Abstract
The occurrence of musculoskeletal tissue injury or disease and the subsequent functional impairment is at an alarming rate. It continues to be one of the most challenging problems in the human health care. Regenerative engineering offers a promising transdisciplinary strategy for tissues regeneration based on the convergence of tissue engineering, advanced materials science, stem cell science, developmental biology and clinical translation. Biomaterials are emerging as extracellular-mimicking matrices designed to provide instructive cues to control cell behavior and ultimately, be applied as therapies to regenerate damaged tissues. Biodegradable polymers constitute an attractive class of biomaterials for the development of scaffolds due to their flexibility in chemistry and the ability to be excreted or resorbed by the body. Herein, the focus will be on biodegradable polyphosphazene-based blend systems. The synthetic flexibility of polyphosphazene, combined with the unique inorganic backbone, has provided a springboard for more research and subsequent development of numerous novel materials that are capable of forming miscible blends with poly (lactide-co-glycolide) (PLAGA). Laurencin and co-workers has demonstrated the exploitation of the synthetic flexibility of Polyphosphazene that will allow the design of novel polymers, which can form miscible blends with PLAGA for biomedical applications. These novel blends, due to their well-tuned biodegradability, and mechanical and biological properties coupled with the buffering capacity of the degradation products, constitute ideal materials for regeneration of various musculoskeletal tissues.
Lay Summary
Regenerative engineering aims to regenerate complex tissues to address the clinical challenge of organ damage. Tissue engineering has largely focused on the restoration and repair of individual tissues and organs, but over the past 25 years, scientific, engineering, and medical advances have led to the introduction of this new approach which involves the regeneration of complex tissues and biological systems such as a knee or a whole limb. While a number of excellent advanced biomaterials have been developed, the choice of biomaterials, however, has increased over the past years to include polymers that can be designed with a range of mechanical properties, degradation rates, and chemical functionality. The polyphosphazenes are one good example. Their chemical versatility and hydrogen bonding capability encourages blending with other biologically relevant polymers. The further development of Polyphosphazene-based blends will present a wide spectrum of advanced biomaterials that can be used as scaffolds for regenerative engineering and as well as other biomedical applications.
Keywords: Regenerative engineering, Musculoskeletal, Biodegradable polymers, Dipeptide-based Polyphosphazene, Polyphosphazene Blends
Introduction
Regenerative engineering has been defined as the convergence of advanced materials sciences, stem cell sciences, physics, developmental biology, and clinical translation for the regeneration of complex tissues and organ systems.[1-3]. One of the most important aspects involved in regenerative engineering is the way cells, especially stem cells; react when in contact with biomaterials. Precisely, the use of biomaterials as a supportive template for tissue development is vital to this approach.
In the list of existing biomaterials, biodegradable polymers is top and keeps attracting attentions of many researchers due to their design flexibility in physical, and biological properties to meet the complex requirements for the target applications [4-7]. It is possible to confer specific hydrophilic/hydrophobic entities, biodegradable repeating units, or multifunctional groups [8-10]. In tissue engineering applications, biodegradable polymers such as poly(lactide), poly(glycolide), and their copolymers (PLAGA) are the most commonly used [11-17]. However, the bulk erosion of these materials results in the accumulation of acid in the region of implants. This acidic accumulation has been reported to cause catastrophic failure of structural integrity, and adversely affect biocompatibility both in vitro and in vivo [18-23]. It usually results in inflammatory responses and foreign body reactions [24]. In addition, these acidic degradation products potentially diminish the bioactivity of growth factors[25]. One encouraging way to bypass these limitations is to blend PLAGA with other macromolecules that can buffer the acidic degradation products with a controlled degradation rate [15, 18, 19, 22, 26-28]
Due to their unique buffering degradation products, biodegradable poly(organophosphazenes) (PPHOS) have generated enormous interest and has proved to provide good candidate materials to achieve this objective [18, 29-31]. These are inorganic-organic hybrid polymers with a backbone of alternating phosphorus and nitrogen atoms and with each phosphorus atom bearing two organic side groups [4, 8, 10, 31-36]. Specific characteristics are deliberately fashioned by introduction of controlled amounts of particular organic side groups [37-39]. This has made available a wide array of polyphosphazenes with diverse properties. Chemical and physical properties are largely dependent upon the type of side groups attached to the polyphosphazene backbone. Incorporation of different side groups can alter the degradation rate and mechanical properties of the polymer [4, 9, 28, 32, 39-41]. For example, amino acid ester side groups will instigate hydrolysis within the polymer backbone [5, 9, 32, 40, 42] while there is a retardation of hydrolysis in the presence of hydrophobic phenylphenoxy side groups [4, 8, 15, 19, 43]. Unlike the polyester family, amino acid ethyl ester substituted polyphosphazenes undergo hydrolysis generating non-toxic and buffering degradation products composed mainly of phosphate, ammonia, and corresponding side groups [10, 23, 39, 44-47]. Therefore, the substitution of both amino acid ester groups and hydrophobic groups into the polyphosphazene backbone provides a tremendous influence on the required degradation pattern for a specific application [4, 7, 15, 18, 19, 26, 28, 32, 48, 49]. Furthermore, Laurencin and coworkers have demonstrated that the mechanical properties of the PPHOS can be improved by substituting in bulkier side groups such as phenylphenoxy into the backbone[10, 23, 28, 39, 41, 47, 50].
Despite polyphosphazenes having unique tunability through their side group chemistry, occasionally this is not enough to meet properties requirements for specific biomedical applications and thus polyphosphazene-based blends have also been explored as potential biomaterials [12, 14, 15, 18, 19, 22, 23, 26, 28, 30, 37, 39, 41, 43, 48, 51]. Blending technique is a very cost effective way of producing new polymeric materials with unique properties and without having to go through the rigorous synthetic protocol. It involves the synergistic combination of blend components which offers versatility in optimizing and tailoring properties of the final products. For example, in the blend system of polyetheretherketone/polyetherimide (PEEK/PEI) where PEEK is known for its excellent mechanical properties but suffers from a poor modulus at temperature above its Tg (145C). PEI On the contrary possesses higher Tg (215C) with lower chemical resistance and mechanical properties than PEEK. Blending PEEK and PEI combines the complementary properties of both polymers[52]. Generally, polymer blends can be divided into three types depending on the free energy of mixing: (1) Immiscible polymer blends which are usually made up of two phases and two glass transition temperatures due to the presence of two polymers (2) compatible polymer blends which by physical observation have a single uniform phase due to sufficiently strong interactions between the component polymers. However, two different glass transition temperatures from the individual polymers are observed (3) miscible blends; which have a single-phase structure with a single glass transition temperature that correspond to the average of the two Tgs of the component polymers. Depending on applications, properties of interest are found either in compatible or miscible blends. Both of these blends exhibit a single phase due to strong interaction between component polymers[53, 54]. Blending to obtain a compatible or miscible polymer blend is a complex process that involves the act of mixing two high-molecular weight polymers with different interfacial energies. Therefore, there is need for strong molecular interactions between the polymer chains to allow the fabrication of miscible polymer blends
Previous work has been successful in developing miscible PPHOS-PLAGA blends through hydrogen bond interactions [18]. In Vitro and In Vivo studies on these miscible polyphosphazene-based blends showed promising prospects as they were able to support cell adhesion, and proliferation and show elevated phenotype expression compared to polyesters. The pH profiles of the media in which these blends degraded were much higher than that of the PLAGA's as the polyphosphazene degradation products were able to neutralize the acidic degradation products from the PLAGA. However, majority of these blend systems do not possess requisite mechanical properties needed for bone tissue engineering [5, 9, 18, 19, 23, 29, 39, 41, 43, 46, 47, 50, 55]. It is still a lingering issue to fabricate completely miscible PPHOS-PLAGA blends with appropriate mechanical properties for bone tissue engineering applications. For instance, mechanically endowed phenylphenoxy side group hinders hydrogen bonding needed in forming miscible blends due to its steric bulk [56]. Work on the development and optimization of biodegradable Polyphosphazenes with high strength and hydrogen bonding ability is ongoing. The current work aims to develop dipeptide based PPHOS-PLAGA blends with superior mechanical strength as PEEK and yet degradable.
In this article, we highlight the importance of polyphosphazene-based blends in regenerative engineering, thereby providing fundamental insights into synthetic uniqueness of polyphosphazene. Advances made so far on the development of these unique biomaterials will be discussed with a focus on dipeptide-based Polyphosphazenes, and PPHOS-PLAGA blend systems.
Synthesis of Polyphosphazene – Mechanisms of polymerization
Thermal ring opening polymerization-bulk phase
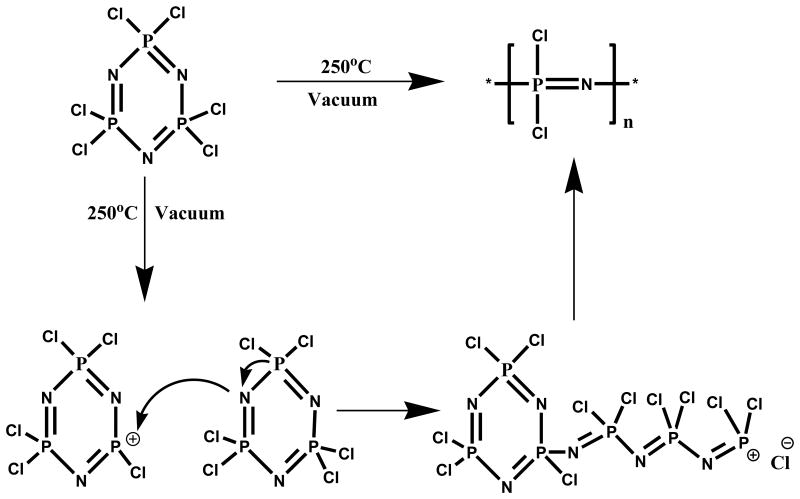
The most widely used route to poly(organo)phosphazenes is via the precursor poly(dichlorophosphazene) (PDCP) developed by Allcock et al.[8, 34, 44, 57]. Polyphosphazenes are commonly synthesized via a two-step reaction process starting from the commercially available cyclic trimer hexachlorocyclotriphosphazene (HCCTP). The first step involves the synthesis of linear PDCP which can be achieved via the controlled thermal ring opening polymerization of cyclic trimer at 250°C under vacuum [4, 10, 15, 21, 32, 37, 39, 40, 42, 44, 49, 56, 58-60]. This reaction is usually carried out under vacuum in a sealed glass tube at high temperatures; typically 250 °C for several hours. Around this temperature, chlorine atoms in HCCTP can be cleaved to give a cationic phosphazenium species which initiates the ring opening of a second ring, thus propagating the polymerization (Fig. 1). Efficient control of temperature is important for complete polymerization since Cl cleavage is minimal at temperature far below 250°C [5, 10, 32, 39, 40, 44, 61]. Significant crosslinking may occur when 250°C is exceeded [10]. Similar to conventional polymerization reactions, the reaction temperature can be lowered to around 200°C by addition of a Lewis acid catalyst, most commonly anhydrous AlCl3. A solution state method that makes use of trichlorobenzene as a solvent, at 214 °C, with CaSO4·2H2O as a promoter and HSO3 (NH2) as a catalyst has been reported [5, 8, 10, 40, 57, 62, 63]. Another route to poly(dichlorophospazene) is by direct reaction of phosphorus pentachloride (PCl5) and ammonium chloride. This method circumvent the use of the hexachlorophosphazene trimer, however the sublimation of PCl5 can pose a problem at the high temperatures required for the ring-opening [5, 8, 10, 40, 44, 57, 62].
Fig. 1. Synthesis of poly (dichlorophospazene): Synthetic Schematics of ring opening polymerization [31].
Ring opening route exhibits high molecular weight and broad polydispersity due to the uncontrollable Cl cleavage in HCCTP [5, 8, 10, 32, 37, 39, 40, 42, 44, 49, 57, 58, 61-66]. Uncontrollable branching is also common in this type of polymerization [10, 39, 67].
Living cationic polymerization
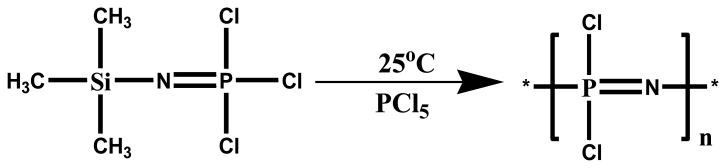
An alternative approach for the synthesis of macromolecular intermediate is by living cationic polymerization of phosphoranimines at ambient temperature. This renders control over the molecular weight of the PDCP and narrow polydispersities is usually obtained. The reaction mechanism involves catalyzed condensation of monomer trichloro(trimethylsilyl)phosphoranimine, (CH3)3Si-N=PCl3 with loss of (CH3)3SiCl (Fig. 2) [10, 32, 40, 42, 61, 63]
Fig. 2. Alternative route to the synthesis of poly(dichlorophospazene): living cationic polymerization of a phosphoranamine monomer[32].
Functionalization of the poly (dichlorophosphazene) precursor
In the second step, the reactive chlorine atoms on the poly(dichlorophosphazene) are replaced via macromolecular substitution reaction with various organic nucleophiles as shown in Fig. 3[5, 8, 10, 32, 37, 39, 40, 42, 44, 49, 57, 58, 61-66]. A few specialized polyphosphazenes, particularly those with hydrolytically sensitive groups such as amino acid ester side groups have been developed extensively as matrices for bone regeneration.
Fig. 3. Macromolecular substitution of PDCP with a wide array of organic substituent groups forming single-substituent polymers (simultaneous replacement of chlorine atoms) or mixed-substituent polymers (sequential substitution of chlorine atoms)[8, 32].
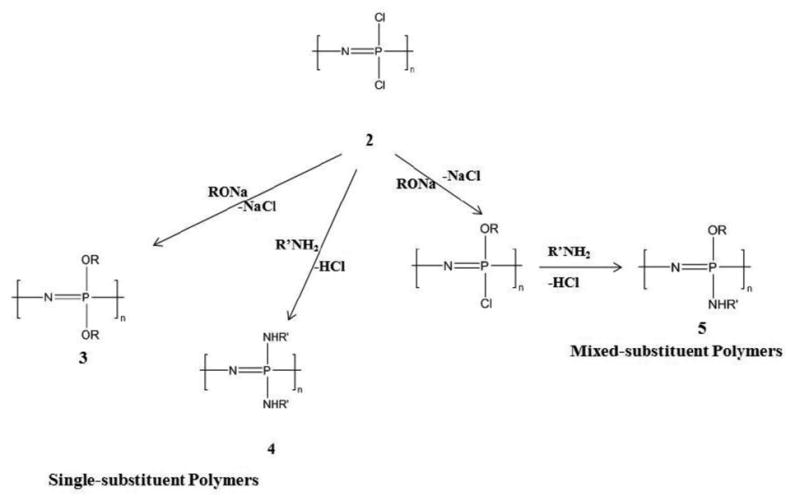
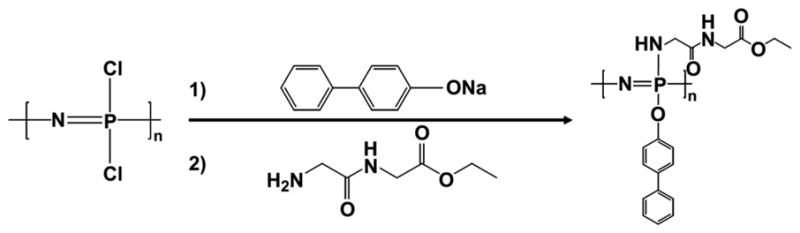
Macromolecular substitution reactions allow the introduction of different side groups that detect the polymer properties in a controlled manner[4, 5, 9, 10, 15, 32, 39, 40, 58, 62, 63, 65, 68, 69]. In addition to single side group substitution, multiple substituents can be covalently linked to the polymer backbone (Fig. 4). For example, different and dissimilar types of side groups can be substituted on the reactive intermediate polymer via sequential substitution, allowing the bulkier group to react first followed by the smaller reactive group in solution phase during substitution[10, 27, 32-35, 57, 61, 62, 70]. The modification of type and ratios of the side chains of the polymer affords the ability to fine-tune degradation rates and physical properties based on these substituents, which is important to the synthesis of a biomaterial suitable for tissue engineering and therapeutic delivery[5, 9, 10, 32, 37, 39, 47, 71-73]. By macromolecular substitution, over 250 different side groups have been covalently linked to the backbone via replacement of the labile chlorine atoms [5, 32, 61].
Fig. 4. Schematic illustrations of a mixed-substituent biodegradable polyphosphazene poly((glycine ethyl glycinato)1(phenyl phenoxy)1phosphazene) (PNGEGPhPh)[18].
Biodegradable Polyphosphazene (Bio-functionalized) and its Blends
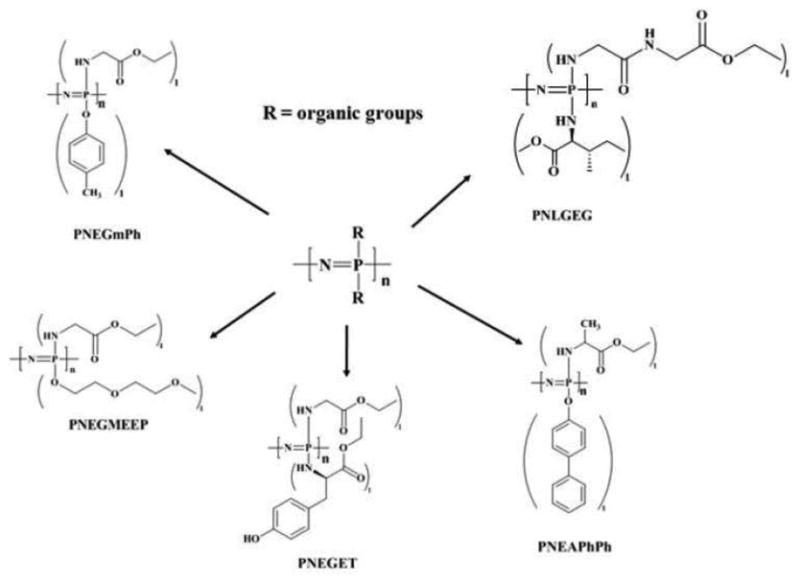
Most synthetic polymers are stable to water. However, polymers that degrade when exposed to aqueous media may be useful for medical devices as in tissue regeneration matrices or controlled drug release. Numerous biodegradable polyphosphazenes have been synthesized by substitution of chlorine atoms of the poly(dichlorophospazene) with hydrolytically labile groups such as amino acid esters, glycolate or lactate ester, imidazole, steroidal residue, and glyceryl[5, 9, 10, 12, 31, 32, 39, 45-47, 61, 63, 65, 66, 70, 71, 74-77]. Table 1 summarizes the physiochemical properties of some of the representative polyphosphazenes used in biomedical applications[5-7, 9, 10, 15, 22, 26-28, 32, 33, 37, 39, 46, 49, 51, 65, 76, 78-84]. Bulkier side groups result in a higher Tg[10, 27, 35, 37, 46, 61, 78, 79].
Table 1.
Illustrations of the effects of side groups on glass transition temperatures (Tg's) and degradation rates of single substituent polyphosphazenes. Table shows the great tunability of physico-chemical properties via modification of side group chemistry[56].
| Polymer | Side Group | Tg(°C) | Degradability |
|---|---|---|---|
| PNEG | Glycine | -40 | Months |
| PNEA | Alanine | -10 | Months |
| PNEL | Leucine | 15.4 | Months |
| PNMV | Valine | 24.8 | Months |
| PNEPhA | Phenylalanine | 41.6 | Months |
| PNGEG | Glycine dipeptide | 56.4 | Weeks |
| PNMEEP | Methoxy ethoxy ethoxy | -84 | Water soluble |
| PNmPh | Methyl Phenoxy | 2.1 | Non-degradable |
poly[bis(glycine ethyl ester)phosphazene](PNEG) *poly[(ethyl alanato)polyphosphazene(PNEA) *poly[(ethyl laucine)phosphazene(PNEL) *poly(methoxyvaline)phosphazene(PNMV)*poly(phenylalanineethylester)phosphazene(PNEPhA)*poly[(glycineethylglycinato)phosphazene(PN GEG)*poly[bis(methoxyethoxyethoxy)phosphazene](PNMEEP)*poly[bis(methylphenoxy)phosphazene] (PNmPh)
Amino-based Polyphosphazenes constitute the largest and most researched category of biodegradable Polyphosphazenes and were first reported by Allcock and Kugel in 1966[78]. Hydrolytic sensitivity and bio-erosion to non-toxic products can be observed in polyphosphazenes that contain amino acid ester units covalently bonded to the backbone through their amino terminus [5, 7, 32, 35, 45, 76, 78]. The rate of hydrolysis generally depends on the hydrophobicity and steric hindrance of the units located at the α-carbon of the amino acid residues[9, 18, 27, 32, 39, 43, 46, 65, 78].
The terminal nitrogen in the amino acid ester unit possesses hydrogen-bonding proton. This is available for interactions with other polymers in polymer blending (Fig. 5). A previous study has capitalized on these hydrogen-bonding capabilities to blend of polyphosphazenes with poly(lactide-co-glycolide) (PLAGA)[5, 18, 19, 22, 23, 41, 43, 48, 69, 85]. In that work, it was discovered that miscible blends can be formed between Polyphosphazenes that contain glycine ethyl ester side groups and PLGA with a lactic to glycolic acid ratio of 50:50 [85]. Miscible blends can also be obtained blending Alanine ethyl ester phosphazene polymers with PLAGA (50:50). However, the presence of the steric methyl group at the α-carbon places some difficulties on the formation of hydrogen bonding with PLAGA[22, 27, 48, 69, 85, 86].
Fig. 5. Illustration of intermolecular hydrogen bonding network between PLAGA and PNGEGPhPh through the glycylglycine dipeptides [18].
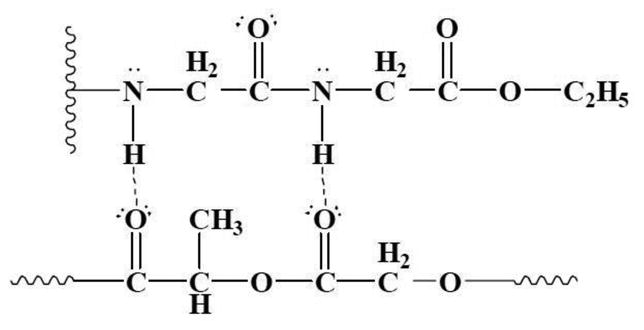
Furthermore, the presence of both hydrolysis sensitive groups and hydrophobic groups in a mixed-substituent polymer offers control over the degradation rate (Fig. 6). Previous studies by Dr. Laurencin and coworkers has reported that the combination of a hydrolytically active group and a hydrophobic group in a 50:50 ratio results in a complete degradation within 12-24 weeks for bone tissue engineering [18].
Fig. 6. Structures of mixed-substituent PPHOS for coordination with PLAGA. The primary selection criterion for side groups was based on its capacity to form miscible blends with PLAGA through intermolecular hydrogen bonding interactions[15].
Degradation Mechanisms
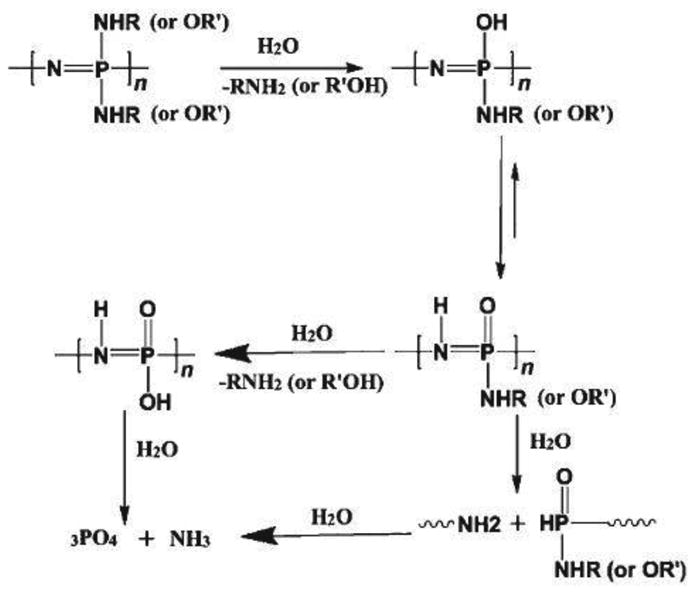
During polymer degradation, the organic side groups on the polyphosphazene are usually the first target. They are attacked by water molecules. After the removal of the side groups from the polymer backbone, P-OH units are formed followed by the migration of the proton from oxygen to nitrogen, which sensitizes the polymer backbone to hydrolysis. The polymer ultimately degrades into nontoxic degradation products comprising mainly of ammonia, phosphoric acid, and the corresponding side groups [5, 18, 26, 30, 45, 61, 66, 87, 88]. A generalized degradation scheme for degradable polyphosphazenes is presented in Fig. 7. For a mixed-substituent polymer, the first target is usually the most hydrolytically sensitive group. It is broken down first from the polyphosphazene backbone and is followed by bulkier side groups such as p-methylphenoxy, p-phenylphenoxy, tyrosine, or pyrrolidone [9, 35, 45, 61, 79]. The degradation products such as ammonium phosphate constitute a natural buffer system in the local microenvironment both in vitro and in vivo [45, 61, 89].
Fig. 7. Pathway to the degradation of biodegradable Polyphosphazenes [61].
Allcock and coworkers investigated the effects of ester groups and α-substituents on the hydrolytic sensitivity of amino acid ester-substituted polyphosphazenes [78]. Methyl, ethyl, tertbutyl, and benzyl esters of glycine-, alanine-, valine-, and phenylalanine-substituted polyphosphazenes were incubated in deionized water, and the molecular weight changes were monitored by gel permeation chromatography analysis. An inverse relationship was observed between the size of the ester group and hydrolytic sensitivity. The hydrolytic sensitivity of the polymer increased with decreasing size of the ester groups decreased [35, 39, 45, 46, 61, 78, 79, 87, 90]. Laurencin et al.[27] demonstrated the possibility of efficiently modifying the degradation rate of ethyl glycinato-substituted polyphosphazene by incorporating a hydrophobic co-substituent, such as a methylphenoxy group. Various studies have shown that the chemistry of Polyphosphazene synthesis can be fine-tuned to control degradation rate to meet the requirements of a specific biomedical application [27, 78, 79]. Ambrosio et al. have designed a novel biodegradable poly[(50% ethyl glycinato)(50% p-methylphenoxy) phosphazene] (PNEGmPh) blend whose degradation products were studied and compared with PLAGA. The media in which the degradation of the polymer blends occurred was monitored for pH changes over time. The blank which consist of medium without any matrix was used as control. No significant differences were observed in pH change of the media in which PNEGmPh degradation occurred over a period of 40 weeks as compared to the control. However, pH of 2.9 was reached at 6 weeks for the media with PLAGA while media from the blend had a pH of 6 at the same time. These data indicated the extent to which the blend controlled the drop in pH of the matrix degradation media [71].
Structure-property Relationship of Polyphosphazene and Its Blends
The properties of any polymer are dependent on the structures of the backbone and the side groups [27, 32-35, 39, 57, 65]. High torsional freedom within the phosphorus-nitrogen backbone and a correspondingly chain flexibility offer a different kinds of polymer properties that range from elastomers, or film or fiber-forming polymers, to rigid solids depending on the side groups. The glass transition temperature of the polymer is greatly affected by the restriction on the rotation of the backbone. Factors such as bulky side group, ionic forces, hydrogen bonding, or coordination interactions decreases molecular mobility within the backbone[5, 6, 10, 22, 35, 39, 62]. As a result of this restriction on rotation, the glass transition temperature increases. Thus, side groups can be selected to tune the glass transition temperature over the range of -100 °C (alkoxy side groups) to 200 °C or higher with adamantylamino groups. The stability of the polymer backbone depends on the side groups [35, 39, 65]. Hydrolytically active side groups attached to the polyphosphazene backbone can hydrolyze. This tendency, which is useful in tissue regeneration and other biomedical processes can provide a calculated means of causing the polymers to break down into phosphate, ammonia, amino acid, and ethanol[32, 39, 45, 46, 63, 65, 78, 90]. On the other hand, groups such as aryloxy, fluoroalkoxy and higher alkoxy possess the tendency to shed water which prevents the hydrolysis of the polymer backbone. Therefore changes in ratios of the two side groups of a mixed-substituted PPHOS with both hydrolysis-sensitizing groups and hydrolysis-retarding groups offers considerable opportunity for having control over the rate of degradation[35]. Furthermore, the hydrogen bonding capability of the PPHOS side groups and the compositional changes of the blend in polyphosphazene-polyester system can have profound influence on the properties of the polyphosphazene-based blend system. Laurencin and co-workers demonstrated the high miscibility of polyphosphazenes with glycine side groups [22, 84, 91]. In that study, a series of glycyl-glycine ethyl ester dipeptide based PPHOS were synthesized and blended with both PLAGA (50:50) and PLAGA (85:15). There was formation of completely miscible blends with all compositions of PLAGA due to increased number of hydrogen sites in dipeptide based PPHOS. It was also found that the Tg for each blend was lower than that of the parent polymers, implying both PPHOS and PLAGA acted as plasticizers for each other in the blend
Suitability of Polyphosphazene blends as biomaterials
Biocompatibility
Scaffold for regenerative engineering must be biocompatible; cells must adhere, function normally, and migrate onto the surface and eventually through the scaffold and begin to proliferate before laying down new matrix. After implantation, the scaffold or tissue engineered construct must induce a negligible immune reaction in order to prevent it from causing severe inflammatory response that might reduce healing or cause rejection by the body [5, 7, 9, 13, 28, 35, 48, 61, 69, 73, 76].
In Vitro Biocompatibility
The biocompatibility of the amino acid ester-substituted polyphosphazenes for tissue-engineering applications was first examined by Laurencin and coworkers who compared rat primary osteoblast adhesion to poly[(ethyl glycinato) phosphazene] (PNEG) with well-known poly(lactic acid-co-glycolic acid) (PLAGA) and poly(anhydrides) [6, 92]. Data from this study showed that the osteoblast cells adhered to the PNEG material to the same extent as the control materials for a period of 8 hours. The results from this study also suggested that cells responded favorably to polyphosphazene materials, especially those with a high ratio of ethyl glycinato substituents and that cell adhesion and proliferation characteristics were not diminished in comparison to tissue culture plate and PLAGA controls. The polymers with 50% and greater of ethyl glycinato substituents demonstrated improved cell growth in comparison to the tissue culture plate and the polymer with 25% ethyl glycinato substitution was only slightly less effective than the tissue culture plate. However, all of these were better than the PLAGA control, which has been widely accepted as a biocompatible material. Studies by Laurencin and coworkers also demonstrated the tissue biocompatibility of biodegradable PPHOS-PLAGA blends [18, 19, 30, 48, 77, 88, 89, 93]. Nair et al. [37]have developed biocompatible blends of PNEA and PLAGA (85:15) at two weight ratios of 25:75 and 50:50. The two blends were characterized for in vitro osteocompatibility. The blend matrices showed significantly higher osteoblast growth rates than Pristine PLAGA after 14 days. By contrast, the significant decrease of cell numbers on PLAGA matrices after 14 day has been attributed to the fast polymer degradation and the subsequent accumulation of acidic degradation products. Increase in the polyphosphazene content in the blend matrix improved cell growth. Also, the ALP activity of blend was found to be significantly higher than PLAGA after 7 days.
In Vivo Biocompatibility
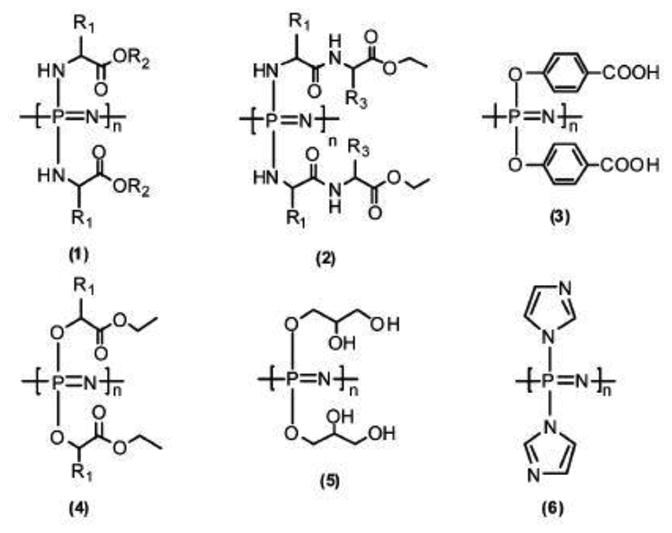
Deng et al. [18] evaluated the in vivo biocompatibility of two weight ratios of dipeptide-based PPHOS to PLAGA blends (25:75 and 50:50) by using a rat subcutaneous model with PLAGA as a control. The tissue responses for both blend implants were minimal, which was characterized by the presence of few neutrophils, erythrocytes, and lymphocytes. PLAGA implants were found to be completely absorbed within 7 weeks. Blend tissue compatibility was characterized by the extent of inflammation and fibrous capsule formation via hematoxylin and eosin (H&E) and Masson's trichrome stain (TRI). Both blend matrices were found to possess better histological responses with thinner fibrous capsules than PLAGA. Therefore, biodegradable polyphosphazenes blends are attractive candidate biomaterials for biomedical applications such as regenerative engineering, tissue engineering and drug delivery (Fig. 8) [9, 18, 76, 89].
Fig. 8. Structures of bio-erodible Polyphosphazene[5].
Biodegradability
Scaffolds and constructs, are not intended as permanent implants. The scaffold must therefore be biodegradable so as to allow cells to produce their own extracellular matrix [73]. The degradation should result in non-toxic products that can be metabolized and excreted by the body, with controllable degradation kinetics to match the rate of bone healing process (12-24 weeks) [18] so that the newly formed biological system compensates the mechanical and mass loss of the degraded matrices.
Polyphosphazenes are attractive because they have been shown to degrade into nontoxic byproducts that are easily metabolized by the body. In the case of an amino acid ester phosphazenes, these hydrolytic degradation products include the amino acid, the corresponding alcohol of the ester, ammonia, and phosphates [35, 39, 45, 46, 78]. Unlike the acidic products produced from the hydrolysis of other polymers, the ammonia and phosphates act as a buffering system and prevent fluctuations in pH, which could otherwise be detrimental to the tissue. Their degradation pattern can also be modified using the side chain chemistry [20, 22, 39, 45, 61, 65].
In Vitro Biodegradation
The self-neutralizing ability with controllable degradation rate is one of the major benefits of the blend system over PLAGA. Deng et al. [51] carried out a study on the degradation of PPHOS-PLAGA in PBS. In that study, the self-neutralizing ability of the blend system was examined by monitoring the pH profile of degradation in PBS. The media was set at pH 7.4, similar to the physiological environment. The blend system showed a higher pH as compared to the pristine PLAGA. This suggests that phosphates and ammonia produced from the polyphosphazene degradation can buffer the acidity that stems from the bulk degradation of PLAGA. This buffering effect can further reduce the acidic catalysis of hydrolysis of PLAGA. Furthermore, degradation over 12 weeks was much slower for blends than the pristine PLAGA (Fig. 9 and 10). It was also observed that the increase in the polyphosphazene composition in the blend resulted in a slower degradation rate. It reaffirms that the polyphosphazene degradation products were able to neutralize the acidic products from PLAGA, and as a result, retard the degradation of both PLAGA and polyphosphazene components in the blend. A 12-week of in vitro degradation led to 70-80% mass loss. [18, 23, 29, 41, 51, 71, 91, 93-95]. Furthermore, by appropriately changing the polymer chemistry and adjusting the composition of the blends, the buffering capacity and the resulting pH environment can be well tuned. The most unique fact about the degradation of the dipeptide PPHOS-PLAGA blends lies in the morphology of the matrix as a function of time. Fig. 9 shows the representative SEM images of blends during in vitro degradation as a function of time. The figures present the unique three dimensional porous structures formed during degradation with pore sizes in the micron range. It has been found that the size of the microspheres formed as well as the pore diameter can be varied by varying the blend composition. These unique in situ created porous structures, which are structurally similar to sintered microsphere matrix, have significant potential for cell in-growth and tissue regeneration [18, 51].
Fig. 9.

Time dependent topographical imaging of the polymeric blends immersed in aqueous media for 12 weeks at 37 °C. Top row: SEM topography of Matrix1 at 0, 4, 7, and 12 weeks of time intervals of in vitro degradation. Spots (e) and (g) show the detailed 3D Spherical structures. Bottom row: SEM topography of Matrix2 under vitro degradation at 0, 4, 7, and 12 weeks. An assemblage of microspheres with interconnected porous structures was evident due to the unique polymer erosion of the blend system[51]
Fig. 10.
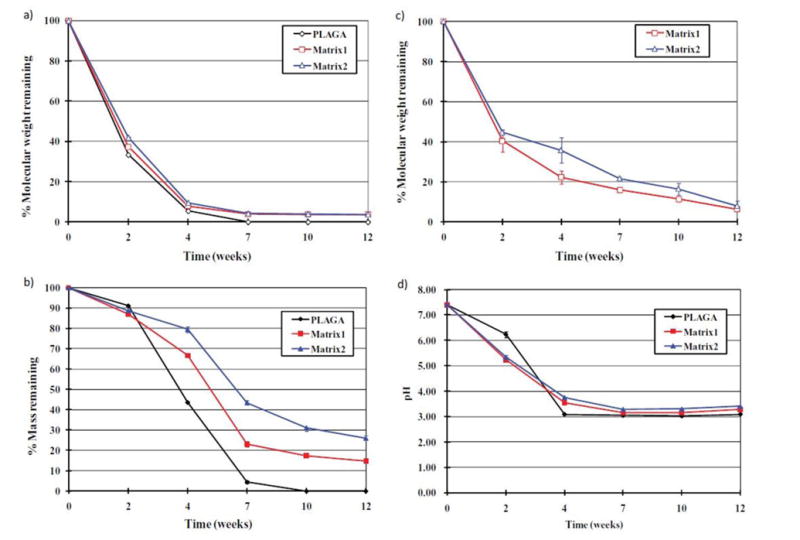
Graphs showing a 12-week study of in vitro degradation of the blend systems in pH 7.4 aqueous media at 37°C(a) Comparison of the molecular weight percentages of PLAGA in pristine PLAGA and the two blend systems after degradation (b) Mass of the residual systems expressed in percentage; (c) Comparison of the molecular weight percentage of polyphosphazene in the residual blends matrices (d) pH profiles of the media during degradation of pristine PLAGA and blend matrices. Slower degradation rate was evident in the blend systems than the pristine PLAGA. The trends for molecular weight changes of the PLAGA and phosphazene reasserted the similarities in the degradations of the two components [51].
In Vivo Biodegradation
In the in vivo degradation study, there was observed similarities in the way molecular weight decreased for both PLAGA and polyphosphazene components in the blend throughout the post-implantation period, which agrees with the in vitro findings. 82-87% porosity which is structurally similar to cancellous bone was seen after a 12-week in vivo degradation [18]. A direct correlation between sphere size and the amount of polyphosphazene in the blend was observed i.e. the sphere size increased with PPHOS composition in the blend. On other hands, compositional changes in polyphosphazene component of the blend did not seem to have effect on the porosity. This is believed to offer an advantage in maintaining a highly porous structure while fine-tuning the pore system for better cell infiltration and tissue in-growth[96]. For example, image analysis has revealed that the average pore size increases with the sphere diameter [11, 96, 97]. Additional surface area and space useful for enhancing cell-material interaction are provided by the intricate porous structure within the polymer spheres. Faster degradation of PLAGA in the blend matrix can facilitate pore formation while the polyphosphazene microspheres maintained the matrix structural integrity during the tissue in-growth. It can be seen in Fig. 11 that the porous structures start forming along the edge of the implants. The micrographs (H&E & TRI staining) also revealed significant collagen tissue in-growth in the porous structure indicating the capability of the structures to serve as an in situ forming three dimensional tissue engineering scaffold.
Fig. 11.
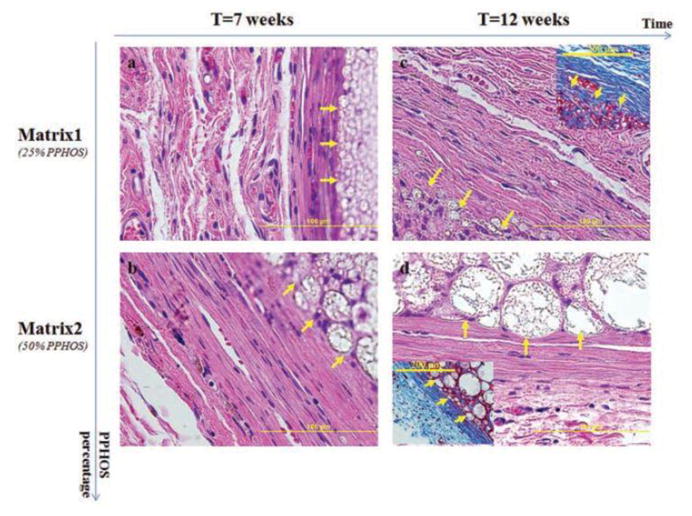
Histological examination reveals the formation of pore system that stems from the arrangement of newly formed polymer spheres. This is capable of accommodating cell infiltration and tissue in-growth within the blend systems. (a, b, H&E): The arrows shows the formation of polymer sphere within Matrix1 and Matrix2 after 7 weeks of implantation, respectively; (c, d, H&E): The arrows show the formation of polymer sphere within Matrix1 and Matrix2 after 12 weeks of implantation, respectively. After 12 weeks of implantation, strong collagen tissue infiltration via in situ formed pores within the matrix was apparent in (C) and (D) with TRI. This reaffirms the availability and enhancement of cell infiltration and collagen tissue in-growth through the formation of in situ 3D interconnected porous structure [51]
Mechanical properties
Ideally, the scaffold should be mechanically competent (e.g. compressive modulus in the range of 20-900 MPa) in order to tolerate the local forces [98]. Producing scaffolds with adequate mechanical properties is one of the great challenges in attempting to engineer bone or cartilage. For these tissues, the implanted scaffold must have sufficient mechanical integrity to function from the time of implantation to the completion of the remodeling process. If the mechanical properties, such as compressive strength and tensile strength, are not comparable to those of natural tissues, problems with mismatch arise which often lead to failure of the tissue-engineered construct [5, 7, 9, 11, 12, 61, 69, 73, 76, 77, 92, 98]. Studies on mechanical properties were carried out by Laurencin and coworkers. The mechanical properties of alanine-based polyphosphazenes were investigated for their application as bone tissue engineering biomaterials. For these studies, polyphosphazenes were compared with the current standard for bone tissue engineering applications, PLAGA (85% lactic acid: 15% glycolic acid). The compressive strengths of PNEA and PNEAmPh were comparable to that of PLAGA (34.9 ± 5.7 MPa). PNEAPhPh on the other hand had a compressive strength that was significantly higher than that of PLAGA due to the presence of the bulky aromatic groups which increases the steric hindrance and decreasing torsion of the polymer backbone. Consequently, these increase the rigidity of the material and modulate its compressive properties [83]. Similar to degradation properties, it can be noted that the mechanical properties of polyphosphazene materials can be tailored based on their proposed applications by changing the side group substituents. For the L-alanine-based polyphosphazene materials, it was shown that increasing the steric bulk of the co-substituent increases both the tensile strength and elasticity of the material with more of an impact being observed as the side chain is changed from a small amino acid such as glycine or alanine ethyl ester in PNEAEG and PNEA respectively, to large aromatic substituents, such as in PNEAmPh and PNEAPhPh. This is because the glass transition temperature and molecular weight of the polymer is affected by the presence of large aromatic ring, which in turn affects the mechanical properties of the material.
Deng et al. [14, 18, 43, 56] characterized the mechanical properties of mixed-substituent biodegradable polyphosphazene poly[(glycine ethyl glycinato)1(phenyl phenoxy)1phosphazene] (PNGEGPhPh) and its blends with a polyester (PLAGA-50% lactic acid: 50% glycolic acid) and compared it to the pristine PLAGA. Two dipeptide-based blends namely 25:75 (Matrix1) and 50:50 (Matrix2) were produced at two different weight ratios of PNGEGPhPh to poly (lactic acid-glycolic acid) (PLAGA). Both blends resulted in higher tensile modulus and strength than the polyester. It was revealed that the tensile modulus and ultimate tensile strength increased significantly with the addition of Polyphosphazene. The above studies showed that a change in the types and ratios of side group chemistries of Polyphosphazene materials and blend compositions can have influence on the mechanical properties [9, 18, 43, 56, 61, 83]. Shan et al. [99]have synthesized a cross-linkable poly (glycine ethyl ester-co-hydroxyethyl methacrylate) phosphazene (PGHP or PNEGHMP) and blended it with PLGA7525 or, poly (L-lactide) (PLLA) using chloroform as a mutual solvent. The photo-crosslinking was then done before solvent removal. The resulting PLGA (or PLLA)/PGHP composite films demonstrated no significant phase separation due to the binding function of the crosslinked PGHP polymeric network. To obtain PLGA7525/PGHP porous composite scaffolds, they used particle-leaching/solvent-casting with photo-crosslinking treatment performed before solvent evaporation. Both crosslinked PLGA (or PLLA)/PGHP films and scaffolds demonstrated a significant improvement in tensile moduli as compared to the corresponding un-crosslinked blends.
Unique Tunability of Degradation and Mechanical Properties
One concern here is that changing the side groups affects not only mechanical properties but also degradation rates and therefore modulating the side chains to obtain suitable mechanical properties may alter the degradation rate of the material. This is undesirable. Thus, further research is needed into other methods to control mechanical properties without having influence on erosion properties. For example, investigation on the effects of different processing methods or scaffold preparation techniques (e.g., electrospinning versus solvent casting and particulate leaching) on mechanical properties might be highly useful [9, 61]. Sequel to this, Deng et al. recently developed a mechanically competent 3D scaffold mimicking the bone marrow cavity and the lamellar structure of bone by orienting electrospun polyphosphazene-polyester blend nanofibers in a concentric manner with an open central cavity (Fig. 12)[43]. The 3D biomimetic scaffold exhibited mechanical attributes similar to native bone. Compressive modulus of the scaffold was found to be within the range of human trabecular bone. When tuned to have desired properties, the concentric open macrostructures of nanofibers that structurally and mechanically mimic the native bone can be a potential scaffold design for accelerated bone healing.
Fig. 12.
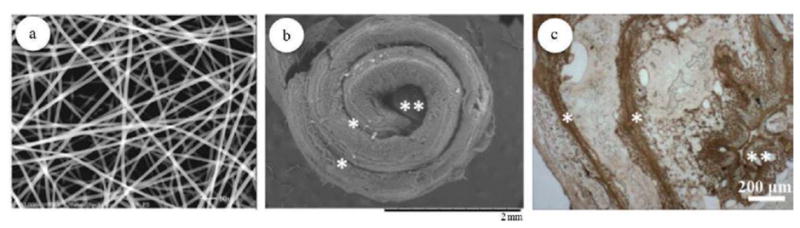
(a) Electrospun polyphosphazene nanofibrous scaffolds; (b) SEM-SE image showing the topographical fixtures of 3D biomimetic scaffolds seeded with cells after 28 days of culture. (c) Immunohistochemical staining for osteopontin (OPN), an important component of the mineralized ECM, showing a homogenous ECM distribution throughout the scaffold architecture at day 28. * indicates interlamellar space, whereas ** indicates central cavity[61].
Applications of Biodegradable Polyphosphazene and Its Blends
Scaffold-based regenerative engineering represents a clinical translational strategy for tissue regeneration. Biodegradable scaffolds play a crucial role in scaffold based regenerative approach and their successful application is dependent on their inherent properties, and compositions [2, 7, 9, 16, 32, 61, 73, 77]. So far, a large number of polyphosphazene-based polymer systems have been developed for use in bone and soft tissue regeneration, as well as in drug delivery [20, 31, 74, 91, 94, 100, 101].
Bone tissue regeneration
Bone and skeletal tissue engineering applications has had the lion's share of the research on biodegradable polyphosphazene. Laurencin and coworkers have extensively studied these materials and their interactions with osteoblast type cells to determine their suitability for bone grafts and implants. In one of the studies[81], a comparison was made between the 3D and 2D matrices of amino acid-based polyphosphazene, on which osteoblast cells was seeded. It was observed that the pores of the 3D constructs resembled, in shape and size, those of natural bone tissue, specifically trabecular bone. They also noted that the 3D polyphosphazene scaffolds were able to promote osteoblast adhesion and proliferation throughout the entire 21-week period of their study, whereas adhesion to the 2D scaffolds was not as effective. Overall, the study was a promising one and showed that polyphosphazene materials were suitable for bone tissue regeneration.
PLGA is renowned for its suitable compressive strength and satisfactory in vitro cell performance, while the material composition suffers from lack of bioactivity, uncontrolled bulk degradation, and acidic degradation products. PPHOS-PLAGA blends will combine the advantages of each parent polymer. Amino acid ester-substituted polyphosphazenes offer a variety of functional groups that allow for controlled degradation into phosphate and ammonia, thereby constituting a natural buffer. A series of studies has been carried out by Deng at el. to evaluate various PPHOS-PLGA blends in the form of thin disks for bone tissue regeneration. In one study, the etheric biodegradable polyphosphazene PNEGMEEP was synthesized and blended with PLAGA at weight ratios of 25:75 and 50:50 (PNEGMEEP to PLGA ratio) using a mutual solvent approach[19]
The blend films, although not completely miscible, were able to nucleate bone-like apatite, degrade into near-neutral pH products, support PRO growth and proliferation, and enhance osteoblast phenotype expression due to the presence of polyphosphazene. In order to improve miscibility, a mixed-substituent biodegradable polyphosphazene PNGEGPhPh was synthesized and blended with PLGA (50:50 lactide to glycolide ratio) at weight ratios of 25:75 and 50:50[18]. Completely miscible PNGEGPhPh-PLGA blends with enhanced mechanical properties were obtained as a result of strong hydrogen bonding between the glycylglycine dipeptide side groups and PLGA. In vitro studies show significantly higher osteoblast growth on the blend films than on the PLGA film. Intriguingly, the novel blend system was able to develop an in situ porous structure, which enabled cell infiltration and collagen tissue ingrowth throughout the void space between spheres as a result of polymer degradation[51]. The versatility of this novel polymer platform is confirmed by the robust tissue growth in the dynamic pore-forming scaffold. This has led to a new strategy in regenerative medicine, developing solid matrices that balance degradation with tissue formation. Furthermore, introduction of extra hydrogen-bonding sites by the incorporation of dipeptide ester side groups to the polyphosphazene backbone has resulted in the formulation of a wide range of miscible PPHOS-PLGA blend materials[91]. These biocompatible and mechanically competent blend systems have shown a great potential for bone regeneration [22, 89, 94]. Polymeric nanofibrous matrices due to their similarity to natural extracellular matrix (ECM) are attractive candidates as bone regenerative scaffolds. Several Polyphosphazene-based nanofibrous scaffolds have been successfully fabricated via an electrospinning process. Deng at el.[43] have designed a mechanically competent 3D scaffold system using electrospun dipeptide-based PPHOS/PLGA (50:50) nanofibers which were orientated in a concentric manner with an open central cavity. The biomimetic scaffold showed a comparable characteristic mechanical behavior to that of native bone. Compressive modulus of the scaffold was found to be within the range of human trabecular bone.
Soft tissue regeneration
Besides bone repair, polyphosphazenes have shown great prospects in the regeneration of other types of tissue such as nerve, vessel, ligament and tendon. These tissues are categorized as soft tissues and their injuries account for 50% of the total musculoskeletal injuries reported in the USA per year. Polyphosphazene materials with improved elastomeric properties are often utilized in this case. Studies on the influence of alkyl ester chain length on mechanical properties and degradation rates have been reported[9, 23, 27, 35, 38, 39, 46, 79, 83, 87, 94, 102, 103]. Nichol et al. [104]investigated the influence of changing alkyl ester chain lengths between five and eight carbons on mechanical properties and degradation rates of L-alanine and L-phenylalanine alkyl ester polyphosphazene materials for their application in tendon and ligament tissue engineering. They determined that the glass transition temperatures (Tg) of the materials decreased with increasing alkyl ester chain length due to increased flexibility of the alkyl side chain and improved elastomeric properties of the polymer. It was also observed that the Tg's of the phenylalanine materials were higher than those of the alanine counterparts which is likely due to the increased bulkiness and steric hindrance of the aromatic side chain that in turn increases the rigidity of the overall polymer. In vivo nerve repair studies were performed using tubes of poly[(ethyl alanato)1.4(imidazolyl)0.6phosphazene] (PEIP) to establish continuity of a completely lacerated sciatic nerve[105]. These scaffolds were found to have degraded extensively at 45 days post implantation, leaving a tissue cable that bridged the lacerated nerve stumps. The PEIP nerve guides appeared to be more biocompatible than silicone tubes that are currently used, as significantly less scar tissue was induced and a thinner fibrotic capsule formed around these guides. Zhang et al.[106] have synthesized a novel electrically-conductive and biodegradable polyphosphazene polymer containing parent aniline pentamer (PAP) and glycine ethyl ester (GEE) as side chains for use in nerve tissue engineering. Conductivity is needed in nerve tissue engineering considering that neural signals are propagated along nerve cells via electrical charges and therefore the polymers that are used to regenerate these tissues must be capable of transmitting waves of electricity. The polymer was formed into thin films for degradation and biocompatibility testing. The poly [(glycine ethyl ester)(aniline pentamer) phosphazene] (PGAP) polymer was shown to have good electroactivity using cyclic voltammetry measurements indicating suitability of the material for propagating neural signals. Thin films of both PGAP and poly[bis(glycine ethyl ester)phosphazene] (PGEE) materials subjected to degradation studies in PBS at 37°C showed a mass loss of ∼ 50% and 70% respectively after 70 days. The mass loss of the PGAP was less than that of the PGEE due to the increased hydrophobicity of the aniline pentamer side chain and therefore decreased rate of hydrolysis since the hydrophobic side chains sterically hinder the approach of water towards the backbone. In vitro cytotoxicity of PGAP to the RSC96 Schwann cells was evaluated using the cell viability assay and was compared to a thin film of poly- DL-lactic acid (PDLLA) as a control since it has extensively been shown to be biocompatible with numerous types of cells. Schwann cells being integral to the peripheral nervous system as supportive cells and neutral signals propagator, RSC96 Schwann cells were selected in this study. The PGAP exhibited no cytotoxicity and improved cell adhesion in comparison to PDLLA. This work shows the versatility of polyphosphazene-based materials as conductive and biodegradable polymers for nerve tissue engineering applications.
Peach et al. [103] investigated the feasibility of using poly[(ethyl alanato)1(p-methyl phenoxy)1]phosphazene (PNEA-mPh) to modify the surface of electrospun PCL nanofiber matrix for their application in tendon tissue engineering. Surface functionalization with PNEA-mPh significantly increased the PCL matrix hydrophilicity. An in vitro study was conducted to examine the effect of surface functionalization with PNEA-mPh on human mesenchymal stem cells (hMSC) adhesion, cell construct infiltration, proliferation and tendon differentiation, as well as long-term cellular construct mechanical properties[38]. Functionalized matrices showed a rough surface morphology and which led to enhanced cell adhesion and infiltration as compared to the uncoated PCL fibers. Furthermore, functionalized matrices supported an enhanced tenogenic differentiation, possessing greater tenomodulin expression and superior phenotypic maturity. Overall, this study was able to show the in vitro biocompatibility of polyphosphazene coated materials towards human mesenchymal stem cells as well as their ability to modulate appropriately the cells' differentiation towards mature tendon cells. To the best of our knowledge, polyphosphazene blends have not been utilized much in soft tissue engineering.
Biodegradable Polyphosphazenes in Drug Delivery
Controlled release and local delivery of therapeutic drugs or growth factors play a key role in tissue engineering approach. Unlike the polyesters that exhibit bulk erosion and the polyanhydrides that undergo surface erosion, polyphosphazenes have the unique capability to exhibit bulk, surface or the combination of both. The side groups attached to the backbone have a big influence on the type of erosion the polymer will undergo [89]. Furthermore, polyphosphazenes are soluble in a wide range of solvents ranging from aqueous to organics. These exceptional features, coupled with flexible degradation kinetics that produces non-toxic degradation products make biodegradable polyphosphazenes attractive candidates for delivering a wide variety of drugs and growth factors[36]. Depending on the polyphosphazene used, drug release from these formulations can be achieved either by diffusion (hydrogels) or erosion (hydrophobic) or combination of both (hydrophilic-hydrophobic)[50, 76, 82, 85, 103, 107-109].
In a study by Orsolini and coworkers, polyphosphazenes poly[(alanine ethyl ester)]phosphazene and poly[80%(phenylalanine ethylester)/20% (imidazolyl)]phosphazene were used to design membranes and microcapsules for the treatment of periodontal diseases. Degradation rate that matched the healing of bone defects was achieved with the polyphosphazene membranes prepared with alanine ethyl ester and imidazole in a molar ratio of 80:20. Effective healing of the rabbit tibia defects was more evident in these membranes than the polytetrafluoroethylene membranes[110].
Laurencin et al. have carried out a study on the development a musculoskeletal delivery system for the controlled release of anti-inflammatory agent colchicine to joints using polyphosphazenes poly[20% (imidazolyl)/80% (p-methylphenoxy)]phosphazene and poly[50% (ethyl glycinato)/50% (p-methylphenoxy)]phosphazene. Colchicine-loaded with an imidazolyl content of 20% and poly[50% (ethyl glycinato)/50% (p-methoxyphenol)]phosphazene were used to carry out polymer degradation and drug release studies. Higher rate of degradation at physiological pH was observed for the 50% ethyl glycinato-substituted polyphosphazene than the 20% for imidazolyl-substituted polymer at physiological pH. Over a 21-day period of the study, the release of colchicine was found to be 20% from the imidazole-substituted polymer and 60% from the ethyl glycinato-substituted polymer. The result confirmed the strong agreement between the colchicine release profiles from the polymers and the degradation of the respective polymers[111].
As aforementioned, blending can be used to manipulate degradation rate and hence impact drugs release profile. Schacht et al. performed a study that investigated the degradation of blends that contain two polyphosphazenes with different rates of degradation as a matrix for controlled drug release. The single-substituent polyphosphazenes that have ethyl glycinate and phenylalanate as side groups as well as their blends were used to study the in vitro release of the antitumor agent mitomycin C (MMC) [112]. Results showed a faster release of MMC from poly[bis(ethylglycinato)]phosphazene than from poly[bis(phenylalanato)]phosphazene. For their blend systems, a direct correlation exist between the release rate and the composition of poly[bis(ethylglycinato)]phosphazene. The release rate increased with increasing ethylglycinato content and decreased with increasing phenylalanato content. The release profile exhibited a diffusion mechanism. The drop in release that is associated with increase in phenylalanato content may be attributed to the presence of a hydrophobic group in the matrix, which would hinder the permeation of water into the matrix.
Conclusion and Future Outlook
Regenerative engineering is a growing and nascent interdisciplinary field, which puts forward a convergence approach to create a regenerative toolbox to move beyond individual tissue repair to the regeneration of complex tissues and organ systems. It aims to completely regenerate complex tissues and organ systems such as a knee or a whole limb. Biodegradable Polyphosphazenes represent polymers with high potentials for regenerative engineering. The synthetic flexibility presents a wide door of opportunities to producing a library of degradable biomaterials with significant prospects for musculoskeletal tissue regeneration. The substitution of certain groups can influence degradation rate and mechanical properties. Polyphosphazene polymers provide a promising matrix platform to create miscible blend systems that mimic the native extracellular macromolecules. Much progress has been made on the development of polyphosphazene blends with polyesters. The tunability and the pH-buffering effect of the polyphosphazene hydrolysis products combined with the high strength of the polyester, allows the development of biomaterials with more useful properties than either of the parent polymers. Nevertheless, several biological and engineering challenges remain for polymeric scaffolds to make a significant clinical impact for musculoskeletal tissue regeneration. The tissues in the human body are incredibly complex with 3D hierarchical structures, anisotropic properties, and cell heterogeneity. The successful translation of this promising technology requires a further understanding of the scaffold design and close collaboration among chemists, biologists, chemical engineers, material scientists, and clinicians.
Table 2. Summary of representative polyphosphazene-based blends used in tissue engineering.
| Polyphosphazene Component | Blend System |
|---|---|
| Poly[(ethyl glycinato) phosphazene] (PNEG) | PNEG/PLAGA |
| Poly[(ethyl alanato)polyphosphazene] (PNEA) | PNEA/PLAGA |
| Poly[(glycine ethyl glycinato)(phenylphenoxy)phosphazene] (PNGEG-PhPh) | PNGEGPhPh/PLAGA |
| Poly[(ethyl alanato) (p-phenyl phenoxy) phosphazene] (PNEA-PhPh) | PNEAPhPh/PLAGA |
| Poly[(ethyl glycinato)(p-methylphenoxy) phosphazene](PNEG-mPh) | PNEGmPh/PLAGA |
| Poly[(ethyl alanato) (p-methylphenoxy) phosphazene] (PNEA-mPh) | PNEAmPh/PLAGA |
| Poly[(ethyl alanato) (ethyl glycinato) phosphazene] (PNEAEG) | PNEAEG/PLAGA |
| Poly[(ethyl glycinato)(hydroxyethylmethacrylate) phosphazene](PNEGH-mP) | PNEGHMP/PLAGA |
| Poly[(ethyl glycinato)(methoxyethoxyethoxy)phosphazene] (PNEGMEEP) | PNEGMEEP/PLAGA |
| Poly[(ethyl alanato)]phosphazene (PNEA) Poly[(ethyl phenylalanato)(imidazolyl)]phosphazene (PNEPhAIL) |
PNEA/PNEPhAIL |
| poly[(ethyl glycinato)(p-methylphenoxy)]phosphazene (PNEG-mPh) poly[(imidazolyl)(p-methylphenoxy)]phosphazene (PNIL-mPh) |
PNEG-mPh/PNIL-mPh |
| poly[bis(ethylglycinato)]phosphazene (PNEG) poly[bis(phenylalanato)]phosphazene (PNPhA) |
PNEG/PNPhA |
References
- 1.Laurencin CT, Khan Y. Regenerative engineering. Science translational medicine. 2012;4(160):160ed169–160ed169. doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- 2.Laurencin CT, Nair LS. Regenerative Engineering: Approaches to Limb Regeneration and Other Grand Challenges. Regenerative Engineering and Translational Medicine. 2015;1(1):1–3. doi: 10.1007/s40883-015-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert WM, Ratner BD, Anderson J, Coury A, Hoffman AS, Laurencin CT, Tirrell D. 2010 Panel on the biomaterials grand challenges. J Biomed Mater Res A. 2011;96(2):275–287. doi: 10.1002/jbm.a.32969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polyphosphazenes for Biomedical Applications (1) Hoboken, US: Wiley; 2009. [Google Scholar]
- 5.Allcock HR, Morozowich NL. Bioerodible polyphosphazenes and their medical potential. Polymer Chemistry. 2012;3(3):578–590. [Google Scholar]
- 6.Laurencin CT, Norman ME, Elgendy HM, El-Amin SF, Allcock HR, Pucher SR, Ambrosio AA. Use of polyphosphazenes for skeletal tissue regeneration. Journal of biomedical materials research. 1993;27(7):963–973. doi: 10.1002/jbm.820270716. [DOI] [PubMed] [Google Scholar]
- 7.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Progress in Polymer Science. 2007;32(8-9):762–798. [Google Scholar]
- 8.Allcock HR. The synthesis of functional polyphosphazenes and their surfaces. Applied organometallic chemistry. 1998;12(10-11):659–666. [Google Scholar]
- 9.Baillargeon AL, Mequanint K. Biodegradable polyphosphazene biomaterials for tissue engineering and delivery of therapeutics. Biomed Res Int. 2014;2014:761373. doi: 10.1155/2014/761373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothemund S, Teasdale I. Preparation of polyphosphazenes: a tutorial review. Chemical Society Reviews. 2016 doi: 10.1039/c6cs00340k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borden M, Attawia M, Khan Y, El-Amin S, Laurencin C. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. Bone & Joint Journal. 2004;86(8):1200–1208. doi: 10.1302/0301-620x.86b8.14267. [DOI] [PubMed] [Google Scholar]
- 12.Cao H, Kuboyama N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46(2):386–395. doi: 10.1016/j.bone.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Carampin P, Conconi MT, Lora S, Menti AM, Baiguera S, Bellini S, Grandi C, Parnigotto PP. Electrospun polyphosphazene nanofibers for in vitro rat endothelial cells proliferation. J Biomed Mater Res A. 2007;80(3):661–668. doi: 10.1002/jbm.a.30999. [DOI] [PubMed] [Google Scholar]
- 14.Deng M, James R, Laurencin CT, Kumbar SG. Nanostructured Polymeric Scaffolds for Orthopaedic Regenerative Engineering. IEEE Transactions on NanoBioscience. 2012;11(1):3–14. doi: 10.1109/TNB.2011.2179554. [DOI] [PubMed] [Google Scholar]
- 15.Deng M, Kumbar SG, Wan Y, Toti US, Allcock HR, Laurencin CT. Polyphosphazene polymers for tissue engineering: an analysis of material synthesis, characterization and applications. Soft Matter 2010. 6(14):3119–3132. [Google Scholar]
- 16.Jabbarzadeh E, Deng M, Lv Q, Jiang T, Khan YM, Nair LS, Laurencin CT. VEGF-incorporated biomimetic poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100B(8):2187–2196. doi: 10.1002/jbm.b.32787. [DOI] [PubMed] [Google Scholar]
- 17.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. Journal of Biomedical Materials Research. 2002;60(4):613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 18.Deng M, Nair LS, Nukavarapu SP, Jiang T, Kanner WA, Li X, Kumbar SG, Weikel AL, Krogman NR, Allcock HR, et al. Dipeptide-based polyphosphazene and polyester blends for bone tissue engineering. Biomaterials. 2010;31(18):4898–4908. doi: 10.1016/j.biomaterials.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Brown JL, Krogman NR, Weikel AL, Allcock HR, Laurencin CT. Biomimetic, bioactive etheric polyphosphazene-poly(lactide-co-glycolide) blends for bone tissue engineering. J Biomed Mater Res A. 2010;92(1):114–125. doi: 10.1002/jbm.a.32334. [DOI] [PubMed] [Google Scholar]
- 20.Ibim SE, Ambrosio AM, Kwon MS, El-Amin SF, Allcock HR, Laurencin CT. Novel polyphosphazene/poly (lactide-co-glycolide) blends: miscibility and degradation studies. Biomaterials. 1997;18(23):1565–1569. doi: 10.1016/s0142-9612(97)80009-9. [DOI] [PubMed] [Google Scholar]
- 21.Krogman NR, Steely L, Hindenlang MD, Nair LS, Laurencin CT, Allcock HR. Synthesis and Characterization of Polyphosphazene-block-polyester and Polyphosphazene-block-polycarbonate Macromolecules. Macromolecules. 2008;41(4):1126–1130. [Google Scholar]
- 22.Krogman NR, Weikel AL, Nguyen NQ, Kristhart KA, Nukavarapu SP, Nair LS, Laurencin CT, Allcock HR. Hydrogen bonding in blends of polyesters with dipeptide-containing polyphosphazenes. Journal of applied polymer science. 2010;115(1):431–437. [Google Scholar]
- 23.Shan D, Huang Z, Zhao Y, Cai Q, Yang X. Improving the miscibility of biodegradable polyester/polyphosphazene blends using cross-linkable polyphosphazene. Biomed Mater. 2014;9(6):061001. doi: 10.1088/1748-6041/9/6/061001. [DOI] [PubMed] [Google Scholar]
- 24.Bostman O, Pihlajamaki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21(24):2615–2621. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 25.Landes CA, Ballon A, Roth C. Maxillary and mandibular osteosyntheses with PLGA and P (L/DL) LA implants: a 5-year inpatient biocompatibility and degradation experience. Plastic and reconstructive surgery. 2006;117(7):2347–2360. doi: 10.1097/01.prs.0000218787.49887.73. [DOI] [PubMed] [Google Scholar]
- 26.Conconi MT, Lora S, Menti AM, Carampin P, Parnigotto PP. In vitro evaluation of poly[bis(ethyl alanato)phosphazene] as a scaffold for bone tissue engineering. Tissue Eng. 2006;12(4):811–819. doi: 10.1089/ten.2006.12.811. [DOI] [PubMed] [Google Scholar]
- 27.Nair LS, Bhattacharyya S, Bender JD, Greish YE, Brown PW, Allcock HR, Laurencin CT. Fabrication and optimization of methylphenoxy substituted polyphosphazene nanofibers for biomedical applications. Biomacromolecules. 2004;5(6):2212–2220. doi: 10.1021/bm049759j. [DOI] [PubMed] [Google Scholar]
- 28.Sethuraman S, Nair LS, El-Amin S, Farrar R, Nguyen MT, Singh A, Allcock HR, Greish YE, Brown PW, Laurencin CT. In vivo biodegradability and biocompatibility evaluation of novel alanine ester based polyphosphazenes in a rat model. J Biomed Mater Res A. 2006;77(4):679–687. doi: 10.1002/jbm.a.30620. [DOI] [PubMed] [Google Scholar]
- 29.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Brown JL, Krogman NR, Weikel AL, Allcock HR, Laurencin CT. Biomimetic, bioactive etheric polyphosphazene-poly(lactide-co-glycolide) blends for bone tissue engineering. Journal of Biomedical Materials Research Part A. 2010;92A(1):114–125. doi: 10.1002/jbm.a.32334. [DOI] [PubMed] [Google Scholar]
- 30.Duan S, Yang X, Mao J, Qi B, Cai Q, Shen H, Yang F, Deng X, Wang S. Osteocompatibility evaluation of poly(glycine ethyl ester-co-alanine ethyl ester)phosphazene with honeycomb-patterned surface topography. J Biomed Mater Res A. 2013;101(2):307–317. doi: 10.1002/jbm.a.34282. [DOI] [PubMed] [Google Scholar]
- 31.Rothemund S, Aigner TB, Iturmendi A, Rigau M, Husar B, Hildner F, Oberbauer E, Prambauer M, Olawale G, Forstner R, et al. Degradable glycine-based photo-polymerizable polyphosphazenes for use as scaffolds for tissue regeneration. Macromol Biosci. 2015;15(3):351–363. doi: 10.1002/mabi.201400390. [DOI] [PubMed] [Google Scholar]
- 32.Allcock HR. The expanding field of polyphosphazene high polymers. Dalton Transactions. 2016;45(5):1856–1862. doi: 10.1039/c5dt03887a. [DOI] [PubMed] [Google Scholar]
- 33.Allcock H. Phosphorus-nitrogen compounds: cyclic, linear, and high polymeric systems. Elsevier; 2012. [Google Scholar]
- 34.Allcock HR. Heteroatom ring systems and polymers. 1967 [Google Scholar]
- 35.Allcock HR. Chemistry and applications of polyphosphazenes. Wiley-Interscience; 2003. [Google Scholar]
- 36.Oredein-McCoy O, Krogman NR, Weikel AL, Hindenlang MD, Allcock HR, Laurencin CT. Novel factor-loaded polyphosphazene matrices: Potential for driving angiogenesis. Journal of microencapsulation. 2009;26(6):544–555. doi: 10.1080/02652040802500473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair LS, Lee DA, Bender JD, Barrett EW, Greish YE, Brown PW, Allcock HR, Laurencin CT. Synthesis, characterization, and osteocompatibility evaluation of novel alanine-based polyphosphazenes. J Biomed Mater Res A. 2006;76(1):206–213. doi: 10.1002/jbm.a.30532. [DOI] [PubMed] [Google Scholar]
- 38.Peach MS, James R, Toti US, Deng M, Morozowich NL, Allcock HR, Laurencin CT, Kumbar SG. Polyphosphazene functionalized polyester fiber matrices for tendon tissue engineering: in vitro evaluation with human mesenchymal stem cells. Biomed Mater. 2012;7(4):045016. doi: 10.1088/1748-6041/7/4/045016. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Krogman NR, Sethuraman S, Nair LS, Sturgeon JL, Brown PW, Laurencin CT, Allcock HR. Effect of side group chemistry on the properties of biodegradable L-alanine cosubstituted polyphosphazenes. Biomacromolecules. 2006;7(3):914–918. doi: 10.1021/bm050752r. [DOI] [PubMed] [Google Scholar]
- 40.Allcock HR. Inorganic—Organic Polymers. Advanced Materials. 1994;6(2):106–115. [Google Scholar]
- 41.Sethuraman S, Nair LS, El-Amin S, Nguyen M-T, Singh A, Krogman N, Greish YE, Allcock HR, Brown PW, Laurencin CT. Mechanical properties and osteocompatibility of novel biodegradable alanine based polyphosphazenes: Side group effects. Acta biomaterialia. 2010;6(6):1931–1937. doi: 10.1016/j.actbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allcock HR, Crane CA, Morrissey CT, Nelson JM, Reeves SD, Honeyman CH, Manners I. “Living” cationic polymerization of phosphoranimines as an ambient temperature route to polyphosphazenes with controlled molecular weights. Macromolecules. 1996;29(24):7740–7747. [Google Scholar]
- 43.Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, Laurencin CT. Biomimetic Structures: Biological Implications of Dipeptide-Substituted Polyphosphazene–Polyester Blend Nanofiber Matrices for Load-Bearing Bone Regeneration. Advanced Functional Materials. 2011;21(14):2641–2651. [Google Scholar]
- 44.Allcock H. Recent advances in phosphazene (phosphonitrilic) chemistry. chemical Reviews. 1972;72(4):315–356. [Google Scholar]
- 45.Allcock H, Fuller T, Matsumura K. Hydrolysis pathways for aminophosphazenes. Inorganic Chemistry. 1982;21(2):515–521. [Google Scholar]
- 46.Allcock HR, Pucher SR, Scopelianos AG. Poly [ (amino acid ester) phosphazenes] as substrates for the controlled release of small molecules. Biomaterials. 1994;15(8):563–569. doi: 10.1016/0142-9612(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 47.Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. Journal of polymer science Part B: polymer physics. 2011;49(12):832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Krogman NR, Singh A, Allcock HR, Laurencin CT. Miscibility and in vitro osteocompatibility of biodegradable blends of poly [(ethyl alanato)(p-phenyl phenoxy) phosphazene] and poly (lactic acid-glycolic acid) Biomaterials. 2008;29(3):337–349. doi: 10.1016/j.biomaterials.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heyde M, Moens M, Van Vaeck L, Shakesheff KM, Davies MC, Schacht EH. Synthesis and characterization of novel poly[(organo)phosphazenes] with cell-adhesive side groups. Biomacromolecules. 2007;8(5):1436–1445. doi: 10.1021/bm060926k. [DOI] [PubMed] [Google Scholar]
- 50.Veronese F, Marsilio F, Caliceti P, De Filippis P, Giunchedi P, Lora S. Polyorganophosphazene microspheres for drug release: polymer synthesis, microsphere preparation, in vitro and in vivo naproxen release. Journal of controlled release. 1998;52(3):227–237. doi: 10.1016/s0168-3659(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 51.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Weikel AL, Krogman NR, Allcock HR, Laurencin CT. In Situ Porous Structures: A Unique Polymer Erosion Mechanism in Biodegradable Dipeptide-based Polyphosphazene and Polyester Blends Producing Matrices for Regenerative Engineering. Adv Funct Mater. 2010;20(17):2743–2957. doi: 10.1002/adfm.201090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramani R, Alam S. Composition optimization of PEEK/PEI blend using model-free kinetics analysis. Thermochimica Acta. 2010;511(1–2):179–188. [Google Scholar]
- 53.Utracki L, Favis B. Polymer alloys and blends. Vol. 4. Marcel Dekker; New York: 1989. [Google Scholar]
- 54.Parameswaranpillai J, Thomas S, Grohens Y. Polymer Blends: State of the Art, New Challenges, and Opportunities. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces. 2014:1–6. [Google Scholar]
- 55.Zhang QS, Yan YH, Li SP, Feng T. Synthesis of a novel biodegradable and electroactive polyphosphazene for biomedical application. Biomed Mater. 2009;4(3):035008. doi: 10.1088/1748-6041/4/3/035008. [DOI] [PubMed] [Google Scholar]
- 56.Deng M. Novel biocompatible polymeric blends for bone regeneration: Material and matrix design and development. 2010 [Google Scholar]
- 57.Allcock HR. Polyphosphazenes: new polymers with inorganic backbone atoms. Science (New York, NY) 1976;193(4259):1214–1219. doi: 10.1126/science.193.4259.1214. [DOI] [PubMed] [Google Scholar]
- 58.Allcock HR, Ambrosio AM. Synthesis and characterization of pH-sensitive poly (organophosphazene) hydrogels. Biomaterials. 1996;17(23):2295–2302. doi: 10.1016/0142-9612(96)00073-7. [DOI] [PubMed] [Google Scholar]
- 59.Chaubal MV, Gupta AS, Lopina ST, Bruley DF. Polyphosphates and other phosphorus-containing polymers for drug delivery applications. Crit Rev Ther Drug Carrier Syst. 2003;20(4):295–315. doi: 10.1615/critrevtherdrugcarriersyst.v20.i4.20. [DOI] [PubMed] [Google Scholar]
- 60.Morozowich NL, Modzelewski T, Allcock HR. Synthesis of Phosphonated Polyphosphazenes via Two Synthetic Routes. Macromolecules. 2012;45(19):7684–7691. [Google Scholar]
- 61.Kumbar S, Laurencin C, Deng M. Natural and synthetic biomedical polymers. Newnes: 2014. [Google Scholar]
- 62.Allcock HR. Recent developments in polyphosphazene materials science. Current Opinion in Solid State and Materials Science. 2006;10(5–6):231–240. [Google Scholar]
- 63.Allcock HR. Expanding Options in Polyphosphazene Biomedical Research In: Polyphosphazenes for Biomedical Applications. John Wiley & Sons, Inc; 2008. pp. 15–43. [Google Scholar]
- 64.Henke H, Wilfert S, Iturmendi A, Brüggemann O, Teasdale I. Branched polyphosphazenes with controlled dimensions. Journal of Polymer Science Part A: Polymer Chemistry. 2013;51(20):4467–4473. doi: 10.1002/pola.26865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krogman NR, Weikel AL, Kristhart KA, Nukavarapu SP, Deng M, Nair LS, Laurencin CT, Allcock HR. The influence of side group modification in polyphosphazenes on hydrolysis and cell adhesion of blends with PLGA. Biomaterials. 2009;30(17):3035–3041. doi: 10.1016/j.biomaterials.2009.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tian Z, Zhang Y, Liu X, Chen C, Guiltinan MJ, Allcock HR. Biodegradable polyphosphazenes containing antibiotics: synthesis, characterization, and hydrolytic release behavior. Polymer Chemistry. 2013;4(6):1826–1835. [Google Scholar]
- 67.Andrianov AK, Svirkin YY, LeGolvan MP. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromolecules. 2004;5(5):1999–2006. doi: 10.1021/bm049745d. [DOI] [PubMed] [Google Scholar]
- 68.Cohen S, Bano MC, Cima LG, Allcock HR, Vacanti JP, Vacanti CA, Langer R. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clin Mater. 1993;13(1-4):3–10. doi: 10.1016/0267-6605(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 69.Deng M, Nair LS, Krogman NR, Allcock HR, Laurencin CT. Polyphosphazenes for Biomedical Applications. John Wiley & Sons, Inc; 2008. Biodegradable Polyphosphazene Blends for Biomedical Applications; pp. 139–154. [Google Scholar]
- 70.Stone DA, Allcock HR. A New Polymeric Intermediate for the Synthesis of Hybrid Inorganic-Organic Polymers. Macromolecules. 2006;39(15):4935–4937. [Google Scholar]
- 71.Ambrosio AMA, Allcock HR, Katti DS, Laurencin CT. Degradable polyphosphazene/poly(α-hydroxyester) blends: degradation studies. Biomaterials. 2002;23(7):1667–1672. doi: 10.1016/s0142-9612(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 72.Morozowich NL, Weikel AL, Nichol JL, Chen C, Nair LS, Laurencin CT, Allcock HR. Polyphosphazenes containing vitamin substituents: synthesis, characterization, and hydrolytic sensitivity. Macromolecules. 2011;44(6):1355–1364. [Google Scholar]
- 73.O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today. 2011;14(3):88–95. [Google Scholar]
- 74.Ambrosio AM, Allcock HR, Katti DS, Laurencin CT. Degradable polyphosphazene/poly(alpha-hydroxyester) blends: degradation studies. Biomaterials. 2002;23(7):1667–1672. doi: 10.1016/s0142-9612(01)00293-9. [DOI] [PubMed] [Google Scholar]
- 75.Andrianov AK. Water-Soluble Polyphosphazenes for Biomedical Applications. Journal of Inorganic and Organometallic Polymers and Materials. 2006;16(4):397–406. [Google Scholar]
- 76.Lakshmi S, Katti DS, Laurencin CT. Biodegradable polyphosphazenes for drug delivery applications. Advanced Drug Delivery Reviews. 2003;55(4):467–482. doi: 10.1016/s0169-409x(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 77.Nukavarapu SP, Kumbar SG, Allcock HR, Laurencin CT. Polyphosphazenes for Biomedical Applications. John Wiley & Sons, Inc; 2008. Biodegradable Polyphosphazene Scaffolds for Tissue Engineering; pp. 117–138. [Google Scholar]
- 78.Allcock H, Fuller T, Mack D, Matsumura K, Smeltz KM. Synthesis of poly [(amino acid alkyl ester) phosphazenes] Macromolecules. 1977;10(4):824–830. [Google Scholar]
- 79.Allcock HR, Pucher SR. Polyphosphazenes with glucosyl and methylamino, trifluoroethoxy, phenoxy, or (methoxyethoxy) ethoxy side groups. Macromolecules. 1991;24(1):23–34. [Google Scholar]
- 80.Cohen S, Baño MC, Cima LG, Allcock HR, Vacanti JP, Vacanti CA, Langer R. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clinical materials. 1993;13(1):3–10. doi: 10.1016/0267-6605(93)90082-i. [DOI] [PubMed] [Google Scholar]
- 81.Laurencin CT, El-Amin SF, Ibim SE, Willoughby DA, Attawia M, Allcock HR, Ambrosio AA. A highly porous 3-dimensional polyphosphazene polymer matrix for skeletal tissue regeneration. Journal of biomedical materials research. 1996;30(2):133–138. doi: 10.1002/(SICI)1097-4636(199602)30:2<133::AID-JBM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 82.Modzelewski T, Wonderling NM, Allcock HR. Polyphosphazene Elastomers Containing Interdigitated Oligo-p-phenyleneoxy Side Groups: Synthesis, Mechanical Properties, and X-ray Scattering Studies. Macromolecules. 2015;48(14):4882–4890. [Google Scholar]
- 83.Sethuraman S, Nair LS, El-Amin S, Nguyen MT, Singh A, Krogman N, Greish YE, Allcock HR, Brown PW, Laurencin CT. Mechanical properties and osteocompatibility of novel biodegradable alanine based polyphosphazenes: Side group effects. Acta Biomater. 2010;6(6):1931–1937. doi: 10.1016/j.actbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weikel AL, Krogman NR, Nguyen NQ, Nair LS, Laurencin CT, Allcock HR. Polyphosphazenes That Contain Dipeptide Side Groups: Synthesis, Characterization, and Sensitivity to Hydrolysis. Macromolecules. 2009;42(3):636–639. [Google Scholar]
- 85.Weikel AL, Owens SG, Morozowich NL, Deng M, Nair LS, Laurencin CT, Allcock HR. Miscibility of choline-substituted polyphosphazenes with PLGA and osteoblast activity on resulting blends. Biomaterials. 2010;31(33):8507–8515. doi: 10.1016/j.biomaterials.2010.07.094. [DOI] [PubMed] [Google Scholar]
- 86.Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, Laurencin CT. Biomimetic Structures: Biological Implications of Dipeptide-Substituted Polyphosphazene–Polyester Blend Nanofiber Matrices for Load-Bearing Bone Regeneration. Advanced Functional Materials. 2011;21(14):2641–2651. [Google Scholar]
- 87.Adibi S. Glycyl-dipeptides: new substrates for protein nutrition. The Journal of laboratory and clinical medicine. 1989;113(6):665–673. [PubMed] [Google Scholar]
- 88.El-Amin SF, Kwon MS, Starnes T, Allcock HR, Laurencin CT. The biocompatibility of biodegradable glycine containing polyphosphazenes: a comparative study in bone. Journal of Inorganic and Organometallic Polymers and Materials. 2006;16(4):387–396. [Google Scholar]
- 89.Kumbar SG, Bhattacharyya S, Nukavarapu SP, Khan YM, Nair LS, Laurencin CT. In Vitro and In Vivo Characterization of Biodegradable Poly(organophosphazenes) for Biomedical Applications. Journal of Inorganic and Organometallic Polymers and Materials. 2006;16(4):365–385. [Google Scholar]
- 90.Allcock HR, Pucher SR, Scopelianos AG. Poly [(amino acid ester) phosphazenes]: synthesis, crystallinity, and hydrolytic sensitivity in solution and the solid state. Macromolecules. 1994;27(5):1071–1075. [Google Scholar]
- 91.Krogman NR, Singh A, Nair LS, Laurencin CT, Allcock HR. Miscibility of bioerodible polyphosphazene/poly (lactide-co-glycolide) blends. Biomacromolecules. 2007;8(4):1306–1312. doi: 10.1021/bm061064q. [DOI] [PubMed] [Google Scholar]
- 92.Laurencin C, Morris C, Pieere-Jaques H, Schwartz E, Zou L. The development of bone bioerodible polymer composites for skeletal tissue regeneration: studies of initial cell adhesion and spread. Tran Orthop Res Soc. 1990;36:383. [Google Scholar]
- 93.Ibim SE, Ambrosio AM, Kwon MS, El-Amin SF, Allcock HR, Laurencin CT. Novel polyphosphazene/poly(lactide-co-glycolide) blends: miscibility and degradation studies. Biomaterials. 1997;18(23):1565–1569. doi: 10.1016/s0142-9612(97)80009-9. [DOI] [PubMed] [Google Scholar]
- 94.Nair LS, Bender JD, Singh A, Sethuraman S, Greish YE, Brown PW, Allcock HR, Laurencin CT. MRS Proceedings. Cambridge Univ Press; 2004. Biodegradable poly [bis (ethyl alanato) phosphazene]-poly (lactide-co-glycolide) blends: miscibility and osteocompatibility evaluations; p. 7. Y9. [Google Scholar]
- 95.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Weikel AL, Krogman NR, Allcock HR, Laurencin CT. In situ Porous Structures: A Unique Polymer Erosion Mechanism in Biodegradable Dipeptide-Based Polyphosphazene and Polyester Blends Producing Matrices for Regenerative Engineering. Advanced Functional Materials. 2010;20(17):2794–2806. doi: 10.1002/adfm.201090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borden M, Attawia M, Khan Y, Laurencin CT. Tissue engineered microsphere-based matrices for bone repair∷ design and evaluation. Biomaterials. 2002;23(2):551–559. doi: 10.1016/s0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 97.Borden M, El-Amin SF, Attawia M, Laurencin CT. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials. 2003;24(4):597–609. doi: 10.1016/s0142-9612(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 98.Thavornyutikarn B, Chantarapanich N, Sitthiseripratip K, Thouas GA, Chen Q. Bone tissue engineering scaffolding: computer-aided scaffolding techniques. Progress in Biomaterials. 2014;3(2):61–102. doi: 10.1007/s40204-014-0026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shan D, Huang Z, Zhao Y, Cai Q, Yang X. Improving the miscibility of biodegradable polyester/polyphosphazene blends using cross-linkable polyphosphazene. Biomedical Materials. 2014;9(6):061001. doi: 10.1088/1748-6041/9/6/061001. [DOI] [PubMed] [Google Scholar]
- 100.Qiu LY. In vitro and in vivo degradation study on novel blends composed of polyphosphazene and polyester or polyanhydride. Polymer international. 2002;51(6):481–487. [Google Scholar]
- 101.Qiu LY, Zhu KJ. Novel blends of poly [bis (glycine ethyl ester) phosphazene] and polyesters or polyanhydrides: compatibility and degradation characteristics in vitro. Polymer international. 2000;49(11):1283–1288. [Google Scholar]
- 102.Ambrosio AM, Sahota JS, Runge C, Kurtz SM, Lakshmi S, Allcock HR, Laurencin CT. Novel polyphosphazene-hydroxyapatite composites as biomaterials. IEEE Eng Med Biol Mag. 2003;22(5):18–26. doi: 10.1109/memb.2003.1256268. [DOI] [PubMed] [Google Scholar]
- 103.Peach MS, Kumbar SG, James R, Toti US, Balasubramaniam D, Deng M, Ulery B, Mazzocca AD, McCarthy MB, Morozowich NL, et al. Design and optimization of polyphosphazene functionalized fiber matrices for soft tissue regeneration. J Biomed Nanotechnol. 2012;8(1):107–124. doi: 10.1166/jbn.2012.1368. [DOI] [PubMed] [Google Scholar]
- 104.Nichol JL, Morozowich NL, Allcock HR. Biodegradable alanine and phenylalanine alkyl ester polyphosphazenes as potential ligament and tendon tissue scaffolds. Polymer Chemistry. 2013;4(3):600–606. [Google Scholar]
- 105.Langone F, Lora S, Veronese FM, Caliceti P, Parnigotto PP, Valenti F, Palma G. Peripheral nerve repair using a poly (organo) phosphazene tubular prosthesis. Biomaterials. 1995;16(5):347–353. doi: 10.1016/0142-9612(95)93851-4. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Q, Yan Y, Li S, Feng T. The synthesis and characterization of a novel biodegradable and electroactive polyphosphazene for nerve regeneration. Materials Science and Engineering: C. 2010;30(1):160–166. [Google Scholar]
- 107.Modzelewski T, Wilts E, Allcock HR. Elastomeric Polyphosphazenes with Phenoxy– Cyclotriphosphazene Side Groups. Macromolecules. 2015;48(20):7543–7549. [Google Scholar]
- 108.Xu J, Zhu X, Qiu L. Polyphosphazene vesicles for co-delivery of doxorubicin and chloroquine with enhanced anticancer efficacy by drug resistance reversal. Int J Pharm. 2016;498(1-2):70–81. doi: 10.1016/j.ijpharm.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Lakshmi S, Katti D, Laurencin C. Biodegradable polyphosphazenes for drug delivery applications. Advanced drug delivery reviews. 2003;55(4):467–482. doi: 10.1016/s0169-409x(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 110.Veronese FM, Marsilio F, Lora S, Caliceti P, Passi P, Orsolini P. Polyphosphazene membranes and microspheres in periodontal diseases and implant surgery. Biomaterials. 1999;20(1):91–98. doi: 10.1016/s0142-9612(97)00104-x. [DOI] [PubMed] [Google Scholar]
- 111.Ibim SM, El-Amin SF, Goad ME, Ambrosio AM, Allcock HR, Laurencin CT. In vitro release of colchicine using poly (phosphazenes): The development of delivery systems for musculoskeletal use. Pharmaceutical development and technology. 1998;3(1):55–62. doi: 10.3109/10837459809028479. [DOI] [PubMed] [Google Scholar]
- 112.Schacht E, Vandorpe J, Lemmouchi Y, Dejardin S, Seymour L. Degradable polyphosphazenes for biomedical applications. Frontiers in Biomedical Polymer Applications of Polymers, Technomic, Lancaster PA. 1998:27–42. [Google Scholar]