and short follow-up. To investigate disease-specific sex and race differences after AMI, we used data from the Cooperative Cardiovascular Project (CCP) to calculate life expectancy and YPLLs after AMI by men and women and blacks and whites. Implemented by the Health Care Financing Administration (now the Centers for Medicare & Medicaid Services) in the mid-1990s, the CCP contains rich, detailed clinical data and now has more than 17 years of follow-up on Medicare beneficiaries.
METHODS
The CCP was a nationwide quality improvement project conducted in the mid-1990s, designed to evaluate and improve the care of AMI patients in the United States (17,18). In brief, the CCP is a prospective cohort study that includes all fee-for-service Medicare beneficiaries discharged from acute-care non-governmental U.S. hospitals, with the principal discharge diagnosis of AMI (International Classification of Diseases, Ninth Revision, Clinical Modification, code 410). Patients were identified between January 1994 and February 1996 using hospital bills (UB-92 claims data) in the Medicare National Claims History File. Patient records were abstracted for detailed clinical information including demographics, medical history, presentation, laboratory, and electrocardiographic data, inhospital events, and treatment. AMI readmissions (code 410.×2) were excluded resulting in 234,771 admissions.
We limited our sample to patients between ages 65 and 90 years with confirmed AMI by standardized criteria. Confirmed AMI was defined as an elevation of creatine kinase-MB (>5% of total creatine kinase), an elevation of lactate dehydrogenase level >1.5 times the upper limit of normal with isoenzyme reversal, or the present of 2 of the following: chest pain prior to admission, a 2-fold elevation in total creatine kinase, and electrocardiographic evidence of AMI (ST-segment elevation or new pathological Q-waves). Patients <65 years of age (n = 17,593), >90 years of age (n = 9,985), and those without clinically confirmed AMI (n = 31,186) were excluded. For patients hospitalized more than once during the study period, we excluded admissions after the first admission (n = 23,773). We also excluded patients who were hospitalized outside the 50 states (n = 1,763) and patients whose records could not be linked to the Medicare Denominator files to ascertain vital status (n = 2,195). Finally, we calculated life expectancy for black and white patients only, because so few patients were classified as other races (n = 9,010). Our final sample size included 146,743 patients. The Yale University institutional review board approved this study.
Outcome Variable
We linked the CCP to Medicare Denominator files from 1994 to 2012 to obtain data on 17-year survival from the time of hospital admission. Denominator files contain demographic and enrollment information on all Medicare fee-for-service and Medicare Advantage beneficiaries in a given year, including age, sex, race, and date of death. Only Medicare fee-for-service patients were used.
Statistical Analyses
Patient characteristics collected during the index admission were compared between men and women using X2 tests and t-tests. Age, sex, and race-specific estimates of life expectancy were calculated using a 3-step process. First, we fit a Cox proportional hazards model that included covariates age, sex, race, and their pairwise interactions. We chose a semiparametric approach, because it provided better fit to the data using AIC and likelihood ratio statistics compared with parametric models such as the Weibull or Gompertz. Proportional hazards assumptions were checked using Schoenfeld residuals, examined graphically and tested formally. Second, we plotted the 17-year expected survival curves from the Cox models for each age, sex, and race combination and then extrapolated the curves to age 100 using exponential models. We chose an exponential model because we lacked information on the shape of the hazard function beyond 17 years and thus opted for a model with a constant hazard that does not make assumptions about changes to the hazard function over time. The constant hazard for the exponential model was specified as the average hazard over the last 2 years of available follow-up. For example, to calculate life expectancy for a 65-year-old white man, we plotted the survival curve for these specific covariate values over 17 years of follow-up and then extrapolated the survival curve using an exponential model over an additional 18 years. Third, mean life expectancy estimates were calculated by adding the areas under the Cox and exponential survival functions. Ninety-five percent confidence intervals for the mean were calculated using the same process for the upper and lower confidence bounds of the expected survival curves.
To estimate YPLLs, we subtracted observed patient survival from age, sex, and race-specific estimates of life expectancy for the overall Medicare population. Life expectancy estimates for the overall Medicare population in 1994–1995 were calculated using the same methods described above with data from the Medicare Denominator Files. For patients in the Cooperative Cardiovascular Patients who survived the 17-year follow-up, we used a multiple imputation approach with 10 repetitions to estimate expected survival. The distribution of expected survival was calculated by adding the area under the exponential portion of the survival curve to the observed 17-year survival for each age, sex, and race combination. Survivors were then assigned a random survival time from this distribution to create a complete dataset. Because the observed differences in YPLLs could be influenced by sex and race differences in life expectancy in the general population, we standardized YPLLs by calculating the percentage of years of potential life lost (or gained) against life expectancy in the Medicare population.
To evaluate whether differences in cardiovascular risk factors, clinical presentation, or treatment might explain the observed sex and racial differences in YPLLs, we used multivariable linear regression with multiple imputations to sequentially adjust for patient medical history (history of congestive heart failure, diabetes mellitus, hypertension, coronary artery disease, and smoking), clinical presentation (anterior AMI, ST-segment elevation AMI, cardiac arrest, Killip class, and renal insufficiency), and treatment (revascularization procedures within 30 days, fibrinolytic therapy during hospitalization, aspirin on arrival, beta-blockers at discharge, and eligibility for core measures). Covariates were selected using a combination of clinical judgment, existing risk models, and significance testing. Patients with missing data on revascularization procedures were included in the model using dummy variables to denote missing data. We modeled the percentage of YPLLs to expected survival rather than absolute YPLLs because these values were standardized against expected population life expectancy and thus more appropriately reflected mortality related to the AMI versus competing causes. We evaluated model assumptions using studentized residuals assessed both graphically and formally. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Of the 146,743 patients with AMI in our cohort, 48.1% were women and 6.4% were black (Table 1). The mean age ± standard deviation at the time of AMI hospitalization was 74.8 ± 6.5 years for white men, 77.2 ± 6.8 years for white women, 74.0 ± 6.4 years for black men, and 75.4 ± 6.7 years for black women. Women and black patients had a greater prevalence of diabetes mellitus, hypertension, and heart failure compared with white men. At the time of hospital presentation, more women were classified as Killip class III or IV than men, and black patients were more likely to demonstrate signs of renal insufficiency and bleeding. Overall, men and white patients were more likely to undergo revascularization procedures and to receive fibrinolytic therapy than women and black patients, respectively.
Table 1.
Characteristics of the Cooperative Cardiovascular Project sample by sex and race (N=146,743)*
| White Men n=72,176 (49.2) |
White Women n=65,239 (44.5) |
Black Men n=3968 (2.7) |
Black Women n=5360 (3.7) |
|
|---|---|---|---|---|
| Age, mean (SD), years | 74.8 (6.5) | 77.2 (6.8) | 74.0 (6.4) | 75.4 (6.7) |
| Medical History | ||||
| Diabetes mellitus, N(%) | 19838 (27.5) | 20977 (32.2) | 1371 (34.6) | 2630 (49.1) |
| Hypertension, N(%) | 39619 (54.9) | 43573 (66.8) | 2872 (66.8) | 4515 (84.2) |
| Congestive heart failure, N(%) | 12668 (17.6) | 15170 (23.3) | 904 (22.8) | 1535 (28.6) |
| History of AMI, CABG, or PCI, N(%) | 30846 (42.7) | 20374 (31.2) | 1421 (35.8) | 1688 (31.5) |
| Current smoker, N(%) | 11515 (16.0) | 9294 (14.3) | 1011 (25.5) | 718 (13.4) |
| Clinical Presentation | ||||
| Cardiac arrest, N(%) | 2960 (4.1) | 2086 (3.2) | 173 (4.4) | 238 (4.4) |
| Anterior AMI, N(%) | 32524 (45.1) | 31119 (47.7) | 1872 (47.2) | 2548 (47.5) |
| Renal insufficiency, N(%) | 8871 (12.3) | 6846 (10.5) | 789 (19.9) | 1017 (19.0) |
| Killip score, N(%) | ||||
| I | 39426 (54.6) | 31511 (48.3) | 1995 (50.3) | 2449 (45.7) |
| II | 8587 (11.9) | 7984 (12.2) | 499 (12.6) | 660 (12.3) |
| III | 22430 (31.1) | 24070 (36.9) | 1389 (35.0) | 2143 (40.0) |
| IV | 1733 (2.4) | 1674 (2.6) | 85 (2.1) | 108 (2.0) |
| ST-elevation AMI, N(%) | 21328 (29.6) | 19001 (29.1) | 1199 (30.2) | 1389 (25.9) |
| Bleeding, N(%) | 12133 (16.8) | 10445 (16.0) | 766 (19.3) | 1021 (19.1) |
| Treatment | ||||
| Any revascularization within 30 days, N(%) | ||||
| Yes | 24999 (34.6) | 16813 (25.8) | 935 (23.6) | 1035 (19.3) |
| No | 44968 (62.3) | 46230 (71.0) | 2919 (73.6) | 4159 (77.6) |
| Missing | 2209 (3.1) | 2106 (3.2) | 114 (2.9) | 166 (3.1) |
| CABG within 30 days, N(%) | ||||
| Yes | 11900 (16.5) | 6708 (10.3) | 407 (10.3) | 404 (7.5) |
| No | 58067 (80.5) | 56425 (86.5) | 3447 (86.9) | 4790 (89.4) |
| Missing | 2209 (3.1) | 2106 (3.2) | 114 (2.9) | 166 (3.1) |
| PCI within 30 days, N(%) | ||||
| Yes | 13803 (19.1) | 10609 (16.3) | 547 (13.8) | 651 (12.2) |
| No | 56164 (77.8) | 52524 (80.5) | 3307 (83.3) | 4543 (84.8) |
| Missing | 2209 (3.1) | 2106 (3.2) | 114 (2.9) | 166 (3.1) |
| Fibrinolytic therapy during hospitalization, N(%) | 14561 (20.2) | 10907 (16.7) | 596 (15.0) | 619 (11.6) |
| Aspirin on arrival (among eligible), N(%) | 43358/55888 (77.6) | 35713/48874 (72.5) | 2212/2975 (74.4) | 2606/3810 (68.4) |
| Beta-blockers on arrival, N(%) | 21642/35550 (60.9) | 17427/29328 (59.4) | 1036/1748 (59.3) | 1270/2285 (55.6) |
Abbreviations: AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CCP, Cooperative Cardiovascular Project; PCI, percutaneous coronary intervention; SD, standard deviation.
Only variables with missing data have “missing” rows. Variables without “missing” have no missing data.
Cardiogenic shock is defined as having a systolic blood pressure <90mmHg on admission.
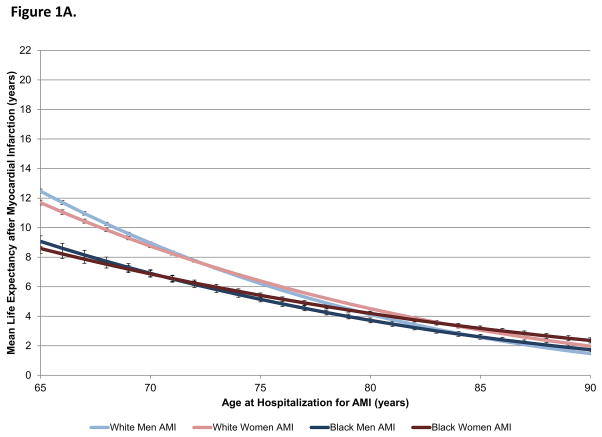
The survival rate after 17 years of follow-up was 8.3% for white men, 6.4% for white women, 5.4% for black men, and 5.8% for black women (7.3% overall). Men and women of the same race had similar life expectancy after AMI at all ages; only minimal differences by sex were observed in the youngest and oldest ages (Figure 1A). In contrast, black patients had lower life expectancies after AMI than white patients at younger ages but comparable life expectancies at older ages.
Figure 1.
Figure 1A. Mean life expectancy after AMI by sex and race.
Mean life expectancy after AMI for white men (light blue), white women (light red), black men (dark blue), and black women (dark red) is shown by age. Sex differences in life expectancy after AMI were minimal but were greatest in the youngest and oldest ages. Racial differences in life expectancy after AMI were greatest in younger patients and became nonsignificant in older patients.
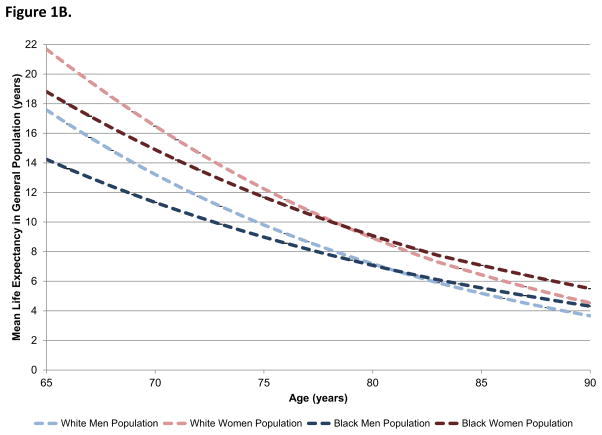
Figure 1B. Mean life expectancy in the general Medicare population by sex and race.
Mean life expectancy for white men (light blue), white women (light red), black men (dark blue), and black women (dark red) in the general Medicare population is shown by age. Women had significantly higher life expectancy than men at all ages. Racial differences in life expectancy were greatest in younger patients.
Compared with AMI patients, more patients in the general Medicare population were female (58.5% vs. 48.1%) and black (8.2% vs. 6.4%). Figure 1B shows the life expectancy estimates for the general Medicare population by age, sex, and race. Compared with the life expectancy estimates shown in Figure 1A, patients in the general Medicare population lived significantly longer than patients with AMI of the same age, sex, and race. In the general population, women had longer life expectancies than men of the same race at all ages. Conversely, black patients had lower life expectancies at younger ages than white patients of the same sex but longer life expectancies in the oldest ages.
Mean YPLLs by sex and race are presented in Figure 2. On average, women lost 1.86 (95% CI 1.74–1.99) more years of life than men across all ages, and black patients lost 0.86 (95% CI 0.12–0.23) more years of life than white patients. However, the magnitude of these differences varied by age and across different combinations of sexes and races. Figure 3 shows the absolute number of YPLLs by age, sex, and race. Across all ages, women lost more years of potential life after AMI than men, although the difference was most pronounced among patients in younger patients. For example, 65-year-old white women lost an average of 9.99 (95% confidence interval (CI) 9.83–10.16) years after AMI compared with only 5.11 (95% CI 4.97–5.26) years in 65-year-old white men. Differences in life years lost by race were less pronounced. Whereas 65-year-old white men lost an average of 5.11 (95% CI 4.97–52.6) years, black men lost 5.17 (95% CI 4.80–5.53) years.
Figure 2. Mean years of potential life lost after AMI overall and by sex and race across all ages.
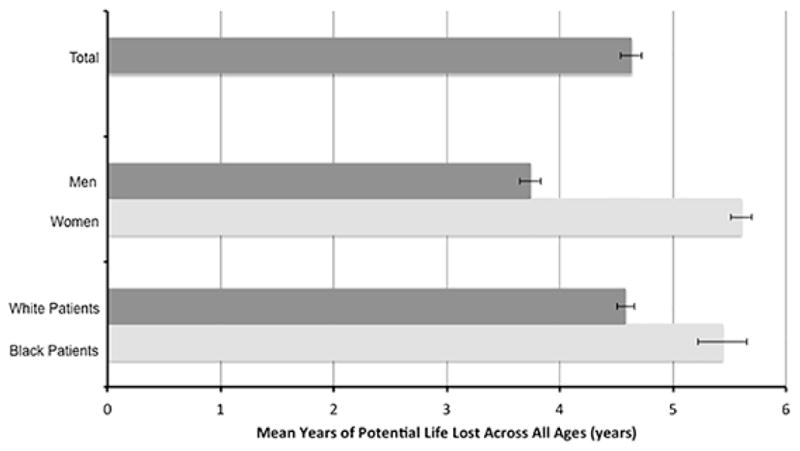
In aggregate, across all ages, women lost more years of potential life after AMI than men, and black patients lost more years than white patients. However, the magnitude of these differences also varied by age.
Figure 3. Years of potential life lost after AMI by sex, race, and age.
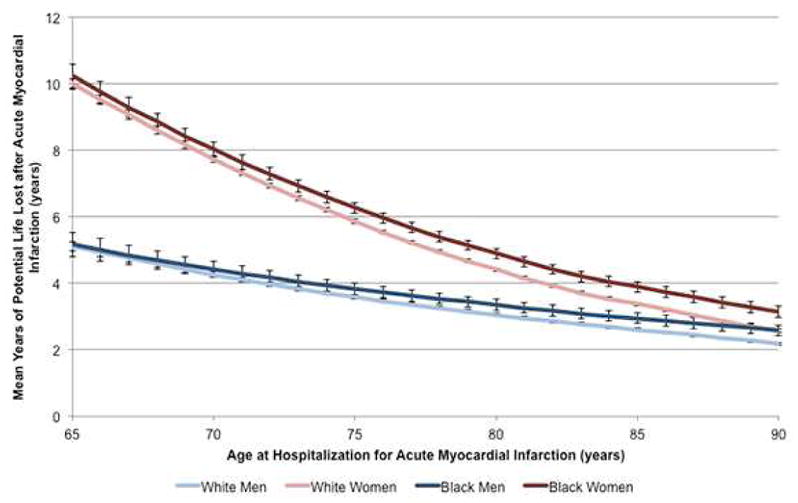
Years of potential life lost after AMI for white men (light blue), white women (light red), black men (dark blue), and black women (dark red) are shown by age. After accounting for sex differences in life expectancy in the general Medicare population, there were large sex differences in years of potential life lost, particularly in younger patients. Racial differences in YPLLs were less pronounced.
The percentages of YPLLs are presented in Figure 4. Among younger patients, black women lost the greatest percentage of YPLLs after AMI, followed by white women then black men (p <0.001 for differences across groups). For example, among patients aged 65 at the time of AMI, black women lost 54.4% (SE, 0.9%) of their potential life after AMI, whereas white men lost only 29.1% (SE, 0.4%) of their potential life. Sex and racial differences in YPLL percentages were greatest among younger ages but became diminished in the oldest patients (p = 0.25 and p = 0.27 for sex and race, respectively).
Figure 4. Percentage of years of potential life lost after AMI by sex and race.
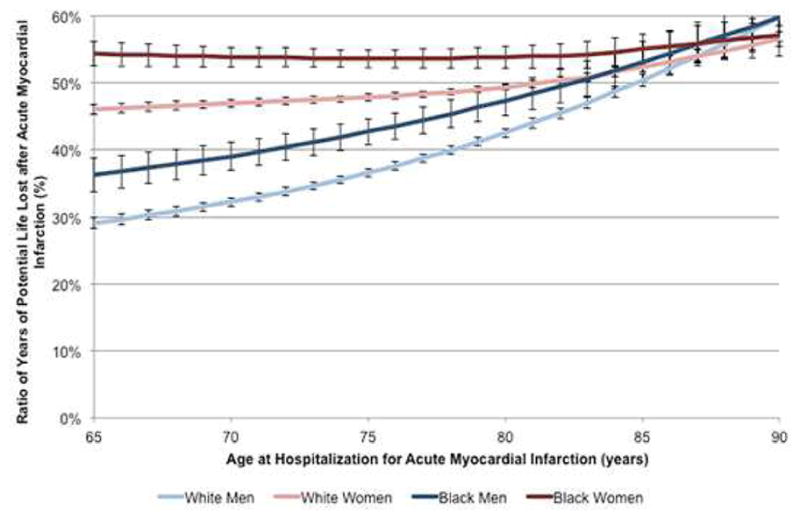
The percentage of YPLLs after AMI to life expectancy in the general population for white men (light blue), white women (light red), black men (dark blue), and black women (dark red) is shown by age. Black women lost the greatest percentage of life-years after AMI, followed by white women and then black men. These differences were most pronounced in younger patients and became similar in very elderly patients.
Both female sex and black race were highly predictive of increased YPLLs in unadjusted analyses (Tables 2 and 3). On average, men lost 41.8% of their remaining life after AMI, whereas women lost 10.5% (SE 0.3%) more of their remaining life than men (52.3% total). Similarly, white patients lost an average of 46.4% of their remaining life after AMI, whereas black patients lost 6.2% (SE 0.6%) more of their remaining life than white patients (52.6% total). After adjustment for demographics, medical history, clinical presentation, and treatment, women still lost an average of 7.8% (SE, 0.3%) more of their remaining life than men; however, the association between black race and percentage of YPLLs switched directions with black patients losing 3.5% (SE, 0.5%) less of their remaining life than white patients. The greatest attenuation in the effect of black race occurred after adjustment for diabetes mellitus, congestive heart failure, clinical presentation, and receipt of revascularization therapies. Additional information on survival rates by age, sex, and race, the expected survival curves, and life expectancy, and YPLL estimates are provided in the Online Appendix.
Table 2.
Unadjusted and adjusted regression coefficients for female sex predicting the percentage of years of potential life lost*
| Model | B (SE) for Female Sex† | p-value |
|---|---|---|
| Unadjusted | 0.105 (0.003) | <0.001 |
| + Age, race | 0.096 (0.003) | <0.001 |
| + Age, race, medical history‡ | 0.084 (0.003) | <0.001 |
| + Age, race, medical history, clinical presentation§ | 0.092 (0.003) | <0.001 |
| + Age, race, medical history, clinical presentation, treatment|| | 0.078 (0.002) | <0.001 |
The intercept for male sex in the unadjusted model was 0.418 (SE 0.002), indicating that men lost on average 41.8% of their remaining life after AMI.
Parameter estimates reflect average difference in life expectancy for women compared with men. For example, prior to adjustment, women lost on average 10.5% more (or 52.3% total) of their remaining life after AMI than men.
Medical history covariates include history of chronic heart failure, diabetes mellitus hypertension, coronary artery disease, and smoking.
Clinical presentation covariates include anterior AMI, ST-segment elevation AMI, cardiac arrest, Killip class, and renal insufficiency.
Treatment covariates include revascularization within 30 days, fibrinolytic therapy, aspirin and beta-blockers at arrival.
Table 3.
Unadjusted and adjusted regression coefficients for black race predicting the percentage of years of potential life lost*
| Model | B (SE) for Black Race† | p-value |
|---|---|---|
| Unadjusted | 0.062 (0.006) | <0.001 |
| + Age, sex | 0.056 (0.006) | <0.001 |
| + Age, sex, medical history‡ | 0.016 (0.005) | 0.004 |
| + Age, sex, medical history, clinical presentation§ | −0.007 (0.005) | 0.150 |
| + Age, sex, medical history, clinical presentation, treatment|| | −0.035 (0.005) | <0.001 |
The intercept for white race in the unadjusted model was 0.464 (SE 0.001), indicating that white patients lost on average 46.4% of their remaining life after AMI.
Parameter estimates reflect average difference in life expectancy for black patients compared with white patients. For example, prior to adjustment, black patients lost on average 6.2% more (or 52.6% total) of their remaining life after AMI than white patients.
Medical history covariates include history of chronic heart failure, diabetes mellitus hypertension, coronary artery disease, and smoking.
Clinical presentation covariates include anterior AMI, ST-segment elevation AMI, cardiac arrest, Killip class, and renal insufficiency.
Treatment covariates include revascularization within 30 days, fibrinolytic therapy, aspirin and beta-blockers at arrival.
DISCUSSION
Sex Differences
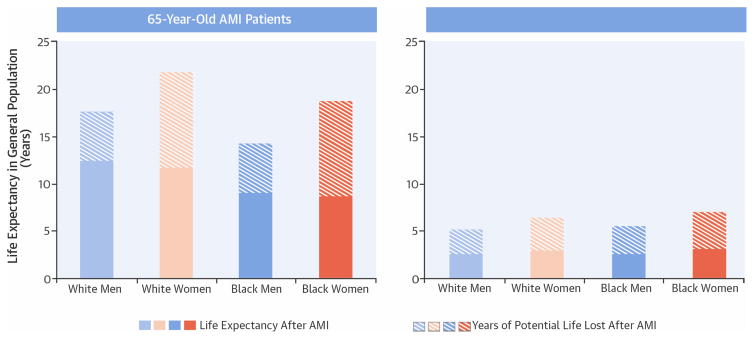
In this study, we found large differences in life expectancy and YPLLs between sexes and races with younger women and black patients having the largest YPLLs (Central Illustration). Men and women of the same race had similar post-AMI life expectancy; however, younger women lost significantly more years of life after AMI compared with men of the same age. Per 1,000 patients hospitalized for AMI, white women lost 1,808 more years than white men and black women lost 2,451 more years than black men; these differences were more pronounced in younger patients.
Central Illustration. Sex, Race, and Life Expectancy after AMI: Life expectancy and YPLLs after AMI in 65- and 85-year-old Patients.
Life expectancy after acute myocardial infarction (AMI) was similar in men and women of the same race. Because women live longer than men in the general population, women lost more years of potential life lost (YPLLs) after AMI. In contrast, black patients had shorter life expectancies after AMI and in the general population. However, they also lost more YPLLs after AMI. Race differences in YPLLs were largely explained by the higher risk factor burden and lower treatment rates in black patients; however, sex differences in YPLLs persisted after adjustment.
By adopting a population perspective, our findings shed new insight on sex differences in survival after AMI in elderly patients. Previous studies have invariably restricted their analyses to patients with AMI and have not considered the entire population experience. As a result, they have generally reported higher crude mortality rates for women after AMI that become attenuated after adjustment for age and clinical factors (7). Like other studies, we found similar life expectancies for men and women after AMI. However, after accounting for differences in expected survival, women lost significantly more years of life than men and remained at a significant survival disadvantage even after multivariable adjustment. For example, we estimated that 65-year-old white men and women are estimated to lose 5.1 and 10.0 years of life after AMI. Sixty-five-year-old white men are expected to live another 17.6 years and white women another 21.7 years, this translates into a 29% reduction in remaining life for men and a 41% reduction for women. Thus, 65-year-old white women are losing 4.9 more years of life, or 12% more, of their remaining life than men. Thus, strategies to avert AMI in women may actually produce more benefit than men.
To our knowledge, only 3 studies have accounted for expected mortality when comparing sex differences in survival after AMI (13–15). Using Cox proportional hazards models with time-dependent covariates, Vaccarino et al. found that AMI was associated with a greater risk of 10-year mortality in women and significantly narrowed women’s typical survival advantage over men (15). Similarly, Peeters et al. conducted a life table analysis of the Framingham Heart Study cohort to estimate life years lost among 60-year-old men and women with a history of AMI (14). Like our study, they reported larger reductions in life expectancy for women than men with a history of AMI (13 versus 9 years, respectively). In contrast, Howland et al. found no difference in 5-year relative survival between men and women after standardizing observed survival against national rates (13). Discrepancies in study findings are likely attributable to differences in study populations, methods, and length of follow-up.
Although we would expect women to live longer than men after AMI due to their life expectancy in the general population, AMI appears to act as an equalizer by negating women’s survival advantage. These sex differences in YPLLs may be due to intrinsic biological and psychosocial factors or disparities in care during or after the index hospitalization. Indeed, the magnitude of the difference in YPLLs between men and women decreased slightly after adjustment for inhospital treatment. Prior studies have found differences in rates of angiography, primary care, and cardiology follow-up in the year following AMI between men and women (19). These findings suggest that part of the sex difference in YPLLs may be attributable to differences in the quality of care received. Although we can speculate as to how much of this difference is due to biological differences between men and women versus disparities in care, it is likely some combination of both. Additional research is needed to disentangle these effects.
Racial Differences
Black patients generally had lower life expectancies post-AMI than white patients, which persisted after accounting for racial differences in population-based life expectancy. On average, black men lost 0.3 more years of life than white men, and black women lost 1.0 more years of life than white women. In relative percentages, this equates to an additional 5% more time lost by black men and women compared with white patients, which suggests that the burden of AMI is higher in black than in white patients. Racial differences in disease burden, however, were largely explained by differences in comorbidities and treatment utilization between black and white patients, adjustment for these factors attenuated racial differences in post-AMI YPLLs.
Our results are consistent with but also extend the prior literature on racial differences in outcomes after AMI. Most studies of racial differences after AMI have reported comparable or lower mortality for black patients in the short-term (20–23), but higher mortality over the long-term as compared with white patients (9–11). Many studies have hypothesized that racial differences in long-term mortality are due to a higher prevalence of comorbid conditions and lower utilization of invasive therapies in black patients (9,22,24–27). Accordingly, we found that black patients had a higher prevalence of diabetes mellitus, hypertension, and heart failure, and they were significantly less likely to receive interventional procedures within 30 days compared with white patients (21.4% vs. 30.7%, p <0.0001).
Implications of Findings
The distinction between disease-specific differences and differences in the broader population is fundamental to understanding how the burden of AMI differs by sex and race and effectively targeting research and interventions. Our observation that women and black patients with AMI are losing more years of life after AMI places a premium on secondary prevention in these groups and underscores the importance of national efforts to ensure equitable delivery of care. Because racial differences in YPLLs after AMI were largely explained by the poorer clinical presentation and lower treatment rates in black patients, efforts to reduce racial differences should target risk factor management and disparities in cardiac care. In contrast, sex differences in YPLLs were not attenuated after multivariable adjustment suggesting the need for additional research to understand this difference, which may be explained by either biological and psychosocial differences or other disparities in care. Strategies that warrant investigation include reducing the higher risk factor burden in women and improving long-term management of heart disease.
Additionally, by accounting for population-based life expectancy in our analyses, we demonstrated relationships between sex, race, and post-AMI survival that could not be detected using traditional mortality estimates. Although most datasets lack the representativeness and length of follow-up afforded by the CCP, several other techniques for adjusting for population differences in life expectancy have been described, which may be feasible for studies with shorter follow-up or smaller sample sizes. Such techniques include “relative survival,” life table methods, parametric survival methods, and Monte Carlo simulations (12,28–33). Regardless of the method selected, studies comparing disease-related survival between patients with different life expectancies should consider the overall population experience in their analyses.
Limitations
We recognize that our analysis has several limitations. First, black patients represented only 6.4% of the cohort, which resulted in small numbers of patients in the older age, sex, and race-specific strata. We modeled the cohort using semiparametric techniques to avoid stratum-specific estimation. Nonetheless, the small sample sizes in some strata may still have limited the predictive power of our models. Second, approximately 10% of patients in the CCP were still alive after 17 years of follow-up, which required extrapolation of the expected survival curves in order to calculate life expectancy. It is possible that using an exponential model may have led to slightly biased estimates. Third, we lacked medical history information for patients in the Medicare Denominator files, and thus were unable to estimate life expectancy in an “AMI-free” population. Using age, sex, and race-specific AMI incidence from the Atherosclerosis Risk in Communities Surveillance Study, we estimate that the prevalence of AMI in the general Medicare population was 14% in white men, 9% in white women, 21% in black men, and 14% in black women (34). Because the average age of coronary heart disease (CHD) onset in men precedes that in women and black patients have a higher incidence of CHD than white patients at all ages, the observed sex differences in life expectancy in the general Medicare population may be partly attributable to the higher prevalence of CHD in men which may, in turn affect the magnitude of sex and race differences in YPLLs we calculated. Fourth, the YPLLs reported in this study may be attributable to factors other than AMI. Because patients with AMI have a greater burden of cardiovascular risk factors than patients in the general population, the higher long-term mortality in patients with AMI may be due, in part, to other comorbidities. Similarly, sex and race differences in YPLLs after AMI may reflect differences in the burden or management of other conditions that are more common in patients with AMI such as diabetes and heart failure. Although it was not possible to adjust for these factors when calculating life expectancy estimates for the general population, we addressed this issue, in part, by adjusting for sex and race differences in the prevalence of these risk factors and comorbidities at baseline. However, it is still possible that the adjusted differences in YPLLs between men and women are attributable to sex differences in the care or outcomes of other AMI-related conditions. Finally, because the quality of care for patients with AMI has improved since the mid-1990s and patients are living longer today, the life expectancy estimates reported in this manuscript may not accurately reflect those of patients today. Additionally, there have been several national efforts over the last two decades to increase awareness of heart disease in women and to reduce racial differences in health outcomes. Nevertheless, data from nationally representative cohorts suggests that although sex and race differences in mortality after AMI have declined over time, there remains a persistent difference (35,36). Although these data would suggest that race and sex differences today are likely smaller than those found in CCP, life expectancy estimates in the general population have also increased by over a year since the mid-1990s (37). Thus, it is difficult to speculate on how the magnitude of these differences has changed over time when standardized again the general population. These limitations are inherent to any study that involved long follow-up.
Although these analyses have their limitations, this study has several strengths that allow us to disentangle disease-specific differences from those in the broader population over the lifespan. Calculation of life expectancy requires complete follow-up over many years, which contemporary datasets currently lack. Although clinical advances and performance improvement efforts have improved the care for patients with AMI since the mid-1990s, life expectancy calculations are necessary to understand how sex and race differences persist and evolve over time and are only possible with long-term follow-up. Additionally, thorough adjustment for differences in patient and hospital characteristics between patients requires detailed clinical information, which only a dataset like CCP can offer.
Conclusions
In conclusion, we found that after adjusting for differences in population-based life expectancy, women and black patients were at significantly higher risk of losing more years of life after AMI. Whereas much of the racial difference in years of potential life lost could be explained by differences in clinical presentation and treatment between black and white patients, this was not true for the observed sex differences.
Supplementary Material
Supplemental Figure 1. Expected survival curves after acute myocardial infarction for patients aged 65 years (A) and 85 years (B) by sex and race.
Supplemental Table 1. Percentage of patients with acute myocardial infarction still living after 17 years of follow-up
Supplemental Table 2. Life expectancy of patients after acute myocardial infarction by age, sex, and race
Supplemental Table 3. Sample characteristics of Medicare fee-for-service enrollees from January, 1994-December, 1995 (N=29,253,643)
Supplemental Table 4. Mean years of potential life lost (YPLLS) after acute myocardial infarction by age, sex, and race
PERSPECTIVES.
Competency in Medical Knowledge
Women and black patients lose more years of life, on average, than men and white patients, respectively, after acute myocardial infarction (AMI). Racial differences are explained by higher comorbidity and lower treatment rates in black patients.
Translational Outlook
Additional research is needed to understand why women face a long-term survival disadvantage after AMI compared to men and to identify targets for secondary prevention linked to survival.
Acknowledgments
The authors acknowledge the assistance of Qualidigm and the Centers for Medicare & Medicaid Services (CMS) in providing data, which made this research possible. The content of this publication does not reflect the views of Qualidigm or CMS, nor does mention of organizations imply endorsement by the U.S. Government. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
Financial Support: Bucholz is supported by an F30 Training grant F30HL120498-01A1 from the National Heart, Lung, and Blood Institute and by NIGMS Medical Scientist Training Program grant T32GM07205. Krumholz is supported by grant U01 HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Normand, Ma, Lin, and Wang report no financial disclosures that contributed to the production of this manuscript.
Abbreviations List
- AMI
acute myocardial infarction
- CCP
Cooperative Cardiovascular Project
- CI
confidence interval
- SE
standard error
- YPLL
years of potential life lost
Footnotes
Disclosures: HMK has received research grants from Medtronic, Inc. (Minneapolis, MN) and Johnson and Johnson (New Brunswick, NJ) through Yale University for the purpose of disseminating clinical trials and chairs the Cardiac Scientific Advisory Board for United Health (Minneapolis, MN). SLN is a member of the Board of Directors of Frontier Science and Technology (Boston, MA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benderly M, Behar S, Reicher-Reiss H, et al. Long-term prognosis of women after myocardial infarction. SPRINT Study Group. Secondary Prevention Reinfarction Israeli Nifedipine Trial. Am J Epidemiol. 1997;146:153–60. doi: 10.1093/oxfordjournals.aje.a009246. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RJ, Gorak EJ, Yarzebski J, et al. A community wide perspective of sex differences and temporal trends in the incidence and survival rates after acute myocardial infarction and out-of-hospital deaths caused by coronary heart disease. Circulation. 1993;87:1947–53. doi: 10.1161/01.cir.87.6.1947. [DOI] [PubMed] [Google Scholar]
- 3.He J, Klag MJ, Whelton PK, et al. Short- and long-term prognosis after acute myocardial infarction in Chinese men and women. Am J Epidemiol. 1994;139:693–703. doi: 10.1093/oxfordjournals.aje.a117059. [DOI] [PubMed] [Google Scholar]
- 4.Isaksson RM, Jansson JH, Lundblad D, et al. Better long-term survival in young and middle-aged women than in men after a first myocardial infarction between 1985 and 2006. An analysis of 8630 patients in the northern Sweden MONICA study. BMC Cardiovasc Disord. 2011;11:1. doi: 10.1186/1471-2261-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang WC, Kaul P, Westerhout CM, et al. Impact of sex on long-term mortality from acute myocardial infarction vs unstable angina. Arch Intern Med. 2003;163:2476–84. doi: 10.1001/archinte.163.20.2476. [DOI] [PubMed] [Google Scholar]
- 6.Machon M, Basterretxea M, Martinez-Camblor P, et al. Sex differences in relative survival and prognostic factors in patients with a first acute myocardial infarction in Guipuzcoa, Spain. Rev Esp Cardiol. 2010;63:649–59. doi: 10.1016/s1885-5857(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 7.Bucholz EM, Butala NM, Rathore SS, et al. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–67. doi: 10.1161/CIRCULATIONAHA.114.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias E. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2012. United States Life tables, 2008. [PubMed] [Google Scholar]
- 9.Iribarren C, Tolstykh I, Somkin CP, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165:2105–13. doi: 10.1001/archinte.165.18.2105. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RH, Marks D, Califf RM, et al. Differences in the clinical features and outcomes in African Americans and whites with myocardial infarction. Am J Med. 2006;119:70, e1–8. doi: 10.1016/j.amjmed.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan J, Schreiber TL, Niraj A, et al. Comparison of five-year outcome in African Americans versus Caucasians following percutaneous coronary intervention. Catheter Cardiovasc Interv. 2008;72:36–44. doi: 10.1002/ccd.21556. [DOI] [PubMed] [Google Scholar]
- 12.Gardner JW, Sanborn JS. Years of potential life lost (YPLL)--what does it measure? Epidemiology. 1990;1:322–9. doi: 10.1097/00001648-199007000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Howland JS, Vaillant HW. Long-term survival of 224 patients with myocardial infarction treated in a community hospital. J Fam Pract. 1980;10:979–83. [PubMed] [Google Scholar]
- 14.Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history. A life course analysis of the original Framingham Heart Study cohort. Eur Heart J. 2002;23:458–66. doi: 10.1053/euhj.2001.2838. [DOI] [PubMed] [Google Scholar]
- 15.Vaccarino V, Berkman LF, Krumholz HM. Long-term outcome of myocardial infarction in women and men: a population perspective. Am J Epidemiol. 2000;152:965–73. doi: 10.1093/aje/152.10.965. [DOI] [PubMed] [Google Scholar]
- 16.Caro JJ, Ishak KJ, Migliaccio-Walle K. Estimating survival for cost-effectiveness analyses: a ase study in atherothrombosis. Value Health. 2004;7:627–35. doi: 10.1111/j.1524-4733.2004.75013.x. [DOI] [PubMed] [Google Scholar]
- 17.Marciniak TA, Ellerbeck EF, Radford MJ, et al. Improving the quality of care for Medicare patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. JAMA. 1998;279:1351–7. doi: 10.1001/jama.279.17.1351. [DOI] [PubMed] [Google Scholar]
- 18.Ramunno LD, Dodds TA, Traven ND. Cooperative Cardiovascular Project (CCP) quality improvement in Maine, New Hampshire, and Vermont. Eval Health Prof. 1998;21:442–60. doi: 10.1177/016327879802100404. [DOI] [PubMed] [Google Scholar]
- 19.Alter DA, Naylor CD, Austin PC, Tu JV. Biology or bias: practice patterns and long-term outcomes for men and women with acute myocardial infarction. J Am Coll Cardiol. 2002;39:1909–16. doi: 10.1016/s0735-1097(02)01892-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Rathore SS, Radford MJ, et al. Racial differences in the use of cardiac catheterization after acute myocardial infarction. N Engl J Med. 2001;344:1443–9. doi: 10.1056/NEJM200105103441906. [DOI] [PubMed] [Google Scholar]
- 21.Jacobi JA, Parikh SV, McGuire DK, et al. Racial disparity in clinical outcomes following primary percutaneous coronary intervention for ST elevation myocardial infarction: influence of process of care. J Interv Cardiol. 2007;20:182–7. doi: 10.1111/j.1540-8183.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 22.Petersen LA, Wright SM, Peterson ED, Daley J. Impact of race on cardiac care and outcomes in veterans with acute myocardial infarction. Med Care. 2002;40:I86–96. doi: 10.1097/00005650-200201001-00010. [DOI] [PubMed] [Google Scholar]
- 23.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994;271:1175–80. [PubMed] [Google Scholar]
- 24.Maynard C, Every NR, Martin JS, Weaver WD. Long-term implications of racial differences in the use of revascularization procedures. Am Heart J. 1997;133:656–62. doi: 10.1016/s0002-8703(97)70167-4. [DOI] [PubMed] [Google Scholar]
- 25.Peterson ED, Shaw LK, DeLong ER, et al. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;336:480–6. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- 26.Spertus JA, Jones PG, Masoudi FA, et al. Factors associated with racial differences in myocardial infarction outcomes. Ann Intern Med. 2009;150:314–24. doi: 10.7326/0003-4819-150-5-200903030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tofler GH, Stone PH, Muller JE, et al. Effects of gender and race on prognosis after myocardial infarction: adverse prognosis for women, particularly black women. J Am Coll Cardiol. 1987;9:473–82. doi: 10.1016/s0735-1097(87)80038-4. [DOI] [PubMed] [Google Scholar]
- 28.Chu PC, Wang JD, Hwang JS, Chang YY. Estimation of life expectancy and the expected years of life lost in patients with major cancers: extrapolation of survival curves under high-censored rates. Value Health. 2008;11:1102–9. doi: 10.1111/j.1524-4733.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 29.Haybittle JL. The use of the Gompertz function to relate changes in life expectancy to the standardized mortality ratio. Int J Epidemiol. 1998;27:885–9. doi: 10.1093/ije/27.5.885. [DOI] [PubMed] [Google Scholar]
- 30.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–55. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 31.Peeters A, Barendregt JJ, Willekens F, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 32.Singh M, Mathew V, Garratt KN, et al. Effect of age on the outcome of angioplasty for acute myocardial infarction among patients treated at the Mayo Clinic. Am J Med. 2000;108:187–92. doi: 10.1016/s0002-9343(99)00429-5. [DOI] [PubMed] [Google Scholar]
- 33.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh JA, Lu X, Ibrahim S, Cram P. Trends in and disparities for acute myocardial infarction: an analysis of Medicare claims data from 1992 to 2010. BMC Med. 2014;12:190. doi: 10.1186/s12916-014-0190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bucholz EM, Butala NM, Rathore SS, et al. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation. 2014;130:757–67. doi: 10.1161/CIRCULATIONAHA.114.009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. [Accessed on April 11, 2015];Life expectancy at birth, at 65 years of age, and at 75 years of age, by race and sex: United States, selected years 1900–2007. http://www.cdc.gov/nchs/data/hus/2010/022.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Expected survival curves after acute myocardial infarction for patients aged 65 years (A) and 85 years (B) by sex and race.
Supplemental Table 1. Percentage of patients with acute myocardial infarction still living after 17 years of follow-up
Supplemental Table 2. Life expectancy of patients after acute myocardial infarction by age, sex, and race
Supplemental Table 3. Sample characteristics of Medicare fee-for-service enrollees from January, 1994-December, 1995 (N=29,253,643)
Supplemental Table 4. Mean years of potential life lost (YPLLS) after acute myocardial infarction by age, sex, and race