Abstract
Objective
To determing the utility of dried blood spot (DBS) PCR in identifying infants with cytomegalovirus infection (CMV)-associated sensorineural hearing loss (SNHL).
Study design
Newborns at 7 U.S. hospitals from March 2007-March 2012 were screened for CMV by saliva rapid culture and/or PCR. Infected infants were monitored for SNHL during the first 4 years of life to determine sensitivity, specificity, positive and negative likelihood ratios of DBS PCR for identifying CMV-associated SNHL.
Results
DBS at birth was positive in 11/26 (42%) children with SNHL at 4 years of age and 72/270 (27%) children with normal hearing (p=0.11). The sensitivity (42.3%, 95% CI 23.4%–63.1%) and specificity (73.3%, 95% CI, 67.6%–78.5%) was low for DBS PCR in identifying children with SNHL at 4 years of age. The positive and negative likelihood ratios of DBS PCR positivity to detect CMV-associated SNHL at 4 years of age were 1.6 (95% CI, 0.97–2.6) and 0.8 (95% CI, 0.6–1.1), respectively. There was no difference in DBS viral loads between children with and without SNHL.
Conclusions
DBS PCR for CMV has low sensitivity and specificity for identifying infants with CMV-associated hearing loss. These findings together with previous reports demonstrate that DBS PCR neither identifies the majority of CMV-infected newborns nor those with CMV-associated SNHL early in life.
Keywords: cytomegalovirus, dried blood spot, hearing loss
Cytomegalovirus (CMV) is an important cause of congenital infection and congenital CMV infection (cCMV) is a significant non-genetic cause of sensorineural hearing loss (SNHL) in children.1–4 Most congenitally infected infants (~90%) do not have obvious clinical abnormalities at birth (asymptomatic cCMV) and therefore, are not identified in the newborn nursery.5,6 However, approximately 15% of children with asymptomatic cCMV develop SNHL.1,7,8 Although CMV-associated SNHL may be present at birth, a substantial proportion of children with cCMV develop late-onset and/or progressive SNHL.1,9 Therefore, most children with cCMV and a significant number of those with CMV-associated SNHL are not identified on routine physical examination or hearing screening in the newborn nursery.
The need to develop rapid and reliable methods to screen newborns for CMV so that infants at increased risk for hearing loss can be identified for targeted monitoring and early intervention has been recognized.10–12 Because dried blood spots (DBS) are collected routinely from all infants in the U.S. for newborn metabolic screening and several initial studies have shown promise, the hope was that DBS polymerase chain reaction (PCR) would facilitate the development of effective strategies to screen all newborns for CMV.13–18 However, in a CMV screening study of 20,446 newborns, we demonstrated that the sensitivity of DBS PCR in identifying infants with cCMV was unacceptably low when compared with saliva rapid culture19 and therefore would not be a suitable screening method. Although DBS PCR failed to identify the majority of infants with cCMV, the possibility that it would detect infants at increased risk for CMV-associated SNHL remained. The present study is aimed at determining the ability of the DBS PCR assay to identify infants with cCMV at risk for disease and sequelae.
METHODS
Between March 2007 and March 2012, infants born at seven US hospitals were enrolled prospectively in the CMV and Hearing Multicenter Screening (CHIMES) study supported by the National Institutes on Deafness and Other Communication Disorders.19,20 Newborn CMV screening was carried out by testing saliva specimens (rapid culture or PCR) and DBS PCR as described previously.19,20 Between March 2007 and May 2008, all DBS specimens collected from screened infants were tested by PCR.19 After May 2008, only DBS specimens from infants who were positive for CMV by rapid culture or PCR of saliva were tested. Demographic information, newborn hearing screening results, saliva and DBS specimens were collected from the screening cohort. Race and ethnicity were self-reported by the parents of the infants and categorized by the NIH definitions for race and ethnicity.21 DBS for this study were collected on a separate card after obtaining the sample for routine newborn metabolic screening without an additional heel stick. The parents/guardians of the screened infants were provided the CMV screening results.
Infants were considered to have symptomatic cCMV if they had any of the following findings in the newborn period: generalized petechial rash, purpuric rash, hepatomegaly, splenomegaly, jaundice with direct bilirubin ≥ 3 mg/dL, unexplained neurologic/CNS abnormalities (e.g., microcephaly, seizures, focal or generalized neurologic deficits), or chorioretinitis. Clinical decisions about evaluation and antiviral treatment of the CMV-infected infants were made by the physicians at each study site and were not part of the CHIMES study. Seventeen infants were treated with antiviral therapy, with nine having SNHL at birth and none having late onset SNHL. The 17 treated infants are included in the cohort for evaluating both DBS positivity and symptomatic status and DBS positivity and SNHL at birth. However, because antiviral therapy has been shown to affect hearing outcome,22 the 17 treated infants were not included in the comparison of DBS positivity and hearing loss at 4 years of age.
Medical records were reviewed for family history of hearing loss or other potential etiologies including congenital malformations that could cause SNHL. In addition, the parents were asked at enrollment about any family history of hearing loss. Also, any new diagnosis possibly related to SNHL was collected from the parents at the follow-up visits. None of the children with SNHL had syndromes or other malformations associated with either SNHL or a family history of SNHL. Local institutional review board approval was obtained at each site.
Follow-up of Children with Congenital CMV Infection
Infants with a positive saliva or DBS screening test were enrolled in the follow-up component of the study to confirm cCMV and to monitor hearing function during the first 4 years of life per study protocol. Infants were considered to have confirmed cCMV if the follow-up urine or saliva sample was positive by rapid culture and/or PCR. After the initial diagnostic audiological evaluation between 3 and 8 weeks of age, the study children were monitored for hearing loss using age and developmentally appropriate audiological testing protocols every 6 months during the first 4 years of life.7
Statistical Analyses
All statistical analyses were performed using SAS software, version 9.3 (SAS Institute). Two-tailed Fisher’s exact test was used to assess the association between DBS positivity and symptomatic infection and hearing outcomes. Viral load levels were compared between the infants with symptomatic infection and asymptomatic infection, and those with and without hearing loss at 4 years of age by the Wilcoxon Rank-Sum test. Sensitivity, specificity, and predictive values for the DBS positivity were calculated using standard methods for proportions and exact 95% confidence limits. Likelihood ratios (LRs) were calculated to summarize the diagnostic accuracy of the DBS PCR. Positive LR was sensitivity/(1-specificity) and the negative LR was (1-sensitivity)/specificity. Confidence intervals (CIs) for LRs were determined using the method described by Simel et al.23
RESULTS
During the study period 100,332 infants were enrolled and screened for cCMV. Screening DBS samples were available from 313/391 (80.1%) infants with confirmed cCMV, and these infants constituted this study population (Figure 1; available at www.jpeds.com). Reasons for unavailability of DBS specimens included: the collection of DBS for the routine metabolic screening before study consent was obtained (72); insufficient blood for an additional study filter card (2); loss of DBS sample (3); and refusal by mother (1). There was no difference in the proportion of infants with symptomatic cCMV and SNHL between those without a screening DBS sample and those with an available DBS. Among the 313 study children, congenital infection was confirmed by rapid culture of saliva or urine samples in 302 infants and by PCR of saliva or urine in the remaining 11 infants.
Figure 1.
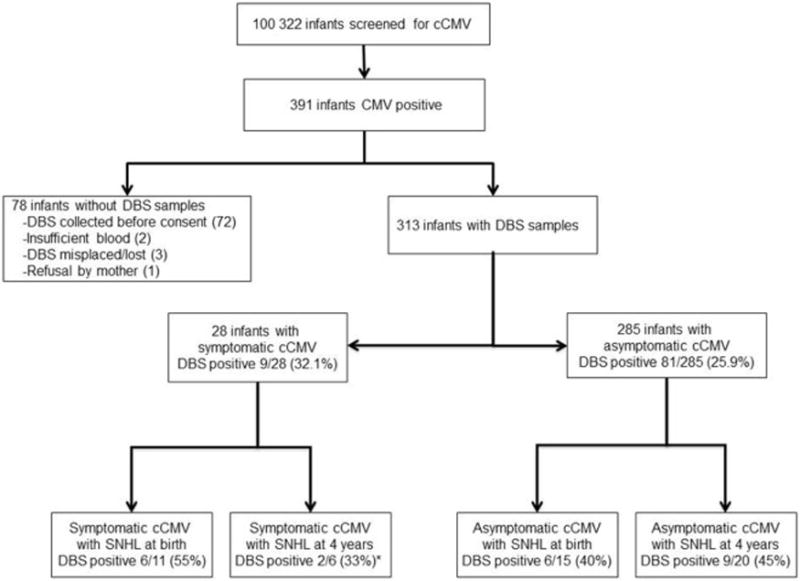
Dried blood spot (DBS) PCR to identify infants with hearing loss in congenital CMV infection. *Infants who received antiviral therapy were excluded from the number of infants with symptomatic cCMV who had hearing loss at 4 years of age.
Of the 313 study children, approximately half were female (48.6%, CI 42.9%–54.3%) and of black race (47.0%, CI 41.3%–52.7%). The racial make-up of the remaining population was 23.0% (CI 18.5%–28.1%) non-Hispanic white, 25.6% (CI 20.8%–30.8%) Hispanic white, 3.2% (CI 1.5%–5.8%) multi-racial and 1.2% (CI 0.35%–3.2%) Asian. Most infants (93.0%, CI 89.6%–95.5%) were from the well-baby nurseries and had public or no insurance (80.8%, CI 76.0%–85.0%). The mean age of DBS sample collection was 2.29 ± 2.19 days of life.
Among the 313 infected infants, 90 DBS samples (28.8%, CI 23.8%–34.1%) were positive for CMV. DBS PCR was positive in 9/28 (32.1%) symptomatic infants compared with 81/285 (25.9%) of infants with asymptomatic cCMV at birth (p=0.7, Figure 1). To determine whether DBS PCR has a role in identifying infants with CMV-associated SNHL at birth, the results of DBS PCR were compared between infants with hearing loss at birth and those with normal hearing (Table). DBS was positive in 12/26 (46%) infants with SNHL at birth compared with 78/287 (27%) children with normal hearing at birth (p=0.07). Among the 285 infants with asymptomatic cCMV, DBS PCR was positive in 6/15 (40%) children with SNHL at birth compared with 75/270 (28.0%, p=0.38) infants with normal hearing. For infants with symptomatic cCMV (n=28), DBS PCR was positive in 6/11 (55%) infants with hearing loss at birth, compared with 3/17 (18%) infants with normal hearing (p=0.10). The sensitivity and specificity of PCR of newborn DBS for detecting infants with SNHL at birth were 46.2% (95% CI, 26.6%–66.6%) and 72.8% (95% CI, 67.3%–77.9%), respectively (Table). The positive likelihood ratio (LR) for DBS positivity was 1.7 (95% CI, 1.1–2.7) and the negative LR was 0.74 (95% CI, 0.5–1.1). The positive predictive value of the DBS PCR for detecting SNHL at birth was 13.3% (95% CI 7.1%–22.1%) and the negative predictive value was 93.7% (95% CI 89.7%–96.5%).
Table 1.
DBS PCR at birth to identify sensorineural hearing loss at birth and at 4 years of age in children with congenital CMV infection
| DBS obtained at birth
| |||||||
|---|---|---|---|---|---|---|---|
| SNHL at birth | Positive | Negative | Total | SNHL at 4 years*† | Positive | Negative | Total |
| Yes | 12 | 14 | 26 | Yes | 11 | 15 | 26 |
| No | 78 | 209 | 287 | No | 72 | 198 | 270 |
| Total | 90 | 223 | 313 | Total | 83 | 213 | 296* |
|
| |||||||
| Other analyses % (95% CI) | |||||||
|
| |||||||
| Sensitivity | 46.2 (26.6–66.6) | 42.3 (23.4–63.1) | |||||
| Specificity | 72.8 (67.3–77.9) | 73.3 (67.6–78.5) | |||||
| Positive likelihood ratio | 1.70 (1.08–2.68) | 1.59 (1.97–2.59) | |||||
| Negative likelihood ratio | 0.74 (0.51–1.06) | 0.79 (0.56–1.1) | |||||
| Positive predictive value | 13.3 (7.1–22.1) | 13.3 (6.8–22.5) | |||||
| Negative predictive value | 93.7 (89.7–96.5) | 93.0 (88.7–96.0) | |||||
17 infants treated with antiviral therapy were removed from the analysis
All children with SNHL at birth also had SNHL at 4 years of age
DBS at birth was positive in 11/26 (42%) children with SNHL at 4 years of age compared with 72/270 (27%) children with normal hearing (p=0.11, Table). Among children with asymptomatic infection (n=277), DBS obtained at birth was positive for CMV in 9/20 (45%) children who developed SNHL by 4 years of age compared with 70/257 (27%) with normal hearing (p=0.12). Among those with symptomatic infection, DBS PCR was positive for CMV in 2/6 (33.3%) of children with SNHL at 4 years of age compared with 2/13 (15.4%) of those with normal hearing (p=0.56). The sensitivity (42.3%, 95% CI 23.4%–63.1%) and specificity (73.3%, 95% CI, 67.6%–78.5%) remain low for DBS PCR in identifying children with SNHL at 4 years of age (Table). The positive and negative likelihood ratios (LR) of DBS PCR positivity to detect CMV-associated SNHL at 4 years of age were 1.59 (95% CI, 0.97–2.6) and 0.79 (95% CI, 0.6–1.1), respectively. The positive and negative predictive values of the DBS PCR for detecting SNHL at 4 years of age were 13.3% (95% CI 6.8%–22.5%) and 93.0% (95% CI 88.7%–96.0%), respectively.
We also examined whether the viral load in DBS is predictive of symptomatic infection and CMV-associated SNHL. There was no difference in median DBS CMV viral load between infants with symptomatic infection (3.0×103 IU/ml, range 3.3×102–6.5×103) and those without clinical findings at birth (2.3×103 IU/ml, range 1.7×102–1.9×105; p=1.00, Figure 2). DBS viral load at birth did not differ between those children with SNHL at birth (3.1 × 103 IU/ml, range 8.3 ×102–1.9 × 105) and those with normal hearing (2.3 × 103 IU/ml, range 1.7 ×102–6.9×104, p=0.51) Similarly, the median DBS viral load was not different between the group of children who developed SNHL by 4 years of age (2.0 × 103 IU/ml, range 8.3 ×102–7.1×104) and those with normal hearing (2.5 × 103 IU/ml, range 1.7 ×102–6.9×104, p=0.44, Figure 2).
Figure 2.
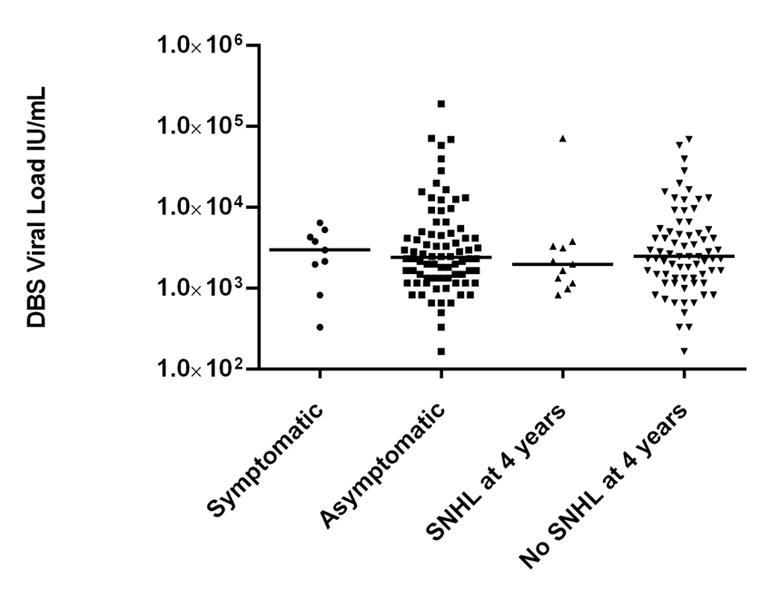
Dried blood spot (DBS) viral load levels at birth in infants with congenital CMV infection. Median viral load values do not differ between infants with symptomatic infection and asymptomatic infection, or between infants that develop SNHL and those with normal hearing.
DISCUSSION
Our findings demonstrate that PCR testing of newborn DBS for CMV DNA has low sensitivity and specificity for identifying infants with CMV-associated hearing loss at birth as well as at 4 years of age. We have previously reported that our PCR method to detect CMV in DBS specimens has low sensitivity (~32%) for identifying infants with cCMV19. Although cCMV is an important non-genetic cause of SNHL in children, most infected infants are not recognized at birth. The identification of predictors of adverse outcome in cCMV are urgently needed, especially for infants with asymptomatic infection at increased risk for SNHL. The findings of the current study together with our previous study demonstrate that DBS PCR is not useful in identifying most congenitally infected infants nor does DBS PCR identify the majority of infants at risk for CMV-associated SNHL. Among infants with asymptomatic cCMV, DBS PCR failed to identify over half of the children who developed SNHL. Furthermore, a positive likelihood ratio close to 1 for both SNHL at birth and at 4 years indicates that a positive DBS result at birth has poor diagnostic accuracy for detecting hearing loss in cCMV. Negative LRs for DBS positivity at birth are not sufficiently small enough to rule out the development of hearing loss in infants with a negative DBS at birth.
In an attempt to identify a marker for hearing outcome, studies have examined the relationship between blood viral load and SNHL in cCMV and reported conflicting findings.24–27. Most of these studies have performed PCR of whole blood to assess viral load, which may be different from DBS viral load. In a retrospective study, Walter et al examined the association between DBS viral load and hearing loss in 39 children with known cCMV. DBS viral load was higher among children with hearing loss (2.69 logs) than those with normal hearing (1.64 logs).27 In contrast, the findings of our large prospective study that included 313 children with cCMV show that median viral load was not different between children with SNHL and those with normal hearing. Of note, in the Walter et al study, DBS PCR was negative in 11/39 infants with cCMV and 5 of the infants with negative DBS developed SNHL.27 These data, along with the current findings, indicate that neither DBS positivity nor viral load levels are useful prognostic markers of hearing outcome in cCMV.
A limitation of this study is that DBS specimens were not available from all infants who tested positive for CMV on newborn screening. However, specimens from most (313/391, 80%) infants with confirmed cCMV including those with asymptomatic infection could be tested. In addition, there were no differences in the frequency of symptomatic infection and hearing loss in children with unavailable DBS compared with the study population. Therefore, unavailability of DBS from approximately 20% of infants is unlikely to have influenced the findings of the study. An additional limitation is that the study children were only monitored for hearing function and therefore, the association between DBS PCR assay and other long-term outcomes such as cognitive and motor deficits could not be determined. However, the frequency of cognitive and motor deficits among children with cCMV is significantly lower than hearing impairment, particularly in children with asymptomatic infection, which will require screening and follow-up of much larger number of children.
Another potential limitation is the sensitivity of our DBS PCR assay to detect cCMV, which is less than 40%.19 The findings of our study show that the sensitivity of DBS PCR in identifying infants with CMV-associated SNHL at birth as well as at 4 years of age remain low (42%). It is possible that the sensitivity of the DBS PCR assay could be improved in the future by utilizing different DNA extraction protocols and by further optimizing the PCR assay. Such an improved DBS PCR method may have a better predictive value for identifying children at increased risk for CMV-associated SNHL. However, even if different extraction protocols or other assay enhancements improve DBS PCR detection in the laboratory, it is essential that these protocols to be evaluated in large screening cohorts of newborns to confirm the increased sensitivity.
In summary, the findings of our study showed the lack of utility of testing of newborn DBS specimens for the identification of infants with CMV-associated SNHL. These findings together with those from our previous report demonstrate that DBS PCR neither identifies the majority of CMV-infected newborns nor those with CMV-associated SNHL early in life.
Acknowledgments
Supported by the National Institute on Deafness and Other Communication Disorders (N01 DC50008, HHS-N-263-2012-00010-C).
Abbreviations
- cCMV
congenital cytomegalovirus infection
- SNHL
sensorineural hearing loss
- DBS
dried blood spot
Appendix
Additional members of the CHIMES Study Group include
University of Alabama at Birmingham Health System: Nitin Arora, MBBS, MPH; Amita Bey, MPH; Belinda Blackstone, MS, CCC-A; Jennifer Blumenthal, MD; Valisa Brown, MPH; Alice Brumbach, MSN; Nazma Chowdhury, MBBS, PhD; Steven Febres-Cordero; Monique Jackson, BS; Mirjam Kempf, PhD; David Kimberlin, MD; Noelle Le Lievre; Faye McCollister, EdD; Emily Mixon, MPH; Misty Purser, BS; and Julie Woodruff, AuD. Carolinas Medical Center: Julie C Courtney; Edith L. Cox, AuD; Nubia Flores; Lisa S. Mohamed, AuD; and Milly E. Ricart. Children’s Hospital of Pittsburgh of UPMC: Noreen Jeffrey, RN; Anne Maracek BA, Gretchen Probst AuD, Diane Sabo, PhD. Saint Peter’s University Hospital: Robert W. Tolan Jr., MD; Melissa Calderon, RNC, BSN,; Maria Class, RN; Charlene Drost, RN; Shona McMahan, RN; Marci Schwab, AuD; Christine Glick, MA, CCC-A; Yunfang Zheng, Sc.D., CCC-A; Caitlin Faccone, AuD, CCC-A, F-AAA: University of Cincinnati and Cincinnati Children’s Hospital Medical Center: Daniel Choo, MD; Kate Catalanotto, RN, BSN, CCRC; Linda Jamison, MSN; Patty Kern, RN; Kurt Schibler, MD; Maureen Sullivan-Mahoney, AuD; and Stacie Wethington, RN, CCRC. University of Mississippi Medical Center: Kathy Irving, AuD; Delia Owens, RN; Suzanne Roark, AuD; Mindy Ware, AuD; Sue Windmill, AuD; Kimberly Ward, AuD; Lauren McNichol, AuD; Lauren Gheber, AuD; Rachel Cooper, AuD; and Victoria Gonzalez, AuD. University of texas Southwestern medical Center at Dallas, Parkland Health & Hospital System and Children’s Medical Center Dallas: Cathy Boatman, MS, CIMI; Joseph B. Cantey, MD; Tiana Delgado, M.S.; Jessica Esquivel; Gregory L. Jackson, MD, MBA; Kathy Katz-Gaynor, BS; Alicia Guzman; April Liehr-Townsley, MA, CCC-A; Amanda Lovering, Au.D.; Asuncion Mejias, MD, PhD; John G. Mistrot, MD; Kristine E. Owen, AuD, CCC-A; Peter S. Roland, MD; Oscar Rosado, MD; Teriann Scheets, Au.D.; Angela G. Shoup, PhD; David Sosa; Jessica Santoyo, BA; Elizabeth K. Stehel, MD; Lizette E. Lee, RN; and Fiker Zeray, RN, MS, CPNP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
We are indebted to the infants and their parents who agreed to take part in this study.
References
- 1.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigations of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11:283–90. [PubMed] [Google Scholar]
- 2.Demmler GJ. Infectious Diseases Society of America and Centers for Disease Control: summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev Infect Dis. 1990;13:315–29. doi: 10.1093/clinids/13.2.315. [DOI] [PubMed] [Google Scholar]
- 3.Goderis J, Keymeulen A, Smets K, Van Hoecke H, De Leenheer E, Boudewyns A, et al. Hearing in Children with Congenital Cytomegalovirus Infection: Results of a Longitudinal Study. J Pediatr. 2016;172:110–5 e2. doi: 10.1016/j.jpeds.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 4.Morton CC, Nance WE. Newborn hearing screening–a silent revolution. N Engl J Med. 2006;354:2151–64. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 5.Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–9. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Britt W. Cytomegalovirus. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious Diseases of the Fetus and Newborn Infant. 7. Philadelphia: W.B. Saunders Company; 2011. pp. 704–53. [Google Scholar]
- 7.Fowler KB, McCollister FP, Dahle AJ, Boppana SB, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–30. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 8.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90:862–6. [PubMed] [Google Scholar]
- 9.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135:60–4. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 10.Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Reviews in medical virology. 2014;24:291–307. doi: 10.1002/rmv.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries JJ, Vossen AC, Kroes AC, van der Zeijst BA. Implementing neonatal screening for congenital cytomegalovirus: addressing the deafness of policy makers. Reviews in medical virology. 2011;21:54–61. doi: 10.1002/rmv.679. [DOI] [PubMed] [Google Scholar]
- 12.Williams EJ, Gray J, Luck S, Atkinson C, Embleton ND, Kadambari S, et al. First estimates of the potential cost and cost saving of protecting childhood hearing from damage caused by congenital CMV infection. Arch Dis Child Fetal Neonatal Ed. 2015;100:F501–6. doi: 10.1136/archdischild-2014-306756. [DOI] [PubMed] [Google Scholar]
- 13.Barbi M, Binda S, Primache V, Caroppo S, Dido P, Guidotti P, et al. Cytomegalovirus DNA detection in Guthrie cards: a powerful tool for diagnosing congenital infection. J Clin Virol. 2000;17:159–65. doi: 10.1016/s1386-6532(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 14.Barbi M, Binda S, Primache V, Luraschi C, Corbetta C. Diagnosis of congenital cytomegalovirus infection by detection of viral DNA in dried blood spots. Clinical and diagnostic virology. 1996;6:27–32. doi: 10.1016/0928-0197(96)00228-0. [DOI] [PubMed] [Google Scholar]
- 15.Johansson PJH, Jonsson M, Ahlfors K, Ivarsson SA, Svanberg L, Guthenberg C. Retrospective diagnosis of congenital cytomegalovirus infection performed by polymerase chain reaction in blood stored on filter paper. Scand J Infect Dis. 1997;29:465–8. doi: 10.3109/00365549709011855. [DOI] [PubMed] [Google Scholar]
- 16.Scanga L, Chaing S, Powell C, Aylsworth AS, Harrell LJ, Henshaw NG, et al. Diagnosis of human congenital cytomegalovirus infection by amplification of viral DNA from dried blood spots on perinatal cards. The Journal of molecular diagnostics: JMD. 2006;8:240–5. doi: 10.2353/jmoldx.2006.050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata M, Takano H, Hironaka T, Hirai K. Detection of human cytomegalovirus DNA in dried newborn blood filter paper. J Virol Methods. 1994;46:279–85. doi: 10.1016/0166-0934(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi Y, Miyagawa H, Wada K, Matsumoto S, Arahori H, Tamura A, et al. CMV DNA detection in dried blood spots for diagnosing congenital CMV infection in Japan. Journal of medical virology. 2006;78:923–5. doi: 10.1002/jmv.20642. [DOI] [PubMed] [Google Scholar]
- 19.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr, Palmer AL, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303:1375–82. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364:2111–8. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health NIo. Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. 2015 [Google Scholar]
- 22.Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372:933–43. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. Journal of clinical epidemiology. 1991;44:763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 24.Forner G, Abate D, Mengoli C, Palu G, Gussetti N. High Cytomegalovirus (CMV) DNAemia Predicts CMV Sequelae in Asymptomatic Congenitally Infected Newborns Born to Women With Primary Infection During Pregnancy. J Infect Dis. 2015;212:67–71. doi: 10.1093/infdis/jiu627. [DOI] [PubMed] [Google Scholar]
- 25.Kawada J, Torii Y, Kawano Y, Suzuki M, Kamiya Y, Kotani T, et al. Viral load in children with congenital cytomegalovirus infection identified on newborn hearing screening. J Clin Virol. 2015;65:41–5. doi: 10.1016/j.jcv.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Ross SA, Novak Z, Fowler KB, Arora N, Britt WJ, Boppana SB. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. Pediatr Infect Dis J. 2009;28:588–92. doi: 10.1097/INF.0b013e3181979a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter S, Atkinson C, Sharland M, Rice P, Raglan E, Emery VC, et al. Congenital cytomegalovirus: association between dried blood spot viral load and hearing loss. Arch Dis Child Fetal Neonatal Ed. 2008;93:F280–5. doi: 10.1136/adc.2007.119230. [DOI] [PubMed] [Google Scholar]


